Abstract
A series of novel indole-2-carboxylate derivatives were synthesized and assayed to determine their in vitro broad-spectrum antiviral activities. The biological results showed that some of the synthesized compounds exhibited potent broad-spectrum antiviral activity. Notably, compound 8f showed the highest SI value (17.1) to Cox B3 virus. Compound 14f showed both potent inhibitory activity against influenza A (IC50=7.53 μmol/L) and the highest SI value (12.1). SAR results showed that the alkyloxy at the 4-position of indole ring was not crucial to the antiviral activities. Incorporation of an acetyl substituent at the amino group disfavored antiviral activity towards RNA viruses.
KEY WORDS: Indole-2-carboxylate, Broad-spectrum antiviral activity, DNA and RNA virus, SAR
Graphical abstract
Compound 8f showed the highest SI value (17.1) to Cox B3 virus. Compound 14f showed both potent inhibitory activity against influenza A (IC50=7.53 μmol/L) and the highest SI value (12.1).
1. Introduction
Viruses are the most common cause of global infectious disease. Viruses with high infection rates and rapid propagation can cause worldwide human and animal pandemics.
Coxsackie B viruses are single-strand RNA viruses; infection with Cox B can cause fever, headache, chest pain and other problems. Cardiac infection with Cox B3 can result in acute myocarditis that is spontaneously resolved or chronic myocarditis with prolonged viral persistence1. Currently, there is no specific treatment or vaccine available for Coxsackie virus infections.
Influenza is a respiratory disease caused by the influenza virus. Despite the extensive effort invested in attempting to control influenza infection, only two classes of drugs are currently clinically available: inhibitors of the M2 protein (e.g., amantadine and rimantadine), and inhibitors of neuraminidase (e.g., zanamivir and oseltamivir). Clinical applications of amantadine and rimantadine have been limited as a consequence of the increasing incidence of adamantane-resistant viruses in the general population2, 3, 4. Furthermore, blockers of the M2 ion channel inhibit only the replication of influenza A virus and are associated with neurological side effects. The neuraminidase inhibitors (zanamivir and oseltamivir) were marketed in 1999 for the treatment and prophylaxis of influenza and have been subsequently stockpiled by many countries for use in the event of a pandemic. Unfortunately, recent studies have identified significant increases in the frequency of oseltamivir-resistant seasonal influenza A (H1N1) in Europe, the United States, Oceania and South Africa5, 6, 7.
Herpes simplex virus types 1 and 2 (HSV-1 and HSV-2) are human herpes viruses belonging to family Herpesviridae8. These types of HSV are responsible for mucocutaneous infections, mainly in immunocompromised patients. HSV-1 provokes orofacial lesions, while HSV-2 causes mucocutaneous genital infections9. Antiviral research on HSV preliminarily focuses on compounds capable of targeting the viral polymerase. Acyclovir, a nucleoside inhibitor of DNA polymerase, was the first selective antiviral agent introduced. Acyclovir is still commonly employed in the treatment of HSV infection10, but the widespread use of this drug has led to the development of viral resistance. Therefore, the search for new drugs against acyclovir-resistant HSV viruses is highly necessary.
Most current antiviral drugs, including those in development, are direct-acting antiviral (DAA) molecules that specifically target viral proteins. These drugs are narrow in spectrum and are vulnerable to the rapid emergence of viral resistance11. The emergence of drug-resistant viruses, especially multidrug-resistant strains, represents a significant problem in current clinical practice that needs to be addressed and should be considered a high priority for new avenues of research12, 13. To fulfill all of these requirements, novel classes of antivirals are needed14. Additionally, due to the high mutation rates that are particularly prevalent in RNA viruses, the lifetime of specific antiviral therapeutics is often severely limited. Broad spectrum antivirals would be one way of circumventing this problem15. Therefore, a crucial need for developing of new agents with novel antiviral mechanisms and broad antiviral activities exists.
Our group has long been engaging antivirus drug research16, 17, 18. Previously, we have done high-throughput screening of self-owned compounds for drugs with anti-influenza activity, and have found a group of indole-2-carboxylate derivatives with potential anti-influenza activity. Analysis proved broad-spectrum antiviral activity of these compounds and suggested the need for further work with these agents. In the current work, a series of indole-2-carboxylate derivatives were synthesized and evaluated for their broad spectrum antiviral activity. Four virus strains, including herpes viruses (HSV-1), Influenza viruses (A/FM/1/47, B/Jifang/13/97) and picorna viruses (Cox B3) were selected to investigate in vitro antiviral activities of the synthesized indole-2-carboxylate derivatives. Antiviral activity experiments in vitro of hepatitis viruses (HBV and HCV) and retrovirus (HIV-1) are not yet complete.
2. Results and discussion
2.1. Chemistry
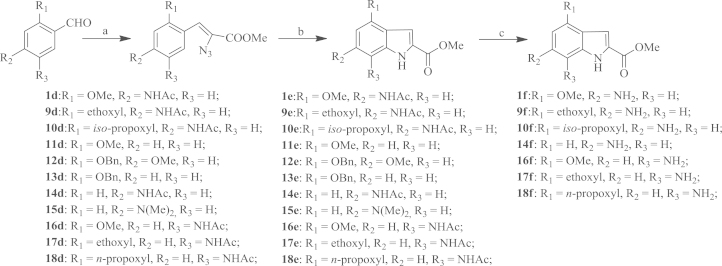
The structure modification in this work was primarily made to the 4, 6 and 7-position of the indole ring. Compounds 1f–10f with substituents at the 4 and 6-position of the indole ring were synthesized and screened for the antiviral activity. Compounds 12e with alkoxyl substituents at the 6-position of indole ring were synthesized and screened for the antiviral activity. Additionally, compounds 11e, 13e, 14f, 15e without amino or alkoxyl group at the 4-position or the 6-position of indole scaffold were designed to probe the importance of alkoxyl group and amino group for the antiviral activity, respectively. Compounds 16f–18f with alkoxyl and amino substituents at both 4 and 7-position were designed to gain an insight into the SAR at those positions.
As shown in Scheme 1, compounds 2f–8f were synthesized according to a reported method with some modifications19. Methyl p-acetaminosalicylate was used as the starting material to react with a variety of alkyl halide affording compounds 2a–8a. When substituted benzyl bromide or allyl bromide was used (2b–4b, 6b), a mild base (anhydrous K2CO3) was potent enough to drive the reaction to completion. However, in the case of saturated alkyl iodide (5b, 7b, 8b), a strong base (NaH) was employed for a reasonable yield. Reduction of compounds 2a–8a by lithium aluminum hydride (LiAlH4) in tetrahydrofuran (THF) yielded corresponding alcohol derivatives 2b–8b, which were further oxidized to afford compounds in the presence of a mild oxidation reagent (BaMnO4). However, the use of BaMnO4 required a prolonged reaction time (24 h) and resulted in moderate yields. The use of an alternative oxidant pyridinium dichromate (PDC)20 reduced the reaction time to 2 h, and compounds 2c–8c were obtained in high yields (85%–90%). During the aldehyde (2c–8c) condensing steps with methyl azidoacetate, the intermediate compounds with high polarity (i.e., small Rf values of TLC) was observed, which were subsequently transformed into compounds 2d–8d. Compounds 2d–8d were not characterized in this work due to their instability. Thermolysis of compounds 2d–8d in boiling xylene afforded the expected indole compounds 2e–8e in 60%–70% yield over two steps. The acid cleavage of acetamide in a boiling hydrogen chloride solution of methanol produced compounds 2f–8f.
