Abstract
Colon cancer is a world-wide health problem and the second-most dangerous type of cancer, affecting both men and women. The modern diet and lifestyles, with high meat consumption and excessive alcohol use, along with limited physical activity has led to an increasing mortality rate for colon cancer worldwide. As a result, there is a need to develop novel and environmentally benign drug therapies for colon cancer. Currently, nutraceuticals play an increasingly important role in the treatment of various chronic diseases such as colon cancer, diabetes and Alzheimer׳s disease. Nutraceuticals are derived from various natural sources such as medicinal plants, marine organisms, vegetables and fruits. Nutraceuticals have shown the potential to reduce the risk of colon cancer and slow its progression. These dietary substances target different molecular aspects of colon cancer development. Accordingly, this review briefly discusses the medicinal importance of nutraceuticals and their ability to reduce the risk of colorectal carcinogenesis.
KEY WORDS: Colon cancer, Nutraceuticals, Therapeutics, Marine organisms, Plant derivatives
Abbreviations: ACC, acetyl CoA carboxylase; ACF, aberrant crypt foci; ACL, ATP-citrate lyase; ASTX, astaxanthin; COX-2, cyclooxygenase 2; DHA, decahexaenoic acid; DMH, 1,2-dimethylhydrazine; DR, death receptor; EGCG, epigallocatechingallate; EPA, eicosapentaenoic acid; FAS, fatty acid synthase; 5-FU, 5-fluorouracil; GADD, growth arrest and DNA damage; HMG-CoA, 3-hydroxy-3-methyl-glutaryl CoA; HUVEC, human umbilical vein endothelial cell; IGF, insulin-like growth factor; IL, interleukin; LDH, lactate dehydrogenase; MMP, matrix metallo-proteins; NF-κB, nuclear factor-kappa B; PRAP, prolactin receptor associated protein; TCA, tricarboxylic acid cycle; TNF, tumor necrosis factor; TRAIL, tumor necrosis factor-related apoptosis-induced ligand; VEGF, vascular endothelial growth factor
Graphical abstract
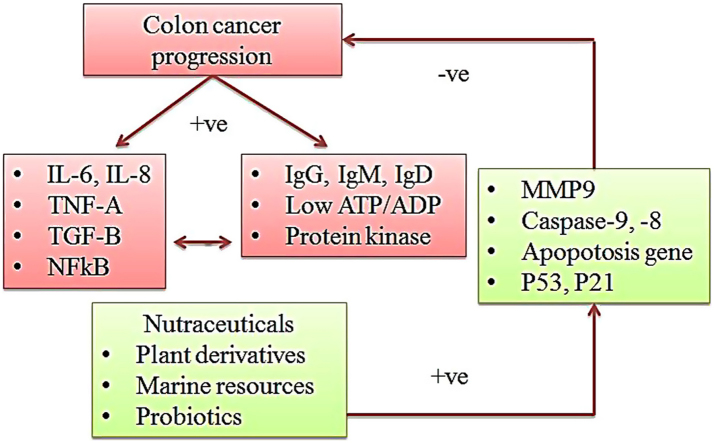
Nutraceuticals are alternative therapeutic agents to control the colon cancer progression. The nutraceuticals stimulate the normal metabolic function in colon cancer cells and regulate the tumor suppressor genes and immunity. These natural products control the over expression of metabolic enzymes and tumor growth factors in colon cancer cell.
1. Introduction
Colon cancer is one of the most dangerous forms of cancer, with potential to spread to distinct parts of the body including liver, lung, ovaries and other gastrointestinal organs. So far, 5-fluorouracil (5-FU) is the first choice for colon cancer treatment, acting as an inhibitor of DNA synthesis1, 2. However, while synthetic chemical anticancer drugs prolong survival, they often have adverse effects and off-target actions. Based on this, nutraceuticals and phytochemicals have been investigated for colon cancer therapeutics3. Nutraceutical is a term derived from nutrition and pharmaceutical, and are sometimes termed “functional foods”4. Nutritional phytochemicals have a strong historical background and significant applications in modern medicine. These compounds are used in medicinal and commercial industries for cosmetics, food aids and additives5.
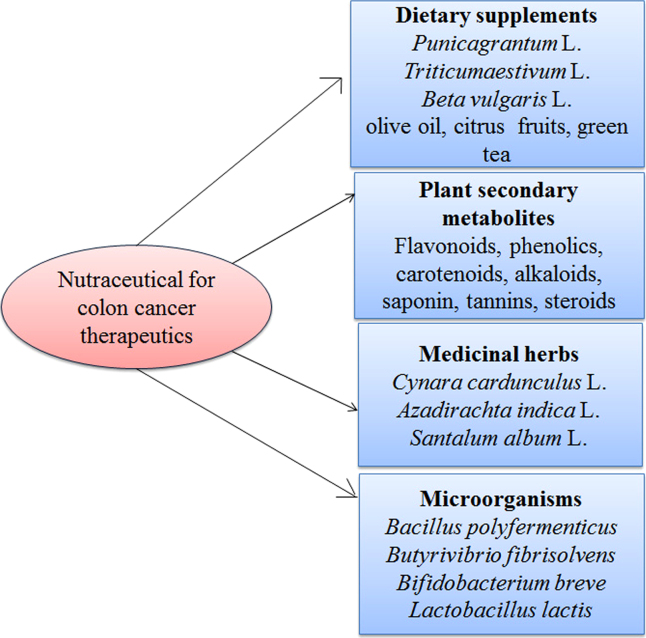
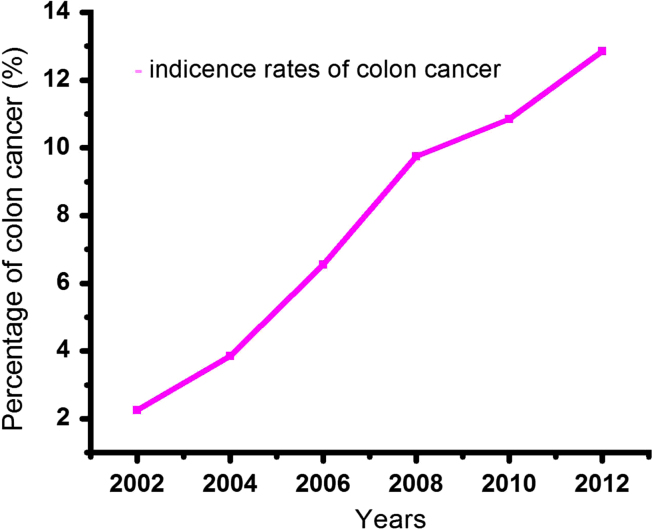
Nutraceuticals have the ability to control the DNA damaging factors in cancer cells and regulate DNA transcription in tumors. They possess numerous therapeutic benefits such as antiobesity effects, cardiovascular effects, antidiabetic effects, immune enhancement, natural antioxidant activity, and anti-inflammatory effects6, 7. Fig. 1 shows different classes of nutraceuticals and their uses. The different stages of colon cancer warrant various treatment options such as chemotherapy, surgery, radiation and phytotherapy. All other forms of cancer therapeutics have significant adverse effects. Plant-derived nutraceuticals are advantageous for the treatment of colon cancer with additional benefit of improving overall health8. These nutritional compounds have provided better treatment and showed fewer adverse effects9. The incidence and mortality rates for colon cancer have been increasing in most of the countries, particularly US, European and part of Asian countries. This increasing incidence of colon cancer appears due to changing dietary constituents, physical activity patterns, as well as genetic influences10. Fig. 2 shows the mortality and incidence rate of colon cancer in 2005–2012.
Figure 1.
Different classes of nutraceuticals and their uses.
Figure 2.
The mortality and incidence rate of colon cancer in the years 2002–2012.
