Abstract
Suppressing sex hormones in women for 1 wk reduces resting energy expenditure (REE). The effects of more chronic suppression on REE and other components of total energy expenditure (TEE), and whether the reduction in REE is specifically due to loss of estradiol (E2), are not known. We compared the effects of 5 mo of sex hormone suppression (gonadotropin releasing hormone agonist therapy, GnRHAG) with placebo (PL) or E2 add-back therapy on REE and the components of TEE. Premenopausal women received GnRHAG (leuprolide acetate 3.75 mg/mo) and were randomized to receive transdermal therapy that was either E2 (0.075 mg/d; n = 24; means ± SD, aged = 37 ± 8 yr, BMI = 27.3 ± 6.2 kg/m2) or placebo (n = 21; aged = 34 ± 9 yr, BMI = 26.8 ± 6.2 kg/m2). REE was measured by using a metabolic cart, and TEE, sleep EE (SEE), exercise EE (ExEE, 2 × 30 min bench stepping), non-Ex EE (NExEE), and the thermic effect of feeding (TEF) were measured by using whole room indirect calorimetry. REE decreased in GnRHAG+PL [mean (95% CI), −54 (−98, −15) kcal/d], but not GnRHAG+E2 [+6 (−33, +45) kcal/d] (difference in between-group changes, P < 0.05). TEE decreased in GnRHAG+PL [−128 (−214, −41) kcal/d] and GnRHAG+E2 [−96 (−159, −32) kcal/d], with no significant difference in between-group changes (P = 0.55). SEE decreased similarly in both GnRHAG+PL [−0.07 (−0.12, −0.03) kcal/min] and GnRHAG+E2 [−0.07 (−0.12, −0.02) kcal/min]. ExEE decreased in GnRHAG+PL [−0.46 (−0.79, −0.13) kcal/min], but not GnRHAG+E2 [−0.30 (−0.65, +0.06) kcal/min]. There were no changes in TEF or NExEE in either group. In summary, chronic pharmacologic suppression of sex hormones reduced REE and this was prevented by E2 therapy.
Keywords: estradiol, premenopausal women, room calorimetry, resistance exercise, gonadotropin releasing hormone
the menopause is associated with accelerated weight and fat gain (21, 28, 40). Studies of both animals and humans suggest that this is related to the loss of ovarian function and, specifically, the loss of estrogens (6, 8, 13, 29, 32, 37, 41, 44). In women, the suppression of sex hormones also appears to adversely affect body fat distribution, by provoking a disproportionate increase in abdominal and visceral fat (6, 8, 32, 44). This increase in abdominal adiposity is of concern, because it may increase the risk of acquiring the metabolic syndrome and associated chronic diseases (i.e., hypertension, coronary heart disease, and noninsulin-dependent diabetes mellitus) (10a). Thus understanding how sex hormones impact body weight regulation and body fat distribution has important public health implications.
The potential system-level mechanisms by which loss of sex hormones disrupts energy balance include reductions in physical activity, energy expenditure (EE), and resting metabolic rate. In rodents, ovariectomy (OVX) has been consistently shown to induce a pronounced and rapid decline in spontaneous physical activity (SPA) (33, 41), which is restored by E2 treatment (25). Decreases in SPA are also observed with disruption of E2 receptor signaling (43). In women followed prospectively, objectively measured physical activity decreased during the menopausal transition (21), although whether this was specifically due to the loss of E2 could not be determined. We (5) and others (1, 22, 23, 35) have found that resting energy expenditure (REE) is lowest during the early follicular phase of the menstrual cycle, when sex hormones are low, and highest during the midluteal phase, when sex hormones are high (5). We have also shown that acute suppression of sex hormones reduces REE below midluteal and early follicular levels (5).
A limitation of our previous study was that sex hormones were suppressed for only 6 days (5). The effects of more chronic suppression (e.g., months) are not known. We also did not previously determine if the reduction in REE was specifically due to the decrease in E2. Furthermore, it is not known if sex hormone suppression affects components of total daily EE (e.g., TEF, EE of exercise, and nonexercise EE) other than REE. In this context, the primary aim of this study was to determine in premenopausal women the effects of chronic sex hormone suppression on total EE (TEE) and its components. The secondary aim was to determine if the decrease in EE is mediated by the loss of circulating E2. Participants underwent 5 mo of gonadotropin releasing hormone agonist (GnRHAG) therapy to chronically suppress ovarian hormones, and were randomized to receive placebo (PL) or E2 add-back therapy. TEE and its components were assessed before and after the intervention using whole room indirect calorimetry. We hypothesized that TEE and its components would be reduced by chronic sex hormone suppression, and that these decreases would be attenuated by E2 therapy. Based on previous research, we anticipated that 5 mo of GnRHAG+PL therapy would cause a decrease in fat-free mass (FFM) (6, 32, 44). Thus as an exploratory aim, some participants in each drug group were also randomized to take part in a progressive resistance exercise training program (GnRHAG+E2+Ex; GnRHAG+PL+Ex). The goal was to generate preliminary data on the effectiveness of exercise to prevent changes in body composition and bone mineral density during ovarian hormone suppression (6, 7, 26, 32, 44).
METHODS
This was a randomized, double-blinded, placebo-controlled trial conducted at the University of Colorado Anschutz Medical Campus (CU-AMC). Procedures followed were in accordance with the ethical standards of and approved by the Colorado Multiple Institutional Review Board and the Scientific Advisory and Review Board of the Clinical and Translational Research Center (CTRC) at CU-AMC. The study was approved on September 22, 2006. The study was registered on ClinicalTrials.gov (NCT00687739) on May 28, 2008. Subject recruitment and enrollment commenced in June 2008, and the last study visit occurred on March 5, 2014.
Subjects and screening procedures.
Volunteers were recruited from the CU-AMC and local communities. Participants were healthy, eumenorrheic premenopausal women (20 to 49 yr). Only nonpregnant, nonlactating women were enrolled. After providing informed written consent, volunteers underwent screening procedures which included a review of medical history (including menstrual cycle history), physical examination, assessment of depressive symptoms, clinical laboratory evaluation (metabolic panel and complete blood count), measurement of bone mineral density, and an exercise stress test (34). Additional exclusion criteria were use of hormonal contraception, oral glucocorticoids, or diabetes medications; smoking; and body mass index (BMI) >39 kg/m2. Those volunteers who met the screening criteria were then instructed on maintaining menstrual cycle calendars over 2 mo to confirm cycle length and ovulation (ClearPlan Easy, Unipath Diagnostics, Waltham, MA). Only women with normal menstrual cycle function (no missed cycles in previous year; cycle length 28 ± 5 days) were enrolled.
