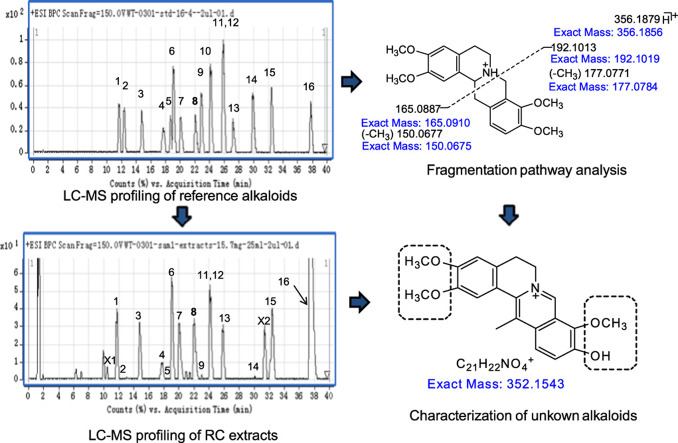
Abstract
Since alkaloids are the major active constituents of Rhizoma corydalis (RC), a convenient and accurate analytical method is needed for their identification and characterization. Here we report a method to profile the alkaloids in RC based on liquid chromatography-tandem quadrupole time-of-flight mass spectrometry (LC–Q-TOF-MS/MS). A total of 16 alkaloids belonging to four different classes were identified by comparison with authentic standards. The fragmentation pathway of each class of alkaloid was clarified and their differences were elucidated. Furthermore, based on an analysis of fragmentation pathways and alkaloid profiling, a rapid and accurate method for the identification of unknown alkaloids in RC is proposed. The method could also be useful for the quality control of RC.
KEY WORDS: LC–Q-TOF-MS/MS, Alkaloid, Fragmentation pathway, Rhizoma corydalis
Graphical abstract
A total of 16 alkaloids in Rhizoma corydalis (RC) belonging to four different classes were identified by comparison with authentic standards based on LC–Q-TOF-MS. Based on the analysis of fragmentation pathways and alkaloid profiling, a rapid and accurate method for the identification of unknown alkaloids in RC is proposed.
1. Introduction
Rhizoma corydalis (RC), known as ‘Yuan-hu’ in China, is prepared from the dried tubers of Corydalis Yanhusuo W.T. Wang. It is a traditional Chinese medicine (TCM) used for hundreds of years to promote blood circulation, reinforce vital energy and act as an analgesic to treat headache, chest pain, epigastric pain, abdominal pain, backache, arthralgia and trauma1. It has also been widely used to treat cardiovascular disease2. As a part of the development of TCM, there is an increasing need to understand the mechanism of action of important therapeutic herbs, such as RC. Previous phytochemical studies have revealed that alkaloids are the main active components of RC, of which 20 alkaloids have been isolated so far3, 4, 5. However, since the alkaloids are difficult to isolate and purify, a rapid and accurate method of identification and characterization is required for the quality control and formulation of this natural medicine6, 7, 8.
Recently liquid chromatography-tandem quadrupole time-of-flight mass spectrometry (LC–Q-TOF-MS/MS) has been frequently applied in the characterization of crude extracts of TCM especially for the minor compounds9, 10, 11, 12, 13. The technique provides accurate mass measurement for both precursor and product ions which gives a higher order mass identification. In this paper, LC–Q-TOF-MS/MS was applied to the analysis of the alkaloid constituents in RC during which 16 alkaloids were identified and divided into four types on the basis of their fragmentation patterns. Furthermore, based on the fragmentation patterns, an analytical method for the identification of unknown alkaloids is proposed.
2. Materials and methods
2.1. Material
HPLC grade methanol and formic acid were purchased from Merck (Germany) and ROE Scientific Inc. (USA), respectively. Water was purchased from the Wahaha Company (China). The Corydaline standard was purchased from Sigma (USA) and standards of the other 15 alkaloids were purchased from Chengdu Herbpurify Co., Ltd. (China). RC was purchased from the Tong-Ren-Tang Pharmaceutical Company (Beijing, China).
2.2. Sample preparation
About 5 g RC was extracted by refluxing for 2 h with 70% ethanol (v/v, 2× 100 mL). After filtration, solvent was removed from the combined extract by rotary evaporation at 60 °C. The residue (15.7 mg) was then reconstituted in 25 mL 70% methanol (v/v) and filtered through a 0.22 µm membrane before analysis. Stock solutions (approximately 0.4 mmol/L) of the 16 alkaloid standards were prepared in 70% methanol (v/v) and diluted 1:100 to yield a mixed solution 4 µmol/L in each compound.
2.3. Chromatography
Analysis was performed using an Agilent 1200 HPLC system (Agilent Technologies, Wilmington, USA) equipped with a binary solvent delivery system, an on-line degasser, an autosampler, a column temperature controller and a photodiode-array detector all controlled by an analytical workstation. Separation was carried out on a T3 Xselect™ column (Waters, Ireland, 100 mm×2.1 mm, 2.5 µm) maintained at 35 °C by gradient elution using water containing 0.03% formic acid as solvent A and methanol containing 0.03% formic acid as solvent B. The linear gradient delivered at 0.2 mL/min increased from 20% B to 40% B over 40 min.