Reactive oxygen species can cause problems in normal cells. Free radicals such as O2– and OH− may increase normal human colonocyte activity and result in the formation colon polyps. Natural antioxidants such as quercetin are derived from fruits and plant resources and can limit the oxidative damage in colon cells. Quercetin belongs to a family of plant-derived flavonoid phytochemicals and is effective for inducing apoptosis in colon cancer cells. Likewise, dietary uses of onion might be able to suppress the proliferation of normal cells. Onion contains high levels of quercetin, which inhibits the effects of colon cancer proliferation in both in vitro and in vivo studies11. Lentinan naturally occurs in the edible mushroom Lentinus edodes. The lentinan compound is known as β-1,3-glucan. It is one of the important drugs used as anticancer agents and is used clinically for colon cancer treatment. Lentinan significantly reduces the formation of colon tumors in an animal model.
Selenium is an important dietary mineral found in broccoli extract, red wine, dietary fiber, pepper, soya, cloves, fenugreek, ginger, apple and other vegetables. Selenium is associated with up to a 50% decrease in the risk for colon cancer12, 13. Yellow mustard oil is synthesized by the brassica family of plants and has been examined for its potential anticancer properties. Mustard contains a complex mixture of long-chain polysaccharides that may play a protective role in colon cancer formation14. Essential oils such as eicosapentanoic acid (EPA), docosahexaenoic acid (DHA) and omega-3 fatty acids are also used to treat and prevent cancer and cardiac diseases. Particularly, the consumption of fish and fish products reduces the risk of colon cancer progression15. Recently developed live micro-organisms such as probiotics are also important dietary supplement for humans. Probiotics balance the mix of intestinal microbes and have beneficial effects on the human digestive system. Environmental factors and dietary habits can alter the colonic bacteria and lactobacilli concentration, and may lead to the formation of polyps and malignant tumors. Hence intake of probiotics may inhibit preliminary cancer cell growth16. The clinically used probiotics may be effective in preventing colon cancer progression in an N-nitroso-N-methylurethene-induced murine colon adenocarcinoma model in mice. Cancer cells from the spleen of treated mice were isolated and purified by flow cytometry for mRNA analysis. The diseased mice were continuously fed probiotics to monitor recovery from epithelial or crypt damage17.
However, the therapeutic benefits of nutraceuticals have not yet been clearly demonstrated. Nonetheless, preliminary characterization and epidemiological studies show that different nutritional packages can control and prevent colon dysfunction. Dietary supplements enhance colon activity and reduce the incidence of colon cancer. These nutraceuticals could be naturally occurring in various plants, bacteria, fungi, algae and marine resources. Hence, this review focuses on important nutraceutical compounds derived from the plant and marine sources as used for treating and controlling colon cancer behavior.
2. Colon cancer and bioenergetics pathways
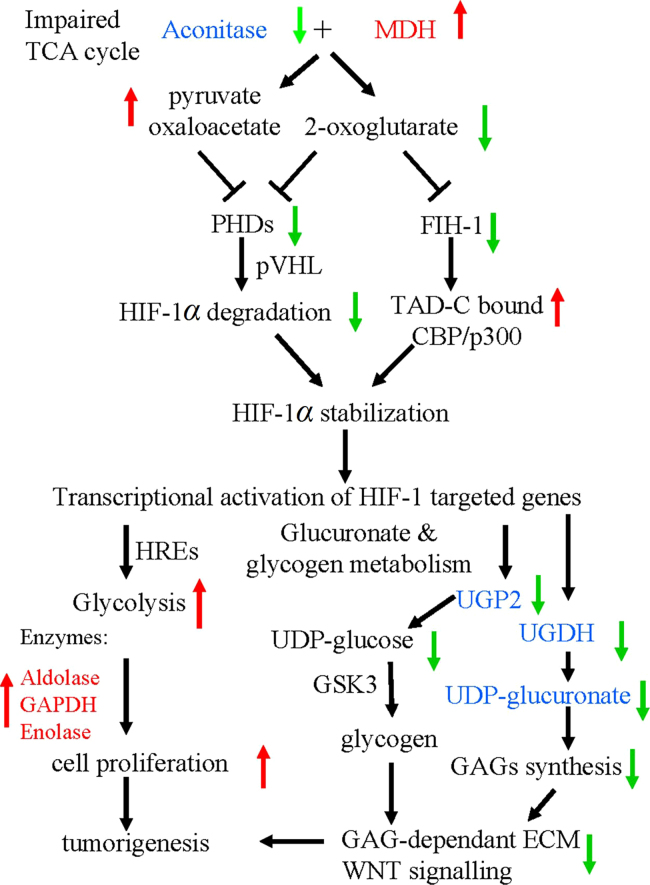
So far, many studies have shown that nutraceutical metabolites can regulate tumor metabolism and growth. The biochemical observations on colon cancer are expressed in abnormal levels of common enzymes and radical changes in the glycolysis pathway. Lactate dehydrogenase (LDH) is a cluster of enzymes involved in energy metabolism. LDH is one of the marker enzymes for the detection of colorectal cancer; it has been shown that increases in the level are associated with the initial stages of colon cancer18. Fig. 3 shows the biochemical metabolic changes in colon cancer cells.
Figure 3.
Biochemical metabolic response of colon cancer cells. The proposed mechanism of colorectal tumorigenesis: MDH, malate dehydrogenase; PHD, prolyl hydroxylase; HRE, hypoxia-responsive element; FIH-1, factor inhibiting HIF-1; TAD-C, C-terminal transcriptional activation domain; UGP2, UDP-glucose pyrophosphorylase 2; GSK3, glycogen synthetase kinase 3; ECM, extracellular matrix; pVHL, von Hippel–Lindau protein; TCA, tricarboxylic acid. Red upward pointing arrows and green downward pointing arrows denote the up-regulation and down-regulation of enzymes of metabolic pathways, respectively. All altered proteins found in the present study are highlighted as follows: up-regulated proteins are in red, and down-regulated proteins are in blue19.
2.1. Alteration in carbohydrate metabolism
Glycolysis, TCA and HMG-CoA metabolic pathways are often altered in cancer cell metabolism. The mevalonate and ketogenesis pathways are increased in tumor cells. Koukourakis et al.20 showed that HMG-CoA reductase expression is very high in human colon cancer. The higher activity of HMG-CoA reductase and high levels of mevalonate-derived metabolites such as isoprenoid compounds are clear evidence of cancer formation. The study showed that important biomolecules are differentially regulated between cancer and normal cells, with significant differences in metabolites of colon cancer tissues. The intermediates of the tricarboxylic acid cycle and lipids were decreased in cancer cells; however, metabolites in the urea cycle, purine, pyrimidine and amino acid synthesis were generally at above normal levels compared to normal colon mucosa21.
2.2. Alterations in fat metabolism
Fatty acid metabolism is a hallmark for finding various chronic diseases including cancer. The altered fatty acid metabolism indicates decreased fat synthesis and increases lipolysis from liver and adipose tissue storage lipids. In cancer the fatty acid oxidation rate observed in colon and gastric cancer is very high. The colon cancer sample was monitored by indirect calorimetry and urinary nitrogen excretion showed significantly higher fat oxidation rates and higher carbohydrate oxidation rates22. The de novo fatty acid synthesis pathway of cancer cells showed abnormal levels of several lipogenic enzymes. The consequent decrease in lipogenesis is advantageous to a further cascade of biosynthetic anabolic activities and cell growth23. In cancer cells, the enzymes ATP-citrate lyase (ACL), acetyl CoA carboxylase (ACC), and fatty acid synthase (FAS) are habitually over expressed relative to normal cells. Particularly, the FAS is used as a cancer biomarker for diagnostic purposes. Fas inhibitors are able to suppress carcinogenesis in in vivo cancer mouse model. Inhibition activates apoptosis in colon and breast cancer cell lines without affecting normal lipogenic tissues24.
