Abstract
Non-Camellia tea is a part of the colorful Chinese tea culture, and is also widely used as beverage and medicine in folk for disease prevention and treatment. In this study, 37 samples were collected, including 33 kinds of non-Camellia teas and 4 kinds of teas (Camellia). Traditional functions of non-Camellia teas were investigated. Furthermore, non-Camellia teas of original plants were characterized and identified by molecular methods. Four candidate regions (rbcL, matK, ITS2, psbA-trnH) were amplified by polymerase chain reaction. In addition, DNA barcodes were used for the first time to discriminate the commercial non-Camellia tea and their adulterants, and to evaluate their safety. This study showed that BLASTN and the relevant phylogenetic tree are efficient tools for identification of the commercial non-Camellia tea and their adulterants. However, some sequences from original plants have not been found and there is a limitation of sequence number of original plants in GenBank. Submitting more original plant sequences to the GenBank will be helpful for evaluating the safety of non-Camellia teas.
KEY WORDS: Non-Camellia tea, Traditional function, Molecular identification, BLASTN, Phylogenetic tree
Graphical abstract
Traditional functions of non-Camellia teas were investigated. Meanwhile, original plants of non-camellia tea were identified by using molecular method. DNA barcoding technology is viable and effective to identify non-Camelliaea tea and evaluate their safety.
1. Introduction
Tea (the leaves from Camellia plants) has been one of the most widely consumed non-alcoholic beverages in the world for thousands of years. It plays important roles in commerce, health, and culture. However, many other kinds of plants have been widely used as tea as well. These are not from Camellia (Theaceae), and are called non-Camellia tea, such as kuding tea, huangqin tea, laoying tea1, 2. More than 20 kinds of non-Camellia tea are reportedly used within the Chinese culture3. Modern pharmacology studies have reported that non-Camellia tea may prevent and/or treat chronic metabolic diseases, by producing hypolipidemic, hypoglycemic, or hypotensive actions. Moreover, most kinds of non-Camellia tea have also been used as medicine for disease prevention and treatment in folklore4, 5, 6.
In recent years, with the increasing international demand for herbal medicines, non-Camellia tea has attracted increasing attention. However, original plants of non-Camellia tea are confused, and some adulterants have begun to appear in the market. Furthermore, fatalities and serious illnesses have occurred after drinking non-Camellia teas, caused by overdose, mislabeled products, or allergic reactions7, 8. For instance, kuding tea is suitable for high blood pressure, body fat or hot body, but not for the person whose body ‘slants cold’ in traditional Chinese medicine theory. According to this theory, a person whose body ‘slants cold’ will receive no improvement from ingestion of kuding tea and symptoms may appear or worsen, including abdominal pain, severe diarrhea and other symptoms9. The significant differences in chemical components among different kinds of the original plants could account for such variations in responses. Finally, undefined compounds in some of these teas may be dangerous to health.
Identification of non-Camellia tea is difficult, partly due to a lack of unified standards. Traditionally, morphological features remain as the main basis of taxonomy10. However, many of these commercial products are dried and processed, rendering the authentication by morphological methods very difficult. When morphological characteristics are absent, a DNA barcoding technique can identify and detect species utilizing one or a few DNA fragments11. DNA barcoding technique is a supplement to traditional authentication method which has been able to solve some identification problems11, 12, 13. In this study, we randomly collected non-Camellia tea from the medicinal material market in China. Using DNA barcoding technique, the original plants from which the teas were derived were identified to (1) explore which DNA regions are better for the authentication of non-Camellia tea traditionally used by Chinese people and (2) evaluate their safety.
2. Materials and methods
2.1. Plant materials
We collected 37 commercialized tea samples, including 33 kinds of non-Camellia tea and 4 kinds of Camellia tea from 16 provinces (Yunnan, Sichuan, Guangxi, etc.) in China during 2012, and recorded the detailed information of these medicinal non-Camellia tea samples, including the local tea name, collecting location and time, and therapeutic effects (Table 1). All the samples were pressed and deposited at the Herbarium of the Institute of Medicinal Plant Development (IMPLAD).
Table 1.
Summary of sample collecting location and time, original plants and traditional function of non-Camellia tea.
| Local commodity name | Collecting location and time | Original plant | Use part | Traditional function | Reference |
|---|---|---|---|---|---|
| Non-Camelliatea | |||||
| Baixue tea | Yunnan Province. May 2012 | Thamnolia vermicularis (Ach.) Asahina | Leaves | Clearing away heat and remove toxic material, relieving cough, reducing sputum, anti-inflammatory. | 14, 15 |
| T. subuliforms (Ehrh.) W. Culb. | |||||
| Big leaf kuding old tea | Guangxi Province June 2012 | Ilex latifolia Thunb. | Leaves | Quenching thirst, improving eyesight, relieving restlessness, refreshing oneself, dissolving phlegm, increasing secretion of urine, relieving sore throat. | 6, 16, 17 |
| I. kaushue S. Y. Hu | |||||
| Big leaf kuding tender tea | Guangxi Province June 2012 | Ilex latifolia Thunb. | Leaves | Quenching thirst, improving eyesight, relieving restlessness, refreshing oneself, dissolving phlegm, increasing secretion of urine, relieving sore throat. | 6, 16, 17 |
| I. kaushue S. Y. Hu | |||||
| Duosuike sweet tea | Sichuan Province May 2012 | Lithocarpus litseifolius (Hance) Chun | Leaves | Reducing fever and causing diuresis, nourishing the liver and kidney, regulating the stomach to descend stomach-qi, moistening the lung to arrest cough. | 18, 19, 20 |
| Fengwei tea | Wenshan, Yunnan Province May 2012 | Elsholtzia bodinieri Vant. | Leaves | Relieving exterior syndrome by dispersion, regulating flow of qi, harmonizing the stomach. | 21 |
| E. heterophylla Diels | |||||
| Gongju tea | Huangshan city, An׳hui Province July 2012 | Chrysanthemum morifolium Ramat | Flowers | Expelling wind and clearing away heat, clearing liver-fire to treat eye disease, and eliminating toxic substances. | 22 |
| Guangxi sweet tea | Guangxi Province June 2012 | Rubus suavissimus S. Lee | Leaves, and branch | Clearing away heat and removing toxic material, promoting the secretion of saliva or body fluid, moistening the lung, relieving a cough, relieving sore throat. | 23, 24, 25 |
