Abstract
An increase in oxygen tension at birth is one of the key signals that initiate pulmonary vasodilation in the fetal lung. We investigated the hypothesis that targeting endothelial nitric oxide synthase (eNOS) to the mitochondrial outer membrane regulates reactive oxygen species (ROS) formation in the fetal pulmonary artery endothelial cells (PAEC) during this transition. We isolated PAEC and pulmonary arteries from 137-day gestation fetal lambs (term = 144 days). We exposed PAEC to a simulated transition from fetal to (3% O2) to normoxic (21%) or hyperoxic (95% O2) postnatal Po2 or to the nitric oxide synthase (NOS) agonist ATP. We assessed the effect of O2 and ATP on eNOS interactions with the mitochondrial outer membrane protein porin and with the chaperone hsp90. We also investigated the effect of decoy peptides that blocked eNOS interactions with porin or hsp90 on PAEC angiogenesis and vasodilator function of pulmonary arteries. Transition of fetal PAEC from 3 to 21% O2 but not to 95% O2 or exposure to ATP increased eNOS association with hsp90 and porin. Decoy peptides that blocked eNOS interactions decreased NO release, increased O2 consumption and mitochondrial ROS levels, and impaired PAEC angiogenesis. Decoy peptides also inhibited the relaxation responses of pulmonary artery rings and dilation of resistance size pulmonary arteries to ATP. The mitochondrial-antioxidant mito-ubiquinone restored the response to ATP in decoy peptide-treated pulmonary arteries. These data indicate that targeting eNOS to mitochondria decreases endothelial oxidative stress and facilitates vasodilation in fetal pulmonary circulation at birth.
Keywords: mitochondrial superoxide, antioxidants, vasodilation, fetal lung, pulmonary circulation
fetal pulmonary vascular resistance (PVR) undergoes a rapid decrease at birth to facilitate gas exchange during postnatal life. The decrease in PVR is primarily mediated by exposure of the fetal lung to higher oxygen tension at birth (18). Endothelial nitric oxide synthase (eNOS) plays a central role in mediating this pulmonary vasodilation at birth (30). Oxygen exposure at birth stimulates eNOS both directly (24) and indirectly by increasing oxidative phosphorylation and release of ATP from fetal red blood cells (12, 13). ATP stimulates NO release (13) by promoting the association of eNOS with the chaperone hsp90, a key determinant of the balance of nitric oxide (NO) and superoxide (O2·−) release from eNOS (14, 20). Oxidative phosphorylation in mitochondria is known to generate O2·− as a byproduct in the vascular cells (1). Despite a dramatic increase in oxygen concentration that occurs in the lung at birth, mechanisms regulating mitochondrial O2·− generation in pulmonary artery endothelial cells (PAEC) during this transition remain unclear.
Some insights into the potential mechanisms come from previous studies of the role of nitric oxide synthase (NOS) in the regulation of mitochondrial oxidative phosphorylation in a number of different cells (7). For example, an alpha isoform of neuronal NOS interacts with and regulates the activity of cytochrome c oxidase in cerebellar neurons (19). An isoform of neuronal NOS was also shown to regulate oxygen consumption and reactive oxygen species (ROS) formation by mitochondria in cardiac myocytes (6). eNOS plays a critical role in decreasing oxygen consumption by isolated rat mesenteric and human umbilical arteries (31). eNOS was also shown to interact with the mitochondrial outer membrane to regulate O2 consumption in human umbilical vein endothelial cells (9). The potential role of eNOS in the regulation of mitochondrial oxidative stress in the fetal pulmonary arteries in response to birth related stimuli, however, is unknown. We investigated the hypothesis that eNOS is targeted to mitochondria to regulate the oxygen consumption and superoxide formation in response to birth-related stimuli in fetal PAEC. We also investigated the role of hsp90 as a chaperone in the regulation of its activity in the mitochondria. The objectives of our studies were to investigate 1) the interaction of eNOS with the mitochondrial outer membrane protein porin and chaperone hsp90 in response to simulated increases in Po2 and to the NOS agonist ATP; and 2) the role of these eNOS interactions with hsp90 and porin in the regulation of NO and ROS formation, PAEC angiogenesis, and vasodilator responses of pulmonary arteries to ATP.
METHODS
Studies were performed in PAEC and pulmonary arteries isolated from 12 fetal lambs at 137 days of gestation (term gestation = 144 days). Protocol for the use of sheep for this study was approved by the Institutional Animal Care and Use Committee of the Medical College of Wisconsin.
Isolation of PAEC.
Fetal lambs were euthanized at 137 days of gestation with an overdose of pentobarbital, and the heart and lungs were removed en bloc following a fetal thoracotomy. The pulmonary arteries were dissected up to third generation branches into the lung and removed (14). Endothelial cells were isolated by 0.1% collagenase digestion and were grown to confluence in DMEM supplemented with 20% fetal calf serum as we described previously (14). The identity of isolated PAEC was verified by positive staining for factor VIII antigen and acetylated LDL uptake (14). PAEC were used before passage 5 for all the studies described. Cells for individual studies were grown in 100-mm tissue culture flasks for immunoprecipitation and O2 consumption studies, six-well plates for NOS activity assays, and four-well chamber slides for mitochondrial targeted hydroethidine (Mito-HE) fluorescence measurement as an indicator of ROS.
Synthesis of eNOS decoy peptides.
Small peptides that mimic specific sequences in eNOS that interact with the client proteins were synthesized as previously described (32). In brief, TSB2 was synthesized to contain a TAT protein transduction domain and 14 amino acids from an eNOS-hsp90 binding domain (310–323) (32, 8). This cell-permeating peptide contains 14 amino acids from the hsp90 binding domain of eNOS (ELVLEVPLEHPTLE), an alanine linker added to the NH2 terminus, and a TAT protein transduction domain (RKKRRQRRR), as described by Rey et al. (22). Our previous studies demonstrated that TSB2 blocks eNOS interaction with hsp90, which uncouples eNOS activity (32). A control peptide that does not block eNOS interaction with hsp90 was generated by substituting the four glutamic acid residues with alanine in TSB2 (32). We also synthesized a peptide that corresponds to AA 628–632 of eNOS (RRKRK), which interacts with porin (9), and designated it as porin decoy peptide (PDP). Our preliminary studies demonstrated that this peptide binds mitochondrial porin and blocks eNOS interaction with porin. The control peptides for PDP were a five-amino acid peptide containing all alanines or substitution of two arginines with alanine (RAKAK).
Isolation and study of conduit pulmonary arteries.