3. Nutraceuticals effective for colon cancer therapeutics
Nutraceuticals are thought to enhance human health and prevent chronic diseases. Fiber content in vegetables and fruits may reduce the risk of colon cancer formation. These food supplements have been proposed as chemo-preventive agents for colon cancer treatment. Also, plant-derived polysaccharides act as protective role in the development of colon lesions25.
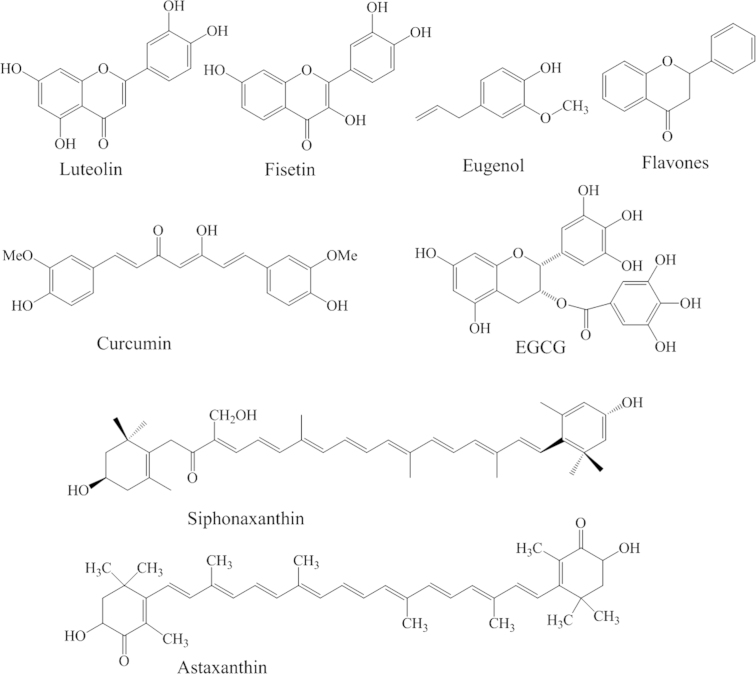
Pro-oxidants are associated with a high risk of cancer. Free radicals can induce posttranscriptional modification in cancer and over express their related proteins. Antioxidant food supplements are an important component for treating and controlling cancer development. Fig. 4 shows clinically important secondary metabolites and their analogous structures. Adequate consumption of dietary nutraceuticals is a reasonable way to maintain health and avert the formation of colon cancers. The most common reasons for the consumption of dietary supplements are to control the symptoms of cancer and to prevent future disease. Dietary supplements such as microalgae, plant derivatives and vegetables are a rich source of vitamins, minerals, amino acids and other micronutrients. These natural sources have a major role in health and disease. Moreover, colon cancer is widely reported as being reduced by the use of various nutraceuticals such as folate, calcium, tomato-soy diet, fiber and vitamins26. Nevertheless, the recommended dosage of nutraceuticals is very important and clearly related to benefit the patients. However, it is necessary to avoid hypervitaminosis, formation of calculi and other obesity-related problems27.
Figure 4.
Structures of clinically important secondary metabolite used in colon cancer treatment.
Recently, nutraceuticals encapsulated by nanoparticles have been developed in nanomedicine. The hybrid systems made of anticancer drugs such as cisplatin, apoferritin, methotrexate and 5-flurouracil have been developed with a drug delivery carrier like layered double hydroxide, protein (LDH) and platinum nanoparticles. The nutraceutical-containing nanoparticles rapidly interact with cancer cells and provide promising anticancer chemotherapy agents with biocompatibility. Some biological agents/nutraceuticals such as antibodies, proteins, polysaccharides aptamers, and peptides have been able to direct nanoparticle targeting to tumor cells. Similarly, targeting nutraceuticals to tumors can enhance their effectiveness: nanoparticles encapsulating curcumin are more effective than the free curcumin, eventually inhibiting NF-κB regulated transcription and angiogenesis28.
3.1. Carotenoids for colon cancer
Carotenoids are a major class of secondary metabolites with many biological activities such as free radical scavenging properties, skin tone improvement and potential for cancer treatment. Generally carotenoids are classified into two main subclasses such as hydrocarbon carotenoids including β-carotene, α-carotene, lycopene and oxycarotenoids which include lutein and zeaxanthin, as well as other compounds. Carotenoids have many applications in the clinical and commercial fields. β-Carotene has been shown to be efficient in controlling cellular damage from free radicals. Secondary metabolites can influence and effectively react with free radicals in the inner part of the cell membrane. The natural compounds have been more effective in maintaining membrane integrity and antimutagenic properties29. The unsaturated nature of lycopene has potential efficiency to provide free radical scavenging activity and inhibit cancer progression. Lycopene is present in various dietary sources such as tomatoes, grapes and papaya. Carotenoids are used for the prevention of colon and gastrointestinal cancer30. Tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) is a protein that induces apoptosis in cancer cells. TRAIL has been targeted in microtubule formation in the cell cycle. Halocynthiaxanthin is a dietary carotenoid and combined with TRAIL can significantly induce apoptosis in DLD-1 colon cancer cells. The combined drug treatment involves important anticancer effects such as poly(ADP-ribose) polymerase cleavage, induction of caspase inhibitors and nuclear condensation. These mechanisms of halocynthiaxanthin have shown the potential to regulate programmed cell death in colon cancer cells. Other phytochemicals such as xanthophyll, astaxanthin, cryoptoxanthin and zeaxanthin metabolites have been used for the treatment of colon cancer31.
3.2. Flavonoids for colon cancer
Luteolin is found in numerous edible plants and vegetables such as green pepper, celery and perilla. Naturally isolated luteolin secondary metabolites are clinically proven to be effective colon cancer agents. In vivo studies showed that luteolin can decrease tumor incidence and multiplicity in dimethyl benzanthracene-induced papillomas. Also, many in vitro studies have reported that luteolin stimulates apoptosis in several cell lines31, 32. Demidenko et al.33 demonstrated that luteolin induced HT-29 cell cycle block, preventing cancer cell growth at the G1/S and G2/M phases. Furthermore, luteolin suppresses the over expression of some antiapoptotic proteins in affected cells and regulates the expression and activity of CDC2 (CDK1) kinase and cyclin B1 proteins, which initiate the G2/M transition phases observed in luteolin-treated colon cancer cell lines. Use of moderate concentrations of luteolin significantly increased cancer cell apoptosis.
Fisetin is a flavonoid natural substance, occurring in many fruits and vegetables including cucumbers. We observed fasting-induced apoptosis of poly(ADP-ribose) cleavage, which is considered a biomarker of apoptosis. Similarly, caspase-3 was activated in fisetin-treated colon cancer cells. Fisetin can modulate the expression of Bcl-2 metabolites to induce the mitochondrial apoptotic pathway in cancer cells and increases the proapoptotic Bak protein in colon cancer cells34.
3.3. Polyphenolic clusters for colon cancer therapeutics
Polyphenols are natural phytochemicals used to treat various viral and fungal diseases. They are derived from various sources such as plants, seaweeds, marine algae and microorganisms. Polyphenols includes different organic constituents such as flavones, flavanols, isoflavones, catechins, epigallocatechin-3 gallate (EGCG) and epicatechins. Numerous plants are reported to have polyphenols in their extracts. Polyphenols isolated from tea plants are well studied for their biological properties. Consumption of green tea protects against several types of chemically induced cancer in animal models35. Green/black tea is one of the most popular and easily affordable drinks in the world. It has important active metabolites and antioxidant compounds to enhance the health benefits. Green tea contains a high amount of antioxidant polyphenols which effectively controlled cancerous growth in both in vitro and in vivo models. Green tea contains many phytochemicals such as heterocyclic amines, flavones and saponins, and can alter the xenobiotic metaboling enzymes. These metabolites induce the signal transduction pathway which leads to induction of apoptosis and cell cycle arrest. Some studies have asserted that the high consumption of black tea is also associated with reducing the risk of digestive track cancers36.
