Abstract
Breathing is more vulnerable to apneas and irregular breathing patterns during rapid eye movement (REM) sleep in both humans and rodents. We previously reported that robust and recurrent recruitment of expiratory abdominal (ABD) muscle activity is present in rats during REM epochs despite ongoing REM-induced muscle atonia in skeletal musculature. To develop a further understanding of the characteristics of ABD recruitment during REM epochs and their relationship with breathing patterns and irregularities, we sought to compare REM epochs that displayed ABD muscle recruitment with those that did not, within the same rats. Specifically, we investigated respiratory characteristics that preceded and followed recruitment. We hypothesized that ABD muscle recruitment would be likely to occur following respiratory irregularities and would subsequently contribute to respiratory stability and the maintenance of good ventilation following recruitment. Our data demonstrate that epochs of REM sleep containing ABD recruitments (REMABD+) were characterized by increased respiratory rate variability and increased presence of spontaneous brief central apneas. Within these epochs, respiratory events that displayed ABD muscle activation were preceded by periods of increased respiratory rate variability. Onset of ABD muscle activity increased tidal volume, amplitude of diaphragmatic contractions, and minute ventilation compared with the periods preceding ABD muscle activation. These results show that expiratory muscle activity is more likely recruited when respiration is irregular and its recruitment is subsequently associated with an increase in minute ventilation and a more regular respiratory rhythm.
Keywords: abdominal, expiration, REM sleep, respiration
respiratory rhythmic activity is highly influenced by sleep states, with the majority of central respiratory disorders occurring during periods of rapid eye movement (REM) epochs, when breathing becomes more irregular and most, but not all, of respiratory and nonrespiratory muscles are affected by REM-induced motor atonia (7, 20, 24). In normal conditions, the main inspiratory pump muscle, the diaphragm, is not affected by REM-induced motor atonia. In contrast, upper airway muscle tone is often depressed, which can lead to recurrent events of obstructive sleep apnea due to reduced patency (collapse) of the airway in humans. Persistence of respiratory modulated activity during REM sleep in intercostal muscles varies across species (24) while activity of expiratory abdominal (ABD) muscles is generally depressed in both experimental animals and humans (17).
Surprisingly, in rodents, it was observed that ABD muscles, which are the primary expiratory muscles, are frequently recruited within epochs of REM sleep (26, 31) or in periods of REM-like states under urethane anesthesia (25, 26). The origin and the mechanisms underlying the recruitment of ABD muscles and its overall effect on ventilation during REM sleep are not yet known. In this study we investigated the occurrence of ABD muscle recruitment within REM epochs during natural sleep in adult rats to better characterize these events, their frequency, and their effect on overall ventilation. We monitored sleep states with a combination of neck electromyogram (EMG) and cortical and hippocampal EEG recordings and measured respiratory muscle activity via implanted diaphragm and ABDEMG electrodes. We recorded rats in multiple sessions across sleep-wake cycles within an electrically insulated recording chamber or inside a whole rat plethysmograph. Our results suggest that expiratory ABD muscle recruitment occurs during REM epochs that are characterized by an increased respiratory variability. Recruitment of ABDEMG activity during REM epochs subsequently reduced respiratory variability and increased tidal volume as well as minute ventilation.
MATERIALS AND METHODS
Animal handling and experimental protocols were approved by the Biosciences Animal Policy and Welfare Committee of the University of Alberta according to the guidelines established by the Canadian Council on Animal Care.
Chronic instrumentation.
Nine male Sprague-Dawley rats (200-250 g) were implanted with EEG and EMG electrodes according to our previously published protocol (26). Briefly, rats were anesthetized with ketamine (90 mg/kg) and xylazine (10 mg/kg). Bipolar EMG electrodes manufactured with multistrand, Teflon-coated stainless steel wires (cat no. AS633, CoonerWire) were inserted into the oblique abdominal (ABD), and diaphragm (DIA) and neck muscles. Wires were tunneled under the skin and attached to an electrical socket (cat no. GS09PLG220, Ginder Scientific) implanted between the shoulder blades.
One week after the initial surgery, rats were again anesthetized by using ketamine/xylazine and positioned in a stereotaxic frame with bregma and lambda coordinates at the same level. Bipolar, multistranded PFA-insulated stainless steel wires (cat no. 793500, A-M Systems) were implanted in the neocortex (nCTX) and hippocampal formation (HPC) according to the following coordinates relative to bregma. For nCTX: anterior-posterior (AP), +2.5; mediolateral (ML), −1.2; dorsoventral (DV), −1.5 to −2.0 mm. For HPC: AP, −3.3; ML, −2.4; DV, −2.5 to −3.0 mm. The distance between the two ends of the wires was kept at ∼1 mm for optimal recordings. For nCTX recordings, the electrodes were lowered in the DV axis just below the position where a marked increase in multiunit discharge was detected via an audio amplifier (model 3300, A-M Systems) connected to the PowerLab acquisition system. This corresponds to the region just below layer V in the nCTX. Similarly, HPC electrodes were lowered below the position of a second audible multiunit discharge, corresponding to the distal apical dendritic layer of CA1 pyramidal cells. Electrodes were then fixed to the skull by jeweler's screws and dental acrylic. Three screws were cemented to the skull to maintain the electrodes in position and soldered with additional wires to be used as additional cortical EEG signal and ground. The wires were then tunneled under the skin and attached to a second electrical socket positioned on the rats' neck.
