Abstract
Growth hormone (GH) plays a central role in regulating somatic growth and in controlling multiple physiological processes in humans and other vertebrates. A key agent in many GH actions is the secreted peptide, IGF-I. As established previously, GH stimulates IGF-I gene expression via the Stat5b transcription factor, leading to production of IGF-I mRNAs and proteins. However, the precise mechanisms by which GH-activated Stat5b promotes IGF-I gene transcription have not been defined. Unlike other GH-regulated genes, there are no Stat5b sites near either of the two IGF-I gene promoters. Although dispersed GH-activated Stat5b binding elements have been mapped in rodent Igf1 gene chromatin, it is unknown how these distal sites might function as potential transcriptional enhancers. Here we have addressed mechanisms of regulation of IGF-I gene transcription by GH by generating cell lines in which the rat Igf1 chromosomal locus has been incorporated into the mouse genome. Using these cells we find that physiological levels of GH rapidly and potently activate Igf1 gene transcription while stimulating physical interactions in chromatin between inducible Stat5b-binding elements and the Igf1 promoters. We have thus developed a robust experimental platform for elucidating how dispersed transcriptional enhancers control Igf1 gene expression under different biological conditions.
Keywords: growth hormone, IGF-I, STAT, gene transcription, epigenetics, chromatin immunoprecipitation
growth hormone (gh) plays a pivotal role in many physiological processes in humans and other species. It is essential for somatic growth, is a key contributor to normal tissue differentiation and repair, and is an important regulator of intermediary metabolism (8, 18, 25, 42). Excessive GH also has been implicated in aging and in the development of certain cancers (4, 40, 49), implying that its activity must be limited in scope and duration to maintain physiological homeostasis, at least in the adult. It is thus important to understand mechanisms of GH action to devise strategies that will enhance its positive physiological effects while limiting its negative impact on human biology and disease.
According to the modern view of the somatomedin hypothesis of GH action, insulin-like growth factor-I (IGF-I), a conserved 70-amino acid secreted protein, is a critical mediator of many of the biological effects of GH, particularly on somatic growth and tissue repair (18, 42). Like GH, IGF-I also is implicated in aging and carcinogenesis (4, 40, 49), and thus a fuller understanding of mechanisms of GH action requires thorough scrutiny of the functions of IGF-I.
Like other cytokines, GH acts through a trans-membrane receptor (the GHR) and engages the JAK - STAT signaling pathway (8). GH binding promotes the receptor-associated activity of Jak2 (7), causing phosphorylation of tyrosine residues on the intracellular part of the GHR (25, 56), and leading to recruitment and activation of STATs 1, 3, 5a, and 5b, and other signaling molecules (25, 56). Despite the contribution of multiple signaling pathways to the biological effects of GH (25, 56), several recent observations, including identification of inactivating mutations in humans (18, 22, 22, 24), results of targeted gene knockouts in mice (50, 52, 53), and molecular and biochemical studies (58), have established that STAT5b is the key signaling agent mediating many of the critical biological effects of GH. For example, GH activates rat Igf1 gene transcription via Stat5b (58), leading to sustained accumulation of Igf1 mRNAs and production of IGF-I (43). Moreover, individuals lacking STAT5B have growth impairment and reduced expression of IGF-I (22). However, the precise biochemical mechanisms by which Stat5b promotes IGF-I gene transcription have not been defined. Unlike other GH-activated and Stat5b-dependent genes, in which critical transcriptional regulatory elements are located within the proximal promoters (12, 61), there are no Stat5b binding sites near either of the two IGF-I gene promoters (12). Although several dispersed GH-inducible STAT5b elements have been mapped in human IGF-I and rodent Igf1 gene chromatin (10, 12, 26, 55, 60), it is unknown whether these binding domains or others function as potential transcriptional enhancers (11, 43).
A key problem preventing detailed dissection of mechanisms of IGF-I gene regulation is the lack of any cell system that robustly expresses IGF-I. Here we have overcome this limitation by generating cell lines in which the rat Igf1 chromosomal locus has been incorporated into the mouse genome. Using these cells we now show that GH-stimulated Stat5b promotes physical interactions between several distal enhancers and the Igf1 promoters to rapidly and potently activate Igf1 gene transcription.
MATERIALS AND METHODS
Materials.
G418 was obtained from Sigma-Aldrich (St. Louis, MO), micrococcal nuclease was from Active Motif (Carlsbad, CA), okadaic acid was from Alexis Biochemicals (San Diego, CA), and TransIT LT1-transfection reagent was from Mirus (Madison, WI). AquaBlock EIA/WIB solution was purchased from East Coast Biologicals (North Berwick, ME), Immobilon-FL membranes were from Millipore (Billerica, MA), and protease inhibitor tablets were from Roche Applied Sciences (Indianapolis, IN). Fetal calf serum and phosphate-buffered saline (PBS) were from Mediatech-Cellgrow (Herndon, VA). Dulbecco's modified Eagle's medium (DMEM), trypsin/EDTA solution, SuperScript III reverse-transcriptase (RT) kit, protein A-Sepharose beads, and SYBR Green Platinum qPCR mix were from Invitrogen-Life Technologies (Carlsbad, CA). The Qia-Quick PCR purification kit was purchased from Qiagen (Valencia, CA), the BCA protein assay kit was from Pierce Biotechnologies (Rockford, IL), and restriction enzymes, buffers, ligases, and polymerases were from New England Biolabs (NEB, Beverly, MA). Recombinant rat GH was purchased from the National Hormone and Pituitary Program, National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK), National Institutes of Health (NIH), and was stored dry in aliquots at −80°C until use, when it was reconstituted at 2.5 mg/ml [∼100 μM]. Primary antibodies were obtained from the following vendors: Santa Cruz Biotechnology (Santa Cruz, CA), Stat5 (C17), RNA polymerase II (H-224), and p300 (C-20); Millipore, phospho-Stat5 (clone 8-5-2); and Sigma-Aldrich, Flag M2 monoclonal and α-tubulin. Secondary antibodies were from Invitrogen (Carlsbad, CA), goat anti-mouse IgG1-Alexa 488, and Rockland Immunochemical (Gilbertsville, PA), goat-anti-rabbit IgG-IR800 and goat anti-mouse IgG-IR680. Custom oligonucleotides were purchased Thermo Scientific (Waltham, MA). Bacterial artificial chromosome (BAC) clone #CH230-116M6 was purchased from Children's Hospital Oakland Research Institute (Oakland, CA), and materials for recombineering were obtained from the National Cancer Institute (Frederick, MD). All other chemicals were reagent grade and were purchased from commercial sources.
Developing recombinant cell lines containing the rat Igf1 chromosomal locus.
BAC clone #CH230-116M6 encodes 241,603 base pairs of rat chromosome 7 and spans coordinates 28.418.684–28.660.287, including the Igf1 gene and flanking DNA at 28.528.240–28.608,863 (these coordinates include the ∼6.4 kb exon 6, most of which is not noted in public rat genome databases; see Ref. 19 for details) (Fig. 1). A DNA cassette encoding the neomycin resistance gene driven by a combined prokaryotic and eukaryotic promoter (derived from plasmid pL452) was inserted into the BAC at position 28.626.630 (Fig. 1) by homologous recombination (recombineering) using Escherichia coli strain SW102 and selection on kanamycin-containing agar plates (28, 62). Modified BAC DNA was purified and the presence of the neomycin resistance cassette and the Igf1 gene and flanking DNA were validated by PCR. The Igf1 locus was incorporated into the genome of mouse C3H10T1/2 cells (ATCC #CCL226) following transfection using Transit LT1 and selection in medium containing G418 at 400 μg/ml. Individual colonies were isolated using cloning cylinders, expanded, checked for an intact Igf1 gene by PCR (Fig. 1 and Table 1), and stored in liquid N2 until use. Transgene copy number was assessed by quantitative (q) PCR, as described (15, 23). A standard curve was constructed with serial dilutions of BAC DNA and rat Igf1-specific primers by plotting the cycle threshold (Ct) vs. amount of input DNA, and the slope and correlation coefficient were determined (see Ref. 14). Results were judged acceptable if r2 was > 0.98.