Curcumin is one of the most important secondary metabolites for anticarcinogenic properties and is already used clinically. Curcumin affects the molecular level of protein expression in colon cancers such as COX-2, VEGF, IL-1, IL-6, IGF and Chemokines. The drug targets the active site of COX-2 in malignant cells and it modulates the action of TNF-α and NF-κB factors. Likewise, curcumin is well known clinically as a chemopreventive agent used during the initiation and progression stages of digestive tract cancer37. Wang et al.38 have explained that curcumin inhibits the secretion of gastrointestinal hormones such as neurotensin. Human colorectal cancer cell line HCT 116 treated with curcumin altered the hormone neurotensin action and induced the IL-8 expression in a time- and dose-dependent manner.
4. Marine nutraceuticals and their derivatives for colon cancer
The marine environment contains an extreme diversity of micro- and macro-organisms. Marine organisms contain structurally diverse bioactive compounds including many that are included in food and health care products. Marine organisms produce novel and pharmacological compounds with fewer adverse effects. Marine-derived bioactive compounds are of current interest to cure several ailments including colon cancers. However, researchers are focusing more on secondary metabolites isolated from marine and other natural sources. Recently natural product research has reported that from 1981 to 2012, 45% of new drugs and 80% drugs approved for anticancer agents were derived from natural products39, 40.
4.1. Acetylapoaranotin
Acetylapoaranotin is a diketopiperazine disulfide that was isolated from marine Aspergillus sp. The acetylapoaranotin chemical structure contains disulfide bridges, which are responsible for the molecule׳s cytotoxicity. The natural acetylapoaranotin compound can induce apoptosis in human colon cancer cells (HCT116) as confirmed by different apoptotic assays such as annexin-V/PI staining and PARP, caspase-3, -8, -9, and Bax cleavage. This compound also significantly inhibits tumor growth in vivo. Choi and researchers41 are evaluating the molecular role of diketopiperazine disulfides in apoptosis of the HCT 116 colon cancer cell line. Acetylapoaranotin has promising activity to regulate the Bcl-2 family of proteins and the proapoptotic protein Bax. Treatment of the cancer cell line HCT 116 with different concentrations of diketopiperazine disulfides was shown to initiate the caspase 8 activation in intrinsic and extrinsic pathways. Various types of marine-derived compounds have numerous biomedical applications such as the neuroprotective properties of seaweeds, chitosan for weight management and dentistry. Also marine plants and animals have a rich source of novel compounds with promising uses in the nutraceutical, medicinal and commercial food industries42. The marine-based nutraceuticals such as glucon, sulphated polysaccharides, peptides and fatty acid immunomodulators have great application in the pharmacological and commercial industries. Seaweed-derived peptides and proteins can modulate the intestinal epithelium cell permeability and consequently enhance the intestinal absorption of macromolecules through the energetic pathways43.
4.2. Astaxanthin
Astaxanthin is one of the carotenoid classes of secondary metabolites, and is abundantly produced by Haematococcus pluvialis, crab and marine animals. The highest content of astaxanthin was found in H. pluvialis, a single-celled green algae. It׳s chemical name is 3,3′-dihydroxy-β,β-carotene-4,4′-dione. Astaxanthin (ASTX) has diverse biological applications to control colon ulcers, inflammation, cancer, and neurological disorders. Astaxanthin is also used as a food supplement to enhance the optimal health for humans44. Treatment of a colon cancer mouse model with astaxanthin was shown to generate normal expression of NF-κB, MMP9, IL-6, TNFα, COX-2, and inhibit proliferation and induce apoptosis. Also, dietary ASTX significantly suppressed the formation of colonic mucosal ulcers and dysplastic crypts in an animal model. NF-κB is a transcription factor which participates in a wide range of cellular roles and cancer pathways. NF-κB factors are critically involved in various signaling mechanisms, particularly which regulate posttranscriptional function. Astaxanthin has specific functional groups to regulate the NF-κB proteins45. Prabhu et al.46 reported that 1,2-dimethylhydrazine (DMH)-induced colon carcinogenesis was markedly reduced by astaxanthin. It has a good chemopreventive effect on lipid peroxidation, antioxidant status, the total number of aberrant crypt foci (ACF), and cell proliferation, and eventually reduced the histological lesions in a rat model.
4.3. Siphonaxanthin
Siphonaxanthin is isolated from the marine green alga Codium fragile and remarkably suppresses cell viability, and induces apoptosis in human leukemia and colon cells47. Siphonaxanthin has significant anti-angiogenic activity by suppressing endothelial cell proliferation and HUVEC tube formation. Although siphonaxanthin had a strong inhibitory effect on micro-vessel formation in an angiogenic model, it might not act through signal transduction by VEGF receptor-2. Ganesan et al.48 reported that siphonaxanthin induces apoptosis in HL-60 cells through caspase-3 activation, which has been associated with the enhancement of GADD45α and DR5 expression levels as well as the suppression of Bcl-2 expression. GADD45α is an important apoptosis regulator that induces cell cycle arrest and DR5 death receptor. Table 1 shows the nutraceuticals targeting colon cancer at the molecular level.
Table 1.
Nutraceuticals effectively control the progression of colon cancer cell.
| Nutraceuticals | Molecular mechanism | Cancer cell line | Ref. |
|---|---|---|---|
| α-Tocopheral | Antiproliferation, oxidative phosphorylation | HCT-116 | 49 |
| Silbinin | Reduced cell growth | HT-29 | 49 |
| Fenugreek | Cytokines, redox reactions | HT-29 | 50 |
| Curcumin (turmeric) | Stimulation of MAPk, Plsk/Akt | HT29 | 51 |
| Iron foods | Transferrin receptor (TfR1) | Cao-2, HCT-116 | 52 |
| Graph seed | Control the signaling related epigenetics, oncogene expression | HCT-116 | 53 |
| Zerumbone | Upregulation of DR4 and DR5 MMP-9, Cdc family | HCT-116 | 54 |
| Allicin | Inhibit the over expression TNF-α family gene | HCT-116 | 55 |
| Rhizochalin | Caspase-3 and PRAP activation | HT-29 | 56 |
| Fucoxanthin | Induce apoptosis | Caco-2, HT-29 | 57 |
| Protein and peptides (milk) | Hypoproliferation of epitheliam | HCT-116 | 58 |
| Garcinol | Inhibition of tyrosine phosphorylation | HT-29 | 59 |
| Xanthohumol | Upregulation of caspase-3, -8, -9 | HT-29 | 60 |
| Boswellic acid | Decrease in cyclin D1 expression | HT-29 | 61 |
| β-Escin | Induced cell cycle arrest at the G1/S phase | HT-29 | 62 |
| Quercetin | Inhibition of cyclin D1 expression | SW 480 | 63 |
| Fisetin | Activation of NF-κB and decrease in cyclin D1 expression | HT-29 | 34 |
5. Dietary supplements for colon cancer prevention
Nutraceutical products and their active metabolites effectively suppress a wide range of colon cancer cell lines, namely HCT-116, HT-29, SW 480, SW 620, CaCo2 and LoVo. In vitro studies of various antioxidant fruits including black raspberry, strawberry, and grape seeds were proven to reduce intestinal tumors64, 65. Garlic has a diverse nutritional profile and is used to treat common and endemic diseases. Garlic contains various dietary ingredients such as organosulfur and S-allylcysteine compounds. It is a main precursor for inhibiting the growth of colon cancer in a clinical model. Rats used for experimental studies by oral administration of garlic extract exhibited decreased multiplication of cancer cells in the initiation stage, but during the late stage the extract was not effective. Also the extract has no clear scientific evidence for cancer preventive efficacy. Garlic extract also has some toxicity, increasing hemolysis and increasing the anemic condition of the patient66. Fenugreek has high content of diosgenin, and it belongs to the steroidal group of saponins. Its cancer-preventative mechanisms have not been fully studied. So far some activity of the fenugreek crude extract supported the anti-proliferation activity against leukemia, colon and breast cancer cell lines67.