Rats were administered an analgesic for 2 days after surgeries (Metacam, 2 mg/kg), provided food and water ad libitum, and maintained on a 12/12 h dark-light cycle. During the first week after surgery rats were habituated to the recording chamber for a couple of 5-h sessions.
Recording procedures.
Recording sessions started 7–9 days after surgery. Rats were connected to tethering cables in the morning (10 AM), and data were collected between 12 and 5 PM. Recordings were made from five rats within an electrically insulated chamber and an additional four rats within a whole body plethysmograph (Buxco Electronics) to further measure respiratory airflow. In those rats, constant airflow (1 liter/min) was delivered through the plethysmograph, and pressure fluctuations relative to a reference chamber were recorded via a differential pressure transducer (model no. DP103-10, Validyne). These values are proportional to tidal volume and therefore indicative of ventilatory changes across natural sleep states. Data obtained from two of these rats have been previously used for sleep data analysis and publication in our previous study (26).
EEG and EMG activity was sampled at 1 kHz by using a PowerLab 16/30 data acquisition system (AD Instruments). Field potential activity from nCTX and HPC was amplified at ×1000 gain and filtered between 0.1 and 500 Hz (amplifier model P511, Grass), while EMG signals were amplified at ×10000 and filtered between 100 and 500 Hz (model 1700, A-M Systems).
Data analysis.
Sleep analysis was carried out on 2–5 h/session, when rats showed consistent and robust sleep behavior. EEG traces from cortex and hippocampus in combination with neck EMG were used to determine sleep and wake states. Periods of immobility were identified in electrographic recordings by low neck EMG tone and irregular activity in the HPC. During sleep, nonREM and REM episodes could be differentiated by the absence or presence of activated patterns of EEG in the nCTX (low voltage fast activity) and HPC (activity in the theta frequency at 4–10 Hz), respectively. Further confirmation was obtained with EMG recordings; a decrease in neck EMG tone was always associated with nonREM to REM transitions. A minimum of 10 s in length was required for a REM epoch to be selected for further analysis of ABD recruitment.
Respiratory parameters were measured on a breath-by-breath basis by using EMG activity from respiratory muscles and airflow traces obtained with whole body plethysmography. For EMG recordings, DIA and ABD traces were rectified and integrated (time constant decay 0.08 s) and normalized to determine phasic activation during either inspiratory or expiratory phase and to calculate peak amplitude of EMG recruitment. Pauses in DIAEMG activity and respiratory flow equal to or longer than 2-s duration were identified as central apneas.
REM epochs were classified as REMABD+ or REMABD− based on the presence or absence of at least three consecutive respiratory events that displayed expiratory ABDEMG activity (i.e., a phasic activation of ABDEMG signal during expiration that was >50% of baseline activity), respectively. NonREM epochs were classified as tonic (T), weakly expiratory (W-EXP), or expiratory (EXP) based on the presence of tonic or expiratory modulated activity in ABD muscles. ABDEMG activity was classified as EXP if peak activity of ∫ABDEMG signal occurred during expiration and was >50% of baseline activity, W-EXP if peak activity of ∫ABDEMG signal occurred during expiration and was <50% of baseline activity. Significant differences in respiratory rate, apnea frequency, and respiratory variability were tested among the three groups by a one-way ANOVA.
The analysis of respiratory frequency, variability, and apnea frequency between REMABD+ and REMABD− epochs was performed by comparing values within the same animals, and a paired t-test was applied to determine significant changes for the selected parameters.
Within REMABD+ epochs, data analysis was performed by calculating average respiratory values [respiratory period, respiratory variability, peak integrated (∫) DIAEMG amplitude, relative tidal volume, and minute ventilation] obtained from the 15 respiratory cycles preceding ABDEMG recruit and comparing the data with the 15 respiratory cycles following the onset of ABDEMG recruitment.
Data values are reported as means ± SE. Respiratory rate variability was assessed by calculating the coefficient of variation (ratio of the standard deviation to the mean) of the respiratory period. Numerical comparisons across conditions were made by using pairwise t-tests and χ2 tests (with a significance level of 0.05).
RESULTS
Sleep and respiratory characteristics in nonREM and REM epochs of adult rats.
