Abstract
Integrity of the immune system is particularly dependent on the availability of zinc. Recent data suggest that zinc is involved in the development of sepsis, a life-threatening systemic inflammation with high death rates, but with limited therapeutic options. Altered cell zinc transport mechanisms could contribute to the inflammatory effects of sepsis. Zip14, a zinc importer induced by proinflammatory stimuli, could influence zinc metabolism during sepsis and serve as a target for therapy. Using cecal ligation-and-puncture (CLP) to model polymicrobial sepsis, we narrowed the function of ZIP14 to regulation of zinc homeostasis in hepatocytes, while hepatic leukocytes were mostly responsible for driving inflammation, as shown by higher expression of IL-1β, TNFα, S100A8, and matrix metalloproteinase-8. Using Zip14 knockout (KO) mice as a novel approach, we found that ablation of Zip14 produced a delay in development of leukocytosis, prevented zinc accumulation in the liver, altered the kinetics of hypozincemia, and drastically increased serum IL-6, TNFα, and IL-10 concentrations following CLP. Hence, this model revealed that the zinc transporter ZIP14 is a component of the pathway for zinc redistribution that contributes to zinc dyshomeostasis during polymicrobial sepsis. In contrast, using the identical CLP model, we found that supplemental dietary zinc reduced the severity of sepsis, as shown by amelioration of cytokines, calprotectins, and blood bacterial loads. We conclude that the zinc transporter ZIP14 influences aspects of the pathophysiology of nonlethal polymicrobial murine sepsis induced by CLP through zinc delivery. The results are promising for the use of zinc and its transporters as targets for future sepsis therapy.
Keywords: zinc transport, sepsis, zinc metabolism, cytokines
sepsis is one of the leading inflammatory diseases, causing millions of deaths annually and producing immense costs for health systems worldwide (22, 28). Despite intense research, the mechanisms underlying sepsis are not completely understood, and treatment is limited to symptomatic approaches, with limited success (44). An association between altered zinc homeostasis and the severity of the inflammatory reactions has been suggested by several studies using various disease models and systems. These include studies of LPS-induced endotoxemia, acute stress, and sepsis in pigs (8, 27), fowl (26, 38), rodents (14, 15, 40), and human subjects (17).
The acute response to endotoxin in vivo includes hypozincemia and altered kinetics of zinc distribution to specific tissues (8). Such changes in body zinc redistribution are believed to be controlled through selective regulation of zinc transport pathways. Two zinc transporter (ZnT) families comprising 24 members (the ZnT family with 10 members and the Zip family with 14 members), exhibiting differential modes of regulation and cell-type expression, alter zinc metabolism to meet dietary and physiological needs (29). On the basis of our previous experiments on the responsiveness of zinc homeostasis to cytokine/hormonal stimuli, we hypothesized that microbial attack would drastically alter expression of specific zinc/Zip transporters in mice. Begum et al. (7) showed that LPS induced a novel gene in monocytes that was subsequently identified as Zip8. Experiments focused on the liver, and use of a quantitative PCR (qPCR) screen of individual transporter gene transcripts showed that Zip14 was the most highly induced transporter in the liver of mice treated with LPS to induce endotoxemia (31). Subsequently, Zip14 was documented to be expressed in multiple tissues of mice under a variety of physiological conditions, including endotoxemia (2, 3, 30). The liver has been a prime target of these investigations. Liver dysfunction is a major factor that contributes to the severity of sepsis (6, 35). This often fatal condition is accompanied by endotoxemia and drastic changes in cytokine production and secretion (1, 13, 23, 47).
On the basis of the responsiveness of Zip14 expression to endotoxin, we hypothesized that this zinc transporter would be induced in the liver during sepsis and, hence, would contribute to the altered zinc homeostasis and functional outcomes observed under such conditions. Using Zip14 knockout (KO) mice as a novel approach, we report that the zinc transporter ZIP14 is a component of the pathway for zinc that contributes to zinc dyshomeostasis during polymicrobial sepsis. Our experiments demonstrate the involvement of ZIP14 in the response to sepsis and the beneficial anti-inflammatory effects of supplemental dietary zinc during sepsis.
MATERIALS AND METHODS
Animals and diets.
Male and female mice of the C57BL/6 strain were used at 8–20 wk of age. Genotypes were Zip14+/+ [wild-type (WT)] and Zip14−/− (KO). Derivation and characterization of mice of the KO genotype are described elsewhere (2, 3). Animals of only one sex were used to generate a specific data set. The mice were fed a commercial rodent diet (Harlan Teklad 7912), except for one series of experiments in which a normal-zinc [zinc-adequate (ZnA), 30 mg Zn/kg] or a zinc-supplemented [high-zinc (ZnH), 180 mg Zn/kg] diet was fed (3). Mice were euthanized by exsanguination via cardiac puncture up to 72 h after cecal ligation-and-puncture (CLP) or sham operation. Isoflurane anesthetic was used for all procedures. Buprenorphine (0.1 mg/kg sc) was used for analgesia as needed. Protocols were approved by the University of Florida Institutional Animal Care and Use Committee.
CLP.
Polymicrobial sepsis was induced by CLP according to established methodology (11). Briefly, a laparotomy was performed under anesthesia, and the cecum was exteriorized and ligated 1 cm from the distal end. The cecum was punctured once with a 27-gauge syringe needle and then returned to the abdominal cavity, and the surgical site was closed with staples. For sham operation, a laparotomy was performed and the cecum was exteriorized, but without ligation or puncture. No mortality resulted from puncture with the 27-gauge needle.
Biochemical analyses.
TNFα and IL-10 levels were measured by enzyme-linked immunosorbent assay (eBioscience, San Diego, CA). IL-6, matrix metalloproteinase (MMP)-9, S100A8, and S100A9 levels were quantified using a customized magnetic multiplexing assay (R & D Systems, Minneapolis, MN). Plasma alanine aminotransferase (ALT) was measured by a colorimetric end-point method as described previously (3). Serum was obtained from blood by centrifugation. For white blood cell (WBC) generation, coagulation was inhibited by addition of EDTA to the blood. Blood erythrocytes were lysed using lysis buffer (0.15 M NH4Cl and 10 mM sodium bicarbonate, pH 7.4). WBC were isolated from the buffy coat and washed twice with PBS; WBC viability was >95%. Zinc concentrations in serum and tissues were measured by flame atomic absorption spectrophotometry and normalized to tissue weight. Tissue was digested in HNO3 prior to assay by flame atomic absorption spectrophotometry. Nonheme iron (NHI) was measured colorimetrically (37).
Isolation of leukocytes and parenchymal cells from liver.
The mice were anesthetized, and the abdomen was wiped with 70% ethanol. The outer skin of the peritoneum was cut to expose the peritoneal cavity. After exsanguination by cardiac puncture, the gallbladder was removed, and the inferior vena cava was severed. The liver was perfused by injection of 10 ml of ice-cold PBS into the hepatic portal vein using a 27-gauge needle. Thereafter, the liver and spleen were removed and placed into ice-cold FACS buffer (PBS and 5% BSA). Single-cell suspensions from the liver were generated by forcing the tissue through a 70-μm cell strainer using the plunger of a 5-ml syringe. An aliquot of this cell suspension was saved for analysis of bacterial load, and the remaining cells were washed twice with ice-cold FACS buffer. The cell pellet was resuspended in 20 ml of isotonic Percoll (33.75%) at room temperature and centrifuged at 700 g for 12 min. Hepatocytes (HP) were collected as a disk-like sheet floating on top of the Percoll gradient, washed in wash buffer (Williams' 43 Medium E and 10 mM HEPES, pH 7.3), and used to generate RNA for expression analysis. The leukocyte-containing pellet was suspended in 4 ml of Tris-buffered NH4Cl (TAC) buffer (17 mM Tris and 140 mM NH4Cl) for 10 min, underlayered with 1 ml of FCS-10 mM EDTA, and centrifuged again. The cells were washed again and then suspended in FACS-EDTA buffer (FACS buffer and 5 mM EDTA) for further analysis; these cells were designated the hepatic leukocyte (HL) fraction. The spleen was passed through a cell strainer using a plunger as described above. An aliquot of the homogenate was used to analyze bacterial load. These methods were adapted from procedures designed by Wang et al. (43). To measure viability, cells were stained with propidium iodide (Sigma-Aldrich) and analyzed using flow cytometry as described elsewhere (19). Viability averaged 90% for HP and HL fractions.
