Abstract
Near infrared spectroscopy (NIRS) has been widely applied in both qualitative and quantitative analysis. There is growing interest in its application to traditional Chinese medicine (TCM) and a review of recent developments in the field is timely. To present an overview of recent applications of NIRS to the identification, classification and analysis of TCM products, studies describing the application of NIRS to TCM products are classified into those involving qualitative and quantitative analysis. In addition, the application of NIRS to the detection of illegal additives and the rapid assessment of quality of TCMs by fast inspection are also described. This review covers over 100 studies emphasizing the application of NIRS in different fields. Furthermore, basic analytical principles and specific examples are used to illustrate the feasibility and effectiveness of NIRS in pattern identification. NIRS provides an effective and powerful tool for the qualitative and quantitative analysis of TCM products.
Key words: Near infrared spectroscopy, Traditional Chinese medicine, Quality control, Fast inspection
Graphical abstract
Chemical identification technology, rapid TLC technology, NIRS technology and microscopy technology are basic parts in fast inspection platform. Advantages of NIRS are respected to solve problems of less sample size, longer test periods and higher cost for quality control of TCM productions.
1. Introduction
Chinese herbs and patent drugs are important parts of traditional Chinese medicine (TCM), the use of which is considerable not only in China but all over the world. Despite this widespread use, there are many confusing aspects related to TCM such as the fact that many similar TCMs produce different pharmacodynamic effects, one TCM can have two or more names and one name can apply to more than one TCM. These problems lead to difficulty in the quality control (QC) of TCM and call for urgent attention1.
In comparison with the Chinese Pharmacopoeia 2005 (ChP2005), drug standards and test methods for TCM have been considerably improved and extended in the Chinese Pharmacopoeia 2010 (ChP2010). Common methods now include histological and morphological examination, thin layer chromatography (TLC), high-performance liquid chromatography (HPLC), gas chromatography (GC), liquid chromatography–mass spectrometry (LC–MS) and gas chromatography–mass spectrometry (GC–MS)2, 3, 4. This more authoritative and comprehensiveness coverage of TCM in the ChP is critical for improving the QC of TCM. However, there are still deficiencies such as the time period of inspection in the ChP is longer than the 28 days recommended by the Institute for Drug Control (IDC) in China.
Another problem is that TCM components are often pretreated by physical or chemical processes before being tested. These procedures are complex and multifarious and some of them such as morphological identification lack accuracy since the results depend on the experience and expertise of the TCM pharmacist undertaking the test5. Thus, for objectivity, rapidity and accuracy of testing, more rational methods are needed.
Since the 1990s, the application of near infrared spectroscopy (NIRS) in fields involving drugs, food, agriculture, the petroleum industry and environmental protection has developed rapidly6, 7. NIRS has many advantages in relation to QC and inspection8 and allows classification, qualitative analysis and quantitative analysis of TCM products. The further development of NIRS will serve to strengthen quality supervision and control of TCM products and regulate markets9.
2. Advantages of NIRS technology
The advantages of NIRS are many with rapidity of analysis being one of the most important10. Thus NIRS combined with appropriate mathematical models and pattern recognition techniques allows analysis of a wide variety of sample types rapidly. Second, NIRS is a non-destructive technique which avoids complex sample preparation by chemical or physical processes. In fact, both solid and liquid samples in different types of packaging stored under different conditions can all be tested without complex pretreatment11 because of the better penetrability of fiber optics used in NIRS. Third, it provides acceptable accuracy in both qualitative and quantitative analysis to meet the requirements of QC and preliminary screening12.
3. Identification of TCM by NIRS
3.1. Qualitative identification
TCM herbs and animal products are subject to numerous complex problems in clinical use. For example, herbs are often adulterated with or replaced by non-therapeutic plants of similar appearance13, 14, 15 because the ability to identify a particular herb is very dependent on the experience of the TCM pharmacist.
Identification based on microscopic examination is frequently applied to TCM powders by looking for the presence of microstructures16, 17, 18. In addition, it is used to examine slices of TCM plant cells. The ChP2005 recorded 620 items requiring identification by microscopy and the number increased to 1253 in the ChP201019. However, identification using microscopy still depends on the experience of the TCM pharmacist whereas NIRS provides a more reliable non-subjective method to identify TCM products. For example, 269 samples of Bai-Zhi (Radix Angelicae Dahuricae) and 350 samples of wild or cultivated Dan-Shen (Radix et Rhizoma Salviae Miltiorrhizae) were identified and classified by NIRS with a accuracy rates of 99% and 95%, respectively20.
Some species of TCM are grown in different regions of China where differences in weather conditions and soil environments lead to variations in quality21. NIRS can then be used to locate the source of a particular sample. For example, Jin-Yin-Hua (Flos Lonicerae Japonicae) is widely planted in the provinces of Henan, Hebei, Hunan, Shandong and Guangxi. Qualitative analysis by NIRS not only identified 22 samples as coming from Henan province with 100% accuracy but also correctly sourced 68 samples from other provinces and sourced only 9 samples incorrectly22.
The use of genuine medicinal materials is very important to the integrity of TCM (Table 1). However, many rare and expensive TCM herbs are often adulterated23. For example, the adulteration of Dong-Chong-Xia-Cao (Cordyceps), one of most important and precious TCM herbs, is a serious problem. Fortunately, NIRS provides a rapid and convenient method to identify Dong-Chong-Xia-Cao with an accuracy rate greater than 95%24. In contrast, Hong-Qu (Rubrum Fermentum) is not a rare material but a commonly used food additive in China and many Asian countries25. The problem here is that many substances are similar in appearance to Hong-Qu making identification by microscopy difficult. In this case, NIRS in combination with cluster analysis was successful in classifying Hong-Qu effectively26, 27.
Table 1.
Quantitative analysis of TCM products by near infrared spectroscopy.
| Province | Genuine medicinal material | Province | Genuine medicinal material |
|---|---|---|---|
| Sichuan and Chongqin | Chuan-Bei-Mu (Bulbus Fritillariae Cirrhosae) | Hubei, Anhui and Jiangsu | Ban-Xia (Rhizoma Pinelliae) |
| Chuan-Xiong (Rhizoma Chuanxiong) | Ge-Gen (Radix Puerariae Lobatae) | ||
| Huang-Lian (Rhizoma Coptidis) | Cang-Zhu (Rhizoma Atractylodis) | ||
| Fu-Zi (Radix Aconiti Lateralis Praeparata) | Tai-Zi-Shen (Radix Pseudostellariae) | ||
| Chuan-Wu (Radix Aconiti) | Dang-Shen (Radix et Rhizoma Salviae Miltiorrhizae) | ||
| Guangzhou, Guangxi and Hainan | Sha-Ren (Fructus Amomi) | Shandong, Hebei, Shanxi and Sha˘nxi | Dang-Shen (Radix et Rhizoma Salviae Miltiorrhizae) |
| Guang-Huo-Xiang (Herba Pogostemonis) | Huang-Qi (Radix Astragali) | ||
| Chuang-Xin-Lian (Herba Andrographis) | Chai-Hu (Radix Bupleuri) | ||
| Jin-Qian-Cao (Herba Lysimachiae) | Huang-Qin (Radix Scutellariae) | ||
| Luo-Han-Guo (Fructus Siraitiae) | Bai-Zhi (Radix Angelicae Dahuricae) | ||
| Yunnan | San-Qi (Radix et Rhizoma Notoginseng) | Hunan, Jiangxi, Fujian and Taiwan | Bai-Bu (Radix Stemonae) |
| Mu-Xiang (Radix Aucklandiae) | Wei-Ling-Xian (Radix et Rhizoma Clematidis) | ||
| Chong-lou (Rhizoma Paridis) | Xu-Chang-Qing (Radix et Rhizoma Cynanchi Paniculati) | ||
| Fu-Ling (Poria) | Ze-Xie (Rhizoma Alismatis) | ||