Scheme 1.
Synthetic route of the target compounds 2f–8f. Reagents and conditions: (a) K2CO3, acetone, R3X (X=Br, I), reflux, 4–8 h, or NaH, DMF, R3X, 80 °C, 8 h; 70%-90%; (b) LiAlH4, dry THF, –5 °C, 2 h, 80%–90%; (c) PDC, CH2Cl2, reflux, 3 h, 90%; (d) N3CH2COOEt, MeONa/MeOH, −15 °C, 1 h, then, 0 °C, 20 h; (e) xylene, reflux, 2 h, 85%; (f) HCl (g)/MeOH, reflux, 3 h, 78%;
As shown in Scheme 2, compounds 1e, 9e–18e with various alkoxyl substituents at 4, 6 and 7 position of the indole scaffold were obtained using a method similar to that employed to synthesize compounds 2e–8e, and compounds 1e, 9e, 10e, 14e, 16e–18e were further deacetylated to afford compound 1f, 9f, 10f, 14f, 16f–18f.
Scheme 2.
Synthetic route of the target compounds 1f, 9f–10f, 14f, 16f–18f. Reagents and conditions: (a) N3CH2COOEt, MeONa/MeOH, −15 °C, 1 h, then, 0 °C, 20 h; (b): xylene, reflux, 2 h, 85%; (c) HCl(g)/MeOH, reflux, 3 h, 78%.
2.2. Antiviral activity
The synthesized compounds were assayed for their broad spectrum antiviral activity towards influenza A, influenza B, HSV-1 and Cox B3 virus in vitro. Specifically, the activity against influenza A and Cox B3 was determined by the cytopathic effect (CPE) inhibitory assay, and oseltamivir and ribavirin (RBV) were used as positive controls, respectively. A total of 22 compounds were evaluated, and the results are summarized in Table 1.
Table 1.
The antiviral activity and cytotoxicity of the compounds.
| Compd. | Cox B3 |
HSV-1 |
A/FM/1/47 |
B/Jifang/13/97 |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TC50 (μmol/L) | 158TCID50 |
TC50 (μmol/L) | 100TCID50 |
TC50 (μmol/L) | 158TCID50 |
TC50 (μmol/L) | 100TCID50 |
|||||
| IC50 (μmol/L) | SI | IC50 (μmol/L) | SI | IC50 (μmol/L) | SI | IC50 (μmol/L) | SI | |||||
| 1e | 763.36 | >254.47 | – | 763.36 | >254.47 | – | 763.36 | 254.47 | 3.0 | 763.36 | 254.47 | 3.0 |
| 2e | 45.59 | >21.92 | – | 45.59 | >21.92 | – | 341.63 | >65.73 | – | NT | NT | NT |
| 8e | 50.69 | >24.38 | – | 50.69 | >24.38 | – | 24.38 | 8.13 | 3.0 | 24.38 | >8.13 | – |
| 11e | 225.46 | 108.39 | 2.1 | 225.46 | >108.39 | – | 225.46 | 62.59 | 3.6 | 225.46 | >108.39 | – |
| 12e | 49.55 | >7.94 | – | 49.55 | >7.94 | – | 92.06 | 23.83 | 3.9 | 92.06 | >23.83 | – |
| 13e | 54.84 | >8.79 | – | 54.84 | >8.79 | – | 136.98 | 26.37 | 5.2 | 136.98 | >26.37 | – |
| 14e | 597.71 | 72.80 | 8.2 | 597.71 | >287.37 | – | 414.44 | 95.78 | 4.3 | 414.44 | >95.78 | – |
| 15e | 212.02 | 44.72 | 4.7 | 212.02 | 101.93 | 2.1 | 711.97 | 101.93 | 7.0 | 711.97 | >101.93 | – |
| 1f | 174.95 | >33.68 | – | 174.95 | >33.68 | – | 174.95 | 33.68 | 5.2 | 174.95 | >33.68 | – |
| 2f | 14.51 | 1.59 | 9.1 | 14.51 | >2.78 | – | 108.64 | >25.12 | – | NT | NT | NT |
| 3f | 16.37 | 4.55 | 3.6 | 16.37 | >7.87 | – | 273.57 | >70.76 | – | NT | NT | NT |
| 4f | 52.91 | >7.58 | – | 52.91 | >7.58 | – | 354.20 | >204.51 | – | NT | NT | NT |
| 5f | 11.75 | >2.71 | – | 11.75 | >8.15 | – | 14.13 | >8.15 | – | NT | NT | NT |
| 6f | 210.33 | 30.12 | 7.0 | 210.33 | >30.12 | – | 130.28 | 30.12 | 4.3 | 130.28 | >30.12 | – |
| 7f | 177.77 | 49.35 | 3.6 | 177.77 | >85.46 | – | 177.77 | >85.46 | - | NT | NT | NT |
| 8f | 122.33 | 7.18 | 17.1 | 122.33 | >28.28 | – | 40.76 | 9.43 | 4.3 | 40.76 | >9.43 | – |
| 9f | 94.96 | >31.67 | – | 94.96 | >31.67 | – | 65.85 | 18.29 | 3.6 | 65.85 | >31.67 | – |
| 10f | 625.85 | >89.60 | – | 625.85 | >89.60 | – | 465.60 | 51.73 | 9.0 | 465.60 | >89.60 | – |
| 14f | 243.26 | 67.53 | 3.6 | 243.26 | >116.95 | – | 90.79 | 7.53 | 12.1 | 90.79 | 13.00 | 7.0 |
| 16f | 145.68 | >33.68 | – | 145.68 | >33.68 | – | 11.23 | >3.73 | – | NT | NT | NT |
| 17f | 18.29 | 10.56 | 1.7 | 31.67 | 10.56 | 3.0 | 31.67 | >10.56 | – | NT | NT | NT |
| 18f | 115.44 | 29.88 | 3.9 | 115.44 | >29.88 | - | 5.77 | >3.31 | – | NT | NT | NT |
| RBV | 8190.01 | 1058.68 | 7.7 | NT | NT | NT | 4766.99 | 2.58 | 1847.8 | 4766.99 | 10.11 | 471.3 |
| ACV | NT | NT | NT | >444.05 | 1.82 | >243.9 | NT | NT | NT | NT | NT | NT |
| Oseltamivir | NT | NT | NT | NT | NT | NT | 4033.29 | 6.43 | 626.9 | 4033.29 | 115.04 | 35.1 |
TC50, 50% cytotoxic concentration; IC50, 50% inhibition concentration; SI, the selectivity index. NT means not tested.
2.2.1. Anti Cox B3 virus activity
As shown in Table 1, 50% of the synthesized compounds showed potent inhibitory activity towards Cox B3 at low micromolar concentrations, especially compounds 2f (IC50=1.59 μmol/L), 3f (IC50=4.55 μmol/L), 8f (IC50=7.18 μmol/L) and 17f (IC50=10.56 μmol/L). The IC50 value of RBV (the positive control) was 1058.68 μmol/L. Of all the compounds tested, compound 8f showed the highest SI value (17.1). Compounds 15e, 6f and 18f exhibited moderate levels of inhibitory activity against the Cox B3 virus, with IC50 values less then 50 μmol/L.
