Abstract
Chronic hepatitis C virus (HCV) infection has become a major public health burden worldwide. Twenty-two sophocarpinic acid or matrine derivatives were synthesized and their anti-HCV activities were evaluated in vitro. The structure-activity analysis revealed that (i) sophocarpinic acids with a D-seco 3-ring structure scaffold were more favorable than matrines with a 4-ring scaffold; (ii) the introduction of an electron-withdrawing group on the phenyl ring in 12-N-benzenesulfonyl Δβγ sophocarpinic acids was beneficial for the antiviral activity against HCV. Among them, compounds 9h and 9j exhibited the most potent inhibitory activities on HCV replication with selectivity indies of 70.3 and 30.9, respectively. Therefore, both were selected as antiviral candidates for further investigation.
KEY WORDS: Sophocarpinic acid, Matrine, Anti-HCV, Antiviral activity, Structure−activity relationship
Graphical abstract
Twenty-two sophocarpinic acid or matrine derivatives were synthesized and their anti-HCV activities were evaluated in vitro. The SAR results were elucidated, and compounds 9h and 9j were selected out as antiviral candidates for further investigations.
1. Introduction
Chronic hepatitis C virus (HCV) infection has become a major public health burden worldwide. The World Health Organization (WHO) reported that over 3% of the global population with approximately 180 million individuals is estimated to be infected with HCV, with a 3–4 million new cases appearing every year globally1, 2. Among all countries, China accommodates the largest HCV-infected population, with more than 41 million people infected, and the incidence rate is rising year by year2. In nearly 85% of the cases, the disease progresses into chronicity, and 30 percent of the cases may progress to liver cirrhosis, end-stage liver disease and hepatocellular carcinoma (HCC)3.
There is no vaccine to prevent HCV infection. The standard therapy for HCV in the clinic is the combination of pegylated-interferon with ribavirin4, 5. The regimen is only effective in approximately 40% of patients infected with HCV genotype 1, the prevalent HCV genotype in the United States, Europe, and China, and is associated with significant side effects3. The introduction of Telaprevir and Boceprevir was temporarily effective with HCV patients, but drug-resistant mutations soon appeared6, 7, 8, 9. Sofosbuvir, a new HCV NS5B RNA polymerase inhibitor approved by FDA in 2013 showed promise in dealing with drug-resistant HCV10. However, a S282T mutation in NS5B was found in genotype 1a, 1b and 2a replicons, and caused a reduced susceptibility to sofosbuvir, again raising the need for new drugs with novel modes of action11.
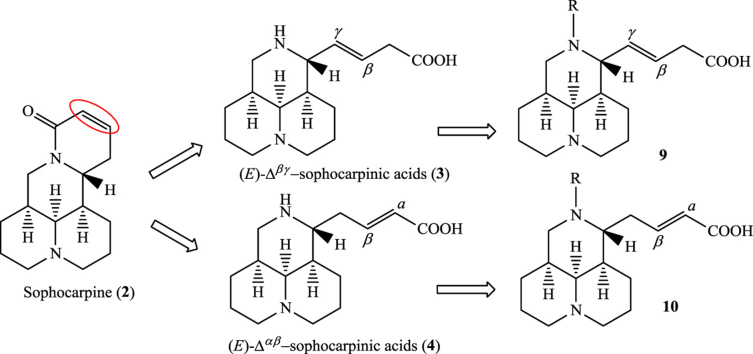
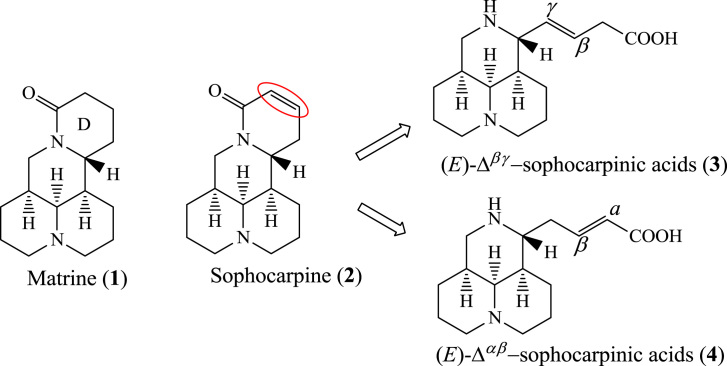
Matrine (1, Fig. 1), a quinolizidine natural product extracted from Sophora flavescens, has been used clinically for HBV treatment for decades and has a novel mechanism of action12, 13. Clinical reports showed that compound 1 was also effective for HCV patients in China14. Bearing a unique 4-ring core scaffold, compound 1 strongly provoked our interest to explore its anti-HCV structure-activity relationship (SAR) in an effort to discover a new chemical entity (NCE) against HCV with a novel mechanism. SAR studies have been carried out in our lab, revealing that the 5S configuration in the core scaffold is beneficial for anti-HCV activity15, 16. In the present study, the 5S configuration was maintained and further SAR analysis was directed toward the carbon-carbon double bond in sophocarpine (2, Fig. 1), another natural product extracted from Sophora flavescens. Based on this strategy, a series of (E)-Δβγ/Δαβ-sophocarpinic acid (3/4, Fig. 1) derivatives bearing a D-seco 3-ring scaffold were synthesized and evaluated for their anti-HCV activities.