| Hongxue tea | Yunnan Province May 2012 | Lethariella cashmeriana Korw. | Leaves | Clearing heart-fire to regain consciousness, relieving pain, hyperlipidemia, anti-fatigue, anti-inflammatory. | 14, 15, 26, 27 |
| L. cladonioides (Nyl.) Krog; | |||||
| L. sernanderi (Motyka) Obermayer; | |||||
| L. zahlbruckneria (Dr.) Krog. | |||||
| Huangqin tea | Inner Mongolia Autonomous Region September 2012 | Scutellaria baicalensis Georgi; | Herbs | Heat-clearing and damp-drying drug, purging fire for removing toxin, anti-inflammatory, promoting digestion. | 3, 28 |
| S. scordifolia Fisch. ex Schrank; | |||||
| S. amoena. C. H. Wright; | |||||
| S. viscidula Bunge | |||||
| Jiaogulan tea | Hunan Province July 2012 | Gynostemma pentaphyllum (Thunb.) Makino | Leaves | Anti-fatigue, anti-hypoxia, enhancing immu-neity, hyperglycemic and hypolipidemic. | 29, 30, 31 |
| Kuqiao tea | Liangzhou, Sichuan Province May 2012 | Fagopyrum tataricum (L.) Gaertn. | Seeds | Hyperglycemic, hypolipidemic, enhancing immunity, et al. | 32 |
| Laoying tea | Guangyuan, Sichuan Province May 2012 | Litsea coreana Levl. Var. lanuginose | Leaves | Diabetes, expelling dampness, anti-diarrhea, stop burping, promoting digestion, et al. | 33, 34, 35, 36 |
| Actinodaphne cupularis (Hemsl.) Gemble | |||||
| Liangwang tea | Yunnan Province May 2012 | Nothopanax delavayi (Franch.) Harms ex Diels | Leaves, and flowers | Clearing away heat and removing toxic material, relaxing muscles and bones, promoting digestion, et al. | 37 |
| Luobuhongma tea | Xinjiang Uygur Autonomous Region July 2012 | Apocynum venetum L. | Leaves, and flower | Hypotensive, anti-radiation, anti-aging, preventing bronchitis and cold. | 38, 39, 40 |
| Luobubaima tea | Xinjiang Uygur Autonomous Region July 2012 | Apocynum hendersonii Hook. | Leaves, and flowers | Reducing fever and causing diuresis, flat liver resting to restore energy, hypotensive, hypolipidemic, anti-inflammatory, anti-anaphylaxis. | 39, 41 |
| Luohan tea | Guangxi Province June 2012 | Engelhardtia roxburghiana. Wall. | Leaves | Clearing away heat and removing toxic material, engendering liquid and allaying thirst, relieving summer-heat, removing dampness. | 42 |
| Lvluohua tea | Tibet Autonomous Region July 2012 | Epipremnum aureum (Linden & André) G. S. Bunting | Flowers | Hypoglycemic, anti-bacterial, anti-inflammatory, hypotensive. | 43, 44 |
| Mabiancao tea | Shanxi Province June 2012 | Verbena officinalis L. | Herbs | Clearing away heat and removing toxic material, promoting blood circulation to induce menstrual, diuretic swelling, preventing attack of malaria. | 45 |
| Niubaiteng sweet tea | Guangxi Province June 2012 | Hedyotis hedyotidea (DC.) Merr. | Stems, leaves | Clearing away heat, dispelling wind, eliminating dampness, detumescence detoxification, et al. | 46, 47 |
| Paraguay tea | Bei Jing August 2012 | Ilex paraguariensis St. Hilaire. | Leaves | Curing dyspepsia, antiobesity effect. | 48, 49, 50 |
| Qingqianliu tea | Jiangxi Province August 2012 | Cyclocarya paliurus (Batal.) Iljinsk. | Leaves | Engendering liquid and allaying thirst, clearing away heat and removing toxic material, enhancing physical strength, prolonging life. | 51, 52, 53 |
| Sishi tea | Wuyuan, Jiangxi Province August 2012 | Scoparia dulcis L. | Herbs | Dispelling wind and relieving cough, clearing away heat, removing dampness by dieresis. | 54 |
| Shen tea | Yunnan Province May 2012 | Clerodendranthus spicatus (Thunb.) C. Y. Wu ex H. W. Li | Leaves | Clearing heat and expelling damp, removal of stone and increasing secretion of urine. | 55 |
| Shiliang tea | Guangxi Province June 2012 | Chimonanthus salicifolius S. Y. H; | Leaves | Dispelling wind to relieve exogenous syndrome, regulating qi-flowing for strengthening spleen, anti-diarrhea. | 56, 57 |
| C. Zhejiangensis M.C. Liu; | |||||
| C. nitens Oliv. | |||||
| Shiya tea | Guangxi Province June 2012 | Adinandra nitida Merr. ex Li | Leaves | Engendering liquid and allaying thirst, anti-inflammatory, clearing away heat and removing toxic material. | 58, 59, 60 |
| Small leaf kuding tea | Sichuan Province. May 2012 | Ligustrum robustum (Roxb.) Blume | Leaves | Cooling and refreshing antipyretic, dieresis. | 6, 17, 61 |
| Tianyeju tea | Yunnan Province May 2012 | Stevia rebaudiana Bertoni | Leaves | Helping to produce saliva and slake thirst, hypotensive, hypoglycemic. | 62 |
| Vine tea | Zhangjiajie, Hunan Province June 2012 | Ampelopsis grossedentata (Hand.-Mazz) W. T. Wang | Stems, leaves | Clearing away heat and removing toxic material, diminishing inflammation and relieving sore throat, hypotensive and hypolipidemic. | 63, 64 |
| Xiangsiteng tea | Guangxi Province June 2012 | Abrus precatorius Linn. | Stems, leaves | Helping to produce saliva, moistening lung, clearing heat, induce diuresis diuresis. | 65, 66 |
| Xiangfeng tea | Hebei Province July 2012 | Chimonanthus salicifolius S. Y. Hu | Leaves | Promoting digestion, treating liver-stomach disharmony, et al. | 57 |
| Yaowang tea | Xi׳an, Shangxi Province July 2012 | Potentilla fruticosa L. | Leaves, flowers | Clearing away heat, invigorating the stomach, regulating the menstrual function. | 67 |
| P. glabra Lodd. var. mandshurica | |||||
| Yeju tea | Zhejiang Province August 2012 | Chrysanthemum indicum L. | Flowers | Clearing away heat and removing toxic material, dispersing wind and heat, dispersing blood stasis, improving eyesight, hypotensive, et al. | 62 |
| Zhegu tea | Wanning, Hainan Province. August 2012 | Mallotus oblongifolius (Miq.) Muell. Arg | Leaves | Neutralizing the greasy, promoting digestion, eliminating summer-heat, prevention and treatment of common cold. | 68 |
| Traditional tea | |||||
| Black tea | Fujian Province March 2012 | Camellia sinensis | Leaves | Strengthening tendons and bones, anti-fatigue, preventing cold. | 69 |
| Green tea | Chongqing Province June 2012 | Camellia sinensis | Leaves | Refreshing oneself, resolving phlegm, promoting digestion, inducing diuresis, detoxify. | 6 |
| Tieguanyin tea | Fujian Province March 2012 | Camellia sinensis | Leaves | Exciting the brain, inducing diuresis strong heart, anti-aging, anticancer and detoxification. | 70 |
| Xihulongjing tea | Hangzhou, Zhejiang Province August 2012 | Camellia sinensis | Leaves | Refreshing oneself, resolving phlegm, promoting digestion, inducing diuresis, detoxify. | 6 |
2.2. DNA barcoding
Four candidate barcodes (rbcL, matK, psbA-trnH and ITS2) were selected based on previous barcoding studies71, 72, 73. We isolated the total genomic DNA from approximately 100 mg of dried powder from each sample using the cetyl trimethylammonium bromide method.74 Extracted DNA was stored in sterile microcentrifuge tubes at −20 °C.
