Abstract
Obesity is one of the major risk factors for asthma. Previous studies have demonstrated that free fatty acid levels are elevated in the plasma of obese individuals. Medium- and long-chain free fatty acids act as endogenous ligands for the free fatty acid receptors FFAR1/GPR40 and FFAR4/GPR120, which couple to Gq proteins. We investigated whether FFAR1 and FFAR4 are expressed on airway smooth muscle and whether they activate Gq-coupled signaling and modulate airway smooth muscle tone. We detected the protein expression of FFAR1 and FFAR4 in freshly dissected native human and guinea pig airway smooth muscle and cultured human airway smooth muscle (HASM) cells by immunoblotting and immunohistochemistry. The long-chain free fatty acids (oleic acid and linoleic acid) and GW9508 (FFAR1/FFAR4 dual agonist) dose-dependently stimulated transient intracellular Ca2+ concentration ([Ca2+]i) increases and inositol phosphate synthesis in HASM cells. Downregulation of FFAR1 or FFAR4 in HASM cells by small interfering RNA led to a significant inhibition of the long-chain free fatty acids-induced transient [Ca2+]i increases. Oleic acid, linoleic acid, or GW9508 stimulated stress fiber formation in HASM cells, potentiated acetylcholine-contracted guinea pig tracheal rings, and attenuated the relaxant effect of isoproterenol after an acetylcholine-induced contraction. In contrast, TUG-891 (FFAR4 agonist) did not induce the stress fiber formation or potentiate acetylcholine-induced contraction. These results suggest that FFAR1 is the functionally dominant free fatty acid receptor in both human and guinea pig airway smooth muscle. The free fatty acid sensors expressed on airway smooth muscle could be an important modulator of airway smooth muscle tone.
Keywords: free fatty acid, FFAR1, FFAR4, Gq-coupled receptor, airway smooth muscle
epidemiological studies have indicated that obesity is one of the risk factors for asthma (42). Asthma symptoms in obese individuals tend to be more severe and do not respond as well to treatment (10). Although the changes in adipose-derived inflammatory molecules, including TNF-α, leptin, and adiponectin, may contribute to asthma in obesity (1), the mechanistic basis for the relationship between obesity and asthma is poorly elucidated (41). Plasma free fatty acids (FFAs) levels have been reported to be elevated in obesity because the increased amount of adipose tissue mass releases more FFAs and FFAs clearance may be reduced (3). In the last decade, FFAR1 (GPR40), FFAR2 (GPR43), FFAR3 (GPR41), FFAR4 (GPR120, also known as O3FAR1), and GPR84 have been de-orphanized and recognized as the receptors for FFAs (5, 6, 22, 25, 46). Among them, FFAR1 and FFAR4 are recognized as Gq-coupled receptors and are activated by endogenous medium- and long-chain FFAs (5, 22, 25). FFAR1 is expressed in pancreatic β-cells (5), breast cancer cell lines (21, 48), and the central nervous system (33) and contributes to physiological functions, including insulin secretion from pancreatic β cells (25). Recently, it has been reported that protein expression of FFAR1 is strongly increased in pancreatic islets of “hyperlipidemic” Zucker diabetic (fa/fa) rats (28). In contrast, FFAR4 is expressed in the intestine, adipocytes, macrophages, and central nervous system and contributes to the secretion of glucagon-like peptide-1, adipocyte differentiation, and anti-inflammatory effects (8, 22, 35). In the airways, FFAR1 has been identified on human bronchial epithelial cells and is reported to induce cell proliferation (18). However, the functional expression of these free fatty acid receptors has never been described on the airway smooth muscle itself.
Long-chain FFAs can activate Gq-coupled FFAR1 and FFAR4 to increase intracellular Ca2+ concentration ([Ca2+]i) (5, 16, 40, 47). In airway smooth muscle, activation of Gq-coupled receptors such as the M3 muscarinic and tachykinin receptors induce inositol 1,4,5-trisphosphate (IP3) accumulation, which mobilizes Ca2+ from the sarcoplasmic reticulum, leading to enhanced Ca2+ oscillations and airway smooth muscle contraction (2, 15, 29). Furthermore, the peroxisome proliferator-activated receptor (PPAR)-γ ligand rosiglitazone stimulated FFAR1 expressed on human bronchial epithelial cells and induced transient [Ca2+]i increases (18). These findings led us to hypothesize that functional FFAR1 and/or FFAR4 on airway smooth muscle cells could respond to medium- and long-chain fatty acids and promote airway smooth muscle contraction through classical Gq-coupled receptor mediated signal transduction.
In the present study, the expression of FFAR1 and FFAR4 was assessed on native human and guinea pig airway smooth muscle tissue and cultured human airway smooth muscle (HASM) cells. In addition, the effects of long-chain FFAs on transient [Ca2+]i mobilization, inositol phosphate synthesis, actin polymerization, and ex vivo contractile tone were assessed to confirm their physiological role in airway smooth muscle.
METHODS
Materials.
Lysates of human brain cerebral cortex obtained from BD Biosciences were used as positive protein controls on immunoblots. Pertussis toxin was obtained from Calbiochem. Total RNA from whole human brain was purchased from Clontech. Protease inhibitor cocktail III was obtained from EMD Biosciences. Alexa Fluor 488-conjugated DNase I, antibiotic-antimycotic, DMEM/F12 medium, fetal bovine serum (FBS), fluo-4 AM, Opti-MEM Pluronic F-127, ProLong gold antifade reagent, and rhodamine-conjugated phalloidin were obtained from Life Technologies. myo-[3H]inositol (20 Ci/mmol) was obtained from MP Biomedicals. GW9508 was obtained from Tocris Bioscience. All other chemicals were obtained from Sigma unless otherwise stated.
Cell culture.
Primary cultured HASM cells were obtained from Lonza. Cells were grown in DMEM/F12 culture medium, supplemented with 10% FBS and an antibiotic-antimycotic mix (100 U/ml penicillin G sodium, 100 μg/ml streptomycin sulfate, 0.25 μg/ml amphotericin B) at 37°C in an atmosphere of 5% CO2-95% air.
Isolation of smooth muscle from human and guinea pig trachea.
All human airway smooth muscle studies were reviewed by Columbia University's Institutional Review Board and deemed not human subjects research under 45 CFR 46. Human trachea were obtained from discarded regions of healthy donor lungs harvested for lung transplantation at Columbia University. Human tissue was transported to the laboratory in cell culture medium as described previously (31).
All guinea pig studies were approved by the Columbia University Institutional Animal Care and Use Committee. These studies were also reviewed by the Committee on the Ethics of Animal Experiments at the Tohoku University School of Medicine, and they were carried out in accordance with both the Guidelines for Animal Experiments issued by the Tohoku University and The Law (No. 105) and Notification (No. 6) issued by the Japanese government. Adult male Hartley guinea pigs (∼400 g body weight) were deeply anesthetized by intraperitoneal pentobarbital (100 mg/kg). The chest cavity was opened, and the guinea pigs were exsanguinated. The entire trachea and whole brain (positive control for immunoblot) were surgically removed and immersed in cold (4°C) Krebs-Henseleit (KH) buffer solution (composition in mM: 118 NaCl, 5.6 KCl, 0.5 CaCl2, 0.2 MgSO4, 25 NaHCO3, 1.3 NaH2PO4, 5.6 d-glucose) with 10 μM indomethacin (DMSO vehicle final concentration of 0.01%). The exteriors of either the human or guinea pig trachea were carefully dissected free of adherent connective tissue under a microscope. Epithelium was left intact for immunohistochemistry but removed for organ bath experiments and immunoblotting.
Western blot analysis.
Freshly dissected native human and guinea pig airway smooth muscle and guinea pig whole brain were homogenized (Tekmar Ultra Turrax T25 high-speed homogenizer set at top speed for 30 s) in ice-cold (4°C) buffer (50 mM Tris, 10 mM HEPES, pH 7.4, 1 mM EDTA with a 1:200 dilution of protease inhibitor cocktail III, 1 mM Na3VO4, 1 mM NaF). The homogenate was filtered through 125-μm Nitex mesh and centrifuged twice at 500 g for 15 min. The supernatant was transferred into new tubes and centrifuged at 50,000 g for 30 min at 4°C. The final membrane pellet was resuspended in the same buffer and stored at −80°C after protein concentration determinations. Confluent cultures of HASM cells were rinsed twice with ice-cold phosphate-buffered saline (PBS) and mechanically scraped from the surface of T75 culture flasks or 6-well plates in the presence of protease inhibitor cocktail III. Cells were pelleted (500 g, 10 min, 4°C) and lysed in ice-cold lysis buffer (50 mM Tris·HCl, pH 8.0, 1% Nonidet P-40, 0.5% sodium deoxycholate, 0.1% SDS, 150 mM NaCl, 1 mM EDTA with 1:200 dilution of protease inhibitor cocktail III, 1 mM Na3VO4, 1 mM NaF). Lysed cells were centrifuged (15,000 g, 15 min, 4°C), and an aliquot of the supernatant was subjected to protein analysis and stored at −80°C. Each sample was solubilized by heating at 95°C for 10 min in sample buffer (final concentrations: 50 mM Tris·HCl, pH 6.8, 2.5% SDS, 6% glycerol, 2.5% 2-mercaptoethanol, and bromophenol blue) before use. Membrane lysates were electrophoresed (7.5% or 10% Mini-Protean TGX precast gel; Bio-Rad) and transferred to polyvinylidene difluoride (PVDF) membranes using a Trans-Blot Turbo transfer system (Bio-Rad). The PVDF membranes were blocked for 1 h at room temperature with 5% ECL prime membrane blocking reagent (RPN418; GE Healthcare) in TBS with 0.1% Tween 20 (TBST) and were then probed with antibodies directed against the FFAR1 protein (rabbit monoclonal 1:1,000, 3393-1; Epitomics) or the FFAR4 protein (rabbit polyclonal 1:1,000, LS-C185366; LifeSpan Biosciences) overnight at 4°C. After three washes with TBST, membranes were incubated for 1 h at room temperature with horseradish peroxidase (HRP)-conjugated secondary anti-rabbit antibodies (1:5,000, NA934V; GE Healthcare) diluted in 1% membrane blocking reagent in TBST. The signals from the immunoreactive bands were detected using ECL prime (GE Healthcare) according to the manufacturer's recommendations, and the signal was captured using a chemiluminescent image analyzer (LAS 4000 Mini; GE Healthcare). The same PVDF membranes were stripped and reprobed with the antibody against the GAPDH protein (rabbit monoclonal 1:1,000, no. 5174; Cell Signaling Technology) to demonstrate the variation in protein loading on the gels.
Isolation of RNA and reverse transcriptase-polymerase chain reaction.