A total of 59.6 h were used for the analysis of respiratory pattern and ABDEMG recruitment across sleep states in nine rats. Rats spent 44.0 ± 2.9%, 46.8 ± 2.4%, and 9.2 ± 1.8% of their time in awake, nonREM, and REM states, respectively. During nonREM sleep (1,352 epochs), ABDEMG activity was either tonic (T, 78.6 ± 4.7% of the epochs, where amplitude of nonrespiratory modulated activity varied between minimal to robust tonic EMG activity), expiratory phasic (EXP, 15.4 ± 4.0%; i.e., peak ∫ABDEMG activity was in expiration and >50% of baseline activity), or weakly expiratory phasic (W-EXP, 6.0 ± 1.1%; i.e., peak ∫ABDEMG activity was in expiration but <50% of baseline activity). Examples of these patterns of activity are displayed in Fig. 1. Most frequently, the pattern of activity persisted with the same characteristics through the whole nonREM epoch. When brief arousals occurred, changes in overall amplitude of ∫ABDEMG activity often occurred (either increased or decreased) likely due to changes in posture. During nonREM epochs we did not observe any apneas or respiratory disturbances. When breathing rate and respiratory variability were analyzed in four sleep recording sessions from four different rats, no significant changes were observed in breathing rate (T: 112 ± 2 breaths per minute, BPM; EXP 110 ± 2 BPM; W-EXP 110 ± 1 BPM; P = 0.5) or coefficient of variability (T: 0.13 ± 0.02; EXP 0.13 ± 0.02; W-EXP 0.14 ± 0.03; P = 0.99) across the three patterns of ABDEMG activity.
Fig. 1.
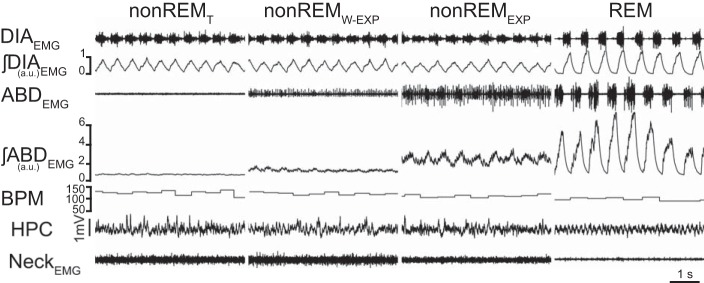
Abdominal muscle activity in nonREM sleep. Brief traces of diaphragm (DIA) electromyogram (EMG), ∫DIAEMG, abdominal (ABD) EMG, ∫ABDEMG, respiratory rate (breaths per minute, BPM), hippocampal activity (HPC), and neck EMG activity during three adjacent nonREM epochs and one REM epoch. Integrated trace of ABDEMG activity demonstrates that during nonREM sleep ABDEMG activity is either tonic with variable amplitude (nonREMT), weakly expiratory modulated (nonREMW-EXP), or expiratory modulated (nonREMEXP). Panel at right shows robust expiratory modulated ABDEMG recruitment in a REM epoch in the same rat.
REM epochs longer than 10 s were used to identify periods that displayed recruitment of ABDEMG activity. We identified an average of 33.3 ± 6.8 REM epochs per rat (n = 9) which displayed an average length of 47.9 ± 3.2 s. REM epochs either terminated with an arousal (81.5 ± 6.1%) or with a transition into nonREM sleep (18.5 ± 6.1%). During REM epochs, breathing rate was 101.3 ± 2.9 BPM (n = 9). Within REM epochs we observed a total of 44 apneas in eight of the nine rats analyzed (0.22 ± 0.07 apneas/min), with an average duration of 2.7 ± 0.1 s.
REM epochs displaying abdominal recruitment are associated with increased breathing variability.
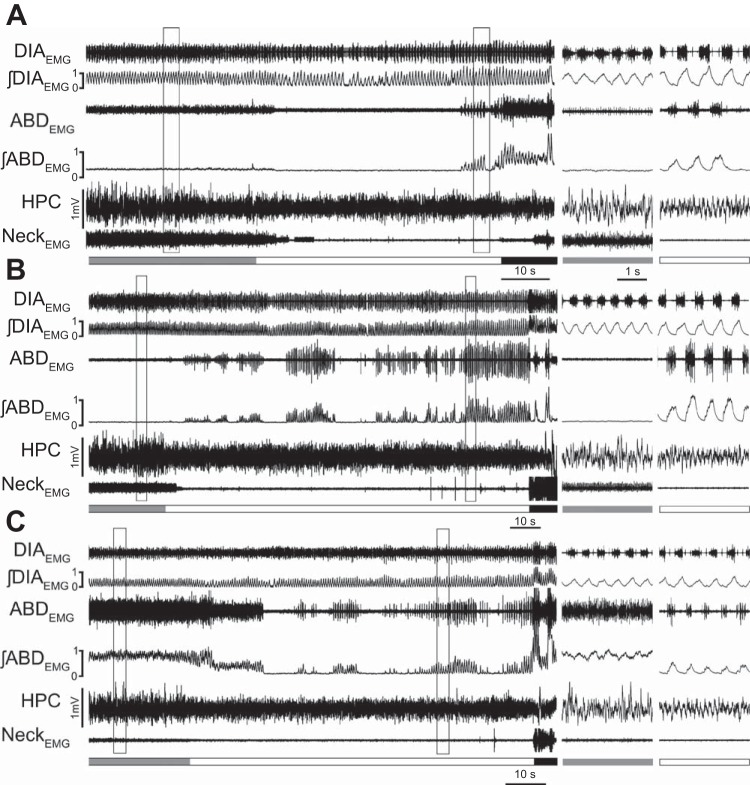
We analyzed in detail expiratory-related ABDEMG recruitment across REM epochs. In 147 out of 300 REM epochs (n = 9), we identified one or more events of ABDEMG recruitment that lasted for more than three consecutive breaths (Fig. 2). Within these REM epochs, the majority of respiratory events were characterized by a progressive decrease in tonic (Fig. 2A) or expiratory phasic (Fig. 2C) ABDEMG activity at REM onset, followed by a period of reduced/absent tonic ABDEMG activity. The gradual reduction in ABDEMG activity was associated with the characteristic loss of muscle tone observed in the neck muscle and typical of REM-induced muscle atonia (28, 30, 34). In some cases, tonic activity of ABDEMG was minimal and no further decrease was observed with REM sleep onset (Fig. 2B). After a variable time from the inception of the REM epoch (21.4 ± 2.8 s in 102 REM epochs), rhythmic and expiratory-related activation of ABDEMG activity was observed. REMABD+ epochs displayed an average of 2.1 ± 0.3 bouts of ABDEMG recruitment, which lasted for 14.7 ± 2.0 respiratory cycles (n = 102 epochs in 9 rats).
