Abstract
Cutaneous acetylcholine (ACh)-mediated dilation is commonly used to assess microvascular function, but the mechanisms of dilation are poorly understood. Depending on dose and method of administration, nitric oxide (NO) and prostanoids are involved to varying extents and the roles of endothelial-derived hyperpolarizing factors (EDHFs) are unclear. In the present study, five incremental doses of ACh (0.01-100 mM) were delivered either as a 1-min bolus (protocol 1, n = 12) or as a ≥20-min continuous infusion (protocol 2, n = 10) via microdialysis fibers infused with 1) lactated Ringer, 2) tetraethylammonium (TEA) [a calcium-activated potassium channel (KCa) and EDHF inhibitor], 3) L-NNA+ketorolac [NO synthase (NOS) and cyclooxygenase (COX) inhibitors], and 4) TEA+L-NNA+Ketorolac. The hyperemic response was characterized as peak and area under the curve (AUC) cutaneous vascular conductance (CVC) for bolus infusions or plateau CVC for continuous infusions, and reported as %maximal CVC. In protocol 1, TEA, alone and combined with NOS+COX inhibition, attenuated peak CVC (100 mM Ringer 59 ± 6% vs. TEA 43 ± 5%, P < 0.05; L-NNA+ketorolac 35 ± 4% vs. TEA+L-NNA+ketorolac 25 ± 4%, P < 0.05) and AUC (Ringer 25,414 ± 3,528 vs. TEA 21,403 ± 3,416%·s, P < 0.05; L-NNA+ketorolac 25,628 ± 3,828%.s vs. TEA+L-NNA+ketorolac 20,772 ± 3,711%·s, P < 0.05), although these effects were only significant at the highest dose of ACh. At lower doses, TEA lengthened the total time of the hyperemic response (10 mM Ringer 609 ± 78 s vs. TEA 860 ± 67 s, P < 0.05). In protocol 2, TEA alone did not affect plateau CVC, but attenuated plateau in combination with NOS+COX inhibition (100 mM 50.4 ± 6.6% vs. 30.9 ± 6.3%, P < 0.05). Therefore, EDHFs contribute to cutaneous ACh-mediated dilation, but their relative contribution is altered by the dose and infusion procedure.
Keywords: calcium-activated potassium channels, laser-Doppler flowmetry, endothelium, nitric oxide, skin
acetylcholine (ach)-mediated vasodilation has been studied extensively in the skin and is commonly used as a test of endothelial microvascular function (27, 50). However, much disparity has been observed in regard to the mechanisms underlying the response. Depending on the dose and route of administration (i.e., microdialysis or iontophoresis), prostanoids may (14, 16, 22, 25, 38) or may not (4, 34) be involved, and nitric oxide (NO) is involved to varying extents, from almost not at all (22, 26, 38) to contributing almost 50% of the response (25, 33). However, regardless of the dose or delivery method, a large portion of the response remains unexplained.
Endothelial-derived hyperpolarizing factors (EDHFs) are involved in ACh-mediated dilation in other vascular beds (12, 49) and in the cutaneous vasodilatory responses to other stimuli (8, 30). The contribution of one type of EDHF, epoxyeicosatrienoic acids (EETs), to ACh-mediated dilation in human skin has been investigated previously, but with mixed results. One study showed no effect of the cytochrome P450 epoxygenase-inhibitor sulfaphenazole (delivered via cutaneous microinjection) (29) and another showed intra-arterial sulfaphenazole significantly attenuated dilation, but to no greater extent than either NO synthase (NOS) or cyclooxygenase (COX) inhibition (37), leaving much of the dilation still unexplained. Many other types of EDHFs exist and are known to contribute to vasodilation in other vascular beds. The majority of EDHFs elicit vasodilation through calcium-activated potassium (KCa) channels found on the endothelium and vascular smooth muscle (15). As such, we sought to test the hypothesis that EDHFs account for the NOS- and COX-independent portion of ACh-mediated vasodilation using the KCa channel inhibitor tetraethylammonium (TEA).
Because the contributions of NO and prostanoids vary depending on the dose of ACh, we expected the contribution of EDHF to also vary. Therefore, we examined a dose response to 0.01, 0.1, 1, 10, and 100 mM ACh. The length of time of ACh delivery has also varied in previous studies, particularly those utilizing the microdialysis technique. Some studies have delivered bolus doses [e.g., 1-min infusion via microdialysis at 2 μl/min followed by allowing skin blood flow (SkBF) to return to normal in between doses] (14, 16, 22, 25, 38), whereas others have used continuous infusions, varying in length of time of infusion from 5 to 20+ min (4, 25, 33, 34). In the present study, we sought to compare both methods of delivery.
In protocol 1, we delivered five incremental bolus doses of ACh, allowing SkBF to return to baseline in between doses, and in protocol 2, we continuously infused the five doses of ACh, also in an incremental order. We hypothesized that with both methods of infusion, EDHFs would contribute a smaller percentage of the hyperemia at lower doses, and a greater extent at higher doses, because combined inhibition of NO and prostanoids appears to block a larger percentage of the response at lower doses compared with higher doses, based on previous reports [see Medow et al. (33) for an example in which they reported the contribution of NOS and COX in response to 0.001 to 100 mM ACh]. Furthermore, we hypothesized that the relative contribution of EDHFs would be greater with continuous ACh delivery compared with bolus delivery because we believed prolonged exposure to ACh could result in a reduction in the contribution of NO to vasodilation, as observed with prolonged local heating (20), resulting in upregulation of EDHF-mediated dilation.
METHODS
Subjects.
Twenty-two subjects (11 men, 11 women) participated in the study. Prior to participation, all subjects gave oral and written informed consent. All protocols were approved by the Institutional Review Board at the University of Oregon and were conducted in accordance with the guidelines set forth by the Declaration of Helsinki. All subjects were young (age 22 ± 1 yr), healthy (BMI 22.0 ± 0.4 kg/m), nonsmokers, and were not taking any prescription medications with the exception of combined hormonal contraceptives. All female subjects were studied during menses or during the placebo phase if they were taking contraceptives to minimize the effects of the female sex hormones (7).
On the study day, subjects reported to the laboratory having fasted for at least 4 h and having refrained from all over-the-counter medications, including nonsteroidal anti-inflammatory drugs, for at least 24 h, and alcohol and caffeine for at least 12 h. Upon arrival, subject height and weight were recorded, and female subjects were required to provide a negative pregnancy test.
Instrumentation.