| Tian-Ma (Rhizoma Gastrodiae) | Zhi-Shi (Fructus Aurantii Immaturus) | ||
| Guizhou | Tian-Dong (Radix Asparagi) | Neimenggu | Suo-Yang (Herba Cynomorii) |
| Huang-Jing (Rhizoma Polygonati) | Gan-Cao (Radix et Rhizoma Glycyrrhizae) | ||
| Ba-Ji (Rhizoma Bletillae) | Ma-Huang (Herba Ephedrae) | ||
| Du-Zhong (Cortex Eucommiae) | Rou-Cong-Rong (Herba Cistanches) | ||
| Wu-Zhu-Yu (Fructus Euodiae) | Yin-Yang-Huo (Folium Epimedii) | ||
| He׳nan | Huai-Di-Huang (Radix Rehmanniae) | Xizang | Hu-Huang-Lian (Rhizoma Picrorhizae) |
| Huai-Niu-Xi (Raix Achyranthis bidentatae) | Zang-Mu-Xiang (Radix Aucklandiae) | ||
| Huai-Shan-Yao (Rhizoma Dioscoreae) | Xue-Lian-Hua (Saussurea Involucrata) | ||
| Huai-Ju-Hua (Flos Chrysanthemi) | Mao-He-Zi (Fructus Terminaliae Billericae) | ||
| Tian-Hua-Feng (Radix Trichosanthis) | She-Xiang (Rhizoma Belamcandae) | ||
| Zhejiang | Zhe-Bei-Mu (Bulbus Fritillariae Thunbergii) | Xinjiang | Xue-Lian-Hua (Saussurea Involucrata) |
| Bai-Zhu (Rhizoma Atractylodis Macrocephalae) | A-Wei (Resina Ferulae) | ||
| Yan-Hu-Suo (Rhizoma Corydalis) | Zi-Cao (Radix Arnebiae) | ||
| Shan-Zhu-Yu (Fructus Corni) | Gan-Cao (Radix et Rhizoma Glycyrrhizae) | ||
| Hang-Bai-Ju (Flos Chrysanthemi) | Zi-Ran (Cuminum Cyminum) | ||
| Heilongjiang, Jilin and Liaoning | Ren-shen (Radix et Rhizoma Ginseng) | Drug from sea | Zhen-Zhu (Margarita) |
| Xi-Xin (Radix et Rhizoma Asari) | Shi-Jue-Ming (Concha Haliotidis) | ||
| Fang-Feng (Radix Saposhnikoviae) | Hai-Piao-Qiao (Endoconcha Sepiae) | ||
| Wu-Wei-Zi (Fructus Schisandrae chinensis) | Mu-Li (Concha Ostreae) | ||
| Long-Dan (Radix et Rhizoma Gentianae) | Hai-Ma (Hippocampus) | ||
| Gansu and Ningxia | Da-Huang (Radix et Rhizoma Rhei) | Import from abroad | Xi-Yang-Shen (Radix Panacis quinquefolii) |
| Dang-Gui (Radix Angelicae sinensis) | Ru-Xiang (Olibanum) | ||
| Qin-Jiao (Radix Gentianae macrophyllae) | Chen-Xiang (Resinatum Aquilariae Lignum) | ||
| Qiang-Huo (Radix et Rhizoma Notopterygii) | Pang-Da-Hai (Semen Sterculiae Lychnophorae) | ||
| Gou-Qi-Zi (Fructus Lych) | Mo-Yao (Myrrha) | ||
Grinding is frequently used in the preparation of TCM as part of the extraction and purification of desired components from crude materials. As a result, TCM herbs and animal products in the form of powders lose their significant characteristics making identification difficult. In addition, different TCM herbs have similar shape, color and microscopic features28. For example, Bai-Zhi (Radix Angelicae Dahuricae), Ye-Ge (Puerariae Lobatae), Cang-Zhu (Rhizoma Atractylodis), Bai-Shao (Radix Paeoniae Alba) and Dang-Gui (Radix Angelicae Sinensis) display only subtle differences in appearance29 and, after grinding, become even more difficult to distinguish. However, using principal component analysis (PCA) and cluster analysis to classify NIRS data allowed TCM powders with indistinguishable appearance to be identified and classified as accurately as by HPLC. NIRS is now considered the technique of choice for the QC of Chinese patent medicines30, 31, 32.
3.2. Pattern recognition technology
A NIR spectrum incorporates a large amount of information and includes overlapping and interconnected signals. As a result, pattern recognition is an important approach to reduce the number of variables. Pattern recognition is classified as either unsupervised or supervised33. Cluster analysis, PCA and discriminant analysis belong to the unsupervised class whereas latent projection, k-nearest neighbor algorithm (KNN), Fisher linear discriminant analysis (KLDA) and artificial neural networks (ANNs) belong to the supervised class.
3.2.1. Cluster analysis
Cluster analysis is frequently used to identify and classify TCM products34. Based on groups of variables, samples can be classified to different resources35, 36. TCMs are multicomponent systems and, in the case where the components in samples give distinct differences in NIR spectra, samples can be classified using cluster analysis37, 38. In fact, cluster analysis is a convenient method to simplify data from multicomponent systems for data analysis and data mining.
3.2.2. Principal component analysis
The large number of variables in TCM makes their analysis difficult39. PCA is a mathematical procedure to reduce the dimensions of data by linear fitting40. It produces a group of new variables which represent the primary information of the original variables without any data loss41. For example, in the NIR spectra of Jin-Yin-Hua (Flos Lonicerae Japonicae) and Huang-Qin (Radix Scutellariae), the two main raw materials in Yin-Huang oral liquid and Shuang-Huang-Lian oral liquid are a large number of signals from baicalin, chlorogenic acid and other components. To simplify the analysis, dimensions of the data were linearly reduced to calculate cumulative contribution rates and score plots42. Results presented on two- or three-dimensional maps effectively demonstrated differences in quality.
3.2.3. k-Nearest neighbor algorithm
k-Nearest neighbor algorithm (KNN) is a non-parametric method to classify objects based on closest training examples in the feature space. It ranks the contributions of neighbors in terms of their closeness to the object. In applying a combination of NIRS and KNN to samples of Dang-Shen (Radix et Rhizoma Salviae Miltiorrhizae), a common TCM herb in China, a mathematical model was established which was able to classify samples from different resources with an accuracy rate of 94%43.
3.2.4. Latent projection
Methods based on quantitative calibration and pattern recognition are established on the basis of latent projection. Partial least squares (PLS)44, partial least-squares discriminant analysis (PLS-DA)45 and soft independent modeling of class analogy (SIMCA)46 were all developed from latent projection. Latent projection has been used in the identification and classification of TCM products by NIRS. For instance, PLS-DA was applied to classify 600 samples of Bing-Lang (Semen Arecae) processed by six different methods45. Moreover, SIMCA and PLS-DA combined with NIRS have been used to identify Ci-Wu-Jia (Radix et Rhizoma Acanthopanacis Senticosi Seu Caulis)46 grown in different geographical areas.
3.2.5. Artificial neural networks
ANNs are a new information processing method applied to TCM47, 48. Many simple neurons connected to each other make up a complex network that can compute values by feeding information through the network49. The radial basis function ANN (RB-ANN)50, 51 and back-propagation ANN (BP-ANN) 52 are common applications used to overcome some of the disadvantages of NIRS such as broad spectral range, poor signal strength and overlapping signals53. ANN reduces interference and noise and has good nonlinear conversion capability to effectively avoid prediction error54. The combination of NIRS and ANN is particularly useful for the identification of TCMs with indistinguishable features55.
3.2.6. Specific spectrum method
Direct comparison of a sample spectrum with the spectrum of an authentic specimen is an important method for qualitative analysis of a TCM product. It is similar to fingerprinting as applied in chromatography and spectroscopy56, 57, 58. Identification of signals characteristic of a particular component can then be applied in the QC of TCM57, 58, 59. NIRS provides just as much information as HPLC and is superior in that it can be applied to most drug preparations with minimal sample preparation, even to the extent of allowing analysis in aluminum–plastic packaging60. Using appropriate wavelength ranges and optimization methods are important in establishing specific spectral features for use in QC.
4. Quantitative analysis of TCM products
Both qualitative and quantitative analysis by NIRS require the development of a mathematical model. For medicines with relatively simple composition containing high purity components, establishing a mathematical model for NIRS is relatively straightforward61 and provides good results in quantitative analysis61, 62, 63, 64. However, for analysis of TCM by NIRS, establishing a model is more difficult because they contain many components at low concentration. Thus, quantitative analysis of TCM by NIRS is still in the development and validation stage65, 66.