Details of the methods for isolation and study of pulmonary arteries from fetal lambs in tissue bath were reported previously (14, 15). Third-fifth generation intrapulmonary arteries with an internal diameter of 300–500 μM were dissected and isolated from the lung. The arteries were cut into rings 1 mm in length, suspended with stainless steel hooks in water-jacketed chambers, and changes in ring tension were measured using standard tissue bath techniques as we described previously (15). Rings were stretched to a passive tension of 0.8 g and were preconstricted with 10−6-10−7 M norepinephrine. The tension reached with norepinephrine constriction for each ring was normalized to 100%, and the percent change from this tension with each dose of ATP was expressed as means ± SD. Vessel rings were incubated with TSB2 (10−5 M) to block the interaction of eNOS with hsp90 or PDP (10−5 M) to block eNOS interaction with porin or their corresponding control peptides for 30 min. Relaxation responses to 10−8-10−3 M doses of ATP, a NOS agonist, were then determined. In some studies, rings treated with TSB2 or PDP were also treated with 10−9 M concentration of mitochondrial targeted ubiquinone (Mito-Q), a mitochondrial specific antioxidant, to determine whether an increase in mitochondrial oxidative stress in response to decoy peptides impairs the relaxation response to ATP.
Isolation and study of pressurized resistance pulmonary arteries.
Fifth to seventh generation intralobar pulmonary arteries with an internal diameter of 100–150 μm were dissected under a microscope from lung tissue and were connected to glass pipettes that taper to an outside diameter of 70 μm. The vessels were tied in place after tying off side branches and superfused with Kreb's buffer, which was aerated to maintain physiologic acid base balance and oxygenation at 37°C. The vessels were cannulated and pressurized to 20–25 mmHg. This pressure reflects the transmural pressure for resistance arterioles in fetal lamb lungs (21). Pressure was continuously monitored via a fluid-filled pressure transducer attached to the outflow line. The lumen diameter of the vessel was monitored and changes were studied as we described previously (14). After an equilibration period of 30–60 min, reactivity of the vessel was confirmed by constriction with 30 mM KCl. Vessels were preconstricted with 10−6 M norepinephrine before testing the dilator responses. The presence of intact endothelium was confirmed by evaluation of vasodilator response to acetylcholine (10−5 M). Treatment of vessels with eNOS decoy peptides (10−5 M) or control peptide (10−5 M) was done by halting the superfusion system for 30 min. The dose-response relationship to ATP was studied with intraluminal infusion of the agent at 10−6 to 10−3 M doses. Our previous studies demonstrated minimal and maximal responses to these doses of ATP in control pulmonary arteries (14). Response to ATP was also assessed in some vessels treated with both decoy peptides and the mitochondrial targeted antioxidant Mito-Q (10−9 M).
Immunoprecipitation studies.
PAEC were grown in 100-mm flasks in DMEM with 20% FCS. PAEC were grown at 3% oxygen-5% CO2 to simulate the oxygen tension of the fetus for 48 h. Cells were then transitioned to 21% O2-5% CO2 or 95%O2-5% CO2 for 1 h or continued at 3% O2. In some studies, PAEC were exposed to buffer containing the NOS agonist ATP (10−5 M) or to buffer alone for 15 min. At the end of the study, supernatant was aspirated and cells were detached using TrypLE Express reagent (Invitrogen, Grand Island, NY). Mitochondria were isolated from the cells using commercially available reagents (Pierce mitochondrial isolation kit; Thermo Scientific, Rockford, IL). eNOS was immunoprecipitated from the mitochondrial isolate and the immunoprecipitate was immunoblotted for eNOS, hsp90, and mitochondrial porin using techniques previously described (14). The ratios of hsp90/eNOS and porin/eNOS were calculated from the radiogram band densities.
In some studies, PAEC were incubated with decoy peptides or their control peptides (10−5 M) for 2 h in media containing 5% serum, followed by mitochondrial isolation. Porin was then immunoprecipitated from mitochondrial isolate, followed by blotting for eNOS, hsp90, and porin to determine the effects of decoy peptides on eNOS interactions with porin or hsp90.
O2 consumption measurements.
PAEC were detached from confluent 100-mm flasks and treated with buffer alone or buffer containing decoy peptides (10−5 M) or the NOS inhibitor NG-nitro-l-arginine methyl ester (l-NAME; 10−4 M) for 1 h. Cells (2 × 106) were then placed in a sealed chamber containing mitochondrial respiration medium (0.5 mM EGTA, 3 mM MgCl2, 20 mM taurine, 10 mM KH2PO4, 20 mM K-HEPES, 200 mM sucrose, and 1 mg/ml BSA) and a Clark type platinum electrode (Oxygraph). Addition of 10 mM succinate, 5 mM ADP and 10 ng of digitonin ensured the availability of substrate, stimulation of respiration, and permeability of cell membrane, respectively. The chamber was then sealed and O2 concentration was continuously recorded over 5 min. Oxygen consumption rates were expressed as nanomoles of O2 consumed per minute per 106 cells and were compared between different groups (Graphpad Prism version 5; Graphpad Software, La Jolla, CA). The effect of eNOS decoy peptides on the O2 consumption by the cells was compared with the effects of l-NAME, which inhibits NO production.
NO levels in PAEC.
PAEC were grown to confluence in six-well plates and were incubated with decoy peptides (10−5 M) or control peptides (10−5 M) for 1 h followed by exposure to the NOS agonist ATP for 15 min. PAEC in some wells were incubated with the NOS antagonist NG-monomethyl-l-arginine (l-NMMA; 10−4 M). The supernatant was then collected and NO2minus + NO3minus levels were estimated using ozone chemiluminescence as described (14). The levels were normalized to protein concentration in each well (14, 3).
Mitochondrial ROS levels.
Estimation of mitochondrial ROS levels was done using mitochondrial targeted Mito-HE (MitoSOX Red; Life Technologies). Confluent PAEC in four-well chamber slides were treated with eNOS decoy peptides or control peptides for 1 h. PAEC were then treated with ATP for 15 min and Mito-HE reagent (10−5 M) was added during the final 10 min of the incubation. Then cells were fixed and fluorescence was visualized (excitation 528 nm/emission 565 nm), as previously described (4). Imaging of cells was done using a fluorescent microscope (NIKON Eclipse 600; Nikon Instruments, Melville, NY) equipped with a SPOT RT Slide camera and software. Mito-HE fluorescence in the images was quantified with MetaVue software (Universal Imaging, Downingtown, PA). Change in mitochondrial ROS levels during transition to normoxia and hyperoxia was also estimated by addition of Mito-HE reagent (10−5 M) to the cells, 45 min after transition to room air or 95% O2. Cells in selected wells were pretreated with cell permeable, polyethylene glycol-superoxide dismutase (PEG-SOD; Sigma-Aldrich, St Louis, MO) at 100 U/ml before the addition of Mito-HE reagent. Fluorescence imaging was done 15 min after the addition of Mito-HE reagent.
Effect of eNOS decoy peptides on PAEC angiogenesis.
We evaluated the effects of eNOS decoy and control peptides on tube formation, cell migration during monolayer scratch recovery, and cell apoptosis as in vitro measures of PAEC angiogenesis function. We also determined if the effect of decoy peptides on PAEC angiogenesis is related to an increase in oxidative stress. The individual protocols are described below.
Tube formation by PAEC.