Total RNA was extracted from freshly dissected native human airway smooth muscle using TRIzol reagent (Ambion) and from primary cultured HASM cells using the RNeasy mini kit (Qiagen) according to the manufacturer's recommendations. Total RNA from whole human brain was used as a positive control. RNA was transcribed into cDNA using the SuperScript VILO cDNA synthesis kit (Life Technologies) as per the manufacturer's instructions. PCR was performed using Advantage 2 PCR kits (Clontech) with sense and antisense primers corresponding to the FFAR4 transcript variant 1 and variant 2 (Table 1). Two-step PCR was performed with a Mastercycler ep Gradient S thermal cycler (Eppendorf) for all PCR reactions, and all reactions included an initial denaturation step at 94°C for 1 min followed by 40 cycles of denaturation (94°C for 10 s) and annealing/extension at 72°C for 1 min. The primers were designed to anneal in two separate exons spanning a large intron to ensure that amplified PCR products resulted from amplified cDNA and not contaminating genomic DNA. PCR products were electrophoresed on 5% nondenaturing polyacrylamide gel in Tris-acetate-EDTA buffer. The gel was stained with ethidium bromide and visualized using ultraviolet illumination. The gel image was captured with a PowerShot A570 digital camera (Canon).
Table 1.
Primer sequences for FFAR4 transcript variants
| Target | Primer Sequence | Amplicon Size, bp |
|---|---|---|
| Human FFAR4 transcript variant 1 (NM_181745) | FP: 5′-CCT CCT GGA TGC AAG AGC TGT CGT-3′ | 165 |
| RP: 5′-ATG AAG AAG GAG ACC ATG AGG AGG AAG A-3′ | ||
| Human FFAR4 transcript variant 2 (NM_001195755) | FP: 5′-TGC ACA CTG ATT TGG CCC ACC ATT-3′ | 141 |
| RP: 5′-CCT CTT CCT TGA TGC CTT TGT GAT CTG TA-3′ |
FFAR4, free fatty acid receptor 4. GenBank accession number is given in parentheses.
Immunohistochemistry.
Human tracheal rings were fixed with 4% paraformaldehyde-1% glutaraldehyde in 0.1 M phosphate buffer for 4 h at 4°C. Guinea pig tracheal rings were fixed with 4% paraformaldehyde in 0.1 M phosphate buffer for 24 h at 4°C. Tracheal rings were paraffin embedded, sectioned (10 μm), dewaxed in xylene, and rehydrated in a graded alcohol series to water. Heat-mediated antigen retrieval was performed with Tris-EDTA buffer (10 mM Tris Base, 1 mM EDTA, pH 9.0) for 2 min using a pressure cooker. Endogenous peroxidase was blocked using 3% hydrogen peroxide for 15 min at room temperature. Sections were blocked with 10% normal goat serum for 30 min. After sections were washed with PBS with 0.1% Triton X-100 (PBST), an avidin biotin blocking kit (Vector Laboratories) was used (in 10% normal goat serum in PBS) to block endogenous biotin. Slides were rinsed with PBST and incubated overnight at 4°C in primary antibody against the FFAR1 protein (rabbit polyclonal 1:500, sc-32905; Santa Cruz Biotechnology) or the FFAR4 protein (rabbit polyclonal 1:2,000, LS-C185366; LifeSpan Biosciences) in 2% normal goat serum in PBST. Different antibodies were used to detect the FFAR1 protein in immunoblot and immunohistochemistry experiments because 1) the antibody used for immunoblot (3393-1; Epitomics) is not recommended for immunohistochemistry in paraffin-embedded tissues, and 2) the antibody used for immunohistochemistry (sc-32905; Santa Cruz Biotechnology) has been used previously for immunohistochemistry in paraffin-embedded tissues (9). A parallel tracheal ring section was incubated with a rabbit isotype IgG antibody as a negative control. After overnight incubation at 4°C, slides were washed three times with PBST and primary antibodies were detected using biotinylated anti-rabbit antibodies (Vector Laboratories) at a concentration of 1:100. After incubation with ABC-HRP complex (Vector Laboratories) for 30 min, the antigen antibody complex was then visualized with the DAB peroxidase substrate kit (Vector Laboratories). Sections were counterstained with hematoxylin (Vector Laboratories), dried, and coverslipped using Poly-mount (Polysciences).
Small interfering RNA transfection.
The predesigned small interfering (si)RNA targeting human FFAR1 (Silencer Select predesigned siRNA no. s194466), human FFAR4 (predesigned to target both FFAR4 transcript variant 1 and variant 2; Silencer Select predesigned siRNA no. s50347), and a nontargeting siRNA (as negative control; no. 4390843) were obtained from Ambion. HASM cells cultured in DMEM/F12 growth medium (supplemented with 10% FBS) without antibiotics were grown in 6-well plates (seeded in 2.5 ml of the growth medium) or 96-well culture plates (seeded in 0.1 ml of the growth medium) until they reached 50% confluence. Cells were then transfected with the predesigned siRNA targeting human FFAR1 or FFAR4 or the nontargeting siRNA using Lipofectamine RNAiMAX (Life Technologies) in serum-free Opti-MEM according to the manufacturer's instructions. Briefly, HASM cells grown in 6-well plates were seeded in the presence of 30 pmol of siRNA and 5 μl of Lipofectamine RNAiMAX in 500 μl of Opti-MEM. Final medium volume was 3 ml, and a final siRNA concentration was 10 nM. HASM cells grown in 96-well plates were seeded in the presence of 1.2 pmol siRNA and 0.2 μl of Lipofectamine RNAiMAX in 20 μl of Opti-MEM. Final medium volume was 120 μl, and a final siRNA concentration was 10 nM. At 24 h after transfection, the antibiotic-free medium was replaced with standard growth medium. Once the cells reached confluence (2–3 days after transfection), the cells were lysed to assess FFAR1 or FFAR4 expression by immunoblot analysis or were used for measurements of [Ca2+]i.
Measurement of [Ca2+]i.
Confluent HASM cells in 96-well plates were incubated in modified Hanks' balanced salt solution (HBSS; in mM: 138 NaCl, 5.3 KCl, 2.5 CaCl2, 0.4 MgSO4, 0.49 MgCl2, 0.34 Na2HPO4, 4.2 NaHCO3, 0.44 KH2PO4, 5.5 dextrose, 20 HEPES, pH 7.4) in 100 μl/well containing 5 μM fluo-4 AM (DMSO vehicle final concentration of 0.5%), 0.05% Pluronic F-127 (DMSO vehicle final concentration of 0. 25%), and 2.5 mM probenecid for 45 min at 37°C. Once the cells were loaded, the cells were washed twice with modified HBSS containing 2.5 mM probenecid and left for an additional 30 min at room temperature to allow complete deesterification of the intracellular fluo-4 AM esters. This buffer was exchanged (100 μl/well) just before the measurement of fluorescence was started. The fluorescence was then continuously recorded every 5 s at wavelengths of 485-nm excitation and 528-nm emission using a fluorescence microplate reader (Appliskan; Thermo Fisher Scientific). Duplicate wells were simultaneously measured, and values were averaged for each data point. After a stable baseline was established for the first 2 min, long-chain FFAs (oleic acid or linoleic acid; 0.1–10 μM), a synthetic agonist of both FFAR1 and FFAR4 (GW9508; 0.1–10 μM), vehicle (0.1% DMSO in HBSS), bradykinin (1 μM), acetylcholine (1 μM), or histamine (10 μM) was added with the autoinjector to the HASM cells, and the fluorescence intensity was recorded for 10 min. In separate experiments, cells were pretreated with inhibitors [U-73122 (5 μM; 10 min), an inhibitor of phospholipase C-β (PLC-β); xestospongin C (20 μM; 30 min), a cell-permeable inhibitor of the IP3 receptor; ryanodine (100 μM; 10 min), which blocks ryanodine receptors at higher concentrations (27, 37, 44)] or vehicle (modified HBSS) before addition of the FFAR agonists. For experiments using pertussis toxin (PTX; 100 ng/ml; 4 h), cells were pretreated with PTX for 3 h before they were loaded with fluo-4 AM so that the PTX pretreatment duration would be 4 h. The cells then were exposed to long-chain FFAs (oleic acid or linoleic acid; 10 μM) or GW9508 (10 μM), and the fluorescence intensity was recorded for 10 min. In all studies the fluorescence intensity following FFA agonist treatments is presented as the change (ΔF) from baseline fluorescence (Fo).
Inositol phosphate assays.
Synthesis of total [3H]inositol phosphates was measured in confluent HASM cells in 24-well tissue culture plates as described previously (24, 26). Briefly, after overnight loading with myo-[3H]inositol (10 μCi/ml, 20 Ci/mmol) in inositol-free and serum-free DMEM (GIBCO), each well was washed three times (37°C, 500 μl of HBSS with 10 mM LiCl). Incubation of cells with long-chain FFAs (oleic acid or linoleic acid; 10 μM) or GW9508 (10 μM) in a final volume of 300 μl at 37°C for 30 min was performed. Reactions were terminated and total [3H]inositol phosphates recovered by chromatography (24).
Filamentous-to-globular actin ratio measurements.
Basal and agonist-induced ratios of filamentous (F) and globular (G) actin were determined as previously described (23, 45). Briefly, HASM cells on an 8-chamber microscope slide were exposed to oleic acid (10 μM), GW9508 (10 μM), TUG-891 (5 μM), or vehicle (0.05% DMSO) for 10 min. After the exposure, the cells were fixed with 4% paraformaldehyde for 15 min at room temperature and washed 3 times with PBS. After permeabilization (0.2% Triton X-100 in PBS for 5 min) and blocking (1% bovine serum albumin in 0.1% Triton X-100 in PBS for 15 min), cells were stained with rhodamine (540-nm excitation, 565-nm emission)-conjugated phalloidin (1 U/ml) and Alexa Fluor 488 (495-nm excitation, 519-nm emission)-conjugated DNase I (10 μg/ml) in 300 μl of 1% bovine serum albumin in PBS in the dark for 20 min. After cells were washed twice with PBS, a coverslip was mounted with Prolong gold antifade reagent and visualized with an inverted fluorescent microscope (DMI-4000; Leica). Digitized images were captured with MetaMorph software (Molecular Devices). To standardize the fluorescence intensity measurements among experiments, we optimally adjusted the duration of image capture (300 ms), the image intensity gain, the image enhancement, and the image black level and kept them constant. An increase in the F- to G-actin ratio (F/G actin) indicated an increase in stress fiber formation.
Organ bath study.