Fig. 2.
Abdominal muscle recruitment during transition from nonREM to REM sleep. Long traces recording of diaphragm (DIAEMG) and abdominal (ABDEMG) muscles, their integrated EMG activity, hippocampal activity (HPC), and neck EMG activity in REM epochs in which ABD muscle activity is recruited. A, B, and C represent three epochs recorded from the same rat in which ABDEMG activity in nonREM sleep is either tonic (A, B) or expiratory modulated. With REM onset, tonic (A) and phasic (C) ABDEMG activity is progressively reduced in association with a reduction of neck EMG activity and expression of theta frequency in the HPC. In cases in which tonic ABDEMG activity is minimal (B), no further decrease in ABDEMG was observed with REM sleep onset. Details of EMG and EEG traces during nonREM and REM epochs (black boxes) are displayed in panels at right. Schematic blocks at the bottom of the Figs. indicate time spent in nonREM (grey), REM (white), and wakefulness (black).
This activity was not associated with awakening, as confirmed by cortical and hippocampal EEG and neckEMG activities, and neither was it associated with phasic REM states since there were no incidences of phasic contractions of neck muscles (Fig. 2). In the remaining cases (45 epochs), phasic ABDEMG activity was present in the preceding nonREM epoch and persisted through the REM epoch.
Initially, we compared REM epochs that displayed ABDEMG recruitment (REMABD+) with similar epochs that did not display ABDEMG recruitment (REMABD−) within the same rat (n = 9; Fig. 3). REMABD+ epochs were significantly longer in duration (64.0 ± 5.5 s; n = 147) compared with REMABD− (33.1 ± 3.2 s; n = 153; P = 0.002). Breathing rate in REMABD+ epochs was 100.8 ± 2.3 BPM, with a CV of 0.27 ± 0.04, whereas breathing rate in REMABD− epochs was 105.1 ± 4.6 BPM, with a CV of 0.17 ± 0.01. While respiratory rate was lower in epochs with ABD recruitment, this was not significant (P = 0.17); however, the CV of respiratory rate was significantly higher during REMABD+ compared with REMABD− (P = 0.04). Out of the 44 apneas identified during REM epochs, 10 apneas (2.8 ± 0.3 s in length) were present in REMABD− and 34 apneas (2.6 ± 0.1 s in length; P = 0.3) were present in REMABD+ [χ2(1) = 13.091; P = 3 × 10−4]. Thus ABD muscle recruitment was more likely during REM epochs that showed apneas. To examine the timing of abdominal events in relation to apneas, we defined a time window following the apneas (10 s) in REMABD+ epochs. We found 27 associated events in which ABD recruitment occurred either during or after apnea occurrence, and 7 nonassociated ABD events in which the following ABD muscle recruitment occurred >10 s from the end of apnea (n = 5; 23.5 ± 5.1 s ABDEMG onset delay) or no ABD muscle recruitment was observed until the end of the REM epoch (n = 2). These were significant based on another χ2-test [χ2(1) = 11.765; P = 6 × 10−4].
Fig. 3.