Subjects rested in a semisupine position. Four microdialysis fibers (MD-2000 Linear Microdialysis Probe; BASi, West Lafayette, IN; 30 kDa cutoff membrane) were inserted into the dermal layer of skin on the ventral side of the left forearm at least 4 cm apart using aseptic technique. Fibers were introduced using a 25-gauge needle through which the fibers were threaded. The needle was removed once the fibers were in place. Fibers had a 1-cm-long semipermeable membrane, which was centered between the entry and exit points (∼2.5 cm apart). Fibers were secured with tape, attached to the outlet port of switch cocks (CMA 110 Liquid Switch; CMA Microdialysis, Kista, Sweden), and continuously infused with lactated Ringer solution at a rate of 2 μl/min (CMA 102 Microdialysis Pump; CMA) until the infusion of study drugs (see Study protocol). A period of at least 75 min was allowed for the trauma associated with needle insertion to dissipate prior to commencement of the study protocol.
Skin red blood cell flux was assessed using laser-Doppler flowmetry probes (MoorLab; Moor Instruments, Devon, UK), which were seated in the center of local heaters. The local heaters cover ∼0.78 cm2 of tissue and were centered over each microdialysis fiber.
A blood pressure cuff was placed on the brachium of the nonexperimental arm to monitor arterial blood pressure via brachial oscillation (Dinamap ProCare 100; GE Medical Systems, Tampa, FL).
Study protocol.
Each site was randomly selected to receive one of the following: 1) lactated Ringer (control); 2) 50 mM tetraethylammonium (TEA; Sigma-Aldrich, St. Louis, MO) to inhibit KCa channels and thus the actions of EDHFs; 3) combined 10 mM N-nitro-l-arginine (L-NNA; Sigma-Aldrich) and 10 mM ketorolac (Keto; Sigma-Aldrich) to inhibit NOS and COX, respectively; and 4) combined TEA+L-NNA+Keto to observe the effects of KCa channel blockade in the presence of NOS and COX inhibition. Investigational new drug (IND) approvals were obtained through the FDA to administer these drugs via cutaneous microdialysis (IND 124,754). These drug concentrations were selected as they have previously been shown to be the minimum dose capable of fully inhibiting each respective pathway (22, 25, 30, 33). L-NNA was used instead of the more commonly used NG-nitro-l-arginine methyl ester (L-NAME) because L-NAME can act as a muscarinic receptor antagonist (9). Our laboratory has also shown 50 mM TEA to be specific to KCa channels when administered via microdialysis, with minimal effects on other types of potassium channels (6). Specific inhibitors of large-conducting (B)KCa channels, such as charybdotoxin and iberiotoxin, were not used because these are toxic to humans (39). All drugs were dissolved in lactated Ringer solution. Drugs were infused via microdialysis for at least 75 min before the start of ACh infusions. A 5-min baseline was recorded prior to ACh infusions.
Five concentrations of ACh (Sigma-Aldrich; purity ≥99% by thin-layer chromatography) were delivered in ascending order: 0.01 mM, 0.1 mM, 1 mM, 10 mM, and 100 mM. ACh was dissolved in lactated Ringer solution, in combination with the corresponding inhibitory agents for each site to avoid interruption of inhibition. Infusions were achieved by switching the stopcock. Use of the stopcock allows a precise dose to be delivered and prevents any interruptions in the flow of the drugs. For protocol 1 (bolus infusions; n = 12), infusions were delivered for 1 min and were staggered by 2 min between each of the four sites. Doses at each site were separated by at least 20 min, or as long as necessary for blood flow to return to baseline values. For protocol 2 (continuous infusion, n = 10), infusions were delivered until a plateau in SkBF was observed for at least 5 min. Fifteen to 20 min of continuous infusion was usually sufficient. Once a plateau was reached, the stopcock was switched to the next concentration without interruption.
Once all infusions were completed, 56 mM sodium nitroprusside (SNP; Nitropress, Ciba Pharmaceuticals, East Hanover, NJ) was infused, and local temperature was increased to 43.5°C using the local heaters to ensure maximal flux had been achieved. In two subjects, we tested the ability of SNP to produce maximal dilation. SNP was infused until a stable plateau in SkBF was obtained (about 20 min), and then the local temperature was increased.
Data analysis.
Data were stored and analyzed off-line using a data acquisition system (Windaq; Dataq Instruments, Akron, OH). Cutaneous vascular conductance (CVC) was calculated as laser-Doppler red blood cell flux divided by mean arterial pressure (MAP). All measurements were normalized to maximal CVC, as determined by infusion of 56 mM SNP and local heating to 43.5°C, and presented as a percentage of maximal CVC (%CVCmax).
Vasodilation to bolus infusion of ACh was characterized by the peak CVC and by the total time and area under the curve (AUC) of the hyperemic response above baseline. At each site, peak CVC was measured as the average CVC over a 30-s time interval. For calculating AUC and total time of the hyperemic response, the start point was determined as the first inflection in CVC above baseline, which consistently occurred at ∼240 s after the stopcock was switched over to begin each infusion of ACh. The end point was determined as the time when CVC had stabilized back at baseline values. Baseline was calculated as the CVC averaged over 60 s immediately before and 60 s immediately following the hyperemic response. When no visible change in CVC was detected (e.g., with double or triple blockade at lower concentrations of ACh), peak CVC was measured at the same time as the control site and a time period equal to that at the control site was used for determination of AUC.
Vasodilation to continuous infusion of ACh was characterized by plateau CVC, which occurred after approximately 15–20 min of continuous ACh infusion at each dose and was defined as a period of at least 5 min of stable CVC. CVC was averaged over at least 2 min toward the end of the plateau, just before moving on to the next dose of ACh.
Unfortunately, we were unable to obtain complete dose-response relationships and so were not able to compare the data using traditional ED50 techniques.
Data are presented as means ± SE.
Statistical analysis.
Data were compared for each type of infusion (bolus or continuous) for statistical differences using a two-way repeated measures ANOVA with factors of inhibitory drug site (lactated Ringer, TEA, L-NNA+Keto, and TEA+L-NNA+Keto) and ACh dose (0.01, 0.1, 1, 10, and 100 mM). When significant main effects were detected, significant differences between paired variables across inhibitory drug sites and ACh doses were determined using a Student-Newman-Keul's post hoc test. Maximal flux and baseline data from each of the two protocols were pooled for each inhibitory drug site. Baseline CVC and maximal flux were compared across drug sites using one-way repeated measures ANOVA with a Student-Newman-Keul's post hoc test. For all statistical analyses, the level of significance was set to α = 0.05.