All studies were approved by Columbia University's Institutional Animal Care and Use Committee. Force measurements were performed on closed guinea pig tracheal rings suspended in organ baths as previously described (26). Briefly, Hartley male guinea pigs (∼400 g body weight) were anesthetized with pentobarbital (100 mg/kg ip), and the tracheas were removed promptly and dissected into closed rings comprising 2 cartilaginous rings from which mucosa, connective tissue, and epithelium were removed. Silk threads were tied to the rings such that the threads were at each end of the posterior aspect of the ring (lacking in cartilage) ∼180° from one another. One thread was attached to a fixed point at the bottom of a 4-ml organ bath (Radnoti Glass Technology), and the opposing thread was attached to a Grass FT03 force transducer (Grass-Telefactor) coupled to a computer via Biopac hardware and Acknowledge 7.3.3 software (Biopac Systems) for continuous digital recording of muscle tension. The rings were suspended in 4 ml of KH buffer solution (composition in mM: 118 NaCl, 5.6 KCl, 0.5 CaCl2, 0.2 MgSO4, 25 NaHCO3, 1.3 NaH2PO4, 5.6 d-glucose) with 10 μM indomethacin (DMSO vehicle final concentration of 0.01%), which was continuously bubbled with 95% O2-5% CO2 at pH 7.4, 37°C. The rings were equilibrated at 1 g of isotonic tension for 1 h with new KH buffer added every 15 min. All rings were precontracted with 10 μM N-vanillylnonanamide (capsaicin analog) and then 2 cycles of cumulatively increasing concentrations of acetylcholine (0.1–100 μM) with extensive buffer washes between and after those 2 cycles with resetting of the resting tension to 1.0 g. Tetrodotoxin (1 μM) and pyrilamine (10 μM) were added to the buffer in the baths to eliminate the confounding effects of airway nerves and histamine receptors. After the stable baseline, tracheal rings were contracted with acetylcholine (an individual EC50 was calculated for each ring). After the achievement of a stable contraction (typically 15 min) with acetylcholine (EC50), long-chain FFAs (oleic acid or linoleic acid; 20 μM), GW9508 (20 μM), or their vehicle (0.1% DMSO) were added to the buffer in the baths. In separate experiments, after a stable acetylcholine (EC50)-induced contraction (typically 5–10 min), long-chain FFAs (oleic acid or linoleic acid; 20 μM), GW9508 (20 μM), TUG-891 (20 μM), or their vehicle (0.1% DMSO) were added to the buffer 7 min before cumulatively increasing concentrations of β-adrenoceptor agonist isoproterenol (0.5 nM-1 μM) were added in half-log increments at 7-min intervals.
Statistical analysis.
Measurements of [Ca2+]i and inositol phosphate synthesis were performed in >100 cells in cell culture plates, and each of these experiments was performed on at least 4 different passages of human airway smooth muscle cells. In F/G actin ratio measurements, the fluorescence intensities were calculated from a view containing >15 cells. The n values for these cellular data represent the number of days on which the experiments were conducted on separate cell culture plates. In the organ bath studies on guinea pig, nine guinea pigs were used for experiments. The n values indicated in the results and figure legends represent the number of tissue samples. The data were analyzed with the two-tailed paired t-test when comparing means between two groups or with repeated-measures ANOVA followed by the Bonferroni posttest when comparing multiple groups, using GraphPad Instat 3 for Macintosh software (GraphPad Software). Data are means ± SE; P < 0.05 was considered significant.
RESULTS
Immunoblot and RT-PCR analysis of FFAR1 and FFAR4 in airway smooth muscle.
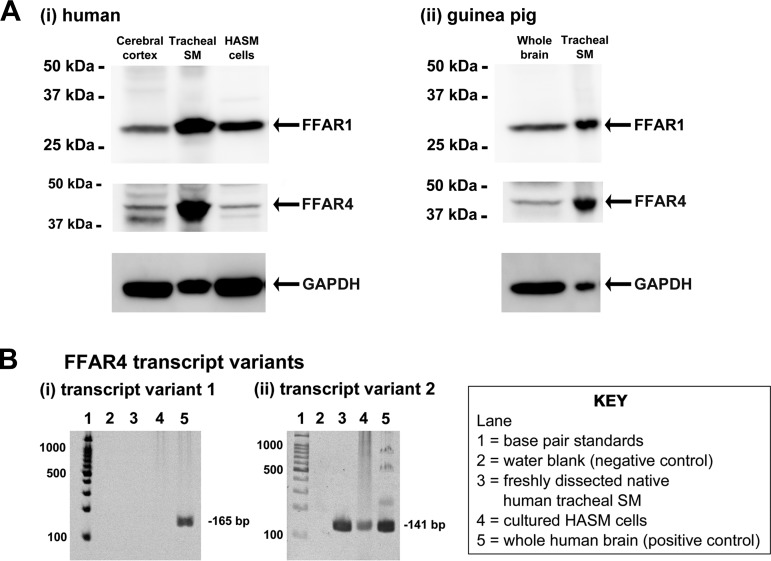
Initially, we examined whether FFAR1 and FFAR4 were expressed in both human and guinea pig airway smooth muscle. Lysates prepared from freshly dissected native human airway smooth muscle tissue, primary cultured HASM cells, and freshly dissected native guinea pig airway smooth muscle tissue were subjected to immunoblot analysis using specific anti-FFAR1 or anti-FFAR4 antibodies. The immunoreactive bands of appropriate molecular mass for FFAR1 (31 kDa) and FFAR4 (42 kDa) were identified in freshly dissected native human and guinea pig airway smooth muscle, primary cultured HASM cells, human brain cerebral cortex (positive control), and guinea pig whole brain (positive control) (Fig. 1A). Because the FFAR4 protein has two isoforms (FFAR4-GPR120L and FFAR4-GPR120S) and an FFAR4 antibody that can distinguish between these two isoforms is not available, we assessed the mRNA expression for the FFAR4 transcript variant 1 (mRNA for FFAR4-GPR120L protein) and FFAR4 transcript variant 2 (mRNA for FFAR4-GPR120S protein) in freshly dissected native human airway smooth muscle tissue and cultured HASM cells. Total RNA from whole brain was used as a positive control. Messenger RNA encoding the FFAR4 transcript variant 2 was detected in freshly dissected native human airway smooth muscle tissue and cultured HASM cells. In contrast, the FFAR4 transcript variant 1 was not detected in freshly dissected native human airway smooth muscle tissue and cultured HASM cells despite the detection in whole human brain RNA (Fig. 1B).
Fig. 1.
A: representative gel images of immunoblot analyses using antibodies against the free fatty acid receptor 1 (FFAR1) and FFAR4 using total protein prepared from human (i) and guinea pig (ii) tissues: human brain cerebral cortex (50 μg), freshly dissected native human tracheal airway smooth muscle (SM; 100 μg), primary cultured human airway smooth muscle (HASM) cells (100 μg), guinea pig whole brain (150 μg), and freshly dissected native guinea pig tracheal SM (100 μg). Reprobing of blots for GAPDH was performed to demonstrate relative lane loading. B: representative gel images of RT-PCR analyses of total RNA using primers specific for human FFAR4 transcript variant 1 (i) and variant 2 (ii). Total RNA extracted from freshly dissected human tracheal SM or cultured HASM cells was analyzed. Lane 1, base pair standards; lane 2, negative control water blank; lane 3, total RNA from freshly dissected native human tracheal SM; lane 4, total RNA from primary cultured HASM cells; lane 5, total RNA from whole human brain (positive control).
Immunohistochemical detection of FFAR1 and FFAR4 expression in guinea pig airway smooth muscle.
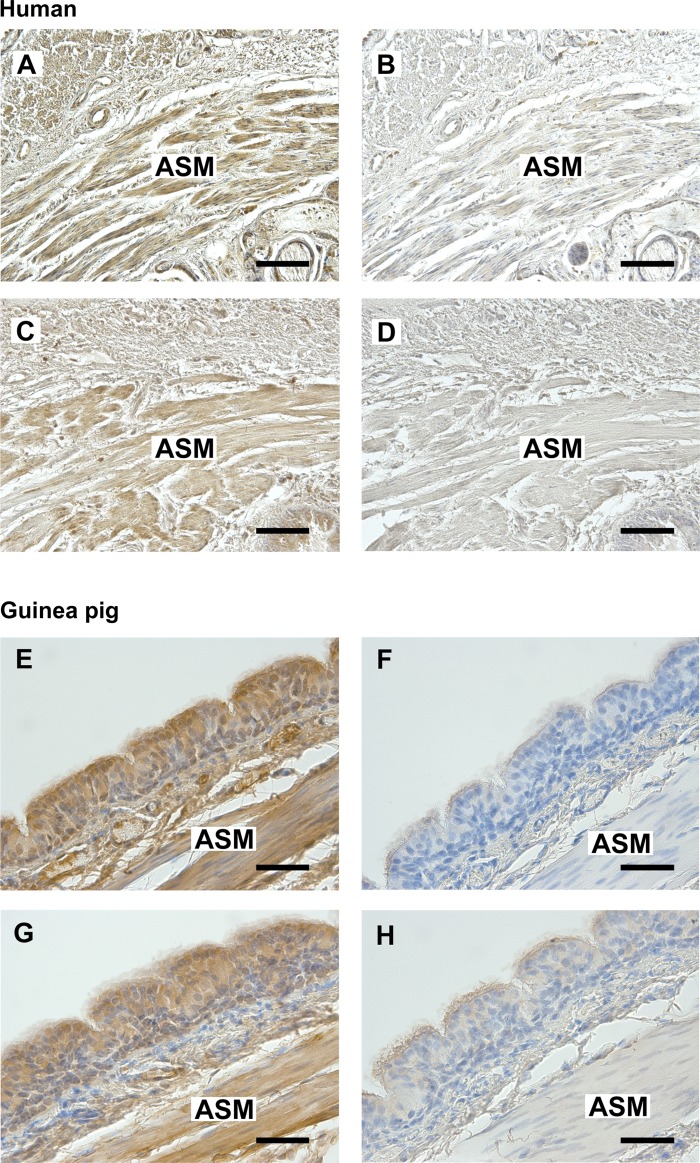
To confirm the localization of both FFAR1 and FFAR4 protein in airway smooth muscle, immunohistochemistry was performed using a rabbit polyclonal antibody that recognizes FFAR1 or FFAR4 in paraffin-embedded sections of both human and guinea pig tracheal rings. Specific immunohistochemical staining of FFAR1 (Fig. 2, A and E) and FFAR4 (Fig. 2, C and G) was obtained throughout the smooth muscle layer of both human and guinea pig trachea (indicated by brown color). Consecutive sections processed with an isotype-specific rabbit IgG negative control primary antibody produced no staining (Fig. 2, B, D, F, and H).
Fig. 2.
A and C: representative photomicrographs of immunohistochemical staining of FFAR1 (A) or FFAR4 (C) in paraformaldehyde-glutaraldehyde-fixed human trachea. E and G: representative photomicrographs of immunohistochemical staining of FFAR1 (E) or FFAR4 (G) in paraformaldehyde-fixed guinea pig trachea. B and D: anti-rabbit IgG isotype negative control in serial section of human trachea. F and H: anti-rabbit IgG isotype negative control in serial section of guinea pig trachea. All sections were counterstained with hematoxylin. Calibration bars: A–D, 100 μm; E–H, 50 μm. ASM, airway smooth muscle. Images are representative of at least 3 independent immunohistochemical analyses from both human and guinea pig trachea.
Long-chain FFAs and GW9508 induced transient [Ca2+]i increases in HASM cells.