2.4. Mass spectrometry
MS was performed on an Agilent 6520 series TOF mass spectrometer equipped with a dual electrospray ionization (ESI) source operated in the positive ion mode. Nitrogen was used as sheath and auxiliary gas and helium as collision gas. Capillary voltage was 3500 V. The drying gas temperature was set at 350 °C with a flow rate of 10.0 L/min and the nebulizing pressure was 30 psi. Mass scan data were collected in the range m/z 100–500 and recorded in centroid. Mass spectra were internally calibrated in real time using a solution containing two reference compounds with m/z 922.00980 (C18H18O6N3P3F24) and 121.0509 (C5H4N4) continuously delivered by an Agilent isocratic pump into the ESI source at approximately 0.01 mL/min. MS/MS experiments were performed with a CID collision energy of 35 eV.
3. Results and discussion
3.1. Fragmentation patterns of alkaloid standards
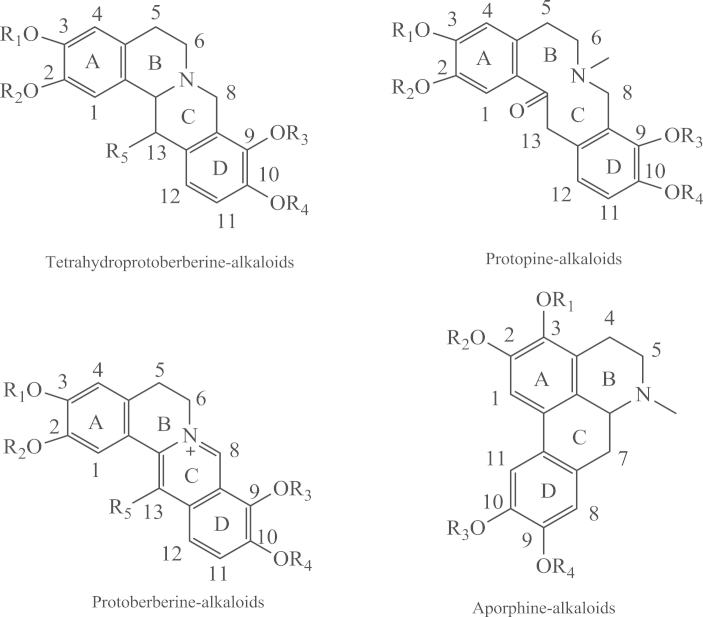
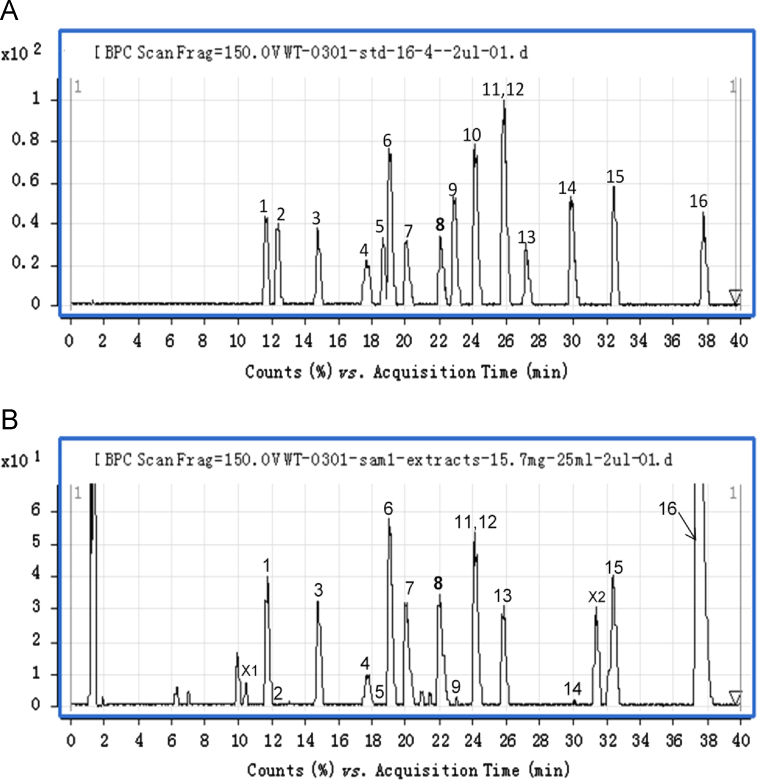
RC contains many alkaloids which are mainly responsible for its bioactivity. Based on their basic heterocyclic nuclei, these alkaloids can be divided into four main groups viz the protoberberines, tetrahydroprotoberberines, protopines and aporphines14 (Fig. 1). Among these, the tetrahydroprotoberberines, aporphines and protopines are tertiary alkaloids whereas the protoberberines are quaternary alkaloids15. Fig. 2 shows the base peak current (BPC) profiles of the RC extract and the mixed solution of alkaloid standards. A total of 16 alkaloids belonging to the four groups were eventually identified in the RC extract by comparing fragmentation patterns and chromatographic retention times with those of the standards15, 16, 17. Based on an analysis of the fragmentation pathway of the different alkaloids in RC, a number of unknown alkaloids could also be identified (Table 1).