Given that compounds 14e, 14f and 15e showed anti-Cox B3 activity, we concluded that the 4-alkyloxy substituent of the indole ring was not crucial to antiviral activity. Relocation of the 6-amino substituent to the 7-position retained activity. Acetyl substituent at the amino group (1e and 2e) disfavored the antiviral activities.
2.2.2. Anti HSV-1 virus activity
HSV-1 was less sensitive to the presently-synthesized compounds compared to influenza A and Cox B3 virus. Only compounds 15e and 17f showed activity towards HSV-1.
2.2.3. Anti-influenza virus activity
As shown in Table 1, the majority of the synthesized compounds exhibited potent antiviral activity towards influenza A/FM/1/47 virus. This was particularly true for compounds 8e (IC50=8.13 μmol/L), 8f (IC50=9.43 μmol/L) and 14f (IC50=7.53 μmol/L). These IC50 values were comparable to those of the positive control drugs RBV (IC50=2.58 μmol/L) and oseltamivir (IC50=6.43 μmol/L). Of all the compounds tested, compound 14f showed both potent inhibitory activity against influenza A (IC50=7.53 μmol/L) and the highest SI value (12.1). Compounds 12e, 13e, 1f, 6f and 10f exhibited moderate levels of inhibitory activity against the A/FM/1/47 strain of the influenza virus, with IC50 values less then 52 μmol/L.
The compounds exhibiting activity against the A/FM/1/47(H1N1) strain of the influenza virus were further evaluated for their potential antiviral activity against strain of the influenza B virus, also using the CPE method. Compounds 1e and 14f also displayed antiviral activity against the influenza B strain. Compound 14f (IC50=13.00 μmol/L) was found to be the most effective compound against the B/Jingfang/13/97 strain of the influenza virus, with an IC50 value comparable to that of the positive control RBV (IC50=10.11 μmol/L).
In terms of the structure-activity relationships, substitution of the benzyloxy (2f–4f) for the alkyloxy (8f–10f) at the 4-position of the indole ring decreased the anti-influenza A activity. Replacement of the amino group at the 6-position of the indole ring (1f, 9f) with hydrogen (11e, 16f and 17f) resulted in a dramatic decrease in the anti-influenza A activity. Thus, the 6-amino group in the indole ring is indispensable to the antiviral activities. Additionally, introduction of the acetyl substituent at the amino group (1e and 2e) disfavored the anti-influenza A activity. Given that compounds 14e, 14f and 15e showed anti-influenza A activity, we concluded that the alkyloxy at the 4-position of indole ring was not crucial to the anti-influenza A activities. In addition, as the activity of compound 8f was superior to that of 10f, it can be concluded that a more bulky alkyloxy substituent at the 4-position of indole ring favored the anti-influenza A activity.
3. Conclusions
In summary, a total of 22 indole-2-carboxylate derivatives were designed, synthesized and screened for antiviral activities towards influenza A, influenza B, HSV-1 and Cox B3. In general, the target compounds were more effective against RNA viruses (influenza A and Cox B3) than against the DNA virus (HSV-1). The majority of the synthesized compounds simultaneously exhibited potent activity against the influenza A and Cox B3 viruses. A similar trend was observed among the SARs of the synthesized compounds towards the RNA virus. For example, the alkyloxy at the 4-position of indole ring was not crucial to the antiviral activities. Acetyl substituent at the amino group also disfavored the antiviral activities. Notably, compounds 8f and 14f showed potent antiviral activity towards the RNA virus at low micromolar concentrations. Compound 8f showed the highest SI value (17.1) to Cox B3 virus. Compound 14f showed both potent inhibitory activity against influenza A (IC50=7.53 μmol/L) and the highest SI value (12.1). The detailed structure optimization of compound 8f and 14f and mechanistic studies are ongoing in our laboratory.
4. Experimental
4.1. Synthesis and characterization
1H NMR and 13C NMR spectra were recorded using TMS as the internal standard in DMSO-d6 or CDCl3 with a Bruker BioSpin GmbH spectrometer. The mass spectra (MS) were recorded on a Thermo Scientific LTQ ORBITRAP instrument with an ESI mass selective detector. Melting points (m.p.) were determined using an SRS-OptiMelt automated melting point instrument, without correction. Flash column chromatography was performed with silica gel (200–300 mesh) purchased from Qingdao Haiyang Chemical Co., Ltd.
4.1.1. General procedure A for the preparation of 2a–8a
Methyl 4-acetamido-2-hydroxybenzoate (20 mmol) was dissolved in anhydrous DMF (50 mL), followed by the addition of anhydrous K2CO3 (30 mmol) or NaH (30 mmol) and alkylation agents (30 mmol). The mixture was heated to 60 °C for 4–6 h, and the reaction mixture was cooled to room temperature and filtered. The filtrate was concentrated in vacuum, and the resulting residue was recrystallized from ethyl acetate and petroleum ether to afford the target compounds.
Methyl 4-acetamido-2-(benzyloxy)benzoate (2a): white solid, yield, 90%; m.p. 107–108 °C. 1H NMR (400 MHz, CDCl3): δ 2.18 (s, 3H), 3.88 (s, 3 H), 5.18 (s, 2 H), 6.82 (d, 1H, J=8.4 Hz), 7.30 (t, 1H, J=7.6 Hz), 7.38 (t, 2H, J=7.6 Hz), 7.51 (d, 2H, J=7.6 Hz), 7.69 (s, 1H), 7.83 (d, 1H, J=8.4 Hz).
Methyl 4-acetamido-2-(4-fluorobenzyloxy)benzoate (3a): white solid, yield, 85%; m.p. 132–133 °C. 1H NMR (400 MHz, CDCl3): δ 2.20 (s, 3H), 3.88 (s, 3H), 5.15 (s, 2H), 6.76 (dd, 1H, J=8.4, 1.6 Hz), 7.06 (m, 2H), 7.50 (m, 2H), 7.76 (s, 1H), 7.83 (d, 1H, J=8.4 Hz)
Methyl 4-acetamido-2-(4-methoxybenzyloxy)benzoate (4a): white solid, yield, 90%. m.p. 124–125 °C. 1H NMR (400 MHz, DMSO-d6): δ 2.05 (s, 3H), 3.73 (s, 3H), 3.74 (s, 3H), 5.03 (s, 2H), 6.94 (d, 2H, J=8.4 Hz), 7.16 (d, 1H, J=8.4 Hz), 7.40 (d, 2H, J=8.4 Hz), 7.56 (s, 1H), 7.66 (d, 1H, J=8.4 Hz), 10.19 (s, 1H).
Methyl 4-acetamido-2-(cyclohexylmethoxy)benzoate (5a): white solid, yield, 65%; m.p. 132–133 °C. 1H NMR (400 MHz, CDCl3): δ 1.05–1.32 (m, 5H), 1.68–1.90 (m, 6H), 2.23 (s, 3H), 3.83 (d, 2H, J=6.0 Hz), 3.87 (s, 3H), 6.79 (d, 1H, J=8.4 Hz), 7.29 (s, 1H), 7.53 (s, 1H), 7.80 (d, 1H, J=8.4 Hz).