5.1. Honey (eugenol)
Eugenol is a natural compound which is derived from honey and is present in some plant extracts including clove oil, cinnamon, Flos Magnolia, citrus and balm. Eugenol exhibited novel medical applications for curing various chronic diseases. It promotes apoptosis in colon cancer cells68. Eugenol is a potential natural drug against colon cancer. Eugenol stimulates cell division in sub-G1 phase inducing apoptosis in regular time-dependent manner. It acts as a transducer of an apoptosis signal to control the production of non-protein thiols and matrix metallo-proteins (MMP). Eugenol-treated colon cancer cells demonstrated increased p53 activation and proline rich acidic protein (PRAP) cleavage69.
5.2. Omega-3 fatty acids
Fatty acids are long chain hydrocarbons which may vary from 10 to 30 carbons and are a component of lipids. Fatty acids such as saturated fatty acids and unsaturated fatty acids are found in marine fishes, microalgae, seaweeds, fish oil, algae oil and eggs. Omega-3 PUFAs have been broadly studied in clinical and pathological conditions. The consumption of omega-3-PUFAs and nutraceutical foods has been correlated with human health benefits. Omega-3 fatty acids have many clinical benefits, including reducing the risk of tumor growth and metastasis. The highest level of omega-3 fatty acids can alter eicosanoid synthesis and have anti-catabolic effects. The supplementation of these essential fatty acids, eicosapentaenoic acids (EPA), docosahexaenoic acid (DHA), protects against colon and breast cancer70. Hence, it controls weight loss in cancer patients, regulates cytokine production and stabilizes the energy metabolism. Several clinical studies evaluated high fat and low carbohydrate fish oil supplement as a potential therapeutic for colon cancer. Omega-3 fatty acid contains in fish oil supplements, stimulates the immune response and enhances apoptosis in cancer cells71. Fusano et al.72 suggested that n-3 fatty acids have antitumor effects during the initiation stages of colon carcinoma. The omega-3 fatty acids reduce the proliferation of early-stage colonic cancers, which may reduce the progression colorectal polyps and may help protect high-risk individuals from colon cancer.
5.3. Vitamins for colon cancer prevention
Vitamins are playing essential role in cancer prevention and treatment. Folic acid plays a major role in DNA methylation and DNA synthesis. It conjugates with vitamins B6 and B12 in the single carbon methyl cycle. Vitamin B complex treatment was initiated to reduce the risk of colon, rectal and breast cancer73. Similarly, vitamin D receptor molecules are highly expressed in colon cancer cells, and may control the abnormal metastasis and regulate the cell death mechanism in colon cells74. Table 2 illustrates the different dietary nutraceuticals tested in colon cancer preventive medicine.
Table 2.
Different dietary nutraceuticals using for anticolon cancer and preventive medicine
| Secondary metabolites | Sources | Cancer cell lines | Applications | Ref. |
|---|---|---|---|---|
| Oleuropein | Olive tree leaves | Colon cancer cell line | Protect DNA damage | 75 |
| Vitamin D (25-hydroxy vitamin D) | Milk and milk based products | Colon cancer cell line | Reducing the colon cancer development | 76 |
| Procyanidins | Apple fruit | SW 480, SW 620 | Differentiation of apoptosis function | 77 |
| Scallian | Allium fistulosum | CT-26 | Effect of inhibit colorectal tumor growth | 78 |
| Centella asiatica crude extract | Whole plant | Caco-2 colon cancer | Regulate the cell cycle | 79 |
| Salograriolide A | Centaurea ainetensis | HCT-116 | Reduce the colon tumor formation | 80 |
| Avenanthramides | Oats | HCT-116 | Attenuates of colon cancer cells | 81 |
| Brachylaena ramiflora crude extract | Whole plant | HCT-116 | Control the p53 tumor gene | 82 |
| Crude Lentinus edodes extract | Mushroom fruit bodies | HCT-116 | Regulate the apoptosis of cancer cells | 83 |
| Resveratrol | Grapes, wines | HCT-116 | Stimulate p53 gene for apoptosis | 84 |
| Conjugated linolinic acid | Probiotic bacteria | Colorectal cancer | Enhance apoptosis of tumor cells | 17 |
| Crude phenolics extract | Cichorium endlvia. L. | HCT-116 | Induction of IL-3 expression | 35 |
| Garlic crude extract | Allium sativum | HT-29 | Inducing apoptotic cell death | 66 |
6. Future prospective
The finger prints of phytochemical compounds, especially nutraceuticals, are well established for the treatment of colon cancer. Dietary phytochemicals are widely used as pharmaceuticals beneficial for human health and other commercial products. However, the mechanisms of action of nutritional databases need further development and better molecular identification in colon cancer treatment. Also the in vivo data for many potent dietary phytochemicals activities is not yet analyzed. Nevertheless, the scientific community will be focused on naturally occurring nutraceuticals and their application in colon cancer therapeutics in detail. The natural diet contains diverse secondary metabolites such as flavonoids, steroids, sulphur-containing compounds, alkaloids, saponins, phenolic acids, vitamins, minerals and other antioxidant enzymes. These nutraceutical compounds may increase the protection from different factors such as red meat, high alcohol consumption and other drugs. Research has proven that the high intake of nutraceuticals is beneficial in the control of colon cancer generation. Finally, it should be recognized that single or clustered dietary nutraceuticals molecules are contributing to therapeutic action and will be important for future assessment.
Acknowledgment
This work has been supported by the Universiti Malaysia Pahang through the internal research Grant Nos. RDU 120302 and GRS 130336. The first author PK sincerely acknowledges the Institute of Postgraduate Studies for financial support in the form of DSS.
Footnotes
Peer review under responsibility of Institute of Materia Medica, Chinese Academy of Medical Sciences and Chinese Pharmaceutical Association.
