Abstract
Understanding sex differences in the qualitative dimensions of exertional dyspnea may provide insight into why women are more affected by this symptom than men. This study explored the evolution of the qualitative dimensions of dyspnea in 70 healthy, young, physically active adults (35 M and 35 F). Participants rated the intensity of their breathing discomfort (Borg 0-10 scale) and selected phrases that best described their breathing from a standardized list (work/effort, unsatisfied inspiration, and unsatisfied expiration) throughout each stage of a symptom-limited incremental-cycle exercise test. Following exercise, participants selected phrases that described their breathing at maximal exercise from a list of 15 standardized phrases. Intensity of breathing discomfort was significantly higher in women for a given ventilation, but differences disappeared when ventilation was expressed as a percentage of maximum voluntary ventilation. The dominant qualitative descriptor in both sexes throughout exercise was increased work/effort of breathing. At peak exercise, women were significantly more likely to select the following phrases: “my breathing feels shallow,” “I cannot get enough air in,” “I cannot take a deep breath in,” and “my breath does not go in all the way.” Women adopted a more rapid and shallow breathing pattern and had significantly higher end-inspiratory lung volumes relative to total lung capacity throughout exercise relative to men. These findings suggest that men and women do not differ in their perceived quality of dyspnea during submaximal exercise, but subjective differences appear at maximal exercise and may be related, at least in part, to underlying sex differences in breathing patterns and operating lung volumes during exercise.
Keywords: dyspnea, exercise, sex
women have smaller lungs, smaller-diameter airways, weaker respiratory muscles, and a decreased surface area for pulmonary gas exchange relative to height and/or lung size-matched men (12, 23, 31, 34, 45, 49). These sex differences may predispose women to greater respiratory system limitations during exercise. For example, some have suggested that women may be at an increased risk of developing expiratory flow limitation (21, 33) and exercise-induced arterial hypoxemia (24, 43), but this remains controversial (25, 48). Women also have a higher total work of breathing than men for a given level of ventilation (V̇e) (20, 21), which is driven by an increase in flow resistance attributable to their smaller-diameter airways (20). Ultimately, these sex differences in respiratory mechanics result in a higher oxygen cost of breathing in women relative to men during exercise (17). The functional and/or clinical consequences of these sex differences have not been adequately explored. However, it is reasonable to speculate that sex differences in pulmonary anatomy and physiology may explain, at least in part, why women self-report more troublesome activity-related dyspnea across the continuum of health and chronic respiratory diseases relative to men (6, 10, 13, 15, 16, 19, 27, 32, 41, 44).
Dyspnea is a complex sensation of breathing discomfort that consists of qualitatively distinct sensations that vary in intensity (42). Only a handful of physiological studies have attempted to explain the apparent sex differences in activity-related dyspnea (19, 41, 44) by focusing on dyspnea intensity rather than on the qualitative aspects of dyspnea. These studies demonstrate that dyspnea intensity is elevated for a given absolute metabolic or ventilatory requirement in women compared with men. However, these sex differences often disappear when work rate and V̇e are adjusted for differences in body size and/or maximal ventilatory capacity. Thus women use a higher fraction of their ventilatory capacity for any given absolute work rate or V̇e compared with men. Consequently, women will have relatively higher levels of neural respiratory drive and contractile respiratory muscle effort requirements to perform the same standardized physical task as men, with attendant higher-intensity ratings of dyspnea (44).
Dyspnea is, by nature, a highly subjective experience. It is often difficult for individuals to describe how their breathing feels, but healthy individuals and those with chronic cardiorespiratory conditions are able to distinguish different qualitative sensations of dyspnea when asked to select from a list of standardized descriptors (9, 46, 47). Different qualitative descriptors of dyspnea (e.g., work/effort, tightness, and unsatisfied inspiration/air hunger) are associated with distinct mechanisms and afferent pathways (42). To our knowledge, no studies have, first, tracked the evolution of the dominant qualitative dimensions of dyspnea throughout exercise in a relatively large number of men and women and, second, determined whether there are sex differences in the qualitative descriptors of dyspnea at the symptom-limited peak of exercise.
Understanding the impact of sex on the qualitative dimensions of activity-related dyspnea may help explain the consistent observation that women have a higher prevalence and severity of dyspnea than men in both health and disease, particularly in individuals with chronic obstructive pulmonary disease (6, 10, 13, 15, 16, 19, 27, 32, 41, 44). Studying a relatively large group of young healthy men and women is an important first step in understanding the “normal” evolution of exertional dyspnea, which will form the foundation for future studies targeting healthy aging and clinical populations. Accordingly, the primary and secondary aims of the present study were to compare the quality and intensity of dyspnea during symptom-limited incremental cycle exercise in healthy, physically active young men and women and to explore potential physiological mechanisms underlying any observed sex differences.
METHODS
Participants.
A total of 70 participants (35 M and 35 F) participated in this study. Inclusion criteria were as follows: 19–39 yr of age, forced expiratory volume in 1 s (FEV1)/forced vital capacity (FVC) ≥ 80% predicted, body mass index (BMI) >18 and <30 kg/m2, ≥150 min/wk of moderate-to-vigorous physical activity, peak aerobic capacity >80% predicted, ability to read and understand English, and ability to ride an upright stationary bicycle. Participants were excluded if they were current or former smokers, had a history or current symptoms of cardiorespiratory disease, had contraindications to exercise testing, and/or if they were participating in a sport at the provincial, national, or international level at the time of testing. The ventilatory response to exercise is not affected by menstrual cycle phase (3, 14, 30) or oral contraceptive use (8). Thus women were tested randomly throughout their menstrual cycle and were permitted to use oral contraceptives.
Experimental overview.
All experimental procedures and protocols received ethical approval from the University of British Columbia Providence Health Care Research Ethics Board, and participants provided written, informed consent before enrollment. All testing was conducted during one visit. After providing consent, participants completed a medical history, provided the number of hours they routinely engage in moderate-to-vigorous physical activity per week, and completed the Physical Activity Readiness Questionnaire (PAR-Q+). Participants then performed detailed pulmonary function testing followed by a symptom-limited incremental-cycle exercise test. All participants were thoroughly familiarized with testing procedures and symptom scales before completing the tests.
Pulmonary function.
Spirometry, plethysmography, maximum voluntary ventilation (MVV), and maximum respiratory pressures were performed with participants seated at rest according to previous recommendations (1, 36, 50) using a commercially available cardiopulmonary testing system (Vmax Encore 229, V62J Autobox; CareFusion, Yorba Linda, CA). Measurements were expressed in absolute values and as a percentage of predicted normal values (4, 7, 11, 37).