Methyl 4-acetamido-2-(allyloxy)benzoate (6a): white solid, yield, 90%; m.p. 114–115 °C. 1H NMR (400 MHz, DMSO-d6): δ 2.06 (s, 3H), 3.74 (s, 3H), 4.54 (d, 2H, J=6.4 Hz), 5.25 (d, 1H, J=6.4 Hz), 5.51 (d, 1H, J=16.0 Hz), 6.02 (m, 1H), 7.16 (dd, 1H, J=8.4, 1.6 Hz), 7.47 (d, 1H, J=1.6 Hz), 7.66 (d, 1H, J=8.4 Hz), 10.18 (s, 1H).
Methyl 4-acetamido-2-(cyclopropylmethoxy)benzoate (7a): white solid, yield 78%; m.p. 127–128 °C. 1H NMR (400 MHz, CDCl3): δ 0.38 (t, 2H, J=4.8 Hz), 0.59–0.64 (m, 2H), 1.30 (t, 1H, J=6.0 Hz), 2.19 (s, 3H), 3.87 (s, 3H), 3.92 (d, 2H, J=6.4 Hz), 6.79 (d, 1H, J=8.4 Hz), 7.33 (s, 1H), 7.59 (s, 1H), 7.78 (d, 1H, J=8.4 Hz).
Methyl 4-acetamido-2-isobutoxybenzoate (8a): white solid, yield, 70%; m.p. 112–113 °C. 1H NMR (400 MHz, DMSO-d6): δ 1.00 (d, 6H, J=6.8 Hz), 2.01 (s, 3H), 2.03–2.05 (m, 1H), 3.72 (d, 2H, J=7.2 Hz), 3.74 (s, 3H), 7.15 (dd, 1H, J=8.4, 2.0 Hz), 7.44 (d, 1H, J=2.0 Hz), 7.64 (d, 1H, J=8.4 Hz), 10.16 (s, 1H).
4.1.2. General procedure B for the preparation of compounds 2b–8b
A suspension of LiAlH4 (25 mmol) in anhydrous THF (100 mL) was added dropwise a solution of compounds 2a–8a (20 mmol) in anhydrous THF (50 mL), maintaining the reaction temperature blow 0 °C. After completion of the addition, the reaction mixture was warmed up to room temperature for another 2 h, and was cooled again with ice bath. Na2SO4·7H2O was added to quench the reaction, and the resulting mixture was stirred for another 2 h at room temperature. After that, the mixture was filtered, and the filtrate was concentrated in vacuum to give a white solid, which was triturated with ether to afford the target compounds.
N-(3-(Benzyloxy)-4-(hydroxymethyl)phenyl)acetamide (2b): white solid, yield, 85%; m.p. 115–116 °C; 1H NMR (400 MHz, DMSO-d6): δ 2.02 (s, 3 H), 4.48 (d, 2H, J=5.6 Hz), 4.89 (t, 1H, J=5.6 Hz), 5.05 (s, 2 H), 7.12 (dd, 1H, J=1.6, 8.0 Hz), 7.26 (d, 1H, J=8.0 Hz), 7.32–7.46 (m, 6H), 9.87 (s, 1H).
N-(3-(4-Fluorobenzyloxy)-4-(hydroxymethyl)phenyl)acetamide (3b): white solid, yield, 80%; m.p. 124–125 °C. 1H NMR (400 MHz, DMSO-d6): δ 2.01 (s, 3H), 4.46 (d, 2 H, J=5.6 Hz), 4.87 (t, 1H, J=5.6 Hz), 5.02 (s, 2 H), 7.09 (d, 1H, J=7.6 Hz), 7.22 (m, 3H), 7.37 (s, 1H), 7.49 (dd, 2H, J=8.0, 5.6 Hz), 9.86 (s, 1H).
N-(4-(Hydroxymethyl)-3-(4-methoxybenzyloxy)phenyl)acetamide (4b): white solid, yield, 85%. m.p. 140–142 °C. 1H NMR (400 MHz, DMSO-d6): δ 2.01 (s, 3H), 3.75 (s, 3H), 4.43 (d, 2H, J=5.6 Hz), 4.85 (t, 1H, J=5.6 Hz), 4.95 (s, 2 H), 6.93 (d, 2H, J=8.4 Hz), 7.08 (d, 1H, J=8.4 Hz), 7.23 (d, 1H, J=8.4 Hz), 7.36 (d, 3H, J=8.4 Hz), 9.85 (s, 1H).
N-(3-(Cyclohexylmethoxy)-4-(hydroxymethyl)phenyl)acetamide (5b): white solid, yield, 79%; m.p. 112–114 °C. 1H NMR (400 MHz, DMSO-d6): δ 1.03–1.29 (m, 5H), 1.68–1.80 (m, 6H), 2.00 (s, 3H), 3.68 (d, 2H, J=6.0 Hz), 4.42 (d, 2H, J=5.2 Hz), 4.83 (t, 1H, J=5.2 Hz), 7.07 (dd, 1H, J= 8.4, 1.6 Hz), 7.21 (d, 1H, J=8.4 Hz), 7.24 (d, 1H, J=1.6 Hz), 9.82 (s, 1H).
N-(3-(Allyloxy)-4-(hydroxymethyl)phenyl)acetamide (6b): white solid, yield, 75%; m.p. 129–131 °C. 1H NMR (400 MHz, DMSO-d6): δ 2.01 (s, 3H), 4.44 (d, 2H, J=6.4 Hz), 4.49 (d, 2H, J=5.6 Hz), 4.86 (t, 1H, J=5.6 Hz), 5.25 (d, 1H, J=6.4 Hz), 5.40 (d, 1H, J=17.2 Hz), 6.01–6.07 (m, 1H), 7.08 (dd, 1H, J=8.4, 2.0 Hz), 7.23 (d, 1H, J=8.4 Hz), 7.28 (d, 1H, J=1.6 Hz), 9.85 (s, 1H).
N-(3-(Cyclopropylmethoxy)-4-(hydroxymethyl)phenyl)acetamide (7b): white solid, yield, 79%. m.p. 120–121 °C. 1H NMR (400 MHz, DMSO-d6): δ 0.30–0.34 (m, 2H), 0.52-0.57 (m, 2H), 1.19-1.22 (m, 1H), 2.20 (s, 3H), 3.76 (d, 2H, J=6.8 Hz), 4.43 (d, 2H, J=5.6 Hz), 4.83 (t, 1H, J=5.6 Hz), 7.06 (dd, 1H, J=8.4, 1.6 Hz), 7.22 (d, 1H, J=8.4 Hz), 7.25 (d, 1H, J=1.6 Hz), 9.82 (s, 1H).
N-(4-(Hydroxymethyl)-3-isobutoxyphenyl)acetamide (8b): white solid, yield, 85%; m.p. 120–121 °C. 1H NMR (400 MHz, DMSO-d6): δ 0.97 (d, 6H, J=6.8 Hz), 2.00 (s, 3H), 2.02-2.03 (m, 1H), 3.65 (d, 2H, J=6.4 Hz), 4.44 (d, 2H, J=5.6 Hz), 4.84 (t, 1H, J=5.6 Hz), 7.06 (dd, 1H, J=8.4, 2.0 Hz), 7.22 (d, 1H, J=8.4 Hz), 7.26 (d, 1H, J=2.0 Hz), 9.83 (s, 1H).
4.1.3. General procedure C for the preparation of compounds 2c–8c
Compounds 2b-8b (20 mmol) was dissolved in CH2Cl2 (100 mL), and PDC (30 mmol) was added. The resulting mixture was heated under reflux for 2 h. The reaction mixture was cooled and filtered through a thin layer of silica gel, and the filtrate was concentrated in vacuum. The residue was recrystallized from ethyl acetate and petroleum ether to afford the target compounds as white solid.