Experimental design and study procedures.
After screening procedures were completed, eligible volunteers underwent baseline testing; although the goal was to perform these measurements during the early follicular phase (days 2 to 6 after onset of menses) of the menstrual cycle, scheduling restraints prevented this in a few women. Testing included measurements of body composition, REE, and TEE. Body composition, including fat mass (FM) and FFM, was measured by using whole body dual-energy X-ray absorptiometry (Hologic Delphi-W, Hologic, Bedford, MA). At the beginning of the next menstrual cycle, participants began 5 mo of GnRHAG therapy to chronically suppress ovarian function. Participants were randomized to receive transdermal E2 (GnRHAG+E2) or an identical placebo patch (GnRHAG+PL). To reduce the risk of endometrial hyperplasia while also minimizing the exposure to progesterone (10, 39), women randomized to E2 received medroxyprogesterone acetate (5 mg/d, in pill form) for 12 d every other month (end of month 2 and 4, and after completion of follow-up testing). After 5 mo, the measurements of body composition and EE were repeated. At the end of the intervention, participants were asked to rate hot flash frequency (0 = never, 1 = rarely, 2 = occasionally, 3 = regularly, 4 = often, and 5 = almost always) and severity (0 = none, 1 = slight, 2 = mild, 3 = moderate, 4 = severe, and 5 = extreme) over the previous 3 mo.
Sex hormone suppression.
GnRHAG (leuprolide acetate 3.75 mg, Lupron; TAP Pharmaceutical Products, Lake Forest, IL) was delivered by monthly intramuscular injection. A single injection of leuprolide acetate produces an initial stimulation (for up to 3 wk) followed by a prolonged suppression of anterior pituitary gonadotropins and gonadal sex steroids. Repeated monthly dosing maintains ovarian hormone suppression. The rationale for the drug regimen was that this standard clinical dose suppresses ovarian function and results in fat gain after 16 wk of treatment (6, 7, 26, 32, 44). Absence of pregnancy was confirmed before each dosing by a urine pregnancy test.
E2 add-back therapy.
Participants were randomized to receive transdermal E2 (Bayer HealthCare Pharmaceuticals, Berkeley, CA) 0.075 mg/d or placebo patches. The E2 regimen was expected to maintain serum E2 concentrations in the mid to late follicular phase range (∼100 to 150 pg/ml).
Resting energy expenditure.
REE was measured between 0700 and 0900 h, following an overnight fast and 24 h abstention from exercise by using standard indirect calorimetry with the ventilated hood technique (TrueOne 2400, ParvoMedics, Sandy, UT). Prior to the measurement, participants rested quietly for 30 min in a dimly lit, thermoneutral room. Respiratory gas exchange was measured for 30 min, and values from the last 20 min were used to determine REE (38).
Total energy expenditure.
TEE was measured by using whole room indirect calorimetry. Oxygen (O2) and carbon dioxide (CO2) concentrations were measured continuously by using a fuel cell-based dual channel O2 analyzer (FC-2 Oxzilla, Sable Systems, International, Las Vegas, NV) and two infrared CO2 analyzers (CA-10 CO2 Analyzers, Sable Systems, International, Las Vegas, NV), as previously described (24). O2 consumption and CO2 production were calculated in 1-min intervals by using the flow rate and the differences in CO2 and O2 concentrations between entering and exiting air, and minute EE was calculated by using the equations of Jequier et al. (16). TEE was obtained by summing minute values for EE and extrapolating to 24 h values. The accuracy and precision of the system was tested monthly by using propane combustion tests. The average O2 and CO2 recoveries during the study were ≥98.0%.
For 4 days prior to and during the calorimeter study day, subjects consumed a controlled diet provided by the CTRC Bionutrition Core. The energy content (kcal/d) of the diet was REE (kcal/d) × 1.5, and the macronutrient composition was 20% protein, 30% fat, and 50% carbohydrate. On the study day, subjects entered the calorimeter at 0800 h and exited at 0700 h the following morning. Subjects were instructed not to nap or perform any exercise other than that prescribed by the protocol, and to go to bed at the same time during each calorimeter stay. Meals were provided at 0830, 1330, and 1800 h. The distribution of energy intake was 40% at breakfast, 30% at lunch, and 30% at dinner. Each subject was provided the same meals during the baseline and follow-up measurements. Following consumption of the breakfast meal, subjects were instructed to remain resting in bed in a semirecumbent position for the subsequent 4 h (0900 to 1300 h) for the estimation of the TEF. To mimic free-living physical activity, two bouts (30 min) of bench stepping exercise were performed at 1400 and 1530 h. Bench stepping was performed by using an 8 in. bench step, and stepping rate was paced by a metronome set at 72 beats per minute.
Indirect calorimetry data were used to determine the thermic effect of feeding (TEF), exercise EE (ExEE), sleeping EE (SEE), and nonexercise EE (NExEE). ExEE (kcal/min) was calculated as the average EE during the final 20 min of each exercise bout. SEE (kcal/min) was calculated by averaging EE over the period from 0100 to 0400 h. NExEE was calculated as 24 h EE − [(SEE × 1,440) + (ExEE × 60) + (24 h TEF)], where 24 h TEF was estimated as 24 h energy intake × 10% (3).
Exercise intervention to stabilize body composition.
Based on previous research, we anticipated that 5 mo of GnRHAG+PL therapy would cause a decrease in FFM of ∼1.0 kg and an increase in FM of ∼1.5 kg (6, 32, 44). This decrease in FFM would be expected to augment the reduction in REE related to E2 suppression (14). As an exploratory aim, some participants in each drug group were also randomized to take part in a progressive resistance exercise training program (GnRHAG+E2+Ex; GnRHAG+PL+Ex). The progressive resistance exercise intervention began in week 1 of sex hormone suppression, and was performed 4 d/wk for 18 wk, as has been previously described (34). The exercise program was stopped 2 wk prior to follow-up testing to minimize the acute effects of exercise on the study outcomes.
Sex hormones.