The selected regions were amplified by polymerase chain reaction (PCR) on a PCR system 9700 thermocycler (Gene Co., USA). DNA was amplified in 20 µL of reaction mixtures containing 1 U ExTaq polymerase with 10× ExTaq buffer (100 mmol/L pH 8.3 Tris–HCl, 500 mmol/L of KCl) (Takara, China), 1.25 mmol/L of deoxyribonucleotide triphosphate, 0.05 mmol/L of each primer, and 20 ng of template DNA. Primers and reaction conditions used in the present study were listed in Table 2. The amplified products were sequenced in forward directions with the primers used for amplification in the Beijing Genomics Institute (China). Sequences were assembled and aligned using Bioedit Sequence Alignment editor version 7.0.9.
Table 2.
Primers and reaction conditions used in the present study.
| Gene name | Name of primer and primer sequence 5′-3′ | PCR reaction condition |
|---|---|---|
| rbcL | 724R: TCGCATGTACCTGCAGTAGC | 95 °C 5 min |
| 1F: ATGTCACCACAAACAGAAAC | 94 °C 30 s, 56 °C 30 s, 72 °C 100 s, 35 cycles | |
| 72 °C 7 min | ||
| matK | 3F: CGTACAGTACTTTTGTGTTTACGAG | 95 °C 5 min |
| 1R: ACCCAGTCCATCTGGAAATCTTGGTTC | 95 °C 30 s, 52 °C 30 s, 72 °C 100 s, 32 cycles | |
| 72 °C 7 min | ||
| psbA-trnH | trnH: CGCGCATGGTGGATTCACAATCC | 94 °C 4 min |
| psbA: GTTATGCATGAACGTAATGCTC | 94 °C 30 s, 58 °C 45 s, 72 °C 100 s, 32 cycles | |
| 72 °C 7 min | ||
| ITS2 | ITS2F: ATGCGATACTTGGTGTGAAT | 95 °C 5 min |
| ITS2R: GACGCTTCTCCAGACTACAAT | 95 °C 30 s, 56 °C 30 s, 72 °C 100 s, 35 cycles | |
| 72 °C 7 min | ||
2.3. BLASTN and phylogenetic analysis
BLASTN and the nearest distance method were used to identify obtained relative accurate identification of species. First, the measured DNA sequences from non-Camellia tea were determined using BLASTN75 against the NCBI databases to identify the original plants of non-Camellia tea with similarity over 95%. To optimize correct identifications, DNA sequences of four candidate regions (rbcL, matK, ITS2, psbA-trnH) from non-Camellia tea were determined from the best reciprocal hits. In most cases this corresponded to the sequence with the highest BLAST score. Second, in order to find a suitable reference sequence, all of rbcL, matK, psbA-trnH, and ITS2 were extracted from the National Center for Biotechnology Information (NCBI) database according to the names of origin plant of the non-Camellia tea. After cluster and phylogenetic analysis, individual sequences were eliminated because of their ambiguous nucleotides shorter than 100 bp. Finally, the download sequences including 29 rbcL, 26 matK. 22 psbA-trnH, and 29 ITS2 (Table 3) combining with the sequences of commercial non-Camellia tea were used to construct phylogenetic trees by Mega 5.076 and Clustal X77 with a bootstrap value of 1000 replicates, respectively. Preliminary trees were reconciled by setting the bootstrap value greater than 50%, yielding a more credible consensus tree.
Table 3.
The sequence information in GenBank.
| Species | Genbank no. |
|||
|---|---|---|---|---|
| rbcL | matK | psbA-trnH | ITS2 | |
| Abrus precatorius (1–2) | JN407285; JF738654 | JN407125 | JN406972 | AF467015 |
| Actinodaphne cupularis | – | – | – | HQ697213 |
| Apocynum venetum | – | – | – | DQ449485 |
| Ampelopsis grossedentata | JQ182479 | JF953244; | JF437070 | – |
| Camellia sinensis (1–2) | JN654337 | AJ429305; JN654321 | GQ487359 | FJ004887 |
| Chimonanthus nitens | – | – | – | AY786094 |
| Chimonanthus Zhejiangensis | – | AY525341 | – | AY786106 |
| Chimonanthus salicifolius | HQ427177 | HQ427325 | HQ427018 | AY786102 |
| Chrysanthemum x morifolium | – | EU334382; HM989758 | EF091621 | EF091597 |
| Chrysanthemum indicum | JF949971 | – | JF949971 | EF577298; JN315940 |
| Clerodendranthus spicatus | GQ464985 | FJ513161 | FJ513103; GQ464982 | HM595465 |
| Cyclocarya paliurus (1–2) | AY263942; AY147094 | AY147098 | – | AF179583 |
| Dasiphora fruticosa | – | AB458578 | JN044379 | – |
| Engelhardia roxburghiana | – | – | – | AF303801 |
| Epipremnum aureum (1–2) | JQ734504; JN090003 | JN090088 | – | – |
| Fagopyrum tataricum | JN187117 | JF829984 | JQ807577 | AB000339 |
| Gynostemma pentaphyllum | AY968523 | AY968451 | EF621687 | FJ980303 |
| Hedyotis hedyotidea | HM752999 | HM753079 | HM640314; HM640334 | HQ148756 |
| Ilex aquifolium | – | JN896160 | – | – |
| Ilex kaushue | JF942007 | JF954101 | JN044945 | – |
| Ilex latifolia | JF942011 | HQ427289 | JN044949 | AF200592; AY140215 |
| Ilex paraguariensis | FJ394634 | GQ248141 | GQ248322 | FJ394705 |
| Lethariella cashmeriana | – | – | – | AF297743; DQ980014 |
| Lethariella sernanderi | – | – | – | AF297744 |
| Ligustrum robustum | JF942292 | JF954385 | JN045240 | – |
| Litsea coreana | – | – | – | AF272286 |
| Lithocarpus litseifolius | – | EF057121 | – | EF057112 |
| Mallotus oblongifolius | JF738963 | – | – | – |
| Poacynum pictum | – | – | – | DQ451830 |
| Potentilla fruticosa | PFU06818 | – | AM114863 | AF163478 |
| Scutellaria amoena | HQ676585 | JX981408 | HQ680371 | – |
| Scutellaria baicalensis | GQ374130 | JX981417; HQ676586; FJ513169 | HQ680366 | JF421544; FJ609732 |
| Scutellaria scordifolia | HM590110 | HQ839713 | FJ513143 | FJ546875 |
| Scutellaria viscidula | HQ676583 | HQ676587 | HQ680369 | – |
| Scoparia dulcis | JQ593281 | JQ588687; JQ588683 | – | AY963776 |
| Stevia rebaudiana | AY215182 | AY215865 | AY215611 | AB457301 |
| Thamnolia vermicularis | – | – | – | JQ409350 |
| Verbena officinalis (1–3) | HM850444; JF950020; JN893754 | HM850974 | GQ435188; HE966861 | GQ434586 |
–Species without the gene sequence in NCBI.