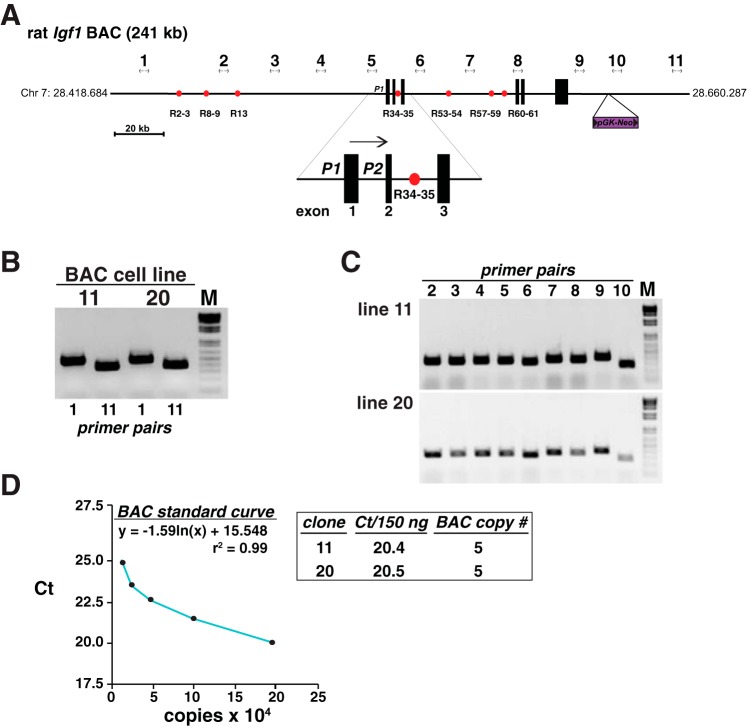
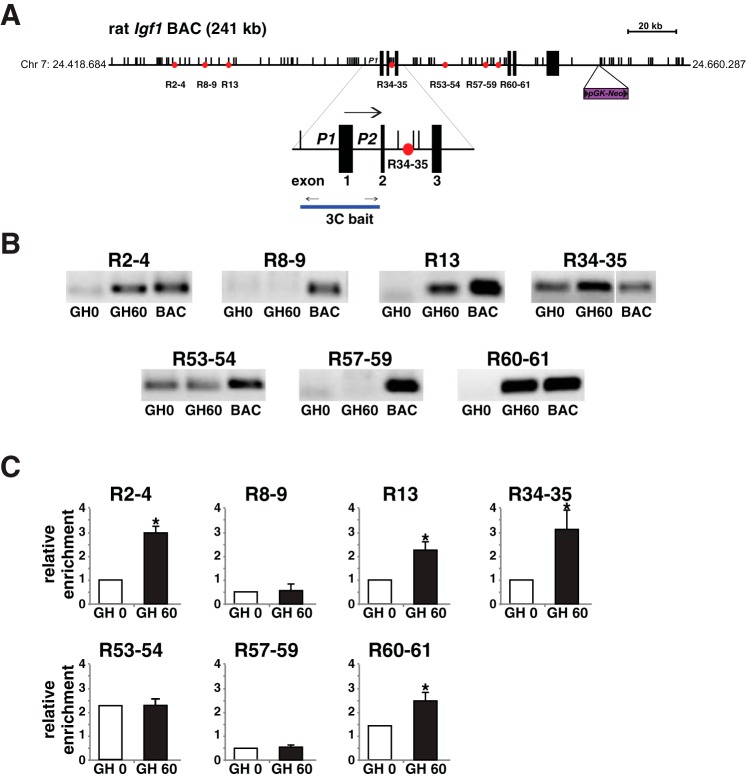
Fig. 1.
Mapping recombinant cell lines incorporating the rat Igf1 locus. A: schematic of rat Igf1 bacterial artificial chromosome (BAC) Ch230-113M6, which consists of 241 kb of rat chromosome 7, including the ∼80 kb 6-exon Igf1 gene, ∼110 kb of 5′-flanking, and ∼51 kb of 3′-flanking DNA. The location of the pGK-Neo selection cassette, which was introduced into the BAC by recombineering (28, 62), is indicated, as are the 7 previously identified growth hormone (GH)-regulated Stat5b-binding elements (red circles) and a scale bar. The enlargement below the main map depicts the two Igf1 promoters, P1 and P2, and adjacent exons 1–3. The horizontal arrow indicates the direction of gene transcription. The locations of the 11 PCR primer pairs used to characterize each clonal cell line are indicated (see Table 1 for DNA sequences). B: results of PCR mapping experiments using genomic DNA isolated from BAC cell lines 11 and 20 and primer pairs 1 and 11. These pairs amplify DNA from the 5′- and 3′-ends of the BAC, respectively. C: results of PCR mapping experiments with genomic DNA from each BAC cell line and primer pairs 2 through 10. Collectively, results in B and C demonstrate that the entire BAC has been incorporated into each recombinant cell line. D: results of copy number determinations for cell lines 11 and 20, using as a standard curve serial dilutions of BAC DNA (see materials and methods for details).
Table 1.
DNA sequences of oligonucleotide primers for mapping BAC cell lines
| Genomic Location (5′-end) | Primer Pair # | Top Strand (5′ to 3′) | Bottom Strand (5′ to 3′) | Amplicon, bp |
|---|---|---|---|---|
| 28.420.673 | 1 | TCTGTGTCTTGAGTTTCCAGAG | CTTAAAGCTGACATGATTTCAAC | 507 |
| 28.461.627 | 2 | GGAGGACTGGCTGGCTATGAACTA | TTCAACAAATGTGGGAAAGTACAA | 330 |
| 28.481.628 | 3 | GTTTGAATGAGGTTAAGCCACTTG | TACTTTCCTCTCTCACTATCTTGA | 333 |
| 28.335.740 | 4 | AAACTCTCTGGCTAGTAGAAGCCT | AATGCTGTTCATAAGATATTGACT | 219 |
| 28.521.628 | 5 | TGGGGAAGAGGATGCTTTTAAAG | GCTTCTGTGACAAGTCTGTGCACT | 326 |
| 28.541.949 | 6 | TCAAGGACGATCTGATGATTCTTG | ACCATGCCCCTAACTTGGATCAGA | 300 |
| 28.561.950 | 7 | ATGAGGAGAAAGGTCATTTCCAACTCC | TGAATACAGTTGGGGTTAATGAAGAGA | 345 |
| 28.582.175 | 8 | GCTCTACACTTTGGTACCTCTTCCTCCTAT | GAGTTGTGCCATTAATCAGGAGTGAAACTATG | 342 |
| 28.606.603 | 9 | TAGTCTTGCCCTTGGTTGTATATGCAACTA | TCTAGAAGAATCTGGTGGGAGAAAAACTAAAGA | 378 |
| 28.621.675 | 10 | TGTAATTACGTTGCATGTAGCTTACACATT | CTAATATCTGACAGAAATACAATGATGGCTG | 258 |
| 28.651.872 | 11 | ACCACCCGTGAGACCCTGGGTCAT | AATCAAAAAGTTTTCAGTCTGTTA | 417 |
BAC, bacterial artificial chromosome.
Analysis of recombinant cell lines.
Igf1-BAC cells were incubated in antibiotic-free DMEM supplemented with 10% fetal bovine serum and 200 μg/ml G418 at 37°C in humidified air with 5% CO2. Cells were co-infected at ∼50% of confluent density with recombinant adenoviruses encoding the mouse GH receptor [(Ad-GHR), multiplicity of infection (MOI) of 200], rat Stat5b (Ad-Stat5b, MOI 200) and a tetracycline-inhibited transcriptional activator (Ad-tTA, MOI 100), as described (58). The latter is required for expression of Stat5b (58). At 20 h after infection, cells were washed with PBS and incubated in serum-free medium for 2 h, followed by addition of serum-free medium and 1% bovine serum albumin, plus vehicle or recombinant rat GH [40 nM] for 15 min to 24 h. Alternatively, cells were co-infected with an adenovirus encoding a constitutively active version of rat Stat5b (Ad-Stat5bCA, MOI 200) plus Ad-tTa (MOI 100) (58) and, at 20 h after infection, were incubated in serum-free medium and 1% bovine serum albumin, with addition of vehicle or the tetracycline analog doxycycline [1 mg/ml] for 24 h.
Animal studies.
Male Sprague-Dawley rats were purchased from Harlan Sprague-Dawley (Indianapolis, IN), after being hypophysectomized at age 7 wk by a transauricular route. Rats were housed at the Oregon Health & Science University (OHSU) Animal Care Facility on a 12 h light-dark schedule with free access to food and water and received care according to NIH guidelines. GH deficiency was established by failure to grow during a >2 wk observation period. Rats subsequently were injected by the intraperitoneal route with vehicle (10 mM NaHCO3) or recombinant rat GH [1.5 mg/kg] and were killed at 1–6 h later. Nuclei, chromatin, RNA, and nuclear proteins were isolated from the liver of individual animals. Adult male mouse liver was a gift from the laboratory of Robert Klein in the Department of Medicine at OHSU. The OHSU Committee on Animal Care and Use approved all animal studies.
Analysis of gene expression.