Figure 1.
The chemical structures of marine (1), sophocarpine (2), (E)-Δβγ sophocarpinic acid (3) and (E)-Δαβ sophocarpinic acid (4).
2. Results and discussion
2.1. Chemistry
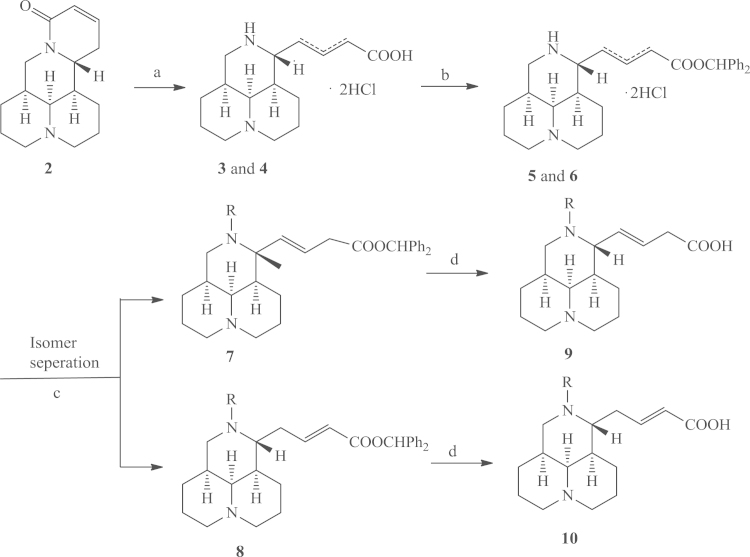
As showed in Scheme 1, compound 2, as the starting material, was hydrolyzed in strong base and formed an isomeric mixture of 3 and 4. The target compounds (E)-12-N-substituted-Δβγ/Δαβ-sophocarpinic acids (9/10) were acquired by using a three-step sequence including carboxyl protection with diphenyldiazomethane as the protective agent, 12-N-alkylation or acylation in the presence of potassium carbonate, and deprotection in m-cresol15, 16, 17, 18, 19, with overall yields of 5%–12%. The regio-isomers were separated before alkylation or acylation.
Scheme 1.
Synthetic routes for N-substituted sophocarpinic acid analogs. Reagents and conditions: (a) 10% KOH/H2O, reflux, 7 h; then 3 mol/L HCl, pH 5–6; (b) diphenyldiazomethane, MeOH/petroleum ether(boiling range 30–60 °C), overnight; (c) (1) substituted benzyl chloride/bromine or benzenesulfonyl chloride, CH2Cl2 or MeCN, K2CO3, r.t., overnight; (2) flash column chromatography; (d) m-cresol, 80 °C, 8–9 h.