Exercise protocol.
Exercise testing was conducted on an electronically braked cycle ergometer (Ergoselect 200P; Ergoline, Bitz, Germany). Tests started with a 6-min steady-state resting period followed by a 1-min warmup of unloaded pedaling and then 25-W increases in work rate (starting at 25 W) every 2 min until volitional exhaustion. Maximal work rate was taken as the highest work rate that could be sustained for ≥30 s.
Symptom evaluation.
Intensity of “breathing discomfort” (hereafter termed dyspnea intensity) was defined as “the sensation of labored or difficult breathing,” and perceived “leg discomfort” was defined as the “sensation of leg muscle fatigue.” These symptoms were evaluated at rest, within the last 30 s of every 2-min stage of exercise, and at peak exercise using the modified 0–10 Borg scale (5). The endpoints of the scale were anchored such that 0 represented “no breathing/leg discomfort” and 10 represented “the most severe breathing/leg discomfort ever experienced or imagined.” In addition to comparing dyspnea intensity ratings at standardized work rates, we computed the slope relating dyspnea intensity to V̇e for each subject using linear regression analysis. The dyspnea intensity/V̇e slope was used as a global index of each subject's dyspnea intensity throughout exercise. Immediately following the symptom intensity ratings, participants were asked to select the phrase(s) that best described their breathing compared with the resting state once during every stage of exercise and at peak exercise using the following three descriptors (28, 29): 1) “My breathing requires more work and effort” (work/effort); 2) “I cannot get enough air in” (unsatisfied inspiration); and 3) “I cannot get enough air out” (unsatisfied expiration). None to all three of the descriptors could be chosen at any one time. Participants were specifically instructed to select the phrase that best described their breathing but were permitted to select more than one phrase only if the phrases applied equally to how their breathing felt. The onset of unsatisfied inspiration and unsatisfied expiration was defined as the lowest work rate in which participants first selected these phrases. Immediately upon exercise cessation, participants were asked to verbalize their main reason(s) for stopping exercise (i.e., breathing discomfort, leg discomfort, combination of breathing and legs, or some other reason). In the minutes following exercise, participants were given a modified questionnaire from Simon et al. (46) consisting of a list of 15 qualitative descriptors of breathing discomfort. Participants were instructed to select all of the descriptors that applied to their breathing sensation at peak exercise.
Cardiorespiratory responses to exercise.
Standard cardiorespiratory measures were recorded breath by breath and averaged over 30-s epochs at rest and during exercise. Heart rate, blood pressure, and oxygen saturation were monitored using a heart rate monitor (Polar T34; Polar Electro, Kempele, Finland), manual sphygmomanometer, and pulse oximeter, respectively. Operating lung volumes were derived from serial inspiratory capacity (IC) maneuvers performed at rest and during exercise as previously described (18). Briefly, end-expiratory lung volume (EELV) was calculated as the difference between total lung capacity (TLC) and IC. End-inspiratory lung volume (EILV) was calculated as the sum of EELV and tidal volume (VT). All standard cardiorespiratory measurements were averaged between 1:00 and 1:30 of each 2-min exercise stage and were subsequently linked with symptom ratings and IC-derived measurements made between 1:30 and 2:00 of each 2-min exercise stage. This approach was used to avoid contamination in breathing pattern responses that might occur while participants rated their symptoms and performed IC maneuvers.
Statistical analysis.
Comparisons between men and women for baseline characteristics and peak exercise responses were conducted using unpaired t-tests. Comparisons for selected variables at standardized submaximal work rates were conducted using repeated-measures ANOVA. To determine whether group differences were present at various work rates, the interaction between group and work rate was tested, followed by Bonferroni-adjusted post hoc comparisons when results were significant. Analyses of log-transformed values were also performed on a few variables to determine whether the ANOVA results were sensitive to observed deviations from normality. The original results were reported when conclusions remained unchanged following sensitivity analysis. Sex differences in the reasons for stopping exercise and the qualitative descriptors of dyspnea were analyzed using Fisher's exact test. Statistical significance was set at P < 0.05. Data are presented as means ± SD unless otherwise specified.
RESULTS
Participants.
Baseline subject characteristics are shown in Table 1. As expected, men were taller, heavier, and had larger lung volumes, higher expiratory flows, and stronger respiratory muscles. Both groups were well matched for the majority of pulmonary function parameters when expressed as a percentage of predicted normal values. All but four men and two women were above the lower limit of normal for FEV1/FVC, and all but two men and one woman were above the lower limit of normal for FEV1 (22). Those participants that were below the lower limit of normal were only below by an average of 0.03 for FEV1/FVC and 0.17 l for FEV1. Men and women engaged in the same amount of self-reported moderate-to-vigorous physical activity. Statistically significant group differences were observed for BMI and age, but absolute differences were small.
Table 1.
Baseline subject characteristics
| Men | Women | |
|---|---|---|
| Age, yr | 27 ± 5 | 25 ± 4* |
| Height, cm | 177 ± 6 | 166 ± 6* |
| Mass, kg | 76 ± 8 | 63 ± 8* |
| BMI, kg/m2 | 24 ± 2 | 23 ± 2* |
| Moderate-to-vigorous physical activity, hr/wk | 5.4 ± 4.1 | 4.9 ± 3.0 |
| FEV1/FVC, % (%predicted) | 80 ± 6 (102 ± 8) | 84 ± 5* (105 ± 7) |
| FVC, l (%predicted) | 5.5 ± 0.8 (102 ± 14) | 4.2 ± 0.5* (103 ± 10) |
| FEV1, l (%predicted) | 4.4 ± 0.6 (103 ± 12) | 3.5 ± 0.4*(108 ± 10) |
| PEF, l/s (%predicted) | 10.7 ± 1.1 (111 ± 12) | 7.8 ± 1.1* (115 ± 15) |
| FEF25–75, l/s (%predicted) | 4.2 ± 1.1 (93 ± 22) | 3.8 ± 0.9 (102 ± 22) |
| TLC, l (%predicted) | 7.0 ± 1.1 (102 ± 13) | 5.3 ± 0.6* (101 ± 9) |
| FRC, l (%predicted) | 3.4 ± 0.7 (101 ± 18) | 2.6 ± 0.4* (90 ± 12*) |
| sRaw, cmH2O·s (%predicted) | 6.7 ± 2.0 (145 ± 44) | 6.7 ± 1.7 (176 ± 45*) |
| MIP, cmH2O (%predicted) | 146 ± 37 (114 ± 29) | 111 ± 35* (122 ± 38) |
| MEP, cmH2O (%predicted) | 176 ± 35 (73 ± 15) | 124 ± 34* (79 ± 21) |
| MVV, l/min | 181 ± 20 | 131 ± 19* |
Values are means ± SD.