Total (whole cell) and nuclear RNA were isolated as described previously (2, 13). RNA integrity was assessed by agarose gel electrophoresis and concentrations were determined spectrophotometrically at 260 nm. Total RNA (2 μg) was reverse-transcribed with oligo-dT primers in a final volume of 20 μl using the SuperScript III RT kit (2, 13). The cDNA (0.5 μl) was used as a template for conventional PCR, as described (2, 13). Pilot studies were performed to establish a cycle number for each primer set that achieved amplification in the linear range (22–25 cycles depending on the primers). Primer pairs may be found in Table 2. Products were visualized after agarose gel electrophoresis and staining with ethidium bromide. Results are representative of at least three independent RT-PCR experiments. Nuclear RNA (2.5 μg) was reverse-transcribed with random hexamers in a final volume of 20 μl using the SuperScript III RT kit, and 1 μl of cDNA was used as template for PCR. Primer pairs may be found in Table 3. For conventional PCR, pilot studies established that 20–25 cycles achieved amplification in the linear range for each primer pair, and products were visualized after agarose gel electrophoresis by ethidium bromide staining. Results are representative of three or more independent experiments. For qRT-PCR, first-strand cDNA was diluted 100-fold (25 μl into a final volume of 2.5 ml), and 1 μl was used as template for PCR (the equivalent of 1.0 ng of nuclear RNA input). Quantitative PCR was performed as described (12) using primer sets listed in Table 3 and the Bio-Rad Chromo4 Real-Time PCR detection system. A standard curve was constructed for each primer pair by plotting the Ct vs. the amount of input DNA (log10 scale), and the slope and correlation coefficient for each curve were determined (see Ref. 14). Standards included serial dilutions of rat or mouse genomic DNA (7 concentrations ranging from 0.06 to 40 ng). Primer sets were judged to be acceptable if the slope of the linear part of the curve approximated −3.3 (theoretical maximum efficiency of amplification) and r2 was > 0.98. The Ct for each sample was extrapolated to the standard curve to determine the quantity of nascent transcript (ng). Results for GAPDH were used to normalize samples at each time point.
Table 2.
Primers used for RT-PCR of total RNA
| Gene | Location | DNA Sequence | Amplicon, bp |
|---|---|---|---|
| Rat Igf1 | exon 1 | 5′-CTGCCTCTGTGACTTCTTGAAGG | 353 |
| exon 4 | 5′-GTCTTGGGCATGTCAGTGTGG | ||
| Rat Igf1 | exon 2 | 5′-GTACCAAAATGAGCGCACCTCCA | 354 |
| exon 4 | 5′-GTCTTGGGCATGTCAGTGTGG | ||
| Rat IgfI | exon 3 | 5′-CTCTTCAGTTCGTGTGTGGACC | 214 |
| exon 4 | 5′-GTCTTGGGCATGTCAGTGTGG | ||
| Mouse | exon 3 | 5′-AACTTTGGCATTGTGGAAGG | 223 |
| Gapdh | exon 4 | 5′-ACACATTGGGGGTAGGAACA | |
| Mouse | exon 3 | 5′-CTCTTCAGTTCGTGTGTGGACC | 213 |
| Igf1 | exon 4 | 5′-GTCTTGGGCATGTCAGTGTGG | |
| Mouse | exon 2 | 5′-GATAGCATCCCAGTCAGCA | 246 |
| Igfals | exon 3 | 5′-TGAGTGAAGCCAGACTTGGT | |
| Mouse | exon 3 | 5′-GAATGGAGCGGACAGGAC | 212 |
| Socs2 | exon 4 | 5′-ATCCTGTTTGACTGAG | |
| Mouse S17 | exon 2 | 5′-ATCCCCAGCAAGAAGCTTCGGAACA | 328 |
| exon 3 | 5′-TATGGCATAACAGATTAAACAGCTC |
Table 3.
Primers used for quantitative RT-PCR of nuclear RNA
| Gene | Location | DNA Sequence | Amplicon, bp |
|---|---|---|---|
| Rat Igf1 | exon 1 | 5′-CTGCCTCTGTGACTTCTTGAAGG | 224 |
| intron 1 | 5′-AATGCATTCATCTCCCGGACCTC | ||
| Rat Igf1 | exon 2 | 5′-GTACCAAAATGAGCGCACCTCCA | 316 |
| intron 2 | 5′-CTCCCCATATGTTCTCCATTCCC | ||
| Mouse | exon 2 | 5′-GATAGCATCCCAGTCAGCA | 189 |
| Igfals | intron 2 | 5′-GTTCCGTTCCAGGTGCAGAT | |
| Mouse | exon 2 | 5′-GAATGGAGCGGACAGGAC | 122 |
| Socs2 | intron 2 | 5′- CCAGCTCCCTACCTGTTTGA | |
| Mouse | exon 2 | 5′-AGGAGCGAGACCCCACTAAC | 114 |
| Gapdh | intron 3 | 5′-CAGCTTTCCGGCCACTTAC |
Immunoblotting and immunocytochemistry.
Whole-cell protein lysates were prepared from cells as described (37). Protein samples (15 μg/lane) were resolved by SDS-PAGE, transferred to Immobilon-FL membranes, blocked with 50% AquaBlock solution for 1 h, and incubated sequentially with primary and secondary antibodies. Primary antibodies were used at a dilution of 1:2,000 (anti-Flag M2, anti-phospho-Stat5) or 1:15,000 (anti-α-tubulin), and secondary antibodies at 1:5,000. Results were visualized and images captured using the LI-COR Odyssey and version 3.0 analytical software (Lincoln, NE). For immunocytochemistry, cells were fixed, permeabilized, blocked, and incubated sequentially in blocking buffer with primary antibodies (anti-Flag M2, 1:2,000 dilution) for 18 h and secondary antibodies (goat anti-mouse IgG1-Alexa 488, 1:2,000) for 1 h, as described (38). Nuclei were stained with Hoechst 33258 dye. Images were captured with a Nikon Eclipse Ti-U inverted microscope using the NIS elements 3.1 software. Image analysis was performed with this software.
Quantitative chromatin immunoprecipitation.
For each time point, 1 × 107 nuclei were incubated on a rotating platform in 0.5 ml of DMEM with 1% formaldehyde for 2 min, followed by addition of 0.25 ml of 1 M glycine and incubation for an additional 1 min. After centrifugation at 200 g for 10 min at 20°C, the pellet was washed in PBS and suspended in 0.35 ml of digestion buffer (plus protease inhibitors) for 5 min at 37°C. Micrococcal nuclease was added to a final concentration of 200 U/ml and incubated for 10 min at 37°C, as per protocol from the supplier (Active Motif), and sheared chromatin was extracted, as described (2, 13). In direct comparison with sonication, we found that treatment with micrococcal nuclease generated sheared chromatin that more consistently contained DNA in the size range of 500 ± 100 bp. DNA was isolated from an aliquot, and the sheared chromatin was adjusted to 1 mg/ml of DNA in immunoprecipitation (IP) buffer (50 mM Tris·HCl, 5 mM EDTA, 150 mM NaCl, 0.1% SDS, 1% Triton X-100, pH 8.1 plus protease inhibitors). Chromatin was stored in 100 μl aliquots at −80°C until use. IPs were performed with sheared chromatin containing ∼100 μg of DNA and 2 μg of each antibody, as described (12, 13). DNA was extracted using the QiaQuick PCR purification kit and used as template in qPCR reactions with the primer pairs listed in Table 4 (12, 13) and a Bio-Rad Chromo4 Real-Time PCR detection system. Reactions (20 μl volume) contained 1× SYBR Green mix, 200 nM primers, and quantitative chromatin immunoprecipitation (qChIP)-enriched DNA and were performed in eight-well strips in a 96-well format. Standard curves consisting of 0.01–1.25 ng of genomic DNA were included in each experiment for each primer pair. Values are expressed as percent input necessary to achieve the identical Ct. Results are the means ± SD of three or more independent qPCR experiments from independent IPs.
Table 4.
DNA sequences of Igf1 locus oligonucleotide primers for ChIP assays
| Location (from 5′ end) | Element | Top Strand (5′ to 3′) | Bottom Strand (5′ to 3′) | Amplicon, bp |
|---|---|---|---|---|
| −86264 | R2-4 | TGAATCTCCAGGGATCACACCA | CTCTTGTTGATTTCATCTTTGACCCA | 84 |
| −73096 | R8-9 | GTGAGTCTGTGTTAGTCAGG | GGTTATGTAAGTCTGATAGAG | 93 |
| −62806 | R13 | TGCATTGGCTACCAGGAACTCT | GAGACAGTTGGAAGGAAGGATAGA | 76 |
| +3740 | R34-35 | GGTGAAACCGCTCACCTTGG | TTCTAAGAAGCAGACAGAGG | 103 |
| +26694 | R53-54 | TGGCACATGCCATTGACCAGAT | AAGGGCAGACCCATCTTTCTGA | 102 |
| +43817 | R57-59 | GGCCAATTCATTCGGCAACTGT | TCCTTTCTGAGAACTGGAGACCGT | 87 |
| +49147 | R60-61 | CCCAACTGAGAAGTAGTAGGCT | GAAACAGTCCCGACAACCTATC | 105 |
| −53692 | Neg1 | TGACAGTAGTCCGAGGCTCATT | ACCGTGAGATTTCTTAGGTGGTGC | 82 |
| −10054 | Neg2 | CCAGCCGTTAAACACCAAGAAG | TGCTAGTCCCAGTACAGCATCT | 94 |
| +12555 | Neg3 | AGCGTTCTTCCAAACACCTTGTGC | GGTTGGCTCTGATGGAGTATCT | 75 |
ChIP, chromatin immunoprecipitation.