RESULTS
Bolus infusions.
Bolus infusion of ACh generally resulted in an increase in CVC, followed by a return to baseline, as shown in Figure 1, a representative tracing of vasodilation to 10 mM ACh in one subject. However, at the lower doses of ACh (0.01 and 0.1 mM), only a minimal change in CVC was observed, which was not significantly different from baseline CVC at any site (P = 0.31 and P = 0.22 for 0.01 and 0.1 mM ACh). Higher doses of ACh (1, 10, and 100 mM) produced an incrementally greater change in CVC as evaluated by both peak CVC and AUC. Averaged data for peak CVC and AUC are displayed in Figure 2, A and B, respectively.
Fig. 1.
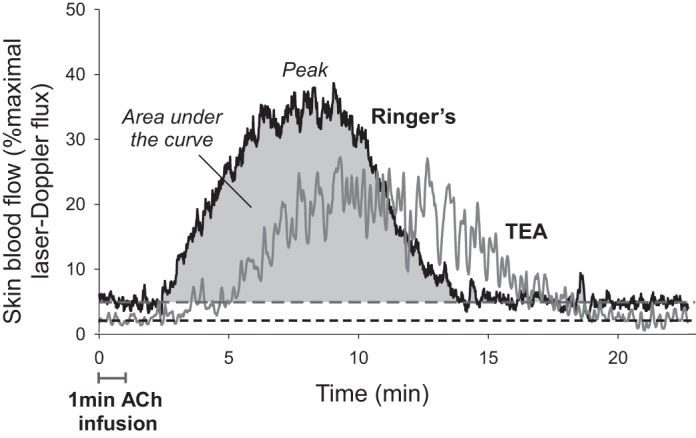
Representative skin blood flow response from one subject to a 1-min bolus infusion of 10 mM acetylcholine (ACh) at microdialysis sites receiving lactated Ringer (black) and tetraethylammonium (TEA; gray). Dashed lines represent baseline skin blood flow for each site.
Fig. 2.
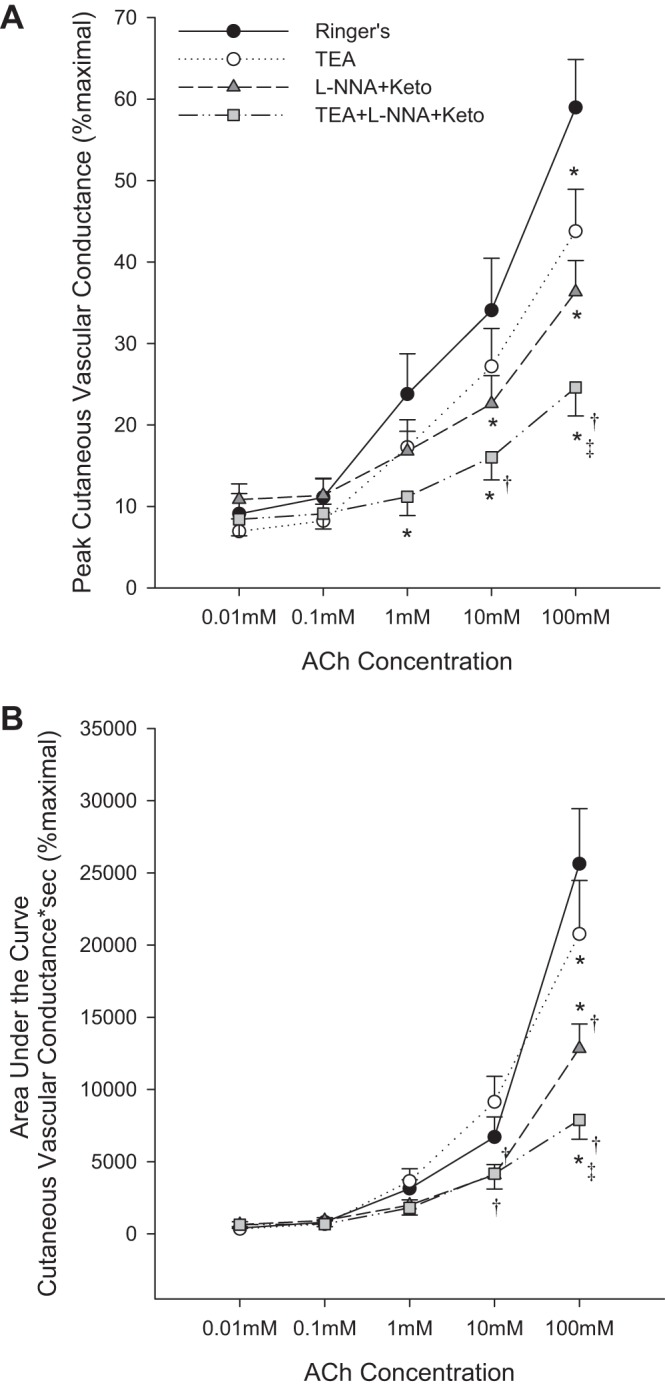
Peak (A) and area under the curve (AUC) (B) cutaneous vascular conductance (CVC) in response to bolus infusions of ACh at the four microdialysis sites. Drugs include tetraethylammonium (TEA), N-nitro-l-arginine (L-NNA), and ketorolac (Keto). Data are means ± SE. *P < 0.05 from Ringer site; †P < 0.05 from TEA site; ‡P < 0.05 from L-NNA+Keto site.
At 1 and 10 mM ACh, TEA had no effect on AUC (P = 0.78 and P = 0.18, respectively), but tended to attenuate peak CVC (P = 0.13 and P = 0.11, respectively). A sample size analysis showed that 114 subjects would be required to reach statistical significance at 10 mM ACh with a power of 0.80 and an α level of 0.05. Interestingly, at these two doses, TEA lengthened the total time of the hyperemic response (shown in Table 1). Thus blockade of KCa channels changed the hyperemic profile, allowing vasodilation to occur to a lesser extent but for a longer period of time, resulting in no difference in AUC. This effect is demonstrated by the representative tracing shown in Figure 1. Conversely, at 100 mM ACh, TEA significantly attenuated both AUC and peak CVC, accounting for 26 ± 13% of the peak dilation above baseline. Furthermore, the total time of the hyperemic response was not longer than at the control site (P = 0.58). Thus the mechanisms behind ACh-mediated dilation differ between 100 mM and the lower doses.