Immunoblotting.
Polyclonal rabbit antibody against ZIP14 was raised in-house as described previously (31). The rabbit IgG fractions were affinity-purified. Liver tissue samples were flash-frozen in liquid nitrogen upon collection. Frozen liver tissue was homogenized in lysis buffer (20 mM Tris·HCl, 1% Triton X-100, 10% glycerol, 137 nM NaCl, and 2 mM EDTA) containing protease inhibitor cocktail (Santa Cruz Biotechnology, Santa Cruz, Santa Cruz, CA) and sodium vanadate as a phosphatase inhibitor. Proteins were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Transfer to a nitrocellulose membrane was confirmed by Ponceau red staining. Immunoreactivity was visualized by enhanced chemiluminescence.
RNA isolation and qPCR.
Liver tissue was collected in RNAlater (Qiagen, Austin, TX) and homogenized in TRIzol reagent (Ambion) using a Bullet Blender (Next Advance, Averill, NY). Cells isolated from liver and blood were placed directly in TRIzol reagent, and RNA was isolated according to the manufacturer's protocol. One microgram of RNA was reverse-transcribed using a high-capacity cDNA reverse transcription kit (Life Technologies, Carlsbad, CA) and diluted 1:40 for PCR. For analysis of Zip4, Zip6, Zip8, Zip10, proliferating cell nuclear antigen (Pcna), hepcidin, and ZnT1 mRNA, primers and probes were used together with TaqMan reagents (Life Technologies). All other genes were analyzed using primers together with SYBR Green reagent (Life Technologies). Relative quantitation was calculated using a standard curve and normalized to TATA-binding protein (TBP) mRNA (2, 3).
Measurement of free intracellular zinc with FluoZin-3 acetoxymethyl ester.
Free zinc was measured as described previously for calcium (19) using 1 μM FluoZin-3 acetoxymethyl ester (Invitrogen). Zinc-dependent fluorescence was analyzed using a flow cytometer (Accuri C6, BD Bioscience, Franklin Lakes, NJ) and Accuri C6 software. The concentration of intracellular labile zinc ([Zn]) was calculated from the mean fluorescence using the formula [Zn] = KD × [(F − Fmin)/(Fmax − F)], where KD for the zinc-FluoZin-3 acetoxymethyl ester complex was 8.9 nM and maximal and minimal fluorescence (Fmax and Fmin) were determined by addition of zinc (100 μM) and pyrithione (50 μM) or N,N,N′,N′-tetrakis(2-pyridylmethyl)ethylenediamine (50 μM), respectively.
Measurement of bacterial load.
Fifty microliters of each sample (whole blood, liver homogenate, and spleen homogenate) were spread onto predried tryptic soy agar plates containing 10% sheep blood. Plates were incubated at 37°C overnight, and the number of colony-forming units was counted.
Statistics.
Statistical significance of experimental results was analyzed using GraphPad Prism version 5 (GraphPad Software, La Jolla, CA). For single comparisons, P < 0.05, P < 0.01, and P < 0.001 are used for data significantly different from the sham-operated control as determined by Student's t-test. For multiple comparisons, significant differences at P < 0.05 were determined by ANOVA/Tukey's test or ANOVA/Bonferroni's test to compare Zip14 KO mice with WT mice and to test the effect of high and low dietary zinc.
RESULTS
Acute sepsis alters zinc homeostasis and coincides with activation of WBC.
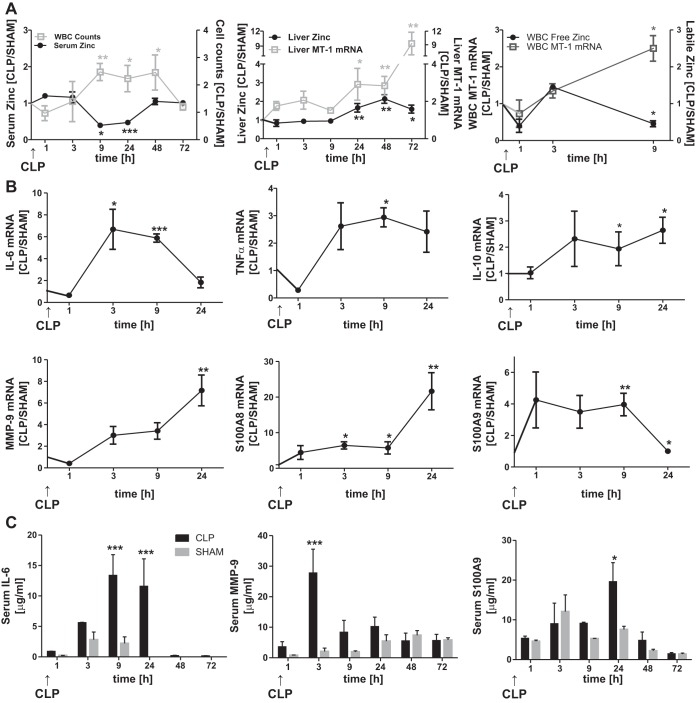
CLP with a 27-gauge needle produced a septic response that included serum hypozincemia and zinc accumulation in the liver compared with sham-operated mice (Fig. 1A). WBC counts during CLP were inversely proportional to the reduction in serum zinc concentration, suggesting a relationship between these processes (Fig. 1A). No significant changes in cell counts in the spleen were observed (data not shown). Induction of liver metallothionein-1 (Mt-1) mRNA was evident in mice after CLP, with increases to ninefold compared with sham-operated animals after 72 h (Fig. 1A). Mt-1 upregulation is indicative of increased cellular zinc accumulation. Increased zinc levels in other organs, including the spleen (data not shown), were detected but were negligible in magnitude compared with levels in the liver. Interestingly, a burst of Mt-1 mRNA abundance in the WBC population was observed 3–9 h after CLP (Fig. 1A). This could result from an influx of intracellular zinc. Labile zinc levels as assessed using FluoZin-3 peaked 3 h after CLP and then decreased significantly compared with levels in sham-operated mice.
Fig. 1.
Cecal ligation-and-puncture (CLP)-induced sepsis alters zinc distribution and gene expression patterns in liver tissue and sepsis markers in serum. Mice were killed 1, 3, 9, 24, 48, and 72 h after sham or CLP surgery. A: time course of zinc concentrations in whole liver and serum and white blood cell (WBC) counts, liver and WBC metallothionein 1 (Mt-1) mRNA expression, and labile intracellular zinc levels. CLP data were normalized to data from sham-operated (SHAM) mice. Values are means ± SE (n = 4–5 mice per group). B: Il-6, Tnfα, Il-10, matrix metalloproteinase-9 (Mmp-9), S100A8, and S100A9 mRNA expression in whole liver. Data were normalized to TATA-binding protein (TBP) mRNA. Values are means ± SE (n = 3–12 mice per group). C: IL-6, MMP-9, and S100A8 concentrations in serum were analyzed using a customized Luminex assay. Values are means ± SE (n = 3–7). *P < 0.05, **P < 0.01, ***P < 0.001 vs. SHAM (by ANOVA/Bonferroni's test).
To more deeply explore the events that are congruent with the transient zinc redistribution following CLP, we monitored the expression of inflammatory mediators and antimicrobial peptides by the liver (Fig. 1B) within the first 24 h following CLP. Liver Il-6 and TNFα mRNA and transcripts for both components of the calprotectin heterodimer (S100A8 and S100A9) in the liver of these mice that underwent CLP surgery were markedly increased (Fig. 1B). In contrast, WBC showed modest changes in Il-6, Tnfα, S100A8, and S100A9 mRNA expression (data not shown). The liver showed significant increases in Il-10 mRNA and Mmp-9 transcripts. These responses are considered anti-inflammatory and antimicrobial, respectively.