3. Results
3.1. Traditional uses
According to the literature (Table 1), original plants of 33 kinds of non-Camellia tea are distributed across 29 genera in 22 families. The most widely used plant portions are leaves (26), followed by flowers (7), herbs (3), stems (3), and the least used plant portions are seeds (1) and branches (1). The investigated non-Camellia teas have a variety of therapeutic applications (Table 1). The non-Camellia teas have been mainly used for three therapeutic effects: (I) heat-clearing tea (20), such as vine tea, qingqiangliu tea, yeju tea; (II) digestant tea (8), such as laoying tea, zhegu tea, liangwang tea; (III) health tea (9), such as jiaogulan tea, kuqiao tea, lvluohua tea.
3.2. DNA extraction, PCR, and sequencing success
All samples were extracted through CTAB method successfully. At the same time, the PCR success rates of rbcL, matK, psbA-trnH and ITS2 were 91.9% (34/37), 75.8% (28/37), 78.4% (29/37) and 100% (37/37), respectively. All the PCR products were sequenced successfully. In all sequences, rbcL sequence lengths ranged from 625 bp to 692 bp; matK sequence lengths ranged from 780 bp to 860 bp. psbA-trnH sequence lengths were from196 bp to 587 bp, and ITS2 sequence lengths were from 426 bp to 506 bp.
3.3. Species identification based on BLASTN
The rbcL, matK, psbA-trnH, and ITS2 sequences of the non-Camellia teas were also blasted against the NCBI database with maximum identity that are greater than 95% using an e-value below 0.0 to determine the difference between the original plants. The closest match in the database was recorded and DNA sequences of non-Camellia tea were determined from the reciprocal best hits.
Taking into account the uncertainties arising from incomplete databases, shared barcodes, ambiguous common names and sequencing without success, BLASTN analysis of the rbcL data showed 32 commercialized samples were assigned to species, one sample to genus, and one sample was not recorded in the GenBank (Table 3). But because rbcL identification ability is limited, only 11 commercialized samples matched with the original plants, including 7 kinds of non-Camellia teas and 4 kinds of traditional tea (Table 4).
Table 4.
BLASTN identification result of non-Camellia tea.
| No. | Commodity tea name | rbcL | matK | psbA-trnH | ITS2 |
|---|---|---|---|---|---|
| BYC-1 | Paraguay tea | Ilex sp. | Ilex aquifolium | Ilex paraguariensis# | Ilex paraguariensis# |
| BYC-2 | Baixue tea | Ampelopsis brevipedunculata | Ampelopsis grossedentata | Ampelopsis grossedentata | Caprifoliaceae |
| BYC-3 | Blank tea | Camellia sinensis# | Camellia sinensis# | Camellia cuspidata | Camellia sinensis# |
| BYC-4 | Big leaf Kuding tender tea | Ilex kaushue# | Ilex aquifolium | Ilex pentagona | Ilex latifolia# |
| BYC-5 | Big leaf Kuding old tea | N | N | N | Ilex latifolia# |
| BYC-6 | Duosuike sweet tea | Quercus nigra | Fagaceae | Quercus phillyraeoides | Lithocarpus sp. |
| BYC-7 | Fengwei tea | Elsholtzia stauntonii | Mosla chinensis | Mosla chinensis | Lamiaceae |
| BYC-8 | Gongju tea | Chrysanthemum mutellinum# | Chrysanthemum×morifolium# | Chrysanthemum indicum | Chrysanthemum morifolium# |
| BYC-9 | Green tea | Camellia sinensis# | Compositae | Camellia cuspidata | Camellia sp. |
| BYC-10 | Guangxi sweet tea | N | N | N | Rubus crataegifolius |
| BYC-11 | Hongxue tea | Panax ginseng | N | N | Scutellaria baicalensis |
| BYC-12 | Huangqin tea | Scutellaria rehderiana | Scutellaria baicalensis# | Scutellaria baicalensis# | Scutellaria baicalensis# |
| BYC-13 | Jiaogulan tea | Gynostemma pentaphyllum# | Gynostemma pentaphyllum# | Gynostemma pentaphyllum# | Gynostemma pentaphyllum# |
| BYC-14 | Kuqiao tea | Panax ginseng | N | N | Stevia rebaudiana |
| BYC-15 | Laoying tea | Litsea japonica | Cinnamomum brenesi | N | Litsea coreana# |
| BYC-16 | Liangwang tea | Macropanax dispermus | Schefflera heptaphylla | Metapanax delavayi# | Metapanax delavayi# |
| BYC-17 | Luobuhongma tea | Apocynum cannabinum | Apocynum cannabinum | Apocynum cannabinum | Apocynum venetum# |
| BYC-18 | Luobubaima tea | Apocynum cannabinum | Apocynum cannabinum | Apocynum cannabinum | Poacynum hendersonii# |
| BYC-19 | Luohan tea | Engelhardtia fenzelii | Alfaropsis roxburghiana# | Unknown | Engelhardtia roxburghiana# |
| BYC-20 | Lvluohua tea | Edgeworthia chrysantha | Thymelaeaceae | Thymelaeaceae | Edgeworthia chrysantha |
| BYC-21 | Mabiancao tea | Verbena bracteata | Verbena rigida | Verbena stricta | Verbena officinalis# |
| BYC-22 | Niubaiteng sweet tea | Hedythyrsus sp. | Hedyotis hedyotidea# | Hedyotis hedyotidea# | Hedyotis hedyotidea# |
| BYC-23 | Qingqianliu tea | Unknown | Cyclocarya paliurus# | Unknown | Cylocarya paliurus# |
| BYC-24 | Sishi tea | Bacopa sp. | N | Gratiola neglecta | Callicarpa poilanei |
| BYC-25 | Shen tea | Clerodendranthus spicatus# | N | N | Orthosiphon aristatus# |
| BYC-26 | Shiliang tea | N | Chimonanthus salicifolius S. Y. H# | Chimonanthus salicifolius# | Chimonanthus salicifolius S.Y. H# |
| BYC-27 | Shiya tea | Panax ginseng | N | N | Adinandra elegans |
| BYC-28 | Tianyeju tea | Stevia rebaudiana# | Stevia rebaudiana# | Stevia rebaudiana# | Stevia rebaudiana# |
| BYC-29 | Tieguanyin tea | Camellia sinensis# | Compositae | Camellia chekiangoleosa | Camellia sp. |
| BYC-30 | Vine tea | Ampelopsis brevipedunculata | Ampelopsis grossedentata# | Ampelopsis grossedentata# | Compositae |
| BYC-31 | Xihulongjing tea | Camellia sinensis# | Camellia sinensis# | Camellia sinensis# | Camellia sinensis# |
| BYC-32 | Xiangsiteng tea | Abrus precatorius# | Abrus precatorius# | Abrus precatorius# | Abrus precatorius# |
| BYC-33 | Xiangfeng tea | Panax ginseng | Chimonanthus salicifolius# | Chimonanthus salicifolius# | Chimonanthus Zhejiangensis# |
| BYC-34 | Small leaf Kuding tea | Panax ginseng | N | Ligustrum robustum# | Ligustrum robustum# |
| BYC-35 | Yaowang tea | Potentilla fruticosa# | Draba lanceolata | N | Dasiphora phyllocalyx |
| BYC-36 | Yeju tea | Chrysanthemum mutellinum | Chrysanthemum×morifolium | Chrysanthemum indicum# | Chrysanthemum indicum# |
| BYC-37 | Zhegu tea | Mallotus sp. | N | Mallotus apelta | Mallotus sp. |
N, Sequencing without success; Unknown, not identification using DNA barcoding. #Identification results are correct.