Table 1.
Total time (in seconds) of the hyperemic response
| ACh concentration | Ringer | TEA | L-NNA+Keto | TEA+L-NNA+Keto |
|---|---|---|---|---|
| 1 mM | 476 ± 62 | 659 ± 43* | 354 ± 24*† | 509 ± 44†‡ |
| 10 mM | 609 ± 78 | 839 ± 64* | 449 ± 33*† | 701 ± 62‡ |
| 100 mM | 1,143 ± 112 | 1,184 ± 69 | 741 ± 66*† | 867 ± 64*† |
Total time of the hyperemic response to increasing bolus doses of acetylcholine (ACh). TEA, tetraethylammonium; L-NNA, N-nitro-l-arginine; Keto, ketorolac. Data are means ± SE
P < 0.05 from Ringer site;
P < 0.05 from TEA site;
P < 0.05 from L-NNA+Keto site.
Combined NOS and COX inhibition significantly attenuated peak CVC to 10 mM and 100 mM ACh. At the highest dose, combined NOS+COX contributed to 37 ± 10% of the peak dilation above baseline. Combined NOS and COX inhibition also attenuated AUC, but this difference became statistically significant only with 100 mM ACh (P = 0.53 and P = 0.33 at 1 and 10 mM, respectively). The total time of the hyperemic response was significantly shortened at all three doses.
TEA in combination with NOS and COX inhibition attenuated peak CVC to 1, 10, and 100 mM ACh. This effect was also significantly attenuated compared with the L-NNA+Keto site at 100 mM. Combined TEA+L-NNA+Keto attenuated AUC to a greater extent than L-NNA+Keto alone only at 100 mM ACh. Total time of the hyperemic response was significantly lengthened compared with the L-NNA+Keto site at 1 and 10 mM ACh (but not at 100 mM ACh, P = 0.09). Despite these effects, a portion of the dilation remained with triple blockade of KCa channels, NOS, and COX.
Continuous infusions.
Continuous infusion of ACh resulted in a vasodilation that reached a plateau within approximately 15–20 min after the start of infusion of each concentration of ACh. The extent of dilation increased incrementally with each concentration of ACh, as shown in Figure 3. Similarly to bolus infusions, very little dilation above baseline was observed at the lower two doses of ACh (0.01 and 0.1 mM, P = 0.59 and P = 0.69 vs. baseline).
Fig. 3.
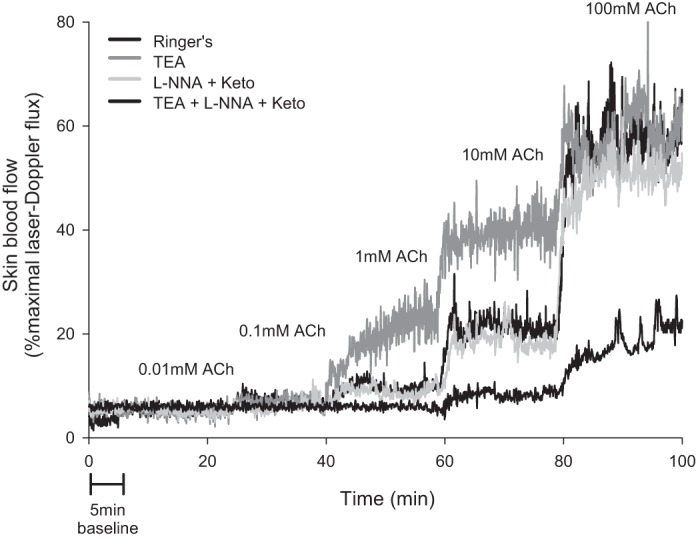
Representative skin blood flow response from one subject to continuous infusions of 0.01, 0.1, 1, 10, and 100 mM ACh at the four microdialysis sites. Drugs include tetraethylammonium (TEA), N-nitro-l-arginine (L-NNA), and ketorolac (Keto).
Blockade of KCa channels with TEA had no effect on plateau CVC at any dose of ACh (P = 0.59 vs. Ringer), nor did combined NOS+COX inhibition (P = 0.75). Triple blockade of KCa channels+NOS+COX significantly attenuated plateau CVC at 10 and 100 mM ACh compared with all other sites. These data are summarized in Figure 4.
Fig. 4.
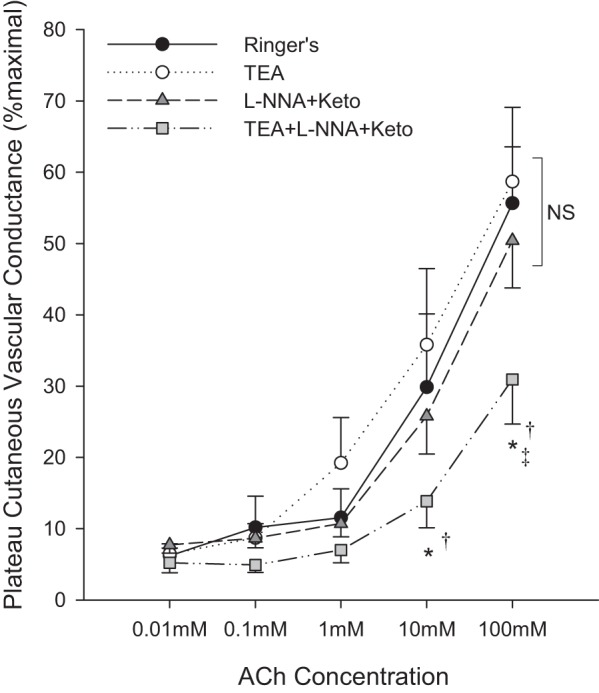
Plateau cutaneous vascular conductance to five continuous infusions of acetylcholine (ACh). Drugs include tetraethylammonium (TEA), N-nitro-l-arginine (L-NNA), and ketorolac (Keto). Data are means ± SE. *P < 0.05 from Ringer site; †P < 0.05 from TEA site; ‡P < 0.05 from L-NNA+Keto site.
Baseline and maximal cutaneous vascular conductance.
Values for baseline and maximal CVC are presented in Table 2. There were no differences in maximal CVC between sites (P = 0.17). TEA attenuated baseline CVC, consistent with previous studies (6, 8). Ketorolac tended to increase baseline CVC, although this was significant only compared with the TEA site (P = 0.11 vs. Ringer).
Table 2.