Blood samples for sex hormones were collected during baseline testing and during week 20 of the intervention. Collection samples were stored at −80°C until analysis. Estrone (E1), E2, and progesterone (P) were determined by radioimmunoassay (Diagnostic Systems Lab, Webster, TX). Respective intra- and interassay CVs were 8.7% and 8.6% for E1, 6% and 11% for E2, and 7.5% and 10.2% for P. Total testosterone (T) was analyzed by chemiluminescence immunoassay (2.1 and 5.1%; Beckman Coulter, Fullerton, CA) and sex hormone-binding globulin by immunoradiometric assay (5.1% and 12%; Diagnostic Systems Laboratory).
Statistical analysis.
The primary analysis compared the GnRHAG+E2 and GnRHAG+PL groups, pooled across exercise status. Baseline differences in all variables between the GnRHAG+E2 and GnRHAG+PL groups were evaluated by using two-group t-tests. Changes within each group in response to intervention were also evaluated with paired t-tests. Differences in change over time between groups were tested by using an ANCOVA model, first with treatment group alone, and again adding FM and FFM to the model. The study was not powered to detect differences among the four treatment groups for the exploratory exercise intervention aim. Therefore, only descriptive statistics and within-group changes are presented for the exercise and nonexercise groups within each drug group. All analyses were done by using SAS 9.3 (SAS Institute, Cary, NC). Data are reported as means ± SD or mean (95% CI), unless otherwise specified. The effects of the interventions on body composition and bone mineral density have been reported (34).
RESULTS
GnRHAG+PL vs. GnRHAG+E2.
As previously reported (34), 79 women were randomized to the two drug groups and 9 participants were lost to follow-up (personal reasons, 4; lack of time, 3; side effects of GnRHAG, 1; and hypertension, 1). Of the 70 women who completed the intervention, 35 were randomized to GnRHAG+E2 and 35 to GnRHAG+PL. Because of scheduling limitations, not all subjects could be studied in the room calorimeter. Thus complete EE data for the current paper were obtained on 24 women in the GnRHAG+PL group and 21 women in the GnRHAG+E2 group. There were no significant differences in the characteristics of the drug groups at baseline, except for testosterone, which was higher in GnRHAG+PL (Table 1). Following the 5-mo intervention, serum E2 was decreased in GnRHAG+PL (−69.5 ± 80.8 pg/ml, P < 0.001) and increased in GnRHAG+E2 (71.8 ± 115.7 pg/ml, P = 0.02, between-group difference in change over time, P < 0.01). Weight remained unchanged in GnRHAG+PL, but increased in GnRHAG+E2 (between-group difference in change over time, P < 0.01). At the end intervention, hot flash frequency (3.2 ± 1.7 vs. 0.6 ± 0.9, P < 0.001) and intensity (2.9 ± 1.4 vs. 0.6 ± 1.4, P < 0.001) were higher in GnRHAG+PL than GnRHAG+E2. The drug treatments differentially affected body composition. FM was unchanged in GnRHAG+PL (−0.2 ± 2.0 kg, P = 0.65) and increased in GnRHAG+E2 (0.7 ± 1.4 kg, P = 0.04; between-group difference in change, P = 0.07). FFM decreased in GnRHAG+PL (−0.5 ± 1.1 kg, P = 0.02) and did not change in GnRHAG+E2 (0.5 ± 1.3 kg, P = 0.85; between-group difference in change, P < 0.01).
Table 1.
Characteristics of participants randomized to gonadotropin releasing hormone agonist therapy plus add-back of placebo or estradiol at baseline and after 5 mo of treatment
| GnRHAG+PL |
GnRHAG+E2 |
|||
|---|---|---|---|---|
| Baseline | 5 mo | Baseline | 5 mo | |
| Age, yr | 37 (8) | 33 (9) | ||
| Body mass index, kg/m2 | 27.3 (6.2) | 27.1 (6.4) | 26.9 (6.2) | 27.4 (6.3)** |
| Weight, kg | 72.6 (14.7) | 71.9 (15.0) | 73.1 (17.6) | 74.4 (12.2)**† |
| Fat mass, kg | 26.1 (11.0) | 25.9 (11.2) | 26.0 (12.2) | 26.7 (12.8)** |
| Fat-free mass, kg | 46.5 (5.6) | 46.0 (5.6)** | 47.1 (6.2) | 47.7 (5.8)† |
| Estrone, pg/ml | 53.8 (19.6)f | 33.1 (23.3)** | 54.0 (14.2)d | 81.2 (34.5)** |
| Estradiol, pg/ml | 90.1 (81.1)e | 20.6 (8.3)** | 55.1 (41.2)c | 124.3 (104.6)**† |
| Progesterone, pg/ml | 1.1 (1.4)e | 0.3 (0.2)** | 0.8 (1.1)c | 0.3 (0.2) |
| Testosterone, pg/ml | 27.6 (8.2)b* | 19.9 (4.4)** | 40.0 (14.5)a | 35.1 (13.6)** |
| SHBG, nmol/l | 54.5 (23.4)f | 46.6 (22.0)** | 40.1 (13.0)d | 44.3 (18.9) |
Values are means ± SD; unless otherwise indicated, n = 24 for the GnRHAG+PL group and n = 21 for the GnRHAG+E2 group. GnRHAG+PL, gonadotropin releasing hormone agonist therapy plus placebo; GnRHAG+E2, gonadotropin releasing hormone agonist therapy plus estradiol; SHBG, sex hormone-binding globulin.
Between-group difference at baseline, P < 0.05;
within-group change, P < 0.05;
between-group difference in change, P < 0.05;
n = 14;
n = 16;
n = 17;
n = 18;
n = 21;
n = 22.