4.1. Model establishment
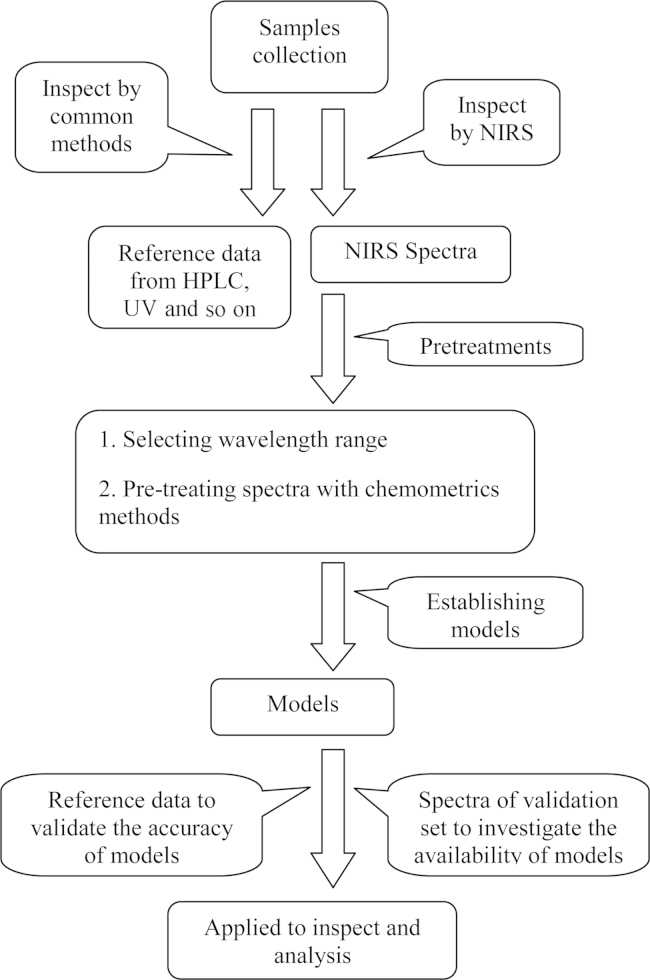
Quantitative analysis by NIRS requires sufficient spectra to make sample measurement sets and validation sets. Sample measurement sets are used to establish the mathematical model after which validation sets are used to test and verify the accuracy of the model. The model is also optimized using samples with known composition67, 68. General steps (Fig. 1) include: (1) pre-quantitative analysis for target compositions, collecting and analyzing sample measurement sets by NIRS; (2) establishing a quantitative model for target compositions and then optimizing the model by NIRS; and (3) using validation sets to verify the accuracy and repeatability of the model.
Figure 1.
A brief summary of the processes involved in model establishment in qualitative and quantitative analysis of TCM products by NIRS.
Having collected sufficient spectra, the two major steps involve selecting the wavelength range and pre-treating spectra69. Selecting a limited wavelength range is important because information derived from the full wavelength range is enormous and the absence of signals in certain regions of the spectra may influence the accuracy of results. Thus it is important to select the wavelength range of positive correlation and reject the range of negative correlation.
Pre-treating spectra is a procedure to optimize data and avoid disturbance due to a changing baseline. Common pretreatments include averaging, smoothing and normalizing using first and second derivative spectra70. The first derivative can eliminate shift errors and the second derivative can eliminate tilt (or rotation) errors71. Other methods to extract information from spectra are also available including multiplicative scatter correction (MSC), Savitzky–Golay method (SG) and standard normal variate (SNV)72.
Improving the model by correction methods is crucial for accurate quantitative analysis. Correction based on multiple linear regression73 and chemical stoichiometry74 is useful to obtain maximum information. Commonly used methods of multiple linear regression are principal component regression (PCR)75, partial least-squares discriminant analysis (PLS-DA) and partial least squares regression (PLS)76. In recent years, optimization methods used for quantitative analysis of TCM by NIRS are shown in Table 2.
Table 2.
NIRS analysis of the partial genuine medicinal materials in China.
| Drug | Component | Wavelength range (cm−1) | Pretreatment method | Reference |
|---|---|---|---|---|
| Taxol injection | Taxol | 9000–4400 | Minus a straight line | 77 |
| Acanthopanax Senticosus Injection | Chlorogenic acid | 8927–8735, 6800–5400, 4700–4300 | First derivative+vector normalization | 78 |
| Syringin | ||||
| Eleutheroside E | ||||
| Acanthopanax Senticosus powder | Syringin | 4601–4246 | First derivative+multiple scattering correction | 79 |
| Cordyceps Sinensis (Berk) Sacc | Glu | 10000–3970 | First derivative+Savitzky–Golay | 80 |
| Arg | 9420–3950 | |||
| Asp | 9050–3855 | |||
| Total amino | 8500–3800 | |||
| Eucommiae Unloads | Pinoresinol diglucoside | 7502–4597 | First derivative+minus a straight line | 81 |
| Yiqing Granule | Baicalin | 6749–4987 | First derivative+multiple scattering correction | 82 |
| Semen Thlaspi | Sinigrin | 7502–5446 | Maximum and minimum normalization | 83 |
| Phellodendron Chinese Schneid | Berberine | 8000–4000 | Second derivative | 84 |
| Radix Scutellariae | Total Flavonoids | 8015–5446 | Maximum and minimum normalization | 85 |
| Baicalin | 6105–4242 | First derivative | ||
| Forsythiae Suspensa | Phillyrin | 9002–4103 | First derivative | 86 |
| Ophiopogon Japonicus | Polysaccharides | 4000–4900, 5100–6900, 7050–10000 | First derivative+Savitzky–Golay+multiple scattering correction | 87 |
| Ligusticum Chuanxiong Hort | Ferulic acid | 7501–6799, 4424–4246 | First derivative | 88 |
| Rehmannia Glutinosa Libosch | Catalpol | 6102–4597 | First derivative+multiple scattering correction | 89 |
| Dioscorea Zingiberensis C.H. | Extracts by ethanol | 5476–7466 | First derivative+standard normalization variate | 90 |
| Rhizoma Dioscoreae | Polysaccharide | 7513–4597 | First derivative+vector normalization | 91 |
| Rhizoma Dioscoreae | Extracts by water | 8717–5446, 4613–4242 | First derivative+vector normalization | 92 |
| Extracts by ethanol | ||||
| Bear Gall powder extracts | Ursodesoxycholic acid | 4500–8500 | First derivative+Savitzky–Golay | 10 |
| Chenodeoxycholic acid | ||||
| Ursolic acid |
4.2. Quantitative analysis of TCM products by NIRS
4.2.1. Single or multiple component systems
Most quantitative tests for TCM follow the ChP and use HPLC or GC. Any new test method intended to replace an existing method must show some advantage and guarantee accuracy. Examples of the use of NIRS for quantitative analysis include determinations of baicalin in Huang-Qin (Radix Scutellariae)85, phillyrin in Lian-Qiao (Fructus Forsythiae)86 and berberine in Huang-Bai (Cortex Phellodendri Chinese)84, In all cases, results were compared with those obtained by HPLC and shown to be accurate.
TCM products generally contain either a single or more than one TCM herb. The former is exemplified by Dan-Shen injection which contains only Dan-Shen (Radix et Rhizoma Salviae Miltiorrhizae) whereas an example of the latter is Fu-Fang-Dan-Shen dripping pills which contain extracts of both Dan-Shen and San-Qi (Radix Notoginseng). The extraction process involves decoction with water, separation, purification and extraction with water, alcohol and other chemical reagents93.
Single component TCM products such as paclitaxel injection94 are readily analyzed by HPLC, UV or NIRS but NIRS has advantages in the QC of such products. It also simplifies test procedures and reduces technical barriers in quantitative analysis77. For example, assay of lutein and β-carotene in cabbage95 and of pinene, methyl salicylate and eugenol in safflower oil96 (commonly used to treat traumatic injury) all gave satisfactory results by NIRS. A more complex example is Tan-Re-Qing injection, a TCM product made up of Huang-Qin (Radix Scutellariae), Xiong-Dan-Fen (Bear gall powder), Shan-Yang-Jiao (Cornu Caprae Hircus), Jin-Yin-Hua (Flos Lonicerae Japonicae) and Lian-Qiao (Fructus Forsythiae). In this case, NIRS spectra of 120 samples were collected97 and the content of chlorogenic acid, caffeic acid, luteoloside, baicalin, ursodesoxycholic acid and chenodeoxycholic acid were determined and shown to be accurate by HPLC.