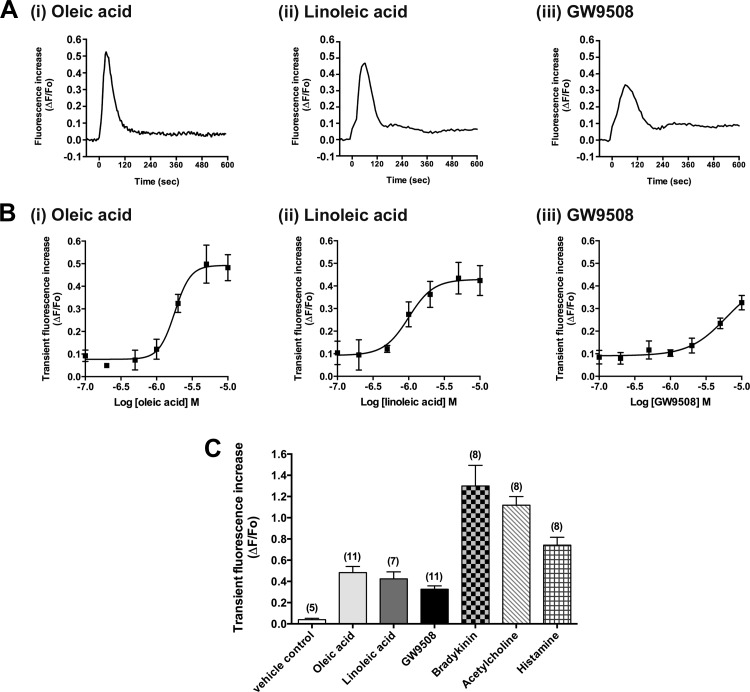
We measured changes of [Ca2+]i in fluo-4 AM-loaded HASM cells following exposure to long-chain FFAs. The long-chain FFAs (oleic acid and linoleic acid; 10 μM) caused a rapid and transient [Ca2+]i increase in HASM cells. Likewise, a synthetic agonist of both FFAR1 and FFAR4 (GW9508; 10 μM) also caused a transient increase in [Ca2+]i (Fig. 3A). These ligands induced transient [Ca2+]i increases in HASM cells in a concentration-dependent manner (Fig. 3B), although these increases were relatively smaller than those induced by other Gq-coupled receptor agonists bradykinin, acetylcholine, or histamine (Fig. 3C). The pEC50 values of oleic acid, linoleic acid, and GW9508 were 5.75 ± 0.08, 6.00 ± 0.13, and 5.22 ± 0.50, respectively.
Fig. 3.
Effects of long-chain free fatty acids (oleic acid or linoleic acid) or a synthetic agonist of both FFAR1 and FFAR4 (GW9508) on intracellular Ca2+ concentrations ([Ca2+]i) in HASM cells. A: representative traces of fluorescence intensity [change in fluorescence (ΔF) from baseline fluorescence (Fo)] illustrating the characteristics of oleic acid (i; 10 μM)-, linoleic acid (ii; 10 μM)-, or GW9508 (iii; 10 μM)-induced [Ca2+]i increases in HASM cells. At time 0, oleic acid, linoleic acid, or GW9508 was injected into the buffer in the wells. B: concentration-dependent effects (0.1–10 μM) of oleic acid, linoleic acid, or GW9508 on transient (peak) fluorescence increases in HASM cells. Data are means ± SE presented as ΔF/Fo; n = 4–11. C: effects of oleic acid (10 μM), linoleic acid (10 μM), GW9508 (10 μM), vehicle (0.1% DMSO), bradykinin (1 μM), acetylcholine (1 μM), or histamine (10 μM) on transient (peak) fluorescence increases in HASM cells. Data are means ± SE presented as ΔF/Fo; n = 5–11 (shown in parentheses).
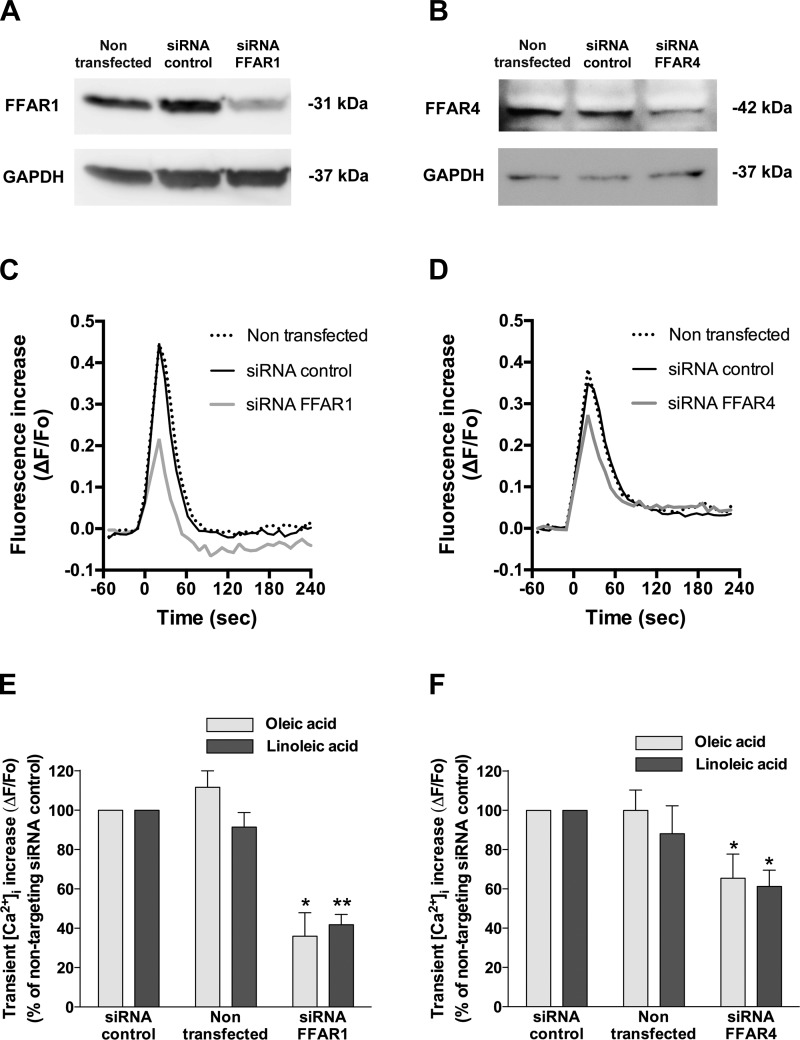
Previous findings have suggested that medium- and long-chain FFAs stimulate FFAR4 as well as FFAR1 (5, 47). To further confirm the involvement of FFAR1 and FFAR4 in FFA-induced transient [Ca2+]i increases, HASM cells were transfected with human FFAR1- or FFAR4-specific siRNA. Immunoblot analyses showed that HASM cells treated with either FFAR1- or FFAR4-specific siRNA displayed a marked decrease in protein expression of FFAR1 or FFAR4, respectively, compared with nontransfected cells or cells treated with nontargeting siRNA (Fig. 4, A and B; n = 3). Knockdown of FFAR1 significantly inhibited the transient [Ca2+]i increases induced by oleic acid (35.95 ± 11.92% of the cells treated with nontargeting siRNA; P < 0.05, n = 5) or linoleic acid (41.78 ± 5.23% of the cells treated with nontargeting siRNA; P < 0.01, n = 5; Fig. 4, C and E). Similarly, knockdown of FFAR4 in HASM cells significantly inhibited the transient [Ca2+]i increases stimulated by oleic acid [65.40 ± 12.36% of the cells treated with nontargeting siRNA; not significant (NS), n = 8] or linoleic acid (61.21 ± 8.33% of the cells treated with nontargeting siRNA; NS, n = 8), although the degree of inhibition was smaller than that induced by FFAR1 knockdown (Fig. 4, D and F). The treatment of HASM cells with nontargeting siRNA did not affect the [Ca2+]i increases induced by oleic acid (97.74 ± 10.56% of nontransfected cells; NS, n = 13) or linoleic acid (114.24 ± 9.58% of nontransfected cells; NS, n = 13). The knockdown of FFAR4 in HASM cells was further confirmed by RT-PCR (data not shown).
Fig. 4.
Involvement of FFAR1 and FFAR4 in transient [Ca2+]i increases induced by long-chain free fatty acids (oleic acid or linoleic acid) in HASM cells. A and B: representative gel images of immunoblot analyses of FFAR1 (A) and FFAR4 (B) protein expression in HASM cells that were transfected with either control nontargeting small interfering RNA (siRNA), FFAR1-specific siRNA, or FFAR4-specific siRNA (targeting for both FFAR4 transcript variant 1 and variant 2) 3 days before analysis. GAPDH was used as loading control. These images are representative of at least 3 independent experiments. C and D: representative traces of fluorescence intensity illustrating the oleic acid (10 μM)-induced [Ca2+]i increases in nontransfected HASM cells, HASM cells transfected with nontargeting siRNA control, or HASM cells transfected with either FFAR1 or FFAR4 siRNA. At time 0, oleic acid was injected to the buffer in the wells. E and F: effect of downregulation of FFAR1 (E; n = 5) or FFAR4 (F; n = 8) by siRNA on oleic acid (10 μM)- or linoleic acid (10 μM)-induced transient [Ca2+]i mobilization in HASM cells. *P < 0.05; **P < 0.01 compared with the HASM cells transfected with nontargeting siRNA control.
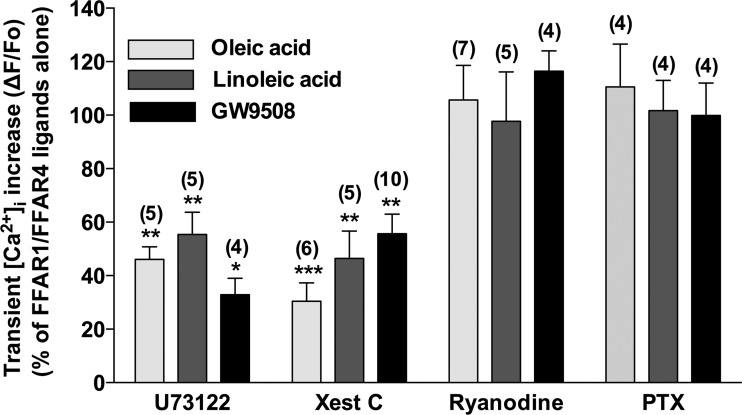
Effect of U-73122, xestospongin C, ryanodine, and PTX on FFAR1/FFAR4-stimulated transient [Ca2+]i increases in HASM cells.
To characterize the intracellular mechanisms of FFAR1/FFAR4-stimulated transient [Ca2+]i mobilization, we first examined whether these ligands stimulated [Ca2+]i mobilization through the classical Gq signaling pathway. The presence of U-73122 (5 μM), an inhibitor of PLC-β, markedly inhibited the transient increases in [Ca2+]i in response to 10 μM oleic acid (46.11 ± 4.68%; P < 0.01, n = 5), linoleic acid (55.40 ± 8.32%; P < 0.01, n = 5), and GW9508 (32.90 ± 6.08%; P < 0.05, n = 4). Similarly, in the presence of xestospongin C (20 μM), a cell-permeable inhibitor of the IP3 receptor, the transient increases in [Ca2+]i in response to 10 μM oleic acid (30.45 ± 6.85%; P < 0.001, n = 6), linoleic acid (46.40 ± 10.22%; P < 0.01, n = 5), and GW9508 (55.67 ± 7.31%; P < 0.01, n = 10) were also significantly inhibited. The ryanodine receptor along with the IP3 receptor are expressed on sarcoplasmic reticulum and participates in Ca2+ release (34). We therefore examined whether the ryanodine receptor takes part in the transient increase in [Ca2+]i stimulated by oleic acid, linoleic acid, or GW9508. The blockade of ryanodine receptor with ryanodine (100 μM), which blocks the ryanodine receptor at this higher concentration (27, 37, 44), did not affect these Ca2+ responses to 10 μM oleic acid (105.68 ± 12.91%; NS, n = 7), linoleic acid (97.68 ± 18.48%; NS, n = 5), or GW9508 (116.44 ± 7.56%; NS, n = 4). The Gi protein as well as the Gq protein also has been shown to be involved in the FFAR1-mediated signaling (21). Moreover, Gi activation releases subunits that crosstalk to activate PLC-β and release IP3, resulting in increases in Ca2+ (13, 30). Therefore, we next examined whether the Gi protein modulates the FFAR1-mediated transient [Ca2+]i increase. Pretreatment of HASM cells with PTX (100 ng/ml), an inhibitor of the Gi protein, did not exert any effect on transient [Ca2+]i increases in response to 10 μM oleic acid (110.55 ± 16.00%; NS, n = 4), linoleic acid (101.69 ± 11.32%; NS, n = 4), or GW9508 (99.89 ± 12.11%; NS, n = 4; Fig. 5).
