Abstract
Despite the application of aggressive surgery, radiotherapy and chemotherapy in clinics, brain tumors are still a difficult health challenge due to their fast development and poor prognosis. Brain tumor-targeted drug delivery systems, which increase drug accumulation in the tumor region and reduce toxicity in normal brain and peripheral tissue, are a promising new approach to brain tumor treatments. Since brain tumors exhibit many distinctive characteristics relative to tumors growing in peripheral tissues, potential targets based on continuously changing vascular characteristics and the microenvironment can be utilized to facilitate effective brain tumor-targeted drug delivery. In this review, we briefly describe the physiological characteristics of brain tumors, including blood–brain/brain tumor barriers, the tumor microenvironment, and tumor stem cells. We also review targeted delivery strategies and introduce a systematic targeted drug delivery strategy to overcome the challenges.
Key words: Barriers targeting, Tumor microenvironment, Tumor cells, Systematic targeted drug delivery
Graphical abstract
In this review, we briefly describe physiological and pathological conditions of brain tumors, including blood–brain/brain tumor barriers, tumor microenvironment and tumor stem cells; we review the corresponding targeting delivery strategies and introduce a systematic targeted drug delivery strategy to overcome current challenges.
1. Introduction
According to the GLOBOCAN 2008, the worldwide cancer incidence of malignant brain tumors is 3.5 per 100,000 people and about 650 people are diagnosed with malignant brain tumors every day1. Brain tumors severely threaten human health due to their fast development and poor prognosis. Glioma, the most frequent primary brain cancer, accounts for 29% of all primary brain and CNS tumors and 80% of malignant brain tumors2. The median overall survival of patients with glioblastoma (GBM) is only 14.6 months after current multimodal treatment–aggressive surgical resection followed by concurrent or sequential radiation and temozolomide chemotherapies3. One reason why poor prognosis and rapid recurrence are associated with this standard therapy is that the infiltrate growth of gliomas makes it difficult for the surgeon to completely remove pathologic or cancer-infiltrated tissues without affecting normal brain functions4. Furthermore, the failure is also ascribed to the side effects of radiotherapy and poor outcome of usual chemotherapy. For many years, researchers have endeavored to deliver therapeutic agents to the tumor region effectively and reduce unnecessary drug accumulation in normal brain and peripheral tissues. For brain tumors, active targeted drug delivery systems have attracted extensive attention in recent decades. Since brain tumors possess many distinctive characteristics from peripheral tumors due to their complicated oncogenesis, many factors must be taken into consideration for effective brain tumor-targeted drug delivery, such as the barriers included in the whole process, the tumor microenvironment, and tumor cells. Now various targets have been exploited to achieve the targeting therapy using nanocarriers. Herein we provide a brief review of several possible targeting delivery strategies for brain tumors.
2. Barriers to targeted drug delivery strategies
The oncogenesis of gliomas is complicated, with various barriers preventing drug from reaching the tumor sites. There are three main barriers for brain tumor treatment: the blood–brain barrier (BBB), the blood–brain tumor barrier (BBTB), and a relatively weak EPR effect. Specific brain tumor development stages require corresponding barrier targeting treatment strategies.
2.1. BBB targeting strategies and related drug delivery systems
At the early stage of brain tumor development and at the infiltration growth region of the tumor, the blood–brain barrier remains intact. The blood–brain barrier, which acts as a natural guard to protect the brain from harmful substances in the blood stream while supplying the brain with the necessary nutrients for proper function, is the key challenge for delivering drugs to brain tumor5. The BBB is a specialized system of capillary endothelial cells which are partially covered by pericytes and basement membrane, and almost fully surrounded by the end feet of astrocytes, preventing approximately 98% of the small molecules and nearly 100% of large molecules including recombinant proteins and genes from being transported into the brain and reaching the tumor sites6, 7. The BBB strictly limits drug transport into the brain by serving as a physical (tight junctions), metabolic (enzymes) and immunological barrier8.
To tackle this challenge, many kinds of active targeting strategies were adopted for developing effective drug delivery systems to the brain. The active targeting systems are mainly divided into absorptive-mediated transcytosis (AMT), transporter-mediated transcytosis, and receptor-mediated endocytosis (RMT)8.
2.1.1. Absorptive-mediated transcytosis
Absorptive-mediated transcytosis provides a means for the delivery of drugs across the BBB by cationic proteins or cell-penetrating peptides (CPPs). It is triggered by electrostatic interactions between the positively charged moieties of the proteins and negatively charged membrane surface regions on the brain endothelial cells9. Typical cationic bovine serum albumin-conjugated, pegylated nanoparticles (CBSA-NP) were prepared by Lu et al.10 for brain targeting. They demonstrated that the permeability of CBSA-NP was about 7.76 times higher than that of BSA-NP, which offered the possibility of delivering therapeutic agents to CNS. It was reported that plasmid pORF-hTRAIL (pDNA)-incorporated CBSA-NP (CBSA-NP-hTRAIL) colocalized with glycoproteins in brain and tumor microvasculature and accumulated in tumor cells at 30 min after i.v. administration to C6 glioma bearing nude mice, via absorptive-mediated transcytosis11. Aclarubicin (ACL)-loaded cationic albumin-conjugated pegylated nanoparticles (CBSA-NP-ACL) could significantly prolong the survival of the intracranial glioblastoma-bearing mice12. Du et al.13 adopted another cationic protein, wheat germ agglutinin (WGA) conjugated to the surface of liposomes and also demonstrated enhanced BBB transport.
Furthermore, alternative AMT-type cell-penetrating peptide (CPP)-based delivery systems show great ability in BBB transport. CPPs have been used to overcome the lipophilic barrier of cellular membranes and deliver a large variety of cargoes, including peptide/proteins, DNA/oligonucleotide, antibodies, imaging agents, toxins, and nanodrug carriers such as liposomes and micelles14. CPPs are heterogeneous in size and sequence and are positively charged. Some share common features such as an amphipathic sequence and the ability to interact with lipid membranes. The CPPs are always derived from natural proteins including the transcription-activating factor Tat, penetratin, and the Syn-B vectors, among which Tat might be the most frequently used15. Qin et al.16 covalently conjugated cell-penetrating peptide TAT (AYGRKKRRQRRR) to cholesterol for preparing doxorubicin-loaded liposomes for glioma therapy. The biodistribution in the brain and heart demonstrated higher efficiency of brain delivery and lower cardiotoxicity. The survival time of the glioma-bearing rats treated with TAT-modified liposomes was significant prolonged16. Moreover, self-assembled polymeric micelles modified with transcriptional activator TAT peptide (TAT-PEG-b-Col) were constructed by Liu et al.17 to successfully deliver antibiotics across the BBB. These studies show the potential of Tat-modified nanoparticle transport into the brain for the diagnosis and treatment of brain diseases18.
2.1.2. Transporter mediated transcytosis
Since there are many kinds of transport systems in the cerebral endothelium that provide the brain with the necessary nutrients and endogenous substances, transporter mediated transcytosis takes advantage of these transport systems as a promising brain targeting strategy. Transporter mediated transcytosis is substrate-selective, so only drugs that closely mimic the endogenous substrates will be taken up and transported into the brain6.