Stability of the mathematical model dictates the accuracy of quantitative analysis by NIRS. It is influenced by a series of factors such as the accuracy of chemical reference values, the number of spectra representative of the model, equipment factors and human factors. A sufficient number of representative samples, strict control of experimental conditions and adoption of appropriate data-processing methods all impact on chemical reference values and accuracy. If sufficient spectra are obtained and there is no perturbation, quantitative analysis of multicomponent systems can be achieved by NIRS. For example, in the analysis of the therapeutically active components in Shan-Zhu-Yu (Fructus Corni), astilbin (regulating the immune system) and ursolic acid (sedation and antibacterial effects)98, NIRS spectra in the wavelength ranges 4638–7659 cm−1 and 8197–9441 cm−1 provided abundant information to give accurate results as confirmed by HPLC.
4.2.2. TCM extracts
Extracts of TCM herbs are the intermediate stage between the original plant and an isolated active component99. In order to reduce any deterioration in therapeutic activity, TCM formulations should be as close to the original plant as possible. However, in practice, physical and chemical pretreatments are required to remove unwanted impurities which can affect the pharmacodynamic effects of the active components. In China, out of more than 5000 TCM products, only 47 extracts are recorded in the ChP2010 including those of hawthorn leaf, scutellaria, gingko leaf, total phenolic acids of salvia, asiatic moonseed and forsythia.
In the extract of Yin-Xing (Ginkgo biloba), flavonoids are important pharmacodynamic constituents100. According to the ChP, total flavonoids are made up of the sum of the total flavonol glycosides and total terpene lactones as determined by HPLC. Total flavonol glycosides are then made up of the sum of the amounts of quercetin, kaempferide and isorhamnetin and total terpene lactones of the amounts of bilobalide, ginkgolide A, ginkgolide B and ginkgolide C. Quality assessment of the extract is a complex task which is accomplished more simply and conveniently by NIRS than by HPLC. Using the wavelength range 1100–2500 nm, pre-treating spectra by SNV and evaluating different regression methods (PCR, PLS and modified PLS) produced an assay with acceptable absolute and relative errors and a reliable accuracy rate101. Another example using NIRS and a PLS algorithm in the regression model is the quantitative analysis of the total phenolic acids in the extracts of Huang-Qi (Radix Salvia) and Dan-Shen (Radix et Rhizoma Salviae Miltiorrhizae)102. NIRS with a PLS model was also applied to analyze the major pharmacodynamic components (polysaccharides and triterpenoids) in the extract of Ling-Zhi (Ganoderma lucidum) and Hei-Ling-Zhi (Ganoderma atrum) and gave accurate results103. Research into the quantitative analysis of the total alkaloids in the extract of Huang-Bai (Cortex Phellodendri) also revealed the superiority and practicality of NIRS104.
5. Analysis of illegally additives in TCM products
The presence of illegal additives in drugs, health care products and cosmetics is a perennial problem that can affect quality and lead to potential harm105, 106, 107. In the case of TCM products, coloring agents are often added. Examples include the addition of golden orange II to Hong-Hua (Flos Carthami)108 to enhance its red color and hide moldiness and discoloration and the addition of auramine O to Huang-Qin (Radix Scutellariae) and Pu-Huang (Pollen Typhae) to enhance their yellow color109. Another example is the addition of abietic acid to Ru-Xiang (Olibanum) and Mo-Yao (Myrrha) to enhance their characteristic smell110.
TCM health care products are widely used in China. Compared with drugs, the supervision of TCM health care products is much less rigorous. For example, sildenafil citrate, sildenafil and phenolphthalein are frequently added to TCM health care products to enhance an effect against male sexual dysfunction111, 112, 113, 114. Also, in cosmetics TCM products aiming to improve effects of treating acne and whitening face, hormones and similar substances are often added115, 116. Similar compounds are often added to Chinese patent drugs. For example, prednisone acetate and dexamethasone acetate are commonly found in preparations for treating cough and asthma117.
TLC is frequently used to assess the presence of illegal additives in TCM products. However, as a technique, it suffers from poor sensitivity and reproducibility. As a result, it often requires HPLC or HPLC–MS to further confirm the presence of illegal additives. In principle, NIRS can detect illegal additives based on correlation coefficient analysis. Spectra are compared to calculate the similarity and characteristic range after which the appropriate threshold of correlation coefficient is adjusted to investigate the correlation118, 119.
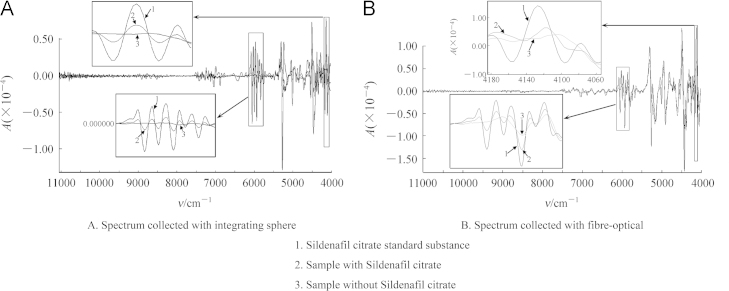
TCM health care products aimed at invigorating kidney function and strengthening “Yang” are used extensively in China. Lu-Rong (Cornu Cervi Pantotrichum), Rou-Cong-Rong (Herba Cistanches), Yin-Yang-Huo (Folium Epimedii), Tu-Si-Zi (Semen Cuscutae), Ba-Ji-Tian (Radix Morindae Officinalis) are also popular TCM products that aim to strengthen male sexual function. However, because their efficacy takes considerable time, drugs, most commonly sildenafil citrate, are often added. To police this phenomenon, NIRS with correlation coefficient analysis is used for rapid screening119. Spectra of sildenafil citrate and samples testing positive and negative for the drug in the wavelength ranges 6070–5800 cm−1 (Fig. 2) and 4170–4070 cm−1 were subject to second derivative and vector normalization. Correlation coefficients of 70% for integrating sphere and 65% for fiber-optical were satisfactory and analysis by LC–MS indicated an accuracy as high as 90%. NIRS can also be used to investigate the presence of illegal additives in foods such as in the control of melamine in milk120.
Figure 2.
Analysis of sildenafil citrate illegally added to TCM products by derivative NIRS.
6. Application of NIRS to fast inspection
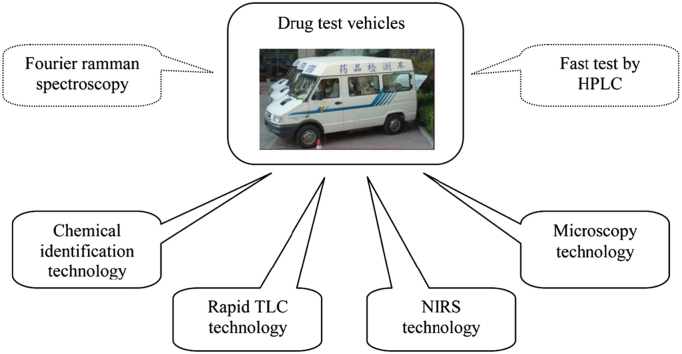
A platform for fast inspection is based on drug identification and other information produced by NIRS, rapid TLC, chemical identification and microscopy. It aims to control the quality of drugs through random inspection (Fig. 3)121, 122 carried out by personnel operating a drug testing vehicle. Mobility and rapidity are key features of the fast inspection platform in TCM supervision123, 124.
Figure 3.
The main components that make up a fast inspection platform. Chemical identification, TLC, NIRS and microscopy are basic technologies in a drug test vehicle. In some parts of China, because of special requirements, Fourier-transform Raman spectroscopy and HPLC are also included.
During a surprise inspection, the advantages of NIRS are important to overcome problems of small sample size, a prolonged testing period and high cost. It can be applied without destroying aluminum packing and to a wide range of formulations including powders, tablets and capsules125. However, NIRS has some serious disadvantages for fast inspection such as poorer sensitivity and stability compared with HPLC. It also suffers from environmental effects, unreliable sampling and poor technical ability on the part of the operatives. Nevertheless, on the whole the accuracy of NIRS is satisfactory for routine analysis126.
Because adulteration of TCM is becoming an increasing problem, attention has been focused on the safety of TCM products on the market. Microscopy, chemical reaction tests, TLC, Fourier-transfer Raman spectroscopy and NIRS can all form part of a fast inspection platform but individual techniques still have problems to overcome. Thus, TLC is useful to back-up the results of NIRS127 but in practice it poses difficulties not only in selecting the best type of plate, solvent system and developing agent but in obtaining clear and well resolved spots. Similarly, chemical reaction tests lack specificity and can only identify the class of substance, e.g., flavonoids and saponins. It also is only useful to support the results obtained by NIRS.