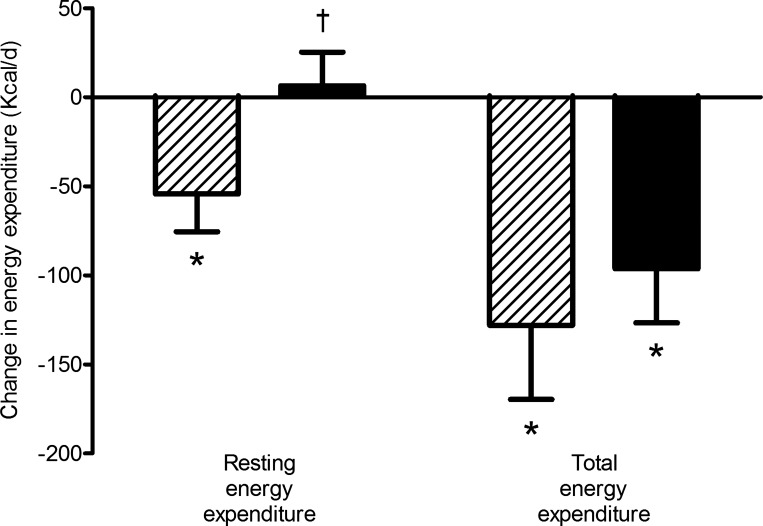
At baseline, there were no differences in REE, TEE, or the components of TEE between the drug groups (Table 2). Following the 5-mo intervention, mean REE decreased significantly by −3.7% in the GnRHAG+PL group, and this was prevented by E2 therapy (Fig. 1). The between-group difference in change in REE remained after adjusting for changes in FM and FFM. In contrast to REE, mean TEE decreased significantly in both GnRHAG+PL (−5.7%) and GnRHAG+E2 (−4.3%) (Fig. 1). The between-group difference in change was not significant (P = 0.55). The decreases in TEE remained significant after adjusting for changes in FM and FFM and did not differ between groups.
Table 2.
Total energy expenditure and its components measured in the room calorimeter at baseline and after 5 mo of treatment in participants randomized to gonadotropin releasing hormone agonist therapy plus add-back of placebo or estradiol
| GnRHAG+PL |
GnRHAG+E2 |
|||
|---|---|---|---|---|
| Baseline | 5 mo | Baseline | 5 mo | |
| Resting EE, kcal/d | 1,455.4 (181.4) | 1,401.1 (167.9)*,† | 1,456.1 (167.9) | 1,462.6 (169.8) |
| Total EE, kcal/d | 2,226.0 (321.7) | 2,098.0 (307.7)* | 2,255.7 (270.7) | 2,159.5 (299.6)* |
| Sleep EE, kcal/min | 1.02 (0.15) | 0.95 (0.16)* | 1.05 (0.12) | 0.97 (0.10)* |
| Exercise EE, kcal/min | 5.47 (1.21) | 5.01 (1.24)* | 5.48 (1.18) | 5.18 (1.41) |
| Thermic effect of feeding, kcal/min | 0.41 (0.11) | 0.45 (0.12) | 0.41 (0.11) | 0.46 (0.14) |
| Nonexercise EE, kcal | 197.2 (110.3) | 216.9 (83.2) | 182.7 (53.7) | 223.9 (108.1) |
Values are means ± SD; GnRHAG+PL, n = 24; GnRHAG+E2, n = 21. EE, energy expenditure.
Within-group change, P < 0.05;
between-group difference in change, P < 0.05.
Fig. 1.
Changes (unadjusted) in resting energy expenditure and total energy expenditure (kcal/d) in GnRHAG+PL (hatched bars) and GnRHAG+E2 (solid bars). *Within-group change, P < 0.05; †between-group difference in change, P < 0.05.
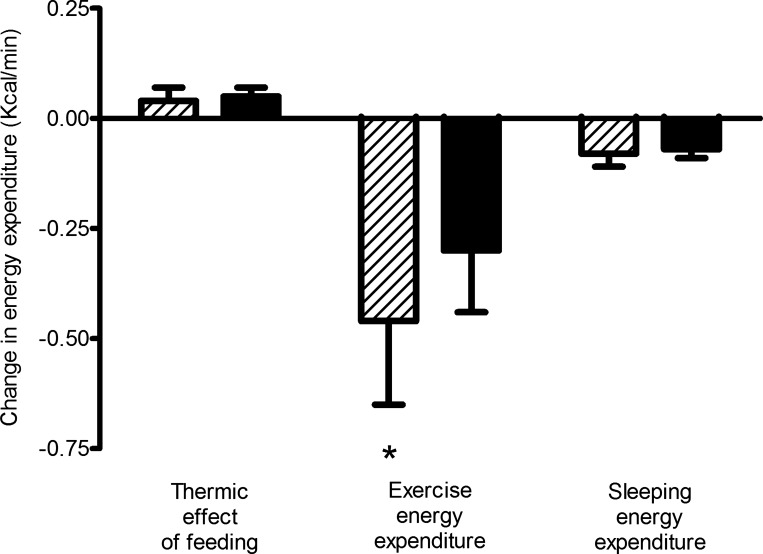
There were no changes in 4-h TEF in response to the intervention in either group, with or without adjustment for changes in FM and FFM (Fig. 2). Mean SEE decreased significantly in both GnRHAG+PL (−6.8%) and GnRHAG+E2 (−6.7%); the difference between groups was not significant (P = 0.81), even after adjusting for changes in FM and FFM. Conversely, mean ExEE decreased in GnRHAG+PL (−8.4%), but did not change significantly in GnRHAG+E2 (−5.5%); the difference in change in ExEE between groups was not significant (P = 0.49). After adjustment for changes in FM and FFM, the decrease in ExEE was significant in both groups, but the difference in change between groups was not (P = 0.76). There were no significant changes in NExEE in either GnRHAG+PL or GnRHAG+E2, even after adjustment for changes in body composition.
Fig. 2.
Changes (unadjusted) in 4-h thermic effect of feeding, exercise energy expenditure, and sleeping energy expenditure (kcal/min) GnRHAG+PL (hatched bars) and GnRHAG+E2 (solid bars). *Within-group change, P < 0.05.
Effects of resistance exercise training.
The changes in body weight within each drug group were similar for the exercisers and nonexercisers, but it appeared that resistance exercise differentially affected changes in body composition within each group (Table 3). In GnRHAG+PL women who did not perform resistance exercise, there was a reduction in FFM, with little change in FM. However, in GnRHAG+PL women who performed resistance exercise, FFM was preserved and there was a modest reduction in FM. In GnRHAG+E2 women who did not perform resistance exercise, there was an increase in FM, and no change in FFM. In GnRHAG+E2 women who performed resistance exercise, there was no change in FM; on average, these women increased FFM, although the confidence interval was large. These changes were similar to those previously reported for the entire cohort (34).
Table 3.