Glucose transporters (GLUT), which facilitate the transport of glucose from the blood to the brain, have a broad prospective use in brain targeting. Liposomes that incorporated a mannose derivative were able to cross the BBB via the glucose transporter GLUT1 in mouse brain19. Qin et al.20 synthesized a new glycosyl derivative of cholesterol as a material for preparing novel liposomes to overcome the ineffective delivery of normal drug formulations to brain by targeting the glucose transporters on the BBB. Pharmacokinetic and distribution experiments demonstrated that this novel brain drug delivery system possessed good brain targeting ability. Another important transport system is the choline transporter which binds positively charged quaternary ammonium groups or simple cations21. Fenart et al.22 examined the ability of 60 nm nanoparticles coated with quaternary ammonium ligands to cross a cell culture model of the blood–brain barrier (BBB) formed by a coculture of bovine brain capillary endothelial cells and rat astrocytes. The coated nanoparticles were able to cross an in vitro model of the BBB (bovine BCEC). Their passage through the endothelial cell monolayer was three- or four-fold higher than that of uncoated nanoparticles, without any modification of paracellular permeability, suggesting that the enhancement was mediated by the BBB choline transporter.
2.1.3. Receptor mediated transcytosis
Generally, for absorptive-mediated transcytosis the specificity of this BBB targeting strategy is poor since the cationic proteins or CPPs can bind with any negatively charged cell membrane constituents. Moreover, the potential toxicity and immunogenicity of cationic proteins or CPPs limit their use. The transporter-mediated transcytosis is substrate selective, in that only drugs that closely mimic the endogenous carrier substrates will be taken up and transported into the brain. Receptor-mediated transcytosis is considered one of the most mature strategies for brain targeted drug delivery with the characteristics of high specificity, selectivity and affinity, although the ligand may have an effect on homeostasis and natural ligands may compete with the drug ligand to reduce targeting efficiency. Since many kinds of receptors are expressed on the capillary endothelium of the brain, such as transferrin receptor (TfR), the low density lipoprotein receptor (LDLR), the insulin receptor and nicotinic acetylcholine receptors, targeting ligands, including endogenous ligands and ligands based on phage display or structure-guided design, have been exploited to facilitate receptor-mediated BBB transport of drug delivery systems.
One of the most widely characterized receptor-mediated transcytosis systems for brain targeting is the transferrin receptor (TfR), which is highly expressed on endothelial cells of the BBB23. A transferrin-conjugated drug delivery system for BBB delivery has been developed. Zhang et al.24 prepared transferrin-modified paclitaxel-loaded polyphosphoester hybrid micells (TPM), and in vitro and in vivo brain-targeting efficiencies were evaluated. It was demonstrated that TPM exhibited stronger anti-glioma activity and the mean survival time of mice bearing intracranial U87 MG glioma was significantly prolonged. However, Tf is likely not an ideal brain delivery ligand since the Tf-modified targeted drug delivery system would have to compete with the natural ligand25. As an alternative, a mouse monoclonal antibody (MAb) against the rat TfR, OX26, has been extensively studied. When OX26 is coupled with the liposomes, transferrin receptor-mediated targeting of daunomycin to the rat brain was achieved by using an immunoliposome-based drug delivery system26.
Another common receptor, the low-density lipoprotein (LDL) receptor-related protein (LRP), has been reported to mediate transport of various ligands conjugated to nanocarriers across the BBB. Aprotinin is a LRP ligand and its BBB transport ability was evaluated using an in vitro model of the BBB and in situ brain perfusion. Its transcytosis across bovine brain capillary endothelial cell monolayers was at least 10-fold greater than that of holo-transferrin27. Angiopep, derived from aprotinin with the Kunitz domains of human proteins, exhibited even higher transcytosis capacity and parenchymal accumulation. Sun et al.28 reported angiopep-2 modified cationic liposomes for the efficient co-delivery of a therapeutic gene with paclitaxel to the brain. After treatment with liposomes, the median survival time of brain tumor-bearing mice was significantly longer than that of other groups, making it a promising drug delivery strategy against glioma.
Nicotinic acetylcholine receptors (nAChRs) are a kind of ligand-gated ion channel that is widely expressed in the brain including the brain capillary endothelial cells29. Since they bind the second loop of the three-finger snake toxin with high affinity and selectivity, nAChRs could be exploited to facilitate BBB crossing and intracranial transport of drug delivery systems. Kumar et al.30 reported that a 29-amino acid peptide derived from rabies virus glycoprotein-RVG29 enabled transvascular siRNA delivery to the brain via nAChRs and provided a safe and noninvasive approach for the delivery of therapeutic agents across the BBB. Therefore, nAChR-mediated brain targeting may be a promising strategy for the intracranial transport of drug delivery systems. It was previously reported that a 16 amino acid peptide CDX, which was derived from the loop II region of the snake neurotoxin candoxin, exhibited high binding affinity to nicotine acetylcholine receptors. It was shown that this peptide enabled brain-targeted drug delivery when conjugated to micelles and significantly prolonged the survival time of intracranial glioblastoma-bearing mice31.
2.2. BBTB targeting strategies and related drug delivery systems
The blood–brain tumor barrier (BBTB), similar to blood–brain barrier (BBB), is located between brain tumor tissues and microvessels formed by highly specialized endothelial cells (ECs), limiting the paracellular delivery of most hydrophilic molecules to tumor tissue32. With the development of brain tumors, tumor cells begin to invade the surrounding normal brain tissue. Only when the tumor cell clusters grow to a certain volume will BBB be damaged and BBTB be formed. The blood–tumor barrier (BTB) of malignant solid tumors growing in peripheral tissues is typically more permeable than the BBTB of similar malignant solid tumors growing in the brain33, 34. The physiologic upper limit of pore size in the BTB of malignant solid tumor-orthotopic RG-2 rat gliomas microvasculature is approximately 12 nm35.
With the deterioration of brain tumors, angiogenesis and gradual impairment of BBB, BBTB becomes the main obstacle of drug delivery nanosystems. At this stage, tumor neovasculature has formed to support the growth of gliomas. The abnormality of microvessels enhances the permeability of BBTB, whereas the cranial microenvironment and/or the gliomas specificity make malignant gliomas less permeable36, 37. Even though the BBB is compromised in malignant gliomas, the permeability differs from other regions. The infiltrating gliomas, especially around the tumor edge, still utilize the available brain vasculature and the BBB still limits glioma-targeted transport of chemotherapeutic agents37. Hence, the receptors present on the BBB/BBTB provide a chance for gliomas-targeted drug delivery at this stage. The strategies proposed for BBTB targeting are mainly based on receptors high expressed on the tumor such as epidermal growth factor receptors and integrin.
The adhesion receptor integrin, which is overexpressed on tumor neovasculture and glioblastoma U87 cells, was identified as a marker of angiogenic vascular tissue38, 39, 40. Particularly, integrin αvβ3 expression is prominent in malignant glioma but not overexpressed on normal brain cells. As the ligands for integrin, cyclic arginine–glycine–aspartic acid (RGD) peptide and its analogs have been widely investigated for the glioma-targeted drug delivery. Herein, the integrin–RGD interaction still provides a promising BBTB-targeted drug delivery strategy. Our group has designed c(RGDyK)-modified polyethylene glycol–polyethylenimine (PEG–PEI) nanoparticles for intracranial glioblastoma-targeted gene delivery. They exhibited high binding affinity with U87 cells and facilitated targeted gene delivery against intracranial glioblastoma in vivo compared to the PEG–PEI gene carrier without RGD modification. It was demonstrated that the therapeutic efficacy of pORF-hTRAIL of this gene carrier was also enhanced as evidenced by a significantly prolonged survival of intracranial glioblastoma-bearing nude mice. These results demonstrated the therapeutic feasibility of this gene delivery system for brain glioblastoma treatment through mediation of integrin αvβ341. Zhan et al.42 reported a cyclic RGD peptide conjugated PEG–PLA micelle for the intracranial gliomas targeted chemotherapy, and the median survival time of mice bearing intracranial U87MG tumor xenografts was significantly prolonged after treated by c(RGDyK)–PEG–PLA–PTX micelles, suggesting that RGD motif is efficient for targeted drug delivery to integrin αvβ3-overexpressed glioblastoma.