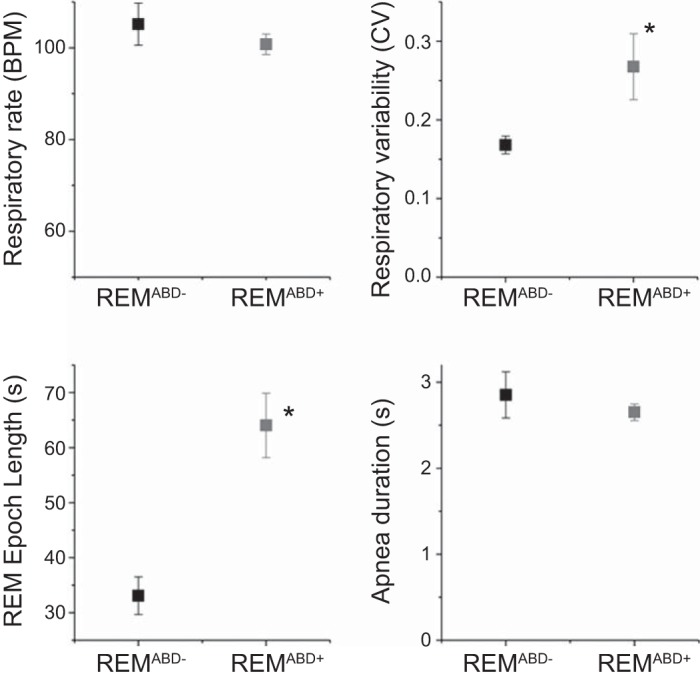
REMABD+ epochs are longer and display increased respiratory variability compared with REMABD− epochs. Averaged pooled respiratory rate (BPM), respiratory variability (coefficient of variation of the period, CV), length of REM epochs (in seconds), and duration of apneas (in seconds) in REM epochs that display (REMABD+; black) or not (REMABD−; grey) expiratory ABD muscle recruitment. REMABD+ epochs are usually longer, and they are characterized by an increased respiratory variability. *Statistical significance (P < 0.05) between values.
Recruitment of ABDEMG activity in REMABD+ epochs reduces breathing variability and increases tidal volume and minute ventilation.
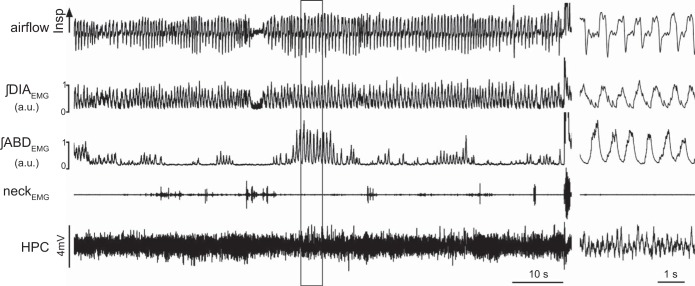
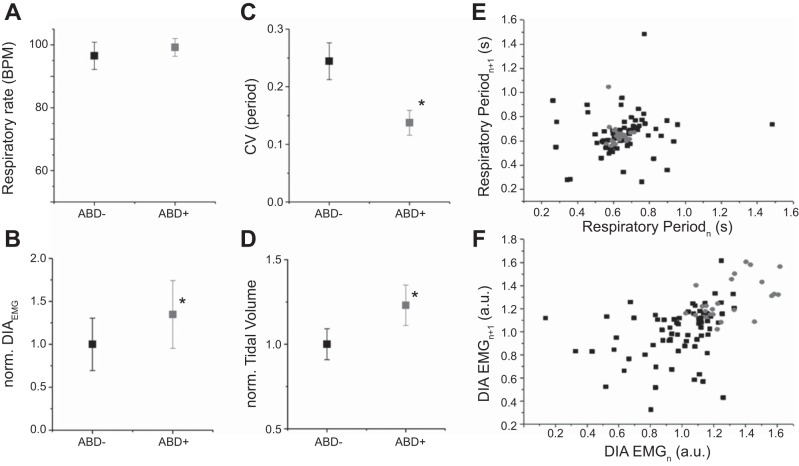
We investigated in further detail REMABD+ epochs and analyzed the respiratory characteristics in the 15 breaths preceding ABDEMG activity recruitment (Figs. 4 and 5) and compared these with the 15 breaths that immediately followed the onset of ABDEMG recruitment to determine if expiratory ABD activity had an effect on respiratory characteristics. Sixty-three events within seven rats were identified and analyzed further. Prior to ABDEMG recruitment, respiratory rate was 96.5 ± 4.3 BPM, with a CV of 0.24 ± 0.03 (n = 63). During ABDEMG recruitment, while respiratory rate was increased, this was not significantly different from the preceding period (99.2 ± 2.8 BPM; P = 0.2); however, CV was significantly reduced (0.14 ± 0.02; P = 0.01; Fig. 5). An example of the period distribution during a whole REMABD+ epoch is represented in the Poincare plot of Fig. 5E. Note the strong restabilization (shown as clustering) of respiratory period during ongoing ABDEMG recruitment. These results suggest that ABDEMG activity is associated with a significant reduction in respiratory variability. Within these epochs we also analyzed DIAEMG activity and relative tidal volume. ABDEMG recruitment was associated with changes in DIAEMG activity: we observed an overall increase in peak amplitude of ∫DIAEMG of 30.2 ± 11.1% (P = 0.04; n = 63 events in 5 rats; Fig. 5, B and F) but no significant change in the variability of the signal amplitude (P = 0.07; n = 63 events in 5 rats). In four rats (32 events) tested inside plethysmograph chambers we also measured respiratory flow and observed an overall significant increase in relative tidal volume of 23.2 ± 7.4% (P = 0.026) and in minute ventilation of 31.8 ± 12.6% (P = 0.03) during ABDEMG recruitment compared with the period preceding ABDEMG recruitment (n = 32 events obtained from 4 rats).