NIRS is more reliable than TLC and chemical reaction tests but accuracy depends on the quality of the model78. In routine screening, a sample which fails an NIRS test should be examined according to the official ChP method. False positives always appear in practice but further inspection following the ChP2010 prevents the problem. False positive results suggest that a model established by NIRS may be flawed and requires improving by enlarging the sample size and sampling region or changing the wavelength region128. This should then improve the stability and applicability of the model.
7. Conclusions
Experience in testing and maintaining the quality of TCM products has developed continuously as their use has grown. To improve and apply routine inspection technologies, higher efficiency of test methods is vital. NIRS has many advantages relevant to daily inspection of TCM products such as its rapidity and non-destructive nature. NIRS technology is developing and improving continuously and its wider application to the quantitative and qualitative analysis of TCM products will certainly improve their quality control and safety in clinical use.
Footnotes
Peer review under responsibility of Institute of Materia Medica, Chinese Academy of Medical Sciences and Chinese Pharmaceutical Association.
References
- 1.Li W. Analysis of factors of impact on the quality of Chinese herbal pieces and countermeasures. Chin Pharm Aff. 2009;23:264–266. [Google Scholar]
- 2.Wu Q.N., Qian D.W., Duan J.A. Discussion on quality changes mechanism of Chinese medicinal materials during storage. J Chin Mater Med. 2010;35:1904–1908. doi: 10.4268/cjcmm20101431. [DOI] [PubMed] [Google Scholar]
- 3.Liu D.J., Xue J., Wu X.B. Advances in determination of multi-residue pesticides in traditional Chinese medicine by GC–MS. J Chin Mater Med. 2011;36:396–400. [PubMed] [Google Scholar]
- 4.Hu X.W. Qualitative and quantitative determination of illegally added acid orangeII in croci stigma by UPLC. China Pharm. 2011;20:40–41. [Google Scholar]
- 5.Long F., Li H.J., Li P. Application of new technologies and methods in morphological and microscopic identification of Chinese materia medica. J Chin Mater Med. 2012;37:1076–1080. [PubMed] [Google Scholar]
- 6.Osborne B.G., Fearn T. Longman Scientific & Technical Press; United States: 1986. Near infrared spectroscopy in food analysis. [Google Scholar]
- 7.Williams P.C., Norris K.H. American Association of Cereal Chemists Press; United States: 1987. Near infrared technology in agriculture and food industries. [Google Scholar]
- 8.Cen H.Y., He Y. Theory and application of near infrared reflectance spectro-scopy in determination of food quality. Trends Food Sci Technol. 2007;18:72–83. [Google Scholar]
- 9.Shi C.X., Yang Y.W., Guo Z.X., Zhu G.G., Yuan Y.G. Application of near-infrared spectroscopy in quality control of Chinese materia medica. J Chin Mater Med. 2005;36:1731–1733. [Google Scholar]
- 10.Li W.L., Liu S.Y., Xue D.S., Qu H.B. Rapid analysis of bear gall powder extracts with near infrared diffused reflectance spectroscopy. Chin Pharmacol J. 2010;45:1500–1503. [Google Scholar]
- 11.Xing J.S., Zhang X.B. Development of a near-infrared method for rapid determination of ampicillin capsules. Chin J Pharm Anal. 2010;30:2408–2411. [Google Scholar]
- 12.Hou S.R., Feng Y.C., Hu C.Q. Development of a near infrared method for rapid determination water content in ceftriaxone sodium for injection. Chin J Pharm Anal. 2008;28:936. [Google Scholar]
- 13.Liu Y.M., Chen K.L. Several common types of counterfeit and inferior drugs in Chinese medicinal materials market. J Chin Mater Med. 2012;37:1089–1092. [PubMed] [Google Scholar]
- 14.Chen K.L., Lei Q.M. Identification of Chinese medicinal materials after extraction. Chin Pharmacol J. 2002;37:463. [Google Scholar]
- 15.Li Z.S., Mu J., Dong L.R., Yin M., Shi M.Z. Situation analysis of 30 kinds of false and bad Chinese medicines (pieces) Yunnan J Tradit Chin Med Mater. 2009;30:29–31. [Google Scholar]
- 16.Liu M.M., Li F. Microscopic identification of new technologies and applications in progress in the identification of Chinese crude drugs. J Liaoning Univ Tradit Chin Med. 2011;13:50–52. [Google Scholar]
- 17.Jiang H., Chen X. The quickly identifying TCM-crop by hemi-micro shape and properties. Chin J Ethnomed Ethnopharm. 2008;10:10–12. [Google Scholar]
- 18.Jiang H. The identifying TCM-crop by hemi-micro technology. Chin J Ethnomed Ethnopharm. 2008;4:54–56. [Google Scholar]
- 19.The State Pharmacopoeia Committee of China . Chinese Medicine Science and Technology Press; Beijing: 2010. The pharmacopoeia of the People׳s Republic of China. [Google Scholar]
- 20.Liu S.H., Zhang X.G., Zhou Q., Sun S.Q. Determination of geographical origins of Chinese medical herbs by NIR and pattern recognition. Spectrosc Spectr Anal. 2006;26:629–632. [PubMed] [Google Scholar]
- 21.Zhang Q.H., Zhu Z.W., Li J. Research progress on chemical composition and cultivation of TCM qinghao. Chin Med Her. 2011;8:10–12. [Google Scholar]
- 22.Li W.H., Cheng Z.W., Wang Y.F., Qu H.B. Quality control of Lonicerae japonicae Flos using near infrared spectroscopy and chemometrics. J Pharm Biomed. 2013;72:33–39. doi: 10.1016/j.jpba.2012.09.012. [DOI] [PubMed] [Google Scholar]
- 23.Zhao Q.C., Du Z.C., Zhang L., Song Q.Y., Shi G.B. Determination content of ursolic acid in 5 kinds of Chinese herbal medicine by HPLC. Chin Tradit Herb Drug. 2009;40:1821–1823. [Google Scholar]
- 24.Wang G.L., Shi Y., Wei Y.H., Wang H.C., Xu X.J., Lin R.C. Application of NIR analysis in identification of genuine cordyceps. Chin Tradit Herb Drug. 2006;37:1569–1571. [Google Scholar]
- 25.Li D., Liu Y., Xie J., Yuan W.P., Chen Z.D. Modern research on Chinese traditional medicine monascus rice: origin, taxology, and pharmacognosy of monascus. China Pharm. 2009;18:1–2. [Google Scholar]
- 26.Liu L.L., Xing W.X., Jia N., Lin P.Y., Mi H.M., Wu Y.T. Identification of red kojie with elustering analysis by near-infrared diffuse reflectance spectrometry. Acad J Second Mil Med Univ. 2002;23:1230–1232. [PubMed] [Google Scholar]
- 27.Xing W.X., Liu L.L., Jia N., Lin P.Y., Mi H.M., Wu YT. Identification of red kojic from different habitats with clustering analysis by near-infrared diffuse reflectance spectrometry. J Chin Med Mater. 2001;24:561–563. [PubMed] [Google Scholar]
- 28.Duan L.Q., Cui B.H., Liu S.F. Identification of Chinese medicine pieces with similar appearance of formulations drug decoction. Chin J Clin Ration Drug Use. 2010;3:75–76. [Google Scholar]
- 29.Ding N.Y., Li W., Feng X.W., Zhu Z.L. Classification and identification of Chinese traditional medicines with NIR diffuse reflection. Comput Appl Chem. 2008;25:499–502. [Google Scholar]
- 30.Zeng H.J., Han Y. Establishment of near infrared qualitative model for tongxinluo capsules. Chin Pharm Aff. 2011;25:373–374. [Google Scholar]
- 31.Chen J.Q., Li Q.H., Li Q. The study of NIR detection on tiandantongluo capsules. Chin Pharm Aff. 2011;25:36–38. [Google Scholar]
- 32.Xu D.L., Ling G.L. Construction of NIR models for determination of water in hard capsule of Chinese traditional medicine. Chin J Pharm Anal. 2010;30:2170–2172. [Google Scholar]