EGFR, a member of ErbB family of receptors, is a transmembrane tyrosine kinase normally expressed in epithelial, mesenchymal, and neuronal tissues43, 44. Overexpression of the epidermal growth factor receptor (EGFR) on the BBTB makes it a promising target for therapy45. EGF and anti-EGFR monoclonal antibody are the commonly used EGFR ligands mediating glioma-targeted therapy. Fondell et al.46 adopted epidermal growth factor to target the EGFR and developed a new two-step targeting strategy to transport a recently synthesized daunorubicin derivative into cancer cell nuclei using PEG-stabilized targeting liposomes named “Nuclisome-particles”. Tsutsui et al.47 constructed a novel drug delivery system using hybrid bionanocapsules (BNCs) conjugated with anti-human EGFR antibody and confirmed the specific delivery of these BNCs to the brain tumor in the in vivo brain tumor model. Anti-human EGFR antibody-conjugated immunoliposomes also were prepared by Feng et al.48 and successfully delivered sodium borocaptate into EGFR-overexpressing gliomas in an animal model of brain tumors.
2.3. EPR effect-based strategies and related drug delivery systems
As the brain tumor develops, the enhanced permeability and retention effect (EPR effect) appears even though it is significantly weaker in the cranial microenvironment than in peripheral tumors, which enables nanosystems with suitable particle size to enter the brain tumor via endothelial gaps on the microvessels of brain tumors42, 49, 50. Some of the nanoscale drug delivery systems have been developed to make use of the glioma EPR effect for tumor targeting. Huang et al.51 have developed a tumor-targeting nanoparticle system which possesses passive tumor target ability via the enhanced permeability and retention effect. This system was able to prolong the survival time of intracranial U87MG tumor-bearing nude mice.
3. Tumor microenvironment
Studies in developmental biology and tumor progression have demonstrated that the extracellular microenvironment is not simply a passive structural element in which cells reside, but is an interactive partner that can be altered by and can alter cellular processes and responses52.
3.1. Lowered extracellular pH
Lowered extracellular pH (pHe) is a common characteristic of various solid tumors, including brain tumors, which is caused by the high rate of glycolysis in cancer cells, under both aerobic and anaerobic conditions53, 54. It has been reported that the tumor pHe is around 5.7–7.2 depending upon tumor histology, tumor volume, and tumor location, and is lower than normal tissue55, 56. Low extracellular pH may be an important factor inducing more aggressive cancer phenotypes. During recent decades many approaches have been developed to target various tumors by pHe, including a shielding/deshielding mechanism or a pH-triggered drug release mechanism. Huang and coworkers have developed a tumor targeting nanoparticle system (called dtACCP) that responds to the lowered tumor pHe and unregulated matrix metalloproteinase 2 (MMP2) expression in the tumor microenvironment. The nanoparticles are modified with an activatable cell-penetrating peptide that is quenched by a pH-sensitive masking peptide, linked by a MMP2 substrate. Once the modified nanoparticles reach the glioblastoma microenvironment, the negative charge of the masking peptide will be eliminated due to the lowered pHe, while the linker is cleaved by MMP2, exposing CPP to drive the nanoparticle׳s internalization into the tumor cells51, 57. This shielding/deshielding mechanism makes it feasible to develop a lowered tumor pHe-triggered drug delivery system, increasing target drug accumulation at the tumor site and lowering the cytotoxicity to the normal tissue.
3.2. Angiogenesis
With the deterioration of brain tumors, angiogenesis occurs with new vessels developing from pre-existing ones. Angiogenesis is essential for glioma tumor growth and metastasis. The angiogenesis targeting delivery system is capable of specifically delivering anti-angiogenic therapeutics to the tumor neovasculature while minimizing systematic toxicity. Therefore, receptors highly expressed during tumor angiogenesis, such as epidermal growth factor receptors and integrin, provide tumor-targeted drug delivery strategies as mentioned in the BBTB targeting strategies section. Currently, antiangiogenic therapy has been restricted to targeting endothelial cells. It was shown that endothelial cells–pericytes signaling networks could also contribute to tumor angiogenesis and metastasis, suggesting that pericytes could be another promising complementary target for cancer treatment. The activated pericytes are capable of stabilizing blood vessels, providing EC survival signals and protecting ECs from VEGF withdrawal, leading to pericyte-mediated resistance to antiangiogenic therapies58, 59. Guan et al.60 developed a pericyte-targeting drug delivery system, TH10 peptide (TAASGVRSMH)-conjugated nanoparticles loaded with docetaxel (TH10-DTX-NP) that can target the NG2 proteoglycan highly expressed in tumor vascular pericytes. Enhanced antitumor effects were achieved, revealing the potency and significance of targeting tumor vascular pericytes using nano-DDS in antiangiogenic cancer therapy. It was reported that doxorubicin-loaded liposomes modified with peptide ligands were constructed for the combined therapy of targeting both tumor pericytes and ECs, displaying enhanced anti-tumor effects and prolonged survival in human neuroblastoma-bearing mice. These results clearly demonstrate that this combined targeting strategy was more effective for destruction of tumor vasculature than either monotherapy61.
3.3. Vasculogenic mimicry
Vasculogenic mimicry (VM) occurs when highly invasive tumor cells form vascular channels for transporting plasma and red blood cells to provide the supply required for tumor growth and metastasis themselves. The channels formed by VM, which can be stained by Periodic Acid-Schiff (PAS), are composed of basement membranes and lined by tumor cells, and no endothelial cells are found on the inner wall62. In 1999, Maniotis and colleagues63 found that tissue sections from aggressive human intraocular (uveal) and metastatic cutaneous melanomas generally lacked evidence of significant necrosis and contained patterned networks of interconnected loops of extracellular matrix. Red blood cells were detected within these vascular channel networks and no endothelial cells were identified by microscopy or an immunohistochemical panel of endothelial cell markers. From then on, research on vasculogenic mimicry had attracted much more attention and provoked the further thinking about its molecular regulatory mechanism and its application in tumor therapy64, 65.
With further research, VM has been observed in several malignant tumor types such as breast cancer, liver cancer, glioma, ovarian cancer, melanoma, prostate cancer, and others66, 67, 68, 69. Vasculogenic mimicry was previously reported in human glioblastoma tissues and human glioma cell-line xenografts70. Yue and Chen71 examined 45 astrocytomas (including 15 World Health Organization grade II cases, 15 grade III cases, and 15 grade IV cases) for CD34 endothelial cell markers and periodic acid-Schiff (PAS) staining, among which CD34-negative, PAS-positive patterns of VM were observed in malignant astrocytomas. Hence VM provides new insights for the therapeutic intervention of tumor-associated vasculature.