Results obtained for mRNA expression were comparable to significant increases in serum IL-6, MMP-9, and S100A9 proteins following CLP (Fig. 1C). Levels of S100A8, TNFα, and IL-10 were also elevated in the serum of CLP mice but did not reach statistical significance (data not shown). Protein levels of all mediators peaked between 3 and 24 h after CLP and returned to basal levels after 48 h. These results underline the differential contribution of the liver and WBC to the inflammatory response and levels of serum mediators during sepsis.
The response of parameters (tissue and serum zinc and Mt-1 mRNA expression) examined following CLP with a single puncture using a 25-gauge needle was greater (data not shown). Some mortality was observed when the 25-gauge needle was used. Hence, a single puncture with the 27-gauge needle, where no mortality was observed, was used in all the experiments reported here.
Zip14 expression in the liver is strongly elevated throughout sepsis.
CLP induced a transient wave of changes in ZnT mRNA in the liver. Of all 14 Zips and 10 zinc transporters tested, maximal increases in Zip4, Zip6, Zip10, and Zip14 mRNAs were detected at 1, 9, 24, and 72 h post-CLP, respectively (Fig. 2A). Changes in liver Zip8 mRNA were minimal over the entire 72-h period post-CLP. The increases in Zip4, Zip6, and Zip10 expression were transient and returned to near-normal levels by 72 h. In contrast, Zip14 mRNA increased by 9 h after CLP and was the only transporter mRNA that was constantly elevated in CLP animals compared with sham-operated animals throughout the 72-h period post-CLP (Fig. 2A). Western blots confirmed upregulation for ZIP14 protein (Fig. 2B). Expression of the other transporters only slightly changed at the protein level (data not shown). Therefore, these data suggest that ZIP14 is upregulated to sustain hepatic zinc transport during sepsis.
Fig. 2.
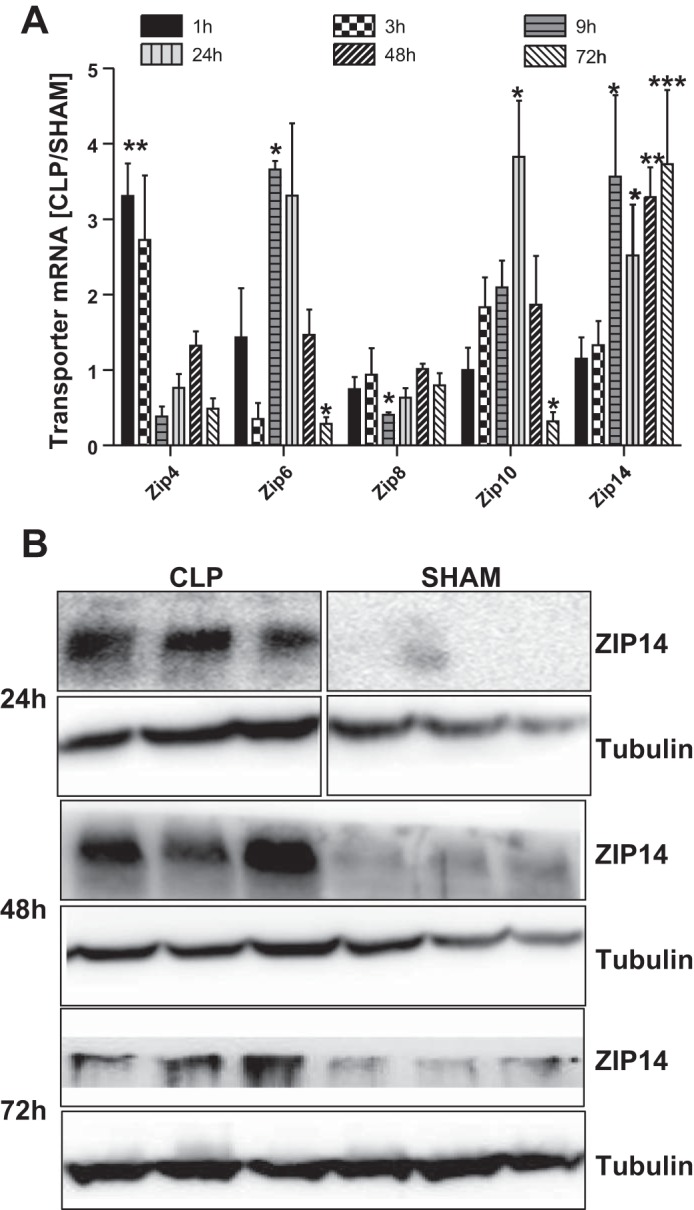
CLP-induced elevation of liver zinc is correlated with elevated Zip14 expression. Mice were killed 1, 3, 9, 24, 48, and 72 h after sham or CLP operation. A: whole liver Zip4, Zip6, Zip8, Zip10, and Zip14 mRNA expression. Data were normalized to TBP mRNA. Values are means ± SE (n = 3–12 mice per group). *P < 0.05, **P < 0.01, ***P < 0.001 (by ANOVA/Bonferroni's test). B: Western analysis of ZIP14 expression in liver of CLP and sham-operated mice 24, 48, and 72 h after surgery. Boxes around CLP and sham blots at 24 h indicate that noncontiguous lanes were derived from the same gel. Blots are representative of results from multiple experiments.
Differences in zinc homeostasis, cytokines, and host defensive mediators during sepsis involve HP and HL.
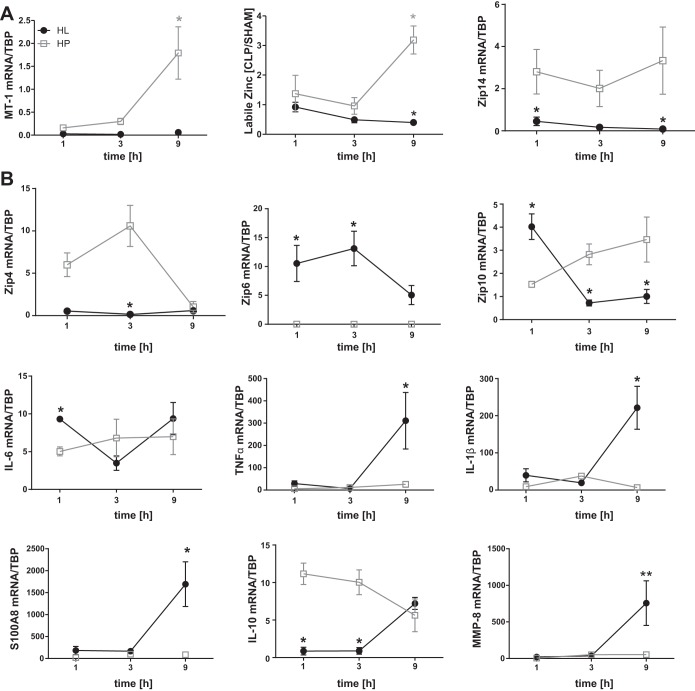
The observation of a sequential induction of zinc transporter and cytokine transcripts in total liver RNA extracts raises the question about the contributions of individual cell types. To answer this question, HP (parenchymal cells) and HL were separated and analyzed for relative expression of transcripts for markers of zinc homeostasis, cytokines, and host defensive factors. Purity of the isolated cell populations was established through expression of the lineage markers hepcidin (HP) and F4/80 (HL) as assessed using qPCR. Viability of ∼90% was confirmed using propidium iodine staining (data not shown). Higher expression of Mt-1 and Zip14 mRNA and a higher intracellular labile zinc content were found in HP than HL (Fig. 3A). This finding, along with the increase in total liver zinc concentrations after CLP (Fig. 1A), demonstrates the significance of HP in zinc redistribution to the liver during sepsis. While Zip4 mRNA appeared to be primarily expressed by HP, Zip6 mRNA expression was restricted to HL. Both cell types expressed Zip10 following CLP (Fig. 3B). Transcripts for the inflammatory markers Tnfα, Il-1β, and S100A8 were elevated in HL by 9 h post-CLP. Il-10 mRNA expression in HP was initially high, but by 9 h, levels were comparable to those in HL. Il-6 was expressed by both cell types (Fig. 3B). Mmp-8 mRNA expression was examined and found to be higher in HL than HP after CLP. These data suggest that, in this in vivo model of polymicrobial sepsis, HP functions to regulate zinc homeostasis and also contributes to inflammation via IL-6 and IL-10 production.