Figure 1.
Chemical structures of the four types of alkaloids in RC.
Figure 2.
Base peak currents (BPC) of the solution of standards (A) and extract of RC (B). Peak identities: 1, Tetrahydrocolumbamine; 2, Tetrahydrojatrorrhizine; 3, Protopine; 4, Allocryptopine; 5, Demethyleneberberine; 6, Tetrahydropalmatine; 7, Coptisine; 8, Glaucine; 9, Canadine; 10, Corydaline; 11, Columbamine; 12, Jatrorrhizine; 13, Worenine; 14, Berberine; 15, Palmatine; 16, Dehydrocorydaline.
Table 1.
LC–Q-TOF-MS data of alkaloids from RC.
| No. | Name | RT (min) | Formula | Mass (m/z) |
Error | Structure pattern | Fragmentation pattern | |
|---|---|---|---|---|---|---|---|---|
| Calculated | Observed | |||||||
| 1 | Tetrahydrocolumbamine | 11.8 | C20H23NO4 | 342.17 | 342.1724 | 7 | Tetrahydroprotoberberine-alkaloids | RDA |
| 2 | Tetrahydrojatrorrhizine | 12.5 | C20H23NO4 | 342.17 | 342.1724 | 7 | Tetrahydroprotoberberine-alkaloids | RDA |
| 3 | Protopine | 14.8 | C20H19NO5 | 354.1336 | 354.1358 | 6.2 | Protopine-alkaloids | RDA and H2O loss |
| 4 | Allocryptopine | 17.7 | C21H23NO5 | 370.1649 | 370.1671 | 5.9 | Protopine-alkaloids | RDA and H2O loss |
| 5 | Demethyleneberberine | 18.5 | C19H18NO4+ | 324.123 | 324.1248 | 5.6 | Protoberberine-alkaloids | CO and CH3 loss |
| 6 | Tetrahydropalmatine | 19.1 | C21H25NO4 | 356.1856 | 356.1879 | 6.4 | Tetrahydroprotoberberine-alkaloids | RDA |
| 7 | Coptisine | 20.1 | C19H14NO4+ | 320.0917 | 320.0934 | 5.3 | Protoberberine-alkaloids | CO loss |
| 8 | Glaucine | 22.1 | C21H25NO4 | 356.1856 | 356.1878 | 6.5 | Aporphine-alkaloids | NH2CH3 loss |
| 9 | Canadine | 23 | C20H21NO4 | 340.1549 | 340.1568 | 5.6 | Tetrahydroprotoberberine-alkaloids | RDA |
| 10 | Corydaline | 24.2 | C23H31NO4 | 370.2013 | 370.2033 | 5.4 | Tetrahydroprotoberberine-alkaloids | RDA |
| 11 | Columbamine | 25.1 | C20H20NO4+ | 338.1387 | 338.1414 | 8 | Protoberberine-alkaloids | CO and CH3 loss |
| 12 | Jatrorrhizine | 25.9 | C20H20NO4+ | 338.1387 | 338.1414 | 8 | Protoberberine-alkaloids | CO and CH3 loss |
| 13 | Worenine | 27.2 | C20H16NO4+ | 334.1074 | 334.1089 | 4.5 | Protoberberine-alkaloids | CO loss |
| 14 | Berberine | 30.1 | C20H18NO4+ | 336.123 | 336.1251 | 6.2 | Protoberberine-alkaloids | CO and CH3 loss |
| 15 | Palmatine | 32.4 | C21H22NO4+ | 352.1543 | 352.1568 | 7.1 | Protoberberine-alkaloids | CO and CH3 loss |
| 16 | Dehydrocorydaline | 37.5 | C22H24NO4+ | 366.17 | 366.1723 | 6.3 | Protoberberine-alkaloids | CO and CH3 loss |
| 17 | X1 | 10.3 | C21H25NO4 | 356.1856 | 356.1877 | 6.4 | Tetrahydroprotoberberine-alkaloids | RDA |
| 18 | X2 | 31.3 | C21H22NO4+ | 352.1543 | 352.1568 | 7.1 | Protoberberine-alkaloids | CO and CH3 loss |
3.1.1. Tetrahydroprotoberberine alkaloids
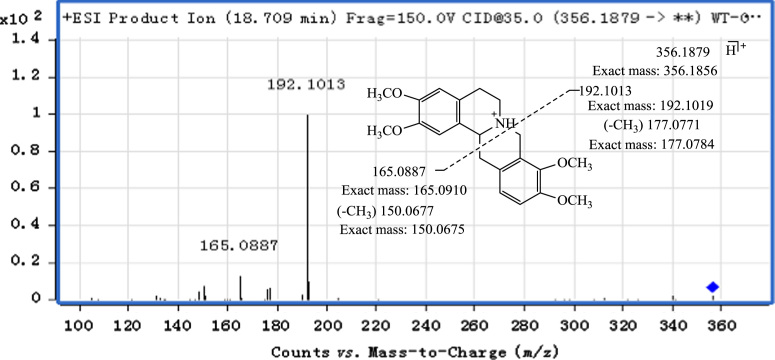
The retro-Diels–Alder (RDA) reaction is a characteristic fragmentation pathway of the tetrahydroprotoberberine and protopine alkaloids. In the MS/MS spectrum of the tetrahydroprotoberberine alkaloid, tetrahydropalmatine (Fig. 3), the predominant ions at m/z 192.1013 and 165.0887 result from RDA C-ring opening to form tetrahydroisoquinoline fragment ions. Fragment ions arising from elimination of the methyl radical from the two predominant ions could also be observed at m/z 177.0771 and m/z 150.0677 respectively but with lower abundance.