After overnight thaw at 4°C, 50 μl of Matrigel were carefully pipetted into each well of a 96-well plate. Matrigel was allowed to solidify in the incubator for 2 h at 37°C. PAEC were treated with either eNOS decoy peptides or control peptides (all at 10−5 M) for 1 h in DMEM, and the treated PAEC (2 × 104) were then added on top of the Matrigel. PAEC in Matrigel were incubated at 37°C and an oxygen concentration of 21% with 5% CO2 for 4 h as previously reported (27–29). In some experiments, PAEC treated with decoy peptides were also treated with the antioxidant n-acetyl cysteine (10−4 M) or the mitochondrial targeted antioxidant Mito-Q (10−9 M) to determine if oxidative stress contributes to impaired angiogenesis in these cells.
Cell migration.
Monolayer scratch recovery test was used to determine the effects of decoy peptides on cell migration after injury (29). Confluent PAEC in six-well plates were switched to DMEM supplemented with 0.5% FCS for 2 h, and two scratches were created by 1-ml pipette-tip in each well. The detached PAEC in the well were washed away twice by DMEM with 0.5% FCS. The medium was then switched to DMEM with 5% FBS. PAEC in selected wells were treated with control or eNOS decoy peptides (10−5 M) with/without 10−4 M NAC or 10−9 M Mito-Q during the 2-h incubation in DMEM, before creating the scratch. NAC or Mito-Q was added to some wells to evaluate the role of reactive oxygen species generation induced by decoy peptides during the 2-h incubation. After 24 h, four randomly selected fields per scratch line were pictured using an inverted microscope (Olympus IX50). The gap distance between recovery frontlines was obtained for comparison.
Cell apoptosis.
Apoptosis was determined by the method reported by Sgonc et al. (23) with some modifications as reported before (29). PAEC (3 × 105) in DMEM with 20% FCS were plated onto each chamber of four-well chamber slide and incubated at 37°C in 21% O2 and 5% CO2 for 16 h. PAEC were treated with decoy peptides or scrambled sequence peptides (10−5 M), with or without NAC (10−4 M) or Mito-Q (10−9 M) for the last 2 h of incubation. At the end of incubations, PAEC were fixed in DPBS containing 4% formalin (pH 7.4) for 1 h followed by 3% H2O2 in methanol treatment for 10 min at 15–25°C. After being washed with DPBS, the fixed PAEC were permeabilized with 0.1% Triton X-100 for 2 min on ice and then treated with labeling mixture for 60 min at 37°C in the dark. Before being stained with DAB, the cells were pretreated with peroxidase converter solution for 30 min at 37°C. After hematoxylin-eosin counterstain, the number of brown nuclei was counted under microscope (×200) and corrected against all nuclei to calculate the percentage of apoptotic cells.
Statistical analysis.
All the data are shown as means ± SD. We compared data between different treatment groups by one-way ANOVA using Graphpad Prism (Graphpad Software). When a significant difference (P < 0.05) was found, Newman-Keuls post hoc test was done to determine where the differences were.
RESULTS
Association of eNOS with hsp90 and porin.
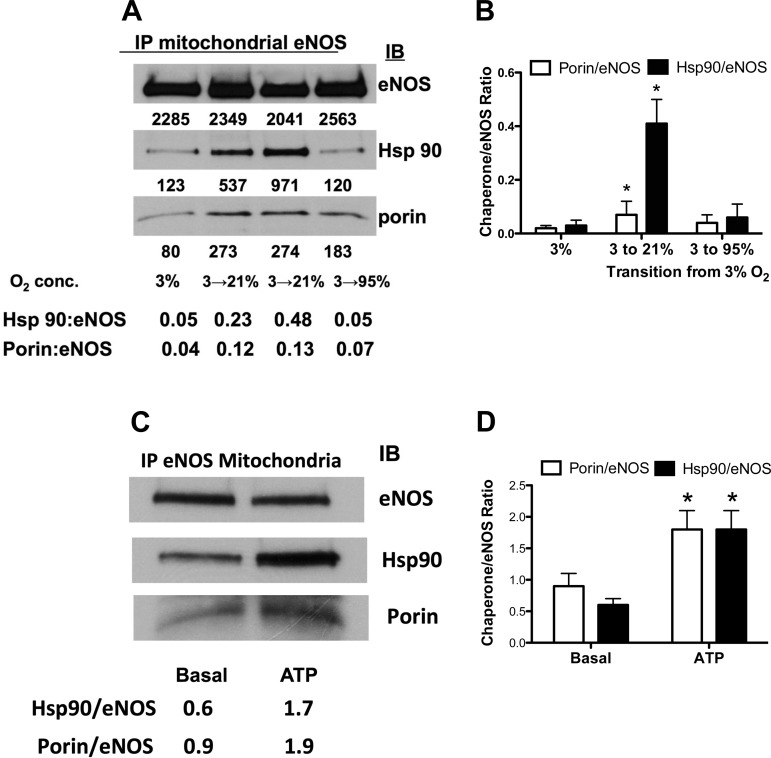
Transition of PAEC from fetal to normoxic postnatal O2 concentration resulted in an increase in the association of eNOS with hsp90 and porin in the mitochondrial isolates, compared with isolates from PAEC kept at 3% O2 (Fig. 1, A and B). Transition to 95% O2 resulted in a decrease of this eNOS association with hsp90 and porin (Fig. 1, A and B). Exposure of PAEC to ATP (10−5 M) also resulted in a significant increase in the association of eNOS with hsp90 and porin in mitochondrial isolates (Fig. 1, C and D).
Fig. 1.
Effect of oxygen tension and ATP on the interaction of endothelial nitric oxide synthase (eNOS) with hsp90 and porin in mitochondria. Endothelial NOS was immunoprecipitated (IP) from mitochondrial isolates of pulmonary artery endothelial cells (PAEC) exposed to 3, 21, or 95% O2 for 1 h after being cultured in 3% O2 (A). IB, immunoblot. Representative blot and summary data (B) show increase in eNOS interactions with client proteins after transition to 21% and decrease with 95% O2 (means ± SD for n = 5). ATP also stimulated increases in eNOS interactions with porin and hsp90 as shown in representative blot (C) and summary data (D, for n = 5). *P < 0.05, from 3% O2 (B) or from basal level (D) by ANOVA and Newman Keuls post hoc test.
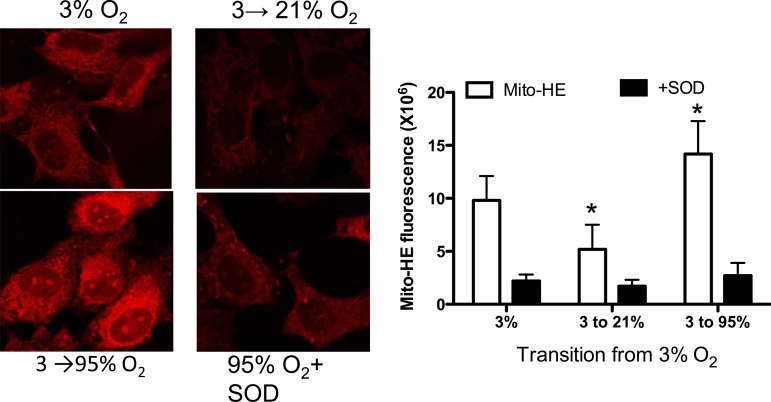
Change in mitochondrial ROS levels.