The matK analysis data showed 24 commercialized samples were assigned to species, 4 to family. As one common primer of DNA barcoding, matK is suitable for genera identification. In this report, only 13 commercialized samples matched the designation of the original plants: 11 kinds of non-Camellia teas and 2 kinds of traditional teas (Table 4).
BLASTN analysis of the psbA-trnH data indicated 26 samples were assigned to species, 1 to family, and 2 were not recorded in GenBank. Only 13 commercialized samples matched the designation of the original plants: 12 kinds of non-Camellia teas and 1 kind of traditional tea (Table 4).
The ITS2 BLASTN result indicated 30 commercialized samples were assigned to species, 4 to genera, 3 to family. Of these, 23 commercialized samples matched the designation of the original plants: 21 kinds of non-Camellia teas and 2 kinds of traditional teas (Table 4).
In summary, using one or more DNA data by BLASTN analysis, 23 non-Camellia teas were assigned to their original plants successfully.
3.4. Species identification based on phylogenetic tree
Some original plants of the non-Camellia tea listed on labels lacked GenBank records. So the reference databases only comprised 29 rbcL, 26 matK, 22 psbA-trnH and 29 ITS2 sequences obtained by downloading all the sequences that yielded an e-value of 0.0 in the initial BLAST searches, and the measured DNA sequences comprised of 34 rbcL, 28 matK, 32 psbA-trnH and 37 ITS2. All the sequences were also used to construct phylogenetic trees using Mega 5.0 and Clustal X, with a bootstrap value of 1000 replicates, respectively. Moreover, we reconciled preliminary trees by setting the bootstrap value greater than 50% to yield a more credible consensus tree.
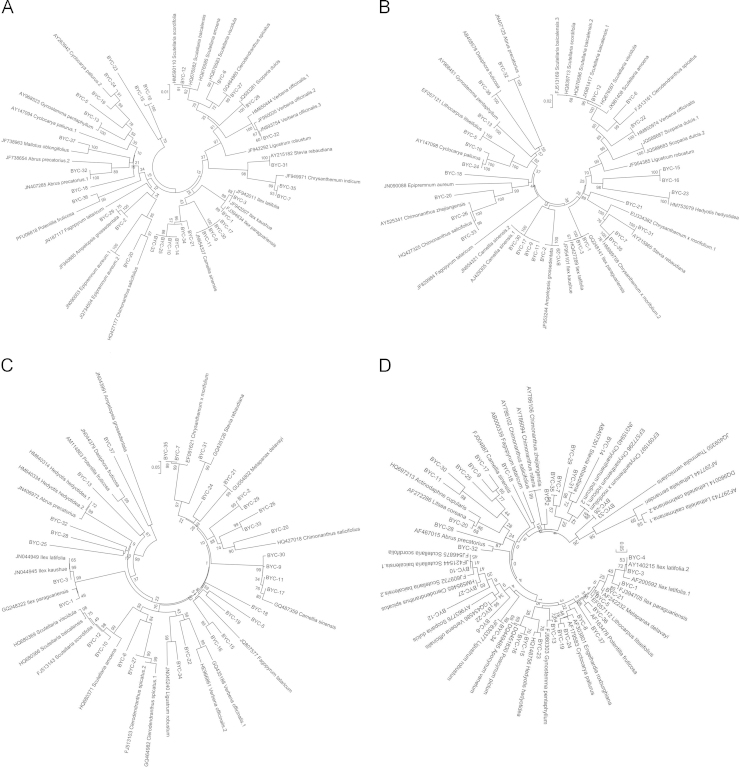
In the rbcL, matK, psbA-trnH and ITS2 tree, 13 (39%), 10 (30%), 8 (30%) and 12 (36%) commercialized samples of identification result accord with the original plants of non-Camellia tea, respectively (Fig. 1).
Figure 1.
Phylogenetic trees constructed by DNA barcoding sequences. A, rbcL; B, matK; C, psbA-trnH; D, ITS2.
4. Discussion
4.1. Non-Camellia tea of misidentification and potential risk
Non-Camellia tea has been widely used in China for centuries. However, correct identification of some of these teas has remained a problem. According to our investigation, some non-Camellia teas from several original plants are used wildly in different regions. For instance, Huangqin tea comes from at least four original plants of the Scutellaria genus, including Scutellaria baicalensis, S. scordifolia, S. amoena, S. viscidula, etc. They were distributed in more than ten provinces in China. However, it is not easy to distinguish differing species of the same genus. Based on experience, the villagers generally seek to collect the herb that has the similar morphological species. Even some people collected other genus species to use as Huangqin tea, such as Dracocephalum rupestre. Therefore, it is of great importance to establish an unequivocal identification system for quality control of non-Camellia tea for safety and optimum therapeutic use.
4.2. Accuracy of authentication based on BLASTN and phylogenetic tree
In our BLASTN results, 11, 11, 12 and 21 non-Camellia teas were identified by rbcL, matK, psbA-trnH and ITS2, respectively. That means the ITS2 have more different loci than chloroplast regions. Of course, the plant species lacking GenBank records should not be ignored.
In all phylogenetic trees, yeju tea, gongju tea and all downloaded species from Chrysanthemum were grouped in a clade with strong support, but both samples did not match with their original plants. It means that yeju tea, gongju tea can be identified at genera level by a signal DNA barcoding marker. At the same time, the possibility of mixed-use between yeju tea and gongju tea also should be considered. The identification of genera also existed in Ilex (paraguay tea and big leaf kuding tea) in rbcL and ITS2 trees. However, additional data are needed for further authentication. Chimonanthus (shiliang tea and xiangfeng tea) became more interesting and different because of the diverse plant materials of shiliang tea. Further research with broader sampling of these species will advance the identification work.