Significantly different from men, P < 0.05.
BMI, body mass index; FEV1, forced expiratory volume in 1 s; FVC, forced vital capacity; PEF, peak expiratory flow; FEF25–75, forced expiratory flow between 25 and 75% of FVC; TLC, total lung capacity; FRC, functional residual capacity; sRaw, specific airways resistance; MIP, maximal inspiratory pressure; MEP, maximal expiratory pressure; MVV, maximum voluntary ventilation.
Peak exercise responses are shown in Table 2. Compared with women, men had higher absolute work rates and V̇o2 values at peak exercise. Both groups were above predicted values for V̇o2 peak, with women achieving slightly higher percentage predicted values than men. On average, men and women both achieved respiratory exchange ratios >1.10 and near maximum heart rates and peak Borg ratings, indicating that maximal effort was exerted to a similar degree in both groups.
Table 2.
Maximal exercise responses
| Men | Women | |
|---|---|---|
| Work rate, W | 276 ± 51 | 213 ± 39* |
| VO2, ml·kg−1·min−1 | 54 ± 8 | 47 ± 7* |
| VO2, l/min | 4.0 ± 0.7 | 2.9 ± 0.5* |
| VO2, % predicted | 124 ± 18 | 136 ± 26* |
| VCO2, l/min | 4.5 ± 0.6 | 3.2 ± 0.5* |
| RER | 1.12 ± 0.07 | 1.11 ± 0.05 |
| VE, l/min | 141 ± 22 | 103 ± 18* |
| VT, l | 2.90 ± 0.55 | 2.08 ± 0.33* |
| Fb, breaths/min | 50 ± 9 | 50 ± 9 |
| VE/VCO2 | 31 ± 3 | 32 ± 3 |
| VE/VO2 | 35 ± 4 | 35 ± 4 |
| PETCO2, mmHg | 35 ± 3 | 35 ± 3 |
| Estimated VD/VT | 0.08 ± 0.03 | 0.12 ± 0.02* |
| ΔIC, l | 0.46 ± 0.45 | 0.27 ± 0.25* |
| EELV, %TLC | 48 ± 6 | 50 ± 5 |
| EILV, %TLC | 89 ± 5 | 89 ± 5 |
| IRV, l | 0.73 ± 0.32 | 0.56 ± 0.29* |
| VT/IC, % | 80 ± 8 | 79 ± 10 |
| VE/MVV, % | 79 ± 12 | 78 ± 10 |
| HR, beats/min | 184 ± 9 | 184 ± 10 |
| HR, %predicted | 96 ± 5 | 95 ± 5 |
| O2 pulse, ml/beat | 22 ± 4 | 16 ± 2* |
| SpO2, % | 96 ± 2 | 97 ± 3 |
| Breathing discomfort, Borg scale | 7.3 ± 2.5 | 7.2 ± 2.2 |
| Dyspnea intensity/VE slope | 0.06 ± 0.02 | 0.09 ± 0.04* |
| Leg discomfort, Borg scale | 8.9 ± 1.6 | 7.8 ± 2.0* |
Values are means ± SD.
Significantly different from men, P < 0.05.
VO2, oxygen consumption; VCO2, carbon dioxide production; RER, respiratory exchange ratio; VE, minute ventilation; VT, tidal volume; Fb, breathing frequency; VE/VCO2, ventilatory equivalent for carbon dioxide; VE/VO2, ventilatory equivalent for oxygen; PETCO2, partial pressure of end-tidal CO2; VD/VT, dead space to tidal volume ratio; ΔIC, change in inspiratory capacity from resting values; EELV, end-expiratory lung volume; EILV, end-inspiratory lung volume; TLC, total lung capacity; IRV, inspiratory reserve volume; HR, heart rate; SpO2, oxygen saturation by pulse oximetry. VO2 prediction equation was from Jones et al. (26).
Reasons for stopping exercise and dyspnea intensity.
There were no sex differences in reasons for stopping exercise (Fig. 1). The majority of men and women terminated exercise due to either leg discomfort alone (46% and 63% of men and women, respectively, P > 0.05) or in combination with breathing discomfort (40% and 20% of men and women, respectively, P > 0.05). Women had significantly higher dyspnea intensity/V̇e slopes compared with men (Table 2). Figure 2 shows dyspnea intensity ratings in men and women during exercise. Women had significantly higher dyspnea intensity ratings at standardized submaximal work rates. Dyspnea intensity ratings were also elevated in women for a given V̇e relative to men, but group differences disappeared when V̇e was expressed as a percentage of MVV.
Fig. 1.
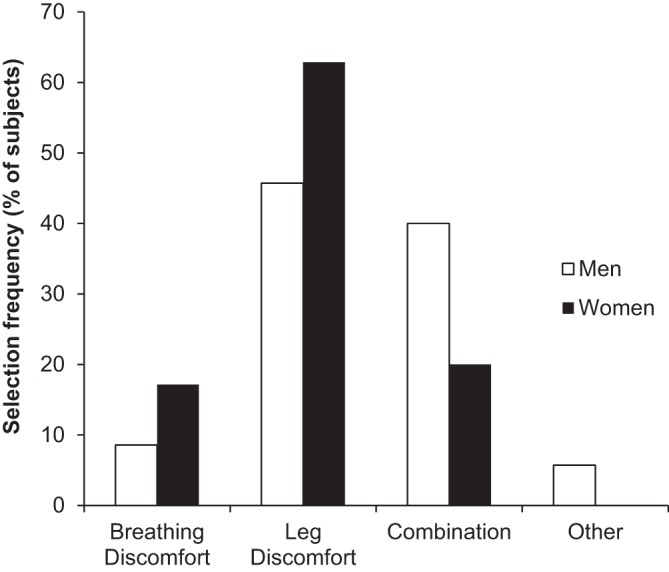
Reasons for stopping exercise. No statistically significant sex differences were observed.
Fig. 2.
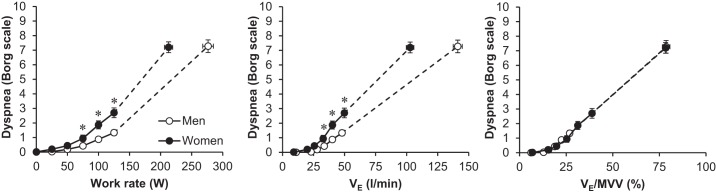
Dyspnea intensity responses to exercise. Values are means ± SE. Statistical comparisons of dyspnea between men and women for a given minute ventilation (Ve) were only performed at the 75-, 100-, and 125-W work rates because VE was not significantly different between groups at these intensities. *Significantly different from men, P < 0.05. MVV, maximum voluntary ventilation.