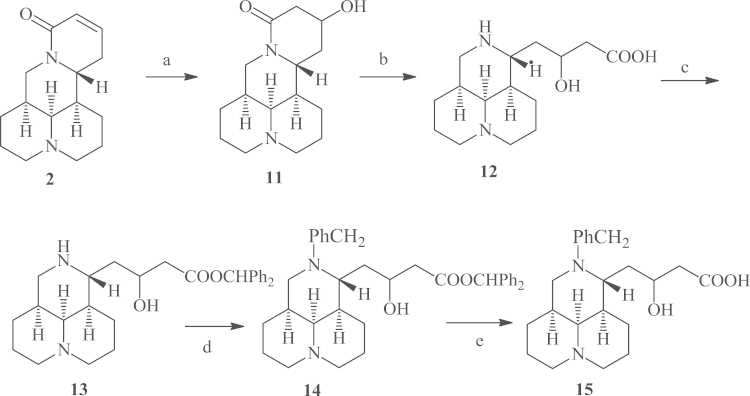
To gain sophocarpinic acid derivatives without the double bond, 2 was refluxed in aqueous base as illustrated in Scheme 2 to gain the key intermediate 11, which was hydrolyzed in strong base to form a 3-ring scaffold product 12, followed by a three-step sequence including carboxyl protection via diphenyldiazomethane, 12-N-alkylation in the presence of potassium carbonate and deprotection in m-cresol to gain the desired product 15 in an overall yield of 16.1%15, 16, 17, 18, 19. Matrine derivatives 16a-d (Table 1) as a class of anti-HBV agents were prepared as previously reported17.
Scheme 2.
Synthetic routes for 12-N-benzyl-β-hydroxylmatrinic acid (15). Reagents and conditions: (a) 5% KOH/H2O, reflux, 8 h; then 3 mol/L HCl, pH 5–6; (b) 10% KOH/H2O, reflux, 7 h; then 3 mol/L HCl, pH 5–6; (c) diphenyldiazomethane, MeOH/petroleum ether (boiling range 30–60 °C), overnight; (d) benzyl bromide, MeCN, K2CO3, r.t., overnight; (e) m-cresol, 80 °C, 8–9 h.
Table 1.
SAR for anti-HCV activity of matrine analogues.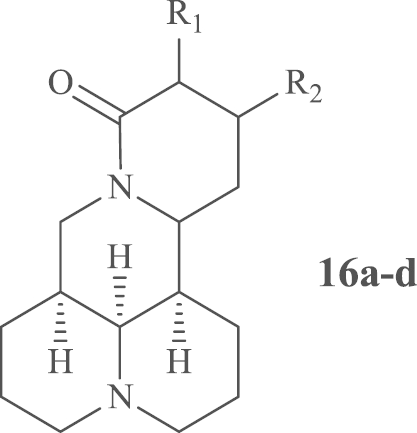
| No | R1 | R2 | TC50 (μg/mL) a | EC50 (μg/mL)b | SIc |
|---|---|---|---|---|---|
| 1 | H | H | >1000 | 98.04 | >10.2 |
| 16a | H | OCH2CH3 | >1000 | 104.62 | >9.6 |
| 16b | OCH2Ph | OCH2Ph | 49.87 | 5.60 | 8.9 |
| 16c | OH | OCOCH2Cl | 54.90 | >37.04 | <1.5 |
| 16d | H | NHCH3 | 543.70 | 31.07 | 17.5 |
| RBV | 2000 | 292.46 | 6.84 |
Cytotoxic concentration required to inhibit Vero cell growth by 50%.
Concentration required to inhibit CVB3 growth by 50%.
Selectivity index values equaled to TC50/EC50.
2.2. SAR analysis for anti-HCV activity in vitro
All the synthesized compounds were tested for their anti-HCV activity and cytotoxicity in Huh7.5 cells using specific real-time RT-PCR assay, as described in our previous publication20. Anti-HCV activity was evaluated by measuring both EC50 (for anti-HCV activity) and TC50 (for cytotoxicity) values. As a key indication, the selectivity index (SI) was calculated as a ratio of TC50 to EC50. Anti-HCV activity of a given compound was estimated by combining its EC50 value with its SI. Twenty-two sophoridinic acid or sophoridine analogs and their anti-HCV effectiveness are shown in Table 1, Table 2.
Table 2.