N-(3-(Benzyloxy)-4-formylphenyl)acetamide (2c): white solid, yield, 90%; m.p. 126-128 °C. 1H NMR (400 MHz, DMSO-d6): δ 2.06 (s, 3H), 5.21 (s, 2H), 7.21 (d, 1H, J=8.4 Hz), 7.35 (t, 1H, J=7.6 Hz), 7.42 (t, 2H, J=7.6 Hz), 7.51 (d, 2H, J=7.6 Hz), 7.65 (s, 1H), 7.68 (d, 1H, J=8.4 Hz), 10.25 (s, 1H), 10.34 (s,1H).
N-(3-(4-Fluorobenzyloxy)-4-formylphenyl)acetamide (3c): white solid, yield, 87%; m.p. 158-160 °C. 1H NMR (400 MHz, CDCl3): δ 2.22 (s, 3H), 5.16 (s, 2H), 6.69 (d, 1H, J=8.0 Hz), 7.08 (m, 2H), 7.35 (s, 1H), 7.43 (dd, 2H, J=8.4, 5.6 Hz), 7.79 (d, 1H, J=8.0 Hz), 7.93 (s, 1H), 10.34 (s, 1H).
N-(4-Formyl-3-(4-methoxybenzyloxy)phenyl)acetamide (4c): white solid, yield, 86%. m.p. 158-160 °C. 1H NMR (400 MHz, DMSO-d6): δ 2.08 (s, 3H), 3.74 (s, 3H), 5.11 (s, 2H), 6.94 (d, 2H, J=8.4 Hz), 7.18 (d, 1H, J=8.4 Hz), 7.42 (d, 2H, J=8.4 Hz), 7.63 (d, 1H, J=8.4 Hz), 7.69 (s, 1H), 10.20 (s, 1H), 10.33 (s, 1H).
N-(3-(Cyclohexylmethoxy)-4-formylphenyl)acetamide (5c): white solid, yield, 90%, m.p. 156-157 °C. 1H NMR (400 MHz, DMSO-d6): δ 1.06-1.27 (m, 5H), 1.70-1.83 (m, 6H), 2.07 (s, 3H), 3.85 (d, 2H, J=6.0 Hz), 7.17 (d, 1H, J=8.4 Hz), 7.53 (s, 1H), 7.62 (d, 1H, J=8.4 Hz), 10.22 (s, 1H), 10.30 (s, 1H).
N-(3-(Allyloxy)-4-formylphenyl)acetamide (6c): white solid. yield, 90%; m.p. 121-122 °C. 1H NMR (400 MHz, DMSO-d6): δ 2.08 (s, 3H), 4.65 (d, 2H, J=6.8 Hz), 5.30 (d, 1H, J=6.4 Hz), 5.48 (d, 1H, J=16.0 Hz), 6.05-6.12 (m, 1H), 7.17 (dd, 1H, J=8.4, 1.6 Hz), 7.58 (d, 1H, J=1.6 Hz), 7.64 (d, 1H, J=8.4 Hz), 10.24 (s, 1H), 10.32 (s, 1H).
N-(3-(Cyclopropylmethoxy)-4-formylphenyl)acetamide (7c): white solid, yield, 82%. m.p. 152–153 °C. 1H NMR (400 MHz, DMSO-d6): δ 0.37 (t, 2H, J=4.8 Hz), 0.56-0.60 (m, 2H), 1.26-1.29 (m, 1H), 2.07 (s, 3H), 3.92 (d, 2H, J=6.8 Hz), 7.15 (d, 1H, J=8.4 Hz), 7.55 (s, 1H), 7.62 (d, 1H, J=8.4 Hz), 10.24 (s, 1H), 10.30 (s, 1H).
N-(4-Formyl-3-isobutoxyphenyl)acetamide (8c): white solid, yield, 84%, m.p. 133-134 °C. 1H NMR (400 MHz, DMSO-d6): δ 1.00 (d, 6H, J=6.8 Hz), 2.07 (s, 3H), 2.10-2.11 (m, 1H), 3.82 (d, 2H, J=6.4 Hz), 7.15 (d, 1H, J=8.4 Hz), 7.56 (s, 1H), 7.63 (d, 1H, J=8.4 Hz), 10.24 (s, 1H), 10.31 (s, 1H).
4.1.4. General procedure D for the preparation of compounds 1e–18e
To a solution of MeONa (30 mmol) in MeOH (80 mL) were added aromatic aldehydes (20 mmol) and methyl 2-azidoacetate (200 mmol) in anhydrous THF (80 mL) at –15 °C, and the mixture was stirred for another 4 h. After that, the reaction was quenched by the addition of saturated ammonium chloride solution, and extracted with ethyl acetate (3×100 mL). The combined organic layer was washed with brine, and dried over anhydrous MgSO4, filtered and concentrated, and the residue was triturated with MeOH to give the intermediate compounds 1d–18d as yellow solids. The crude compounds 1d–18d were dissolved in xylene, and heated under reflux for 2 h. After cooling, white solids were crystallized. Filtration afforded the target compounds.
Methyl 6-acetimidamido-4-methoxy-1H-indole-2-carboxylate (1e): white solid, yield, 84%. m.p. 237–239 °C. 1H NMR (400 MHz, DMSO-d6): δ 2.05 (s, 3H), 3.82 (s, 3H), 3.85 (s, 3H), 6.76 (s, 1H), 7.01 (s, 1H), 7.60 (s, 1H), 9.93 (s, 1H), 11.77 (s, 1H). HRMS (ESI+): found 263.10238 (Calcd. for C13H15O4N2 [M+H]+: 263.10263).
Methyl 6-acetamido-4-(benzyloxy)-1H-indole-2-carboxylate (2e): white solid, yield, 60%; m.p. 264-265 °C. 1H NMR (400 MHz, DMSO-d6): δ 2.04 (s, 3H), 3.83 (s, 3H), 5.18 (s, 2H), 6.78 (s, 1H), 7.07 (s, 1H), 7.36 (t, 1H, J=7.6 Hz), 7.42 (t, 2H, J =7.6 Hz), 7.51 (d, 2H, J=7.6 Hz), 7.62 (s, 1H), 9.92 (s, 1H), 11.79 (s, 1H). 13C NMR (126 MHz, DMSO-d6): δ 24.62, 52.05, 69.50, 95.76, 105.79, 115.05, 125.86, 127.83, 128.27, 128.93, 137.45, 138.16, 139.37, 152.81, 161.90, 168.60. HRMS (ESI+): found 339.13379 (Calcd. for C19H19O4N2 [M+H]+: 339.13393).
Methyl 6-acetamido-4-(4-fluorobenzyloxy)-1H-indole-2-carboxylate (3e): white solid, yield, 60%; m.p. 273-273 °C. 1H NMR (400 MHz, DMSO-d6): δ 2.03 (s, 3H), 3.82 (s, 3H), 5.15 (s, 2H), 6.78 (s, 1H), 7.05 (s, 1H), 7.23 (t, 2H, J=8.4 Hz), 7.55 (dd, 2H, J= 5.6, 8.4 Hz), 7.60 (s, 1H), 9.92 (s, 1H), 11.79 (s, 1H). HRMS (ESI+): found 357.12429 (Calcd. for C19H18O4N2F [M+H]+: 357.12451).