Qualitative descriptors of dyspnea during exercise.
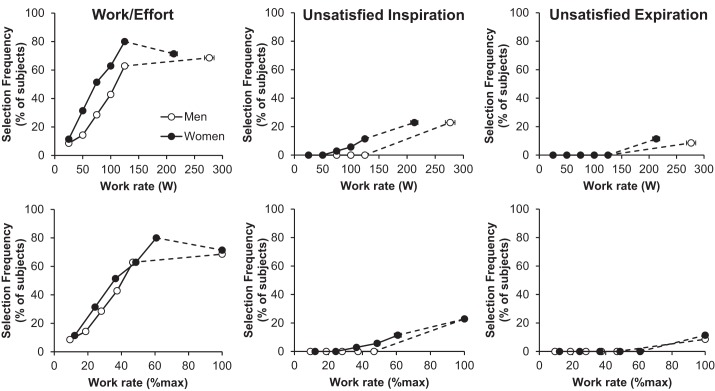
The selection frequencies of qualitative descriptors of dyspnea throughout exercise are shown in Fig. 3. The dominant qualitative descriptor was the sensation of increased work/effort in both men and women throughout submaximal and maximal exercise. A small percentage of participants selected unsatisfied inspiration (men = 23% and women = 23%) and unsatisfied expiration (men = 9% and women = 11%) at maximal exercise with no differences between sexes. A total of 40% of female participants and 29% of male participants selected unsatisfied inspiration, whereas only 14% of females and 14% of males selected unsatisfied expiration at some point during the exercise test. The onset of unsatisfied inspiration in those that selected this phrase occurred at a significantly lower absolute work rate (166 ± 54 vs. 258 ± 62 W, P < 0.001) and V̇e (74 ± 30 vs. 123 ± 36 l/min, P < 0.01) but at a similar relative work rate (80 ± 21 vs. 90 ± 17% Wmax, P > 0.05) and V̇e/MVV (57 ± 22 vs. 67 ± 22%, P > 0.05) in women vs. men, respectively. The onset of unsatisfied expiration in those that selected this phrase also occurred at a significantly lower absolute work rate (180 ± 21 vs. 240 ± 38 W, P < 0.05) and V̇e (74 ± 17 vs. 126 ± 33 l/min, P < 0.05) and tended to occur at a lower relative work rate (78 ± 14 vs. 90 ± 7 %Wmax, P = 0.13) and V̇e/MVV (56 ± 14 vs. 74 ± 20 %, P = 0.14) in women vs. men, respectively.
Fig. 3.
Selection frequency of the 3 dyspnea descriptor phrases throughout submaximal and maximal exercise: 1) “My breathing requires more work and effort” (work/effort); 2) “I cannot get enough air in” (unsatisfied inspiration); and 3) “I cannot get enough air out” (unsatisfied expiration). No statistically significant sex differences were observed at any work rate.
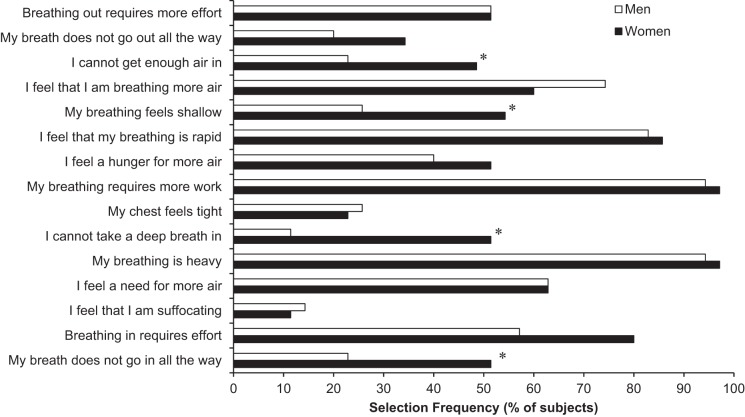
Figure 4 shows the selection frequency of the 15 qualitative descriptors from the standard questionnaire that was administered immediately after exercise. A significantly higher percentage of women selected phrases describing inspiratory difficulty/unsatisfied inspiration (“My breath does not go in all the way,” “I cannot take a deep breath in,” and “I cannot get enough air in”) and shallow breathing (“My breathing is shallow”) compared with men. In an exploratory analysis, we compared all baseline subject characteristics and peak exercise responses in the women that selected “I cannot take a deep breath in” to the women that did not select this phrase. We analyzed this phrase specifically because it showed the greatest difference between sexes among the phrases alluding to inspiratory difficulty/unsatisfied inspiration. A similar analysis was performed comparing those women that selected “My breathing feels shallow” to those that did not. No statistically significant differences were observed between these female subgroups for any baseline descriptive characteristics (e.g., pulmonary function, anthropometrics, etc.) or peak exercise variables.
Fig. 4.
Selection frequency of qualitative descriptors of dyspnea at maximal exercise. *Significantly different from men, P < 0.05.
Ventilatory responses.
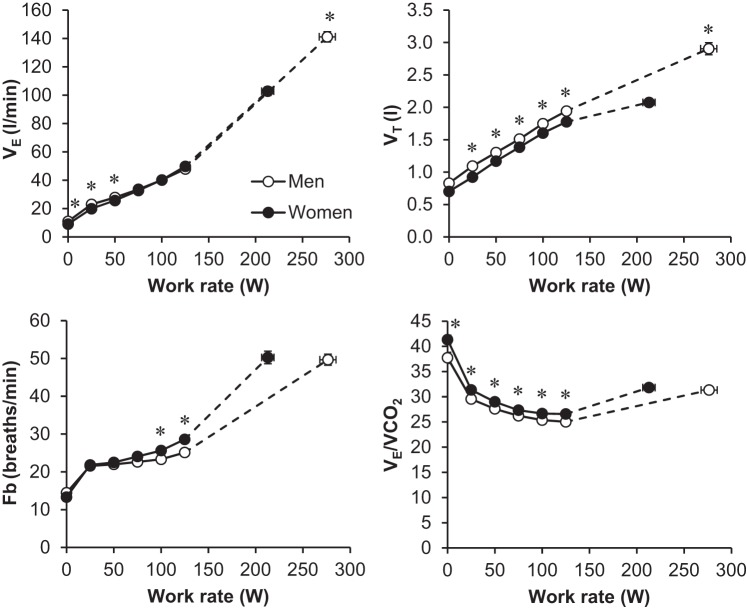
Ventilatory and breathing pattern responses are shown in Fig. 5. Men achieved a significantly higher V̇e and VT at maximal exercise compared with women. Women had a higher breathing frequency and lower VT during exercise at standardized absolute work rates and to achieve the same absolute V̇e, particularly at 100 and 125 W (Fig. 5). The ventilatory equivalent for carbon dioxide was modestly but consistently elevated in women throughout submaximal exercise intensities. Figure 6 shows operating lung volumes as a function of increasing work rate, V̇e, and V̇e/MVV. Women had higher EILV (%TLC) for any given absolute work rate and V̇e, but group differences disappeared when V̇e was expressed as a percentage of MVV.