Baseline and maximal vascular conductance
| Baseline CVC, % maximal | Maximal CVC, mV/MAP·100 | |
|---|---|---|
| Ringer | 5.1 ± 0.7 | 253 ± 19 |
| TEA | 3.8 ± 0.5* | 230 ± 18 |
| L-NNA+Keto | 6.8 ± 0.7† | 258 ± 23 |
| TEA+L-NNA+Keto | 4.9 ± 0.7 | 214 ± 18 |
Data are means ± SE.
P < 0.05 from Ringer site;
P < 0.05 from TEA site.
DISCUSSION
The major findings of the present study are that EDHFs contribute to ACh-mediated dilation in the skin, but to an extent that varies depending on the dose and length of infusion (bolus vs. continuous). The relative contribution of NOS/COX to the hyperemic response also varies with dose and length of infusion. Importantly, a portion of the response was found to be NOS/COX and KCa channel-independent.
Roles of EDHFs.
When administering ACh in bolus doses, blockade of KCa channels with TEA inhibited peak dilation, alone and in combination with L-NNA+Keto, but it had no effect on AUC except at the highest dose of ACh. Furthermore, the total time of the hyperemic response was increased significantly at both 1 and 10 mM ACh. TEA alone had no effect on plateau CVC when administering ACh continuously. Taken together, these data suggest that EDHFs may be more important in the earlier phases of vasodilatory responses or that they may play a role in the speed at which relaxation of the blood vessels occurs. Hyperpolarization of the vascular smooth muscle caused by activation of KCa channels generally results in activation of inward-rectifying potassium (KIR) channels, which serve to amplify the hyperpolarizing signal and rapidly conduct it along the length of the cell membrane (36), suggesting a short-term modulatory role for EDHFs to more rapidly control the state of relaxation or contraction of the smooth muscle. In support of this notion, EDHFs have been shown to play a more transient role in vasodilation in some vascular beds (10, 18). In the human cutaneous hyperemic response to gradual local heating, administration of TEA alone delays the rate of dilation, but it has little effect on the extent of dilation (11). Thus when KCa channels are blocked, dilation may still occur due to production of NO, prostanoids, and/or KCa-independent dilatory factors, but more time is required to achieve dilation.
Roles of NOS and COX.
There has been much disparity in the methods used for studying ACh-mediated vasodilation in the skin. As such, reports of the relative contributions of NO and prostanoids to the response has varied greatly. In the present study, we sought to address the wide variability in reports by administering ACh at various doses and for different lengths of time (bolus vs. continuous infusion). Although it was statistically significant only at the highest dose, we observed that combined NOS and COX inhibition attenuated both the peak and AUC hyperemic responses to bolus infusions of ACh, such that NOS+COX contributed to approximately 35–40% of the vasodilation above baseline. This is less than what others have reported when a bolus dose of ACh was delivered via microdialysis (16, 22), although a dose of 0.1375 mM ACh was used in both of those studies. When infusing ACh continuously, we observed no effect of combined NOS and COX inhibition. This finding conflicts with the findings of Medow et al. (33), although the contribution of NOS+COX to continuous infusion of ACh that those authors observed was only minimal, particularly at 100 mM ACh.
The differing roles of NOS+COX between bolus and continuous infusions of ACh may be due to cross talk between NOS, COX, and EDHFs. EDHF-dependent dilation is often greater in the absence of NOS and COX, as demonstrated by the results of the current study. NOS can inhibit the release of EDHFs from the endothelium (3), thus allowing greater EDHF release in the presence of NOS inhibition. Blockade of COX enzymes may also facilitate greater EDHF production by allowing more arachidonic acid to be metabolized through the epoxygenase and lipoxygenase pathways (42). Perhaps the greater length of ACh infusion allowed for augmentation of EDHF release to occur to a greater extent during the continuous infusion protocol compared with the bolus infusions. However, this does contradict the transient nature of EDHFs discussed in the previous section. Hyperpolarization can occur in a sustained fashion (5), which perhaps occurs to a greater extent when NOS+COX are blocked.
Cross talk may also offer another explanation as to why TEA was able to attenuate ACh-mediated dilation during bolus infusions, but not during continuous infusions. EETs have been shown to induce NO-dependent dilation in mouse mesenteric arteries (19). Thus EDHF-dependent dilation can occur even in the presence of KCa channel blockade, and this may occur to a greater extent the longer the time of ACh infusion.
NOS, COX, and KCa-independent dilation.
With both bolus and continuous infusions of ACh, a portion of the hyperemic response was NOS-, COX-, and KCa-independent (by approximately 30–40%). It is possible this is due to incomplete blockades of these pathways using the selected drugs, although it is more likely that other mechanisms are involved, either under normal conditions or as redundant pathways that are activated when NOS, COX, and KCa channels are blocked. We did not explore the role of ATP-sensitive potassium (KATP) channels in the current study, which are known to contribute to vasodilation in other vascular beds and can be stimulated by some types of EDHFs independently of KCa channel activation; namely, EETs (51) and hydrogen sulfide (52). Hojs et al. (21) demonstrated a reduction in laser-Doppler flux in response to iontophoretic application of ACh following microinjection of glibenclamide, a KATP channel inhibitor. This finding suggests a role for KATP channels in ACh-mediated dilation, however, glibenclamide also has other effects, namely on prostaglandin-induced vasodilation, the Na+-K+-ATPase pump, and on other potassium channels (40). It is also questionable that vasodilation via KATP channels could account for the entirety of the NOS-, COX-, and KCa-independent dilation as the density of KATP channels in the microvasculature is quite low (40). A role for the sensory nerves, independent of the endothelium, is also possible. For example, release of calcitonin gene-related peptide (CGRP) from the sensory nerves has been shown to attenuate ACh-mediated dilation in isolated perfused rat kidney (2) and mesenteric arteries (46).
Considerations for dosing and presentation of data.
In the present study, ACh elicited minimal dilation at 0.01 and 0.1 mM and also elicited less dilation to 100 mM ACh compared with what other researchers have reported, when presented as a percentage of maximal dilation. Values from others have been in the range of 85%CVCmax to 95%CVCmax (17, 33, 43, 45). We believe the most likely explanation for the difference lies in the methods used to obtain maximal CVC values. Studies that have reported near maximal vasodilation to 100 mM ACh used only SNP to elicit maximal dilation, whereas we used heating to 43.5°C in addition to infusion of SNP in the present study. We have previously reported that SNP alone does not consistently maximize CVC in sites that have received ketorolac (32), and it is possible that SNP alone is also not sufficient to produce maximal dilation when ACh has been infused. For example, ACh has been shown to exert delayed and prolonged effects on smooth muscle in rat aortas (47), specifically on cyclic GMP (48). As such, these prolonged effects may interact with the ability of SNP to produce vasodilation. To test this, in two subjects we infused SNP for 30 min after the completion of ACh infusions. After a stable plateau in skin blood flux was observed, we then locally heated the skin to 43.5°C. In both subjects and in all drug sites, skin blood flux increased substantially with the additional heating compared with SNP alone. Thus we believe future studies should utilize both local heating and SNP infusion when obtaining maximal dilation following ACh infusion.