SAR for anti-HCV activity of sophocarpinic acid derivatives.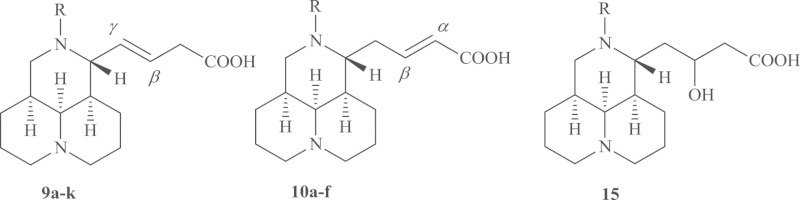
| Compd. | R | TC50 (μg/mL)a | EC50 (μg/mL)b | SIc |
|---|---|---|---|---|
| 1 | >1000 | 98.04 | >10.2 | |
| 9a | –CH2PhCH3-o | 726.98 | 155.66 | 4.7 |
| 9b | –CH2Ph | >1000 | 49.3 | 20.3 |
| 9c | –CH2PhBr- p | 357.41 | 24.36 | 14.7 |
| 9d | –SO2PhNO2-p | 380.7 | 74.0 | 5.14 |
| 9e | –SO2Ph | >1000 | 257.55 | >3.9 |
| 9f | –SO2PhCH3-p | >1000 | 112.06 | >8.9 |
| 9g | –SO2PhOCH3-p | >1000 | 165.21 | > 6.1 |
| 9h | –SO2PhCF3-p | 530.07 | 7.54 | 70.3 |
| 9i | –SO2PhCl-o | 876.08 | 134.58 | 6.5 |
| 9j | –SO2PhCN-m | 123.08 | 3.98 | 30.9 |
| 9k | –SO2Ph(NHCOCH3)-p | 65.77 | 2.80 | 23.5 |
| 10a | –CH2PhNO2- p | >1000 | 52.82 | >18.9 |
| 10b | –SO2Ph | >1000 | 59.65 | >16.8 |
| 10c | –SO2PhCH3-p | 171.29 | 25.02 | 6.8 |
| 10d | –SO2PhCl-o | 404.64 | 241.27 | 1.68 |
| 10e | –SO2Ph(NHCOCH3)-p | 552.03 | 222.06 | 2.5 |
| 10f | –SO2PhOCH3-p | 700.4 | 291.8 | 2.4 |
| 15 | –CH2Ph | >1000 | >333.3 | 3 |
| RBV | 2000 | 292.46 | 6.84 |
Cytotoxic concentration required to inhibit Vero cell growth by 50%.
Concentration required to inhibit CVB3 growth by 50%.
Selectivity Index values equaled to TC50/EC50.
The SAR study for anti-HCV activity was initially focused on the influence of the substitutions on ring D while the 4-ring scaffold was unchanged. As indicated in Table 1, matrine compounds 16a-c afforded similar or lower SI values compared to compound 1, while compound 16d bearing a methylamino showed a higher SI value, which hinted that suitable substitutions on ring D could enhance the activity against HCV.
Table 2 disclosed the SAR analysis on the D-meso 3-ring scaffold derivatives. The compounds bearing a carbon-carbon double bond on the side chain, specifically, (E)-12-N-substituted-Δβγ-sophocarpinic acids (9a–k) and their isomers (E)-12-N-substituted -Δαβ-sophocarpinic acids (10a–f), were evaluated in this study. First, the influence of the substituents on the 12-nitrogen atom in the (E)-Δβγ-sophocarpinic acids was examined. The results showed that 12-N-benzyl derivatives 9a–c showed anti-HCV activities similar to or lower than compound 1, and most of the compounds in the 12-N-benzenesulfonyl series (9d–k) had low activities against HCV, except that compound 9h and 9j showed significantly higher activity than compound 1 with SI values of 70.3 and 30.9, respectively. The results suggested that the introduction of CF3 or CN on the sulfonylphenyl ring might be beneficial for the anti-HCV activity.
In the case of the (E)-Δαβ-sophocarpinic acids derivatives 10a–f, most showed decreased or similar anti-HCV activities as compared to compound 1, regardless of the electron-donating or electron-withdrawing groups on the phenyl ring, which hinted that a Δβγ analog could do better than its Δαβ isomer in anti-HCV area. The introduction of OH at the double bond could decrease anti-HCV activity, as compared with product 15 and compound 9b.
All together, it appeared that a D-meso 3-ring structure scaffold was more favorable than a 4-ring scaffold, and the introduction of an electron-withdrawing group on the phenyl ring in 12-N-benzenesulfonyl Δβγ-sophocarpinic acid derivatives was beneficial for anti-HCV activity.