Fig. 5.
Ventilatory responses to exercise. Values are means ± SE. *Significantly different from men, P < 0.05. VT, tidal volume; Fb, breathing frequency; VE/VCO2, ventilatory equivalent for carbon dioxide.
Fig. 6.
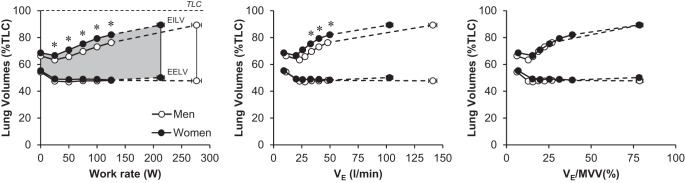
Operating lung volumes during exercise. Gray shaded region represents the VT [i.e., the difference between end-expiratory lung volume (EELV) and end-inspiratory lung volume (EILV)]. Values are means ± SE. Statistical comparisons of operating lung volumes between men and women for a given Ve were only performed at the 75-, 100-, and 125-W work rates because Ve was not significantly different between groups at these intensities. *Significantly different from men, P < 0.05. TLC, total lung capacity.
DISCUSSION
This is the first study to comprehensively evaluate sex differences in both the intensity and qualitative dimensions of exertional dyspnea in a relatively large group of healthy, young, physically active men and women. The main findings of this study are fourfold: 1) women had higher dyspnea intensity ratings than men attributable in large part to their relatively reduced maximum ventilatory capacity; 2) perceived work/effort of breathing was the dominant qualitative descriptor throughout submaximal and maximal exercise in both men and women; 3) more women selected dyspnea descriptors related to inspiratory difficulty/unsatisfied inspiration and shallow breathing relative to men at volitional exhaustion; 4) women adopted a more rapid and shallow breathing pattern and have higher EILV relative to TLC compared with men at absolute submaximal work rates, which may partially explain sex differences in their qualitative descriptors of dyspnea.
Dyspnea is a debilitating and distressing sensation that can have a dramatic impact on quality of life. A growing body of evidence suggests that women with asthma and chronic obstructive pulmonary disease self-report more dyspnea during activities of daily living than their male counterparts even after matching for disease severity (10, 15, 16, 27, 32). The mechanisms underlying this sex difference have only recently been investigated in a handful of physiological studies (19, 41, 44). These studies demonstrate that women have higher dyspnea intensity ratings during exercise for a given absolute work rate, V̇o2, and V̇e; however, dyspnea intensity ratings become similar between men and women when the data are normalized to account for sex differences in body mass or maximal ventilatory capacity. Indeed, our study has confirmed these observations (Fig. 2). The present data coupled with the work of others (19, 41, 44) demonstrate that sex differences in the intensity of perceived dyspnea during exercise are related, at least in part, to the smaller lungs, narrower airways, and weaker respiratory muscles that manifest as a relatively reduced maximal ventilatory capacity in women relative to men. Thus, to perform the same standardized physical task, women will utilize a higher fraction of their ventilatory capacity and will be more likely to experience ventilatory constraints at lower absolute work rates and levels of V̇e relative to men. To build on these observations, new mechanistic insight into sex differences in dyspnea can be gained by evaluating the qualitative dimensions of dyspnea in men and women during exercise.
Unique to the present investigation was our detailed assessment of sex differences in the qualitative dimensions of exertional dyspnea. To characterize the qualitative evolution of dyspnea during exercise, we asked each subject to select the phrase(s) that best described their breathing at each standardized submaximal work rate and at peak exercise (Fig. 3). This approach has been used previously in patients with asthma (28) and chronic obstructive pulmonary disease (29); however, to our knowledge, we are the first to use this method in healthy participants. The results demonstrate that perceived work/effort is the dominant qualitative descriptor of dyspnea in both men and women throughout exercise. This finding supports the hypothesis that the proximate source of dyspnea in both healthy young men and women is the awareness of increased central respiratory motor command output and attendant central corollary discharge. These findings also support the hypothesis advanced by Schaeffer et al. (44) that, during submaximal exercise, sex differences in the intensity of perceived dyspnea likely reflect women's relatively higher neural respiratory drive and contractile respiratory muscle effort requirements to support a given absolute work rate and/or ventilation. It is noteworthy that a small percentage of participants in the present study selected unsatisfied inspiration and unsatisfied expiration during exercise with no differences between sexes. There was a tendency, however, for women to select unsatisfied inspiration and unsatisfied expiration at a slightly lower percentage of peak work rate and at a lower V̇e/MVV, but this did not reach statistical significance. Our approach to tracking the evolution of dyspnea during exercise was unique because participants were asked to select only the phrase(s) that best described their breathing. Thus selecting work/effort did not necessarily mean that they were not experiencing unsatisfied inspiration but that work/effort (and not unsatisfied inspiration) was the dominant qualitative descriptor. To round off our qualitative study of exertional dyspnea, we used a second tool to comprehensively characterize breathing sensation at maximal exercise.
At the end of exercise, we administered a well-established questionnaire listing different descriptors of dyspnea, as used previously in healthy and clinical populations (38–40) and as shown in Fig. 4. A significantly greater proportion of women selected phrases related to shallow breathing and inspiratory difficulty/unsatisfied inspiration. These results demonstrate important sex differences in the sensory experience of dyspnea and potentially suggest that women experience more distressing or unpleasant forms of dyspnea at exhaustion. This is an important observation because these sex differences are emerging even in young, healthy, and physically active men and women. It seems reasonable to speculate that these sex differences in the quality of perceived dyspnea might become amplified when age and/or chronic cardiorespiratory disease are superimposed on women's biologically smaller lungs, narrower airways, and weaker respiratory muscles (19). We speculated, therefore, that previous studies reporting higher levels of activity-related dyspnea in women (10, 15, 16, 27, 32) may be related, at least in part, to hitherto unexplored sex differences in the qualitative dimensions of dyspnea. More specifically, individuals who are mechanically predisposed to developing more distressing forms of dyspnea may self-report higher levels of dyspnea compared with those that experience less distressing or unpleasant forms of dyspnea although this requires further detailed investigation.