Until now little has been known about the molecular mechanisms involved in VM. It was reported that aggressive VM-positive tumors exhibited higher expression of the basement membrane extracellular matrix (ECM) component laminin 5γ2, MMP1, MMP2, MMP9 and membrane type-1-matrix/metalloproteinase72. Paulis et al.73 considered the VE-cadherin/EphA2/MMP-2 as one of the key VM signaling pathways. Besides, the ischemia and hypoxia effects of the local tumor microenvironment also have an impact on the formation of VM channels69. Many researchers involved in VM studies have been trying to treat tumors based on an anti-VM therapy. But almost all VM targeting therapies have been performed in vitro. Hess et al.74 adopted general inhibitors of protein tyrosine kinases with transient knockout of EphA2 to abrogate the ability of tumor cells to form tubular structures. Downregulating VE-cadherin, using antibodies against human MMPS and the laminin 5γ2 chain are also possible strategies to inhibit VM75. Francescone and colleagues75 found that vascular channels of VM in GBM were composed of mural-like tumor cells that strongly express VEGF receptor 2 (Flk-1). Therefore, the identification of Flk-1 as a key factor regulating VM could offer a novel therapeutic target for GBM treatment.
It is necessary to combine both classic anti-angiogenesis and anti-VM in anti-tumor therapies. Tumor cells, which line in the inner surface of VM channels, are highly malignant and have high plasticity. They are directly exposed to blood flow and readily allow tumor cells to migrate and metastasize to other organs. With traditional anti-angiogenesis therapy, the vasculogenic mimicry could also nourish the tumor with nutrients and promote metastasis. As a result, multiple antivascular approaches, including targeting VM and angiogenesis together, may represent the best possible regimen in the treatment of tumors. Since VEGF receptors are highly expressed on angiogenic cells and the VM of GBM, a corresponding ligand-mediated drug delivery system may be utilized for the tumor targeting therapy75.
4. Tumor cells
Two alternative models have been put forward to explain how tumors initiate and develop. One is the stochastic model, proposing that tumor cells are heterogeneous, and virtually any of them can function as a tumor-initiating cell. Another one is the hierarchical model, encouraged by increasing experimental data, which hypothesizes that only a small subpopulation of tumor stem cells can induce tumorigenesis76, 77. The normal tumor cells, accounting for most of the tumor tissue, are incapable of tumorigenesis and could be killed by common chemotherapeutic drugs, while cancer stem cells have the ability to self-renew, spending most of their time in the G0 phase of the cell division cycle. Cancer stem cells are likely to share many of the properties of normal stem cells that include a long lifespan, relative quiescence, resistance to drugs and toxins through the expression of several ATP-binding cassette (ABC) transporters, an active DNA-repair capacity, and a resistance to apoptosis77. In recent years, brain tumor stem cells have been reportedly identified and isolated, and the concept of cancer stem cells was extended to brain tumors78, 79. The brain tumor stem cells (BTSC) are capable for self-renewal, multipotency, and induction of tumorigenesis. BTSC exhibited remarkable resistance to chemotherapy due to their relative quiescence, active DNA repair regulatory systems and high expression of ABC transporters80, 81. Thus, for brain tumor targeted strategies, cancer stem cell targeting is essential. Recently, several stem cell targeting drug delivery systems have been constructed. Zhang et al.82 prepared mitochondrial targeting liposomes showing the strongest efficacy in treating MCF-7 cancer cells and cancer stem cells in vitro and in treating relapsed tumor cells in mice. Nevertheless, brain tumor stem cells targeting nanocarriers are still under research.
CD133, a 120 kDa cell-surface protein, is considered as a marker of normal human neuronal precursors and could be used for the enrichment of tumor stem-like cells from brain tumors83, 84. Sun and colleagues85 obtained a specific mouse CD133 binding peptide named LS7 (LQNAPRS) by phage-displayed peptide library technology, which could be used to target cancer stem cells. Nestin, a 200–240 kDa intermediate filament protein, is also the most commonly used marker for the isolation and study of brain tumor stem cells. Beck et al.86 identified an effective Nestin targeted peptide (AQYLNPS) through the phage display technology, providing a promising opportunity to design a BTSC-targeted drug delivery system. BTSC and neuronal stem cells share many characteristics including these two markers. Up to now, research on BTSC-targeted drug delivery system is only in its infancy, and specific targets are still needed for the BTSC targeted drug delivery systems.
5. Conclusions
In actual research practice, several targeting strategies have been combined to achieve better therapeutic effects. One common dual targeting drug delivery system was the combination of trans-BBB targeting and brain tumor cell targeting in two ways. The first was dual-targeting moiety modification such as transferrin (Tf) and wheat germ agglutinin (WGA)87, and p-aminophenyl-α-d-mannopyranoside(MAN) and transferrin (Tf)88; the second one is a single-targeting moiety that targets both BBB and tumor cells, such as angiopep-2 targeting to LRP overexpressed on both BBB and glioma cells89, 90. Another dual targeting drug delivery system was combining trans-BBTB targeting with brain tumor cell targeting. For instance, a single targeting moiety (RGD) was utilized to target both BBTB and tumor cells42. Anti-angiogenesis and anti-tumor combination treatments gained much attention because of the potential for dual inhibition of both tumor proliferation and tumor invasion. Gao et al. modified nanoparticles with interleukin-13 peptide, targeting GBM cells and RGD targeting the neovasculature to construct a neovasculature and tumor cell dual targeting delivery, which demonstrated a clear anti-tumor effect in vitro and in vivo83.
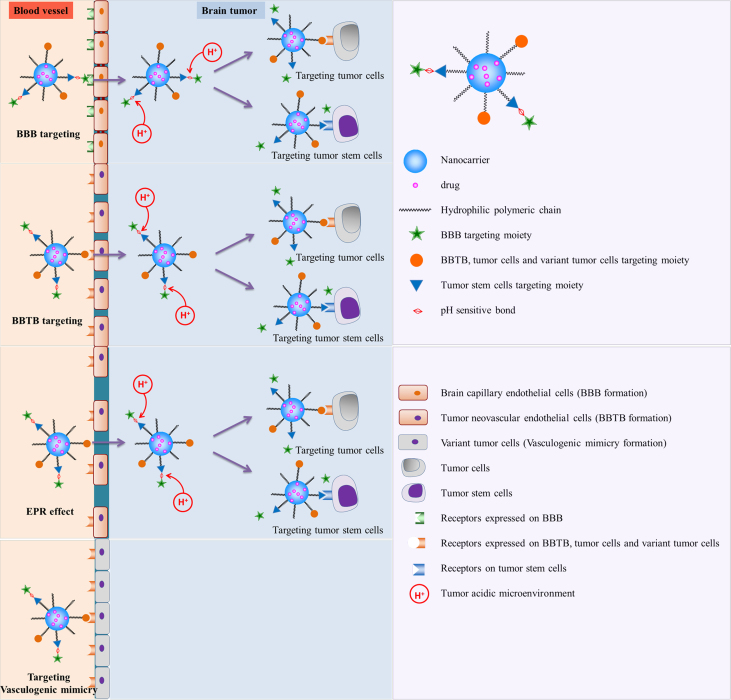
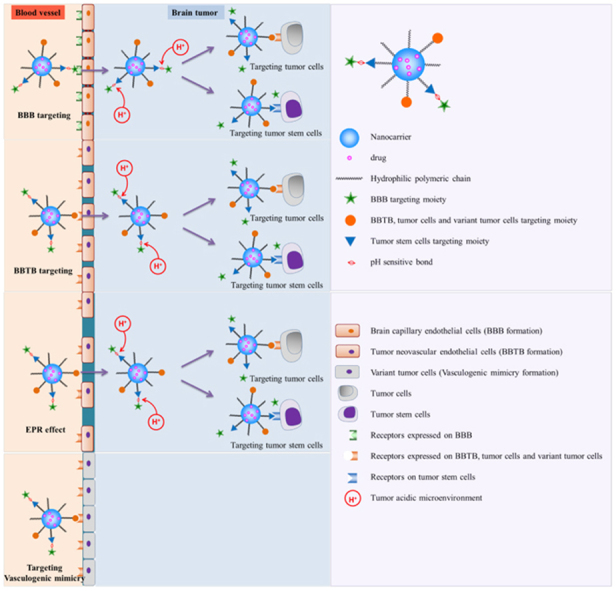
Taken together, although current brain tumor-targeted drug delivery systems have achieved significant advances in various stages of brain tumors, there are still limitations of the current drug therapy. As a consequence of these challenges, a series of overall targeting drug delivery systems called “systematic targeted drug delivery” or “whole-process targeting” (WPT) were introduced to try to make the best of every potential target for more effective treatment of brain tumors48. The “systematic targeted drug delivery” or “whole-process targeting” is recognized as an effective brain tumor targeted drug delivery strategy which considers all the possible targets including barriers, microenvironment and cancer cells. At the early stage of a brain tumor or around the tumor edge of the infiltrating glioma after BBTB formation, the tumor is nourished by the normal brain capillary network. Nanocarriers modified with ligands targeting the BBB and tumor cells are needed. With the development of the tumor, tumor neovasculature has formed to support the growth of gliomas. The BBTB and tumor cells are the main target goals. Along with the deterioration of the brain tumor, the EPR effect in brain tumor tissues appears and the nano-drug delivery vesicles enter the tumor tissue via passive targeting. Furthermore, vasculogenic mimicry, lowered extracellular pH and tumor stem cells provide possible complementary targets which should be taken into consideration for better therapy of the brain tumors (Fig. 1).