Figure 3.
MS/MS spectrum and fragmentation of tetrahydropalmatine.
Furthermore, it can be seen that if the substituent at position-13 is a methyl group, the RDA reaction generates ions by a different fragmentation pathway. For example, in the MS/MS spectrum of corydaline (Fig. 4), ions at m/z 192.1027 and 179.1061 are formed by the same RDA reaction as for tetrahydropalmatine but the ions at m/z 205.1112 and 165.0914 are not. It was first considered that these ions were formed by the RDA reaction of the B ring, but, based on high resolution MS/MS data, they are more likely formed by the RDA reaction of a compound produced by a rearrangement of the C ring in one or several steps to a new seven membered ring structure (Fig. 4). It is noteworthy that this tetrahydroisoquinoline fragment ion is not only characteristic but also provides structural information about this type of alkaloid. It is always the most abundant product ion in the MS/MS spectra of these alkaloids and probably reflects the substitution pattern in the A ring. For example, the product ion with m/z 192, the most abundant ion in both tetrahydropalmatine and corydaline, is indicative of two methoxy substituents in the A ring.
Figure 4.
MS/MS spectrum and fragmentation of corydaline.
3.1.2. Protopine alkaloids
The protopine alkaloids can fragment by the RDA reaction, and in comparison with the tetrahydroberberine alkaloids, it also can undergo another characteristic fragmentation pathway. Selecting protopine as an example, fragment ion at m/z 206.0823 and 149.0603 in the MS/MS spectrum are generated by RDA C ring opening (Fig. 5), but given the presence of hydroxyl groups, the product ions at m/z 336.1209 and m/z 188.0721 are probably formed by loss of H2O from the molecular ion and from the m/z 206.0823 ion. This loss of H2O fragmentation pathway could be used to distinguish between the protopine and tetrahydroprotoberberine alkaloids.
Figure 5.
MS/MS spectrum and fragmentation pathway of protopine.
3.1.3. Protoberberine alkaloids
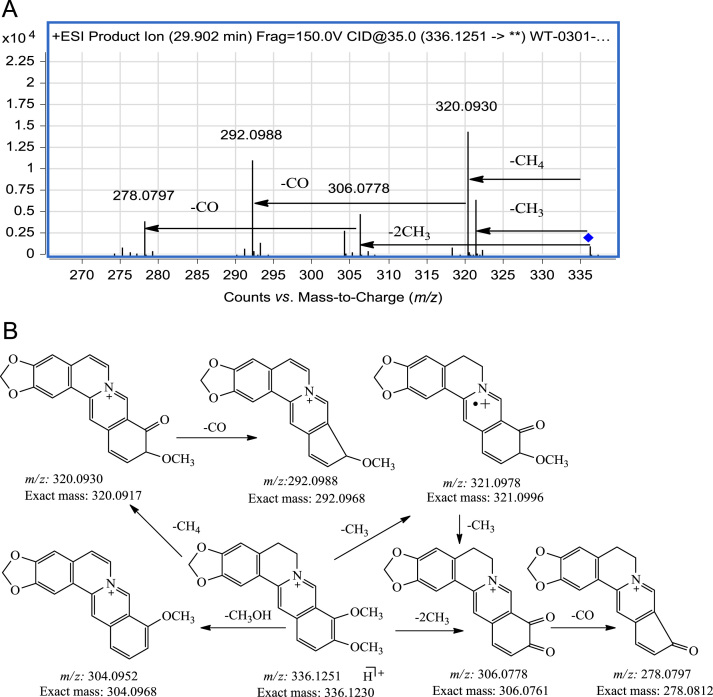
The protoberberines are benzyltetrahydroisoquinoline alkaloids and their conjugated planar C ring does not undergo the RDA reaction18, 19. In the MS/MS spectrum of berberine (Fig. 6), the major product ions appear at m/z 320.0930 and 321.0978 and correspond to elimination of the methyl radical and CH4 respectively from the methoxy substituent. The ion at m/z 306.0778 is formed by continuous elimination of two methyl radicals and the ion at m/z 304.0952 is formed by loss of CH3OH from the precursor ion. The ions at m/z 292.0988 and 278.0797 are then formed by loss of CO from the ions at m/z 320.0930 and m/z 306.0778, respectively, and this sequential loss of a methyl radical and CO is the characteristic fragmentation pathway of this kind of alkaloid. This fragmentation behavior also occurs in other protoberberine alkaloids such as palmatine, jatrorrhizine, dehydrocorydaline and demethyleneberberine. The results demonstrate that if methoxy groups are present at C-9, C-10 or C4, C5, this fragmentation pathway is quite characteristic.