Laoying tea is from Litsea coreana var. lanuginose (Lauraceae) and Actinodaphne cupularis (Hemsl.) Gemble (Lauraceae), and the major original plant is the former. In the ITS2 tree, all downloaded sequences of species from Lauraceae were in the same clade with the BYC-20 as sister group, and the commercial sample has much closer relationship with L. coreana. This means that laoying tea can be most accurately identified by ITS2.
Finally, some non-Camellia teas, such as hongxue tea, baixue tea, lvluohua tea and kuqiao tea, were not accurately matched to their original plants in all trees, indicating significant errors associated with the accuracy of DNA barcording among these species. The limited data in all trees among these species probably contribute to these errors. These results show that commercial non-Camellia teas should be identified with more accurate DNA barcoding sequences and broader sampling techniques.
4.3. Safety evaluation based on BLASTN and phylogenetic tree
Consumers have become more interested in the beneficial effects of tea to improve health. However, non-Camellia tea is not easily identified by morphological characteristics in the market. Adulteration and misidentification are common in the non-Camelliaea teas market, which might be malgenic or lethal. Several kinds of non-Camelliaea tea (e.g., Verbena officinalis L.) are considered abortifacients, and, if unknowingly consumed by a pregnant woman, could cause miscarriage. Luobuma tea from Apocynum (Apocynaceae) also is difficult to morphologically distinguish from some toxic plants in Apocynaceae. In our study, V. officinalis L. was accurately identified by DNA barcoding. Luobuhongma tea and luobubaima tea were identified at the genera level by BLASTN and both samples were grouped in a branch separated with other kinds of non-Camelliaea tea. However, we did note that DNA barcoding technology can׳t identify some of non-Camellia teas, such as fengwei tea, vine tea and sishi tea. This is because there are only a limited number of these sequences from original plants in GenBank. As a consequence, it is not easy to evaluate the safety of non-Camelliaea teas. More original plant sequences need to be submitted to GenBank in order to improve the safety of non-Camellia teas
5. Conclusions
Non-Camellia tea has been ingested for centuries for cultural and health purposes. These teas have been used to protect health and prevent diseases, such as cancer, hyperlipidemia, hypertension and hyperglycemia. In recent years, with the development and utilization of non-Camellia tea, only few non-Camellia teas have been developed into beverages, such as jiaogulan tea78 and kuding tea79. But published data concerning the toxicity of some kinds of non-Camellia tea are very limited; the pharmacological activity and mechanisms of action for most kinds of non-Camellia tea have not been systematically studied. Additional research on all of these aspects of non-Camellia tea is needed.
In this study, molecular results revealed that DNA barcoding technology is a viable and effective method to identify non-Camelliaea tea. DNA barcoding technology can offer an effective method to help provide more accurate ingredient labels to consumers, thereby helping improve the safety of food and botanicals80. This is particularly pertinent in an increasingly global economy where longer and more complex market chains increase distances between suppliers and consumers, and where regulatory agencies are becoming more stringent with food and botanicals81, 82.
Acknowledgment
This research was financially supported by the “Twelfth Five-year Plan” Program supported by the Ministry of Science and Technology (2012BAI28B02).
Footnotes
Peer review under responsibility of Institute of Materia Medica, Chinese Academy of Medical Sciences and Chinese Pharmaceutical Association.
Contributor Information
Minhui Li, Email: li_minhui@aliyun.com.
Peigen Xiao, Email: xiaopg@public.bta.net.cn.
References
- 1.He Z.D., Peng Y., Xiao P.G. Science Press; Beijing: 2010. Kudingcha research and development. p. 1–25. [Google Scholar]
- 2.Xiao W., Peng Y., Xu L.J., He C.N., Liu Y.Z., Xiao P.G. Preliminary exploration of the diversity of the Chinese tea culture. Mod Chin Med. 2011;13:60–62. [Google Scholar]
- 3.He C.N., Peng Y., Xiao W., Lin M.H., Cui Z.H., Yan Y. Huang qin tea of application history and research status. Mod Chin Med. 2011;13(3–7):19. [Google Scholar]
- 4.Ni H.C., Li J., Jin Y., Cheng W.M., Peng L., Zhang J.Y. Effects of total flavonoids of Litsea coreana level on nonalcoholic steatohepatitis. Chin Pharmacol Bull. 2006;22:591–594. [Google Scholar]
- 5.Chen X.J., Chen X.F., Li M., Huang S.L. Total flavone of Ampelopsis grossedentata lipid-lowering effect research. Guangxi J Trad Chin Med. 2001;5:52–54. [Google Scholar]
- 6.Li L., Xu L.J., Peng Y., Shi R., Xiao P. Comparison of green tea and four other kind of teas. China J Chin Mater Med. 2011;36:5–10. [PubMed] [Google Scholar]
- 7.Hsu C.K., Leo P., Shastry D., Meggs W., Weisman R., Hoffman R.S. Anticholinergic poisoning associated with herbal tea. Arch Intern Med. 1995;155:2245–2248. [PubMed] [Google Scholar]
- 8.Cupp M.J. Humana Press; Totowa: 2000. Toxicology and clinical pharmacology of herbal products. [Google Scholar]
- 9.Guo S.Z. Kuding tea: not everyone should drink. Fam Med. 2009;7:43. [Google Scholar]
- 10.Heinrich M. The identification of medicinal plants. J Ethnopharmacol. 2007;111:440. [Google Scholar]
- 11.Schindel D.E., Miller S.E. DNA barcoding a useful tool for taxonomists. Nature. 2005;435:17. doi: 10.1038/435017b. [DOI] [PubMed] [Google Scholar]
- 12.Godfray H.C.J. Towards taxonomy׳s ׳glorious revolution׳. Nature. 2002;420:461. doi: 10.1038/420461a. [DOI] [PubMed] [Google Scholar]