Figure 1.
Brain tumor “systematic targeted drug delivery”.
Research on “systematic targeted drug delivery” is a promising approach to the treatment of brain tumors. It will help to fill in the gaps that remain in fully understanding the relationship between the physiological and pathological conditions of brain tumors, providing a basis for various targeted delivery systems. We believed that systematic targeted drug delivery strategies for brain tumors that consider characteristics of brain tumor growth will provide promising ways to treat brain tumor in the future.
Acknowledgments
This work was supported by the National Basic Research Program of China (973 Program, No. 2013CB932500) and the National Natural Science Foundation of China (No. 81273458).
Footnotes
Peer review under responsibility of Institute of Materia Medica, Chinese Academy of Medical Sciences and Chinese Pharmaceutical Association.
References
- 1.Ferlay J., Shin H.R., Bray F., Forman D., Mathers C., Parkin D.M. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127:2893–2917. doi: 10.1002/ijc.25516. [DOI] [PubMed] [Google Scholar]
- 2.Dolecek T.A., Propp J.M., Stroup N.E., Kruchko C. CBTRUS statistical report: primary brain and central nervous system tumors diagnosed in the United States in 2005–2009. Neuro Oncol. 2012;14:v1–49. doi: 10.1093/neuonc/nos218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stupp R., Mason W.P., van den Bent M.J., Weller M., Fisher B., Taphoorn M.J.B. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. New Engl J Med. 2005;352:987–996. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 4.Sanai N., Berger M.S. Glioma extent of resection and its impact on patient outcome. Neurosurgery. 2008;62:753–764. doi: 10.1227/01.neu.0000318159.21731.cf. [DOI] [PubMed] [Google Scholar]
- 5.Daneman R. The blood–brain barrier in health and disease. Ann Neurol. 2012;72:648–672. doi: 10.1002/ana.23648. [DOI] [PubMed] [Google Scholar]
- 6.de Boer A.G., Gaillard P.J. Drug targeting to the brain. Ann Rev Pharmacol Toxicol. 2007;47:323–355. doi: 10.1146/annurev.pharmtox.47.120505.105237. [DOI] [PubMed] [Google Scholar]
- 7.Wong A.D., Ye M., Levy A.F., Rothstein J.D., Bergles D.E., Searson P.C. The blood-brain barrier: an engineering perspective. Front Neuroeng. 2013;6:7. doi: 10.3389/fneng.2013.00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pardridge W.M. Drug targeting to the brain. Pharm Res. 2007;24:1733–1744. doi: 10.1007/s11095-007-9324-2. [DOI] [PubMed] [Google Scholar]
- 9.Hervé F., Ghinea N., Scherrmann J.M. CNS delivery via adsorptive transcytosis. AAPS J. 2008;10:455–472. doi: 10.1208/s12248-008-9055-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lu W., Tan Y.Z., Hu K.L., Jiang X.G. Cationic albumin conjugated pegylated nanoparticle with its transcytosis ability and little toxicity against blood–brain barrier. Int J Pharm. 2005;295:247–260. doi: 10.1016/j.ijpharm.2005.01.043. [DOI] [PubMed] [Google Scholar]
- 11.Lu W., Sun Q., Wan J., She Z.J., Jiang X.G. Cationic albumin–conjugated pegylated nanoparticles allow gene delivery into brain tumors via intravenous administration. Cancer Res. 2006;66:11878–11887. doi: 10.1158/0008-5472.CAN-06-2354. [DOI] [PubMed] [Google Scholar]
- 12.Lu W., Wan J., Zhang Q., She Z.J., Jiang X.G. Aclarubicin-loaded cationic albumin-conjugated pegylated nanoparticle for glioma chemotherapy in rats. Int J Cancer. 2007;120:420–431. doi: 10.1002/ijc.22296. [DOI] [PubMed] [Google Scholar]
- 13.Du J., Lu W.L., Ying X., Liu Y., Du P., Tian W. Dual-targeting topotecan liposomes modified with tamoxifen and wheat germ agglutinin significantly improve drug transport across the blood-brain barrier and survival of brain tumor-bearing animals. Mol Pharm. 2009;6:905–917. doi: 10.1021/mp800218q. [DOI] [PubMed] [Google Scholar]
- 14.Gupta B., Levchenko T.S., Torchilin V.P. Intracellular delivery of large molecules and small particles by cell-penetrating proteins and peptides. Adv Drug Deliver Rev. 2005;57:637–651. doi: 10.1016/j.addr.2004.10.007. [DOI] [PubMed] [Google Scholar]
- 15.Banks W.A., Robinson S.M., Nath A. Permeability of the blood–brain barrier to HIV-1 Tat. Exp Neurol. 2005;193:218–227. doi: 10.1016/j.expneurol.2004.11.019. [DOI] [PubMed] [Google Scholar]
- 16.Qin Y., Chen H.L., Zhang Q.Y., Wang X.X., Yuan W.M., Kuai R. Liposome formulated with TAT-modified cholesterol for improving brain delivery and therapeutic efficacy on brain glioma in animals. Int J Pharm. 2011;420:304–312. doi: 10.1016/j.ijpharm.2011.09.008. [DOI] [PubMed] [Google Scholar]
- 17.Liu L.H., Venkatraman S.S., Yang Y.Y., Guo K., Lu J., He B.P. Polymeric micelles anchored with TAT for delivery of antibiotics across the blood–brain barrier. Pept Sci. 2008;90:617–623. doi: 10.1002/bip.20998. [DOI] [PubMed] [Google Scholar]
- 18.Zou L.L., Ma J.L., Wang T., Yang T.B., Liu C.B. Cell-penetrating peptide-mediated therapeutic molecule delivery into the central nervous system. Curr Neuropharmacol. 2013;11:197–208. doi: 10.2174/1570159X11311020006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Umezawa F., Eto Y. Liposome targeting to mouse brain: mannose as a recognition marker. Biochem Biophs Res Commun. 1988;153:1038–1044. doi: 10.1016/s0006-291x(88)81333-0. [DOI] [PubMed] [Google Scholar]
- 20.Qin Y., Fan W., Chen H.L., Yao N., Tang W.W., Tang J. In vitro and in vivo investigation of glucose-mediated brain-targeting liposomes. J Drug Target. 2010;18:536–549. doi: 10.3109/10611861003587235. [DOI] [PubMed] [Google Scholar]
- 21.Lockman P.R., Allen D.D. The transport of choline. Drug Dev Ind Pharm. 2002;28:749–771. doi: 10.1081/ddc-120005622. [DOI] [PubMed] [Google Scholar]