Fig. 5.
Effects of pretreatment with U-73122 (5 μM), xestospongin C (Xest C; 20 μM), ryanodine (100 μM), or pertussis toxin (PTX; 100 ng/ml) on peak increase in [Ca2+]i stimulated by ligands for FFAR1/FFAR4 (oleic acid, linoleic acid, or GW9508; 10 μM each) in HASM cells. Data are means ± SE presented as a percentage of the ligand (10 μM)-stimulated fluorescence increases in the absence of inhibitors. *P < 0.05; **P <0.01; ***P < 0.001 compared with FFAR1/FFAR4 ligands (10 μM) alone. Numbers of experiments are shown in parentheses.
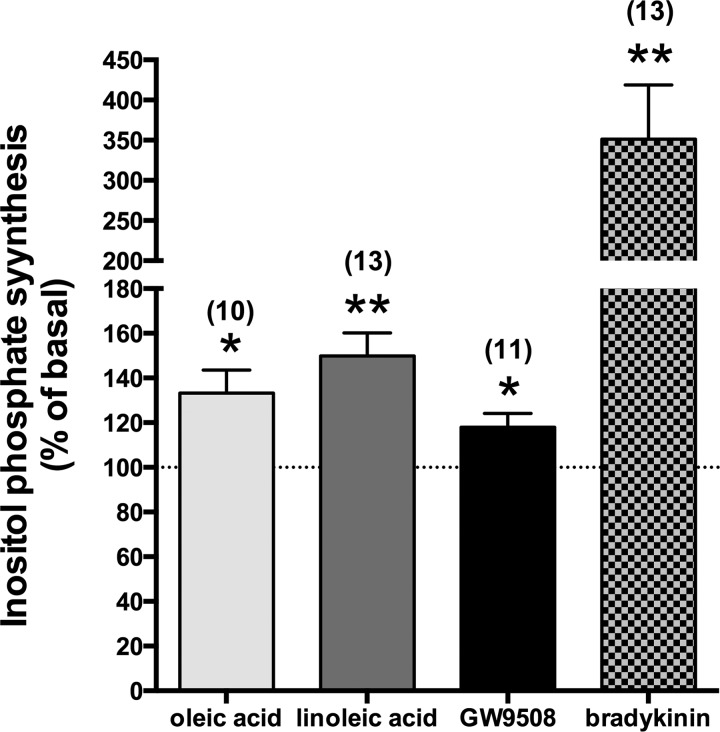
Long-chain FFAs and GW9508 induced inositol phosphate synthesis in HASM cells.
The evidence that the PLC-β inhibitor U-73122 and IP3 receptor antagonist xestospongin C inhibited the transient [Ca2+]i increases stimulated by oleic acid, linoleic acid, or GW9508 led us to examine whether these ligands stimulate inositol phosphate synthesis in HASM cells. Long-chain FFAs (oleic acid or linoleic acid; 10 μM each) and GW9508 (10 μM) significantly increased inositol phosphate synthesis (oleic acid: 133.28 ± 10.30% of basal level; P < 0.05, n = 10; linoleic acid: 149.83 ± 10.28% of basal level; P < 0.01, n = 13; GW9508: 117.92 ± 6.20% of basal level; P < 0.05, n = 11), although these effects were smaller than those induced by 1 μM bradykinin (351.40 ± 67.30% of basal level; P < 0.01, n = 13; Fig. 6), which is a potent stimulant for inositol phosphate synthesis in HASM cells (29).
Fig. 6.
Effects of ligands for FFAR1/FFAR4 (oleic acid, linoleic acid, or GW9508; 10 μM each) on the classical Gq-coupled receptor agonist bradykinin (1 μM) on the synthesis of inositol phosphate (shown as a percentage of basal level) in HASM cells. *P < 0.05; **P < 0.01 compared with basal level. Data are means ± SE. Numbers of experiments are shown in parentheses.
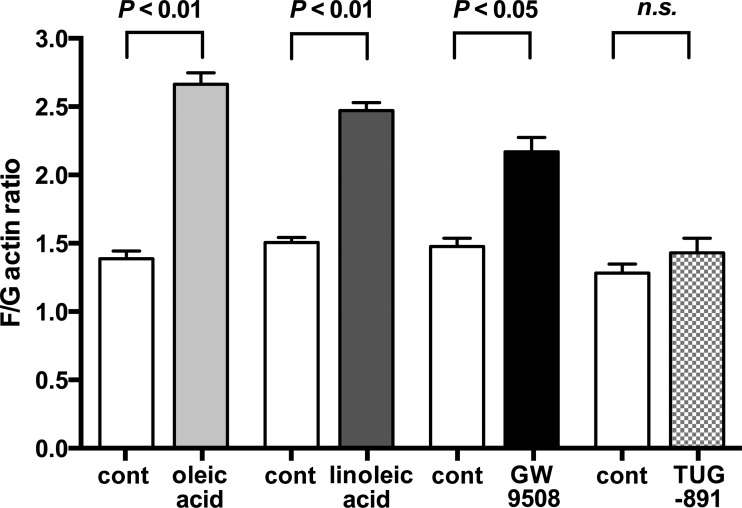
Long-chain FFAs and GW9508 induced actin reorganization in HASM cells.
In airway smooth muscle, activation of Gq-coupled receptors induces IP3 accumulation, which mobilizes Ca2+ from sarcoplasmic reticulum, leading to airway smooth muscle contraction (15, 29). Actin reorganization is a process required for smooth muscle contraction. Therefore, we examined the effects of the long-chain FFAs (oleic acid and linoleic acid), the dual agonist of FFAR1/FFAR4 (GW9508), and the highly selective agonist of FFAR4 (TUG-891) on actin reorganization in HASM cells. Oleic acid (10 μM), linoleic acid (10 μM), and GW9508 (10 μM) induced actin reorganization, whereas the specific FFAR4 agonist TUG-891 (5 μM) did not induce the reorganization (Fig. 7).
Fig. 7.
Fluorescent staining ratio of filamentous and globular actin (F/G actin) in HASM cells under untreated (cont) conditions or treated with oleic acid (10 μM; n = 6), linoleic acid (10 μM; n = 7), GW9508 (10 μM; n = 5), or TUG-891 (5 μM; n = 7). Data are means ± SE. An increase in F/G actin indicates an increase in filamentous actin, which is related to smooth muscle cell contraction.
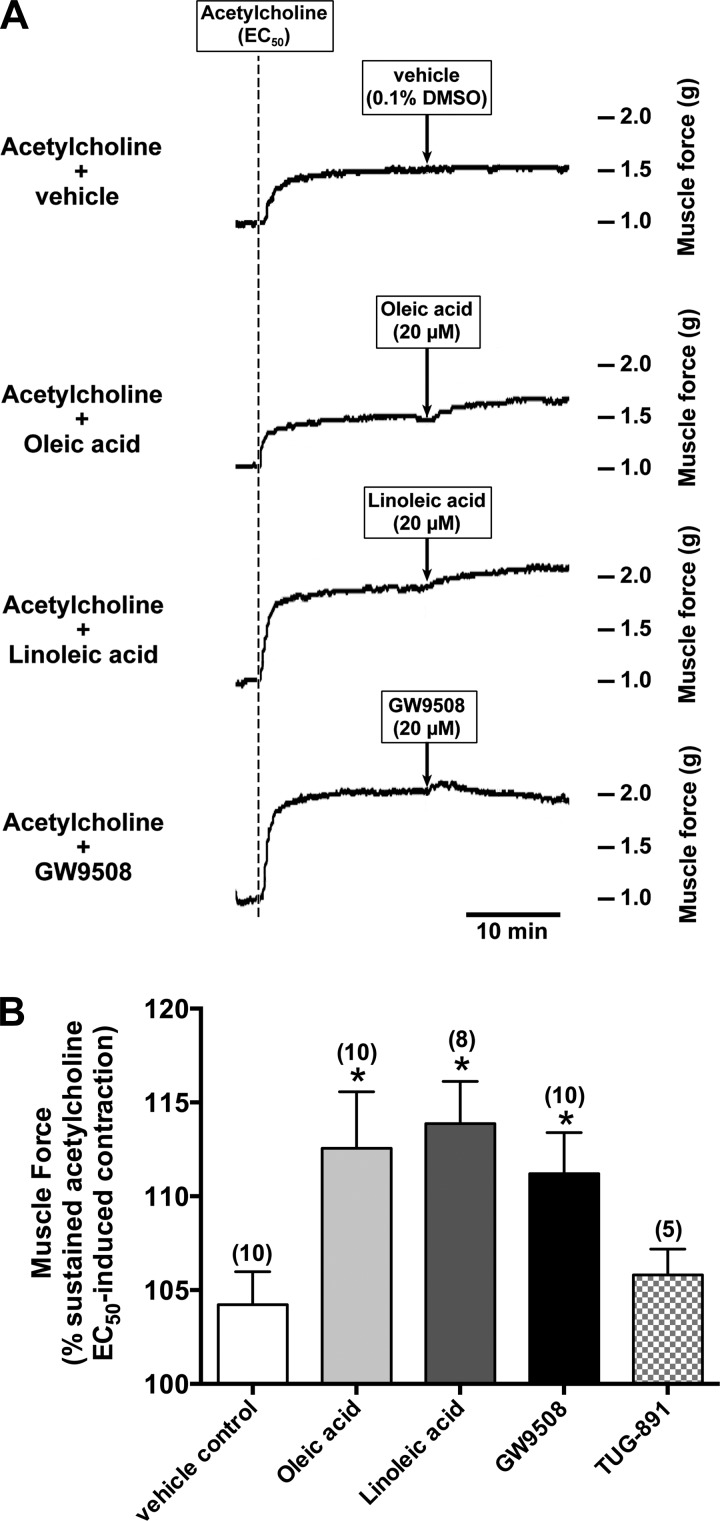
Long-chain FFAs and GW9508 potentiated acetylcholine-induced guinea pig airway contraction.