Fig. 4.
Abdominal muscle recruitment during a REM epoch is associated with an increased respiratory flow. Long traces recording of respiratory flow, integrated EMG activity of diaphragm (∫DIAEMG) and abdominal (∫ABDEMG) muscles, neck EMG activity, and hippocampal activity (HPC) during a REMABD+ epoch. Note the increased respiratory flow (both inspiratory and expiratory) during ABDEMG recruitment. Details of EMG and EEG traces during ABD muscle recruitment during REM sleep (black box) are displayed in panel at right.
Fig. 5.
ABDEMG recruitment within REMABD+ epochs significantly increases respiratory stability, peak ∫DIAEMG activity, and tidal volume. A–D: comparison of respiratory rate, normalized ∫DIAEMG peak amplitude, coefficient of variation of respiratory period, and normalized tidal volume between respiratory cycles preceding ABD activation (black) and respiratory cycles displaying ABDEMG activation within the same REM epoch (grey). Note reduction in CV and increase in ∫DIAEMG peak amplitude and tidal volume during expiratory ABDEMG recruitment. E: Poincare' plots for respiratory periods before ABD activation (black) and during rhythmic ABD activation (grey) recorded in a single REMABD+ epoch. Note clustering of inspiratory periods during ABD recruitments. F: Poincare' plots for ∫DIAEMG peak amplitude before ABD activation (black) and during rhythmic ABD activation (grey) recorded in a single REMABD+ epoch. Note the increase and clustered distribution of DIAEMG activity during ABD recruitment.
DISCUSSION
This study demonstrates that in adult rats ABDEMG recruitment during REM epochs is associated with preceding periods of increased respiratory variability and that the onset of expiratory ABDEMG activity is associated with a reduction in respiratory variability and an increase in minute ventilation.
REM epochs are characterized by the presence of central activation of cortical and hippocampal activity, ponto-geniculo-occipital waves, complete motor atonia in most skeletal muscles, and occurrence of phasic muscle twitches in the cranial muscles of jaw, tongue, and eye (32). Respiratory activity during REM epochs is usually characterized by irregularities in breathing rate, hyperpnea followed by periods of shallow ventilation, and frequent occurrence of apneas in both humans and rodents (3, 7, 12, 20). Respiratory variability during REM epochs has been postulated to be the consequence of an imbalance between excitatory and inhibitory inputs to the respiratory motor network. This effect is likely of central origin since variability is not dependent on activation of chemoreceptors, or upon vagal or thoracic afferents (24). In fact, during this time, responses to chemical stimuli (central and peripheral chemoreception) and other respiratory reflexes may be reduced and brainstem respiratory networks operate independently from the automatic and metabolic mechanisms that are present during nonREM sleep (5, 13, 22).
Another key feature of REM sleep is muscle atonia. Lack of muscular tone in REM sleep has been attributed to the activation of central inhibitory GABAergic and glycinergic mechanisms, although dysfacilitation of excitatory inputs may also be involved (6, 9, 21, 30, 32–34).
We recently reported that REM epochs in rodents frequently display recruitment of ABDEMG muscle activity (26). Here, we have investigated in further detail the respiratory characteristics of REM epochs in spontaneously breathing rats, comparing epochs demonstrating or not demonstrating ABDEMG recruitment to establish if expiratory activity is either a contributor to respiratory variability and apnea occurrence, or it is correlated with improved respiratory stability.
Our current results indicate that under normal breathing conditions, recruitment of expiratory ABDEMG activity during nonREM sleep does not affect respiratory rate, respiratory variability, or occurrence of apneas. In contrast, REM epochs displaying robust ABDEMG recruitment were characterized by an overall increase in respiratory period variability and an increase in number of spontaneous central apneas compared with REM epochs where no expiratory ABD was recorded. In addition, when we analyzed in detail REMABD+ epochs and compared the periods immediately preceding ABD recruitment with the periods of ongoing ABD recruitment, we observed an increased respiratory variability prior to ABDEMG recruitment. In the presence of ABDEMG recruitment, peak amplitude of ∫DIAEMG activity, tidal volume, and minute ventilation increased compared with values recorded prior to recruitment. These results indicate that recruitment of expiratory ABDEMG in REM epochs is associated with improvement in overall ventilation during REM epochs.
In behaving and anesthetized rats, abdominal expiratory-related activity is potentiated by central and peripheral chemosensory stimulation, exercise, and the stimulation of the brainstem expiratory oscillator, the paraFacial Respiratory Group (pFRG) (1, 16, 17, 23, 27). Recent progress on the mechanisms that control respiratory rhythmogenesis suggest that active contraction of expiratory muscles may be generated by an independent conditional oscillator located in the brainstem, pFRG (8, 14, 18, 27). pFRG neurons send projections to expiratory bulbospinal neurons in the caudal medulla that provide two thirds of the depolarization needed for ABD motoneurons to reach threshold and drive expiratory ABD muscle activity (4, 19, 29). Optogenetic stimulation of pFRG in anesthetized rats induces recruitment of expiratory ABD muscles, active expiration, and increase in minute ventilation in urethane anesthetized rats (27). In addition, recruitment of expiratory ABD muscles occurs when rodents are subjected to an increased respiratory drive, such as hypercapnia and hypoxia (11, 15, 16), or upon stimulation of a major chemosensitive site, the retrotrapezoid nucleus (RTN) (1, 2).