- 13.Hebert P.D.N., Gregory T.R. The promise of DNA barcoding for taxonomy. Systematic Biol. 2005;54:852–859. doi: 10.1080/10635150500354886. [DOI] [PubMed] [Google Scholar]
- 14.Fu H., Wang L.S., Chen Y.H., Liao R. A study on nutritional components of two different Lichen teas from Yunnan. Nat Prod Res Develop. 2005;17:340–343. [Google Scholar]
- 15.Zhang Y.B., Zhang Y.M., Cui K.C. The ornamental value, development and utilization of Xue tea. Spec Econ Anim Plant. 2012;4:31. [Google Scholar]
- 16.Chen S.K., Feng Y.X. Vol. 45. Science Press; Beijing: 1999. Aquifoliaceae. (Flora reipublicae popularis sinicae). p. 105–10. [Google Scholar]
- 17.Gu J., Xu L.J., Peng Y., Xiao W., Chen Z.D., Xiao P.G. Investigation of original plants of Kuding tea products and the character identification. J Chin Med Mater. 2011;34:196–199. [Google Scholar]
- 18.Xie Z.W., Fang C.S., Zhu Z.Y. People׳s Medical Publishing House; Beijing: 1989. The compilation of Chinese herbal medicine. p. 256–9. [Google Scholar]
- 19.Huang C.J., Zhang Y.T., Xu Y.C. Vol. 22. Science Press; Beijing: 1998. Fagaceae. (Flora reipublicae popularis sinicae). p. 201–2. [Google Scholar]
- 20.Yang C.Y., Chen Q.B. Extraction and content׳s determination of Lithoarpus Polystachyus Rehd׳s Lavonoids. Guangdong Chem Indus. 2011;2:152–154. [Google Scholar]
- 21.Editorial Board of Zhong Hua Ben Cao . Vol. 19. Science Press; Shanghai: 1999. (Zhong Hua Ben Cao). p. 34–5. [Google Scholar]
- 22.Liu J.Q., Shen Q.Q., Liu J.S., Wu D.L., Wang J.T. Studies on the chemical constituents from Chrysanthemum morifolium Ramat. China J Chin Mater Med. 2001;26:547–548. [PubMed] [Google Scholar]
- 23.Li S.G. Sweet tea, a new Rubus (Rosaceae) from Guangxi. Guihaia. 1981;1:17–19. [Google Scholar]
- 24.Wang J.X., Lü H.C. Studies on the chemical constituents of Rubus suavissimus S. Lee. J Chin Med Maters. 2007;30:800–802. [PubMed] [Google Scholar]
- 25.Wu L.C., Huang G.H. Quality determination of quercetin and kaempferol in Rubus suavissimus S. Lee from Guangxi by HPLC. Chin Pharm Affair. 2008;22:571–573. 602. [Google Scholar]
- 26.Zhao C., Zhang X.H. Study on the effects of Hongxue tea mixture on small Rats׳ anti-fatigue. Yunan J Trad Chin Med Mater Med. 2005;26:27–29. [Google Scholar]
- 27.Zhan J.F., Lu S.M., Qu G.F., Sun L., Meng Z.Y., Cao Q.E. Analysis of volatile components in Hongxue tea and Baixue tea by SDE–GC–MS method. Nat Prod Res Dev. 2008;20:657–661. [Google Scholar]
- 28.Wu Z.Y., Li X.W. Vol. 65. Science Press; Beijing: 1977. Lamiaceae. (Flora reipublicae popularis sinicae). p. 192–199, 232. [Google Scholar]
- 29.Chen S.K. Vol. 73. Science Press; Beijing: 1986. Cucurbitaceae. (Flora reipublicae popularis sinicae). p. 265–77. [Google Scholar]
- 30.Chen Y.S., Chen H.D. Human immortality-Gynostemma tea. China Tea Process. 1996;1:36–38. [Google Scholar]
- 31.Yu C.D., Chen Z.L., Mei Q.X. Progress of studies on pharmacological effect of Gynostemma Pentaphyllam tea. Lishizhen Med Mater Med Res. 2008;19:2296–2298. [Google Scholar]
- 32.Gong F.Y. Comparison and analysis on the content of total flavonoids in Liangshan Tartary Buckwheat tea. Hubei Agric Sci. 2011;50:3811–3814. [Google Scholar]
- 33.Yang X.J., Huang P.H. Vol. 31. Science Press; Beijing: 1982. Lauraceae. (Flora reipublicae popularis sinicae). p. 201, 202, 296. [Google Scholar]
- 34.Li T.S. Studies on investigation and exploitation of the resources of Laoyin tea. J Guizhou Tea. 1995;84:10–13. [Google Scholar]
- 35.Yu J.P., Gu L.Q. The chemical constituents of Laoying tea from Guizhou. J Plant Res Environ. 2001;10:61–62. [Google Scholar]
- 36.Li J., Zhang J. Advances in studies of Litsea coreana Levl.var. J Sichuan Agric Univ. 2005;23:248–252. [Google Scholar]
- 37.Hou F. Resource development and utilization of Nothopanax delavayi in Chengjiang. For Prod Spec Chin. 2006;2:60–61. [Google Scholar]
- 38.Jiang Y., Li B.T. Vol. 63. Science Press; Beijing: 1977. Apocynaceae. (Flora reipublicae popularis sinicae). p. 157–9. [Google Scholar]
- 39.Zhang W.M., Xiao Z.C., Gu G.P., Zhang G.L., Qian X.S. Resources utilization of Apocynum and its classification. Chin Wild Plant Res. 2006;25:15–19. [Google Scholar]
- 40.Wang J., Xu L., Zhang W.M., Liu Q.T., Gu G.P., Lu C.M. Quality comparison on the Apocynum venetum tea collected in different periods. J Anhui Agric Sci. 2010;38:1235–1237. [Google Scholar]
- 41.Qiang W., Zhang Y.C., Liu X.H., Song J.P. Analysis of volatile components of Apoacynum venetum leaves by HSGC–MS. Xinjiang J Trad Chin Med. 2009;27:46–48. [Google Scholar]
- 42.Wei L.T., Feng J., Mo S.R., Lan M.X. Anti-cancer effect of the extract from the leaves of Ilex kudingcha C.J.Tseng and Engelhardia roxburghiana Wall. in vitro. China Med Her. 2013;10:18–19. [Google Scholar]
- 43.Han L., Guo X.L., Feng Y.F., Lin M.N. Study on the chemical constituents of volatile oil from Lvluohua. Chin J Ethnomed Ethnopharm. 2009;9:148–149. [Google Scholar]
- 44.Zhu Q., Zhang K., Du Z.Y., Xu X.T., Jiang Y.F., Yu X.H. Study on inhibitive effect of extracts from Scindapsus aureus on α-glucosidase and its antioxidant activity. J Chin Med Mater. 2009;32:89–92. [PubMed] [Google Scholar]
- 45.Editorial Board of Zhong Hua Ben Cao . Vol. 18. Science Press; Shanghai: 1991. (Zhong Hua Ben Cao). p. 592. [Google Scholar]
- 46.Xin N., Zhu H., Liao Y.K. Pharmacognostic identification of four sweet teas in Guangxi. J Chin Med Mater. 2003;26:549–552. [Google Scholar]
- 47.Huang C.Y., Liao Y.K., Lin A.P. Pharmacognostic identification of dried root and stem of Hedyotis hedyotidea. Chin Trad Herbal Drugs. 2001;32:549–551. [Google Scholar]
- 48.Gugliucci A. Antioxidant effects of Ilex paraguariensis: induction of decreased oxidability of human LDL in vivo. Biochem Biophy Res Commun. 1996;224:338–344. doi: 10.1006/bbrc.1996.1030. [DOI] [PubMed] [Google Scholar]