- 33.Li Y.Z., Min S.G., Liu X. Study on the methods and applications of near-infrared spectroscopy chemical pattern recognition. Spectrosc Spectr Anal. 2007;27:1299–1303. [PubMed] [Google Scholar]
- 34.Wang L.L., Wang C., Pan Z.F., Sun Y., Zhu X.Y. Application of pyrolysis-gas chromatography and hierarchical cluster analysis to the discrimination of the Chinese traditional medicine Dendrobium candidum wall. ex lindl. J Anal Appl Pyrolysis. 2011;90:13–17. [Google Scholar]
- 35.Bock H.H. Probabilistic models in cluster analysis. Comput Stat Data Anal. 1996;23:5–28. [Google Scholar]
- 36.Xu Y.Q., Huang H., Zhou Q., Zhou H.T., Hu S.L., Sun S.Q. The application of fingerprint infrared spectra and clustering analysis in the discrimination of geographical origin of Paeonia lactiflora pall. Chin J Anal Chem. 2003;31:5–9. [Google Scholar]
- 37.Guo L.P., Huang L.Q., Huck C. Near infrared spectroscopy (NIRS) technology and its application in geoherbs. J Chin Mater Med. 2009;34:1751–1757. [PubMed] [Google Scholar]
- 38.Liu M.Q., Zhou D.C., Xu X.Y., Sun Y.J., Zhou X.L., Han L. Clustering analysis applied to near-infrared spectroscopy analysis of Chinese traditional medicine. Spectrosc Spectr Anal. 2007;27:1985–1988. [PubMed] [Google Scholar]
- 39.Li X.Q., Sun X.H., Cai S., Ying X.X., Li F.M. Investigation on the chemical constituents and variation of the flower buds of lonicera species by UPLC-ESI-MS/MS and principle component analysis. Acta Pharm Sin. 2009;44:895–904. [PubMed] [Google Scholar]
- 40.Roggo Y., Duponchel L., Ruckebusch C., Huvenne J.P. Statistical tests for comparison of quantitative and qualitative models developed with near infrared spectral data. J Mol Struct. 2003;654:253–262. [Google Scholar]
- 41.Yuan Y.F., Tao Z.H., Liu J.X., Tian C.H., Wang G.W., Li Y.Q. Identification of Cortex Phellodendri by Fourier-transform infrared spectroscopy and principal component analysis. Spectrosc Spectr Anal. 2011;31:1258–1261. [PubMed] [Google Scholar]
- 42.Wang N., Sun D., Dong H.P. Quick identification of shuanghuanglian oral liquid and yinhuang oral liquid by AOTF-near infrared spectroscopy principal component analysis. Chin Pharm. 2009;20:2355–2357. [Google Scholar]
- 43.Li B.X., Wei Y.H., Duan H.G., Xi L.L., Wu X.A. Discrimination of the geographical origin of Codonopsis pilosula using near infrared diffuse reflection spectroscopy coupled with random forests and k-nearest neighbor methods. Vib Spectrosc. 2012;62:17–22. [Google Scholar]
- 44.Lu H.Y., Wang S.S., Cai R., Meng Y., Xie X., Zhao W.J. Rapid discrimination and quantification of alkaloids in corydalis tuber by near-infrared spectroscopy. J Pharm Biomed. 2012;59:44–49. doi: 10.1016/j.jpba.2011.09.037. [DOI] [PubMed] [Google Scholar]
- 45.Fu H.Y., Huang D.C., Yang T.M., She Y.B., Zhang H. Rapid recognition of Chinese herbal pieces of areca catechu by different concocted processes using Fourier transform mid-infrared and near-infrared spectroscopy combined with partial least-squares discriminant analysis. Chin Chem Lett. 2013;24:639–642. [Google Scholar]
- 46.Lucio-Gutiérrez J.R., Coello J., Maspoch S. Application of near infrared spectral fingerprinting and pattern recognition techniques for fast identification of eleutherococcus senticosus. Food Res Int. 2011;44:557–565. [Google Scholar]
- 47.Tang D., Li W., Xu Y., Wu Y.L., Wu Q.Y. Research on artificial neural network method of pogostemon cablin fingerprint analysis. J Chin Med Mater. 2004;27:534–536. [Google Scholar]
- 48.Wang L.Q., Fan Q., Yi Z.K., Wang Y.W. Identification of different ephedra plants using HPLC fingerprints in combination with back-propagation artificial neural network and discriminant analysis. J Southwest China Normal Univ. 2012;37:73–77. [Google Scholar]
- 49.Warren M., Pitts W. A logical calculus of the ideas immanent in nervous activity. Bull Math Biol. 1943;5:115–133. [PubMed] [Google Scholar]
- 50.Liang X.F., Xiong N.X., Yang L.T., Zhang H., Park J.H. A compensation scheme of fingerprint distortion using combined radial basis function model for ubiquitous services. Comput Commun. 2008;31:4360–4366. [Google Scholar]
- 51.Yang D.Z., An Y.Q., Jiang X.L., Tang D.Q., Gao Y.Y., Zhao H.T. Development of a novel method combining HPLC fingerprint and multi-ingredients quantitative analysis for quality evaluation of traditional Chinese medicine preparation. Talanta. 2011;85:885–890. doi: 10.1016/j.talanta.2011.04.059. [DOI] [PubMed] [Google Scholar]
- 52.Lachenbruch P., Goldstein M. Hafner Press; United States: 1979. Discriminant analysis biometrics. [Google Scholar]
- 53.Qi X.M., Zhang L.D., Du X.L., Song Z.J., Zhang Y., Xu S.Y. Quantitative analysis using NIR by building PLS-BP model. Spectrosc Spectr Anal. 2003;23:870–872. [Google Scholar]
- 54.Hou J., Chen G.S., Wang Z.P. Development of artificial neural network and its application in multivariate calibration. J Anal Sci. 2001;17:68–74. [Google Scholar]
- 55.Gao H.B., Xiang B.R., Li R., Liu H. Rapid, non-destructive quantitative analysis of nicotinic acid tablets by NIR combined with wavelet transform and artificial neural network. J China Pharm Univ. 2006;37:326–329. [Google Scholar]
- 56.Zhou J.L., Qi L.W., Li P. Quality control of Chinese herbal medicines with chromatographic fingerprint. Chin J Chromatogr. 2008;26:153–159. doi: 10.1016/s1872-2059(08)60011-5. [DOI] [PubMed] [Google Scholar]
- 57.Ni L.J., Zhang L.G., Hou J., Shi W.Z., Guo M.L. A strategy for evaluating antipyretic efficacy of Chinese herbal medicines based on UV spectrafingerprints. J Ethnopharmacol. 2009;124:79–86. doi: 10.1016/j.jep.2009.04.006. [DOI] [PubMed] [Google Scholar]
- 58.Tian R.T., Xie P.S., Liu H.P. Evaluation of traditional Chinese herbal medicine: chaihu (Bupleuri Radix) by both high-performance liquid chromatographic and high-performance thin-layer chromatographic fingerprint and chemometric analysis. J Chromatogr A. 2009;1216:2150–2155. doi: 10.1016/j.chroma.2008.10.127. [DOI] [PubMed] [Google Scholar]
- 59.Zhang J.L., Cui M., He Y., Yu H.L., Guo D.A. Chemical fingerprint and metabolic fingerprint analysis of danshen injection by HPLC–UV and HPLC–MS methods. J Pharm Biomed. 2005;36:1029–1035. doi: 10.1016/j.jpba.2004.09.009. [DOI] [PubMed] [Google Scholar]
- 60.Huang B.K., Huang L.Q., Qin L.P., Zhao Z.X., Lu F., Zheng HC. Fingerprints clustering analysis of four valeriana medicinal plants by near infrared method. J Chin Med Mater. 2008;31:1494–1496. [Google Scholar]
- 61.Liu X.P., Feng Y.C., Hu C.Q., Ding L. Construction of universal quantitative models for determination of cefradine capsules. Chin J Pharm Anal. 2008;28:722–726. [Google Scholar]
- 62.Zou W.B., Feng Y.C., Song D.Q., Hu C.Q. Construction of a universal quantitative model for the determination of azithromycin in granules using near-infrared diffuse reflectance spectroscopy. Chin Pharmacol J. 2012;21:459–467. [Google Scholar]
- 63.Pang H.H., Feng Y.C., Zhang X.B., Xiang B.R. Construction of universal quantitative models for the determination of cefoperazone sodium/sulbactam sodium for injection from different manufacturers using near-infrared reflectance spectroscopy. Spectrosc Spectr Anal. 2006;17:22–29. [PubMed] [Google Scholar]