Transition of PAEC from 3 to 21% O2 was associated with a significant decrease in mitochondrial ROS levels in the cells, indicated by Mito-HE fluorescence (Fig. 2, A and B). Mito-HE fluorescence increased significantly during the transition from 3 to 95% O2 in PAEC and this increase was attenuated in the presence of PEG-SOD, indicating that an increase in mitochondrial ROS levels occur during hyperoxia (Fig. 2, A and B). These data correlate with the immunoprecipitation studies above, which demonstrate an increase in the association of eNOS with porin and hsp90 during normoxia, but not with hyperoxia.
Fig. 2.
Effect of transition from fetal (3%) to normoxic (21%) or hyperoxic (95%) oxygen tension on mitochondrial targeted hydroethidine (Mito-HE) fluorescence. The increase in fluorescence by hyperoxia was quenched by cell permeable polyethylene glycol superoxide dismutase (SOD) at 100 U/ml, indicating that reactive oxygen species contribute to increase in fluorescence. Summary data are shown on the right as means ± SD for 5 experiments. *P < 0.05, from 3% O2 by ANOVA and Newman-Keuls post hoc test.
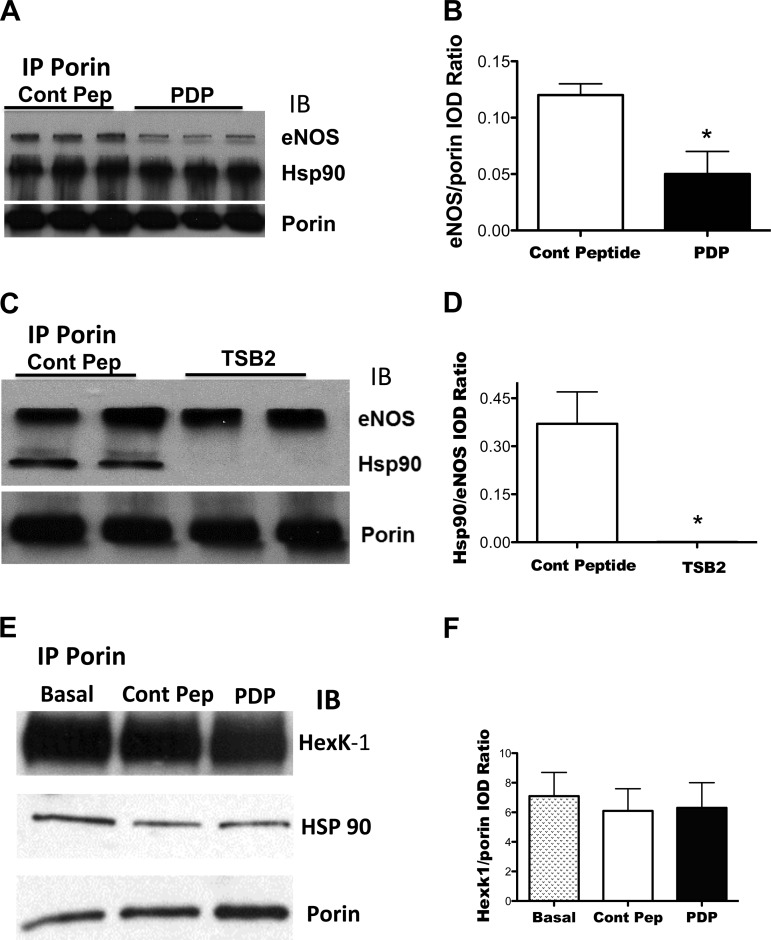
Effect of decoy peptides on the association of eNOS with porin and Hsp90.
Incubation of PAEC with PDP decreased the association of eNOS with porin in the mitochondrial isolate, compared with isolates from PAEC incubated with control sequence peptide (Fig. 3, A and B). Incubation with PDP or the control peptide did not alter the association of porin with Hsp90 (Fig. 3, A and E) or with hexokinase-1, which normally interacts with porin (25) (Fig. 3, E and F). TSB2 decreased the association of eNOS with hsp90 in the mitochondrial isolate, compared to incubation with its control peptide (Fig. 3, C and D). TSB2 did not alter the association of eNOS with porin (Fig. 3C). These data suggest that the two decoy peptides we used were specific for the interaction of eNOS with either porin or hsp90 in the PAEC.
Fig. 3.
Effect of decoy peptides on eNOS interactions with porin (A and B), Hsp90 (C and D), and hexokinase-1 (E and F). Mitochondrial isolates were obtained from PAEC incubated with either control or decoy peptides (10−5M) and porin was immunoprecipitated. The IP was blotted for eNOS, Hsp90, porin, and hexokinase-1. Decoy peptide for eNOS domain that interacts with porin (PDP) decreased the interaction of eNOS with porin (A and B) but did not alter eNOS interaction with Hsp90 (A). TSB2, which inhibits eNOS interaction with Hsp90, decreased eNOS interaction with Hsp90, without altering eNOS interaction with porin in mitochondria (C and D). Control peptide used for porin decoy peptide (control Pep) or porin decoy peptide (PDP) did not alter the interaction of porin with Hsp90 or with hexokinase-1 (E: representative blot; F: summarized data). *P < 0.05, from control peptide (Cont Pep)-treated cells by ANOVA and Newman Keuls test for n = 4 for all experiments.
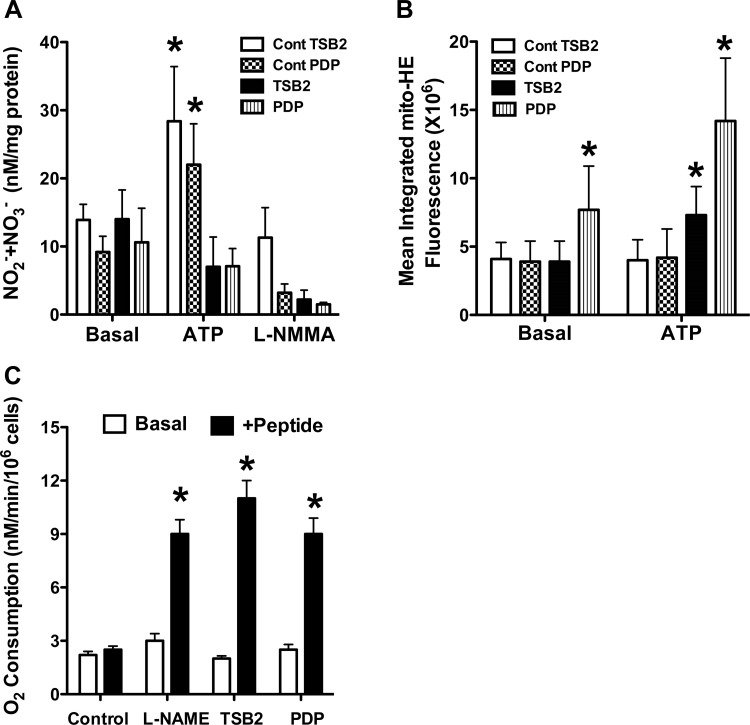
Effect of decoy peptides on the NO and mitochondrial ROS levels.