References
- 1.Donaldson M.S. Nutrition and cancer: a review of the evidence for an anti-cancer diet. Nutr J. 2000;3:19–21. doi: 10.1186/1475-2891-3-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Margovich M.J., Morris J., Brown V., Ellis J., Logothetis B., Weber R. Nutraceutical use in late-stage cancer. Cancer Metastasis Rev. 2010;29:503–510. doi: 10.1007/s10555-010-9240-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Davenport D.J., Roudebush P. The use of nutraceuticals in cancer therapy. NAVC Proc Small Animal-Oncol. 2006;20:7–11. doi: 10.1016/j.cvsm.2003.09.001. [DOI] [PubMed] [Google Scholar]
- 4.Ricciardiello L., Bazzoli F., Fogliano V. Phytochemicals and colorectal cancer prevention – myth or reality? Nat Rev Gastroenterol Hepatol. 2011;8:592–596. doi: 10.1038/nrgastro.2011.149. [DOI] [PubMed] [Google Scholar]
- 5.Slattery M.L., Curtin K.P., Edwards S.L., Schaffer D.M. Plant foods, fiber, and rectal cancer. Am J Clin Nutr. 2004;79:274–281. doi: 10.1093/ajcn/79.2.274. [DOI] [PubMed] [Google Scholar]
- 6.Gerson M. The cure of advanced cancer by diet therapy: a summary of 30 years of clinical experimentation. Physiol Chem Phys. 1978;10:449–464. [PubMed] [Google Scholar]
- 7.Holt P.R. Dairy foods and prevention of colon cancer: human studies. J Am Coll Nutr. 1999;18:379 S–391SS. doi: 10.1080/07315724.1999.10718902. [DOI] [PubMed] [Google Scholar]
- 8.Prakash D., Gupta C., Sharma G. Importance of phytochemicals in nutraceuticals. J Chin Med Res Develop. 2012;1:70–78. [Google Scholar]
- 9.Pandey N., Meena R.P., Rai S.K., Pandey-Rai S. Medicinal plants derived nutraceuticals: a re-emerging health aid. Intl J Pharma Bios. 2011;2:419–423. [Google Scholar]
- 10.Center M.M., Jemal A., Ward E. International trends in colorectal cancer incidence rates. Cancer Epidemiol Biomarkers Prev. 2009;18:1688–1694. doi: 10.1158/1055-9965.EPI-09-0090. [DOI] [PubMed] [Google Scholar]
- 11.Potter J.D. Colorectal cancer: molecules and populations. J Natl Cancer Inst. 1999;91:916–932. doi: 10.1093/jnci/91.11.916. [DOI] [PubMed] [Google Scholar]
- 12.Finley J.W., Davis C.D., Feng Y. Selenium from high selenium broccoli protects rats from colon cancer. J Nutr. 2000;130:2384–2389. doi: 10.1093/jn/130.9.2384. [DOI] [PubMed] [Google Scholar]
- 13.Rajamanickam S., Agarwal R. Natural products and colon cancer: current status and future prospects. Drug Dev Res. 2008;69:460–471. doi: 10.1002/ddr.20276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nobili S., Lippi D., Witort E., Donnini M., Bausi L., Mini E. Natural compounds for cancer treatment and prevention. Pharmacol Res. 2009;59:365–378. doi: 10.1016/j.phrs.2009.01.017. [DOI] [PubMed] [Google Scholar]
- 15.Pandey M., Verma R.K., Saraf S.A. Nutraceuticals: new era of medicine and health. Asian J Pharma Clin Res. 2010;3:11–15. [Google Scholar]
- 16.Uccello M., Malaguarnera G., Basile F., D׳agata V., Malaguarnera M., Bertino G. Potential role of probiotics on colorectal cancer prevention. BMC Surg. 2012;12:S1–35. doi: 10.1186/1471-2482-12-S1-S35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shmuely H., Domniz N., Cohen D. Probiotics in the prevention of colorectal cancer. Curr Colorectal Cancer Rep. 2013;9:31–36. [Google Scholar]
- 18.Caruso M.G., Notarnicola M. Biochemical changes of mevalonate pathway in human colorectal cancer. Anticancer Res. 2005;25:3393–3397. [PubMed] [Google Scholar]
- 19.Bi X.Z., Lin Q.S., Foo T.W., Joshi S., You T., Shen H.M. Proteomic analysis of colorectal cancer reveals alterations in metabolic pathways: mechanism of tumorigenesis. Mol Cell Proteom. 2006;5:1119–1130. doi: 10.1074/mcp.M500432-MCP200. [DOI] [PubMed] [Google Scholar]
- 20.Koukourakis M.I., Giatromanolaki A., Harris A.L., Sivridis E. Comparison of metabolic pathways between cancer cells and stromal cells in colorectal carcinomas: a metabolic survival role for tumor-associated stroma. Cancer Res. 2006;66:632–637. doi: 10.1158/0008-5472.CAN-05-3260. [DOI] [PubMed] [Google Scholar]
- 21.Zhang R.F., Humphreys I., Sahu R.P., Shi Y., Srivastava S.K. In vitro and in vivo induction of apoptosis by capsaicin in pancreatic cancer cells is mediated through ROS generation and mitochondrial death pathway. Apoptosis. 2008;13:1465–1478. doi: 10.1007/s10495-008-0278-6. [DOI] [PubMed] [Google Scholar]
- 22.Cerella C., Radogna F., Dicato M., Diederich M. Natural compounds as regulators of the cancer cell metabolism. Int J Cell Biol. 2013 doi: 10.1155/2013/639401. 639401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Menendez J.A., Decker J.P., Lupu R. In support of fatty acid synthase (FAS) as a metabolic oncogene: extracellular acidosis acts in an epigenetic fashion activating FAS gene expression in cancer cells. J Cell Biochem. 2005;94:1–4. doi: 10.1002/jcb.20310. [DOI] [PubMed] [Google Scholar]
- 24.Mills G.B., Moolenaar W.H. The emerging role of lysophosphatidic acid in cancer. Nat Rev Cancer. 2003;3:582–591. doi: 10.1038/nrc1143. [DOI] [PubMed] [Google Scholar]
- 25.Espín J.C., García-Conesa M.T., Tomás-Barberán F.A. Nutraceuticals: facts and fiction. Phytochemistry. 2007;68:2986–3008. doi: 10.1016/j.phytochem.2007.09.014. [DOI] [PubMed] [Google Scholar]
- 26.Hubner R.A., Houlston R.S. Folate and colorectal cancer prevention. Br J Cancer. 2009;100:233–239. doi: 10.1038/sj.bjc.6604823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.González-Sarrías A., Larrosa M., García-Conesa M.T., Tomás-Barberán F.A., Espín J.C. Nutraceuticals for older people: facts, fictions and gaps in knowledge. Maturitas. 2013;75:313–334. doi: 10.1016/j.maturitas.2013.05.006. [DOI] [PubMed] [Google Scholar]
- 28.Nair H.B., Sung B., Yadav V.R., Kannappan R., Chaturvedi M.M., Aggarwal B.B. Delivery of anti-inflammatory nutraceuticals by nanoparticles for the prevention and treatment of cancer. Biochem Pharmacol. 2010;80:1833–1843. doi: 10.1016/j.bcp.2010.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Slattery M.L., Benson J., Curtin K., Ma K.N., Schaeffer D., Potter J.D. Carotenoids and colon cancer. Am J Clin Nutr. 2000;71:575–582. doi: 10.1093/ajcn/71.2.575. [DOI] [PubMed] [Google Scholar]
- 30.Miller E.C., Hadley C.W., Schwartz S.J., Erdman J.W., Boileau T.W.M., Clinton S.K. Lycopene, tomato products, and prostate cancer prevention. Have we established causality? Pure Appl Chem. 2002;74:1435–1441. [Google Scholar]
- 31.Yoshida T., Maoka T., Das S.K., Kanazawa K., Horinaka M., Wakada M. Halocynthiaxanthin and peridinin sensitize colon cancer cell lines to tumor necrosis factor-related apoptosis-inducing ligand. Mol Cancer Res. 2007;5:615–625. doi: 10.1158/1541-7786.MCR-06-0045. [DOI] [PubMed] [Google Scholar]