Changes in response to 5 mo of gonadotropin releasing hormone agonist therapy plus add-back of placebo or estradiol, with or without resistance exercise training
| GnRHAG+PL |
GnRHAG+E2 |
|||
|---|---|---|---|---|
| No exercise (n = 16) | Exercise (n = 8) | No exercise (n = 16) | Exercise (n = 5) | |
| Weight, kg | −0.6 (−1.8,+0.7) | −1.0 (−2.5,+0.5) | 1.1 (+0.3,+1.9) | 1.6 (−2.2,+5.4) |
| Fat mass, kg | 0.2 (−0.9,+1.4) | −1.0 (−2.2,+0.2) | 0.9 (+0.1,+1.6) | 0.1 (−1.7,+1.9) |
| Fat-free mass, kg | −0.8 (−1.3,-0.3) | 0.1 (−0.9,+0.9) | 0.2 (−0.3,+0.8) | 1.5 (−0.8,+3.7) |
| Estradiol, pg/ml | −82.3 (−141.9,−22.7)a | −48.8 (−77.7,−19.7) | 44.6 (−6.9,+96.2)a | 199.0 (−200.0,+598.0)b |
| REE, kcal/d | −37.8 (−88.7,+13.0) | −87.2 (−185.9,+11.4) | 14.6 (−34.8,+64.0) | −19.3 (−95.1+56.4) |
| TEE, kcal/min | −108.8 (−201.1,−16.5) | −166.5 (−388.1,+55.1) | 92.7 (−167.1,−18.4) | −107.4 (−298.3,+83.5) |
Values are means (95% CI). GnRHAG+PL, GnRH agonist treatment + placebo; GnRHAG+E2, GnRH agonist treatment + estradiol; REE, resting energy expenditure; TEE, total energy expenditure.
n = 13;
n = 4.
Despite these different effects on body composition, resistance exercise appeared to have little to no effect on REE or TEE in either GnRHAG+E2 or GnRHAG+PL. REE and TEE decreased in both treatment arms in women who exercised. These decreases were not significant (all CIs cross 0), and the CIs were all wide because of the relatively small number of subjects in each group. Nonetheless, these data do not suggest a protective effect of resistance exercise training on the decreases in REE and TEE that occur with GnRHAG+PL. The changes in TEF (+0.02 to −0.10 kcal/min), SEE (−0.6 to −0.10 kcal/min), ExEE (−0.2 to −0.5 kcal/min), and NExEE (−12 to −46 kcal/d) were small and in the same direction in exercising and nonexercising women within each drug group.
DISCUSSION
We studied the effects of 5 mo of sex hormone suppression plus placebo or E2 therapy on TEE and its components. To our knowledge, this was the first study to investigate the regulation of EE by E2 in a controlled manner in humans. Our results demonstrated that chronic sex hormone suppression reduced REE, SEE, ExEE, and TEE, and that E2 therapy attenuated the decreases in REE and ExEE. If our model of pharmacologic suppression of ovarian function mimics the effects of menopause on bioenergetics, the decreases in EE may contribute, at least in part, to the propensity for fat gain across the menopause transition.
Suppression of sex hormones has been shown to cause increases in FM, with a preferential accumulation of visceral adiposity (6, 8, 32, 44). This fat accumulation pattern is mirrored during the menopause (21, 28, 40). Furthermore, several large randomized controlled trials have demonstrated that women on hormone therapy (HT) gain less weight and FM then placebo-treated women (9, 15, 17, 36), and that the attenuated fat gain in women on HT is primarily from the trunk region (11, 15, 18, 20), suggesting a protective effect of estrogens. The results of the current study did not confirm an effect of E2 to protect against excess fat accumulation. In fact, the GnRHAG+E2 group had an increase in FM, whereas the GnRHAG+PL group did not. Based on previous studies of the effects of 16 to 24 wk of GnRHAG therapy on body composition (6, 7, 26, 32, 44), the expected increase in FM after 20 wk of GnRHAG+PL therapy was 1.0 to 1.5 kg; the change in this group was −0.2 kg. The previous studies of GnRHAG therapy did not evaluate the effects of adding back E2 (6, 7, 26, 32, 44), so it is possible that fat gains would have been even larger with GnRHAG+E2, which would be consistent with our finding. However, if E2 promotes fat gain in women, this would be inconsistent with the plethora of evidence from basic (e.g., estrogen receptor-α knockout mice) (13, 27, 43) and preclinical (e.g., OVX with vs. without E2 therapy) (12, 25, 31, 33, 41, 42) studies documenting that the loss of estrogens promotes excess fat gain. An alternative explanation for our findings is that, because weight gain was discussed during the consenting process for the current study, it is possible that the results were influenced by behavioral modifications. This may not have been the case in previous studies because they involved patients undergoing GnRHAG therapy for a clinical indication (e.g., treatment of endometriosis or uterine fibroids) (6, 7, 26, 32, 44).
Several lines of evidence suggest that the decrease in endogenous E2 is the mechanistic trigger that disrupts energy balance and causes weight gain. For example, replacing E2 in rodents after OVX attenuates the weight and fat gain that occurs with OVX (30, 42). Furthermore, disrupting E2 signaling by knocking out estrogen receptor α causes excessive weight and fat gain (13, 27). However, how lack of E2 causes weight gain is not understood. There is strong preclinical evidence from studies of OVX vs. OVX+E2 that lack of E2 causes a decrease in TEE through reductions in physical activity and resting metabolic rate (12, 25, 33, 41). It has also been demonstrated that EE is reduced when ERα signaling in hypothalamic steroidogenic factor-1 neurons is disrupted (43). Whether such effects of E2 also occur in humans is not well understood. In women, REE varies across the menstrual cycle and is lowest during the early follicular phase, when ovarian hormones are low (1, 5, 22, 23, 35). However, in these studies, it was not possible to isolate the potential effects of E2 from other factors (e.g., progesterone). Our results are consistent with preclinical studies (33, 41, 43) and provide the first mechanistic evidence in women, demonstrating that E2 add-back ameliorates the decrease in REE in response to suppression of sex hormones.
We previously demonstrated that suppression of sex hormones for only 6 days also reduced REE below levels measured during the early follicular (−35 kcal/d) and midluteal (−71 kcal/d) phases of the menstrual cycle (5). In the current study most, but not all, women were studied in the early follicular phase, and the magnitude of reduction in REE (−54 kcal/d) was consistent with the previous study. The decrease in REE in GnRHAG+PL was similar with and without adjustment for changes in body composition, suggesting that the changes in REE were independent of changes in body composition. If sustained, the decrease in REE would be expected to increase the propensity for weight gain. In our previous study, we could not determine if the reduction in REE was specifically due to reductions in E2. In the current study, E2 therapy prevented the decline in REE observed in GnRHAG+PL. Thus we conclude that reductions in E2 are mechanistically linked to the reduction in whole body REE. However, the tissue- or cellular-level mechanisms that mediate this change remain unknown. Previous work by our group has demonstrated that muscle sympathetic nerve activity (MSNA) is reduced during acute ovarian hormone suppression (4). Interestingly, MSNA was restored by progesterone but not E2 add-back. Whether these same results would be observed with a longer period of ovarian function or in a larger cohort is not known. Understanding how the different ovarian hormones impact metabolic rate in various tissues is an area that warrants future investigation.