Fig. 3.
Functions influencing hepatic zinc metabolism and the hepatic immune response are cell-specific. Mice were killed 1, 3, and 9 h after CLP, and hepatocytes (HP) and hepatic leukocytes (HL) were separated by Percoll gradient centrifugation. A: Mt-1 and Zip14 mRNA expression. Labile intracellular zinc concentration was assessed via flow cytometry using FluoZin-3. Data were normalized to TBP mRNA. B: Zip4, Zip6, Zip10, Il-6, Tnfα, Il-1β, S100A8, Il-10, and Mmp-8 mRNA expression. Data were normalized to TBP mRNA. Values are means ± SE (n = 3–7 mice per group). *P < 0.05, **P < 0.01 vs. HP for each time point (by ANOVA/Bonferroni's test).
Zinc homeostasis in response to sepsis is significantly changed in Zip14 KO mice.
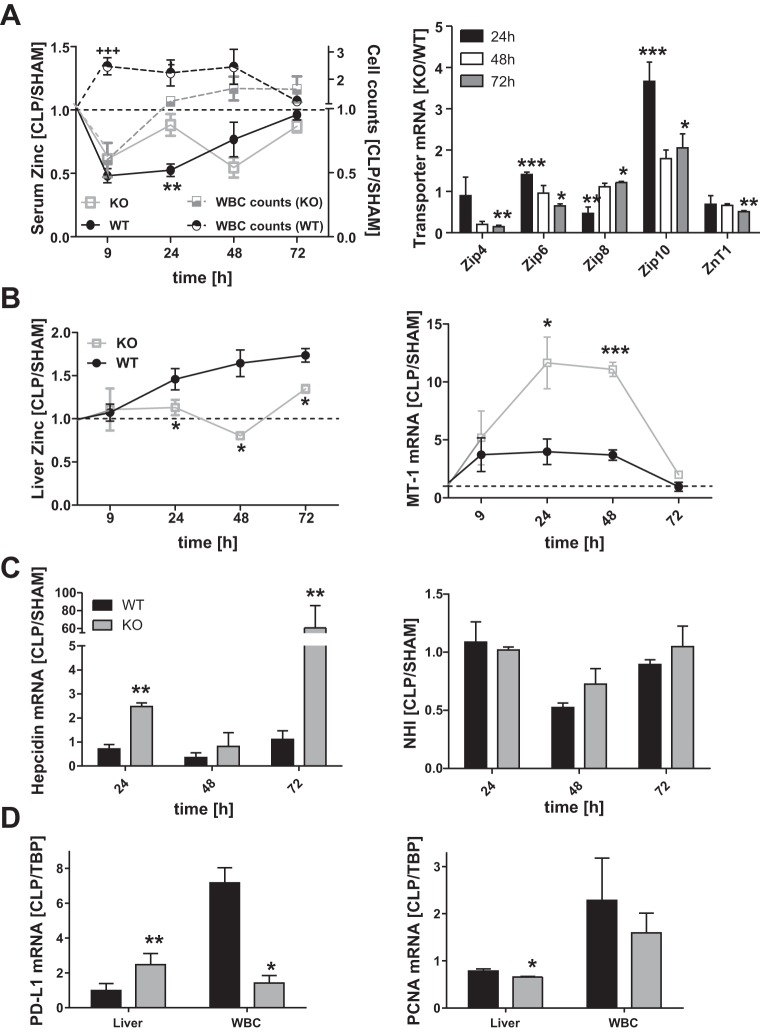
The availability of the Zip14 KO mouse strain allowed us to examine the role of ZIP14-mediated zinc redistribution in the response to CLP-induced sepsis. The response of serum zinc concentration in the KO mice following CLP was different from that in the WT mice. Specifically, serum zinc concentration was significantly depressed (P < 0.01) only at 24 h after CLP in the KO mice (Fig. 4A). The delayed response in mice when ZIP14 is not produced suggests that the initial hypozincemia of polymicrobial sepsis is the result of a compensatory mechanism involving other zinc transporters (Fig. 4A). In support of this hypothesis, levels of Zip4, Zip6, and Zip10 mRNA expression were significantly elevated in the KO mice compared with the WT mice at 24 h post-CLP (Fig. 4A). The increase in WBC counts in the WT mice was inversely related to the extent of hypozincemia. The WBC counts in blood from the KO mice did not closely follow the serum zinc levels, however (Fig. 4A). This suggests that hypozincemia influences the level of circulating WBC. In contrast, ablation of Zip14 prevented the accumulation of total liver zinc in the WT mice after CLP (Fig. 4B). The Zip14 ablation caused an induction of Mt-1 mRNA following CLP, suggesting that either the KO mice retained a pool of zinc that activated metal-responsive transcription factor 1 (MTF-1) or the induction is mediated by cytokines, e.g., the increase in circulating IL-6. The necessity of ZIP14 for hepatic zinc accumulation (Fig. 4A) suggests that ZIP14 may function in intracellular processing/utilization of zinc, as well as uptake at the cell surface. Surprisingly, hepcidin mRNA expression was significantly stronger in KO animals after CLP than in WT animals (Fig. 4C). The high hepcidin mRNA levels in the KO mice, perhaps a reflection of elevated IL-6, are not consistent with the similar levels of NHI in liver of both genotypes (Fig. 4D). Apoptosis of liver cells, based on the marker programmed death-ligand 1 (Pd-l1) mRNA, was significantly increased in the septic KO animals and was decreased in WBC (Fig. 4C). On the other hand, liver cell proliferation, based on Pcna mRNA, was significantly decreased in the liver of KO animals. It was lower, but not significantly, in WBC as well.
Fig. 4.
Zinc transfer to liver in Zip14 knockout (KO) mice and altered zinc homeostasis. Zip14 KO and wild-type (WT) mice were killed 9, 24, 48, and 72 h after CLP or sham operation. A: serum zinc concentrations and WBC counts and Zip4, Zip6, Zip8, Zip10, and zinc transporter 1 (ZnT1) mRNA expression in liver. Data were normalized to TBP mRNA and sham-operated animals, and the quotient for KO/WT was calculated. B: liver zinc and Mt-1 mRNA expression. C: liver hepcidin mRNA expression and non-heme iron (NHI) concentration. D: programmed death-ligand 1 (Pd-l1) and proliferating cell nuclear antigen (Pcna) mRNA expression in liver and WBC. Values are means ± SE (n = 3 mice per group). *P < 0.05, **P < 0.01, ***P < 0.001 vs. KO (by ANOVA/Bonferroni's test).
Knockout of Zip14 results in altered cytokine production, but not sepsis progression.