- 32.Lim D.Y., Jeong Y., Tyner A.L., Park J.H.Y. Induction of cell cycle arrest and apoptosis in HT-29 human colon cancer cells by the dietary compound luteolin. Am J Physiol Gastrointest Liver Physiol. 2007;292:G66–G75. doi: 10.1152/ajpgi.00248.2006. [DOI] [PubMed] [Google Scholar]
- 33.Demidenko Z.N., Blagosklonny M.V. Flavopiridol induces p53 via initial inhibition of Mdm2 and p21 and, independently of p53, sensitizes apoptosis-reluctant cells to tumor necrosis factor. Cancer Res. 2004;64:3653–3660. doi: 10.1158/0008-5472.CAN-04-0204. [DOI] [PubMed] [Google Scholar]
- 34.Suh Y., Afaq F., Johnson J.J., Mukhtar H. A plant flavonoid fisetin induces apoptosis in colon cancer cells by inhibition of COX-2 and Wnt/EGFR/NF-κB-signalling pathways. Carcinogenesis. 2009;30:300–307. doi: 10.1093/carcin/bgn269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Alshehri A. Molecular and biochemical evaluation of anti-proliferative effect of (Cichorium endivia L.) phenolic extracts on cancer cell line HCT-116. Academic J Cancer Res. 2012;5:53–60. [Google Scholar]
- 36.Yang G., Zheng W., Xiang Y.B., Gao J., Li H.L., Zhang X.L. Green tea consumption and colorectal cancer risk: a report from the Shanghai Men׳s Health Study. Carcinogenesis. 2011;32:1684–1688. doi: 10.1093/carcin/bgr186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Huang M.T., Lou Y.R., Ma W., Newmark H.L., Reuhl K.R., Conney A.H. Inhibitory effects of dietary curcumin on forestomach, duodenal and colon carcinogenesis in mice. Cancer Res. 1994;54:5841–5847. [PubMed] [Google Scholar]
- 38.Wang X.F., Wang Q.D., Ives K.L., Evers B.M. Curcumin inhibits neurotensin-mediated interleukin-8 production and migration of HCT116 human colon cancer cells. Clin Cancer Res. 2006;12:5346–5355. doi: 10.1158/1078-0432.CCR-06-0968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sawadogo W.R., Schumacher M., Teiten M.H., Cerella C., Dicato M., Diederich M. A survey of marine natural compounds and their derivatives with anti-cancer activity reported in 2011. Molecules. 2013;18:3641–3673. doi: 10.3390/molecules18043641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gupta S.C., Kim J.H., Prasad S., Aggarwal B.B. Regulation of survival, proliferation, invasion, angiogenesis, and metastasis of tumor cells through modulation of inflammatory pathways by nutraceuticals. Cancer Metastasis Rev. 2010;29:405–434. doi: 10.1007/s10555-010-9235-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Choi E.J., Park J.S., Kim Y.J., Jung J.H., Lee J.K., Kwon H.C. Apoptosis inducing effect of diketopiperazine disulfides produced by Aspergillus sp. KMD 901 isolated from marine sediment on HCT116 colon cancer cell lines. J Appl Microbiol. 2011;110:304–313. doi: 10.1111/j.1365-2672.2010.04885.x. [DOI] [PubMed] [Google Scholar]
- 42.Kim SK. Marine nutraceuticals: prospects and perspectives. Basel:CRC Press; 2013, p. 327–64
- 43.Barrow C, Shahidi F. Marine nutraceuticals and functional foods Basel: CRC Press; 2007, p. 237–89.
- 44.Yang Y., Kim B., Lee J.Y. Astaxanthin structure, metabolism, and health benefits. J Hum Nutr Food Sci. 2013;1:1003–1014. [Google Scholar]
- 45.Nagendraprabhu P., Sudhandiran G. Astaxanthin inhibits tumor invasion by decreasing extracellular matrix production and induces apoptosis in experimental rat colon carcinogenesis by modulating the expressions of ERK-2, NF-κB and COX-2. Invest New Drugs. 2011;29:207–224. doi: 10.1007/s10637-009-9342-5. [DOI] [PubMed] [Google Scholar]
- 46.Prabhu P.N., Ashokkumar P., Sudhandiran G. Antioxidative and antiproliferative effects of astaxanthin during the initiation stages of 1,2-dimethyl hydrazine-induced experimental colon carcinogenesis. Fundam Clin Pharmacol. 2009;23:225–234. doi: 10.1111/j.1472-8206.2009.00669.x. [DOI] [PubMed] [Google Scholar]
- 47.Ganesan P., Noda K., Manabe Y., Ohkubo T., Tanaka Y., Maoka T. Siphonaxanthin, a marine carotenoid from green algae, effectively induces apoptosis in human leukemia (HL-60) cells. Biochim Biophys Acta. 2011;1810:497–503. doi: 10.1016/j.bbagen.2011.02.008. [DOI] [PubMed] [Google Scholar]
- 48.Ganesan P., Matsubara K., Ohkubo T., Tanaka Y., Noda K., Sugawara T. Anti-angiogenic effect of siphonaxanthin from green alga, Codium fragile. Phytomedicine. 2010;17:1140–1144. doi: 10.1016/j.phymed.2010.05.005. [DOI] [PubMed] [Google Scholar]
- 49.Sharma R. Nutraceuticals and nutraceutical supplementation criteria in cancer: a literature survey. Open Nutraceuticals J. 2009;2:92–106. [Google Scholar]
- 50.Post-White J., Ladas E.J., Kelly K.M. Advances in the use of milk thistle (Silybum marianum) Integr Cancer Ther. 2007;6:104–109. doi: 10.1177/1534735407301632. [DOI] [PubMed] [Google Scholar]
- 51.Chen J.Z. Prevention of obesity-associated colon cancer by (−)-epigallocatechin-3 gallate and curcumin. Transl Gastrointest Cancer. 2012;1:243–249. [Google Scholar]
- 52.Sorokin L.M., Morgan E.H., Yeoh G.C. Transformation-induced changes in transferrin and iron metabolism in myogenic cells. Cancer Res. 1989;49:1941–1947. [PubMed] [Google Scholar]
- 53.Kaur M., Tyagi A., Singh R.P., Sclafani R.A., Agarwal R., Agarwal C. Grape seed extract upregulates p21 (Cip1) through redox-mediated activation of ERK1/2 and posttranscriptional regulation leading to cell cycle arrest in colon carcinoma HT29 cells. Mol Carcinog. 2011;50:553–562. doi: 10.1002/mc.20739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yodkeeree S., Sung B., Limtrakul P., Aggarwal B.B. Zerumbone enhances TRAIL-induced apoptosis through the induction of death receptors in human colon cancer cells: evidence for an essential role of reactive oxygen species. Cancer Res. 2009;69:6581–6589. doi: 10.1158/0008-5472.CAN-09-1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mo S.J., Son E.W., Rhee D.K., Pyo S. Modulation of TNF-α-induced ICAM-1 expression, NO and H2O2 production by alginate, allicin and ascorbic acid in human endothelial cells. Arch Pharm Res. 2003;26:244–251. doi: 10.1007/BF02976837. [DOI] [PubMed] [Google Scholar]
- 56.Khanal P., Kang B.S., Yun H.J., Cho H.G., Makarieva T.N., Choi H.S. Aglycon of rhizochalin from the Rhizochalina incrustata induces apoptosis via activation of AMP-activated protein kinase in HT-29 colon cancer cells. Biol Pharm Bull. 2011;34:1553–1558. doi: 10.1248/bpb.34.1553. [DOI] [PubMed] [Google Scholar]
- 57.Hosokawa M., Kudo M., Maeda H., Kohno H., Tanaka T., Miyashita K. Fucoxanthin induces apoptosis and enhances the antiproliferative effect of the PPARgamma ligand, troglitazone, on colon cancer cells. Biochim Biophys Acta. 2004;1675:113–119. doi: 10.1016/j.bbagen.2004.08.012. [DOI] [PubMed] [Google Scholar]
- 58.Parodi P.W. A role for milk proteins and their peptides in cancer prevention. Curr Pharm Des. 2013;13:813–828. doi: 10.2174/138161207780363059. [DOI] [PubMed] [Google Scholar]