To evaluate whether acetylcholine-induced airway smooth muscle contraction was potentiated by long-chain FFAs and GW9508, guinea pig tracheal rings were contracted with acetylcholine (EC50) followed by the long-chain FFAs (oleic acid or linoleic acid; 20 μM), the selective agonist of FFAR1/FFAR4 (GW9508; 20 μM), and the highly selective agonist of FFAR4 (TUG-891; 20 μM). Acetylcholine-induced contraction in guinea pig tracheal rings were significantly potentiated by oleic acid (112.56 ± 3.00% of sustained acetylcholine EC50-induced contraction; P < 0.05 compared with vehicle control, n = 10), linoleic acid (113.87 ± 2.24% of sustained acetylcholine EC50-induced contraction; P < 0.05 compared with vehicle control, n = 8), and GW9508 (111.19 ± 2.20% of sustained acetylcholine EC50-induced contraction; P < 0.01 compared with vehicle control, n = 10). Both oleic acid and linoleic acid induced a sustained potentiation of contractile tone, whereas GW9508 induced a transient potentiation of contractile tone. In contrast, TUG-891 did not significantly potentiated acetylcholine-induced contraction (105.80 ± 1.38% of sustained acetylcholine EC50-induced contraction; NS compared with vehicle control, n = 5; Fig. 8).
Fig. 8.
Ligands for FFAR1/FFAR4 (oleic acid, linoleic acid, or GW9508) potentiated acetylcholine-induced contractions in guinea pig tracheal rings. A: representative tension tracing in guinea pig tracheal ring illustrating potentiation of acetylcholine (EC50) contraction by the natural ligands for FFAR1/FFAR4 (oleic acid or linoleic acid; 20 μM each) or the_FFAR1/FFAR4-selective agonist GW9508 (20 μM). B: oleic acid, linoleic acid, or GW9508 significantly potentiated the acetylcholine (EC50)-induced contraction, whereas the highly selective FFAR4 agonist TUG-891 (20 μM) did not potentiate the contraction. Data are means ± SE. *P < 0.05 compared with vehicle (0.1% DMSO) control. Numbers of experiments are shown in parentheses.
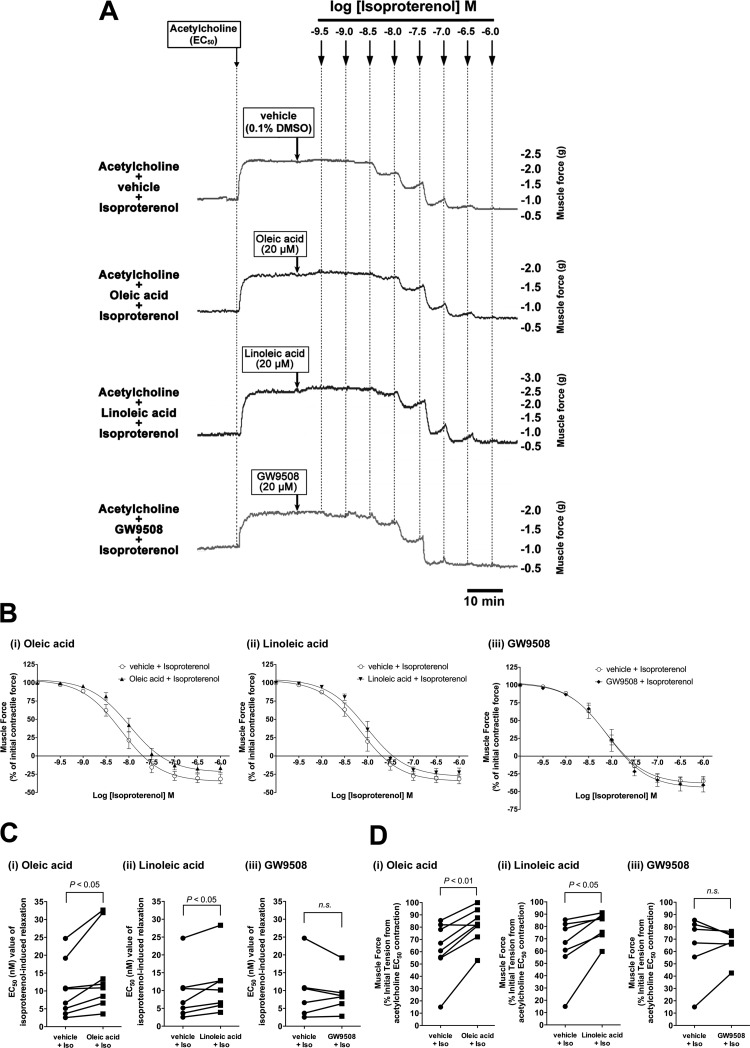
Long-chain FFAs attenuated isoproterenol-induced airway relaxation after acetylcholine-induced guinea pig airway contraction.
In asthmatic individuals, β-adrenoceptor agonists are the mainstay for clinical relaxation of constricted airway smooth muscle. To evaluate whether the activation of FFAR1/FFAR4 affects β-adrenoceptor agonist-induced airway smooth muscle relaxation after an acetylcholine-induced contraction, guinea pig tracheal rings were contracted with acetylcholine (EC50) followed by the long-chain FFAs (oleic acid or linoleic acid; 20 μM each) or GW9508 (20 μM), followed by relaxation induced by cumulatively increasing concentrations of isoproterenol. After contraction with acetylcholine, a significant rightward shift in the EC50 for the isoproterenol dose-response relaxation curve occurred in the presence of oleic acid (EC50 = 14.93 ± 3.94 nM with isoproterenol + oleic acid vs. 10.39 ± 2.77 nM with isoproterenol + vehicle; P < 0.05, n = 8) and linoleic acid (EC50 = 11.47 ± 3.16 nM with isoproterenol + linoleic acid vs. 9.14 ± 2.86 nM with isoproterenol + vehicle; P < 0.05, n = 7). However, GW9508 (20 μM) did not exert a significant effect on isoproterenol-induced relaxation (EC50 = 9.07 ± 2.24 nM with isoproterenol + GW9508 vs. 9.82 ± 3.29 nM with isoproterenol + vehicle; NS, n = 6; Fig. 9, A–C). The magnitude of the relaxation achieved by isoproterenol at 10−8.5 M was significantly attenuated by oleic acid or linoleic acid (oleic acid: muscle force = 81.45 ± 5.08% of initial acetylcholine-induced force for oleic acid + isoproterenol vs. 62.34 ± 7.94% for vehicle + isoproterenol, n = 8, P < 0.01; linoleic acid: muscle force = 80.35 ± 4.32% of initial acetylcholine-induced force for linoleic acid + isoproterenol vs. 63.41 ± 9.08% for vehicle + isoproterenol; P < 0.05, n = 7), whereas GW9508 did not exert significant effect on the relaxation (muscle force = 66.65 ± 5.09% of initial acetylcholine-induced force for GW9508 + isoproterenol vs. 63.85 ± 10.74% for vehicle + isoproterenol, NS, n = 6; Fig. 9D).
Fig. 9.
A: representative tension tracing in guinea pig tracheal rings after an acetylcholine (EC50) contraction followed by increasing concentrations of the β-adrenoceptor agonist isoproterenol in the presence of oleic acid (20 μM), linoleic acid (20 μM), GW9508 (20 μM), or their vehicle (0.1% DMSO). B: isoproterenol concentration-response relaxation curves in the presence of vehicle (0.1% DMSO) or oleic acid (20 μM; n = 8), linoleic acid (20 μM; n = 7), or GW9508 (20 μM; n = 6). C: EC50 value of isoproterenol-induced relaxation of an acetylcholine (EC50) contraction in the presence of oleic acid (20 μM; n = 8), linoleic acid (20 μM; n = 7), or GW9508 (20 μM; n = 6) compared with vehicle (0.1% DMSO). D: muscle force remaining after isoproterenol (10−8.5 M)-induced relaxation of acetylcholine (EC50) contraction in the presence of oleic acid (20 μM; n = 8), linoleic acid (20 μM; n = 7), or GW9508 (20 μM; n = 6) compared with vehicle control.
DISCUSSION
In the present study, we demonstrate for the first time that FFAR1 and FFAR4 are expressed in both human and guinea pig airway smooth muscle tissue and cultured HASM cells in primary culture. Activation of these fatty acid receptors by the long-chain FFAs or GW9508 (selective agonist for FFAR1 and FFAR4) increased [Ca2+]i in HASM cells in a dose-dependent manner, and the transient [Ca2+]i increases were primarily mediated via classical Gq protein signaling mechanisms. Furthermore, long-chain FFAs induced stress fiber formation, potentiated acetylcholine-induced airway smooth muscle contraction, and attenuated the relaxant effect of isoproterenol after an acetylcholine-induced contraction.
FFAs, especially the long-chain FFAs, are important in the development of many metabolic diseases including obesity, diabetes, and atherosclerosis (49). Recent evidence has shown that both FFAR1 and FFAR4 work as the sensor of medium- to long-chain FFAs (5, 22, 25, 47). In the airways, mRNA encoding FFAR1 has been demonstrated in human lung (5) and human bronchial epithelial cells (18). Furthermore, Einstein et al. (12), using expression microarrays, has revealed that mRNA encoding both FFAR1 and FFAR4 are expressed in HASM cells. In the present study, immunoblot analyses revealed the protein expression of both FFAR1 and FFAR4 in both human and guinea pig airway smooth muscle. Detection of mRNA by RT-PCR further revealed that the FFAR4 transcript variant 2 was the only variant of FFAR4 detected in human airway smooth muscle. Immunohistochemical analysis further confirmed the protein expression of both FFAR1 and FFAR4 on the airway smooth muscle layer of both human and guinea pig. Although FFAR1 and FFAR4 have been reported to be colocalized in taste buds (7) and intestine (11, 22), our findings further suggest that these receptors are also colocalized in airway smooth muscle itself.
Molecular identification of both FFAR1 and FFAR4 on airway smooth muscle itself led us to question whether these receptors could modulate airway smooth muscle tone. We first sought to assess its ability to increase intracellular Ca2+, which leads to airway smooth muscle contraction. FFAR1 and FFAR4 are classified as Gq-coupled receptors and are activated by medium- and long-chain FFAs (5, 22, 25). In airway smooth muscle cells, the activation of Gq-coupled receptors (e.g., neurokinin receptors, muscarinic M3 receptor, and histamine H1 receptor) stimulates intracellular [Ca2+]i increases (29, 32). Long-chain FFAs stimulate both FFAR1 and FFAR4 and evoke transient [Ca2+]i increases in pancreatic β-cells (5, 16, 40) and breast cancer cell lines (21, 48). Consistent with these previous findings, the present study showed that long-chain FFAs (oleic acid and linoleic acid) and a synthetic agonist of both FFAR1 and FFAR4 (GW9508) evoked transient [Ca2+]i increases in HASM cells in a dose-dependent manner. Furthermore, in HASM cells with reduced FFAR1 or FFAR4 expression induced by specific siRNAs, transient [Ca2+]i increases evoked by long-chain FFAs were significantly inhibited compared with nontransfected HASM cells or cells transfected with nontargeting siRNA. Briscoe et al. (4, 5) showed that long-chain FFAs stimulated [Ca2+]i in human embryonic kidney (HEK)-293 cells expressing FFAR1, and the range of their pEC50 values was 4.39–5.94. Itoh et al. (25) also showed that EC50 values of either oleic acid- or linoleic acid-stimulated [Ca2+]i rise in Chinese hamster ovary (CHO) cells expressing FFAR1 were 2.0 ± 0.3 or 1.8 ± 0.1 μM, respectively. These pEC50 values are similar to our findings for oleic acid (pEC50 = 5.75 ± 0.08) or linoleic acid (pEC50 = 6.00 ± 0.13) for [Ca2+]i increases in HASM cells. Previous studies showed that total plasma concentrations of FFAs in humans are 90-2,500 μM, and the highest values appear during fasting, strenuous exercise, or diabetes (38, 43). However, 99% or more of FFAs are bound to serum albumin (43), and the unbound form of FFAs is capable of interacting with FFAR1 to increase [Ca2+]i (16, 25). In addition, it has been reported that the effective concentration of unbound FFAs to release insulin in a pancreatic β-cell line is 10 μM (14, 25). These findings suggest that physiological serum concentrations of FFAs could stimulate [Ca2+]i mobilization in HASM cells.