ATP stimulation increased the NO2− + NO3− levels in PAEC treated with control sequence peptides (Fig. 4A). Inhibition of the increase in NO2− + NO3− levels during ATP exposure by l-NMMA suggests that eNOS is the source of increased NO2− + NO3− levels. Incubation of PAEC with eNOS decoy peptides to block its association with hsp90 (via TSB2) or porin (via PDP) attenuated the NO2− + NO3− production in response to ATP stimulation (Fig. 4A). The decoy peptides also increased basal and stimulated PAEC Mito-HE fluorescence, which was attenuated by PEG-SOD, suggesting that mitochondrial ROS levels were increased by decoy peptides (Fig. 4B).
Fig. 4.
Effect of decoy peptides on NO and mitochondrial ROS levels and O2 consumption by PAEC. Increase in NO2− + NO3− levels in response to ATP was attenuated by decoy peptides targeting eNOS-porin (PDP) or eNOS-Hsp90 (TSB2) interactions (A). l-NMMA (10-4M) attenuated the increase in NO2− + NO3− levels in response to ATP, indicating that NO release accounts for the increase in NO2− + NO3− levels. PDP increased Mito-HE fluorescence at basal level and both TSB2 and PDP increased the fluorescence in response to ATP (B). Control peptides had no effect on basal O2 consumption (C); both decoy peptides (10−5 M) and NG-nitro-l-arginine methyl ester (l-NAME; 10−4M) increased the O2 consumption rate from basal level. *P < 0.05, from basal level for n = 6 for all 3 studies.
Effect of decoy peptides on PAEC O2 consumption.
Both TSB2 and PDP increased the rate of O2 consumption by PAEC. The increase in O2 consumption was similar to the effect of the NOS inhibitor l-NAME (Fig. 4C), suggesting a loss of NO regulation of mitochondria when PAEC are treated with decoy peptides. These data are consistent with the studies above showing that decoy peptides increase the mitochondrial ROS levels in PAEC. They are also consistent with previous studies that demonstrated a role for NO in modulating O2 consumption (16).
Effect of decoy peptides on the vasodilator response of conduit and resistance size pulmonary arteries.
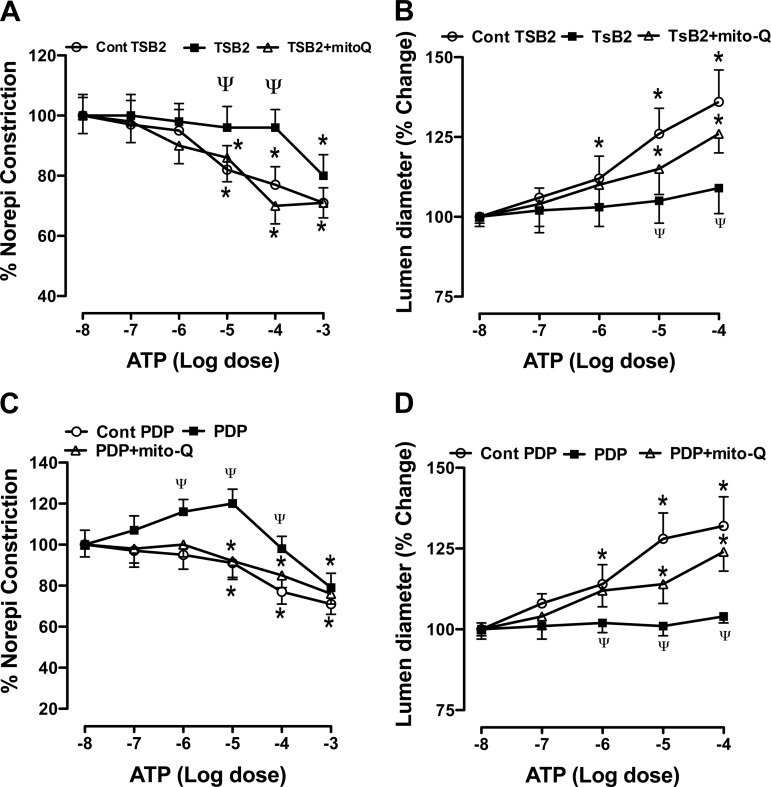
ATP induced a dose-dependent relaxation of the conduit size pulmonary artery rings (Fig. 5, A and C). Both TSB2 and PDP attenuated the relaxation responses of pulmonary artery rings to ATP (Fig. 5, A and C). The mitochondrial targeted O2·− scavenger Mito-Q improved the relaxation responses to ATP in decoy peptide treated rings. ATP also induced a dose-dependent dilation of resistance size pulmonary arteries (Fig. 5, B and D). This vasodilator response to ATP was attenuated by the decoy peptides. Mito-Q enhanced the vasodilator response to ATP in decoy peptide-treated vessels (Fig. 5, B and D). These data suggest that an increase in mitochondrial oxidative stress occurs and impairs vasodilator responses when eNOS interactions with hsp90 and porin are blocked. These data are consistent with the effects of decoy peptides on the mitochondrial ROS observed in Fig. 4B.
Fig. 5.
Effect of decoy peptides that block eNOS interactions with Hsp90 (TSB2) and porin (PDP) on the response to ATP in conduit size (A and C) and resistance size (B and D) pulmonary arteries. TSB2 (10−5 M) attenuated the relaxation response of conduit size pulmonary artery rings (A) and the dilator response of resistance size arterioles (B). The attenuated response was improved by Mito-Q (10−9 M). PDP (10−5 M) similarly attenuated the response to ATP in pulmonary artery rings and resistance size arterioles (C and D); mitochondrial-targeted ubiquinone (Mito-Q; 10−9 M) improved this attenuated response. *P < 0.05, from −8 M dose of ATP, and ΨP < 0.05, from control peptide group, by ANOVA and Newman Keuls test for n = 8 for ring studies and n = 6 for resistance size artery studies.
Effect of decoy peptides on the in vitro PAEC angiogenesis.