- 22.Fenart L., Casanova A., Dehouck B., Duhem C., Slupek S., Cecchelli R. Evaluation of effect of charge and lipid coating on ability of 60-nm nanoparticles to cross an in vitro model of the blood-brain barrier. J Pharmacol Exp Ther. 1999;291:1017–1022. [PubMed] [Google Scholar]
- 23.Ponka P., Lok C.N. The transferrin receptor: role in health and disease. Int J Biochem Cell B. 1999;31:1111–1137. doi: 10.1016/s1357-2725(99)00070-9. [DOI] [PubMed] [Google Scholar]
- 24.Zhang P.C., Hu L.J., Yin Q., Zhang Z.W., Feng L.Y., Li Y.P. Transferrin-conjugated polyphosphoester hybrid micelle loading paclitaxel for brain-targeting delivery: synthesis, preparation and in vivo evaluation. J Control Release. 2012;159:429–434. doi: 10.1016/j.jconrel.2012.01.031. [DOI] [PubMed] [Google Scholar]
- 25.Qian Z.M., Li H.Y., Sun H.Z., Ho K. Targeted drug delivery via the transferrin receptor-mediated endocytosis pathway. Pharmacol Rev. 2002;54:561–587. doi: 10.1124/pr.54.4.561. [DOI] [PubMed] [Google Scholar]
- 26.Huwyler J., Wu D., Pardridge W.M. Brain drug delivery of small molecules using immunoliposomes. Proc Natl Acad Sci USA. 1996;93:14164–14169. doi: 10.1073/pnas.93.24.14164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Demeule M., Régina A., Che C., Poirier J., Nguyen T., Gabathuler R. Identification and design of peptides as a new drug delivery system for the brain. J Pharmacol Exp Ther. 2008;324:1064–1072. doi: 10.1124/jpet.107.131318. [DOI] [PubMed] [Google Scholar]
- 28.Sun X.Y., Pang Z.Q., Ye H.X., Qiu B., Guo L.R., Li J.W. Co-delivery of pEGFP-hTRAIL and paclitaxel to brain glioma mediated by an angiopep-conjugated liposome. Biomaterials. 2012;33:916–924. doi: 10.1016/j.biomaterials.2011.10.035. [DOI] [PubMed] [Google Scholar]
- 29.Abbruscato T.J., Lopez S.P., Mark K.S., Hawkins B.T., Davis T.P. Nicotine and cotinine modulate cerebral microvascular permeability and protein expression of ZO-1 through nicotinic acetylcholine receptors expressed on brain endothelial cells. J Pharm Sci. 2002;91:2525–2538. doi: 10.1002/jps.10256. [DOI] [PubMed] [Google Scholar]
- 30.Kumar P., Wu H.Q., McBride J.L., Jung K.E., Kim M.H., Davidson B.L. Transvascular delivery of small interfering RNA to the central nervous system. Nature. 2007;448:39–43. doi: 10.1038/nature05901. [DOI] [PubMed] [Google Scholar]
- 31.Zhan C.Y., Li B., Hu L.J., Wei X.L., Feng L.Y., Fu W. Micelle-based brain-targeted drug delivery enabled by a nicotine acetylcholine receptor ligand. Angew Chem Int Ed Engl. 2011;50:5582–5585. doi: 10.1002/anie.201100875. [DOI] [PubMed] [Google Scholar]
- 32.Ningaraj N.S., Rao M., Hashizume K., Asotra K., Black K.L. Regulation of blood-brain tumor barrier permeability by calcium-activated potassium channels. J Pharmacol Exp Ther. 2002;301:838–851. doi: 10.1124/jpet.301.3.838. [DOI] [PubMed] [Google Scholar]
- 33.Monsky W.L., Carreira C.M., Tsuzuki Y., Gohongi T., Fukumura D., Jain R.K. Role of host microenvironment in angiogenesis and microvascular functions in human breast cancer xenografts: mammary fat pad versus cranial tumors. Clin Cancer Res. 2002;8:1008–1013. [PubMed] [Google Scholar]
- 34.Roberts W.G., Delaat J., Nagane M., Huang S., Cavenee W.K., Palade G.E. Host microvasculature influence on tumor vascular morphology and endothelial gene expression. Am J Clin Pathol. 1998;153:1239–1248. doi: 10.1016/s0002-9440(10)65668-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sarin H., Kanevsky A.S., Wu H., Sousa A.A., Wilson C.M., Aronova M.A. Physiologic upper limit of pore size in the blood-tumor barrier of malignant solid tumors. J Transl Med. 2009;7:51. doi: 10.1186/1479-5876-7-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schlageter K.E., Molnar P., Lapin G.D., Groothuis D.R. Microvessel organization and structure in experimental brain tumors: microvessel populations with distinctive structural and functional properties. Microvasc Res. 1999;58:312–328. doi: 10.1006/mvre.1999.2188. [DOI] [PubMed] [Google Scholar]
- 37.Zhan C.Y., Lu W.Y. The blood-brain/tumor barriers: challenges and chances for malignant gliomas targeted drug delivery. Curr Pharm Biotechnol. 2012;13:2380–2387. doi: 10.2174/138920112803341798. [DOI] [PubMed] [Google Scholar]
- 38.Brooks P.C., Clark R.A., Cheresh D.A. Requirement of vascular integrin αvβ3 for angiogenesis. Science. 1994;264:569–571. doi: 10.1126/science.7512751. [DOI] [PubMed] [Google Scholar]
- 39.Kumar C.C., Armstrong L., Yin Z., Malkowski M., Maxwell E., Ling H. Targeting integrins αvβ3 and αvβ5 for blocking tumor-induced angiogenesis. Adv Exp Med Biol. 2000;476:169–180. [PubMed] [Google Scholar]
- 40.Kim S., Bell K., Mousa S.A., Varner J.A. Regulation of angiogenesisin in vivo by ligation of integrin α5β1 with the central cell-binding domain of fibronectin. Am J Clin Pathol. 2000;156:1345–1362. doi: 10.1016/s0002-9440(10)65005-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhan C.Y., Meng Q.G., Li Q.H., Feng L.L., Zhu J.H., Lu W.Y. Cyclic RGD–polyethylene glycol-polyethylenimine for intracranial glioblastoma-targeted gene delivery. Chem-Asian J. 2012;7:91–96. doi: 10.1002/asia.201100570. [DOI] [PubMed] [Google Scholar]
- 42.Zhan C.Y., Gu B., Xie C., Li J., Liu Y., Lu W.Y. Cyclic RGD conjugated poly(ethylene glycol)-co-poly(lactic acid) micelle enhances paclitaxel anti-glioblastoma effect. J Control Release. 2010;143:136–142. doi: 10.1016/j.jconrel.2009.12.020. [DOI] [PubMed] [Google Scholar]
- 43.Yano S., Kondo K., Yamaguchi M., Richmond G., Hutchison M., Wakeling A. Distribution and function of EGFR in human tissue and the effect of EGFR tyrosine kinase inhibition. Anticancer Res. 2002;23:3639–3650. [PubMed] [Google Scholar]