Figure 6.
MS/MS spectrum and fragmentation pathway of berberine.
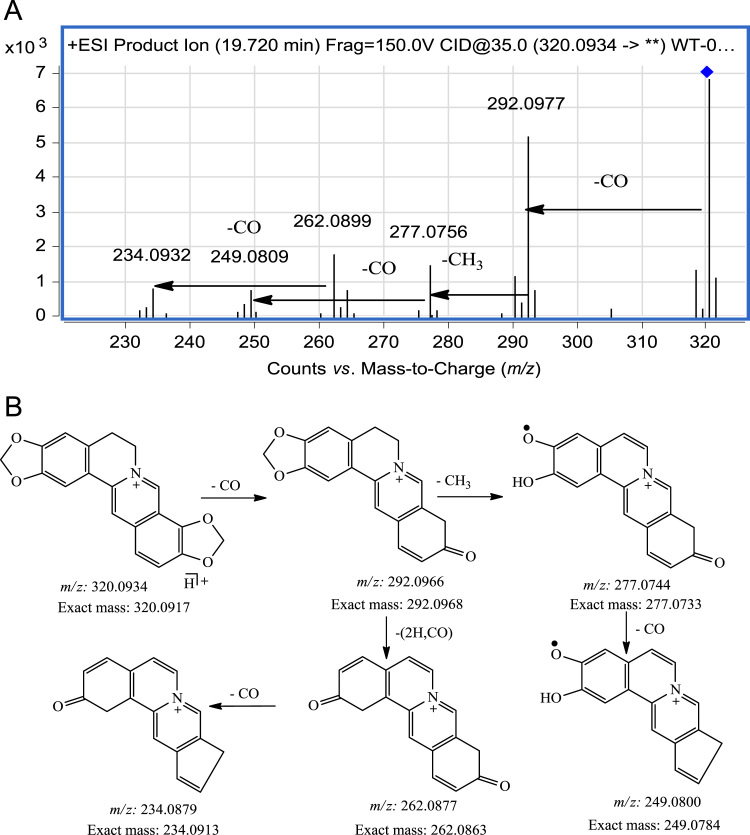
When there are no methoxy groups present in the alkaloid as in coptisine and worenine, the fragmentation pathway is different from that of other protoberberine alkaloids. Thus, in the MS/MS spectrum of coptisine (Fig. 7), the base product ion at m/z 292.0966 is formed by loss of CO from the precursor ion while in other protoberberine alkaloids, the base ion is formed by loss of CH4 from the precursor ion. The ion at m/z 277.0744 is then formed by loss of CH3 from the base product ion at m/z 292.0966 and the ion at m/z 249.0800 is formed by loss of CO from the ion at m/z 277.0744. The ion at m/z 262.0877 is formed by the loss of 2H and one CO from the ion at m/z 292.0966. The fragmentation pathway of worenine is similar to that of coptisine with the only difference being the loss of the CH3 fragment at position 13.
Figure 7.
MS/MS spectrum (A) and fragmentation pathway (B) of Coptisine.
3.1.4. Aporphine alkaloids
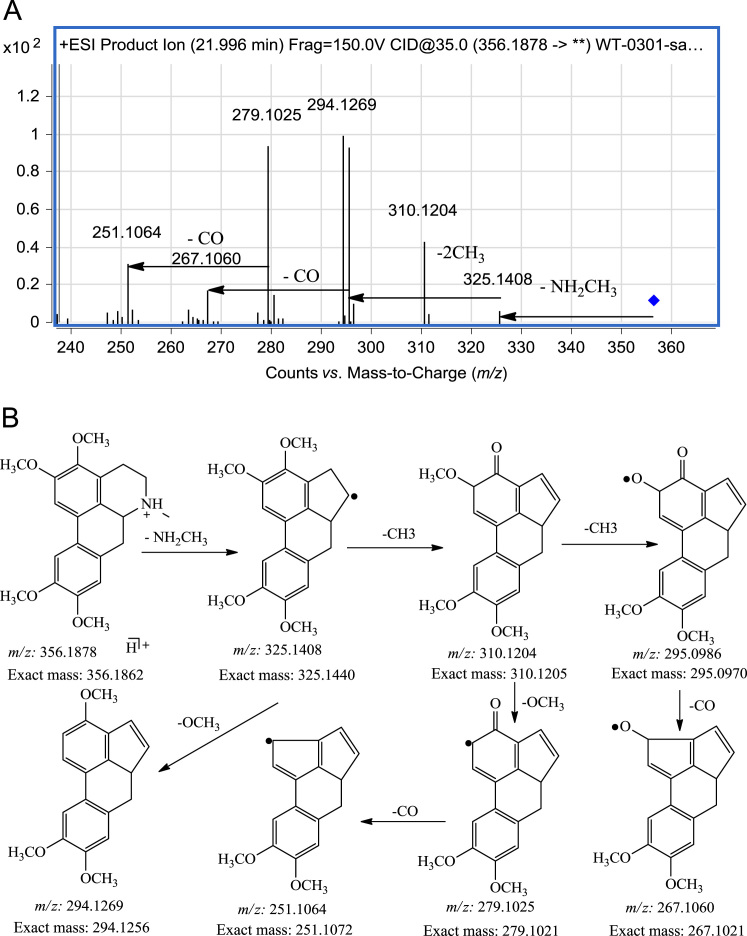
Aporphine alkaloids are not highly represented in RC but glaucine is present with high abundance. The major structural feature of this kind of alkaloid is the distribution of the C and B rings. They do not undergo the RDA reaction and are thus easily distinguished from the protopine and tetrahydroprotopine alkaloids. Although similar in structure, the fragmentation pathways of the protoberberine alkaloids also show differences with that of glaucine. Thus in the MS/MS spectrum of glaucine (Fig. 8), an important product ion at m/z 325.1430 was observed which was thought to be formed by loss of a methoxy radical20, 21. However, based on high resolution MS/MS data, the ion is actually generated by loss of NH2CH317, since the ion formed by loss of a methoxy radical would be at m/z 325.1672. This characteristic ion represents a unique fragmentation pathway and gives rise to most of the other product ions of glaucine. The ion at m/z 294.1269 is formed by loss of the methoxy radical from the ion at m/z 325.1408 which can also lose one or two methyl radicals to generate the ions at m/z 310.1204 and m/z 295.0986, respectively. The ion at m/z 310.1204 can lose a methoxy radical to form an m/z 279.1025 ion which could further lose CO to form the m/z 251.1064 ion. The ion at m/z 267.1060 is formed by loss of CO from the ion at m/z 295.0986.