A secondary aim of the study was to determine whether suppressing sex hormones affected TEE and its components. Although we observed significant decreases in TEE, SEE, and ExEE in response to GnRHAG+PL, the changes were not different from those in response to GnRHAG+E2. The results were not influenced by changes in body composition. The decreases in TEE and SEE in the GnRHAG+PL group were consistent with changes in a prospective study of women through the menopause transition (21), but the absence of between-group differences in the changes was surprising, especially given the between-group differences in changes in REE and FFM. In future studies, it would be desirable to measure TEE during free-living conditions, rather than in a room calorimeter, and over more than a single day.
Given the decrease in REE in the GnRHAG+PL group, the lack of an effect on SEE was surprising. It is plausible that sleep was disrupted in the GnRHAG+PL group (19), which could have increased metabolic rate and masked the true effects on sex hormone suppression on SEE. Although we did not obtain a measure of sleep quality during the calorimeter stays, women in the GnRHAG+PL arm reported a greater frequency and intensity of hot flashes at the end of the intervention, which could contribute to sleep disturbances. It is also worth noting that SEE at baseline was slightly higher than REE in both groups (Table 2). We speculate that differences in the approaches used to measure REE and SEE contributed to this. SEE was measured during the calorimeter stay between 0100 and 0400 h. There was no monitoring to ensure that participants were actually sleeping during this time. In contrast, REE was measured upon exiting the calorimeter in the morning by using a metabolic cart for increased precision over a short time interval. REE measurement was highly controlled, and subjects were monitored to be sure they remained awake and motionless. We also observed that SEE decreased in both groups over time and was, indeed, lower than REE at 5 mo. We have observed this in previous serial measurements (Melanson, unpublished data), and hypothesize that this reflects acclimation to sleeping in the unfamiliar setting of the room calorimeter. If metabolic rate was, indeed, increased at night in response to GnRHAG+PL because of sleep disruptions, this may explain why the difference between the groups in the change in REE (−54 kcal/d in GnRHAG+PL vs. +6 kcal/d in GnRHAG+E2; difference of 60 kcal/d) was greater than the change in TEE (−128 kcal/d in GnRHAG+PL vs. −96 kcal/d in GnRHAG+E2; difference of 32 kcal/d).
The decrease in ExEE in both groups was also unexpected, because weight remained unchanged in GnRHAG+PL and slightly increased in GnRHAG+E2. The energy cost of the exercise performed in this study (bench stepping) is determined by body weight and stepping rate. Although the women were asked to match stepping cadence to a metronome, whether this occurred was not verified. Further, we did not have an objective measurement of whether they completed the entire prescribed bout of exercise. Thus it is possible that the decrease in ExEE was the result of decreases in stepping rate and/or exercise time rather than to the suppression of sex hormones. Understanding how suppression of sex hormones impacts exercise EE and, perhaps, the motivation to exercise remains another area for future investigation.
An exploratory aim of the primary study was to determine if resistance exercise training could attenuate the effects of suppressing sex hormones on changes in body composition. As stated a priori, the study was not powered to detect differences between exercise and nonexercise groups within each drug arm, and only a subset of women in each group was randomized to exercise. The intent was to generate preliminary evidence regarding whether exercise could prevent or attenuate the changes in body composition and bioenergetics that occur in response to sex hormone suppression. Because of the small number of subjects in the exercise groups, these data should be interpreted only as preliminary findings to guide future studies. As previously reported (34), the resistance exercise program preserved FFM and attenuated gains in FM in women on GnRHAG+PL. In women on GnRHAG+E2, the resistance exercise program attenuated the gains in FM and lead to a modest increase in FFM. Despite the preservation of FFM, resistance exercise training had no effect on REE, TEE, or the components of TEE. However, because the study was not powered to evaluate the effects of exercise, no conclusions can be drawn and results should be used only to guide future investigations.
Strengths of the current study included the randomized controlled design, double blinding, robust experimental control over the sex hormone milieu by using a pharmacological approach, add-back of estradiol or placebo, and the use of a room calorimeter to measure TEE and its components in a highly controlled manner. We also studied premenopausal women, which allowed us to isolate the independent effects hormone deficiency from those of age or menopause. However, limitations of GnRHAG therapy as a model for menopause are that changes in sex hormones more abrupt and gonadotropins are suppressed rather than elevated. Also, we tested only a single dose of E2, delivered transdermally, with only intermittent exposure to oral progesterone.
In conclusion, chronic pharmacologic suppression of sex hormones reduced REE and this was prevented by E2 therapy. Other components of EE, such as SEE and ExEE, may also be regulated by E2, but confirmation of this will require more rigorous measurement approaches than those used in the current trial. Adjusting the changes in EE for changes in FM and FFM had little effect on the results. If bioenergetic responses to the pharmacologic suppression of ovarian function are similar to the responses to menopause, the decreases in REE and ExEE may contribute to the propensity for fat gain across the menopause. Finally, although our preliminary data suggested that resistance exercise may not be an effective strategy to prevent the decrease in EE in response to ovarian suppression, this must be further evaluated.
GRANTS
This work was supported by National Institutes of Health Grants R01 AG-018198; P30 DK-048520; and UL1 TR-001082, T32 AG000279, and F32 AG046957.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
E.L.M. and K.L.S. performed experiments; E.L.M., P.W., and W.M.K. analyzed data; E.L.M., K.M.G., P.W., M.E.W., R.S.S., and W.M.K. interpreted results of experiments; E.L.M. prepared figures; E.L.M. drafted manuscript; E.L.M., K.M.G., K.L.S., P.W., M.E.W., R.S.S., and W.M.K. edited and revised manuscript; E.L.M., K.M.G., K.L.S., P.W., M.E.W., R.S.S., and W.M.K. approved final version of manuscript; R.S.S. and W.M.K. conception and design of research.