- 44.Spano J.P., Fagard R., Soria J.C., Rixe O., Khayat D., Milano G. Epidermal growth factor receptor signaling in colorectal cancer: preclinical data and therapeutic perspectives. Ann Oncol. 2005;16:189–194. doi: 10.1093/annonc/mdi057. [DOI] [PubMed] [Google Scholar]
- 45.Ohgaki H., Kleihues P. Genetic pathways to primary and secondary glioblastoma. Am J Clin Pathol. 2007;170:1445–1453. doi: 10.2353/ajpath.2007.070011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fondell A., Edwards K., Ickenstein L.M., Sjöberg S., Carlsson J., Gedda L. Nuclisome: a novel concept for radionuclide therapy using targeting liposomes. Eur J Nucl Med Mol I. 2010;37:114–123. doi: 10.1007/s00259-009-1225-7. [DOI] [PubMed] [Google Scholar]
- 47.Tsutsui Y., Tomizawa K., Nagita M., Michiue H., Nishiki T., Ohmori I. Development of bionanocapsules targeting brain tumors. J Control Release. 2007;122:159–164. doi: 10.1016/j.jconrel.2007.06.019. [DOI] [PubMed] [Google Scholar]
- 48.Feng B., Tomizawa K., Michiue H., Miyatake S., Han X., Fujimura A. Delivery of sodium borocaptate to glioma cells using immunoliposome conjugated with anti-EGFR antibodies by ZZ-His. Biomaterials. 2009;30:1746–1755. doi: 10.1016/j.biomaterials.2008.12.010. [DOI] [PubMed] [Google Scholar]
- 49.Liu Y., Lu W.Y. Recent advances in brain tumor-targeted nano-drug delivery systems. Expert Opin Drug Del. 2012;9:671–686. doi: 10.1517/17425247.2012.682726. [DOI] [PubMed] [Google Scholar]
- 50.Hobbs S.K., Monsky W.L., Yuan F., Roberts W.G., Griffith L., Torchilin V.P. Regulation of transport pathways in tumor vessels: role of tumor type and microenvironment. Proc Natl Acad Sci USA. 1998;95:4607–4612. doi: 10.1073/pnas.95.8.4607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Huang S.X., Shao K., Liu Y., Kuang Y.Y., Li J.F., An S. Tumor-targeting and microenvironment-responsive smart nanoparticles for combination therapy of antiangiogenesis and apoptosis. ACS Nano. 2013;7:2860–2871. doi: 10.1021/nn400548g. [DOI] [PubMed] [Google Scholar]
- 52.Seftor R.E., Hess A.R., Seftor E.A., Kirschmann D.A., Hardy K.M., Margaryan N.V. Tumor cell vasculogenic mimicry: from controversy to therapeutic promise. Am J Clin Pathol. 2012;181:1115–1125. doi: 10.1016/j.ajpath.2012.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Engin K., Leeper D.B., Cater J.R., Thistlethwaite A.J., Tupchong L., McFarlane J.D. Extracellular pH distribution in human tumours. Int J Hyperth. 1995;11:211–216. doi: 10.3109/02656739509022457. [DOI] [PubMed] [Google Scholar]
- 54.van Sluis R., Bhujwalla Z.M., Raghunand N., Ballesteros P., Alvarez J., Cerdán S. In vivo imaging of extracellular pH using 1H MRSI. Magn Reson Med. 1999;41:743–750. doi: 10.1002/(sici)1522-2594(199904)41:4<743::aid-mrm13>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 55.Zhang X.M., Lin Y.X., Gillies R.J. Tumor pH and its measurement. J Nucl Med. 2010;51:1167–1170. doi: 10.2967/jnumed.109.068981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lee E.S., Gao Z.G., Bae Y.H. Recent progress in tumor pH targeting nanotechnology. J Control Release. 2008;132:164–170. doi: 10.1016/j.jconrel.2008.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Huang S.X., Shao K., Kuang Y.Y., Liu Y., Li J.F., An S. Tumor targeting and microenvironment-responsive nanoparticles for gene delivery. Biomaterials. 2013;34:5294–5302. doi: 10.1016/j.biomaterials.2013.03.043. [DOI] [PubMed] [Google Scholar]
- 58.Raza A., Franklin M.J., Dudek A.Z. Pericytes and vessel maturation during tumor angiogenesis and metastasis. Am J Hematol. 2010;85:593–598. doi: 10.1002/ajh.21745. [DOI] [PubMed] [Google Scholar]
- 59.Erber R., Thurnher A., Katsen A.D., Groth G., Kerger H., Hammes H.P. Combined inhibition of VEGF and PDGF signaling enforces tumor vessel regression by interfering with pericyte-mediated endothelial cell survival mechanisms. FASEB J. 2004;18:338–340. doi: 10.1096/fj.03-0271fje. [DOI] [PubMed] [Google Scholar]
- 60.Guan Y.Y., Luan X., Xu J.R., Liu Y.R., Lu Q., Wang C. Selective eradication of tumor vascular pericytes by peptide-conjugated nanoparticles for antiangiogenic therapy of melanoma lung metastasis. Biomaterials. 2014;35:3060–3070. doi: 10.1016/j.biomaterials.2013.12.027. [DOI] [PubMed] [Google Scholar]
- 61.Loi M., Marchiò S., Becherini P., Di Paolo D., Soster M., Curnis F. Combined targeting of perivascular and endothelial tumor cells enhances anti-tumor efficacy of liposomal chemotherapy in neuroblastoma. J Control Release. 2010;145:66–73. doi: 10.1016/j.jconrel.2010.03.015. [DOI] [PubMed] [Google Scholar]
- 62.Folberg R., Maniotis A.J. Vol. 112. 2004. Vasculogenic mimicry; pp. 508–525. (APMIS). [DOI] [PubMed] [Google Scholar]
- 63.Maniotis A.J., Folberg R., Hess A., Seftor E.A., Gardner L.M., Pe׳er J. Vascular channel formation by human melanoma cells in vivo and in vitro: vasculogenic mimicry. Am J Clin Pathol. 1999;155:739–752. doi: 10.1016/S0002-9440(10)65173-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bissell M.J. Tumor plasticity allows vasculogenic mimicry, a novel form of angiogenic switch: a rose by any other name? Am J Clin Pathol. 1999;155:675–679. doi: 10.1016/S0002-9440(10)65164-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Barinaga M. New type of blood vessel found in tumors. Science. 1999;285:1475. doi: 10.1126/science.285.5433.1475. [DOI] [PubMed] [Google Scholar]
- 66.Shirakawa K., Tsuda H., Heike Y., Kato K., Asada R., Inomata M. Absence of endothelial cells, central necrosis, and fibrosis are associated with aggressive inflammatory breast cancer. Cancer Res. 2001;61:445–451. [PubMed] [Google Scholar]