Methyl 6-acetimidamido-4-(4-methoxyphenoxy)-1H-indole-2-carboxylate (4e): pale yellow solid, yield, 80%. m.p. 268-270 °C. 1H NMR (400 MHz, DMSO-d6): δ 2.03 (s, 3H), 3.76 (s, 3H), 3.81 (s, 3H), 5.08 (s, 2H), 6.77 (s, 1H), 6.95 (d, 1H, J=8.4 Hz), 7.01 (s, 1H), 7.42 (d, 1H, J=8.4 Hz), 7.59 (s, 1H), 9.91 (s, 1H), 11.77 (s, 1H). HRMS (ESI+): found 391.12626 (Calcd. for C20H20O5N2Na [M+Na]+: 391.12644).
Methyl 6-acetamido-4-(cyclohexylmethoxy)-1H-indole-2-carboxylate (5e): pale yellow solid, yield, 63%; m.p. 253-255 °C. 1H NMR (400 MHz, DMSO-d6): δ 1.04-1.30 (m, 5H), 1.65-1.75 (m, 6H), 2.02 (s, 3H), 3.81 (s, 3H), 3.82 (d, 2H, J=6.0 Hz), 6.63 (s, 1H), 7.01 (s, 1H), 7.57 (s, 1H), 9.87 (s, 1H), 11.74 (s, 1H). HRMS (ESI+): found 345.18094 (Calcd. for C19H25O4N2 [M+H]+: 345.18088).
Methyl 6-acetamido-4-(allyloxy)-1H-indole-2-carboxylate (6e): pale yellow solid, yield, 62%; m.p. 200-201 °C. 1H NMR (400 MHz, DMSO-d6): δ 2.01 (s, 3H), 3.82 (s, 3H), 4.61 (d, 2H, J=5.2 Hz), 5.28 (d, 1H, J=6.4 Hz), 5.45 (d, 1H, J=17.2 Hz), 6.06-6.14 (m, 1H), 6.68 (s, 1H), 7.03 (s, 1H), 7.59 (s, 1H), 9.90 (s, 1H), 11.77 (s, 1H). HRMS (ESI+): found 289.11805 (Calcd. for C15H17O4N2 [M+H]+: 289.11828).
Methyl 6-acetimidamido-4-cyclopropoxy-1H-indole-2-carboxylate (7e): Pale white solid, yield, 84%. m.p. 240-241 °C. 1H NMR (400 MHz, DMSO-d6): δ 0.35-0.39 (m, 2H), 0.57-0.60 (m, 2H), 1.30-1.33 (m, 1H), 2.02 (s, 3H), 3.82 (s, 3H), 3.88 (d, 2H, J=6.8 Hz), 6.64 (s, 1H), 7.01 (s, 1H), 7.56 (s, 1H), 9.87 (s, 1H), 11.74 (s, 1H). HRMS (ESI+): found 325.11577 (Calcd. for C16H18O4N2Na [M+Na]+: 325.11588).
Methyl 6-acetamido-4-isobutoxy-1H-indole-2-carboxylate (8e): white solid, yield, 66%; m.p. 107–108 °C. 1H NMR (400 MHz, DMSO-d6): δ 1.01 (d, 6H, J=6.8 Hz), 2.03 (s, 3H), 2.06-2.11 (m, 1H), 3.78 (d, 2H, J=6.4 Hz), 3.82 (s, 3H), 6.65 (s, 1H), 7.01 (s, 1H), 7.57 (s, 1H), 9.88 (s, 1H), 11.74 (s, 1H). 13C NMR (150 MHz, DMSO-d6): δ 19.52, 24.62, 28.19, 52.02, 74.13, 95.33, 105.74, 114.99, 125.69, 138.26, 139.34, 153.31, 161.93, 168.58. HRMS (ESI+): found 305.14938 (Calcd. for C16H21O4N2 [M+H]+: 305.14958).
Methyl 6-acetimidamido-4-ethoxy-1H-indole-2-carboxylate (9e): white solid, yield, 76%. m.p. 205-207 °C. 1H NMR (400 MHz, DMSO-d6): δ 1.33 (s, 3H), 2.02 (s, 3H), 3.87 (s, 3H), 3.95 (s, 2H), 6.78 (s, 1H), 7.06 (s, 1H), 7.65 (s, 1H), 9.63 (s, 1H), 11.70 (s, 1H). HRMS ( ESI+ ): found 277.11863, (Calcd. for C14H17O4N2 [M+H]+: 277.11828).
Methyl 6-acetimidamido-4-isopropoxy-1H-indole-2-carboxylate (10e): pale white solid, yield, 72%. m.p. 196-197 °C. 1H NMR (400 MHz, DMSO-d6): δ 1.38 (s, 6H), 2.03 (s, 3H), 3.88 (s, 3H), 4.04 (s, 1H), 6.88 (s, 1H), 7.12 (s, 1H), 7.63 (s, 1H), 9.69 (s, 1H), 11.65 (s, 1H). HRMS (ESI+): found 291.13396 (Calcd. for C15H19O4N2 [M+H]+: 291.13393).
Methyl 4-methoxy-1H-indole-2-carboxylate (11e): white solid, yield, 65%; m.p. 143-145 °C. 1H NMR (400 MHz, DMSO-d6): δ 3.86 (s, 3H), 3.88 (s, 3H), 6.53 (d, 1H, J=7.6 Hz), 7.02 (d, 1H, J=7.6 Hz), 7.09 (s, 1H), 7.18 (t, 1H, J=7.6 Hz), 11.92 (s, 1H). 13C NMR (126 MHz, DMSO-d6): δ 52.36, 55.34, 100.05, 105.36, 105.99, 118.37, 126.23, 126.33, 139.21, 154.28, 162.05. HRMS (ESI+): found 206.08092 (Calcd. for C11H12O3N [M+H]+: 206.08117).
Methyl 4-(benzyloxy)-6-methoxy-1H-indole-2-carboxylate (12e): white solid, yield, 85%. m.p. 182-183 °C. 1H NMR (400 MHz, DMSO-d6): δ 3.74 (s, 3H), 3.81 (s, 3H), 5.20 (s, 2H), 6.28 (s, 1H), 6.46 (s, 1H), 7.03 (s, 1H), 7.32 (t, 1H, J=7.2 Hz), 7.39 (t, 2H, J=7.2 Hz), 7.48 (d, 2H, J=7.2 Hz), 11.69 (s, 1H). HRMS (ESI+): found 312.12275 (Calcd. for C18H18O4N [M+H]+: 312.12303).
Methyl 4-(benzyloxy)-1H-indole-2-carboxylate (13e): white solid, yield, 82%. m.p. 192-193 °C. 1H NMR (400 MHz, DMSO-d6): δ 3.86 (s, 3H), 5.24 (s, 2H), 6.63 (d, 1H, J=7.6 Hz), 7.03 (d, 1H, J=7.6 Hz), 7.13 (s, 1H), 7.16 (t, 1H, J=7.6 Hz), 7.36 (t, 1H, J=7.6 Hz), 7.41 (t, 2H, J=7.6 Hz), 7.52 (d, 2H, J=7.6 Hz), 11.94 (s, 1H). HRMS (ESI+): found 282.11227 (Calcd. for C17H16O3N[M+H]+: 282.11247).