Chromatin-conformation and capture assays.
These studies followed published protocols (2, 31). Nuclei from 1 × 108 cells were fixed by addition of 1.0 ml of lysis buffer (10 mM HEPES, 1.5 mM MgCl2, 10 mM KCl, 0.5 mM DTT, 1% Triton X-100, pH 7.9) with protease inhibitors and 2% formaldehyde for 2–3 min at 15°C and quenched by addition of glycine to 0.125 M for 5 min on ice. Following two washes with ice-cold lysis buffer, nuclei were resuspended in 1.0 ml of 1.2× NEB CutSmart restriction enzyme buffer (100 mM NaCl, 50 mM Tris·HCl, 10 mM MgCl2, 1 mM DTT, pH 7.9) containing 0.3% SDS and incubated at 37°C for 1 h with shaking at 1,000 RPM. Triton X-100 was added to a 1.8% final concentration, and samples were incubated for 1 h at 37°C with shaking followed by addition of 1,500 U of AflII and incubation for 16 h at 37°C and 1,000 RPM. After addition of SDS to 1.3% for 30 min at 65°C, each sample was mixed with 20 ml of 1× DNA ligation buffer. Triton X-100 was added to 1%, and nuclei were incubated for 1 h at 37°C and 400 RPM. After equilibration at 16°C, T4 DNA ligase was added (1,000 units), followed by incubation for 5 h at 16°C with slow agitation. After addition of proteinase K (300 μg for 16 h at 65°C) and RNase A (3 μl of a 100 mg/ml solution for 30 min at 37°C), DNA was isolated by phenol-chloroform extraction and ethanol precipitation, and dissolved in 1 ml of 10 mM Tris·Cl, 1 mM Na2EDTA, pH 7.9. DNA concentrations were measured with a NanoDrop 2000c UV-Vis spectrophotometer (Thermo Scientific) and adjusted to 50 ng/μl. Two-stage PCR reactions were performed using the primers listed in Table 5. For qualitative analysis, 1 μl of DNA was used in the first PCR step in a reaction volume of 20 μl with 100 nM of primer pairs for 25 cycles. For the second step, 0.5 μl of product from the first PCR stage served as template in a reaction volume of 20 μl with 100 nM of primer pairs for 30 cycles. Control templates were generated by ligation of AflII-digested BAC CH230-113M6 DNA, which was stored in aliquots at −80°C at a concentration of 100 ng/μl. Results were visualized by ethidium bromide staining after electrophoresis through 1% agarose gels. Positives were confirmed by DNA sequencing. For qPCR studies, 1 μl of DNA was used in an initial PCR step in a reaction volume of 20 μl with 100 nM of primer pairs for 10 cycles, and 0.5 μl of product from the first PCR reactions were used in the second stage.
Table 5.
DNA sequences of oligonucleotide primers for 3C assays
| Element | PCR1 (5′ to 3′) | PCR2 (5′ to 3′) | Amplicon PCR2, bp |
|---|---|---|---|
| R2-4 | TGAATTGTTTGAGGTTTGTTG | CTAGTATGTGGTCTGTTTTAG | 123 |
| R8-9 | GTTACAGACAACTCAGCAAATAT | TAAATCATCTTACAGCAGTTGC | 124 |
| R13 | TTCAAAGTGTCAAAACTGAAC | TCCGTCCTTGCTTCCAAACCA | 119 |
| R34-35 | AATGTATGGGGAAGTAACCCCCAT | TGTGGAAGCTCTTTTTTTTCTGAA | 127 |
| R53-54 | GTTGGGGATTTAGCTCAGT | TAGAGCACAAGGCCCTGGGTT | 120 |
| R57-59 | AGATAAGAAATGCCCTTCAAAAGC | ATGGTCCAGTGATCTCCTTCCTCA | 119 |
| R60-61 | TGAGATGGGTGTGGGCTGAGCACA | GGATCAGAGAGGTTAGCGTCACAC | 121 |
| promoter | ATACCTGCCTGGGTGTCCAAATGT | AACTAGATGCTTTCACAAACCCCA | — |
3C, chromatin conformation and capture.
Statistical analysis.
Data are presented as means ± SD. Statistical significance was determined using a paired Student's t-test. Results were considered statistically significant when P < 0.05.
RESULTS
Developing a chromosomally integrated rat Igf1 transgene.
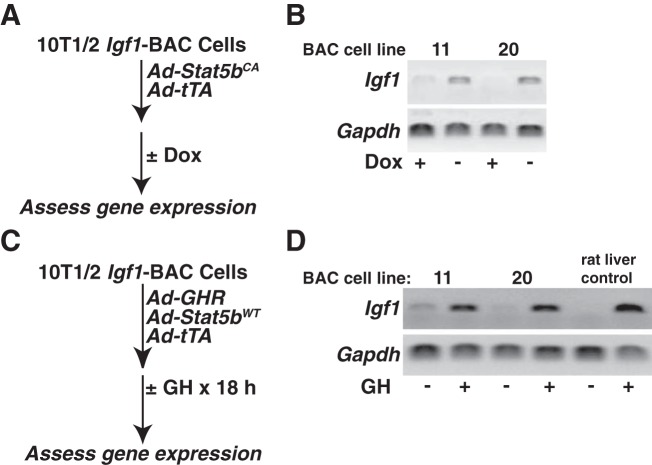
There are few cell-based experimental systems available to study IGF-I gene regulation. Of the primary cells or cell lines that produce measureable amounts of IGF-I, very few respond even minimally to trophic factors such as GH (3, 45), a key agent involved in control of IGF-I production in vivo (27, 29). As a consequence, despite work over many years, progress has been incomplete in understanding the mechanisms responsible for control of IGF-I biosynthesis. To address this problem, we have developed recombinant cell lines in which the rat Igf1 locus has been integrated in chromatin in the mouse C3H10T1/2 cell line. To generate the transgene, we first modified the bacterial artificial chromosome, BAC CH230-113M6, by incorporating a selectable marker for neomycin resistance, encoded by pGK-Neo, into a position ∼18 kb downstream from the 3′-end of Igf1 (Fig. 1A). CH230-113M6 contains the 80 kb rat Igf1 gene, including both promoters and all 6 exons (19), and ∼110 kb of 5′- and ∼51 kb of 3′-flanking DNA (Fig. 1A). Transgenic DNA was integrated into C3H10T1/2 cells after selection with G418, followed by isolation of individual clones, expansion of cells, and testing for an intact Igf1 locus by PCR, using primer pairs that spanned the entire 241 kb DNA fragment (Fig. 1). Two of six cell lines screened had intact BAC inserts (Fig. 1, B and C), and both had a copy number per haploid genome of 5 (Fig. 1D). Both lines showed robust production of Igf1 mRNA by RT-PCR after cells were transduced with a recombinant adenovirus encoding a constitutively active version of Stat5b, which is expressed in the absence of doxycycline (58) (Fig. 2, A and B). Both cell lines also exhibited induction of Igf1 mRNA in response to GH treatment after cells were transduced with adenoviruses for the mouse GHR and rat Stat5b, while minimal Igf1 mRNA was detected in the absence of GH (Fig. 2, C and D). The level of Igf1 transcripts observed in GH-treated cells was comparable to that detected in rat liver RNA after acute GH exposure for 6 h (Fig. 2D).
Fig. 2.
Analysis of recombinant cell lines incorporating the rat Igf1 locus. A: experimental plan to test responsiveness to Stat5b of the rat Igf1 gene in individual BAC-containing cell lines (Dox, doxycycline). B: results of RT-PCR experiments for rat Igf1 (primers span exons 3 and 4) or mouse Gapdh mRNA (see Table 2) using RNA isolated from the listed recombinant cell lines transduced with constitutively active rat Stat5b and tTA and treated ± Dox for 18 h. C: experimental plan to test GH responsiveness of the rat Igf1 gene in individual BAC-containing cell lines. D: results of RT-PCR experiments using RNA isolated from the listed recombinant cell lines transduced with the mouse GH receptor, rat Stat5b, and tTA, and treated with vehicle or recombinant rat GH [40 nM] for 18 h. Liver RNA from GH-deficient rats treated with vehicle or recombinant rat GH [1.5 mg/kg] for 6 h represents a negative and positive control respectively for GH responsiveness.
Rapid and robust induction of Igf1 gene transcription by GH.