ACKNOWLEDGMENTS
We are grateful to the nursing, bionutrition, core laboratory, information systems, and administrative staffs of the Clinical and Translational Research Center (CTRC) and Energy Balance Core of the Nutrition and Obesity Research Center for their support of the study. We also acknowledge the members of our research group who carried out the day-to-day activities for the project. Finally, we thank the women who volunteered to participate in the study for their time and efforts.
REFERENCES
- 1.Bisdee JT, James WP, Shaw MA. Changes in energy expenditure during the menstrual cycle. Br J Nutr 61: 187–199, 1989. [DOI] [PubMed] [Google Scholar]
- 3.Bloesch D, Schutz Y, Breitenstein E, Jequier E, Felber JP. Thermogenic response to an oral glucose load in man: comparison between young and elderly subjects. J Am Coll Nutr 7: 471–483, 1988. [DOI] [PubMed] [Google Scholar]
- 4.Day DS, Gozansky WS, Bell C, Kohrt WM. Acute sex hormone suppression reduces skeletal muscle sympathetic nerve activity. Clin Auton Res 21: 339–345, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Day DS, Gozansky WS, Van Pelt RE, Schwartz RS, Kohrt WM. Sex hormone suppression reduces resting energy expenditure and β-adrenergic support of resting energy expenditure. J Clin Endocrinol Metab 90: 3312–3317, 2005. [DOI] [PubMed] [Google Scholar]
- 6.Douchi T, Kuwahata R, Yamasaki H, Yamamoto S, Oki T, Nakae M, Nagata Y. Inverse relationship between the changes in trunk lean and fat mass during gonadotropin-releasing hormone agonist therapy. Maturitas 42: 31–35, 2002. [DOI] [PubMed] [Google Scholar]
- 7.Douchi T, Kuwahata T, Yoshimitsu N, Iwamoto I, Yamasaki H, Nagata Y. Changes in serum leptin levels during GnRH agonist therapy. Endocr J 50: 355–359, 2003. [DOI] [PubMed] [Google Scholar]
- 8.Dumesic DA, Abbott DH, Eisner JR, Herrmann RR, Reed JE, Welch TJ, Jensen MD. Pituitary desensitization to gonadotropin-releasing hormone increases abdominal adiposity in hyperandrogenic anovulatory women. Fertil Steril 70: 94–101, 1998. [DOI] [PubMed] [Google Scholar]
- 9.Espeland MA, Stefanick ML, Kritz-Silverstein D, Fineberg SE, Waclawiw MA, James MK, Greendale GA. Effect of postmenopausal hormone therapy on body weight and waist and hip girths. Postmenopausal Estrogen-Progestin Interventions Study Investigators. J Clin Endocrinol Metab 82: 1549–1556, 1997. [DOI] [PubMed] [Google Scholar]
- 10.Ettinger B, Selby J, Citron JT, Vangessel A, Ettinger VM, Hendrickson MR. Cyclic hormone replacement therapy using quarterly progestin. Obstet Gynecol 83: 693–700, 1994. [PubMed] [Google Scholar]
- 10a.Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) (Adult Treatment Panel III). JAMA 285: 2486–2497, 2001. [DOI] [PubMed] [Google Scholar]
- 11.Gambacciani M, Ciaponi M, Cappagli B, De Simone L, Orlandi R, Genazzani AR. Prospective evaluation of body weight and body fat distribution in early postmenopausal women with and without hormonal replacement therapy. Maturitas 39: 125–132, 2001. [DOI] [PubMed] [Google Scholar]
- 12.Gorzek JF, Hendrickson KC, Forstner JP, Rixen JL, Moran AL, Lowe DA. Estradiol and tamoxifen reverse ovariectomy-induced physical inactivity in mice. Med Sci Sports Exerc 39: 248–256, 2007. [DOI] [PubMed] [Google Scholar]
- 13.Heine PA, Taylor JA, Iwamoto GA, Lubahn DB, Cooke PS. Increased adipose tissue in male and female estrogen receptor-α knockout mice. Proc Natl Acad Sci U S A 97: 12729–12734, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Heymsfield SB, Gallagher D, Wang Z. Body composition modeling. Application to exploration of the resting energy expenditure fat-free mass relationship. Ann N Y Acad Sci 904: 290–297, 2000. [DOI] [PubMed] [Google Scholar]
- 15.Jensen LB, Vestergaard P, Hermann AP, Gram J, Eiken P, Abrahamsen B, Brot C, Kolthoff N, Sorensen OH, Beck-Nielsen H, Nielsen SP, Charles P, Mosekilde L. Hormone replacement therapy dissociates fat mass and bone mass, and tends to reduce weight gain in early postmenopausal women: a randomized controlled 5-year clinical trial of the Danish Osteoporosis Prevention Study. J Bone Miner Res 18: 333–342, 2003. [DOI] [PubMed] [Google Scholar]
- 16.Jequier E, Acheson K, Schutz Y. Assessment of energy expenditure and fuel utilization in man. Ann Rev Nutr 7: 187–208, 1987. [DOI] [PubMed] [Google Scholar]
- 17.Kanaya AM, Herrington D, Vittinghoff E, Lin F, Grady D, Bittner V, Cauley JA, Barrett-Connor E. Glycemic effects of postmenopausal hormone therapy: the Heart and Estrogen/progestin Replacement Study A randomized, double-blind, placebo-controlled trial. Ann Intern Med 138: 1–9, 2003. [DOI] [PubMed] [Google Scholar]
- 18.Kohrt WM, Ehsani AA, Birge SJ Jr. HRT preserves increases in bone mineral density and reductions in body fat after a supervised exercise program. J Appl Physiol 84: 1506–1512, 1998. [DOI] [PubMed] [Google Scholar]
- 19.Kravitz HM, Zhao X, Bromberger JT, Gold EB, Hall MH, Matthews KA, Sowers MR. Sleep disturbance during the menopausal transition in a multi-ethnic community sample of women. Sleep 31: 979–990, 2008. [PMC free article] [PubMed] [Google Scholar]
- 20.Kristensen K, Pedersen SB, Vestergaard P, Mosekilde L, Richelsen B. Hormone replacement therapy affects body composition and leptin differently in obese and non-obese postmenopausal women. J Endocrinol 163: 55–62, 1999. [DOI] [PubMed] [Google Scholar]