Figure 8.
MS/MS spectrum and fragmentation pathway of Glaucine.
3.2. Investigation of unknown alkaloids in RC
Based on analysis of fragmentation pathways, the structural characterization of unknown alkaloids in RC was investigated15, 17, 22. The procedure can be divided into three main steps. First, the RDA fragmentation pathway was used to distinguish the tetrahydroproberberine and protopinealkaloids. Second, the protopine alkaloids were distinguished from the tetrahydroproberberine alkaloids by the presence of fragments due to loss of H2O. Moreover, the sequential loss of CH3 and CO or the loss of NH2CH3 can be used to discriminate between the protoberberine and aporphine alkaloids. Finally, based on the literature3, 14, 15, 17, the substituents in alkaloids exhibit some characteristics. The main substitution sites at positions 2, 3, 9, 10 and 13 are occupied mainly by methylenedioxy, hydroxy, methoxy and methyl groups and, based on the mass of the substituents and product ions, the identities of some unknown alkaloids could be tentatively assigned.
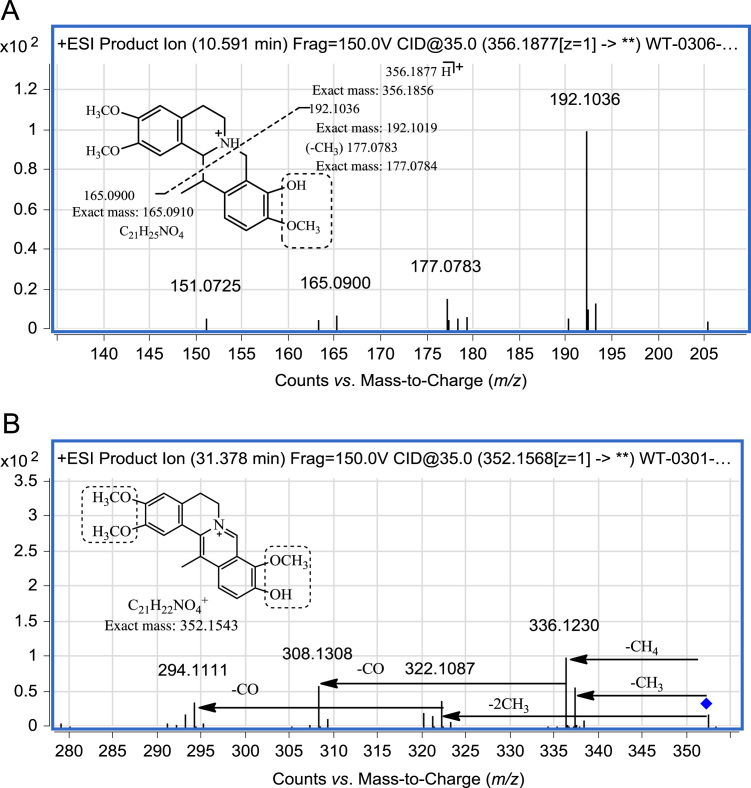
For example, in the MS/MS spectrum of the ion at m/z 356.1877 (Fig. 9A), the product ions at m/z 192.1036 and 165.0900 were obviously formed by the RDA reaction and no fragment due to loss of H2O was observed. Therefore the compound could be identified as a tetrahydroprotoberberine alkaloid. Comparison with the MS/MS spectrum of tetrahydropalmatine, it could be shown that compound X1 was an isomer with similar structure. As mentioned above, for the most abundant ion at m/z 192.1036, the substituents in the A ring should be the same as in tetrahydropalmatine. It could then be inferred that two methoxy groups are present at positions 2,3 respectively and one methyl group is present at the 13 position. Moreover, hydroxyl and methoxyl groups should be present at positions 9 and 10 of the D ring, respectively. Fig. 9A shows one possible structure of X1 but the actual structure may be an isomer with a different distribution of substituents.