Determining the precise mechanisms underlying the observed sex differences in qualitative descriptors of dyspnea remains a challenge because differences could reflect physiological and/or psychological factors. In a subgroup analysis, we were not able to identify any statistically significant differences in baseline characteristics between the women that selected the phrases “I cannot take a deep breath in” and “My breathing feels shallow” compared with the women that did not select these phrases. Nevertheless, the present study may provide some mechanistic insight from a physiological perspective based on the breathing pattern and operating lung volume data. For example, we observed differences in VT and breathing frequency between sexes, indicating that women adopt a more rapid and shallow breathing pattern to perform the same standardized physical task and to achieve similar levels of V̇e at higher work rates (Fig. 5), a finding consistent with other studies (19, 41, 44). This observation may partially explain why women were more likely to describe their breathing as shallow at the end of exercise. The EILV response (Fig. 6) suggests that women also have a tendency to breathe closer to their TLC (i.e., closer to the upper alinear extreme of the sigmoid pressure-volume curve of the respiratory system) relative to men, at least at similar absolute submaximal work rates and levels of V̇e. The greater VT constraints in the setting of a higher V̇e/V̇co2 response (Fig. 5) may be associated with greater dissociation/uncoupling between respiratory neural drive and the mechanical output of the respiratory system in women vs. men, particularly near the limits of exercise tolerance. Collectively, these factors may explain, at least in part, why women were more likely to select phrases related to inspiratory difficulty/unsatisfied inspiration at maximal exercise. However, this remains speculative because a direct causal relationship cannot be determined based on our experimental design. Future studies are required to directly manipulate operating lung volumes in women to determine whether there are important sensory consequences. The higher selection frequency of phrases related to inspiratory difficulty/unsatisfied inspiration may also be related to unmeasured differences in the mechanical work of breathing. Recent evidence suggests that the inspiratory resistive work of breathing is elevated in women (20) because of their smaller-diameter airways (45). Whether women sense the increased resistive work remains to be determined. Thus future studies are needed to determine whether there is a link between the inspiratory resistive work of breathing, airway size, and qualitative descriptors of dyspnea in women.
Methodological considerations.
There are some limitations to this study that must be acknowledged. First, we tested young, healthy, normal-weight, and physically active adults. As such, our results cannot be extrapolated to sedentary individuals or those that are elderly, overweight/obese, and/or have chronic cardiorespiratory disorders. We intentionally recruited physically active younger adults rather than elite athletes or sedentary individuals because we believe that this population represents the “normal” dyspnea response to exercise. In other words, our results are not confounded by deconditioning in sedentary individuals or by the very high ventilatory requirements of elite athletes. Thus our study represents an important starting point so that future studies can examine sex differences in the qualitative dimensions of exertional dyspnea across the continuum of age, cardiorespiratory fitness, and disease severity. Second, our groups had statistically significant differences in age, BMI and %predicted V̇o2 max. While statistically different, the absolute differences between groups for age and BMI were small and unlikely to have an impact on our outcomes. The group differences in %predicted V̇o2 max may have influenced some of our results. However, the observed group differences in the sensory intensity and qualitative dyspnea responses were, if anything, underestimated by having women with modestly higher relative fitness levels than men. Third, we did not include the assessment of unpleasantness or the emotional response to dyspnea in this study. The recently developed Multidimensional Dyspnea Profile, which evaluates sensory intensity, unpleasantness, sensory qualities, and emotional responses (2, 35), may shed additional light on sex differences in dyspnea across the spectrum of health and chronic respiratory diseases in future studies. Fourth, we did not formally collect a pregnancy history from our female subjects. It is possible that a previous pregnancy could alter the intensity and/or affective dyspnea response to exercise compared with nulliparous women. Nevertheless, most women in our study were young university students, the majority of whom (66%) were using oral contraceptives or intrauterine devices. Finally, the reliability of the dyspnea descriptors during exercise has not been systematically investigated. However, in a recent study, Laveneziana et al. (29) conducted incremental and constant work rate exercise tests on separate days in patients with chronic obstructive pulmonary disease and found remarkably similar dyspnea descriptor selections using identical methods as in the present study. This suggests, albeit indirectly, that participants can reliably select these phrases, independent of the exercise protocol.
Conclusions.
This study is the first to provide a detailed characterization of the complex sensory intensity and qualitative dyspnea responses to progressive exercise in a relatively large number of healthy, young, normal-weight, and physically active men and women. We have shown that perceived work/effort of breathing is the dominant qualitative descriptor throughout exercise in both sexes. However, women were more likely than men to report sensations of inspiratory difficulty/unsatisfied inspiration and of shallow breathing at the end of exercise. These sex differences may be related to differences in breathing patterns and the behavior of dynamic operating lung volumes (particularly EILV) during exercise. These findings in healthy adults may have important clinical implications when inherent sex differences in pulmonary anatomy and physiology are superimposed on an aging and/or diseased respiratory system. In this regard, sex differences in the qualitative dimensions of exertional dyspnea may help explain why women with chronic respiratory conditions consistently report more dyspnea than their male counterparts despite similar levels of disease severity (10, 15, 16, 27, 32); further studies are necessary to determine the presence and nature of such relationships.
GRANTS
This research was supported by a Discovery Grant from the Natural Sciences and Engineering Research Council of Canada and an Infrastructure Grant from the Canada Foundation for Innovation. J. Cory was supported by a Canadian Institutes for Health Research Health Professional Student Research Award and the UBC Faculty of Medicine Summer Student Research Program. M. Schaeffer was supported by fellowships from the University of British Columbia and British Columbia Lung Association. D. Jensen was supported by a Chercheurs-Boursiers Junior 1 salary award from the Fonds de Recherche du Québec-Santé and by a William Dawson Research Scholars Award (McGill University). J. Guenette was supported by a Scholar Award from the Michael Smith Foundation for Health Research and a New Investigator Award from the Providence Health Care Research Institute and St. Paul's Hospital Foundation.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: J.M.C., M.R.S., S.S.W., A.H.R., B.A., R.B., M.L., C.L., and B.M. performed experiments; J.M.C., M.R.S., S.S.W., J.H.P., B.A., R.B., M.L., C.L., B.M., and J.A.G. analyzed data; J.M.C., M.R.S., S.S.W., D.J., and J.A.G. interpreted results of experiments; J.M.C. and J.A.G. drafted manuscript; J.M.C., M.R.S., S.S.W., A.H.R., D.J., and J.A.G. edited and revised manuscript; J.M.C., M.R.S., S.S.W., A.H.R., J.H.P., B.A., R.B., M.L., C.L., B.M., D.J., and J.A.G. approved final version of manuscript; J.A.G. conception and design of research; J.A.G. prepared figures.