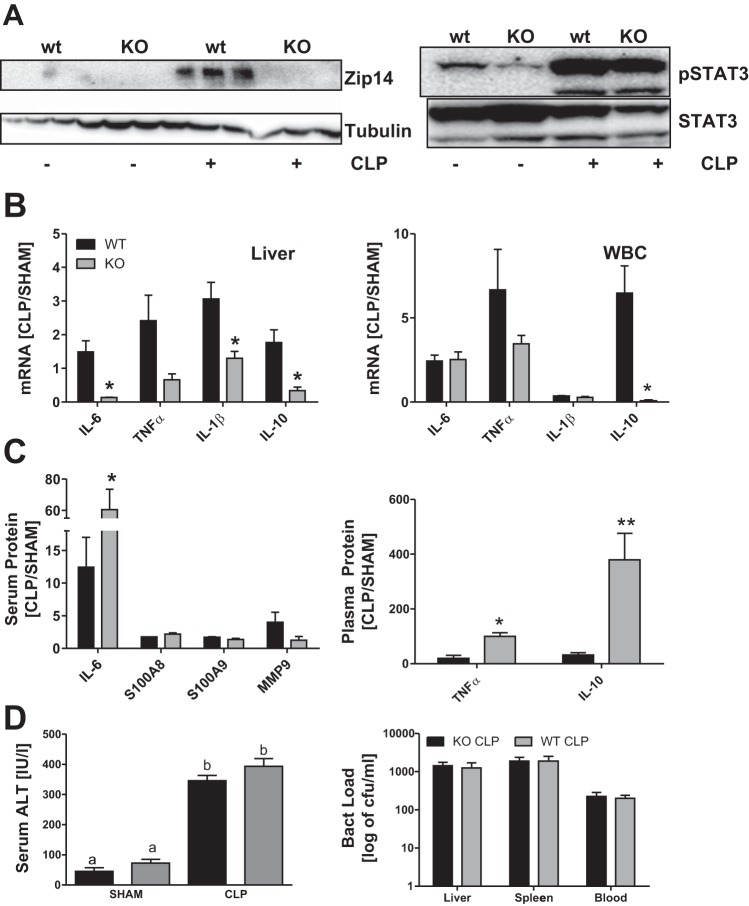
Western analysis proved that ZIP14 is not produced in the liver of the KO mice in response to CLP. In contrast, cell activation, indicated by STAT3 phosphorylation, was comparably enhanced in both genotypes (Fig. 5A). Analysis of cytokine mRNA expression in the liver revealed a decrease in Tnfα and significant decreases in Il-6, Il-1β, and of Il-10 in the KO mice (Fig. 5B). In contrast, the proinflammatory response by WBC from the KO mice remained unchanged, except Il-10 mRNA expression was significantly decreased. Most surprisingly, plasma levels of IL-6, IL-10, and TNFα were significantly higher in septic KO mice than in the WT animals, despite lower mRNA expression (Fig. 5C). Increased inflammatory markers in serum suggest a disadvantage during sepsis in the KO mice. No changes were detected for serum ALT or bacterial load in liver, spleen, or blood, however (Fig. 5D). In addition, none of the KO animals died or showed signs of more severe disease than the WT mice. Hence, the Zip14 null mutation did not appear to enhance the progression of mild sepsis over the time course of our study.
Fig. 5.
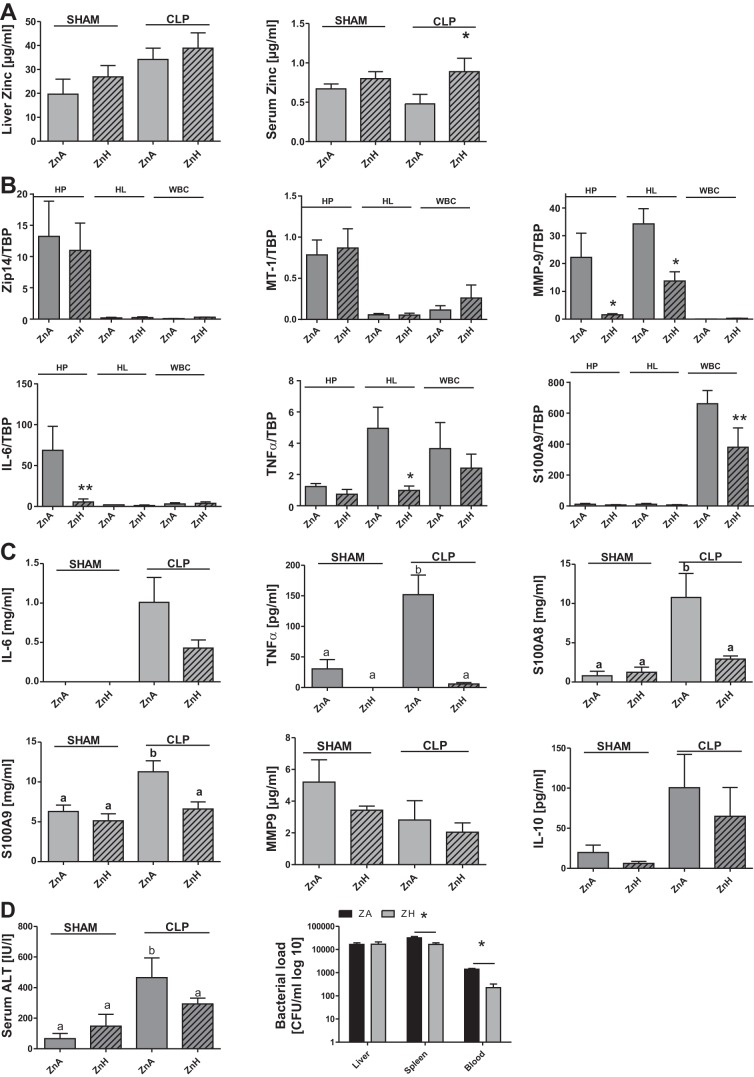
Zip14 ablation alters the hepatic inflammatory response to sepsis. Zip14 KO and WT mice were killed 24 h after CLP or sham operation. A: Western analysis of ZIP14 expression and STAT3 phosphorylation (pSTAT3) in liver of CLP and sham-operated mice. Blots are representative of results from 3 independent experiments; tubulin and STAT3 were used as loading controls. B: Il-6, Tnfα, Il-1β, and Il-10 mRNA expression in liver and WBC. Data were normalized to TBP mRNA. Values are means ± SE (n = 3 mice per group). C: IL-6, MMP-9, S100A8, and S100A9 concentrations in serum analyzed using a customized Luminex assay and TNFα and IL-10 plasma concentrations analyzed by ELISA. Values are means ± SE (n = 3 mice per group). D: serum alanine aminotransferase (ALT) activity (n = 3–4 mice per group) and bacterial load [colony-forming units (cfu)/ml] of liver homogenate, spleen homogenate, and whole blood (n = 3 mice per group). Values are means ± SE. *P < 0.05 vs. KO [by ANOVA/Bonferroni's test (B and C) and ANOVA/Tukey's test (D)]. Significantly different values do not share the same letter (a, b).
Zinc supplementation reduces sepsis progression.
The Zip14 KO model provided new and valuable information on the role of zinc transport to and within the liver during sepsis. To more directly characterize the effects of zinc in mild sepsis, we fed WT mice the ZnA or ZnH diet for 1 wk prior to CLP. Figure 6A illustrates an increase in liver zinc in sham-operated mice fed the ZnH diet and a greater increase by 24 h after CLP. The significant drop in serum zinc following CLP was prevented in the ZnH diet-fed mice compared with the ZnA diet-fed mice. However, no significant changes in Zip14 and Mt-1 mRNA expression were observed in HP, HL, and WBC from mice fed the ZnA diet compared with those fed the ZnH diet (Fig. 6B). Mmp-9 mRNA expression was significantly decreased in HP and HL and negligible in WBC. The ZnH diet significantly decreased Il-6 mRNA in HP, Tnfα mRNA in HL, and S100A9 mRNA in WBC, demonstrating the anti-inflammatory effect of zinc (Fig. 6B). We found significantly decreased levels of TNFα, S100A8, and S100A9 in serum from ZnH diet-fed mice compared with ZnA diet-fed mice (Fig. 6C). Moreover, IL-6, MMP-9, and IL-10 were decreased, but not significantly. In concordance with the ameliorated inflammatory response, ALT levels in the serum were significantly decreased in the ZnH diet-fed mice. Interestingly, we also found a lower bacterial load in spleen and blood from mice fed the ZnH diet. These results clearly point to a benefit of prior zinc supplementation for the outcomes in this mouse model of sepsis.
Fig. 6.