Similarly to FFAR1, knockdown of the FFAR4-GPR120S isoform with siRNA partially but significantly inhibited the FFA-evoked transient [Ca2+]i, although the degree of inhibition was smaller than that induced by FFAR1 knockdown. FFAR4 protein has two isoforms (FFAR4-GPR120L and FFAR4-GPR120S), and FFAR4-GPR120S is the dominant isoform for both oleic acid- and GW9508-induced [Ca2+]i mobilization in HEK-293 cells expressing GPR120 (47). In our findings, the FFAR4 transcript variant 2 (mRNA for FFAR4-GPR120S protein) was the dominant isoform in human airway smooth muscle. Taking these findings together, long-chain FFAs increase [Ca2+]i in HASM cells through both FFAR1 and FFAR4-GPR120S.
Previous studies have suggested that human FFAR1 is coupled to both Gqα and Giα proteins in a human breast cancer cell line (48). Conversely, in pancreatic β-cells, FFAR1 solely coupled to Gqα, and an endogenous agonist such as linoleic acid promoted PLC activation by the Gqα protein through FFAR1 (5, 16). In the present study, transient [Ca2+]i increases induced by long-chain FFAs or the FFAR1/FFAR4-selective agonist GW9508 were markedly inhibited by both the PLC inhibitor U-73122 and the IP3 receptor antagonist xestospongin C, whereas the blockade of Giα protein by PTX was without effect. In addition, long-chain FFAs and GW9508 significantly stimulated inositol phosphate synthesis in HASM cells. These results taken together suggest that the FFAR1/FFAR4 receptors are solely coupled to the Gqα protein in HASM cells, rather than to the Giα protein. In smooth muscle, the ryanodine receptor as well as the IP3 receptor mediates Ca2+ release from sarcoplasmic reticulum (34). However, in HASM cells, transient [Ca2+]i increases induced by neurokinins (whose receptors also couple to the Gqα protein) were not impaired by blockade of the ryanodine receptor with high concentrations of ryanodine (29). Similarly, in the present study, FFAR1/FFAR4-activated transient [Ca2+]i increases in HASM cells were not inhibited by blockade of the ryanodine receptor. These findings suggest that FFAR1/FFAR4-stimulated transient [Ca2+]i increases are predominantly mediated via the classical Gq-PLC/IP3 pathway.
Because the activation of Gq-coupled receptors expressed in airway smooth muscle induces airway contraction (19), we further examined whether the Gq-coupled FFAR1/FFAR4 receptors could induce stress fiber formation and modulate airway smooth muscle tone. As expected, activation of FFAR1 with oleic acid, linoleic acid, or GW9508 significantly induced stress fiber formation and additional airway contraction following an acetylcholine (EC50)-induced contraction. Furthermore, when the long-chain FFAs were added after the acetylcholine-induced contraction, the relaxant effect of isoproterenol was significantly attenuated, indicating that FFA hampers airway relaxation. In contrast, activation of FFAR4 with TUG-891 did not exert any effect on stress fiber formation and acetylcholine-induced contraction. Collectively, these results suggest that although both FFAR1 and FFAR4 are expressed on airway smooth muscle and modulate [Ca2+]i FFAR1 is the dominant sensor for FFA-induced contraction.
Although GW9508 by itself induced additional airway constriction after acetylcholine contraction, GW9508 did not significantly attenuate isoproterenol-induced airway relaxation. Similarly, in the present study, transient [Ca2+]i increases in HASM cells stimulated by GW9508 were less potent than those stimulated by oleic acid and linoleic acid. Collectively, these findings suggest that GW9508 was less potent than long-chain FFAs in airway smooth muscle. In MIN6 cells, [Ca2+]i increase induced by other long-chain free fatty acid linolenic acid (100 μM) was greater than induced by GW9508 (100 μM) (20). Briscoe et al. (4) also showed that the maximum response of GW9508 in [Ca2+]i increase in HEK-293 cells expressing FFAR1 was smaller than those in response to long-chain FFAs, including linoleic acid, and reported that linoleic acid was more efficacious because GW9508 only appeared to be a partial agonist at FFAR1. Ou et al. (36) also reported that GW9508 exerts a partial agonist effect to regulate blood glucose. These findings suggest that long-chain FFAs had more potent effects than GW9508 on FFAR1. It is also possible that the effects of long-chain FFAs do not rely exclusively on FFAR1 in airway smooth muscle. For example, nuclear receptor PPAR-δ is involved in long-chain FFA-mediated intracellular signaling (39). It is also well accepted that the effects of FFAs are attributed to their intracellular metabolism to long-chain acyl-CoA esters, which stimulate Ca2+ release from sarcoplasmic reticulum in skeletal muscle cells (17). In pancreatic β-cells, both FFAR1-mediated and intracellular metabolite-mediated signaling pathways contributed to the increase in [Ca2+]i by linoleic acid stimulation, and the transient initial increase in [Ca2+]i was due to FFAR1-mediated Ca2+ release from intracellular Ca2+ stores, whereas the long-lasting second-phase increase in [Ca2+]i was mediated via the intracellular metabolite signaling pathway (50). In airway smooth muscle, the initial [Ca2+]i transient is responsible for tension development, and Ca2+ oscillations are required for sustained airway contraction (2). In our study, airway constriction induced by FFAs was long lasting, whereas that induced by GW9508 was transient. These findings suggest that FFAR1/FFAR4-mediated transient [Ca2+]i increases may contribute to the initiation of tension development in airway smooth muscle and that another, unidentified signaling mechanism is required for the sustained effect of FFAs on increased airway smooth muscle contraction. However, limitations of the present study are that we did not examine 1) whether the metabolites of FFAs increase [Ca2+]i in HASM cells, and 2) whether stimulation of FFAR1/FFAR4 by long-chain FFAs and GW9508 induces Ca2+ oscillations in HASM cells. Further studies are required to further define the precise intracellular mechanisms by which long-chain FFAs induce airway smooth muscle contraction.
In summary, we have demonstrated for the first time that functional FFAR1 and FFAR4 are expressed in both human and guinea pig airway smooth muscle. Although activation of FFAR1 or FFAR4 produced transient [Ca2+]i increases through the classical Gq pathway, FFAR1 is the sole receptor for airway smooth muscle contraction. These findings suggest that the long-chain free fatty acid sensor FFAR1 on airway smooth muscle could be an important regulator of airway smooth muscle tone and might play a pivotal role linking obesity to asthma.
GRANTS
This work was supported by National Institute of General Medical Sciences Grant GM-065281 (to C. W. Emala Sr.), Japan Society for the Promotion of Science Grants-in-Aid 24689072 (to K. Mizuta) and 23792311 (to F. Mizuta), and a research grant from Takeda Science Foundation (to K. Mizuta).
DISCLOSURES
No conflicts of interest, financial or otherwise are declared by the authors.
AUTHOR CONTRIBUTIONS
K.M. and C.W.E. conception and design of research; K.M., Y.Z., F.M., H.H., T.S., and C.W.E. performed experiments; K.M., Y.Z., F.M., H.H., T.S., and C.W.E. analyzed data; K.M., Y.Z., F.M., H.H., T.S., and C.W.E. interpreted results of experiments; K.M. and C.W.E. prepared figures; K.M., E.M., and C.W.E. drafted manuscript; K.M., E.M., and C.W.E. edited and revised manuscript; K.M., Y.Z., F.M., H.H., T.S., E.M., and C.W.E. approved final version of manuscript.