- 67.Sood A.K., Seftor E.A., Fletcher M.S., Gardner L.M., Heidger P.M., Buller R.E. Molecular determinants of ovarian cancer plasticity. Am J Clin Pathol. 2001;158:1279–1288. doi: 10.1016/S0002-9440(10)64079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sharma N., Seftor R.E.B., Seftor E.A., Gruman L.M., Heidger P.M., Cohen M.B. Prostatic tumor cell plasticity involves cooperative interactions of distinct phenotypic subpopulations: role in vasculogenic mimicry. Prostate. 2002;50:189–201. doi: 10.1002/pros.10048. [DOI] [PubMed] [Google Scholar]
- 69.Zhang S.W., Zhang D.F., Sun B.C. Vasculogenic mimicry: current status and future prospects. Cancer Lett. 2007;254:157–164. doi: 10.1016/j.canlet.2006.12.036. [DOI] [PubMed] [Google Scholar]
- 70.Niclou S.P., Danzeisen C., Eikesdal H.P., Wiig H., Brons N.H.C., Poli A.M.F. A novel eGFP-expressing immunodeficient mouse model to study tumor-host interactions. FASEB J. 2008;22:3120–3128. doi: 10.1096/fj.08-109611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yue W.Y., Chen Z.P. Does vasculogenic mimicry exist in astrocytoma? J Histochem Cytochem. 2005;53:997–1002. doi: 10.1369/jhc.4A6521.2005. [DOI] [PubMed] [Google Scholar]
- 72.Seftor R.E., Seftor E.A., Koshikawa N., Meltzer P.S., Gardner L.M., Bilban M. Cooperative interactions of laminin 5 gamma2 chain, matrix metalloproteinase-2, and membrane type-1-matrix/metalloproteinase are required for mimicry of embryonic vasculogenesis by aggressive melanoma. Cancer Res. 2001;61:6322–6327. [PubMed] [Google Scholar]
- 73.Paulis Y.W.J., Soetekouw P.M.M.B., Verheul H.M.W., Tjan-Heijnen V.C.G., Griffioen A.W. Signalling pathways in vasculogenic mimicry. BBA-Rev Cancer. 2010;1806:18–28. doi: 10.1016/j.bbcan.2010.01.001. [DOI] [PubMed] [Google Scholar]
- 74.Hess A.R., Seftor E.A., Gardner L.M., Carles-Kinch K., Schneider G.B., Seftor R.E. Molecular regulation of tumor cell vasculogenic mimicry by tyrosine phosphorylation: role of epithelial cell kinase (Eck/EphA2) Cancer Res. 2001;61:3250–3255. [PubMed] [Google Scholar]
- 75.Francescone R., Scully S., Bentley B., Yan W., Taylor S.L., Oh D. Glioblastoma-derived tumor cells induce vasculogenic mimicry through Flk-1 protein activation. J Biol Chem. 2012;287:24821–24831. doi: 10.1074/jbc.M111.334540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Reya T., Morrison S.J., Clarke M.F., Weissman I.L. Stem cells, cancer, and cancer stem cells. Nature. 2001;414:105–111. doi: 10.1038/35102167. [DOI] [PubMed] [Google Scholar]
- 77.Vescovi A.L., Galli R., Reynolds B.A. Brain tumour stem cells. Nat Rev Cancer. 2006;6:425–436. doi: 10.1038/nrc1889. [DOI] [PubMed] [Google Scholar]
- 78.Ignatova T.N., Kukekov V.G., Laywell E.D., Suslov O.N., Vrionis F.D., Steindler D.A. Human cortical glial tumors contain neural stem‐like cells expressing astroglial and neuronal markers in vitro. Glia. 2002;39:193–206. doi: 10.1002/glia.10094. [DOI] [PubMed] [Google Scholar]
- 79.Galli R., Binda E., Orfanelli U., Cipelletti B., Gritti A., de Vitis S. Isolation and characterization of tumorigenic, stem-like neural precursors from human glioblastoma. Cancer Res. 2004;64:7011–7021. doi: 10.1158/0008-5472.CAN-04-1364. [DOI] [PubMed] [Google Scholar]
- 80.Bao S.D., Wu Q.L., McLendon R.E., Hao Y.L., Shi Q., Hjelmeland A.B. Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature. 2006;444:756–760. doi: 10.1038/nature05236. [DOI] [PubMed] [Google Scholar]
- 81.Dean M., Fojo T., Bates S. Tumour stem cells and drug resistance. Nat Rev Cancer. 2005;5:275–284. doi: 10.1038/nrc1590. [DOI] [PubMed] [Google Scholar]
- 82.Zhang L., Yao H.J., Yu Y., Zhang Y., Li R.J., Ju R.J. Mitochondrial targeting liposomes incorporating daunorubicin and quinacrine for treatment of relapsed breast cancer arising from cancer stem cells. Biomaterials. 2012;33:565–582. doi: 10.1016/j.biomaterials.2011.09.055. [DOI] [PubMed] [Google Scholar]
- 83.Singh S.K., Hawkins C., Clarke I.D., Squire J.A., Bayani J., Hide T. Identification of human brain tumour initiating cells. Nature. 2004;432:396–401. doi: 10.1038/nature03128. [DOI] [PubMed] [Google Scholar]
- 84.Singh S.K., Clarke I.D., Terasaki M., Bonn V.E., Hawkins C., Squire J. Identification of a cancer stem cell in human brain tumors. Cancer Res. 2003;63:5821–5828. [PubMed] [Google Scholar]
- 85.Sun J.M., Zhang C., Liu G.B., Liu H., Zhou C.P., Lu Y.X. A novel mouse CD133 binding-peptide screened by phage display inhibits cancer cell motility in vitro. Clin Exp Metastasis. 2012;29:185–196. doi: 10.1007/s10585-011-9440-6. [DOI] [PubMed] [Google Scholar]
- 86.Beck S., Jin X., Yin J.L., Kim S.H., Lee N.K., Oh S.Y. Identification of a peptide that interacts with Nestin protein expressed in brain cancer stem cells. Biomaterials. 2011;32:8518–8528. doi: 10.1016/j.biomaterials.2011.07.048. [DOI] [PubMed] [Google Scholar]
- 87.He H., Li Y., Jia X.R., Du J., Ying X., Lu W.L. PEGylated poly (amidoamine) dendrimer-based dual-targeting carrier for treating brain tumors. Biomaterials. 2011;32:478–487. doi: 10.1016/j.biomaterials.2010.09.002. [DOI] [PubMed] [Google Scholar]
- 88.Ying X., Wen H., Lu W.L., Du J., Guo J., Tian W. Dual-targeting daunorubicin liposomes improve the therapeutic efficacy of brain glioma in animals. J Control Release. 2010;141:183–192. doi: 10.1016/j.jconrel.2009.09.020. [DOI] [PubMed] [Google Scholar]
- 89.Demeule M., Currie J.C., Bertrand Y., Ché C., Nguyen T., Régina A. Involvement of the low-density lipoprotein receptor-related protein in the transcytosis of the brain delivery vector Angiopep-2. J Neurochem. 2008;106:1534–1544. doi: 10.1111/j.1471-4159.2008.05492.x. [DOI] [PubMed] [Google Scholar]
- 90.Xin H.L., Jiang X.Y., Gu J.J., Sha X.Y., Chen L.C., Law K. Angiopep-conjugated poly(ethylene glycol)-co-poly(epsilon-caprolactone) nanoparticles as dual-targeting drug delivery system for brain glioma. Biomaterials. 2011;32:4293–4305. doi: 10.1016/j.biomaterials.2011.02.044. [DOI] [PubMed] [Google Scholar]