Zinc supplementation limits hyperinflammation and positively influences the antimicrobial response. Mice were fed a zinc-adequate (ZnA) or a zinc-supplemented [high-zinc (ZnH)] diet for 7 days and killed 24 h after CLP or sham operation. A: liver and serum zinc concentrations. B: Zip14, Mt-1, Mmp-9, Il-6, Tnfα, and S100A9 mRNA expression in HP, HL, and WBC. Values were normalized to TBP mRNA. C: IL-6, MMP-9, S100A8, and S100A9 concentrations in serum analyzed using a customized Luminex assay and TNFα and IL-10 plasma concentrations analyzed by ELISA. D: serum ALT and bacterial load of liver homogenate, spleen homogenate, and whole blood. Values are means ± SE (n = 3–5 mice per group) (A and B). *P < 0.05, **P < 0.01 vs. SHAM (by ANOVA/Bonferroni's test) (C and D). Significantly different values do not share the same letter (a, b).
DISCUSSION
The inflammatory acute-phase response is a complex physiological process involving various cell types and proinflammatory, as well as anti-inflammatory, phases. The ultimate goal is clearance of the insult, usually a pathogen or injury, and reestablishment of homeostasis. If homeostasis is not reestablished, as during sepsis, tissue damage, organ failure, and death are the final consequences (1, 13, 23). In this report we connect inflammation of sepsis to changes in zinc homeostasis. The murine model used here focuses primarily on sepsis up to 24 h post-CLP, when leukocytosis occurs and inflammatory stimuli, including IL-6, IL-1β, and TNFα, increase. Zinc is needed for liver metabolism and protection; however, the exact role of this micronutrient in the immune response to sepsis is unknown.
Unique to this study is the identification of a cascade of Zip4, Zip6, and Zip10 mRNA expression in the liver of septic mice. These changed on a temporal basis and were cell type-specific, but they played only a minor role in generating hypozincemia. Zip14 was the major transporter responsible for zinc redistribution during sepsis. Therefore, results from this polymicrobial sepsis model are in agreement with results from LPS-induced endotoxemia (2, 30, 31) and HP in vitro (30, 31). In addition, we narrowed the main function in regulation of zinc homeostasis to HP, while HL were mostly responsible for driving inflammation, as shown by higher expression of Il-1β, Tnfα, S100A8, and Mmp-8. While these factors have been shown to be elevated during sepsis, their origin has not been assigned to specific cell types of the liver, nor were they previously related to zinc redistribution.
Of significance was the inverse correlation of WBC in the blood to the decrease in serum zinc. This finding supports the hypothesis that hypozincemia is an activating signal for the immune system (21). Not only the number, but also the activity, of WBC was altered during sepsis, most importantly increasing expression of proinflammatory mediators. Associated with the acute changes in zinc distribution in vivo was increased Tnfα, Il-1β, calprotectin, Il-10, Mmp-8, and Mmp-9 mRNA expression in WBC.
The high level of Il-6 expression by liver cells, but not WBC, suggests that this cytokine functions in an autocrine fashion to generate the rapid and strong responses during sepsis, which makes it a valuable target for sepsis therapy (6, 35). A similar Il-6 expression profile was observed for HL. The uptake of high amounts of zinc by HP may deprive other cell populations of zinc in the systemic circulation. Our evidence in support of this idea is the increase in Mt-1 mRNA and labile zinc concentration in HP compared with HL (Fig. 3A). Of note were high levels of Tnfα and Il-1β expression in HL, indicating that restriction of zinc availability might be necessary for induction of specific cytokine expression in leukocytes. The possibility that systemic zinc deficiency may activate cytokine expression/secretion consequences has been suggested by several in vitro studies (21).
To approach an explanation for the significance of zinc uptake into the liver during sepsis, we analyzed Zip14 KO and WT mice. As previously reported for LPS stimulation of Zip14 expression in liver (3), STAT3 phosphorylation increased after CLP. The activation may be similar in KO and WT mice and requires further analysis. Knockout of Zip14 expression eliminated the robust increase in hepatic zinc accumulation in the WT mice following CLP. The hypozincemic response in the WT mice over 48 h after CLP was less in the KO mice, and a different WBC response pattern was evident. Notably, the null mutation prevented the increase in WBC for ≥24 h after induction of sepsis by CLP. We interpret this to indicate a greater metabolic flux of plasma zinc to various cells, including liver HP, in early sepsis. With time, in the KO model the WBC population is able to overcome that transient zinc deficit, and the number of WBC in the systemic circulation increases. Further research is needed to define the mechanism.
The very robust increase in MT-1 and hepcidin in the KO mice following CLP was somewhat surprising, as both are usually directly connected to major changes in zinc and iron homeostasis, respectively. In vitro and in vivo studies have shown ZIP14 can transport non-transferrin-bound iron and manganese under some circumstances (4, 18, 32). Previously, we demonstrated that elevated liver hepcidin expression is a characteristic of the Zip14 KO phenotype (2). In studies using the Zip14 KO model, but focused on the intestine, increased Mt-1 mRNA expression was observed, as was extensive accumulation of labile zinc in endosomes, with modest increases in total tissue zinc content (20). The high level of Mt-1 mRNA expression in the liver observed here suggests an abnormally high level of MTF-1 activation in HP of KO mice. In vitro evidence suggests that the hepcidin gene may be regulated by MTF-1 (5). Alternatively, since hepcidin is upregulated by IL-6 (34), the high serum IL-6 levels in the Zip14 KO mice would yield greater-than-normal hepcidin expression. We propose that a high level of hepcidin mRNA expression does not reflect higher-than-normal circulating hepcidin and would explain why the hepatic NHI concentrations were not different in the WT or KO mice with the CLP model used here. The role of hepcidin in sepsis has been viewed as generally protective (48).
Clinical evidence from human studies suggests that lower plasma TNFα, IL-6, and IL-10 levels correlate with survival in severely septic patients (47). Similarly, plasma TNFα correlates with severity of sepsis in human patients (12). Our murine sepsis data show that both HP and HL contribute to systemic IL-6 levels, while HL contribute to systemic TNFα and IL-10. Survival was not an issue in our experiments, since relatively mild conditions were used. Comparison of Tnfα, Il-6, and Il-10 transcripts in liver cells of KO vs. WT mice after CLP with serum protein levels shows opposite effects. This could relate to the times of RNA sampling vs. serum sampling. Expression by splenocytes would be an explanation as well. Alternatively, mechanisms mediating resolution of the inflammatory response, including expression of antagonists to proinflammatory mediators, such as IL-1 receptor antagonist, IL-6 receptor, and TNF receptor, might be disturbed. These possibilities remain to be tested. Nevertheless, the marked increases in serum TNFα, IL-6, and IL-10 suggest that progression of sepsis is influenced by Zip14 expression. More extensive studies are needed to evaluate the physiological role of ZIP14 in a severe sepsis model. In addition, in future studies the influence of sex needs to be investigated using this mutant model. Nevertheless, our data support the concept that zinc, as transported by ZIP14, influences cytokine levels through an as-yet-unidentified mechanism during sepsis. Our finding that supplemental dietary zinc generally reduces cytokine levels in CLP mice supports this hypothesis.
Evidence from genome expression assays indicates that metallothionein and Zip8 are among highly upregulated genes in nonsurvivors of pediatric septic shock (46). Those patients exhibited hypozincemia, while surviving patients had normal serum zinc levels. Those findings support the idea that a zinc-related component is involved in the severity of sepsis. At first glance, accumulation of high amounts of zinc might be surprising, as it could be the perfect environment also for bacterial growth. However, our data reveal that most of the zinc is directly taken up by HP, possibly causing a transient zinc deficiency in the intracellular space and blood vessels supplying the liver. On the basis of serum ALT levels, the highly compromised transfer of zinc to the liver in the KO mice did not lead to cell damage. However, greater Pd-l1 expression supports the notion that HP are in a protective metabolic state (49).