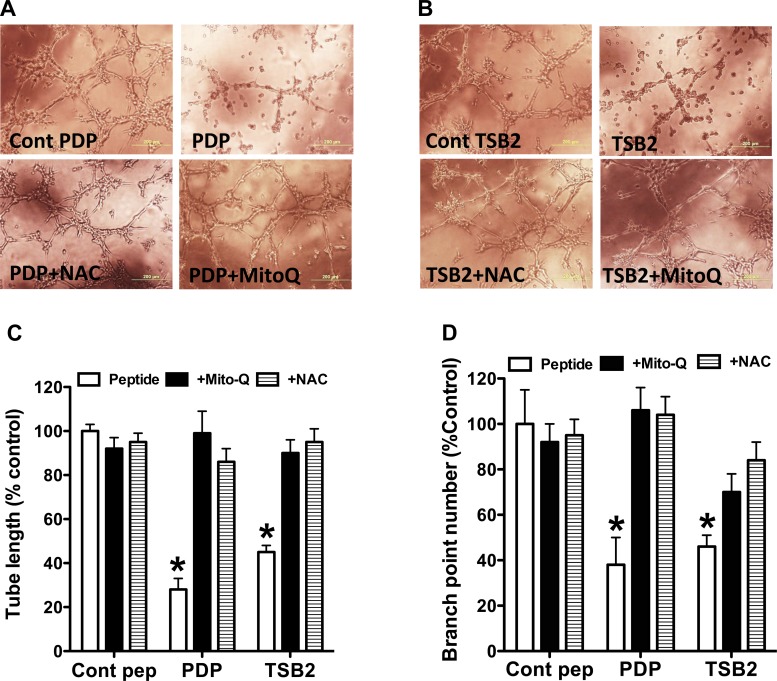
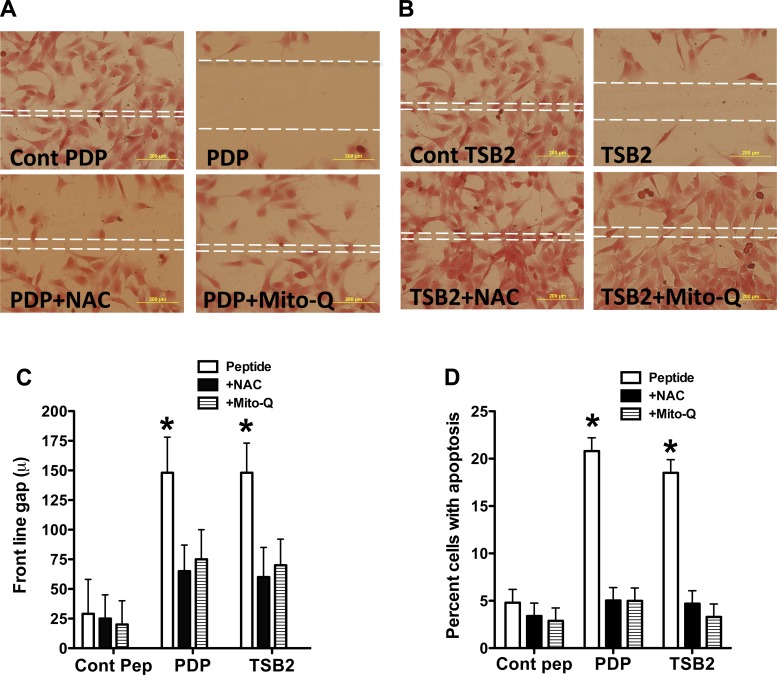
Both TsB2 and PDP attenuated in vitro tube formation by PAEC with decreases in tube length and branch point numbers (Fig. 6). The antioxidants NAC and Mito-Q rescued tube formation in PAEC cultures treated with decoy peptides (Fig. 6). The decoy peptides also decreased cell migration during scratch recovery, resulting in larger gaps for treated cells compared with PAEC treated with control peptides (Fig. 7, A–C). NAC and Mito-Q rescued cell migration in PAEC treated with decoy peptides (Fig. 7, A–C). The decoy peptides increased the percentage of apoptotic cells, while NAC and Mito-Q reduced the number of apoptotic cells in decoy peptide-treated PAEC (Fig. 7D). These data are consistent with the observation that decoy peptides increase mitochondrial oxidative stress and decrease NO production in PAEC.
Fig. 6.
Effect of decoy peptides on tube formation by PAEC in Matrigel. Decoy peptides blocking eNOS interactions with porin (PDP; A) and Hsp90 (TSB2; B) attenuated the tube formation by PAEC in Matrigel. Summarized data are shown for tube length (C) and branch point number (D) as means ± SD from 6 experiments. Both N-acetyl cysteine (NAC) at 10−4 M and mitochondrial-targeted Mito-Q at 10−9 M restored the tube formation in decoy peptide treated cells, suggesting that increase in oxidative stress by decoy peptides impairs tube formation. *P < 0.05, from control peptide-treated cells by ANOVA and Newman Keuls test.
Fig. 7.
Effect of decoy peptides blocking eNOS interactions with porin (PDP; A) and Hsp90 (TSB2; B) on PAEC migration into scratch margins. The frontline gap is indicated by broken lines in the center. Both PDP and TSB2 reduced PAEC migration into the scratch area, increasing gap distance. NAC (10−4 M) and Mito-Q (10−9 M) restored the migration of cells, suggesting that oxidative stress contributed to impaired cell migration with decoy peptides. Summarized data are shown for front line gap (C) as means ± SD from 6 experiments. Both PDP and TSB2 also increased apoptosis of PAEC (D). NAC and Mito-Q attenuated the apoptosis caused by the decoy peptides. *P < 0.05, from control peptide by ANOVA and Newman Keuls test.
DISCUSSION
Our study demonstrated that eNOS regulates PAEC mitochondrial ROS levels during exposure to oxygen or ATP by modulating oxygen consumption and ROS formation. Regulation of mitochondrial ROS by eNOS plays an important role in the availability of NO. Maintaining redox balance and NO levels in the cell facilitates the angiogenesis function of PAEC and the vasodilator response of pulmonary arteries to physiologic stimuli. Our data also suggest that high O2 concentrations decrease the targeting of eNOS to the mitochondrial outer membrane and increase ROS production during the transition.
Previous studies demonstrated that eNOS interacts with hsp90 upon activation by agonists (10, 20). Thus hsp90 plays an important role in the coupled activity of eNOS (20). Whether hsp90 also plays a role in the targeting of eNOS to subcellular compartments remains unknown. Here we hypothesized that hsp90-eNOS interactions are involved in the mitochondrial targeting of eNOS and/or in the function of the targeted eNOS. Our data show that hsp90 interactions with eNOS and porin increase during the transition of PAEC to normoxic environment and during exposure to ATP. Data supporting this mechanism come from our studies showing that TSB2 disrupts hsp90 interaction with eNOS. TSB2 also increases mitochondrial O2 consumption and ROS levels while decreasing NO production.
Subcellular targeting of eNOS was previously shown to play an important role in cell biology (11). Thus interaction of eNOS with a voltage-dependent anion channel has been previously shown to promote coupled eNOS function in cultured endothelial cells (5, 26). Gao et al. (9) demonstrated that eNOS interacts with the mitochondrial outer membrane protein porin to regulate O2 consumption in HUVEC. Disruption of the association led to an increase in O2 consumption in these cells (9). Victor et al. (31) demonstrated that O2 consumption by isolated rat mesenteric arteries or mouse aorta increases after treatment with the NOS inhibitor N(G)-nitro-L-arginine (l-NA). Basal O2 consumption was also higher in vessels and in renal cortical slices isolated from eNOS knockout mice (2, 31). The mechanism by which NO decreases mitochondrial O2 consumption has been studied previously. Interaction of NO with the heme site of cytochrome c oxidase is believed to decrease the affinity of complex-IV for O2 (7). Additional mechanisms, including nitrosylation of electron transport chain complex proteins, may play a role in the regulation of O2 consumption by NO (2, 31). Although NOS inhibition may decrease O2 utilization by NOS, loss of NO clamp on mitochondrial oxygen consumption results in greater increase in O2 consumption by the cells (7).