3. Conclusions
In searching for novel anti-HCV agents, 22 sophocarpinic acid or matrine derivatives were synthesized and evaluated for their anti-HCV activities in vitro with 1 as the lead. Among these derivatives, compounds 9h and 9j exhibited the most potent antiviral activities against HCV with SI values of 70.3 and 30.9, respectively. SAR revealed that (i) sophocarpinic acids with a D-seco 3-ring structure scaffold are more favorable than matrines with a 4-ring scaffold; (ii) introduction of an electron-withdrawing group on the phenyl ring in 12-N-benzenesulfonyl substituted Δβγ-sophocarpinic acid derivatives is beneficial for activity. In addition, compounds 9h and 9j showed satisfactory activity against coxsackievirus type B3 (CVB3) and CVB6 in our earlier study, plus good pharmacokinetic profiles with low toxicity in vivo19. All together, 9h and 9j are highly recommended to be further developed as broad-spectrum antiviral drug candidates.
4. Experimental
4.1. Chemistry
Melting point (mp) was obtained with an MPA 100 OptiMelt automated melting point system (Stanford Research Systems, California, USA) and uncorrected. 1H NMR spectra was performed on a Varian Inova 400 MHz spectrometer (Varian, San Francisco, CA) or 500 MHz spectrometer (AV500-III, Brvker, Swiss), with Me4Si as internal standard. ESI high-resolution mass spectra (HRMS) were recorded on an AutospecUItima-TOF mass spectrometer (Micromass UK Ltd., Manchester, UK). Flash chromatography was performed on CombiflashRf 200 (Teledyne, Nebraska, USA), particle size 0.038 mm.
Compound 2 with purity over 98.5% was purchased from the Yanchi Dushun Biological and Chemical Co., Ltd. (Shanxi, China) and the Ningxia Zijinghua Pharmacy Co., Ltd. (Ningxia, China). Target compounds 9b-c, 9e–k and 10b–f were prepared as a family of anti-CVB3 inhibitors19.
4.1.1. General procedures for 9 and 10
Compound 2 (12.3 g, 50 mmol, 1 equiv.) was added to a solution of KOH (33.6 g, 600 mmol) in H2O (300 mL). The reaction mixture was heated and refluxed for 7 h and then stirred at room temperature overnight. The reaction solution was cooled with an ice–water bath and was acidified with HCl (3 mol/L) to pH 5–6. The solvent was removed in vacuo, and the residue was recrystallized in methanol to give an isomer mixture of 3 and 4.
A mixture of diphenylmethanone hydrazone (14.7 g, 75 mmol, 1.5 equiv.) and electrolytic manganese dioxide (13.04 g, 150 mmol, 3 equiv.) in petroleum ether (boiling range 30–60 °C) was heated at reflux for 0.5 h, and the mixture was filtered. The filtrate was added to the solution of 3 and 4 in methanol, and the mixture was then stirred overnight at room temperature. A corresponding isomer mixture of 5 and 6 was obtained and was used directly in the next reaction without further purification.
Anhydrous K2CO3 (3.5 equiv.) and benzyl bromine, or sulfonyl chloride (1 equiv.) were added to the solution of 5 and 6 in dichloromethane or MeCN (50 mL), and the reaction solution was then stirred at room temperature until TLC analysis showed completion of the reaction. The reaction mixture was filtered and the filtrate was evaporated to afford a mixture of 7 and 8, which were obtained by splitting of the mixture with flash column chromatography on silica gel with ethyl acetate and cyclohexane as the eluents. Compound 7 was then dissolved in m-cresol (10 mL), and the reaction mixture was stirred at 80 °C for 8–9 h. It was then cooled, and methylisobutylketone (30 mL) was added. The resulting solution was extracted with H2O (50 mL×3), and the combined extracts were evaporated to afford the crude compound, which was purified by flash column chromatography on silica gel with dichloromethane and methanol as the eluents, to afford 9. Compound 10 was obtained from compound 8 by the same method.
(E)-12-N-(o-Methylbenzyl)-Δβγ- sophocarpinic acid (9a): white solid (0.6 g, 6.5%), mp 98–100 °C; 1H NMR (400 MHz, CD3OD): δ 7.13−7.31 (m, 4H), 5.95−6.06 (m, 1H), 5.30 (dd, 1H, J=9.2, 15.2 Hz), 4.46 (m, 1H), 3.03−3.10 (m, 3H), 2.99 (d, 2H, J=7.2 Hz), 2.82−2.93 (m, 2H), 2.42−2.61 (m, 3H), 2.31−2.33 (m, 3H), 2.19 (s, 1H), 1.87−2.03 (m, 3H), 1.41−1.79 (m, 7H); HRMS: calcd. for C23H33N2O2 (M+H)+ 369.2542, found 369.2529.