- 64.Shen Y., Pan Y., Liu Q., Xiang J.Z. Qualitative and quantitative assay of amoxicillin capsules by NIR diffuse reflectance spectroscopy. Chin J Pharm Anal. 2005;25:385–389. [Google Scholar]
- 65.Shi C.X., Yang Y.W., Guo Z.X., Zhu G.G. Quantitative analysis on Radix Salviae miltiorrhizae by NIR. J Chin Med Mater. 2006;29:897–899. [PubMed] [Google Scholar]
- 66.Zhang Y., Xie Y.F., Song F.R., Liu Z.Q., Cong Q., Zhao B. Quantitative analysis of berberine in processed coptis by near-infrared diffuse reflectance spectroscopy. Chem Res Chin Univ. 2008;24:717–721. [Google Scholar]
- 67.Qu N., Li X.S., Dou Y., Mi H., Guo Y., Ren Y. Nondestructive quantitative analysis of erythromycin ethylsuccinate powder drug via short-wave near-infrared spectroscopy combined with radial basis function neural networks. Eur J Pharm Sci. 2007;31:156–164. doi: 10.1016/j.ejps.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 68.Cho C.H., Woo Y.A., Kim H.J., Chung Y.J., Chang S.Y., Chung H. Rapid qualitative and quantitative evaluation of deer antler (cervus elaphus) using near-infrared reflectance spectroscopy. Microchem J. 2001;68:189–195. [Google Scholar]
- 69.Pang H.H., Feng Y.C., Hu C.Q., Xiang B.R. Construction of universal quantitative models for determination of cefoperazone sodium for injection from different manufacturers using near infrared reflectance spectroscopy. J Chin Pharm Sci. 2008;26:2214–2218. [PubMed] [Google Scholar]
- 70.Lei D.Q., Hu C.Q., Feng Y.C., Feng F. Feasibility of the extended application of near infrared universal quantitative models. Acta Pharm Sin. 2010;45:1421–1426. [PubMed] [Google Scholar]
- 71.Svante W., Henrik A., Fredrik L., Jerker Ö. Orthogonal signal correction of near-infrared spectra. Chemom Intell Lab Syst. 1998;44:175–185. [Google Scholar]
- 72.Ni Y.N., Lin W. Near-infrared spectra combined with partial least squares for pH determination of toothpaste of different brands. Chin Chem Lett. 2011;22:1473–1476. [Google Scholar]
- 73.Gimet R., Luong A.T. Quantitative determination of polymorphic forms in a formulation matrix using the near infra-red reflectance analysis technique. J Pharm Biomed. 1987;5:205–211. doi: 10.1016/0731-7085(87)80024-9. [DOI] [PubMed] [Google Scholar]
- 74.Hui Y.N., Feng W.X., Wei T., Lü X.Q., Song J., Zhao S.S. Adjustment of coordination environment of Ln3+ ions to modulate near-infrared luminescent properties of Ln3+ complexes. Inorg Chem Commun. 2011;14:200–204. [Google Scholar]
- 75.Yip W.L., Gausemel I., Sande S.A., Dyrstad K. Strategies for multivariate modeling of moisture content in freeze-dried mannitol-containing products by near-infrared spectroscopy. J Pharm Biomed. 2012;70:202–211. doi: 10.1016/j.jpba.2012.06.043. [DOI] [PubMed] [Google Scholar]
- 76.Jarkko H., Leppämäki M., Paatero E., Pentti M. Monitoring the kinetics of the ion-exchange resin catalysed esterification of acetic acid with ethanol using near infrared spectroscopy with partial least squares (PLS) model. Chemom Intell Lab Syst. 1998;44:341–352. [Google Scholar]
- 77.Xu T., Zhou M., Wang L.L., Ye L.M., Chen C., Tang Y. Determination of taxol in taxol injection using near infrared transmission spectroscopy. J Biomed Eng. 2009;26:982–984. [PubMed] [Google Scholar]
- 78.Liu Y.Y., Hu C.Q., Hang Y.J. Discussion on spectral wavelength selection principles and the strategy for eliminating the solvent interference in the near infrared quantitative models for determination of Acanthopanax senticosus injection. Sci China Chem. 2010;40:1664–1673. [Google Scholar]
- 79.Xu S.Q., Zhang R., Liu H.Q., Zhang H., Yan M.X., Zhang XR. Determination of syringoside content in the bark of Acanthopanax senticosus (Rupr.et Maxim.) harms by near infrared spectrum. Spec Wild Econ Anim Plant Res. 2012;4:54–57. [Google Scholar]
- 80.Shi Y., Wang G.L., Lin R.C. NIR determination of amino acid from Cordyceps sinensis (berk) sacc. Chin J Pharm Anal. 2007;27:90–92. [Google Scholar]
- 81.Li W., Sun S.Q., Qin J.P., Yi Y.H., Yang M.H. Quantitative determination of pinoresinol diglucoside in eucommiae unloads by NIRS. J Chin Mater Med. 2010;35:3318–3321. [PubMed] [Google Scholar]
- 82.Li Y.Y., Bai Y., Chen Z.H., Fan K.F. Quantitative analysis on yiqing granule from different manufacturers by near infrared diffuse reflectance spectroscopy. Chin J Pharm Anal. 2009;29:1126–1129. [Google Scholar]
- 83.Wang L.L., Chen C., Zhou M., Wang J.Z., Luo X., Huang G. Determination of sinigrin in Semen Thlaspi from Sichuan and Tibet using near infrared diffuse reflectance spectroscopy. Spectrosc Spectr Anal. 2009;29:2673–2676. [PubMed] [Google Scholar]
- 84.Zhou M., Wang T.Z., Ye L.M., Chen C., Huang G., Wu Y.W. Determination of berberine in Phellodendron chinese schneid from Sichuan using near infrared diffuse reflectance spectroscopy. Spectrosc Spectr Anal. 2007;27:1527–1530. [Google Scholar]
- 85.Huang Q.Q., Pan R.L., Wei J.H., Wu Y.W., Zhang L.D. Determination of baicalin and total flavonoids in Radix Scutellariae by near infrared diffuse reflectance spectroscopy. Spectrosc Spectr Anal. 2009;29:2425–2428. [PubMed] [Google Scholar]
- 86.Zhang W., Bai Y., Wang X., Chen Z.H. Determination of phillyrin in the extractum of forsythiae suspense by near-infrared diffuse reflectance spectroscopy. Chin J Hosp Pharm. 2010;30:1018–1021. [Google Scholar]
- 87.Wang Y., Qin M.J., Qi J., Yu B.Y., Tang L. Analysis of polysaccharides contents in Ophiopogon japonicus by NIR. Spectrosc Spectr Anal. 2009;29:2677–2680. [PubMed] [Google Scholar]
- 88.Wang X.M., Jiao L., Liu X.L., Li H. Quantitative analysis of ferulic acid in Ligusticum chuanxiong hort by near infrared diffuse reflectance spectroscopy. Chin J Pharm Anal. 2011;31:1016–1019. [Google Scholar]
- 89.Xu M.C. Rapid determination of the contents of catalpol in Rehmannia Glutinosa libosch by NIR. J Chin Med Mater. 2011;34:1072–1074. [Google Scholar]
- 90.Xie C.X., Zuo C.F., Lei J.W., Bai Y. Quantitative analysis on extracts by ethanol from Dioscorea zingiberensis C.H. Wright by near-infrared diffuse reflection spectroscopy. Chin J Mod Appl Pharm. 2012;29:931–935. [Google Scholar]
- 91.Bai Y., Gong H.Y., Song R.L., Bai Y. Rapid determination of the content of polysaccharides in Rhizoma Dioscoreae by NIR. Chin Tradit Herb Drug. 2010;32:110–112. [Google Scholar]
- 92.Bai Y., Gong H.Y., Song R.L., Li S., Chen Z.H. Quantitative analysis on extracts by water and ethanol in Rhizoma Dioscoreae by near-infrared diffuse reflection spectroscopy. Chin J Mod Appl Pharm. 2010;27:163–167. [Google Scholar]
- 93.Liu M.Y., Wang B.C. Advances in new technologies applying to Chinese materia medica extraction. Chin Tradit Herb Drug. 2010;41:169–175. [Google Scholar]
- 94.Pan L.L., Dong J., Wang S.L., Su D.S. Preparation and preliminary evaluation of physical stability of the paclitaxel injection. J Shenyang Pharm Univ. 2004;21:101–104. [Google Scholar]