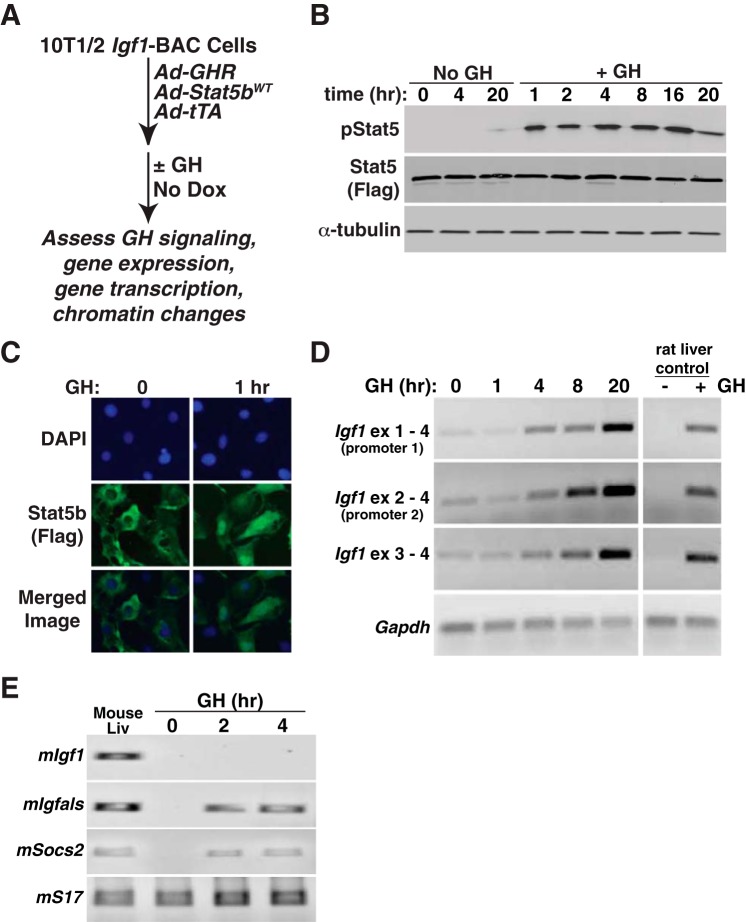
Having identified recombinant cell lines with regulated expression of Igf1 mRNA, we next studied the rate and extent of the response to GH, with a focus on transcriptional activation, since GH stimulates Igf1 gene transcription in vivo (5, 58). We first performed time course studies, harvesting RNA from 1 to 20 h after exposure of cells to a midphysiological level of GH in rats [40 nM] (48) (Fig. 3). Under these conditions, hormone treatment caused prolonged activation of the GHR, as evidenced by sustained stimulation of tyrosine phosphorylation of Stat5b for more than 16 h (Fig. 3B), nuclear accumulation of Stat5b (Fig. 3C), and induction of Igf1 mRNA beginning by 4 h after GH exposure and continuing for at least 20 h (Fig. 3D). By 8 h after GH addition to cells, the amount of Igf1 mRNA was nearly equivalent to that measured in the liver of GH-deficient rats at 4 h after systemic injection of GH, and this level of accumulation of Igf1 mRNA was greatly exceeded by the 20 h time point (Fig. 3D). In contrast to results with C3H10T1/2 cells encoding the rat Igf1 transgene, endogenous mouse Igf1 gene expression was not activated by GH and Stat5b, although both Igfals and Socs2, which, like Igf1, are dependent on active Stat5b to mediate the GH response, were rapidly stimulated to levels equivalent to those observed in mouse liver (Fig. 3E).
Fig. 3.
Rapid induction of Igf1 gene expression by GH in a BAC-containing cell line. A: experimental plan outlining steps to test Igf1 gene regulation in response to GH and Stat5b in 10T1/2 Igf1-BAC clone 11. B: time course of appearance of tyrosine phosphorylated Stat5b (pStat5), total Stat5b (using antibody to Flag), and α-tubulin, as assessed by immunoblotting after addition of vehicle (No GH) or recombinant rat GH [40 nM] to clone 11 cells. C: GH treatment causes the nuclear accumulation of Stat5b in the nucleus of 10T1/2 Igf1-BAC clone 11, as detected by immunocytochemistry. DAPI was used to stain nuclei. D: time course of accumulation of Igf1 and Gapdh mRNAs, as assessed by RT-PCR using RNA isolated at different times after GH addition to cells. Liver RNA from GH-deficient rats treated acutely with vehicle or a single injection of recombinant rat GH [1.5 mg/kg] serves as both negative and positive controls for GH-responsiveness. E: time course of appearance of mouse Igf1, Igfals, Socs2, and S17 mRNAs, as assessed by RT-PCR using RNA isolated at different times after GH addition to cells. Liver RNA from intact adult male mice serves as a positive control.
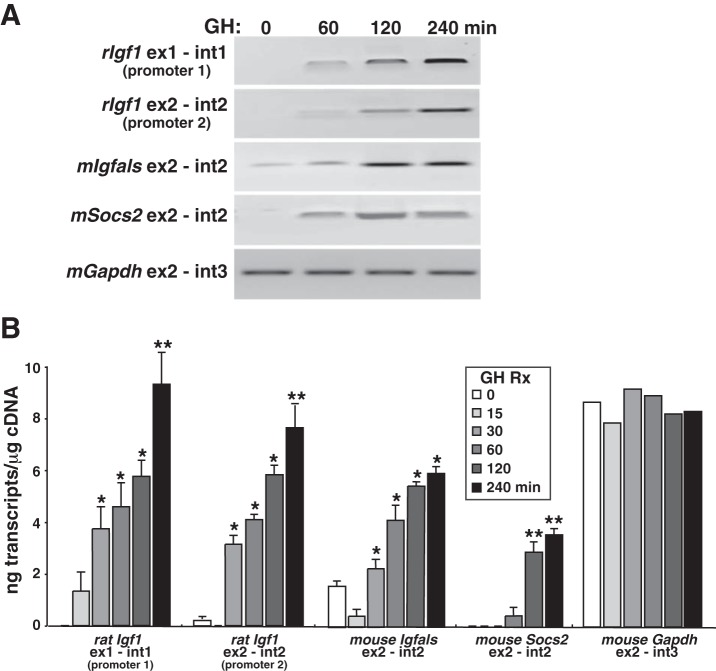
Tandem promoters govern Igf1 gene expression, and each promoter controls a unique 5′-exon (Fig. 1A and Ref. 43). In rodents, promoter 1 is active in all cells in which Igf1 is produced (1, 19, 21), while promoter 2 has a more restricted distribution, directing Igf1 gene transcription in only a few tissues in vivo, including the liver, the organ with the highest level of Igf1 mRNA in vivo (1, 19, 21). As seen in Fig. 3D, Igf1 mRNAs containing each unique 5′-exon were both increased in abundance after exposure to GH. To address the issue of differential promoter usage more directly, we assessed Igf1 gene transcription in response to GH by measuring nascent nuclear transcripts by qRT-PCR with several different exon-intron primer sets at various times after hormone exposure (Fig. 4). Igf1 gene transcription was low in the absence of GH but rose >10-fold within 30 min after hormone addition to cell culture medium (Fig. 4B). Transcriptional stimulation of the Igf1 transgene was comparable to the induction of two endogenous GH- and Stat5b-dependent mouse genes, Igfals and Socs2 (61). At 4 h after GH administration, the level of Igf1 transcription from each promoter was comparable to the rate of transcription of Gapdh, a highly expressed gene that is not regulated by GH (Fig. 4, A and B). Taken together, results in Figs. 3 and 4 show that transcription from both Igf1 promoters is rapidly and robustly induced by GH in transgenic cells and support the idea that we have developed a useful model system to dissect mechanisms of Igf1 gene regulation by GH.
Fig. 4.
Rapid induction of Igf1 gene transcription by GH and Stat5b in a BAC-containing cell line. A: representative experiment showing time course of appearance of nascent transcripts for the rat Igf1 gene and for several mouse genes, as assessed by RT-PCR using nuclear RNA isolated at different times after GH addition to clone 11 cells. B: quantitative analysis of time course of accumulation of nascent transcripts from rat Igf1 promoters 1 and 2 and for mouse Igfals, Socs2, and Gapdh, measured at different times after addition of GH to clone 11 cells, as assessed by qRT-PCR. Primer sets are listed in Table 3 (means ± SD of n = 5 independent experiments; *P < 0.01; **P < 0.001 vs. time 0).
Evaluating potential GH-activated transcriptional enhancers in the rat Igf1 locus.
Using a combination of bioinformatics and biochemical and molecular studies, we previously identified several regions dispersed throughout the rat Igf1 locus that possessed epigenetic properties associated with transcriptional enhancers (13). Each of these seven DNA elements was shown to bind Stat5b in vivo in rat liver chromatin in response to GH-activated signals and also were found to bind transcriptional cofactors, including RNA polymerase II, p300, and the Mediator complex (13). We additionally showed that each element functioned as a GH-activated transcriptional regulator in promoter-reporter assays in cultured cells, although with different potencies, and this activity was dependent on intact Stat5b binding sequences (13, 54).