(E)-12-N-(p-Nitrobenzenesulfony)-Δβγ-sophocarpinic acid (9d): white solid (2.2 g, 9.8%), mp 176−179 °C; 1H NMR (400 MHz, CD3OD): δ 8.36 (d, 2H, J=8.8 Hz), 7.99 (d, 2H, J=8.8 Hz), 5.44−5.47 (m, 2H), 3.76 (dd, 1H, J=5.0, 11.2 Hz), 3.42−3.55 (m, 1H), 3.29−3.37 (m, 1H), 3.07−3.15 (m, 1H), 2.93 (d, 2H, J=5.0 Hz), 2.79 (d, 1H, J=11.2 Hz), 2.68−2.69 (m, 1H), 2.50−2.54 (m, 1H), 1.90−2.11 (m, 3H), 1.30−1.83 (m, 8H); HRMS: calcd. for C21H28N3O6S (M+H)+ 450.1693, found 450.1710.
(E)-12-N-(p-Nitrobenzyl)-Δαβ-sophocarpinic acid (10a): white solid (0.95 g, 9.5%), mp 139−141 °C; 1H NMR (400 MHz, CD3OD): δ 8.12−8.15 (m, 2H), 7.51−7.57 (m, 2H), 6.97−7.04 (m, 1H), 5.85 (d, 1H, J=15.0 Hz), 4.23 (d, 1H, J=14.8 Hz), 3.27−3.47 (m, 4H), 2.90−3.01 (m, 3H), 2.79−2.85 (m, 1H), 2.47−2.78 (m, 3H), 2.19−2.22 (m, 1H), 1.93−2.08 (m, 2H), 1.65−1.88 (m, 7H); HRMS: calcd. for C22H30N3O4 (M+H)+ 400.2236, found 400.2217.
4.1.2. Procedures for 12-N-benzyl-β-hydroxyl sophocarpinic acid (15)
To a solution of KOH (33.6 g, 0.6 mol) in water (600 mL) was added 2 (12.3 g, 0.05 mol) with stirring at room temperature. The reaction mixture was refluxed for 8 h, then cooled down to 0 °C and neutralized with 3 mol/L HCl. After concentrated in vacuo, MeOH (150 mL) was added, insoluble solid was removed by filtration, and the filtrate was concentrated under reduced pressure. The residue was purified with flash column chromatography on silica gel using CH2Cl2/MeOH as eluent to give 11 (6.2 g, 47%) as white solid.
Compound 11 (1 equiv.) was added to a solution of KOH (10%) in water. The reaction mixture was refluxed for 9 h, and then stirred at room temperature overnight. The reaction solution was cooled in ice–water bath, and acidified with HCl (3 mol/L). The solvent was removed in vacuo and the residue was dissolved in methanol to give a corresponding solution of crude 12.
A mixture of diphenylmethanone hydrazone (14.7 g, 75 mmol, 1.5 equiv.) and electrolytic manganese dioxide (13.04 g, 150 mmol, 3 equiv.) in petroleum ether (boiling range 30–60 °C) was heated at reflux for 0.5 h, and the mixture was filtered. The filtrate was added to the solution of 13 in methanol, and the mixture was then stirred at room temperature until the purple color disappeared, and then filtered. The resulting filtrate was evaporated under reduced pressure to dryness. The residue was washed with petroleum ether to afford crude compound 13 which was used directly in the next step without further purification.