However, other explanations exist for why our data differ from those reported by others. First, ACh is a highly hygroscopic drug. Although we purchased a high-purity drug (≥99%) and were careful to store it under dry, air-tight conditions, it is possible our drug absorbed moisture while in storage, thus lowering its purity and therefore concentrations of ACh that were delivered via microdialysis. However, we used the same drug, stored under the same conditions, in another study in our laboratory in which we used SNP only to obtain maximal CVC values to 100 mM ACh and observed dilation in the range of 80%CVCmax to 90%CVCmax (31), indicating the different methods for obtaining maximal SkBF are more likely the reason for the differences between our data and those of other researchers. However, we unfortunately cannot rule out this limitation in the present study. Regardless, our conclusions that the relative contributions of the NOS, COX, and EDHF pathways differ at higher vs. lower doses of ACh and between bolus vs. continuous infusions are still valid. Second, it is possible some tachyphylaxis may have been occurring such that the higher doses of ACh were less effective. By the time we infused 100 mM, the microvasculature had been exposed to ACh (and other inhibitory drugs) for more than 2 h. However, others who have used the same dose-response protocol have not reported this effect (33, 43).
One possible way to circumvent the challenges in obtaining true maximal CVC values that has commonly been used in mechanistic studies is to present data as a percentage of or change from baseline. However, many of the drugs infused in these types of studies affect baseline vascular conductance, as observed with TEA in the present study. Thus normalization of data to baseline may disguise the true underlying physiology and may be an inappropriate way of expressing data unless it is clear there are no differences in baseline across drug sites/preparations.
Finally, many studies have utilized curve-modeling approaches to characterize ACh-mediated dilation, including one study in the cutaneous microvasculature (43), although that study did not investigate any effects of inhibitory drugs. Unfortunately in the present study, we were not able to obtain a full dose-response and so could not perform these analyses. Studies using isolated vessels have shown a reduction in EC50 to ACh in the presence of KCa channel inhibitors (13, 24, 35), which is consistent with our data across the doses of ACh we used. This further suggests the human cutaneous circulation mirrors generalized microvascular function.
Limitations.
There was a large degree of variability in the response. For example, vasodilation to bolus 100 mM ACh at the control site ranged from 24%CVCmax to 75%CVCmax. Vasodilation to continuous infusion of 100 mM ACh was even greater, ranging from 35%CVCmax to 98%CVCmax. Furthermore, the difference in vasodilation to 100 mM ACh between the control and TEA sites in protocol 2 ranged from the TEA site being 16%CVCmax higher to 30%CVCmax lower than the control site. Variability was also observed in the L-NNA+Keto and TEA+L-NNA+Keto sites, although to a lesser extent. Considerable inter-site variability in ACh-mediated dilation has been reported previously (an intersite coefficient of variation of ∼15%) (41). Interindividual variability appears to be even greater and should be considered in future studies. We did not observe any differences in responses to ACh between men and women. However, inclusion of both sexes may have contributed to the degree of variability.
We did not separately isolate the NO and prostaglandin pathways. Although others have previously isolated these pathways during cutaneous administration of ACh, we would have been better able to compare the contribution of all three endothelial pathways across the different doses and lengths of infusion had we also administered NO and prostaglandin blockade independently. However, isolation of these pathways in addition to EDHFs, plus investigating the combined effects, would have required at least six microdialysis fibers, which is not feasible to perform in human subjects. As such, we chose to focus on the effects of EDHF blockade in the present study.
Perspectives.
ACh has commonly been used to assess endothelial function, thus making it critical for fully understanding the mechanisms that underlie ACh-mediated dilation. Such understanding allows us to investigate alterations in these pathways under various disease states and identify potential avenues for therapeutic interventions. In humans, the skin offers an ideal microvascular bed in which to pharmacologically investigate these pathways. It is not only easily accessible, but impairments in cutaneous vascular function generally mirror systemic disease progression. For example, ACh-mediated dilation has been shown to be impaired in the skin of chronic smokers (16), and individuals with hypertension (44) or coronary artery disease (1, 23), among others. Studies that have utilized microdialysis have found reduced NO bioavailability to account for much of the impairment, but not all (16), suggesting a role of EDHFs.
The present study provides a framework for the future study of these pathways in clinical populations, and in particular, highlights the importance of carefully designing experimental protocols to best answer the questions at hand. Based on the data in the present study, bolus infusions of ACh at concentrations ≥1 mM may be the most appropriate method of studying ACh-mediated dilation in the skin (when using the fiber type and rate of infusion as in the present study), particularly for those interested in EDHF-mediated dilation. This method appears to involve more consistent responses, and importantly, the independent roles of EDHFs would have been missed if only continuous infusions of ACh had been used. Bolus infusions also allow for the return of SkBF to baseline between doses so as to attain a measurement of AUC in addition to peak hyperemia, which has been shown to be more telling of vascular health in recent reports (28). Finally, it is critical to obtain maximal SkBF values in these studies; thus we strongly recommend using combined local heating with SNP infusion in studies utilizing microdialysis.
GRANTS
Supported by National Heart, Lung, and Blood Institute Grant HL-081671 and by the Ken and Kenda Singer Endowment.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
V.E.B., N.F., and C.T.M. conception and design of research; V.E.B. and N.F. performed experiments; V.E.B. analyzed data; V.E.B., N.F., and C.T.M. interpreted results of experiments; V.E.B. prepared figures; V.E.B. drafted manuscript; V.E.B., N.F., and C.T.M. edited and revised manuscript; V.E.B., N.F., and C.T.M. approved final version of manuscript.
ACKNOWLEDGMENTS
We sincerely thank the subjects for their participation in the study.