ACKNOWLEDGMENTS
We thank our subjects for their enthusiastic participation.
REFERENCES
- 1.American Thoracic Society and European Respiratory Society. ATS/ERS Statement on respiratory muscle testing. Am J Respir Crit Care Med 166: 518–624, 2002. [DOI] [PubMed] [Google Scholar]
- 2.Banzett RB, O'Donnell CR, Guilfoyle TE, Parshall MB, Schwartzstein RM, Meek PM, Gracely RH, Lansing RW. Multidimensional dyspnea profile: an instrument for clinical and laboratory research. Eur Respir J 45: 1681–1691, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beidleman BA, Rock PB, Muza SR, Fulco CS, Forte VA Jr, Cymerman A. Exercise VE and physical performance at altitude are not affected by menstrual cycle phase. J Appl Physiol 86: 1519–1526, 1999. [DOI] [PubMed] [Google Scholar]
- 4.Black LF, Hyatt RE. Maximal respiratory pressures: normal values and relationship to age and sex. Am Rev Respir Dis 99: 696–702, 1969. [DOI] [PubMed] [Google Scholar]
- 5.Borg GA. Psychophysical bases of perceived exertion. Med Sci Sports Exerc 14: 377–381, 1982. [PubMed] [Google Scholar]
- 6.Bowden JA, To TH, Abernethy AP, Currow DC. Predictors of chronic breathlessness: a large population study. BMC Public Health 11: 33, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Briscoe WA, Dubois AB. The relationship between airway resistance, airway conductance and lung volume in subjects of different age and body size. J Clin Invest 37: 1279–1285, 1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bryner RW, Toffle RC, Ullrich IH, Yeater RA. Effect of low dose oral contraceptives on exercise performance. Br J Sports Med 30: 36–40, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chang AS, Munson J, Gifford AH, Mahler DA. Prospective use of descriptors of dyspnea to diagnose common respiratory diseases. Chest 148: 895–902, 2015. [DOI] [PubMed] [Google Scholar]
- 10.Chhabra SK, Chhabra P. Gender differences in perception of dyspnea, assessment of control, and quality of life in asthma. J Asthma 48: 609–615, 2011. [DOI] [PubMed] [Google Scholar]
- 11.Crapo RO, Morris AH, Clayton PD, Nixon CR. Lung volumes in healthy nonsmoking adults. Bull Eur Physiopath Respir 18: 419–425, 1982. [PubMed] [Google Scholar]
- 12.Crapo RO, Morris AH, Gardner RM. Reference values for pulmonary tissue volume, membrane diffusing capacity, and pulmonary capillary blood volume. Bull Eur Physiopath Respir 18: 893–899, 1982. [PubMed] [Google Scholar]
- 13.Currow DC, Plummer JL, Crockett A, Abernethy AP. A community population survey of prevalence and severity of dyspnea in adults. J Pain Symptom Manage 38: 533–545, 2009. [DOI] [PubMed] [Google Scholar]
- 14.De Souza MJ, Maguire MS, Rubin KR, Maresh CM. Effects of menstrual phase and amenorrhea on exercise performance in runners. Med Sci Sports Exerc 22: 575–580, 1990. [DOI] [PubMed] [Google Scholar]
- 15.de Torres JP, Casanova C, Hernandez C, Abreu J, Aguirre-Jaime A, Celli BR. Gender and COPD in patients attending a pulmonary clinic. Chest 128: 2012–2016, 2005. [DOI] [PubMed] [Google Scholar]
- 16.de Torres JP, Casanova C, Montejo de Garcini A, Aguirre-Jaime A, Celli BR. Gender and respiratory factors associated with dyspnea in chronic obstructive pulmonary disease. Respir Res 8: 18, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dominelli PB, Render JN, Molgat-Seon Y, Foster GE, Romer LM, Sheel AW. Oxygen cost of exercise hyperpnoea is greater in women compared with men. J Physiol 593: 1965–1979, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guenette JA, Chin RC, Cory JM, Webb KA, O'Donnell DE. Inspiratory capacity during exercise: Measurement, analysis, and interpretation. Pulm Med 2013: 956081, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guenette JA, Jensen D, Webb KA, Ofir D, Raghavan N, O'Donnell DE. Sex differences in exertional dyspnea in patients with mild COPD: physiological mechanisms. Respir Physiol Neurobiol 177: 218–227, 2011. [DOI] [PubMed] [Google Scholar]
- 20.Guenette JA, Querido JS, Eves ND, Chua R, Sheel AW. Sex differences in the resistive and elastic work of breathing during exercise in endurance-trained athletes. Am J Physiol Regul Integr Comp Physiol 297: R166–R175, 2009. [DOI] [PubMed] [Google Scholar]
- 21.Guenette JA, Witt JD, McKenzie DC, Road JD, Sheel AW. Respiratory mechanics during exercise in endurance-trained men and women. J Physiol 581: 1309–1322, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hankinson JL, Odencrantz JR, Fedan KB. Spirometric reference values from a sample of the general US population. Am J Respir Crit Care Med 159: 179–187, 1999. [DOI] [PubMed] [Google Scholar]
- 23.Harms CA. Does gender affect pulmonary function and exercise capacity? Respir Physiol Neurobiol 151: 124–131, 2006. [DOI] [PubMed] [Google Scholar]
- 24.Harms CA, McClaran SR, Nickele GA, Pegelow DF, Nelson WB, Dempsey JA. Exercise-induced arterial hypoxaemia in healthy young women. J Physiol 507: 619–628, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hopkins SR, Barker RC, Brutsaert TD, Gavin TP, Entin P, Olfert IM, Veisel S, Wagner PD. Pulmonary gas exchange during exercise in women: effects of exercise type and work increment. J Appl Physiol 89: 721–730, 2000. [DOI] [PubMed] [Google Scholar]
- 26.Jones NL, Makrides L, Hitchcock C, Chypchar T, McCartney N. Normal standards for an incremental progressive cycle ergometer test. Am Rev Respir Dis 131: 700–708, 1985. [DOI] [PubMed] [Google Scholar]