Figure 9.
MS/MS spectrum and fragmentation pathway of unknown alkaloids X1 (A) and X2 (B).
In the MS/MS spectrum of the ion at m/z 352.1568 (Fig. 9B), the sequential loss of a methyl radical and CO could be observed which is the characteristic fragmentation of a proberberine alkaloid. The alkaloid X2 was identified as a proberberine alkaloid and isomer of palmatine. Based on the structure of palmaltine, it could be inferred that X2 contains a methyl group at position13 and a hydroxyl group in the A or D ring. Fig. 9B shows one possible structure of X2.
The differentiation of isomers is a major challenge in deducing the actual structures of unknown alkaloids in RC by this method. The major problem is that the same substituents can be ortho to each other at different sites in the molecule. The product ions of structures containing such substituents have the same molecular weight and determination of structure then depends on the fragmentation pattern alone23. Thus the definite identification of these alkaloids by LC-MS requires further study.
4. Conclusions
This paper reports the fragmentation pathways of alkaloids in RC determined using LC–Q-TOF-MS/MS. A total of 16 alkaloids were identified and classified into four types based on their heterocyclic nuclei. The fragmentation pathways of each type were elucidated and their differences evaluated. Furthermore, a strategy to identify unknown alkaloids in RC was proposed. LC–Q-TOF-MS/MS provides a rapid and accurate method to identify and characterize the alkaloids in RC which could be useful in its quality control and in the search for new alkaloids.
Acknowledgments
The work was funded by the National Natural Science Foundation of China (Nos. 81202950 and 81102795) and National Key Project of the Scientific and Technical Supporting Programs of China (No. 2012ZX10004301-608).
Footnotes
Peer review under responsibility of Institute of Materia Medica, Chinese Academy of Medical Sciences and Chinese Pharmaceutical Association.
References
- 1.Wang C., Guo Z., Zhang J., Zeng J., Zhang X., Liang X. High-performance purification of quaternary alkaloids from Corydalis yanhusuo W. T. Wang using a new polar-copolymerized stationary phase. J Sep Sci. 2011;34:53–58. doi: 10.1002/jssc.201000625. [DOI] [PubMed] [Google Scholar]
- 2.Ling H., Wu L., Li L. Corydalis yanhusuo rhizoma extract reduces infarct size and improves heart function during myocardial ischemia/reperfusion by inhibiting apoptosis in rats. Phytother Res. 2006;20:448–453. doi: 10.1002/ptr.1875. [DOI] [PubMed] [Google Scholar]
- 3.Yan X.J., Zhou J.J., Xie G.R. Chemical Industry Press; Beijing: 2004. Traditional Chinese medicines: molecular structures, natural sources and applications. [Google Scholar]
- 4.Huang Q.Q., Bi J.L., Sun Q.Y., Yang F.M., Wang Y.H., Tang G.H. Bioactive isoquinoline alkaloids from Corydalis saxicola. Planta Med. 2012;78:65–70. doi: 10.1055/s-0031-1280126. [DOI] [PubMed] [Google Scholar]
- 5.Jha R.N., Pandey M.B., Singh A.K., Singh S., Singh V.P. New alkaloids from Corydalis species. Nat Product Res. 2009;23:250–255. doi: 10.1080/14786410801996390. [DOI] [PubMed] [Google Scholar]
- 6.Wang C., Wang S., Fan G., Zou H. Screening of antinociceptive components in Corydalis yanhusuo W.T. Wang by comprehensive two-dimensional liquid chromatography/tandem mass spectrometry. Anal Bioanal Chem. 2010;396:1731–1740. doi: 10.1007/s00216-009-3409-1. [DOI] [PubMed] [Google Scholar]
- 7.Liu Q., Zhou B., Wang X., Ke Y., Jin Y., Yin L. Establishment of a search library about benzylisoquinoline alkaloids based on selective separation on the binaphthyl column and standard analysis on C18 column. J Sep Sci. 2012;35:3317–3325. doi: 10.1002/jssc.201200605. [DOI] [PubMed] [Google Scholar]
- 8.Ma H., Wang Y., Guo T., He Z., Chang X., Pu X. Simultaneous determination of tetrahydropalmatine, protopine, and palmatine in rat plasma by LC-ESI-MS and its application to a pharmacokinetic study. J Pharm Biomed Anal. 2009;49:440–446. doi: 10.1016/j.jpba.2008.11.019. [DOI] [PubMed] [Google Scholar]
- 9.Glauser G., Schweizer F., Turlings T.C., Reymond P. Rapid profiling of intact glucosinolates in Arabidopsis leaves by UHPLC-QTOFMS using a charged surface hybrid column. Phytochem Anal. 2012;23:520–528. doi: 10.1002/pca.2350. [DOI] [PubMed] [Google Scholar]