Methyl 6-acetamido-1H-indole-2-carboxylate (14e): white solid, yield, 70%; m.p. 235-237 °C. 1H NMR (400 MHz, DMSO-d6): δ 2.05 (s, 3H), 3.83 (s, 3H), 7.07 (s, 1H), 7.10 (d, 1H, J=8.8 Hz), 7.52 (d, 1H, J=8.8 Hz), 8.01 (s, 1H), 9.95 (s, 1H), 11.75 (s, 1H). HRMS (ESI+): found 233.09207 (Calcd. for C12H13O3N2 [M+H]+: 233.09188).
Methyl 6-(dimethylamino)-1H-indole-2-carboxylate (15e): brown solid, yield, 67%; m.p. 177–178 °C. 1H NMR (400 MHz, DMSO-d6): δ 2.91 (s, 6H), 3.80 (s, 3H), 6.56 (s, 1H), 6.74 (dd, 1H, J=8.8, 2.0 Hz), 6.99 (s, 1H), 7.43 (d, 1H, J=8.8 Hz), 11.34 (s, 1H). 13C NMR (126 MHz, DMSO-d6): δ 41.22, 51.83, 93.62, 108.90, 111.03, 119.10, 122.57, 124.94, 139.97, 149.58, 161.97. HRMS (ESI+): found 219.11261 (Calcd. for C12H15O2N2 [M+H]+: 219.11280).
4.1.5. General procedure E for the preparation of compounds 1f–10f, 14f, 16f–18f
A solution of compounds 1e–3e, 5e–10e, 14e, 16e–18e (10 mmol) in MeOH (50 mL) presaturated with hydrogen chloride was heated under reflux for 2 h. After cooling, the solution was poured into saturated sodium hydrogen carbonate (200 mL), and extracted with ethyl acetate (3 × 80 mL). The combined organic layer was dried over MgSO4. Filtered and concentrated, the residue was recrystallized from anhydrous EtOH to afford the target compounds.
Methyl 6-amino-4-methoxy-1H-indole-2-carboxylate (1f): pale yellow solid, yield, 78%. m.p. 202-203 °C. 1H NMR (400 MHz, DMSO-d6): δ 3.84 (s, 3H), 3.88 (s, 3H), 6.40 (s, 1H), 6.92 (s, 1H), 7.06 (s, 1H), 9.49 (br, 2H), 12.01 (s, 1H). HRMS (ESI+ ): found 221.09183 (Calcd. for C11H13O3N2 [M+H]+: 221.09207).
Methyl 6-amino-4-(benzyloxy)-1H-indole-2-carboxylate (2f): pale yellow solid, yield, 75%; m.p. 222-223 °C. 1H NMR (400 MHz, DMSO-d6): δ 3.78 (s, 3H), 5.12 (s, 2H), 5.16 (s, 2H), 6.06 (s, 1H), 6.14 (s, 1H), 6.95 (s, 1H), 7.36 (t, 1H, J=7.6Hz),7.41 (t, 2H, J=7.6 Hz), 7.49 (d, 2H, J=7.6 Hz), 11.19 (s, 1H). HRMS (EI+): found 296.1146 (Calcd. for C17H16O3N2 [M]+: 296.1161).
Methyl 6-amino-4-(4-fluorobenzyloxy)-1H-indole-2-carboxylate (3f): pale yellow solid, yield, 70%; m.p. 254-256 °C. 1H NMR (400 MHz, DMSO-d6): δ 3.84 (s, 3H), 5.21 (s, 2H), 6.49 (s, 1H), 6.94 (s, 1H), 7.12 (s, 1H), 7.24 (t, 2H, J=8.4 Hz), 7.56 (dd, 2H, J=5.6, 8.4 Hz), 9.54 (br, 2H), 12.04 (s, 1H). 13C NMR (126 MHz, DMSO-d6): δ 52.15, 69.05, 97.38, 105.60, 115.72, 115.89, 127.13, 130.17, 130.23, 133.03, 138.45, 153.26, 153.57, 161.34, 161.74, 163.28. HRMS (ESI+): found 315.11399 (Calcd. for C17H16O3N2F [M+H]+: 315.11395).
Methyl 6-amino-4-(4-methoxybenzyloxy)-1H-indole-2-carboxylate (4f): pale yellow solid, yield, 61%; m.p. 270-272 °C. 1H NMR (400 MHz, DMSO-d6): δ 3.83 (s, 3H), 3.89 (s, 3H),5.13 (s, 2H), 6.28 (s, 1H), 6.77 (s, 1H), 7.09 (s, 1H), 7.28 (t, 2H, J=8.0 Hz), 7.50 (dd, 2H, J=5.2, 8.0 Hz), 9.61 (br, 2H), 11.99 (s, 1H). HRMS (ESI+): found 327.13388 (Calcd. for C18H19O3N2 [M+H]+: 327.13393).
Methyl 6-amino-4-(cyclohexylmethoxy)-1H-indole-2-carboxylate (5f): pale yellow solid, yield, 73%; m.p. 240-242 °C. 1H NMR (400 MHz, CDCl3): δ 1.08-1.31 (m, 5H), 1.65-1.85 (m, 6H), 3.83 (s, 3H), 3.87 (d, 2H, J=5.6 Hz), 6.34 (s, 1H), 6.82 (s, 1H), 7.06 (s, 1H), 9.18 (br, 2H), 11.92 (s, 1H). 13C NMR (126 MHz, DMSO-d6): δ 25.78, 26.49, 29.52, 37.12, 52.36, 73.44, 85.82, 96.48, 105.59, 115.00, 116.73, 126.96, 138.44, 154.10, 161.77. HRMS (ESI+): found 303.17031 (Calcd. for C17H23O3N2 [M+H]+: 303.17032).
Methyl 4-(allyloxy)-6-amino-1H-indole-2-carboxylate (6f): pale yellow solid, yield, 65%; m.p. 230-231 °C. 1H NMR (400 MHz, DMSO-d6): δ 3.85 (s, 3H), 4.68 (d, 2H, J=5.2Hz), 5.30 (d, 1H, J=6.4 Hz), 5.48 (d, 1H, J=17.2 Hz), 6.06-6.16 (m, 1H), 6.46 (s, 1H), 6.99 (s, 1H), 7.11 (s, 1H), 9.75 (br, 2H), 12.08 (s, 1H). 13C NMR (126 MHz, DMSO-d6): δ 52.14, 69.05, 97.11, 100.16, 105.58, 117.14, 118.26, 127.25, 133.59, 138.45, 153.59, 161.69. HRMS (ESI+): found 247.10758 (Calcd. for C13H15O3N2 [M+H]+: 247.10772).
Methyl 6-amino-4-cyclopropoxy-1H-indole-2-carboxylate (7f): pale yellow solid, yield, 90%. m.p. 251-253 °C. 1H NMR (400 MHz, DMSO-d6): δ 0.37-0.40 (m, 2H), 0.58-0.62 (m, 2H), 1.29-1.34 (m, 1H), 3.84 (s, 3H), 3.94 (d, 2H, J=6.8 Hz), 6.38 (s, 1H), 6.91 (s, 1H), 7.07 (s, 1H), 9.50 (br, 2H), 12.00 (s, 1H). HRMS (ESI+): found 261.12305 (Calcd. for C14H17O3N2 [M+H]+: 261.12337).