- 21.Lovejoy JC, Champagne CM, de Jonge L, Xie H, Smith SR. Increased visceral fat and decreased energy expenditure during the menopausal transition. Int J Obes (Lond) 32: 949–958, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Matsuo T, Saitoh S, Suzuki M. Effects of the menstrual cycle on excess postexercise oxygen consumption in healthy young women. Metabolism 48: 275–277, 1999. [DOI] [PubMed] [Google Scholar]
- 23.Meijer GA, Westerterp KR, Saris WH, ten Hoor F. Sleeping metabolic rate in relation to body composition and the menstrual cycle. Am J Clin Nutr 55: 637–640, 1992. [DOI] [PubMed] [Google Scholar]
- 24.Melanson EL, Ingebrigtsen JP, Bergouignan A, Ohkawara K, Kohrt WM, Lighton JR. A new approach for flow-through respirometry measurements in humans. Am J Physiol Regul Integr Comp Physiol 298: R1571–R1579, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mitsushima D, Takase K, Funabashi T, Kimura F. Gonadal steroids maintain 24 h acetylcholine release in the hippocampus: organizational and activational effects in behaving rats. J Neurol 29: 3808–3815, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nowicki M, Adamkiewicz G, Bryc W, Kokot F. The influence of luteinizing hormone-releasing hormone analog on serum leptin and body composition in women with solitary uterine myoma. Am J Obstet Gynecol 186: 340–344, 2002. [DOI] [PubMed] [Google Scholar]
- 27.Ohlsson C, Hellberg N, Parini P, Vidal O, Bohlooly YM, Rudling M, Lindberg MK, Warner M, Angelin B, Gustafsson JA. Obesity and disturbed lipoprotein profile in estrogen receptor-α-deficient male mice. Biochem Biophys Res Commun 278: 640–645, 2000. [DOI] [PubMed] [Google Scholar]
- 28.Pasquali R, Casimirri F, Labate AM, Tortelli O, Pascal G, Anconetani B, Gatto MR, Flamia R, Capelli M, Barbara L. Body weight, fat distribution and the menopausal status in women. The VMH Collaborative Group. Int J Obes Relat Metab Disord 18: 614–621, 1994. [PubMed] [Google Scholar]
- 29.Pedersen SB, Bruun JM, Kristensen K, Richelsen B. Regulation of UCP1, UCP2, and UCP3 mRNA expression in brown adipose tissue, white adipose tissue, and skeletal muscle in rats by estrogen. Biochem Biophys Res Commun 288: 191–197, 2001. [DOI] [PubMed] [Google Scholar]
- 30.Pighon A, Barsalani R, Yasari S, Prud'homme D, Lavoie JM. Does exercise training prior to ovariectomy protect against liver and adipocyte fat accumulation in rats? Climacteric 13: 238–248, 2010. [DOI] [PubMed] [Google Scholar]
- 31.Pighon A, Gutkowska J, Jankowski M, Rabasa-Lhoret R, Lavoie JM. Exercise training in ovariectomized rats stimulates estrogenic-like effects on expression of genes involved in lipid accumulation and subclinical inflammation in liver. Metabolism 60: 629–639, 2011. [DOI] [PubMed] [Google Scholar]
- 32.Revilla R, Revilla M, Villa LF, Cortes J, Arribas I, Rico H. Changes in body composition in women treated with gonadotropin-releasing hormone agonists. Maturitas 31: 63–68, 1998. [DOI] [PubMed] [Google Scholar]
- 33.Rogers NH, Perfield JW 2nd, Strissel KJ, Obin MS, Greenberg AS. Reduced energy expenditure and increased inflammation are early events in the development of ovariectomy-induced obesity. Endocrinology 150: 2161–2168, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shea KS, Gavin KM, Melanson EL, Gibbons E, Stavros A, Wolfe P, Kittleson J, Vondracek S, Gozansky WS, Schwartz RS, Wierman ME, Kohrt WM. Body composition and bone mineral density after ovarian hormone suppression with and without estradiol add-back. Menopause. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Solomon SJ, Kurzer MS, Calloway DH. Menstrual cycle and basal metabolic rate in women. Am J Clin Nutr 36: 611–616, 1982. [DOI] [PubMed] [Google Scholar]
- 36.Utian WH, Gass ML, Pickar JH. Body mass index does not influence response to treatment, nor does body weight change with lower doses of conjugated estrogens and medroxyprogesterone acetate in early postmenopausal women. Menopause 11: 306–314, 2004. [DOI] [PubMed] [Google Scholar]
- 37.Wade GN, Gray JM, Bartness TJ. Gonadal influences on adiposity. Int J Obes 9 Suppl 1: 83–92, 1985. [PubMed] [Google Scholar]
- 38.Weir JB. New methods for calculating metabolic rate with special reference to protein metabolism. J Physiol 109: 1–9, 1949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Williams DB, Voigt BJ, Fu YS, Schoenfeld MJ, Judd HL. Assessment of less than monthly progestin therapy in postmenopausal women given estrogen replacement. Obstet Gynecol 84: 787–793, 1994. [PubMed] [Google Scholar]
- 40.Wing RR, Matthews KA, Kuller LH, Meilahn EN, Plantinga PL. Weight gain at the time of menopause. Arch Intern Med 151: 97–102, 1991. [PubMed] [Google Scholar]
- 41.Witte MM, Resuehr D, Chandler AR, Mehle AK, Overton JM. Female mice and rats exhibit species-specific metabolic and behavioral responses to ovariectomy. Gen Comp Endocrinol 166: 520–528, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wohlers LM, Spangenburg EE. 17Beta-estradiol supplementation attenuates ovariectomy-induced increases in ATGL signaling and reduced perilipin expression in visceral adipose tissue. J Cell Biochem 110: 420–427, 2010. [DOI] [PubMed] [Google Scholar]
- 43.Xu Y, Nedungadi TP, Zhu L, Sobhani N, Irani BG, Davis KE, Zhang X, Zou F, Gent LM, Hahner LD, Khan SA, Elias CF, Elmquist JK, Clegg DJ. Distinct hypothalamic neurons mediate estrogenic effects on energy homeostasis and reproduction. Cell metabolism 14: 453–465, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yamasaki H, Douchi T, Yamamoto S, Oki T, Kuwahata R, Nagata Y. Body fat distribution and body composition during GnRH agonist therapy. Obstet Gynecol 97: 338–342, 2001. [DOI] [PubMed] [Google Scholar]