- 10.Han L., Boakye-Yiadom M., Liu E., Zhang Y., Li W., Song X. Structural characterisation and identification of phenylethanoid glycosides from Cistanches deserticola Y.C. Ma by UHPLC/ESI-QTOF-MS/MS. Phytochem Anal. 2012;23:668–676. doi: 10.1002/pca.2371. [DOI] [PubMed] [Google Scholar]
- 11.Quirantes-Pine R., Lozano-Sanchez J., Herrero M., Ibanez E., Segura-Carretero A., Fernandez-Gutierrez A. HPLC-ESI-QTOF-MS as a powerful analytical tool for characterising phenolic compounds in olive-leaf extracts. Phytochem Anal. 2013;24:213–223. doi: 10.1002/pca.2401. [DOI] [PubMed] [Google Scholar]
- 12.Sun J., Lin L.Z., Chen P. Study of the mass spectrometric behaviors of anthocyanins in negative ionization mode and its applications for characterization of anthocyanins and non-anthocyanin polyphenols. Rapid Commun Mass Spectrom. 2012;26:1123–1133. doi: 10.1002/rcm.6209. [DOI] [PubMed] [Google Scholar]
- 13.Wang J., van der Heijden R., Spijksma G., Reijmers T., Wang M., Xu G. Alkaloid profiling of the Chinese herbal medicine Fuzi by combination of matrix-assisted laser desorption ionization mass spectrometry with liquid chromatography-mass spectrometry. J Chromatogr A. 2009;1216:2169–2178. doi: 10.1016/j.chroma.2008.11.077. [DOI] [PubMed] [Google Scholar]
- 14.Cong Puzhu L.S., editor. Natural organic mass spectrometry. China Medical Science and Technology Press; Beijing: 2003. [Google Scholar]
- 15.Zhang J., Jin Y., Dong J., Xiao Y., Feng J., Xue X. Systematic screening and characterization of tertiary and quaternary alkaloids from Corydalis yanhusuo W.T. Wang using ultra-performance liquid chromatography-quadrupole-time-of-flight mass spectrometry. Talanta. 2009;78:513–522. doi: 10.1016/j.talanta.2008.12.002. [DOI] [PubMed] [Google Scholar]
- 16.Li W., Yang M., Zheng Y. Fragmentation investigation of seven arylnaphthalide lignans using liquid chromatography/tandem quadrupole time-of-flight mass spectrometry. Rapid Commun Mass Spectrom. 2012;26:950–956. doi: 10.1002/rcm.6189. [DOI] [PubMed] [Google Scholar]
- 17.Jeong E.K., Lee S.Y., Yu S.M., Park N.H., Lee H.S., Yim Y.H. Identification of structurally diverse alkaloids in Corydalis species by liquid chromatography/electrospray ionization tandem mass spectrometry. Rapid Commun Mass Spectrom. 2012;26:1661–1674. doi: 10.1002/rcm.6272. [DOI] [PubMed] [Google Scholar]
- 18.Ren L., Xue X., Zhang F., Xu Q., Liang X. High performance liquid chromatography-mass spectrometry analysis of protoberberine alkaloids in medicine herbs. J Sep Sci. 2007;30:833–842. doi: 10.1002/jssc.200600246. [DOI] [PubMed] [Google Scholar]
- 19.Wu W., Song F., Yan C., Liu Z., Liu S. Structural analyses of protoberberine alkaloids in medicine herbs by using ESI-FT-ICR-MS and HPLC–ESI-MSn. J Pharm Biomed Anal. 2005;37:437–446. doi: 10.1016/j.jpba.2004.11.026. [DOI] [PubMed] [Google Scholar]
- 20.Ding B., Zhou T., Fan G., Hong Z., Wu Y. Qualitative and quantitative determination of ten alkaloids in traditional Chinese medicine Corydalis yanhusuo W.T. Wang by LC–MS/MS and LC–DAD. J Pharm Biomed Anal. 2007;45:219–226. doi: 10.1016/j.jpba.2007.06.009. [DOI] [PubMed] [Google Scholar]
- 21.Hong Z., Zhao L., Wang X., Le J., Jia J., Chai Y. High-performance liquid chromatography-time-of-flight mass spectrometry with adjustment of fragmentor voltages for rapid identification of alkaloids in rat plasma after oral administration of rhizoma Corydalis extracts. J Sep Sci. 2012;35:1690–1696. doi: 10.1002/jssc.201200126. [DOI] [PubMed] [Google Scholar]
- 22.Xu M.J., Wu B., Ding T., Chu J.H., Li C.Y., Zhang J. Simultaneous characterization of prenylated flavonoids and isoflavonoids in Psoralea corylifolia L. by liquid chromatography with diode-array detection and quadrupole time-of-flight mass spectrometry. Rapid Commun Mass Spectrom. 2012;26:2343–2358. doi: 10.1002/rcm.6361. [DOI] [PubMed] [Google Scholar]
- 23.Ablajan K., Tuoheti A. Fragmentation characteristics and isomeric differentiation of flavonol O-rhamnosides using negative ion electrospray ionization tandem mass spectrometry. Rapid Commun Mass Spectrom. 2013;27:451–460. doi: 10.1002/rcm.6476. [DOI] [PubMed] [Google Scholar]