To the mixture of crude 13 and anhydrous K2CO3 (3 equiv.) in MeCN was added benzyl bromide (1 equiv.). The reaction mixture was stirred at room temperature till the reaction was complete (checked by TLC), then filtered. The filtrate was evaporated in vacuo to give the crude product 14 as an oily residue, which was then dissolved in m-cresol (10 mL), and the reaction mixture was stirred at 80 °C for 8–9 h. It was then cooled, and methylisobutylketone (30 mL) was added. The resulting solution was extracted with H2O (50 mL×3), and the combined extracts were evaporated to dryness, and the residue was purified through flash chromatography over silica gel to give 1.5 g of compound 15. White solid (1.5 g, 16.1%), mp 115−117 °C; 1H NMR (400 MHz, CD3OD): δ 7.26−7.44 (m, 5H), 4.38−4.47 (m, 1H), 4.08−4.13 (m, 1H), 3.72−3.74 (m, 1H), 3.15−3.25 (m, 2H), 2.76−3.18 (m, 3H), 2.59−2.70 (m, 1H), 2.32−2.49 (m, 3H), 1.85−2.25 (m, 6H), 1.39−1.72 (m, 7H); HRMS: calcd. for C22H33N2O3 (M+H)+ 373.2491, found 373.2493.
4.2. Biological methods
4.2.1. Cell culture
Human liver cell line Huh7.5 cells (kindly provided by Vertex Pharmaceuticals, Inc., Boston, MA) were cultured in Dulbecco׳s modified eagle medium (DMEM) supplemented with 10% inactivated fetal bovine serum and 1% penicillin–streptomycin (Invitrogen). Cells were digested with 0.05% trypsin–ethylene diamine tetraacetic acid (EDTA) and split twice a week.
4.2.2. Anti-HCV effect in vitro
Huh7.5 cells were seeded into 96-well or 6-well plates (Costar) at a density of 3×104 cells/cm2. After 24 h incubation, the cells were infected with HCV viral stock (45 IU/cell) and simultaneously treated with the test compounds at various concentrations or solvent as control. The culture medium was removed after 72 h inoculation, the intracellular total RNA (in 96-well plates) was extracted with RNeasy Mini Kit (Qiagen), and total intracellular proteins (in 6-well plates) were extracted with Cyto-Buster Protein Extraction Reagent added with 1 mmol/L protease inhibitor cocktail. The intracellular HCV RNA was quantified with a real time one-step reverse-transcription polymerase chain reaction (RT-PCR).
4.2.3. Cytotoxicity assay
Huh7.5 cells were seeded into 96-well plates (Costar) at a density of 3×104 cells/cm2. After incubated for 24 h, fresh culture medium containing test compounds at various concentrations were added. Seventy-two hours later, cytotoxicity was evaluated with 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT).
Acknowledgments
This work was supported by the National Natural Science Fund for Young Scientists (No. 81302645) and Beijing National Natural Science Fund (No. 7121009).
Footnotes
Peer review under responsibility of Institute of Materia Medica, Chinese Academy of Medical Sciences and Chinese Pharmaceutical Association.
References
- 1.Mohd Hanafiah K, Groeger J, Flaxman AD, Wiersma ST. Global epidemiology of hepatitis C virus infection: new estimates of age-specific antibody to HCV seroprevalence. Hepatology. 2013;57:1333–1342. doi: 10.1002/hep.26141. [DOI] [PubMed] [Google Scholar]
- 2.Fan JH, Wang JB, Jiang Y, Xiang W, Liang H, Wei WQ. Attributable causes of liver cancer mortality and incidence in china. Asian Pac J Cancer Prev. 2013;14:7251–7256. doi: 10.7314/apjcp.2013.14.12.7251. [DOI] [PubMed] [Google Scholar]
- 3.Xiang X, Lu J, Dong Z, Zhou H, Tao W, Guo Q. Viral sequence evolution in Chinese genotype 1b chronic hepatitis C patients experiencing unsuccessful interferon treatment. Infect Genet Evol. 2011;11:382–390. doi: 10.1016/j.meegid.2010.11.011. [DOI] [PubMed] [Google Scholar]
- 4.Melnikova I. Hepatitis C therapies. Nat Rev Drug Discov. 2008;7:799–800. [Google Scholar]
- 5.Zeuzem S. Interferon-based therapy for chronic hepatitis C: current and future perspectives. Nat Clin Pract Gastroenterol Hepatol. 2008;5:610–622. doi: 10.1038/ncpgasthep1274. [DOI] [PubMed] [Google Scholar]