REFERENCES
- 1.Arteaga-Solis E, Zee T, Emala CW, Vinson C, Wess J, Karsenty G. Inhibition of leptin regulation of parasympathetic signaling as a cause of extreme body weight-associated asthma. Cell Metab 17: 35–48, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bergner A, Sanderson MJ. Acetylcholine-induced calcium signaling and contraction of airway smooth muscle cells in lung slices. J Gen Physiol 119: 187–198, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bjorntorp P, Bergman H, Varnauskas E. Plasma free fatty acid turnover rate in obesity. Acta Med Scand 185: 351–356, 1969. [DOI] [PubMed] [Google Scholar]
- 4.Briscoe CP, Peat AJ, McKeown SC, Corbett DF, Goetz AS, Littleton TR, McCoy DC, Kenakin TP, Andrews JL, Ammala C, Fornwald JA, Ignar DM, Jenkinson S. Pharmacological regulation of insulin secretion in MIN6 cells through the fatty acid receptor GPR40: identification of agonist and antagonist small molecules. Br J Pharmacol 148: 619–628, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Briscoe CP, Tadayyon M, Andrews JL, Benson WG, Chambers JK, Eilert MM, Ellis C, Elshourbagy NA, Goetz AS, Minnick DT, Murdock PR, Sauls HR Jr, Shabon U, Spinage LD, Strum JC, Szekeres PG, Tan KB, Way JM, Ignar DM, Wilson S, Muir AI. The orphan G protein-coupled receptor GPR40 is activated by medium and long chain fatty acids. J Biol Chem 278: 11303–11311, 2003. [DOI] [PubMed] [Google Scholar]
- 6.Brown AJ, Goldsworthy SM, Barnes AA, Eilert MM, Tcheang L, Daniels D, Muir AI, Wigglesworth MJ, Kinghorn I, Fraser NJ, Pike NB, Strum JC, Steplewski KM, Murdock PR, Holder JC, Marshall FH, Szekeres PG, Wilson S, Ignar DM, Foord SM, Wise A, Dowell SJ. The orphan G protein-coupled receptors GPR41 and GPR43 are activated by propionate and other short chain carboxylic acids. J Biol Chem 278: 11312–11319, 2003. [DOI] [PubMed] [Google Scholar]
- 7.Cartoni C, Yasumatsu K, Ohkuri T, Shigemura N, Yoshida R, Godinot N, le Coutre J, Ninomiya Y, Damak S. Taste preference for fatty acids is mediated by GPR40 and GPR120. J Neurosci 30: 8376–8382, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cintra DE, Ropelle ER, Moraes JC, Pauli JR, Morari J, Souza CT, Grimaldi R, Stahl M, Carvalheira JB, Saad MJ, Velloso LA. Unsaturated fatty acids revert diet-induced hypothalamic inflammation in obesity. PLoS One 7: e30571, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Del Guerra S, Bugliani M, D'Aleo V, Del Prato S, Boggi U, Mosca F, Filipponi F, Lupi R. G-protein-coupled receptor 40 (GPR40) expression and its regulation in human pancreatic islets: the role of type 2 diabetes and fatty acids. Nutr Metab Cardiovasc Dis 20: 22–25, 2010. [DOI] [PubMed] [Google Scholar]
- 10.Dixon AE, Holguin F, Sood A, Salome CM, Pratley RE, Beuther DA, Celedon JC, Shore SA. An official American Thoracic Society Workshop report: obesity and asthma. Proc Am Thorac Soc 7: 325–335, 2010. [DOI] [PubMed] [Google Scholar]
- 11.Edfalk S, Steneberg P, Edlund H. Gpr40 is expressed in enteroendocrine cells and mediates free fatty acid stimulation of incretin secretion. Diabetes 57: 2280–2287, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Einstein R, Jordan H, Zhou W, Brenner M, Moses EG, Liggett SB. Alternative splicing of the G protein-coupled receptor superfamily in human airway smooth muscle diversifies the complement of receptors. Proc Natl Acad Sci USA 105: 5230–5235, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ethier MF, Madison JM. Adenosine A1 receptors mediate mobilization of calcium in human bronchial smooth muscle cells. Am J Respir Cell Mol Biol 35: 496–502, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Feng DD, Luo Z, Roh SG, Hernandez M, Tawadros N, Keating DJ, Chen C. Reduction in voltage-gated K+ currents in primary cultured rat pancreatic β-cells by linoleic acids. Endocrinology 147: 674–682, 2006. [DOI] [PubMed] [Google Scholar]
- 15.Fryer AD, Jacoby DB. Muscarinic receptors and control of airway smooth muscle. Am J Respir Crit Care Med 158: S154–S160, 1998. [DOI] [PubMed] [Google Scholar]
- 16.Fujiwara K, Maekawa F, Yada T. Oleic acid interacts with GPR40 to induce Ca2+ signaling in rat islet β-cells: mediation by PLC and L-type Ca2+ channel and link to insulin release. Am J Physiol Endocrinol Metab 289: E670–E677, 2005. [DOI] [PubMed] [Google Scholar]
- 17.Fulceri R, Nori A, Gamberucci A, Volpe P, Giunti R, Benedetti A. Fatty acyl-CoA esters induce calcium release from terminal cisternae of skeletal muscle. Cell Calcium 15: 109–116, 1994. [DOI] [PubMed] [Google Scholar]
- 18.Gras D, Chanez P, Urbach V, Vachier I, Godard P, Bonnans C. Thiazolidinediones induce proliferation of human bronchial epithelial cells through the GPR40 receptor. Am J Physiol Lung Cell Mol Physiol 296: L970–L978, 2009. [DOI] [PubMed] [Google Scholar]
- 19.Hall IP. Second messengers, ion channels and pharmacology of airway smooth muscle. Eur Respir J 15: 1120–1127, 2000. [DOI] [PubMed] [Google Scholar]
- 20.Hara T, Hirasawa A, Sun Q, Koshimizu TA, Itsubo C, Sadakane K, Awaji T, Tsujimoto G. Flow cytometry-based binding assay for GPR40 (FFAR1; free fatty acid receptor 1). Mol Pharmacol 75: 85–91, 2009. [DOI] [PubMed] [Google Scholar]
- 21.Hardy S, St-Onge GG, Joly E, Langelier Y, Prentki M. Oleate promotes the proliferation of breast cancer cells via the G protein-coupled receptor GPR40. J Biol Chem 280: 13285–13291, 2005. [DOI] [PubMed] [Google Scholar]
- 22.Hirasawa A, Tsumaya K, Awaji T, Katsuma S, Adachi T, Yamada M, Sugimoto Y, Miyazaki S, Tsujimoto G. Free fatty acids regulate gut incretin glucagon-like peptide-1 secretion through GPR120. Nat Med 11: 90–94, 2005. [DOI] [PubMed] [Google Scholar]
- 23.Hirshman CA, Emala CW. Actin reorganization in airway smooth muscle cells involves Gq and Gi-2 activation of Rho. Am J Physiol Lung Cell Mol Physiol 277: L653–L661, 1999. [DOI] [PubMed] [Google Scholar]
- 24.Hotta K, Emala CW, Hirshman CA. TNF-α upregulates Giα and Gqα protein expression and function in human airway smooth muscle cells. Am J Physiol Lung Cell Mol Physiol 276: L405–L411, 1999. [DOI] [PubMed] [Google Scholar]
- 25.Itoh Y, Kawamata Y, Harada M, Kobayashi M, Fujii R, Fukusumi S, Ogi K, Hosoya M, Tanaka Y, Uejima H, Tanaka H, Maruyama M, Satoh R, Okubo S, Kizawa H, Komatsu H, Matsumura F, Noguchi Y, Shinohara T, Hinuma S, Fujisawa Y, Fujino M. Free fatty acids regulate insulin secretion from pancreatic beta cells through GPR40. Nature 422: 173–176, 2003. [DOI] [PubMed] [Google Scholar]
- 26.Jooste E, Zhang Y, Emala CW. Rapacuronium preferentially antagonizes the function of M2 versus M3 muscarinic receptors in guinea pig airway smooth muscle. Anesthesiology 102: 117–124, 2005. [DOI] [PubMed] [Google Scholar]
- 27.MacMillan D, Chalmers S, Muir TC, McCarron JG. IP3-mediated Ca2+ increases do not involve the ryanodine receptor, but ryanodine receptor antagonists reduce IP3-mediated Ca2+ increases in guinea-pig colonic smooth muscle cells. J Physiol 569: 533–544, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Meidute Abaraviciene S, Muhammed SJ, Amisten S, Lundquist I, Salehi A. GPR40 protein levels are crucial to the regulation of stimulated hormone secretion in pancreatic islets. Lessons from spontaneous obesity-prone and non-obese type 2 diabetes in rats. Mol Cell Endocrinol 381: 150–159, 2013. [DOI] [PubMed] [Google Scholar]
- 29.Mizuta K, Gallos G, Zhu D, Mizuta F, Goubaeva F, Xu D, Panettieri RA Jr, Yang J, Emala CW Sr. Expression and coupling of neurokinin receptor subtypes to inositol phosphate and calcium signaling pathways in human airway smooth muscle cells. Am J Physiol Lung Cell Mol Physiol 294: L523–L534, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mizuta K, Mizuta F, Xu D, Masaki E, Panettieri RA Jr, Emala CW. Gi-coupled gamma-aminobutyric acid-B receptors cross-regulate phospholipase C and calcium in airway smooth muscle. Am J Respir Cell Mol Biol 45: 1232–1238, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mizuta K, Zhang Y, Xu D, Masaki E, Panettieri RA Jr, Emala CW. The dopamine D2 receptor is expressed and sensitizes adenylyl cyclase activity in airway smooth muscle. Am J Physiol Lung Cell Mol Physiol 302: L316–L324, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Murray RK, Kotlikoff MI. Receptor-activated calcium influx in human airway smooth muscle cells. J Physiol 435: 123–144, 1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nakamoto K, Nishinaka T, Matsumoto K, Kasuya F, Mankura M, Koyama Y, Tokuyama S. Involvement of the long-chain fatty acid receptor GPR40 as a novel pain regulatory system. Brain Res 1432: 74–83, 2012. [DOI] [PubMed] [Google Scholar]
- 34.Narayanan D, Adebiyi A, Jaggar JH. Inositol trisphosphate receptors in smooth muscle cells. Am J Physiol Heart Circ Physiol 302: H2190–H2210, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Oh DY, Talukdar S, Bae EJ, Imamura T, Morinaga H, Fan W, Li P, Lu WJ, Watkins SM, Olefsky JM. GPR120 is an omega-3 fatty acid receptor mediating potent anti-inflammatory and insulin-sensitizing effects. Cell 142: 687–698, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ou HY, Wu HT, Hung HC, Yang YC, Wu JS, Chang CJ. Multiple mechanisms of GW-9508, a selective G protein-coupled receptor 40 agonist, in the regulation of glucose homeostasis and insulin sensitivity. Am J Physiol Endocrinol Metab 304: E668–E676, 2013. [DOI] [PubMed] [Google Scholar]
- 37.Pessah IN, Zimanyi I. Characterization of multiple [3H]ryanodine binding sites on the Ca2+ release channel of sarcoplasmic reticulum from skeletal and cardiac muscle: evidence for a sequential mechanism in ryanodine action. Mol Pharmacol 39: 679–689, 1991. [PubMed] [Google Scholar]
- 38.Potter BJ, Sorrentino D, Berk PD. Mechanisms of cellular uptake of free fatty acids. Annu Rev Nutr 9: 253–270, 1989. [DOI] [PubMed] [Google Scholar]
- 39.Ravnskjaer K, Frigerio F, Boergesen M, Nielsen T, Maechler P, Mandrup S. PPARδ is a fatty acid sensor that enhances mitochondrial oxidation in insulin-secreting cells and protects against fatty acid-induced dysfunction. J Lipid Res 51: 1370–1379, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schnell S, Schaefer M, Schofl C. Free fatty acids increase cytosolic free calcium and stimulate insulin secretion from β-cells through activation of GPR40. Mol Cell Endocrinol 263: 173–180, 2007. [DOI] [PubMed] [Google Scholar]
- 41.Shore SA. Obesity, airway hyperresponsiveness, and inflammation. J Appl Physiol 108: 735–743, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shore SA, Johnston RA. Obesity and asthma. Pharmacol Ther 110: 83–102, 2006. [DOI] [PubMed] [Google Scholar]
- 43.Spector AA, Hoak JC. Letter: Fatty acids, platelets, and microcirculatory obstruction. Science 190: 490–492, 1975. [DOI] [PubMed] [Google Scholar]
- 44.Thompson MA, Prakash YS, Pabelick CM. Arachidonate-regulated Ca2+ influx in human airway smooth muscle. Am J Respir Cell Mol Biol 51: 68–76, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Togashi H, Emala CW, Hall IP, Hirshman CA. Carbachol-induced actin reorganization involves Gi activation of Rho in human airway smooth muscle cells. Am J Physiol Lung Cell Mol Physiol 274: L803–L809, 1998. [DOI] [PubMed] [Google Scholar]
- 46.Wang J, Wu X, Simonavicius N, Tian H, Ling L. Medium-chain fatty acids as ligands for orphan G protein-coupled receptor GPR84. J Biol Chem 281: 34457–34464, 2006. [DOI] [PubMed] [Google Scholar]
- 47.Watson SJ, Brown AJ, Holliday ND. Differential signaling by splice variants of the human free fatty acid receptor GPR120. Mol Pharmacol 81: 631–642, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yonezawa T, Katoh K, Obara Y. Existence of GPR40 functioning in a human breast cancer cell line, MCF-7. Biochem Biophys Res Commun 314: 805–809, 2004. [DOI] [PubMed] [Google Scholar]
- 49.Yonezawa T, Kurata R, Yoshida K, Murayama MA, Cui X, Hasegawa A. Free fatty acids-sensing G protein-coupled receptors in drug targeting and therapeutics. Curr Med Chem 20: 3855–3871, 2013. [DOI] [PubMed] [Google Scholar]
- 50.Zhao Y, Wang L, Qiu J, Zha D, Sun Q, Chen C. Linoleic acid stimulates [Ca2+]i increase in rat pancreatic beta-cells through both membrane receptor- and intracellular metabolite-mediated pathways. PLoS One 8: e60255, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]