Our studies in fetal PAEC support and extend these previous observations. We found that interaction with mitochondrial porin allows eNOS to regulate the mitochondrial ROS during the transition of PAEC from low to higher O2 levels and in response to the physiologic NOS agonist ATP. Whether oxidative stress induced by exposure to hyperoxia leads to impairment of this targeting requires further study. Our previous studies suggested that oxidative stress in pulmonary hypertension of the newborn is associated with decreased eNOS interaction with Hsp90. This may be a potential mechanism for the impairment of targeting during hyperoxia; however, this possible mechanism requires further study. Whether ROS generated in the mitochondria during hyperoxia translocate to cytosolic compartment is unclear. Some studies suggested that O2·− can interact with and potentially translocate via the voltage-dependent anion channel (17). Whether this leads to depletion of NO is unclear and requires further investigation.
We used decoy peptides in our study to inhibit the interaction of eNOS with the client proteins hsp90 and porin. The importance of using this approach is that decoy peptides are designed to block protein-protein interactions and not affect the enzyme structure and activity directly. This approach gave us greater confidence in defining how disruption of the interactions alters mitochondrial function. Previous studies have shown that the decoy peptide TSB2 inhibits eNOS-hsp90 interactions and leads to eNOS uncoupling (32). Our results support this observation and suggest that TSB2 increases oxygen consumption and mitochondrial superoxide levels in fetal PAEC. We observed similar effects with PDP on O2 consumption and mitochondrial ROS levels. These data suggest that eNOS regulates mitochondrial oxidative phosphorylation. Using these peptides, we were able to investigate the role of eNOS regulation of mitochondria on two important functions of pulmonary arteries, angiogenesis and vasodilation.
Considerable evidence suggests that eNOS plays a central role in the regulation of endothelial cell function. We previously reported that decreased eNOS function and oxidative stress impair PAEC angiogenesis by a mechanism that is reversed by the BH4 analog sepiapterin or by O2·− scavengers (28, 29). Our current studies show that loss of eNOS association with hsp90 and porin in mitochondria impairs PAEC angiogenesis. Our data suggest that these interactions facilitate the coupled function of eNOS in the mitochondria. These data also suggest that inhibition of angiogenesis in the lungs by hyperoxia may in part be due to loss of eNOS regulation of mitochondrial ROS. This hypothesis requires further investigation.
Another objective of our study was to determine whether the interaction of eNOS with hsp90 and porin modulates NOS-dependent vasodilator response of pulmonary arteries. Our studies show that inhibition of eNOS interactions with client proteins by decoy peptides has led to impaired response of conduit and resistance size pulmonary arteries to ATP, a NOS agonist. The reversal of this impaired vasodilation by the mitochondrial targeted antioxidant Mito-Q suggests a mechanistic role for mitochondrial ROS in this inhibition. Additional support for this mechanism comes from our observation that decoy peptides decrease NO production and increase ROS levels in PAEC.
Our study has some limitations. We used a fluorescent probe, mitochondrial targeted hydroethidine (Mito-HE), to detect reactive oxygen species in the mitochondria of intact cells. Although we used PEG-SOD as a negative control, previous studies suggested that other oxidation products of Mito-HE may lead to artifacts that interfere with the assessment of ROS detection by this method (33). Direct evidence for translocation of O2·− from mitochondria to cytosolic compartment is currently not available. Therefore, it is unclear whether PEG-SOD in our experiments directly quenched mitochondrial ROS to decrease Mito-HE fluorescence in our studies. We also relied on decoy peptides to disrupt the association of eNOS with hsp90 or porin. Although we showed reciprocal specificity of these peptides, their off target effects cannot be excluded.
In conclusion, our data demonstrate that eNOS is targeted to mitochondrial outer membrane, where it interacts with porin during fetal PAEC transition to normoxia. Disruption of this interaction increases mitochondrial oxidative stress and impairs vasodilation. Whether alterations in this mechanism contribute to impaired transition in pulmonary hypertension of the newborn requires further investigation.
GRANTS
This work was supported by National Institutes of Health Grants RO1-HL-057268 and RO3-HD-065841, research grants from Advancing Healthier Wisconsin Foundation and Children's Research Institute, and Muma Endowed Chair in Neonatology at Children's Hospital of Wisconsin.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
G.G.K. and K.A.P. conception and design of research; G.G.K., A.J.A., A.E., and R.-J.T. performed experiments; G.G.K. analyzed data; G.G.K. interpreted results of experiments; G.G.K. and R.-J.T. prepared figures; G.G.K. drafted manuscript; G.G.K. approved final version of manuscript; K.A.P. edited and revised manuscript.
REFERENCES
- 1.Addabbo F, Montagnani M, Goligorsky MS. Mitochondria and reactive oxygen species. Hypertension 53: 885–892, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Adler S, Huang H, Loke KE, Xu X, Tada H, Laumas A, Hintze TH. Endothelial nitric oxide synthase plays an essential role in regulation of renal oxygen consumption by NO. Am J Physiol Renal Physiol 280: F838–F843, 2001. [DOI] [PubMed] [Google Scholar]
- 3.Afolayan AJ, Eis A, Teng RJ, Bakhutashvili I, Kaul S, Davis JM, Konduri GG. Decreases in manganese superoxide dismutase expression and activity contribute to oxidative stress in persistent pulmonary hypertension of the newborn. Am J Physiol Lung Cell Mol Physiol 303: L870–L879, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Afolayan AJ, Teng RJ, Eis A, Rana U, Broniowska KA, Corbett JA, Pritchard K, Konduri GG. Inducible HSP70 regulates superoxide dismutase-2 and mitochondrial oxidative stress in the endothelial cells from developing lungs. Am J Physiol Lung Cell Mol Physiol 306: L351–L360, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alvira CM1, Umesh A, Husted C, Ying L, Hou Y, Lyu SC, Nowak J, Cornfield DN. Voltage-dependent anion channel-2 interaction with nitric oxide synthase enhances pulmonary artery endothelial cell nitric oxide production. Am J Respir Cell Mol Biol 47: 669–678, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dedkova EN, Blatter LA. Characteristics and function of cardiac mitochondrial nitric oxide synthase. J Physiol 587: 851–872, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Elfering SL, Sarkela TM, Giulivi C. Biochemistry of mitochondrial nitric-oxide synthase. J Biol Chem 277: 38079–38086, 2002. [DOI] [PubMed] [Google Scholar]