- 49.Gorzalczany S., Filip R., Alonso M.D.R., Miño J., Ferraro G.E., Acevedo C. Choleretic effect and intestinal propulsion of ‘mate’(Ilex paraguariensis) and its substitutes or adulterants. J Ethnopharmacol. 2001;75:291–294. doi: 10.1016/s0378-8741(01)00179-9. [DOI] [PubMed] [Google Scholar]
- 50.Xiong J.H., Liu Z.H. Research progress in chemical component and pharmacologic activities of Ilex paraguarensis. Guangdong Agric Sci. 2006;12:129–132. [Google Scholar]
- 51.Kuang K.Z., Lu A.M. Vol. 21. Science Press; Beijing: 1979. Juglandaceae. (Flora reipublicae popularis sinicae). p. 19. [Google Scholar]
- 52.Editorial Board of Zhong Hua Ben Cao . Vol. 5. Science Press; Shanghai: 1999. (Zhong Hua Ben Cao). p. 370–1. [Google Scholar]
- 53.Zhou Y.Y., Fu X.X., Shang X.L., Yang W.X., Fang S.Z. Preliminary study on the genetic diversity of germplasm for Cyclocarya paliurus revealed by SRAP markers. Genom Appl Biol. 2011;30:40–46. [Google Scholar]
- 54.Editorial Board of Zhong Hua Ben Cao . Vol. 20. Science Press; Shanghai: 1991. (Zhong Hua Ben Cao). p. 389–91. [Google Scholar]
- 55.Wu Z.Y., Xuan S.J. Vol. 66. Science Press; Beijing: 1977. Lamiaceae. (Flora reipublicae popularis sinicae). p. 574–577. [Google Scholar]
- 56.Li B.T. Vol. 30. Science Press; Beijing: 1977. Calycanthaceae. (Flora reipublicae popularis sinicae). p. 7–10. [Google Scholar]
- 57.Hu C.Y. Xiang Feng tea-Chimonanthus salicifolius S. Y. Hu. Forest Sci Tech. 2005;2:34. [Google Scholar]
- 58.Chen M.Z., Yu J., She G.Z., Zhu Y.K. Analysis of nutritional components and medicinal components in wild Shi Ya Cha. Nat Prod Res Devel. 1996;8:84–86. [Google Scholar]
- 59.Lin L.G. Vol. 50. Science Press; Beijing: 1998. Theaceae. (Flora reipublicae popularis sinicae). p. 28–9. [Google Scholar]
- 60.Liu C.L., Yang X., Bai D.M., Li Y., Xiao H.M. Study on the extraction and biological activities of water soluble polysaccharide from Adinandra Nitride. J Liaoning Univ Trad Chin Med. 2010;12:10–12. [Google Scholar]
- 61.Qiu L.Q., Miao B.M., Chang M.C. Vol. 61. Science Press; Beijing: 1992. pp. 155–156. (Flora reipublicae popularis sinicae). [Google Scholar]
- 62.Editorial Board of Zhong Hua Ben Cao . Vol. 21. Science Press; Shanghai: 1991. (Zhong Hua Ben Cao). p. 801–5, 980, 981. [Google Scholar]
- 63.Li C.L. Vol. 48. Science Press; Beijing: 1998. Vitaceae. (Flora reipublicae popularis sinicae). p. 53. [Google Scholar]
- 64.Luo Z.Y., Fu X.F., Wu M.C. Progress on the study of Ampelopsis Grossedentata. Food Sci. 2005;26:513–517. [Google Scholar]
- 65.Wei Z., Li J.N. Vol. 40. Science Press; Beijing: 1994. (Flora reipublicae popularis sinicae). p. 123–4. [Google Scholar]
- 66.Editorial Board of Zhong Hua Ben Cao . Vol. 11. Science Press; Shanghai: 1999. (Zhong Hua Ben Cao). p. 307–8. [Google Scholar]
- 67.Hu Y.L., Wang W.Y., Li M.H., Xu L.J., Peng Y., Xiao P.G. Research progress in Yaowang Tea. Drugs Clin. 2013;28:236–241. [Google Scholar]
- 68.Liu G.M., Li J.L., Wang X.J., He Q.X. A study on ethnobotany of Mallotus Oblongifolius in Hainan. J Hainan Norm Univ (Nat Sci) 2007;20:167–172. [Google Scholar]
- 69.Hou D.Y., Hui R.H., Liu X.Y., Tian R., Zhu Y.Q. Comparison of the antioxidation effects of green tea, black tea and wulong tea. Food Sci. 2006;27:90–93. [Google Scholar]
- 70.Huang M.T., Zhang J., Wu Y.S. Study on chemical constituents of anxi Tieguanyin Tea. Food Drug. 2008;10:37–38. [Google Scholar]
- 71.Kress W.J., Wurdack K.J., Zimmer E.A., Weigt L.A., Janzen D.H. Use of DNA barcodes to identify flowering plants. Proc Natl Acad Sci USA. 2005;102:8369–8374. doi: 10.1073/pnas.0503123102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Newmaster S.G., Fazekas A.J., Ragupathy S. DNA barcoding in land plants: evaluation of rbcL in a multigene tiered approach. Botany. 2006;84:335–341. [Google Scholar]
- 73.Kress W.J., Erickson D.L. A two-locus global DNA barcode for land plants: the coding rbcL gene complements the non-coding trnH-psbA spacer region. PLoS One. 2007;2:508. doi: 10.1371/journal.pone.0000508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Attitalla I.H. Modified CTAB method for high quality genomic DNA extraction from medicinal plants. Pak J Biol Sci. 2011;14:998–999. [PubMed] [Google Scholar]
- 75.Altschul S.F., Madden T.L., Schaffer A.A., Zhang J., Zhang Z., Miller W. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 1997;25:3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Tamura K., Peterson D., Peterson N., Stecher G., Nei M., Kumar S. MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol. 2011;28:2731–2739. doi: 10.1093/molbev/msr121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Jeanmougin F., Thompson J.D., Gouy M., Higgins D.G., Gibson T.J. Multiple sequence alignment with Clustal X. Trends Biochem Sci. 1998;23:403–405. doi: 10.1016/s0968-0004(98)01285-7. [DOI] [PubMed] [Google Scholar]
- 78.Yu X., Wang D.P., Huang J.P. Preparation of Gynostemma pentaphyllum drink. Food Technol. 2001;4:45,46,48. [Google Scholar]
- 79.Xiao W.J., Yang W.L., Li M.L. Processing technique of kudingcha drink, the screening of material combination. J Hunan Agric Univ (Nat Sci) 2001;27:221–223. [Google Scholar]
- 80.Prince L.M., Parks C.R. Phylogenetic relationships of Teaceae inferred from chloroplast DNA sequence data. Am J Bot. 2001;88:2309–2320. [PubMed] [Google Scholar]
- 81.Ebeler S.E., Takeoda G.R., Winterhalter P. Oxford University Press; Washington, DC: 2007. Authentication of food and wine. p. 364. [Google Scholar]
- 82.Chen S.L., Yao H., Han J.P., Liu C., Song J.Y., Shi L.C. Validation of the ITS2 region as a novel DNA barcode for identifying medicinal plant species. PLoS One. 2010;5:8613. doi: 10.1371/journal.pone.0008613. [DOI] [PMC free article] [PubMed] [Google Scholar]