In our experiments we did not measure arterial levels of Po2 and Pco2; therefore we are unable to correlate increased expiratory ABD muscle recruitment with blood changes of O2 and CO2 levels in rats. A positive correlation is possible but unlikely given the fact that no serious respiratory disturbances (only increased respiratory variability) were consistently observed before occurrence of expiratory ABD muscle recruitment. In addition, central and peripheral chemosensitivity are usually depressed during REM sleep, and therefore an increase in ABD muscle recruitment is likely not a direct consequence of either activation of carotid bodies or central chemoreceptive sites (5, 13, 22).
Our results suggest that during REM epochs a phasic excitatory pathway to the abdominal motoneurons may be activated to generate robust ABD muscle recruitment during expiration. Together with other excitatory mechanisms that increase the drive to inspiratory muscles (10), the potentiation of expiratory modulated activity is likely to contribute to the overall improvement in respiratory stability and minute ventilation.
One potential source of this excitation is the activation of a rhythmic excitatory drive originating from the brainstem pFRG during periods of REM. In resting conditions and during anesthesia, the rodent pFRG is generally silent, but release of inhibition activates late expiratory neurons in the pFRG and promotes active contraction of expiratory muscles and generation of active expiration (14, 27). State-dependent modulation of pFRG neurons and the influence of specific neurotransmitters across states are currently unknown. Alternatively, expiratory ABDEMG recruitment could be generated by a phasic excitatory drive to the expiratory premotoneurons that promote ABD muscle activity if excited (4). Further studies will be necessary to determine the actual contribution of the pFRG expiratory oscillator or other possible excitatory descending pathways to the abdominal muscles across states.
Overall, these results support the hypothesis that an excitatory drive affects the recruitment of the expiratory muscle activity during REM epoch of natural sleep with the overall effect of improving minute ventilation.
GRANTS
S. Pagliardini was supported by CIHR OOGP Bridge Funding for New Investigator and WCHRI Recruitment Grant. C. Andrews was supported by NSERC USRA, WCHRI, and AIHS Undergraduate Summer Studentships.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
C.G.A. and S.P. performed experiments; C.G.A. and S.P. analyzed data; C.G.A. and S.P. interpreted results of experiments; C.G.A. and S.P. edited and revised manuscript; C.G.A. and S.P. approved final version of manuscript; S.P. conception and design of research; S.P. prepared figures; S.P. drafted manuscript.
ACKNOWLEDGMENTS
We thank Drs. C. Dickson and J. Greer and Ms. J. Saini for comments on the manuscript.
REFERENCES
- 1.Abbott SB, Stornetta RL, Coates MB, Guyenet PG. Phox2b-expressing neurons of the parafacial region regulate breathing rate, inspiration, and expiration in conscious rats. J Neurosci 31: 16410–16422, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abbott SB, Stornetta RL, Fortuna MG, Depuy SD, West GH, Harris TE, Guyenet PG. Photostimulation of retrotrapezoid nucleus phox2b-expressing neurons in vivo produces long-lasting activation of breathing in rats. J Neurosci 29: 5806–5819, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aserinsky E. Periodic respiratory pattern occurring in conjunction with eye movements during sleep. Science 150: 763–766, 1965. [DOI] [PubMed] [Google Scholar]
- 4.Bongianni F, Corda M, Fontana GA, Pantaleo T. Chemical activation of caudal medullary expiratory neurones alters the pattern of breathing in the cat. J Physiol 474: 497–507, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burke PG, Kanbar R, Basting TM, Hodges WM, Viar KE, Stornetta RL, Guyenet PG. State-dependent control of breathing by the retrotrapezoid nucleus. J Physiol 593: 2909–2926, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chase MH. Confirmation of the consensus that glycinergic postsynaptic inhibition is responsible for the atonia of REM sleep. Sleep 31: 1487–1491, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Coote JH. Respiratory and circulatory control during sleep. J Exp Biol 100: 223–244, 1982. [DOI] [PubMed] [Google Scholar]
- 8.Feldman JL, Del Negro CA, Gray PA. Understanding the rhythm of breathing: so near, yet so far. Annu Rev Physiol 75: 423–452, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fenik VB, Davies RO, Kubin L. REM sleep-like atonia of hypoglossal (XII) motoneurons is caused by loss of noradrenergic and serotonergic inputs. Am J Respir Crit Care Med 172: 1322–1330, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fraigne JJ, Orem JM. Phasic motor activity of respiratory and non-respiratory muscles in REM sleep. Sleep 34: 425–434, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fregosi RF, Hwang JC, Bartlett D Jr, St John WM. Activity of abdominal muscle motoneurons during hypercapnia. Respir Physiol 89: 179–194, 1992. [DOI] [PubMed] [Google Scholar]