- 59.Liao C.H., Sang S.M., Ho C.T., Lin J.K. Garcinol modulates tyrosine phosphorylation of FAK and subsequently induces apoptosis through down-regulation of Src, ERK, and Akt survival signaling in human colon cancer cells. J Cell Biochem. 2005;96:155–169. doi: 10.1002/jcb.20540. [DOI] [PubMed] [Google Scholar]
- 60.Pan L., Becker H., Gerhäuser C. Xanthohumol induces apoptosis in cultured 40–16 human colon cancer cells by activation of the death receptor- and mitochondrial pathway. Mol Nutr Food Res. 2005;49:837–843. doi: 10.1002/mnfr.200500065. [DOI] [PubMed] [Google Scholar]
- 61.Liu J.J., Huang B.H., Hooi S.C. Acetyl-keto-β-boswellic acid inhibits cellular proliferation through a p21-dependent pathway in colon cancer cells. Br J Pharmacol. 2006;148:1099–1107. doi: 10.1038/sj.bjp.0706817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Patlolla J.M.R., Raju J., Swamy M.V., Rao C.V. β-Escin inhibits colonic aberrant crypt foci formation in rats and regulates the cell cycle growth by inducing p21waf1/cip1 in colon cancer cells. Mol Cancer Ther. 2006;5:1459–1466. doi: 10.1158/1535-7163.MCT-05-0495. [DOI] [PubMed] [Google Scholar]
- 63.Shan B.E., Wang M.X., Li R.Q. Quercetin inhibit human SW480 colon cancer growth in association with inhibition of cyclin D1 and survivin expression through Wnt/β-catenin signaling pathway. Cancer Invest. 2009;27:604–612. doi: 10.1080/07357900802337191. [DOI] [PubMed] [Google Scholar]
- 64.Khan N., Afaq F., Syed D.N., Fisetin Mukhtar H. a novel dietary flavonoid, causes apoptosis and cell cycle arrest in human prostate cancer LNCaP cells. Carcinogenesis. 2008;29:1049–1056. doi: 10.1093/carcin/bgn078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bobe G., Barrett K.G., Mentor-Marcel R.A., Saffiotti U., Young M.R., Colburn N.H. Dietary cooked navy beans and their fractions attenuate colon carcinogenesis in azoxymethane-induced ob/ob mice. Nutr Cancer. 2008;60:373–381. doi: 10.1080/01635580701775142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Delshad A.A., Heshmati M., Ghaini M.H. Garlic extract can induce apoptotic cell death in the human colon adenocarcinoma HT29 cell line. Iran J Pathol. 2010;5:126–131. [Google Scholar]
- 67.Raju J., Bird R.P. Diosgenin a naturally occurring furostanol saponin suppresses 3-hydroxy-3-methylglutaryl CoA reductase expression and induces apoptosis in HCT-116 human colon carcinoma cells. Cancer Lett. 2007;255:194–204. doi: 10.1016/j.canlet.2007.04.011. [DOI] [PubMed] [Google Scholar]
- 68.Seeram N.P., Adams L.S., Zhang Y.J., Lee R., Sand D., Scheuller H.S. Blackberry, black raspberry, blueberry, cranberry, red raspberry, and strawberry extracts inhibit growth and stimulate apoptosis of human cancer cells in vitro. J Agric Food Chem. 2006;54:9329–9339. doi: 10.1021/jf061750g. [DOI] [PubMed] [Google Scholar]
- 69.Jaganathan S.K., Mazumdar A., Mondhe D., Mandal M. Apoptotic effect of eugenol in human colon cancer cell lines. Cell Biol Int. 2011;35:607–615. doi: 10.1042/CBI20100118. [DOI] [PubMed] [Google Scholar]
- 70.Larsson S.C., Kumlin M., Ingelman-Sundberg M., Wolk A. Dietary long-chain 3 fatty acids for the prevention of cancer: a review of potential mechanisms. Am J Clin Nutr. 2004;79:935–945. doi: 10.1093/ajcn/79.6.935. [DOI] [PubMed] [Google Scholar]
- 71.Cockbain A.J., Toogood G.J., Hull M.A. Omega-3 polyunsaturated fatty acids for the treatment and prevention of colorectal cancer. Gut. 2012;61:135–149. doi: 10.1136/gut.2010.233718. [DOI] [PubMed] [Google Scholar]
- 72.Fasano A., Uzzau S. Modulation of intestinal tight junctions by Zonula occludens toxin permits enteral administration of insulin and other macromolecules in an animal model. J Clin Invest. 1997;99:1158–1164. doi: 10.1172/JCI119271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lee K.W., Lee H.J., Surh Y.J., Lee C.Y. Vitamin C and cancer chemoprevention: reappraisal. Am J Clin Nutr. 2003;78:1074–1078. doi: 10.1093/ajcn/78.6.1074. [DOI] [PubMed] [Google Scholar]
- 74.Tangpricha V., Flanagan J.N., Whitlatch L.W., Tseng C.C., Chen T.C., Holt P.R. 25-Hydroxyvitamin D-1α-hydroxylase in normal and malignant colon tissue. Lancet. 2001;357:1673–1684. doi: 10.1016/S0140-6736(00)04831-5. [DOI] [PubMed] [Google Scholar]
- 75.Carrera-Gonzalez M.P., Ramírez-Expósito M.J., Mayas M.D., Martınez-Martos J.M. Protective role of oleuropein and its metabolite hydroxytyrosol on cancer. Trends Food Sci Technol. 2013;31:92–99. [Google Scholar]
- 76.Martínez M.E., Giovannucci E.L., Colditz G.A., Stampfer M.J., Hunter D.J., Speizer F.E. Calcium, vitamin D, and the occurrence of colorectal cancer among women. J Natl Cancer Inst. 1996;88:1375–1382. doi: 10.1093/jnci/88.19.1375. [DOI] [PubMed] [Google Scholar]
- 77.Maldonado-Celis M.E., Bousserouel S., Gossé F., Minker C., Lobstein A., Raul F. Differential induction of apoptosis by apple procyanidins in trail-sensitive human colon tumor cells and derived trail-resistant metastatic cells. J Cancer Mol. 2009;5:21–30. [Google Scholar]
- 78.Arulselvan P., Wen C.C., Lan C.W., Chen Y.H., Wei W.C., Yang N.S. Dietary administration of scallion extract effectively inhibits colorectal tumor growth: cellular and molecular mechanisms in mice. PLoS One. 2012;7:e44658. doi: 10.1371/journal.pone.0044658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bunpo P., Kataoka K., Arimochi H., Nakayama H., Kuwahara T., Ohnishi Y. Centella asiatica extract induces cell cycle arrest in Caco-2 human colon cancer cells. Chiang Mai Med Bull. 2005;44:21–28. [Google Scholar]
- 80.El-Najjar N., Dakdouki S., Darwiche N., El-Sabban M., Saliba N.A., Gali-Muhtasib H. Anti-colon cancer effects of Salograviolide A isolated from Centaurea ainetensis. Oncol Rep. 2008;19:897–904. [PubMed] [Google Scholar]
- 81.Guo W.M., Nie L., Wu D.Y., Wise M.L., Collins F.W., Meydani S.N. Avenanthramides inhibit proliferation of human colon cancer cell lines in vitro. Nutr Cancer. 2010;62:1007–1016. doi: 10.1080/01635581.2010.492090. [DOI] [PubMed] [Google Scholar]
- 82.Karimi M., Conserva F., Mahmoudi S., Bergman J., Wiman K.G., Bykov V.J.N. Extract from Asteraceae Brachylaena ramiflora induces apoptosis preferentially in mutant p53-expressing human tumor cells. Carcinogenesis. 2010;31:1045–1053. doi: 10.1093/carcin/bgq084. [DOI] [PubMed] [Google Scholar]
- 83.Ng M.L., Yap A.T. Inhibition of human colon carcinoma development by lentinan from shiitake mushrooms (Lentinus edodes) J Altern Complement Med. 2002;8:581–599. doi: 10.1089/107555302320825093. [DOI] [PubMed] [Google Scholar]
- 84.Mahyar-Roemer M., Katsen A., Mestres P., Roemer K. Resveratrol induces colon tumor cell apoptosis independently of p53 and precede by epithelial differentiation, mitochondrial proliferation and membrane potential collapse. Int J Cancer. 2001;94:615–622. doi: 10.1002/ijc.1516. [DOI] [PubMed] [Google Scholar]