As all seven putative enhancer elements were present in the rat Igf1 BAC (Fig. 1A), we next examined them for features of GH-regulated enhancers in transgenic cells. We performed a series of qChIP experiments to assess binding of Stat5b, RNA polymerase II, and p300. We found that Stat5b was present at each segment at levels several-fold higher than at other regions that did not contain Stat5 sequences and that at six sites binding was rapidly and potently increased by GH (Fig. 5A). Transcriptional cofactors were detected above background levels at several elements before GH exposure, as found previously in vivo in the liver (13), and in most cases were induced significantly by GH (6/7 for RNA polymerase II, 5/7 for p300) (Fig. 5, B and C). Taken together, these epigenetic data are very similar to observations that we made previously using rat liver chromatin (13) and support the idea that these Stat5b binding elements might be transcriptional enhancers for the rat Igf1 gene.
Fig. 5.
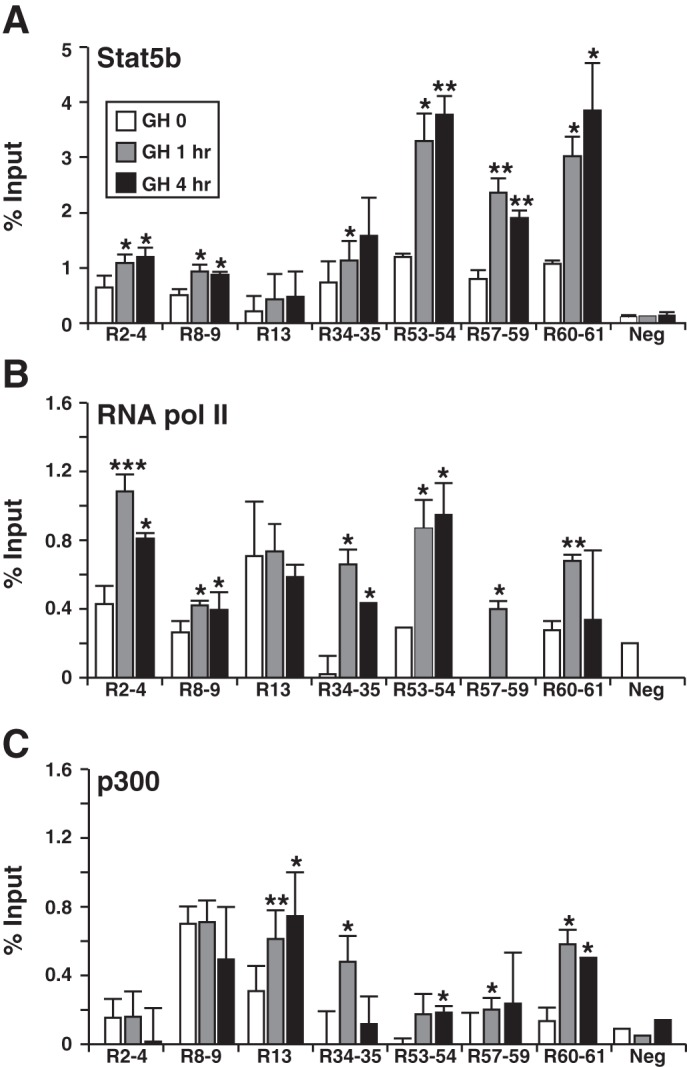
GH induces recruitment of Stat5b, RNA polymerase II, and p300 to potential binding elements in chromatin within the rat Igf1 locus. Results are shown of quantitative chromatin immunoprecipitation experiments using chromatin harvested from rat Igf1-BAC clone 11 after addition to cells of recombinant rat GH [40 nM] for 0, 1 or 4 h. Primer sets are listed in Table 4. All studies were performed by qPCR. A: analysis of binding of Stat5b to the 7 Stat5 binding elements depicted in Fig. 1 plus control primer pairs dispersed throughout the rat Igf1 locus (Neg). B: results of binding of RNA polymerase II (pol II) to the same elements. C: binding of p300 to the same elements. Results reflect the means ± SD of 3–5 independent experiments (*P < 0.05; **P < 0.01; ***P < 0.001 vs. time 0).
Defining enhancer-promoter communication and its regulation by GH.
Enhancers control target gene transcription by transmitting information that stimulates or represses promoter activity (9, 39). This is now thought to occur through direct physical interactions between the enhancer and its cognate promoter (30, 57) and can be demonstrated experimentally by application of the chromatin conformation and capture (3C) assay (16). To determine if individual Stat5b-binding elements in the rat Igf1 locus might function as enhancers, we performed a series of 3C experiments with nuclei isolated prior to or soon after exposure of cells to GH. Nuclei were digested with the restriction endonuclease AflII for these studies, as each Stat5b-binding element and the two Igf1 promoters were flanked by AflII recognition sites (Fig. 6A). We found that 5/7 elements physically associated in chromatin with the DNA fragment containing Igf1 promoters 1 and 2 (3C bait, Fig. 6A). For four elements, R2-4, R13, R34-35, and R60-61, the interaction was induced or was increased after GH treatment, while for R53-54, the association was constant (Fig. 6, B and C). We interpret these results as indicating that at least four of the Stat5b-binding segments in the rat Igf1 locus potentially function as GH-regulated inducible transcriptional enhancers.
Fig. 6.
Identifying GH-induced interactions of Stat5b binding elements with Igf1 gene promoters by chromatin conformation and capture (3C) assay. A: schematic of rat Igf1 BAC CH230-113M6 depicting the ∼80 kb 6-exon Igf1 gene and flanking DNA, locations of 7 putative Stat5b-binding enhancers (circles), and locations of restriction sites for AflII (multiple small vertical lines). A scale bar and the insertion site of the pGK-Neo selection cassette also are illustrated. Below the main map the enlargement depicts the two Igf1 promoters, P1 and P2, and adjacent exons 1–3, and below that the line indicates the 3C “bait” DNA fragment and PCR primers. B: results of 3C experiments measured by semiquantitative PCR for each of the 7 putative Stat5b-binding enhancers studied before and 60 min after addition of recombinant rat GH [40 nM] to cells. The lane marked “BAC” represents a positive control, consisting of an aliquot of BAC DNA that was digested with AflII, and then religated. All positive results were confirmed by DNA sequencing. C: quantitative results of 3C experiments, measured by qPCR for each of the 7 putative Stat5b-binding enhancers studied in B (means ± SD of 3 independent experiments; *P < 0.05 vs. time 0). Primer sets are listed in Table 5 for parts B and C.
DISCUSSION
Genetic studies in humans and mice have determined that the transcription factor Stat5b is a key agent in mediating the GH/IGF-I growth pathway (22, 24, 50, 53), and physiological experiments in rats have established that activated Stat5b can directly and potently stimulate Igf1 gene transcription in the liver (58). Yet the biochemical mechanisms by which GH via Stat5b controls IGF-I gene activity have remained elusive, in large part because of the absence of high-quality experimental systems to study this problem in detail. Here we have developed a GH-stimulated IGF-I gene regulatory system in a recombinant cell line by stably inserting the rat chromosomal Igf1 locus into cultured mouse C3H10T1/2 cells. Transient addition of the GH receptor and Stat5b to these cells leads to robust GH-stimulated induction of endogenous GH- and Stat5b-dependent genes, including mouse Igfals and Socs2, as well as the rat Igf1 transgene, with both the kinetics and magnitude of transcriptional activation being comparable to those observed in vivo in the liver (5, 13, 14). We have used this experimental system to identify GH-stimulated and Stat5b-dependent physical interactions between a cohort of transcriptional enhancers and the Igf1 promoters and suggest that these interactions are responsible for mediating GH-activated Igf1 gene transcription. Our results provide new insights into gene regulation by GH, as they identify the first GH-activated enhancer-promoter communication and present an experimental platform for elucidating how dispersed transcriptional enhancers may control Igf1 gene expression under different physiological and pathological conditions.
The experimental system described here is admittedly artificial and may have been successful in part because of distinctive characteristics of C3H10T1/2 cells, such as their tetraploid chromosomal content (41). Attempts to transduce Cos-7 and HeLa cell lines did not yield clones in which intact BACs could be identified and thus did not produce any lines with GH-activated Igf1 gene expression (data not shown). Another artificial aspect of the studies described here is that the potent effects of GH on rat Igf1 gene transcription may reflect overexpression of the GH receptor and Stat5b, although we did not enhance levels of Jak2, another key component of GH-activated signaling. Nevertheless, our data represent potential new insights into the biology of GH-regulated enhancer function that now may be tested in experimental animals. An analogous approach also may be adapted to the human IGF-I gene, which cannot be studied in vivo.