- 12.Horner RL. Neuromodulation of hypoglossal motoneurons during sleep. Respir Physiol Neurobiol 164: 179–196, 2008. [DOI] [PubMed] [Google Scholar]
- 13.Horner RL, Liu X, Gill H, Nolan P, Liu H, Sood S. Effects of sleep-wake state on the genioglossus vs. diaphragm muscle response to CO(2) in rats. J Appl Physiol 92: 878–887, 2002. [DOI] [PubMed] [Google Scholar]
- 14.Huckstepp RT, Cardoza KP, Henderson LE, Feldman JL. Role of parafacial nuclei in control of breathing in adult rats. J Neurosci 35: 1052–1067, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Iizuka M. Abdominal expiratory muscle activity in anesthetized vagotomized neonatal rats. J Physiol Sci 59: 157–163, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Iizuka M, Fregosi RF. Influence of hypercapnic acidosis and hypoxia on abdominal expiratory nerve activity in the rat. Respir Physiol Neurobiol 157: 196–205, 2007. [DOI] [PubMed] [Google Scholar]
- 17.Iscoe S. Control of abdominal muscles. Prog Neurobiol 56: 433–506, 1998. [DOI] [PubMed] [Google Scholar]
- 18.Janczewski WA, Feldman JL. Distinct rhythm generators for inspiration and expiration in the juvenile rat. J Physiol 570: 407–420, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Janczewski WA, Onimaru H, Homma I, Feldman JL. Opioid-resistant respiratory pathway from the preinspiratory neurones to abdominal muscles: in vivo and in vitro study in the newborn rat. J Physiol 545: 1017–1026, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Krieger J. Respiratory Physiology: breathing in normal subjects. In: Principles and Practice of Sleep Medicine. Philadelphia, PA: Elsevier/Saunders, 2005, p. 232–244. [Google Scholar]
- 21.Kubin L, Davies RO, Pack AI. Control of upper airway motoneurons during REM sleep. News Physiol Sci 13: 91–97, 1998. [DOI] [PubMed] [Google Scholar]
- 22.Lovering AT, Dunin-Barkowski WL, Vidruk EH, Orem JM. Ventilatory response of the cat to hypoxia in sleep and wakefulness. J Appl Physiol 95: 545–554, 2003. [DOI] [PubMed] [Google Scholar]
- 23.Marina N, Abdala AP, Trapp S, Li A, Nattie EE, Hewinson J, Smith JC, Paton JF, Gourine AV. Essential role of Phox2b-expressing ventrolateral brainstem neurons in the chemosensory control of inspiration and expiration. J Neurosci 30: 12466–12473, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Orem J, Kubin L. Respiratory physiology: central neural control. In: Principles and Practice of Sleep Medicine. Philadelphia, PA: Elsevier/Saunders, 2005, p. 213–223. [Google Scholar]
- 25.Pagliardini S, Gosgnach S, Dickson CT. Spontaneous sleep-like brain state alternations and breathing characteristics in urethane anesthetized mice. PLoS ONE 8: e70411, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pagliardini S, Greer JJ, Funk GD, Dickson CT. State-dependent modulation of breathing in urethane-anesthetized rats. J Neurosci 32: 11259–11270, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pagliardini S, Janczewski WA, Tan W, Dickson CT, Deisseroth K, Feldman JL. Active expiration induced by excitation of ventral medulla in adult anesthetized rats. J Neurosci 31: 2895–2905, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Peever J. Control of motoneuron function and muscle tone during REM sleep, REM sleep behavior disorder and cataplexy/narcolepsy. Arch Ital Biol 149: 454–466, 2011. [DOI] [PubMed] [Google Scholar]
- 29.Road JD, Ford TW, Kirkwood PA. Connections between expiratory bulbospinal neurons and expiratory motoneurons in thoracic and upper lumbar segments of the spinal cord. J Neurophysiol 109: 1837–1851, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sakai K, Neuzeret PC. Brainstem neurons responsible for postural, masseter or pharyngeal muscle atonia during paradoxical sleep in freely-moving cats. Arch Ital Biol 149: 325–347, 2011. [DOI] [PubMed] [Google Scholar]
- 31.Sherrey JH, Pollard MJ, Megirian D. Proprioceptive, chemoreceptive and sleep state modulation of expiratory muscle activity in the rat. Exp Neurol 101: 50–62, 1988. [DOI] [PubMed] [Google Scholar]
- 32.Siegel JM. REM sleep. In: Principles and Practice of Sleep Medicine. Philadelphia, PA: Elsevier/Saunders, 2005, p. 120–135. [Google Scholar]
- 33.Soja PJ. Glycine-mediated postsynaptic inhibition is responsible for REM sleep atonia. Sleep 31: 1483–1486, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vetrivelan R, Chang C, Lu J. Muscle tone regulation during REM sleep: neural circuitry and clinical significance. Arch Ital Biol 149: 348–366, 2011. [DOI] [PubMed] [Google Scholar]