REFERENCES
- 1.Agarwal SC, Allen J, Murray A, Purcell IF. Laser Doppler assessment of dermal circulatory changes in people with coronary artery disease. Microvasc Res 84: 55–59. [DOI] [PubMed] [Google Scholar]
- 2.Ay I, Tuncer M. Both endothelium and afferent nerve endings play a role in acetylcholine-induced renal vasodilation. Life Sci 79: 877–882, 2006. [DOI] [PubMed] [Google Scholar]
- 3.Bauersachs J, Popp R, Hecker M, Sauer E, Fleming I, Busse R. Nitric oxide attenuates the release of endothelium-derived hyperpolarizing factor. Circulation 94: 3341–3347, 1996. [DOI] [PubMed] [Google Scholar]
- 4.Berghoff M, Kathpal M, Kilo S, Hilz MJ, Freeman R. Vascular and neural mechanisms of ACh-mediated vasodilation in the forearm cutaneous microcirculation. J Appl Physiol 92: 780–788, 2002. [DOI] [PubMed] [Google Scholar]
- 5.Brayden JE. Membrane hyperpolarization is a mechanism of endothelium-dependent cerebral vasodilation. Am J Physiol Heart Circ Physiol 259: H668–H673, 1990. [DOI] [PubMed] [Google Scholar]
- 6.Brunt VE, Fujii N, Minson CT. No independent, but an interactive, role of calcium-activated potassium channels in human cutaneous active vasodilation. J Appl Physiol 115: 1290–1296, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brunt VE, Miner JA, Meendering JR, Kaplan PF, Minson CT. 17β-estradiol and progesterone independently augment cutaneous thermal hyperemia but not reactive hyperemia. Microcirculation 18: 347–355, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brunt VE, Minson CT. KCa channels and epoxyeicosatrienoic acids: major contributors to thermal hyperaemia in human skin. J Physiol 590: 3523–3534, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Buxton IL, Cheek DJ, Eckman D, Westfall DP, Sanders KM, Keef KD. NG-nitro L-arginine methyl ester and other alkyl esters of arginine are muscarinic receptor antagonists. Circ Res 72: 387–395, 1993. [DOI] [PubMed] [Google Scholar]
- 10.Chen G, Suzuki H, Weston AH. Acetylcholine releases endothelium-derived hyperpolarizing factor and EDRF from rat blood vessels. Br J Pharmacol 95: 1165–1174, 1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Choi PJ, Brunt VE, Fujii N, Minson CT. New approach to measure cutaneous microvascular function: an improved test of NO-mediated vasodilation by thermal hyperemia. J Appl Physiol 117: 277–283, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Coats P, Johnston F, MacDonald J, McMurray JJ, Hillier C. Endothelium-derived hyperpolarizing factor: identification and mechanisms of action in human subcutaneous resistance arteries. Circulation 103: 1702–1708, 2001. [DOI] [PubMed] [Google Scholar]
- 13.Doughty JM, Plane F, Langton PD. Charybdotoxin and apamin block EDHF in rat mesenteric artery if selectively applied to the endothelium. Am J Physiol Heart Circ Physiol 276: H1107–H1112, 1999. [DOI] [PubMed] [Google Scholar]
- 14.Durand S, Tartas M, Bouyé P, Koïtka A, Saumet JL, Abraham P. Prostaglandins participate in the late phase of the vascular response to acetylcholine iontophoresis in humans. J Physiol 561: 811–819, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Félétou M, Vanhoutte PM. EDHF: an update. Clin Sci 117: 139–155, 2009. [DOI] [PubMed] [Google Scholar]
- 16.Fujii N, Reinke MC, Brunt VE, Minson CT. Impaired acetylcholine-induced cutaneous vasodilation in young smokers: roles of nitric oxide and prostanoids. Am J Physiol Heart Circ Physiol 304: H667–H673, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gagnon D, Crandall CG, Kenny GP. Sex differences in postsynaptic sweating and cutaneous vasodilation. J Appl Physiol 114: 394–401, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Harrington LS, Carrier MJ, Gallagher N, Gilroy D, Garland CJ, Mitchell JA. Elucidation of the temporal relationship between endothelial-derived NO and EDHF in mesenteric vessels. Am J Physiol Heart Circ Physiol 293: H1682–H1688, 2007. [DOI] [PubMed] [Google Scholar]
- 19.Hercule HC, Schunck WH, Gross V, Seringer J, Leung FP, Weldon SM, da Costa Goncalves ACh, Huang Y, Luft FC, Gollasch M. Interaction between P450 eicosanoids and nitric oxide in the control of arterial tone in mice. Arterioscler Thromb Vasc Biol 29: 54–60, 2009. [DOI] [PubMed] [Google Scholar]
- 20.Hodges GJ, Kosiba WA, Zhao K, Johnson JM. The involvement of heating rate and vasoconstrictor nerves in the cutaneous vasodilator response to skin warming. Am J Physiol Heart Circ Physiol 296: H51–H56, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hojs N, Strucl M, Cankar K. The effect of glibenclamide on acetylcholine and sodium nitroprusside induced vasodilatation in human cutaneous microcirculation. Clin Physiol Funct Imaging 29: 38–44, 2009. [DOI] [PubMed] [Google Scholar]
- 22.Holowatz LA, Thompson CS, Minson CT, Kenney WL. Mechanisms of acetylcholine-mediated vasodilatation in young and aged human skin. J Physiol 563: 965–973, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.IJzerman RG, de Jongh RT, Beijk MA, van Weissenbruch MM, Delemarre-van de Waal HA, Serné EH, Stehouwer CD. Individuals at increased coronary heart disease risk are characterized by an impaired microvascular function in skin. Eur J Clin Invest 33: 536–542, 2003. [DOI] [PubMed] [Google Scholar]
- 24.Katakam PV, Ujhelyi MR, Miller AW. EDHF-mediated relaxation is impaired in fructose-fed rats. J Cardiovasc Pharmacol 34: 461–467, 1999. [DOI] [PubMed] [Google Scholar]
- 25.Kellogg DL, Zhao JL, Coey U, Green JV. Acetylcholine-induced vasodilation is mediated by nitric oxide and prostaglandins in human skin. J Appl Physiol 98: 629–632, 2005. [DOI] [PubMed] [Google Scholar]
- 26.Khan F, Davidson NC, Littleford RC, Litchfield SJ, Struthers AD, Belch JJ. Cutaneous vascular responses to acetylcholine are mediated by a prostanoid-dependent mechanism in man. Vasc Med 2: 82–86, 1997. [DOI] [PubMed] [Google Scholar]