Dietary zinc deficiency has been shown to accentuate organ damage in a murine model of severe sepsis, resulting in high mortality (25). Conversely, short-term (3 days) dietary zinc supplementation in another model of severe murine sepsis decreased mortality and lowered indexes of sepsis, including bacterial load (36). The mechanism of the influence of zinc on sepsis is not fully known. The MMPs play a role in tissue injury of sepsis (33, 42). Calprotectin (S100A8 and S100A9) inhibits microbial growth through zinc chelation (9). Calprotectin may also inhibit MMPs by binding zinc, which is essential for enzymatic activity (24). Zinc may also inhibit cytokine production through a variety of mechanisms (16, 21). Our very positive effects of zinc supplementation suggest that timing of zinc as a therapy is very important. In a clinical setting with human septic patients, zinc supplementation could be monitored by measurement of peripheral blood mononuclear cell (PBMC) metallothionein mRNA levels (2).
There has been some controversy regarding the value of murine models of human sepsis (39, 41) for which PBMCs were used as a source of the RNA for transcriptome profiling by microarrays. Nevertheless, significant similarities exist, as mRNAs for MMPs, calprotectins, specific cytokines, and nutrient transport proteins in mice and human PBMCs were detected by these profiling experiments (41). Consequently, significant advances continue to be made with the murine CLP-induced sepsis model, for example, the role of IL-3 (44) and TNF receptor shedding from hepatocytes (12). This is particularly the situation in experiments at the organ/tissue level, e.g., the liver, as shown in our present experiments, rather than transcriptome analysis using circulating blood cells.
The focus of this report is on the liver, which is considered a key organ subject to dysfunction in sepsis (6, 35). The experiments presented here, the first with a zinc transporter KO model, suggest that zinc and the zinc transporter ZIP14 influence aspects of the pathophysiology of nonlethal polymicrobial murine sepsis induced by CLP. Ablation of Zip14 produced a delay in development of leukocytosis, prevented zinc accumulation in the liver, altered the kinetics of hypozincemia, and drastically increased serum IL-6, TNFα, and IL-10 concentration following CLP. To identify zinc-responsive factors modulated by acute sepsis, mice were fed the ZnH diet for 1 wk. The ZnH diet reduced cytokines, calprotectins (S100A8 and S100A9), serum ALT, and blood bacterial loads. Supplemental zinc at this level did not appear to produce toxicity. These data indicate that 1) zinc has an anti-inflammatory role during sepsis, in that it attenuates the proinflammatory response, and 2) ZIP14, when located at the cell surface, has an important transport function, in that it transports zinc into cells for sites of action.
GRANTS
This research was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grant RO1 DK-94244 and the Boston Family Endowment Funds of the University of Florida to R. J. Cousins. I. Wessels was supported by Deutsche Forschungsgemeinschaft Fellowship WE 5329/1-1.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
R.J.C. and I.W. developed the concept and designed the research; R.J.C. and I.W. drafted the manuscript; R.J.C. and I.W. edited and revised the manuscript; R.J.C. and I.W. approved the final version of the manuscript; I.W. performed the experiments; I.W. analyzed the data; I.W. prepared the figures.
ACKNOWLEDGMENTS
We acknowledge the scientific contributions and advice of Drs. Tolunay B. Aydemir, Catalina Troche, and Shou-Mei Chang throughout this project. We thank Laura Orta for manuscript preparation.
Present address of I. Wessels: Institute of Immunology, Aachen University Hospital, Aachen, Germany.
REFERENCES
- 1.Angus DC, van der Poll T. Severe sepsis and septic shock. N Engl J Med 369: 840–51, 2013. [DOI] [PubMed] [Google Scholar]
- 2.Aydemir TB, Blanchard RK, Cousins RJ. Zinc supplementation of young men alters metallothionein, zinc transporter, and cytokine gene expression in leukocyte populations. Proc Natl Acad Sci USA 103: 1699–1704, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aydemir TB, Sitren HS, Cousins RJ. The zinc transporter Zip14 influences c-Met phosphorylation and hepatocyte proliferation during liver regeneration in mice. Gastroenterology 142: 1536–1546, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aydemir TB, Chang SM, Guthrie GJ, Maki AB, Ryu MS, Karabiyik A, Cousins RJ. Zinc transporter ZIP14 functions in hepatic zinc, iron and glucose homeostasis during the innate immune response (endotoxemia). PLos One 7: e48679, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Balesaria S, Ramesh B, McArdle H, Baylee HK, Srai SK. Divalent metal dependent regulation of hepcidin expression by MTF-1. FEBS Lett 584: 719–725, 2010. [DOI] [PubMed] [Google Scholar]
- 6.Bauer M, Press AT, Trauner M. The liver in sepsis: patterns of response and injury. Curr Opin Crit Care 19: 123–127, 2013. [DOI] [PubMed] [Google Scholar]
- 7.Begum NA, Kobayashi M, Moriwaki Y, Matsumoto M, Toyoshima K, Seya T. Mycobacterium bovis BCG cell wall and lipopolysaccharide induce a novel gene, BIGM103, encoding a 7-TM protein: identification of a new protein family having Zn-transporter and Zn-metalloprotease signatures. Genomics 80: 630–645, 2002. [DOI] [PubMed] [Google Scholar]
- 8.Chesters KL, Will M. Measurement of zinc flux through plasma in normal and endotoxin-stressed pigs and the effects of Zn supplementation during stress. Br J Nutr 45: 119, 1981. [DOI] [PubMed] [Google Scholar]
- 9.Corbin BD, Seeley EH, Raab A, Feldmann J, Miller MR, Torres VJ, Anderson KL, Dattilo BM, Dunman PM, Skaar EP. Metal chelation and inhibition of bacterial growth in tissue abscesses. Science 319: 962–965, 2008. [DOI] [PubMed] [Google Scholar]
- 10.Cousins RJ, Leinart AS. Tissue-specific regulation of zinc metabolism and metallothionein genes by interleukin. FASEB J 2: 2884–2890, 1988. [DOI] [PubMed] [Google Scholar]
- 11.Cuenca AG, Delano MJ, Kelly-Scumpia KM, Moldawer LL, Efron PA. Cecal ligation and puncture. Curr Protoc Immunol 19: 19 13, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Deng M, Loughran PA, Zhang L, Scott MJ, Billiar TR. Shedding of the tumor necrosis factor (TNF) receptor from the surface of the hepatocytes during sepsis limits inflammation through cGMP signaling. Sci Signal 8: ra11, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Deutschman CS, Tracey KJ. Sepsis: current dogma and new perspectives. Immunity 40: 463–475, 2014. [DOI] [PubMed] [Google Scholar]
- 14.DiSilvestro RA, Cousins RJ. Mediation of endotoxin-induced changes in zinc metabolism in rats. Am J Physiol Endocrinol Metab 247: E436–E441, 1984. [DOI] [PubMed] [Google Scholar]
- 15.Etzel KR, Swerdel MR, Swerdel JN, Cousins RJ. Endotoxin-induced changes in copper and zinc metabolism in the Syrian hamster. J Nutr 112: 2363–2373, 1982. [DOI] [PubMed] [Google Scholar]
- 16.Fraker PJ, King LE. Reprogramming of the immune system during zinc deficiency. Annu Rev Nutr 24: 277–298, 2004. [DOI] [PubMed] [Google Scholar]
- 17.Gaetke LM, McClain CJ, Talwalkar RT, Shedlofsky SI. Effects of endotoxin on zinc metabolism in human volunteers. Am J Physiol Endocrinol Metab 272: E952–E956, 1997. [DOI] [PubMed] [Google Scholar]