Our laboratory has used genomic tools previously to identify and characterize a cohort of seven distinct GH-regulated Stat5b binding domains in hepatic chromatin within the rat Igf1 locus (13) and to show by biochemical assays that they are each functional, at least in the context of plasmid-based promoter-reporter assays in transiently transfected cultured cells (13, 54). Analogous regions have been identified near the mouse Igf1 gene (17, 26) and to a far more limited extent near human IGF-I (55). The most remarkable feature of these chromosomal regions, perhaps besides their dynamic plasticity in chromatin, is that there are so many of them. Although there are other examples in which several enhancers that bind the same transcription factor are associated with a single target gene, in few cases are these enhancers as prevalent or as dispersed as seen in the Igf1 locus. For example, the mouse Rankl gene encodes some distal elements that bind c-fos (6), the β-hemoglobin locus contains sites for GATA transcription factors (35), and the GH locus, sequences for Pou1f1 (47), but these potential enhancers are fewer in number than the Stat5b elements in Igf1. As there appear to be no other genes within ∼150 kb of rat Igf1 on chromosome 7q22 or within ∼200 kb of human IGF-I on chromosome 12q23.2 (Ensemble release 78, December 2014), it seems reasonable to assume that these elements are involved primarily with Igf1 [although ENCODE data for several human cell lines indicate that only some identified interactions between distal elements and promoters map to the nearest gene from the element (46)]. Even though all seven of the Stat5b-binding regions possess some epigenetic properties of transcriptional enhancers (Fig. 5 and Ref. 13), our observations now show that they are not all equivalent. As our novel 3C results demonstrate, only five of the seven segments interact with the two Igf1 promoters, and of these, four show GH-inducible association, while the other one binds constitutively (Fig. 6).
As noted earlier, both GH and IGF-I are pivotal agents in human physiology and disease, as they have positive biological effects on somatic growth and tissue repair (8, 27, 29, 34) but also in a negative way can drive aging and carcinogenesis (4, 40, 59). In both contexts, perhaps some of the GH-regulated Stat5b elements within the IGF-I locus identified here may act as “decoy enhancers” to limit access of hormone-stimulated Stat5b to the more functional promoter-interacting elements, thus reducing the magnitude or duration of GH-stimulated IGF-I gene transcription. Alternatively, as our 3C results represent the outcome of exposure of cells to a brief pulse of a midphysiological dose of GH (48), perhaps the other elements become functional enhancers at higher levels of GH or after more prolonged exposure to hormone-activated signaling. More broadly, perhaps each individual Stat5b-binding domain in the Igf1 locus differs in its activation dynamics or kinetics and/or responds independently to varying hormone doses or exposures, but collectively the cohort of elements function as a high-content GH dose-dependent rheostat. We can now test these and other hypotheses in our experimental system and subsequently in animal models and begin to elucidate the complex regulatory mechanisms by which GH or other factors (20, 32, 33, 36, 44, 51) control IGF-I gene expression.
GRANTS
Support for the studies described in this manuscript is from NIDDK Grant R01 DK-069703-08 (to P. Rotwein).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: D.A., A.M., and P.R. conception and design of research; D.A. and A.M. performed experiments; D.A., A.M., and P.R. analyzed data; D.A., A.M., and P.R. interpreted results of experiments; D.A. and A.M. edited and revised manuscript; P.R. prepared figures; P.R. drafted manuscript; P.R. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank the OHSU DNA Services Core for performing DNA sequencing.
REFERENCES
- 1.Adamo ML, Ben-Hur H, Roberts CT Jr, LeRoith D. Regulation of start site usage in the leader exons of the rat insulin-like growth factor-I gene by development, fasting, and diabetes. Mol Endocrinol 5: 1677–1686, 1991. [DOI] [PubMed] [Google Scholar]
- 2.Alzhanov DT, McInerney SF, Rotwein P. Long-range interactions regulate Igf2 gene transcription during skeletal muscle differentiation. J Biol Chem 285: 38969–38977, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Benbassat C, Shoba LN, Newman M, Adamo ML, Frank SJ, Lowe WLJ. Growth hormone-mediated regulation of insulin-like growth factor I promoter activity in C6 glioma cells. Endocrinology 140: 3073–3081, 1999. [DOI] [PubMed] [Google Scholar]
- 4.Berryman DE, Christiansen JS, Johannsson G, Thorner MO, Kopchick JJ. Role of the GH/IGF-1 axis in lifespan and healthspan: lessons from animal models. Growth Horm IGF Res 18: 455–471, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bichell DP, Kikuchi K, Rotwein P. Growth hormone rapidly activates insulin-like growth factor I gene transcription in vivo. Mol Endocrinol 6: 1899–1908, 1992. [DOI] [PubMed] [Google Scholar]
- 6.Bishop KA, Coy HM, Nerenz RD, Meyer MB, Pike JW. Mouse Rankl expression is regulated in T cells by c-Fos through a cluster of distal regulatory enhancers designated the T cell control region. J Biol Chem 286: 20880–20891, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brooks AJ, Dai W, O'Mara ML, Abankwa D, Chhabra Y, Pelekanos RA, Gardon O, Tunny KA, Blucher KM, Morton CJ, Parker MW, Sierecki E, Gambin Y, Gomez GA, Alexandrov K, Wilson IA, Doxastakis M, Mark AE, Waters MJ. Mechanism of activation of protein kinase JAK2 by the growth hormone receptor. Science 344: 1249783, 2014. [DOI] [PubMed] [Google Scholar]
- 8.Brooks AJ, Waters MJ. The growth hormone receptor: mechanism of activation and clinical implications. Nat Rev Endocrinol 6: 515–525, 2010. [DOI] [PubMed] [Google Scholar]
- 9.Calo E, Wysocka J. Modification of enhancer chromatin: what, how, and why? Mol Cell 49: 825–837, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chia DJ, Ono M, Woelfle J, Schlesinger-Massart M, Jiang H, Rotwein P. Characterization of distinct Stat5b binding sites that mediate growth hormone-stimulated IGF-I gene transcription. J Biol Chem 281: 3190–3197, 2006. [DOI] [PubMed] [Google Scholar]
- 11.Chia DJ. Minireview: mechanisms of growth hormone-mediated gene regulation. Mol Endocrinol 28: 1012–1025, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chia DJ, Rotwein P. Defining the epigenetic actions of growth hormone: acute chromatin changes accompany GH-activated gene transcription. Mol Endocrinol 24: 2038–2049, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chia DJ, Varco-Merth B, Rotwein P. Dispersed chromosomal Stat5b-binding elements mediate growth hormone-activated insulin-like growth factor-I gene transcription. J Biol Chem 285: 17636–17647, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chia DJ, Young JJ, Mertens AR, Rotwein P. Distinct alterations in chromatin organization of the two IGF-I promoters precede growth hormone-induced activation of IGF-I gene transcription. Mol Endocrinol 24: 779–789, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.D'haene B, Vandesompele J, Hellemans J. Accurate and objective copy number profiling using real-time quantitative PCR. Methods 50: 262–270, 2010. [DOI] [PubMed] [Google Scholar]
- 16.Dekker J. The three ‘C’ s of chromosome conformation capture: controls, controls, controls. Nat Methods 3: 17–21, 2006. [DOI] [PubMed] [Google Scholar]
- 17.Eleswarapu S, Gu Z, Jiang H. Growth hormone regulation of insulin-like growth factor-I gene expression may be mediated by multiple distal signal transducer and activator of transcription 5 binding sites. Endocrinology 149: 2230–2240, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Feigerlova E, Hwa V, Derr MA, Rosenfeld RG. Current issues on molecular diagnosis of GH signaling defects. Endocr Dev 24: 118–127, 2013. [DOI] [PubMed] [Google Scholar]
- 19.Hall LJ, Kajimoto Y, Bichell D, Kim SW, James PL, Counts D, Nixon LJ, Tobin G, Rotwein P. Functional analysis of the rat insulin-like growth factor I gene and identification of an IGF-I gene promoter. DNA Cell Biol 11: 301–313, 1992. [DOI] [PubMed] [Google Scholar]
- 20.Hewitt SC, Li Y, Li L, Korach KS. Estrogen-mediated regulation of Igf1 transcription and uterine growth involves direct binding of estrogen receptor alpha to estrogen-responsive elements. J Biol Chem 285: 2676–2685, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hoyt EC, Van Wyk JJ, Lund PK. Tissue and development specific regulation of a complex family of rat insulin-like growth factor I messenger ribonucleic acids. Mol Endocrinol 2: 1077–1086, 1988. [DOI] [PubMed] [Google Scholar]