- 95.Chen X.J., Wu J.G., Zhou S.J., Yang Y.J., Ni X.L. Application of near-infrared reflectance spectroscopy to evaluate the lutein and β-carotene in Chinese kale. J Food Compos Anal. 2009;22:148–153. [Google Scholar]
- 96.Wu Y.W., Sun S.Q., Zhou Q., Leung H.W. Fourier transform mid-infrared (MIR) and near-infrared (NIR) spectroscopy for rapid quality assessment of Chinese medicine preparation honghua oil. J Pharm Biomed. 2008;46:498–504. doi: 10.1016/j.jpba.2007.11.021. [DOI] [PubMed] [Google Scholar]
- 97.Li W.H., Xing L.H., Fang L.M., Wang J., Qu H.B. Application of near infrared spectroscopy for rapid analysis of intermediates of tanreqing injection. J Pharm Biomed. 2010;53:350–358. doi: 10.1016/j.jpba.2010.04.011. [DOI] [PubMed] [Google Scholar]
- 98.Zhang Z. Development of a near-infrared diffuse method for rapid determination of astilbin and ursolic acid in alcohol extract from Cornus officinalis. Anhui Med Pharm J. 2011;15:955–957. [Google Scholar]
- 99.Qu H.B., Ou D.L., Cheng Y.Y. A new quality control method of Chinese medicinal plant extracts. Chin Pharm J. 2006;41:57–60. [Google Scholar]
- 100.Wang Y., Yang Y.F. The research of Ginkgo biloba pharmacology. Chin J Mod Appl Pharm. 2001;18:1. [Google Scholar]
- 101.Hu G.L., Lu X.Y., Wu J.G., Shao S.R., Shi C.H. Direct determination of total flavones in powder of Ginkgo biloba extract by near-infrared diffuse reflection spectroscopy. Chin J Pharm Anal. 2004;24:18–20. [Google Scholar]
- 102.Li W.H., Qu H.B. Rapid quantification of phenolic acids in Radix Salvia miltrorrhiza extract solutions by FT-NIR spectroscopy in transflective mode. J Pharm Biomed. 2010;52:425–431. doi: 10.1016/j.jpba.2010.01.009. [DOI] [PubMed] [Google Scholar]
- 103.Chen Y., Xie M.Y., Zhang H., Wang Y.X., Nie S.P., Li C. Quantification of total polysaccharides and triterpenoids in Ganoderma lucidum and Ganoderma atrum by near infrared spectroscopy and chemometrics. Food Chem. 2012;135:268–275. [Google Scholar]
- 104.Chan C.O., Chu C.C., Mok D.K., Daniel K.W., Chau F.T. Analysis of berberine and total alkaloid content in Cortex Phellodendri by near infrared spectroscopy (NIRS) compared with high-performance liquid chromatography coupled with ultra-visible spectrometric detection. Anal Chim Acta. 2007;592:121–131. doi: 10.1016/j.aca.2007.04.016. [DOI] [PubMed] [Google Scholar]
- 105.Gu B.R., Xu Q., Miao G. UPLC determination of 12 hormones illegally adulterated into traditional Chinese medicines and health products. Chin J Pharm Anal. 2010;30:2185–2187. [Google Scholar]
- 106.Dai X.M., An N., Wu J.M., Li H.Y., Zhang Q.M. Development and validation of HPLC-UV-MS method for the control of four anti-diabetic drugs in suspected counterfeit products. Acta Pharm Sin. 2010;45:347–352. [PubMed] [Google Scholar]
- 107.Zhang C.Y., Li Z.G., Xu J.L., Liu N.Q. Determination of sildenafil citrate illegally mixed into traditional Chinese medicinal preparation by ultra performance liquid chromatography-tandem mass spectrometry. Chin Tradit Pat Med. 2007;29:1315–1318. [Google Scholar]
- 108.Hu Z.Y., Tian J.X., Li X.L. Advances in studies on liquid chromatography-tandem mass spectrometry to analysis of non-target compounds in Chinese materia medica. Chin Tradit Herb Drug. 2011;42:180–184. [Google Scholar]
- 109.Qiu Y.J. Identification of abietic acid illegally added in gujinwan capsules by HPLC. Chin Pharm. 2011;20:39–40. [Google Scholar]
- 110.Ge H.Q., Jia T.Z. Study of auramine O in dyed Radix Scutellariae praepareta and Pollen Typhae by UPLC-Ms/pad. J Tradit Chin Med. 2011;39:1616–1618. [Google Scholar]
- 111.Zhu S.X. Investigation and analysis in “bushen, zhuangyang” health products illegal addition of sildenafil citrate. Chin Pharm Aff. 2004;18:125–126. [Google Scholar]
- 112.Pang X.X., Lu H.C., Zhang Y.H., Wang J.Z. The rapid detection method of silaenafil added in Chinese traditional medicine and health care products. Chin J Hosp Pharm. 2008;28:673–674. [Google Scholar]
- 113.Qiu Y.H., Wang T.J., Li M.F., Pang X.B., Li J. Detection of phenolphthalein illegally mixed into slimming Chinese traditional medicines and health products by the liquid chromatography-quadrupole mass spectrometry method. Chin J Pharm Anal. 2006;26:1609–1611. [Google Scholar]
- 114.Li W.J., Chen X.L., Li W.J., Wei F., Xiao X.Y., Lin R.C. Rapid and direct analysis of sibutramine hydrochloride illegally added in weight-loss healthy food by DART-MS/MS method. Chin Pharm Aff. 2012;26:147–149. [Google Scholar]
- 115.Xi B.B., Guo R.F., He J.F. Analysis of 8 kinds of hormones by reversed phase high performance liquid chromatography in traditional Chinese medicine health care products. Chin Pharm. 2007;16:19. [Google Scholar]
- 116.Wang C.X., Liu G.Y., Ni X.L., Han L., Fang X.M., Zhang S. Determination of glucocorticoids in cosmetics by HPLC-MS/MS. Anal Instrum. 2008;6:23–28. [Google Scholar]
- 117.Zhang C.Y., Li Z.G., Xu J.L. Theophylline, prednisone acetate and dexamethasone acetate illegally mixed into traditional Chinese medicinal preparation for antitussive and antiasthmastics by UPLC-MS. Chin Tradit Pat Med. 2008;30:1326–1330. [Google Scholar]
- 118.Liu T., Feng Y.C., Song D.Q., Hu C.Q. Selection of characteristic spectral bands for the analysis by the NIR correlation coefficient method. J Chin Pharm Sci. 2011;20:83–91. [Google Scholar]
- 119.Wang X.L., Feng Y.C., Hu C.Q. Near infrared correlation coefficient method with characteristic spectral band for the determination of illegal addition of sildenafil citrate in capsules of Chinese traditional medicine. Chin J Anal Chem. 2009;37:1825–1828. [Google Scholar]
- 120.Liu J.W., Zhang B.Y., Li S.F., Wang L.M. Near infrared spectroscopy technique for detecting the melamine of dairy products. J Light Scatt. 2010;22:291–297. [Google Scholar]
- 121.Xu T.Q., Zhang W.H., Sun M., Shi F.L., Zhang T.X., Chen G. Status analysis of rapid detection facilities in health agencies. Chin J Health Insp. 2007;14 (405-7,411) [Google Scholar]
- 122.Lu C.H. The present situation and prospect of rapid drug test. Chin Pharm Aff. 2006;20:652–653. [Google Scholar]
- 123.Jin S.H. Development and role of drug testing vehicles. Chin Pharm Aff. 2007;21:8–11. [Google Scholar]
- 124.Liu B.C., Luo Y.C. The effective operation and experience of drug testing vehicles. Strait Pharm J. 2007;22:189–191. [Google Scholar]
- 125.Chen G.B., Tao Q.F., Hong L.Y., Hu C.Q. Development of quantitative models for determination of simvastatin tablets using near infrared diffuse reflectance spectroscopy. Chin J Pharm Anal. 2009;29:989–993. [Google Scholar]
- 126.Li Y., Yang X.C., Fan F.F. Study on near-infrared model of domperidone tablets in the drug testing vehicles. Chin Pharm Aff. 2010;24:176–177. [Google Scholar]
- 127.Wei F., Cheng X.L., Ma L.Y., Xiao X.Y., Ma S.C., Lin R.C. Discussion on technical requirements of substitution method of reference substance used for drug standards. Drug Stand China. 2012;13:12–15. [Google Scholar]
- 128.Roggo Y., Chalus P., Maurer L., Lema-Martinez C., Edmond A., Jent A. A review of near infrared spectroscopy and chemometrics in pharmaceutical technologies. J Pharm Biomed. 2007;44:683–700. doi: 10.1016/j.jpba.2007.03.023. [DOI] [PubMed] [Google Scholar]