- 27.Khan F, Patterson D, Belch JJ, Hirata K, Lang CC. Relationship between peripheral and coronary function using laser Doppler imaging and transthoracic echocardiography. Clin Sci 115: 295–300, 2008. [DOI] [PubMed] [Google Scholar]
- 28.Kruger A, Stewart J, Sahityani R, O'Riordan E, Thompson C, Adler S, Garrick R, Vallance P, Goligorsky MS. Laser Doppler flowmetry detection of endothelial dysfunction in end-stage renal disease patients: correlation with cardiovascular risk. Kidney Int 70: 157–164, 2006. [DOI] [PubMed] [Google Scholar]
- 29.Lenasi H. The role of nitric oxide- and prostacyclin-independent vasodilatation in the human cutaneous microcirculation: effect of cytochrome P450 2C9 inhibition. Clin Physiol Funct Imaging 29: 263–270, 2009. [DOI] [PubMed] [Google Scholar]
- 30.Lorenzo S, Minson CT. Human cutaneous reactive hyperaemia: role of BKCa channels and sensory nerves. J Physiol 585: 295–303, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lorenzo S, Minson CT. Heat acclimation improves cutaneous vascular function and sweating in trained cyclists. J Appl Physiol 109: 1736–1743, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McCord GR, Cracowski JL, Minson CT. Prostanoids contribute to cutaneous active vasodilation in humans. Am J Physiol Regul Integr Comp Physiol 291: R596–R602, 2006. [DOI] [PubMed] [Google Scholar]
- 33.Medow MS, Glover JL, Stewart JM. Nitric oxide and prostaglandin inhibition during acetylcholine-mediated cutaneous vasodilation in humans. Microcirculation 15: 569–579, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Morris SJ, Shore AC. Skin blood flow responses to the iontophoresis of acetylcholine and sodium nitroprusside in man: possible mechanisms. J Physiol 496, Pt 2: 531–542, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Murphy ME, Brayden JE. Apamin-sensitive K+ channels mediate an endothelium-dependent hyperpolarization in rabbit mesenteric arteries. J Physiol 489, Pt 3: 723–734, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nelson MT, Quayle JM. Physiological roles and properties of potassium channels in arterial smooth muscle. Am J Physiol Cell Physiol 268: C799–C822, 1995. [DOI] [PubMed] [Google Scholar]
- 37.Newton DJ, Davies J, Belch JJ, Khan F. Role of endothelium-derived hyperpolarising factor in acetylcholine-mediated vasodilatation in skin. Int Angiol 32: 312–318, 2013. [PubMed] [Google Scholar]
- 38.Noon JP, Walker BR, Hand MF, Webb DJ. Studies with iontophoretic administration of drugs to human dermal vessels in vivo: cholinergic vasodilatation is mediated by dilator prostanoids rather than nitric oxide. Br J Clin Pharmacol 45: 545–550, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pickkers P, Hughes AD, Russel FG, Thien T, Smits P. Thiazide-induced vasodilation in humans is mediated by potassium channel activation. Hypertension 32: 1071–1076, 1998. [DOI] [PubMed] [Google Scholar]
- 40.Quayle JM, Nelson MT, Standen NB. ATP-sensitive and inwardly rectifying potassium channels in smooth muscle. Physiol Rev 77: 1165–1232, 1997. [DOI] [PubMed] [Google Scholar]
- 41.Sauvet F, Mahé G, Chennaoui M, Langrume C, Vasseur M, Abraham P, Lefthériotis G. Acetylcholine chloride as a potential source of variability in the study of cutaneous vascular function in man. Microvasc Res 82: 190–197, 2011. [DOI] [PubMed] [Google Scholar]
- 42.Shi Y, Ku DD, Man RY, Vanhoutte PM. Augmented endothelium-derived hyperpolarizing factor-mediated relaxations attenuate endothelial dysfunction in femoral and mesenteric, but not in carotid arteries from type I diabetic rats. J Pharmacol Exp Ther 318: 276–281, 2006. [DOI] [PubMed] [Google Scholar]
- 43.Smith CJ, Kenney WL, Alexander LM. Regional relation between skin blood flow and sweating to passive heating and local administration of acetylcholine in young, healthy humans. Am J Physiol Regul Integr Comp Physiol 304: R566–R573, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Smith CJ, Santhanam L, Bruning RS, Stanhewicz A, Berkowitz DE, Holowatz LA. Upregulation of inducible nitric oxide synthase contributes to attenuated cutaneous vasodilation in essential hypertensive humans. Hypertension 58: 935–942, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stapleton JM, Fujii N, McGinn R, McDonald K, Kenny GP. Age-related differences in postsynaptic increases in sweating and skin blood flow postexercise. Physiol Rep 16: 2, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Takenaga M, Kawasaki H, Wada A, Eto T. Calcitonin gene-related peptide mediates acetylcholine-induced endothelium-independent vasodilation in mesenteric resistance blood vessels of the rat. Circ Res 76: 935–941, 1995. [DOI] [PubMed] [Google Scholar]
- 47.Tang EH, Félétou M, Huang Y, Man RY, Vanhoutte PM. Acetylcholine and sodium nitroprusside cause long-term inhibition of EDCF-mediated contractions. Am J Physiol Heart Circ Physiol 289: H2434–H2440, 2005. [DOI] [PubMed] [Google Scholar]
- 48.Taylor SG, Southerton JS, Weston AH, Baker JR. Endothelium-dependent effects of acetylcholine in rat aorta: a comparison with sodium nitroprusside and cromakalim. Br J Pharmacol 94: 853–863, 1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Taylor SG, Weston AH. Endothelium-derived hyperpolarizing factor: a new endogenous inhibitor from the vascular endothelium. Trends Pharmacol Sci 9: 272–274, 1988. [DOI] [PubMed] [Google Scholar]
- 50.Turner J, Belch JJ, Khan F. Current concepts in assessment of microvascular endothelial function using laser Doppler imaging and iontophoresis. Trends Cardiovasc Med 18: 109–116, 2008. [DOI] [PubMed] [Google Scholar]
- 51.Ye D, Zhou W, Lee HC. Activation of rat mesenteric arterial KATP channels by 11,12-epoxyeicosatrienoic acid. Am J Physiol Heart Circ Physiol 288: H358–H364, 2005. [DOI] [PubMed] [Google Scholar]
- 52.Zhao W, Zhang J, Lu Y, Wang R. The vasorelaxant effect of H2S as a novel endogenous gaseous KATP channel opener. EMBO J 20: 6008–6016, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]