- 22.Hwa V, Nadeau K, Wit JM, Rosenfeld RG. STAT5b deficiency: lessons from STAT5b gene mutations. Best Pract Res Clin Endocrinol Metab 25: 61–75, 2011. [DOI] [PubMed] [Google Scholar]
- 23.Joshi M, Keith Pittman H, Haisch C, Verbanac K. Real-time PCR to determine transgene copy number and to quantitate the biolocalization of adoptively transferred cells from EGFP-transgenic mice. Biotechniques 45: 247–258, 2008. [DOI] [PubMed] [Google Scholar]
- 24.Kofoed EM, Hwa V, Little B, Woods KA, Buckway CK, Tsubaki J, Pratt KL, Bezrodnik L, Jasper H, Tepper A, Heinrich JJ, Rosenfeld RG. Growth hormone insensitivity associated with a STAT5b mutation. N Engl J Med 349: 1139–1147, 2003. [DOI] [PubMed] [Google Scholar]
- 25.Lanning NJ, Carter-Su C. Recent advances in growth hormone signaling. Rev Endocr Metab Disord 7: 225–235, 2006. [DOI] [PubMed] [Google Scholar]
- 26.Laz EV, Sugathan A, Waxman DJ. Dynamic in vivo binding of STAT5 to growth hormone-regulated genes in intact rat liver. Sex-specific binding at low- but not high-affinity STAT5 sites. Mol Endocrinol 23: 1242–1254, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Le Roith D, Bondy C, Yakar S, Liu JL, Butler A. The somatomedin hypothesis: 2001. Endocr Rev 22: 53–74, 2001. [DOI] [PubMed] [Google Scholar]
- 28.Lee EC, Yu D, Martinez de Velasco J, Tessarollo L, Swing DA, Court DL, Jenkins NA, Copeland NG. A highly efficient Escherichia coli-based chromosome engineering system adapted for recombinogenic targeting and subcloning of BAC DNA. Genomics 73: 56–65, 2001. [DOI] [PubMed] [Google Scholar]
- 29.LeRoith D. Clinical relevance of systemic and local IGF-I: lessons from animal models. Pediatr Endocrinol Rev 5, Suppl 2: 739–743, 2008. [PubMed] [Google Scholar]
- 30.Levine M, Cattoglio C, Tjian R. Looping back to leap forward: transcription enters a new era. Cell 157: 13–25, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ling JQ, Li T, Hu JF, Vu TH, Chen HL, Qiu XW, Cherry AM, Hoffman AR. CTCF mediates interchromosomal colocalization between Igf2/H19 and Wsb1/Nf1. Science 312: 269–272, 2006. [DOI] [PubMed] [Google Scholar]
- 32.Mak RH, Rotwein P. Myostatin and insulin-like growth factors in uremic sarcopenia: the yin and yang in muscle mass regulation. Kidney Int 70: 410–412, 2006. [DOI] [PubMed] [Google Scholar]
- 33.Matheny RWJ, Nindl BC, Adamo ML. Minireview: Mechano-growth factor: a putative product of IGF-I gene expression involved in tissue repair and regeneration. Endocrinology 151: 865–875, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mauras N. Can growth hormone counteract the catabolic effects of steroids? Horm Res 72, Suppl 1: 48–54, 2009. [DOI] [PubMed] [Google Scholar]
- 35.Miccio A, Blobel GA. Role of the GATA-1/FOG-1/NuRD pathway in the expression of human beta-like globin genes. Mol Cell Biol 30: 3460–3470, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Moyano P, Rotwein P. Mini-review: estrogen action in the uterus and insulin-like growth factor-I. Growth Horm IGF Res 14: 431–435, 2004. [DOI] [PubMed] [Google Scholar]
- 37.Mukherjee A, Rotwein P. Akt promotes BMP2-mediated osteoblast differentiation and bone development. J Cell Sci 122: 716–726, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mukherjee A, Wilson EM, Rotwein P. Insulin-like growth factor (IGF) binding protein-5 blocks skeletal muscle differentiation by inhibiting IGF actions. Mol Endocrinol 22: 206–215, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Plank JL, Dean A. Enhancer function: mechanistic and genome-wide insights come together. Mol Cell 55: 5–14, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pollak M. The insulin and insulin-like growth factor receptor family in neoplasia: an update. Nat Rev Cancer 12: 159–169, 2012. [DOI] [PubMed] [Google Scholar]
- 41.Reznikoff CA, Brankow DW, Heidelberger C. Establishment and characterization of a cloned line of C3H mouse embryo cells sensitive to postconfluence inhibition of division. Cancer Res 33: 3231–3238, 1973. [PubMed] [Google Scholar]
- 42.Rosenfeld RG, Hwa V. The growth hormone cascade and its role in mammalian growth. Horm Res 71, Suppl 2: 36–40, 2009. [DOI] [PubMed] [Google Scholar]
- 43.Rotwein P. Mapping the growth hormone–Stat5b–IGF-I transcriptional circuit. Trends Endocrinol Metab 23: 186–193, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rotwein P, Billiard J, Woelfle J. Molecular physiology of IGF-I expression. J Pediatr Endocrinol Metab 15, Suppl 5: 1455–1458, 2002. [PubMed] [Google Scholar]
- 45.Sadowski CL, Wheeler TT, Wang LH, Sadowski HB. GH regulation of IGF-I and suppressor of cytokine signaling gene expression in C2C12 skeletal muscle cells. Endocrinology 142: 3890–3900, 2001. [DOI] [PubMed] [Google Scholar]
- 46.Sanyal A, Lajoie BR, Jain G, Dekker J. The long-range interaction landscape of gene promoters. Nature 489: 109–113, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shewchuk BM, Liebhaber SA, Cooke NE. Specification of unique Pit-1 activity in the hGH locus control region. Proc Natl Acad Sci USA 99: 11784–11789, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tannenbaum GS, Martin JB. Evidence for an endogenous ultradian rhythm governing growth hormone secretion in the rat. Endocrinology 98: 562–570, 1976. [DOI] [PubMed] [Google Scholar]
- 49.Tatar M, Bartke A, Antebi A. The endocrine regulation of aging by insulin-like signals. Science 299: 1346–1351, 2003. [DOI] [PubMed] [Google Scholar]
- 50.Teglund S, McKay C, Schuetz E, van Deursen JM, Stravopodis D, Wang D, Brown M, Bodner S, Grosveld G, Ihle JN. Stat5a and Stat5b proteins have essential and nonessential, or redundant, roles in cytokine responses. Cell 93: 841–850, 1998. [DOI] [PubMed] [Google Scholar]
- 51.Thissen JP, Underwood LE, Ketelslegers JM. Regulation of insulin-like growth factor-I in starvation and injury. Nutr Rev 57: 167–176, 1999. [DOI] [PubMed] [Google Scholar]
- 52.Tronche F, Opherk C, Moriggl R, Kellendonk C, Reimann A, Schwake L, Reichardt HM, Stangl K, Gau D, Hoeflich A, Beug H, Schmid W, Schutz G. Glucocorticoid receptor function in hepatocytes is essential to promote postnatal body growth. Genes Dev 18: 492–497, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Udy GB, Towers RP, Snell RG, Wilkins RJ, Park SH, Ram PA, Waxman DJ, Davey HW. Requirement of STAT5b for sexual dimorphism of body growth rates and liver gene expression. Proc Natl Acad Sci USA 94: 7239–7244, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Varco-Merth B, Mirza K, Alzhanov DT, Chia DJ, Rotwein P. Biochemical characterization of diverse Stat5b-binding enhancers that mediate growth hormone-activated insulin-like growth factor-I gene transcription. PLoS One 7: e50278, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wang Y, Jiang H. Identification of a distal STAT5-binding DNA region that may mediate growth hormone regulation of insulin-like growth factor-I gene expression. J Biol Chem 280: 10955–10963, 2005. [DOI] [PubMed] [Google Scholar]
- 56.Waters MJ, Hoang HN, Fairlie DP, Pelekanos RA, Brown RJ. New insights into growth hormone action. J Mol Endocrinol 36: 1–7, 2006. [DOI] [PubMed] [Google Scholar]
- 57.Wei Z, Huang D, Gao F, Chang WH, An W, Coetzee GA, Wang K, Lu W. Biological implications and regulatory mechanisms of long-range chromosomal interactions. J Biol Chem 288: 22369–22377, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Woelfle J, Billiard J, Rotwein P. Acute control of insulin-like growth factor-I gene transcription by growth hormone through Stat5b. J Biol Chem 278: 22696–22702, 2003. [DOI] [PubMed] [Google Scholar]
- 59.Woelfle J, Chia DJ, Massart-Schlesinger MB, Moyano P, Rotwein P. Molecular physiology, pathology, and regulation of the growth hormone/insulin-like growth factor-I system. Pediatr Nephrol 20: 295–302, 2005. [DOI] [PubMed] [Google Scholar]
- 60.Woelfle J, Chia DJ, Rotwein P. Mechanisms of growth hormone (GH) action. Identification of conserved Stat5 binding sites that mediate GH-induced insulin-like growth factor-I gene activation. J Biol Chem 278: 51261–51266, 2003. [DOI] [PubMed] [Google Scholar]
- 61.Woelfle J, Rotwein P. In vivo regulation of growth hormone-stimulated gene transcription by STAT5b. Am J Physiol Endocrinol Metab 286: E393–E401, 2004. [DOI] [PubMed] [Google Scholar]
- 62.Zhang Y, Muyrers JP, Testa G, Stewart AF. DNA cloning by homologous recombination in Escherichia coli. Nat Biotechnol 18: 1314–1317, 2000. [DOI] [PubMed] [Google Scholar]