- 18.Girijashanker K, He L, Soleimani M, Reed JM, Li H, Liu Z, Wang B, Dalton T, Nebert DW. Slc39a14 gene encodes ZIP14, a metal/bicarbonate symporter: similarities to the ZIP8 transporter. Mol Pharmacol 73: 1413–1423, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grynkiewicz G, Poenie M, Tsien RY. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem 260: 3440–3450, 1985. [PubMed] [Google Scholar]
- 20.Guthrie GJ, Aydemir TB, Troche C, Martin AB, Chang SM, Cousins RJ. Influence of ZIP14 (slc39A14) on intestinal zinc processing and barrier function. Am J Physiol Gastrointest Liver Physiol 308: G171–G178, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Haase H, Rink L. Functional significance of zinc-related signaling pathways in immune cells. Annu Rev Nutr 29: 133–152, 2009. [DOI] [PubMed] [Google Scholar]
- 22.Hotchkiss RS, Coopersmith CM, McDunn JE, Ferguson TA. The sepsis seesaw: tilting toward immunosuppression. Nat Med 15: 495–497, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hotchkiss RS, Monneret G, Payen D. Sepsis-induced immunosuppression: from cellular dysfunctions to immunotherapy. Nat Rev Immunol 13: 862–874, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Isaksen B, Fagerhol MK. Calprotectin inhibits matrix metalloproteinases by sequestration of zinc. Mol Pathol 54: 289–292, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Knoell DL, Julian MW, Bao S, Besecker B, Macre JE, Leikauf GD, DiSilvestro RA, Crouser ED. Zinc deficiency increases organ damage and mortality in a murine model of polymicrobial sepsis. Crit Care Med 37: 1380–1388, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Koutsos EA, Klasing KC. The acute phase response in Japanese quail (Coturnix coturnix japonica). Comp Biochem Physiol C Toxicol Pharmacol 128: 255–263, 2001. [DOI] [PubMed] [Google Scholar]
- 27.Krones C, Klosterhalfen B, Fackeldey V, Junge K, Rosch R, Schwab R, Stumpf M, Klinge U, Schumpelick V. Deleterious effect of zinc in a pig model of acute endotoxemia. J Invest Surg 17: 249–256, 2004. [DOI] [PubMed] [Google Scholar]
- 28.Lagu T, Rothberg MB, Nathanson BH, Pekow PS, Steingrub JS, Lidenauer KP. The relationship between hospital spending mortality in patients with sepsis. Arch Intern Med 171: 292–299, 2011. [DOI] [PubMed] [Google Scholar]
- 29.Lichten L, Cousins RJ. Mammalian zinc transporters: nutritional and physiologic regulation. Annu Rev Nutr 29: 153–176, 2009. [DOI] [PubMed] [Google Scholar]
- 30.Lichten LA, Liuzzi JP, Cousins RJ. Interleukin-1β contributes via nitric oxide to the upregulation and functional activity of the zinc transporter Zip14 (Slc39a14) in murine hepatocytes. Am J Physiol Gastrointest Liver Physiol 296: G860–G867, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liuzzi JP, Lichten LA, Rivera S, Blanchard RK, Aydemir TB, Knutson MD, Ganz T, Cousins RJ. Interleukin-6 regulates the zinc transporter Zip14 in liver and contributes to the hypozincemia of the acute-phase response. Proc Natl Acad Sci USA 102: 6843–6848, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liuzzi JP, Aydemir F, Nam H, Knutson MD, Cousins RJ. Zip14 (Slc39a14) mediates non-transferrin-bound iron uptake into cells. Proc Natl Acad Sci USA 103: 13612–13617, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Martin G, Asensi V, Montes AH, Collazos J, Alvarez V, Carton JA, Taboada F, Garay EV. Role of plasma matrix-metalloproteases (MMPs) and their polymorphisms (SNPs) in sepsis development and outcome in ICU patients. Nature 4: 5002, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nemeth E, Rivera S, Gabayan V, Keller C, Taudorf S, Pedersen BK, Ganz T. IL-6 mediates hypoferremia of inflammation by inducing the synthesis of the iron regulatory hormone hepcidin. J Clin Invest 113: 1271–1276, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nesseler N, Launey Y, Aninat C, Morel F, Mallédant Y, Seguin P. The liver in sepsis. Crit Care 16: 235, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nowak JE, Harmon K, Caldwell CC, Wong HR. Prophylactic zinc supplementation reduces bacterial load and improves survival in a murine model of sepsis. Pediatr Crit Care Med 13: e323–e329, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rebouche CJ, Wilcox CL, Widness JA. Microanalysis of non-heme iron in animal tissues. J Biochem Biophys Methods 58: 239–251, 2013. [DOI] [PubMed] [Google Scholar]
- 38.Sas B, Bremner I. Effect of acute stress on the absorption and distribution of zinc and on Zn-metallothionein production in the liver of the chick. J Inorg Biochem 11: 67–76, 1979. [DOI] [PubMed] [Google Scholar]
- 39.Seok J, Warren HS, Cuenca AG, Mindrinos MN, Baker HV, Xu W, Richards DR, McDonald-Smith GP, Gao H, Hennessey L, Finnerty CC, Lopez CM, Honari S, Moore EE, Minei JP, Cuschieri J, Bankey PE, Johnson JL, Sperry J, Nathens AB, Billiar TR, West MA, Jeschke MG, Klein MB, Gamelli RL, Gibran NS, Brownstein BH, Miller-Graziano C, Calvano SE, Mason PH, Cobb JP, Rahme LG, Lowry SF, Maier RV, Moldawer LL, Herndon DN, Davis RW, Xiao W, Tompkins RG; Inflammation and Host Response to Injury, Large Scale Collaborative Research Program. Genomic responses in mouse models poorly mimic human inflammatory diseases. Proc Natl Acad Sci USA 110: 3507–3512, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sobocinski PZ, Powanda MC, Canterbury WJ, Machotka SV, Walker RI, Snyder SL. Role of zinc in the abatement of hepatocellular damage and mortality incidence in endotoxemic rats. Infect Immun 15: 950–957, 1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Takao K, Miyakawa T. Genomic responses in mouse models greatly mimic human inflammatory diseases. Proc Natl Acad Sci USA 112: 1167–1172, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vandenbroucke RE, Dejager L, Libert C. The first MMP in sepsis. EMBO Mol Med 3: 367–369, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang N, Strugnell R, Wijburg O, Brodnicki T. Measuring bacterial load and immune responses in mice infected with Listeria monocytogenes. J Vis Exp 54: e3076, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Weber GF, Chousterman BG, He S, Fenn AM, Nairz M, Anzai A, Brenner T, Uhle F, Iwamoto Y, Robbins CS, Noiret L, Maier SL, Zönnchen T, Rahbari NN, Schölch S, Klotzsche-von Ameln A, Chavakis T, Weitz J, Hofer S, Wiegand MA, Nahrendorf M, Weissleden R, Swirski FK. Interleukin-3 amplifies acute inflammation and is a potential therapeutic target in sepsis. Science 347: 1260, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Williams SC. After Xigris, researchers look to new targets to combat sepsis. Nat Med 18: 1001, 2012. [DOI] [PubMed] [Google Scholar]
- 46.Wong HR, Shanley TP, Sakthivel B, Cvijanovich N, Lin R, Allen GL, Thomas NJ, Doctor A, Kalyanaraman M, Tofil NM, Penfil S, Monaco M, Tagavilla MA, Odoms K, Dunsmore K, Barnes M, Aronow BJ; Genomics of Pediatric SIRS/Septic Shock Investigators. Genome level expression profiles in pediatric septic shock indicate a role for altered zinc homeostasis in poor outcome. Physiol Genomics 30: 146–155, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wu HP, Chen CK, Chung K, Tseng JC, Hua C. Serial cytokine levels in patients with severe sepsis. Inflamm Res 58: 385–393, 2009. [DOI] [PubMed] [Google Scholar]
- 48.Zeng CL, Chen QX, Zhang K, Chen QH, Song SW, Fang XM. Hepatic hepcidin protects against polymicrobial sepsis in mice by regulating host iron status. Anesthesiology 122: 374–386, 2015. [DOI] [PubMed] [Google Scholar]
- 49.Zhu W, Bao R, Fan X, Tao T, Zhu J, Wang J, Li J, Bo L, Deng X. PD-L1 blockade attenuated sepsis-induced liver injury in a mouse cecal ligation and puncture model. Mediators Inflamm 2013: 361501, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]