- 27.Katsura H, Yamada K, Wakabayashi R, Kida K. Gender-associated differences in dyspnoea and health-related quality of life in patients with chronic obstructive pulmonary disease. Respirology 12: 427–432, 2007. [DOI] [PubMed] [Google Scholar]
- 28.Laveneziana P, Bruni GI, Presi I, Stendardi L, Duranti R, Scano G. Tidal volume inflection and its sensory consequences during exercise in patients with stable asthma. Respir Physiol Neurobiol 185: 374–379, 2013. [DOI] [PubMed] [Google Scholar]
- 29.Laveneziana P, Webb KA, Ora J, Wadell K, O'Donnell DE. Evolution of dyspnea during exercise in chronic obstructive pulmonary disease: impact of critical volume constraints. Am J Respir Crit Care Med 184: 1367–1373, 2011. [DOI] [PubMed] [Google Scholar]
- 30.Macnutt MJ, De Souza MJ, Tomczak SE, Homer JL, Sheel AW. Resting and exercise ventilatory chemosensitivity across the menstrual cycle. J Appl Physiol 112: 737–747, 2012. [DOI] [PubMed] [Google Scholar]
- 31.Martin TR, Castile RG, Fredberg JJ, Wohl ME, Mead J. Airway size is related to sex but not lung size in normal adults. J Appl Physiol 63: 2042–2047, 1987. [DOI] [PubMed] [Google Scholar]
- 32.Martinez FJ, Curtis JL, Sciurba F, Mumford J, Giardino ND, Weinmann G, Kazerooni E, Murray S, Criner GJ, Sin DD, Hogg J, Ries AL, Han M, Fishman AP, Make B, Hoffman EA, Mohsenifar Z, Wise R. Sex differences in severe pulmonary emphysema. Am J Respir Crit Care Med 176: 243–252, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McClaran SR, Harms CA, Pegelow DF, Dempsey JA. Smaller lungs in women affect exercise hyperpnea. J Appl Physiol 84: 1872–1881, 1998. [DOI] [PubMed] [Google Scholar]
- 34.Mead J. Dysanapsis in normal lungs assessed by the relationship between maximal flow, static recoil, and vital capacity. Am Rev Respir Dis 121: 339–342, 1980. [DOI] [PubMed] [Google Scholar]
- 35.Meek PM, Banzett R, Parshall MB, Gracely RH, Schwartzstein RM, Lansing R. Reliability and validity of the multidimensional dyspnea profile. Chest 141: 1546–1553, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Miller MR, Hankinson J, Brusasco V, Burgos F, Casaburi R, Coates A, Crapo R, Enright P, van der Grinten CP, Gustafsson P, Jensen R, Johnson DC, MacIntyre N, McKay R, Navajas D, Pedersen OF, Pellegrino R, Viegi G, Wanger J. Standardisation of spirometry. Eur Respir J 26: 319–338, 2005. [DOI] [PubMed] [Google Scholar]
- 37.Morris JF, Koski A, Temple WP, Claremont A, Thomas DR. Fifteen-year interval spirometric evaluation of the Oregon predictive equations. Chest 93: 123–127, 1988. [DOI] [PubMed] [Google Scholar]
- 38.O'Donnell DE, Bertley JC, Chau LK, Webb KA. Qualitative aspects of exertional breathlessness in chronic airflow limitation: pathophysiologic mechanisms. Am J Respir Crit Care Med 155: 109–115, 1997. [DOI] [PubMed] [Google Scholar]
- 39.O'Donnell DE, Chau LK, Webb KA. Qualitative aspects of exertional dyspnea in patients with interstitial lung disease. J Appl Physiol 84: 2000–2009, 1998. [DOI] [PubMed] [Google Scholar]
- 40.O'Donnell DE, Hong HH, Webb KA. Respiratory sensation during chest wall restriction and dead space loading in exercising men. J Appl Physiol 88: 1859–1869, 2000. [DOI] [PubMed] [Google Scholar]
- 41.Ofir D, Laveneziana P, Webb KA, Lam YM, O'Donnell DE. Sex differences in the perceived intensity of breathlessness during exercise with advancing age. J Appl Physiol 104: 1583–1593, 2008. [DOI] [PubMed] [Google Scholar]
- 42.Parshall MB, Schwartzstein RM, Adams L, Banzett RB, Manning HL, Bourbeau J, Calverley PM, Gift AG, Harver A, Lareau SC, Mahler DA, Meek PM, O'Donnell DE. An official American Thoracic Society statement: update on the mechanisms, assessment, and management of dyspnea. Am J Respir Crit Care Med 185: 435–452, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Richards JC, McKenzie DC, Warburton DE, Road JD, Sheel AW. Prevalence of exercise-induced arterial hypoxemia in healthy women. Med Sci Sports Exerc 36: 1514–1521, 2004. [DOI] [PubMed] [Google Scholar]
- 44.Schaeffer MR, Mendonca CT, Levangie MC, Andersen RE, Taivassalo T, Jensen D. Physiological mechanisms of sex differences in exertional dyspnoea: role of neural respiratory motor drive. Exp Physiol 99: 427–441, 2014. [DOI] [PubMed] [Google Scholar]
- 45.Sheel AW, Guenette JA, Yuan R, Holy L, Mayo JR, McWilliams AM, Lam S, Coxson HO. Evidence for dysanapsis using computed tomographic imaging of the airways in older ex-smokers. J Appl Physiol 107: 1622–1628, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Simon PM, Schwartzstein RM, Weiss JW, Fencl V, Teghtsoonian M, Weinberger SE. Distinguishable types of dyspnea in patients with shortness of breath. Am Rev Respir Dis 142: 1009–1014, 1990. [DOI] [PubMed] [Google Scholar]
- 47.Simon PM, Schwartzstein RM, Weiss JW, Lahive K, Fencl V, Teghtsoonian M, Weinberger SE. Distinguishable sensations of breathlessness induced in normal volunteers. Am Rev Respir Dis 140: 1021–1027, 1989. [DOI] [PubMed] [Google Scholar]
- 48.Smith JR, Rosenkranz SK, Harms CA. Dysanapsis ratio as a predictor for expiratory flow limitation. Respir Physiol Neurobiol 198: 25–31, 2014. [DOI] [PubMed] [Google Scholar]
- 49.Thurlbeck WM. Postnatal human lung growth. Thorax 37: 564–571, 1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wanger J, Clausen JL, Coates A, Pedersen OF, Brusasco V, Burgos F, Casaburi R, Crapo R, Enright P, van der Grinten CP, Gustafsson P, Hankinson J, Jensen R, Johnson D, Macintyre N, McKay R, Miller MR, Navajas D, Pellegrino R, Viegi G. Standardisation of the measurement of lung volumes. Eur Respir J 26: 511–522, 2005. [DOI] [PubMed] [Google Scholar]