- 8.Fontana J, Fulton D, Chen Y, Fairchild TA, McCabe TJ, Fujita N, Tsuruo T, Sessa WC. Domain mapping studies reveal that the M domain of hsp90 serves as a molecular scaffold to regulate Akt-dependent phosphorylation of endothelial nitric oxide synthase and NO release. Circ Res 90: 866–873, 2002. [DOI] [PubMed] [Google Scholar]
- 9.Gao S, Chen J, Brodsky SV, Huang H, Adler S, Lee JH, Dhadwal N, Cohen-Gould L, Gross SS, Goligorsky MS. Docking of endothelial nitric oxide synthase (eNOS) to the mitochondrial outer membrane: a pentabasic amino acid sequence in the autoinhibitory domain of eNOS targets a proteinase K-cleavable peptide on the cytoplasmic face of mitochondria. J Biol Chem 279: 15968–15974, 2004. [DOI] [PubMed] [Google Scholar]
- 10.Garcia-Cardena G, Fan R, Shah V et al: Dynamic activation of endothelial nitric oxide synthase by HSP90. Nature 392: 821–824, 1998. [DOI] [PubMed] [Google Scholar]
- 11.Jagnandan D, Sessa WC, Fulton D. Intracellular location regulates calcium-calmodulin-dependent activation of organelle-restricted eNOS. Am J Physiol Cell Physiol 289: C1024–C1033, 2005. [DOI] [PubMed] [Google Scholar]
- 12.Konduri GG, Mattei J. Role of oxidative phosphorylation and ATP release in birth related pulmonary vasodilation in fetal lambs. Am J Physiol Heart Circ Physiol 283: H1600–H1608, 2002. [DOI] [PubMed] [Google Scholar]
- 13.Konduri GG, Mital S. Adenosine and ATP cause nitric oxide-dependent pulmonary vasodilation in fetal lambs. Biol Neonate 78: 220–9, 2000. [DOI] [PubMed] [Google Scholar]
- 14.Konduri GG, Ou J, Shi Y, Pritchard KA Jr. Decreased association of HSP90 impairs endothelial nitric oxide synthase in fetal lambs with persistent pulmonary hypertension. Am J Physiol Heart Circ Physiol 285: H204–H211, 2003. [DOI] [PubMed] [Google Scholar]
- 15.Konduri GG, Bakhutashvili I, Eis A, Pritchard K Jr. Oxidant stress from uncoupled nitric oxide synthase impairs vasodilation in fetal lambs with persistent pulmonary hypertension. Am J Physiol Heart Circ Physiol 292: H1812–H1820, 2007. [DOI] [PubMed] [Google Scholar]
- 16.Loke KE, McConnell PI, Tuzman JM, Shesely EG, Smith CJ, Stackpole CJ, Thompson CI, Kaley G, Wolin MS, Hintze TH. Endogenous endothelial nitric oxide synthase-derived nitric oxide is a physiological regulator of myocardial oxygen consumption. Circ Res 84: 840–845, 1999. [DOI] [PubMed] [Google Scholar]
- 17.Madesh M, Hajnóczky G. VDAC-dependent permeabilization of the outer mitochondrial membrane by superoxide induces rapid and massive cytochrome c release. J Cell Biol 155: 1003–1015, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Morin FC 3rd, Egan EA, Ferguson W, Lundgren CE. Development of pulmonary vascular response to oxygen. Am J Physiol 23: H542–H546, 1988. [DOI] [PubMed] [Google Scholar]
- 19.Persichini T, Mazzone V, Polticelli F, Moreno S, Venturini G, Clementi E, Colasanti M. Mitochondrial type I nitric oxide synthase physically interacts with cytochrome c oxidase. Neurosci Lett 384: 254–259, 2005. [DOI] [PubMed] [Google Scholar]
- 20.Pritchard KA, Ackerman AW, Gross ER, Stepp DW, Shi Y, Fontana JT, Baker JE, Sessa WC. Heat shock protein 90 mediates the balance of nitric oxide, and superoxide anion from endothelial nitric oxide synthase. J Biol Chem 276: 17621–17624, 2001. [DOI] [PubMed] [Google Scholar]
- 21.Raj JU, Chen P. Microvascular pressures measured by micropuncture in isolated perfused lamb lungs. J Appl Physiol 61: 2194–2201, 1986. [DOI] [PubMed] [Google Scholar]
- 22.Rey FE, Cifuentes ME, Kiarash A, Quinn MT, Pagano PJ. Novel competitive inhibitor of NAD(P)H oxidase assembly attenuates vascular O2− and systolic blood pressure in mice. Circ Res 89: 408–414, 2001. [DOI] [PubMed] [Google Scholar]
- 23.Sgonc R, Boeck G, Dietrich H, Gruber J, Recheis H, Wick G. Simultaneous determination of cell surface antigens and apoptosis. Trends Genet 10: 41–42, 1994. [DOI] [PubMed] [Google Scholar]
- 24.Shaul PW, Farrar MA, Magness RR. Pulmonary endothelial nitric oxide production is developmentally regulated in the fetus, and newborn. Am J Physiol Heart Circ Physiol 265: H1056–H1063, 1993. [DOI] [PubMed] [Google Scholar]
- 25.Shoshan-Barmatz V, Zakar M, Rosenthal K, Abu-Hamad S. Key regions of VDAC1 functioning in apoptosis induction and regulation by hexokinase. Biochim Biophys Acta 1787: 421–30, 2009. [DOI] [PubMed] [Google Scholar]
- 26.Sun J, Liao JK. Functional interaction of endothelial nitric oxide synthase with a voltage dependent anion channel. Proc Natl Acad Sci USA 99: 13108–13, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Teng RJ, Du J, Afolayan AJ, Eis A, Shi Y, Konduri GG. AMP kinase activation improves angiogenesis in pulmonary artery endothelial cells with in utero pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol 304: L29–L42, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Teng RJ, Du J, Xu H, Bakhutashvili I, Eis A, Shi Y, Pritchard KA Jr, Konduri GG. Sepiapterin improves angiogenesis of pulmonary artery endothelial cells with in utero pulmonary hypertension by recoupling endothelial nitric oxide synthase. Am J Physiol Lung Cell Mol Physiol 301: L334–L345, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Teng RJ, Eis A, Bakhutashvili I, Arul N, Konduri GG. Increased superoxide production contributes to the impaired angiogenesis of fetal pulmonary arteries with in utero pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol 297: L184–L195, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tiktinsky MH, Morin FC 3rd. Increasing oxygen tension dilates fetal pulmonary circulation via endothelium-derived relaxing factor. Am J Physiol Heart Circ Physiol 265: H376–H380, 1993. [DOI] [PubMed] [Google Scholar]
- 31.Victor VM, Nunez C, D'Ocon P, Taylor CT, Esplugues JV, Moncada S. Regulation of oxygen distribution in tissues by endothelial nitric oxide. Circ Res 104: 1178–1183, 2009. [DOI] [PubMed] [Google Scholar]
- 32.Xu H, Shi Y, Wang J, Jones D, Weilrauch D, Ying R, Wakim B, Pritchard KA Jr. A heat shock protein 90 binding domain in endothelial nitric-oxide synthase influences enzyme function. J Biol Chem 282: 37567–37574, 2007. [DOI] [PubMed] [Google Scholar]
- 33.Zielonka J, Kalyanaraman B. Hydroethidine- and MitoSOX-derived red fluorescence is not a reliable indicator of intracellular superoxide formation: another inconvenient truth. Free Radic Biol Med 48: 983–1001, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]