- 6.Susser S, Welsch C, Wang Y, Zettler M, Domingues FS, Karey U. Characterization of resistance to the protease inhibitor boceprevir in hepatitis C virus-infected patients. Hepatology. 2009;50:1709–1718. doi: 10.1002/hep.23192. [DOI] [PubMed] [Google Scholar]
- 7.Sarrazin C, Kieffer TL, Bartels D, Hanzelka B, Müh U, Welker M. Dynamic hepatitis C virus genotypic and phenotypic changes in patients treated with the protease inhibitor telaprevir. Gastroenterol. 2007;132:1767–1777. doi: 10.1053/j.gastro.2007.02.037. [DOI] [PubMed] [Google Scholar]
- 8.Lok AS, Gardiner DF, Lawitz E, Martorell C, Everson GT, Ghalib R. Preliminary study of two antiviral agents for hepatitis C genotype 1. N Engl J Med. 2012;366:216–224. doi: 10.1056/NEJMoa1104430. [DOI] [PubMed] [Google Scholar]
- 9.McCown MF, Rajyaguru S, Kular S, Cammack N, Nájera I. GT-1a or GT-1b subtype-specific resistance profiles for hepatitis C virus inhibitors telaprevir and HCV-796. Antimicrob Agents Chemother. 2009;53:2129–2132. doi: 10.1128/AAC.01598-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Delemos AS, Chung RT. Hepatitis C treatment: an incipient therapeutic revolution. Trends Mol Med. 2014;20:315–321. doi: 10.1016/j.molmed.2014.02.002. [DOI] [PubMed] [Google Scholar]
- 11.Lam AM, Espiritu C, Bansal S, Micolochick Steuer HM, Niu C, Zennou V. Genotype and subtype profiling of PSI-7977 as a nucleotide inhibitor of hepatitis C virus. Antimicrob Agents Chemother. 2012;56:3359–3368. doi: 10.1128/AAC.00054-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li CQ, Zhu YT, Zhang FX, Fu LC, Li XH, Cheng Y. Anti-HBV effect of liposome-encapsulated matrine in vitro and in vivo. World J Gastroenterol. 2005;11:426–428. doi: 10.3748/wjg.v11.i3.426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Long Y, Lin XT, Zeng KL, Zhang L. Efficacy of intramuscular matrine in the treatment of chronic hepatitis B. Hepatobiliary Pancreat Dis Int. 2004;3:69–72. [PubMed] [Google Scholar]
- 14.Zhang JT, Wang W, Duan ZH. Progress of research and application of matrine-type alkaloids. Prog Mod Biomed. 2007;7:451–454. [Google Scholar]
- 15.Du NN, Peng ZG, Bi CW, Tang S, Li YH, Li JR. N-Substituted benzyl matrinic acid derivatives inhibit hepatitis C virus (HCV) replication through down-regulating host heat-stress cognate 70 (Hsc70) expression. PLoS One. 2013;8:e58675. doi: 10.1371/journal.pone.0058675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li YH, Du NN, Peng ZG, Jiang JD, Song DQ. Synthesis and biological evaluation of matrinic acid derivatives as anti-hepatitis C virus agents. Chin Sci Pap. 2014;9:321–326. [Google Scholar]
- 17.Gao LM, Han YX, Wang YP, Li YH, Shan YQ, Li X. Design and synthesis of oxymatrine analogs overcoming drug resistance in hepatitis B virus through targeting host heat stress cognate 70. J Med Chem. 2011;54:869–876. doi: 10.1021/jm101325h. [DOI] [PubMed] [Google Scholar]
- 18.Li X, Zhao WL, Jiang JD, Ren KH, Du NN, Li YB. Synthesis, structure-activity relationship and biological evaluation of anticancer activity for novel N-substituted sophoridinic acid derivatives. Bioorg Med Chem Lett. 2011;21:5251–5254. doi: 10.1016/j.bmcl.2011.07.038. [DOI] [PubMed] [Google Scholar]
- 19.Gao LM, Tang S, Wang YX, Gao RM, Zhang X, Peng ZG. Synthesis and biological evaluation of N-substituted sophocarpinic acid derivatives as coxsackievirus B3 inhibitors. Chem Med Chem. 2013;8:1545–1553. doi: 10.1002/cmdc.201300224. [DOI] [PubMed] [Google Scholar]
- 20.Peng ZG, Fan B, Du NN, Wang YP, Gao LM, Li YH. Small molecular compounds that inhibit hepatitis C virus replication through destabilizing heat shock cognate 70 messenger RNA. Hepatology. 2010;52:845–853. doi: 10.1002/hep.23766. [DOI] [PubMed] [Google Scholar]