Methyl 6-amino-4-isobutoxy-1H-indole-2-carboxylate (8f): pale yellow solid, yield, 68%; m.p. 230–233 °C. 1H NMR (400 MHz, DMSO-d6): δ 1.02 (d, 6H, J=6.8 Hz), 2.08-2.14 (m, 1H), 3.84 (d, 2H, J=6.4 Hz), 3.85 (s, 3H), 6.42 (s, 1H), 6.95 (s, 1H), 7.10 (s, 1H), 9.62 (br, 2H), 12.04 (s, 1H). 13C NMR (126 MHz, DMSO-d6): δ 19.30, 28.20, 52.36, 74.48, 96.49, 105.39, 126.69, 138.44, 149.09, 149.31, 153.90, 161.47. HRMS (ESI+): found 263.13892 (Calcd. for C14H19O3N2 [M+H]+: 263.13902).
Methyl 6-amino-4-ethoxy-1H-indole-2-carboxylate (9f): pale yellow solid, yield, 65%. m.p. 184-186 °C. 1H NMR (400 MHz, DMSO-d6): δ 1.35 (s, 3H), 3.86 (s, 3H), 3.97 (s, 2H), 6.60 (s, 1H), 6.79 (s, 1H), 7.08 (s, 1H), 9.27 (br, 2H), 11.75 (s, 1H). HRMS (ESI+): found 235.10768 (Calcd. for C12H15O3N2 [M+H]+: 235.10772).
Methyl 6-amino-4-isopropoxy-1H-indole-2-carboxylate (10f): pale yellow solid, yield, 70%. m.p. 171-173 °C. 1H NMR (400 MHz, DMSO-d6): δ 1.37 (s, 6H), 3.86 (s, 3H), 4.10 (s, 1H), 6.67 (s, 1H), 6.84 (s, 1H), 7.00 (s, 1H), 9.47 (br, 2H), 11.88 (s, 1H). HRMS (ESI+): found 249.12330 (Calcd. for C13H17O3N2 [M+H]+: 249.12337).
Methyl 6-amino-1H-indole-2-carboxylate (14f): pale yellow solid, yield, 65%; m.p. 240-242 °C. 1H NMR (400 MHz, DMSO-d6): δ 3.87 (s, 3H), 7.01 (s, 1H), 7.19 (s, 1H), 7.40 (s, 1H), 7.72 (d, 1H, J=8.4 Hz), 9.79 (br, 2H), 12.01 (s, 1H). 13C NMR (151 MHz, DMSO-d6): δ 52.27, 107.22, 107.87, 115.73, 123.32, 126.15, 128.63, 129.81, 137.43, 161.87. HRMS (ESI+): found 191.08137 (Calcd. for C10H11O2N2 [M+H]+: 191.08150).
Methyl 7-amino-4-methoxy-1H-indole-2-carboxylate (16f): pale yellow solid, yield, 70%. m.p. 184-185 °C. 1H NMR (500 MHz, CDCl3): δ 3.89-3.99 (m, 8H), 6.32 (d, 1H, J=8.0 Hz), 6.64 (d, 1H, J=8.0 Hz), 7.47 (d, 1H, J=8.0 Hz), 9.04 (s, 1H). HRMS (ESI+): found 221.09209 (Calcd. for C11H13O3N2 [M+H]+: 221.09207).
Methyl 7-amino-4-ethoxy-1H-indole-2-carboxylate (17f): pale yellow solid, yield, 75%. m.p. 138–140 °C. 1H NMR (500 MHz, CDCl3): δ 1.44-1.47 (t, J=7.0 Hz, 3H), 3.94 (s, 3H), 4.10 (q, 2H, J=7.0 Hz), 6.33 (d, 1H, J=8 Hz), 6.59 (d, 1H, J=8.0 Hz), 7.35 (s, 1H), 8.92 (s, 1H). HRMS (ESI+): found 235.10787 (Calcd. for C12H15O3N2 [M+H]+: 235.10772).
Methyl 7-amino-4-propoxy-1H-indole-2-carboxylate (18f): pale yellow solid, yield, 76%. m.p. 130–132 °C. 1H NMR (500 MHz, CDCl3): δ 1.05–1.09 (t, 3H, J=7.0 Hz), 1.82-1.89 (m, 2H), 3.95 (s, 3H), 3.98–4.01 (t, 2H, J=6.5 Hz), 6.34 (d, 1H, J=8.0 Hz), 6.59 (d, 1H, J=7.5 Hz), 7.34 (s, 1H), 8.96 (s, 1H). HRMS (ESI+): found 249.12349 (Calcd. for C13H17O3N2 [M+H]+: 249.12337).
4.2. Antiviral assays
Madin-Darby Canine Kidney (MDCK) cells and Coxsackie viruses (Cox B3 Nancy strain) were purchased from ATCC. Influenza A strains were all obtained from the Institute of Virology, Chinese Academy of Preventive Medicine.
4.2.1. Anti-Coxsackie B3 activity assays
Confluent Vero cells grown in 96-well plates were infected with a median tissue culture infective dose of 100 (100TCID50) Cox B3 viruses. After 1 h of viral adsorption at 37 °C, the monolayers were washed with phosphate buffered saline (PBS) and incubated at 37 °C in the maintenance medium (MEM+2% fetal bovine serum (FBS)) with or without different concentrations of test compounds. Viral cytopathic effect (CPE) was measured when the viral control group reached a level of 4 and the antiviral activity of test compounds was determined by the Reed and Muench analyses.
4.2.2. Anti-influenza assays
Confluent MDCK cells grown in 96-well microplates were infected with influenza A at a median tissue culture infective dose TCID50 of 100. After 2 h of viral adsorption at 37 °C, the monolayers were washed with PBS and incubated at 37 °C in the maintenance medium with or without different concentrations of test compounds. Viral cytopathic effect (CPE) was measured when the viral control group reached a value of 4 and the antiviral activities of the synthesized compounds were determined by Reed and Muench analyses.
4.2.3. Anti-HSV assays in vitro
Confluent Vero cells grown in 96-well microplates were infected with 100TCID50 HSV-1 virus respectively. HEL cells were infected with HCMV. Tests were performed as follows: After 1hr adsorption at 37 °C, the monolayer cells were washed by PBS and incubated at 37 °C in the maintenance medium (MEM+2% FBS) with or without different concentrations of test compounds or positive control drug. CPE was observed when viral control group reached 4+ and the antiviral activity of compound was determined by the Reed & Muench analyses.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (No. 81273439) and the National Mega-project for Innovative Drugs (No. 2012ZX09301002-001-024-02).
Footnotes
Peer review under responsibility of Institute of Materia Medica, Chinese Academy of Medical Sciences and Chinese Pharmaceutical Association.
Contributor Information
Yuhuan Li, Email: yuhuanlibj@126.com.
Zhuorong Li, Email: l-z-r@263.net.
References
- 1.Pinkert S., Klingel K., Lindig V., Dörner A., Zeichhardt H., Spiller O.B. Virus–host coevolution in a persistently coxsackievirus B3-infected cardiomyocyte cell line. J Virol. 2011;85:13409–13419. doi: 10.1128/JVI.00621-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Calatayud L., Lackenby A., Reynolds A., McMenamin J., Phin N.F., Zambon M. Oseltamivir-resistant pandemic (H1N1) 2009 virus infection in England and Scotland, 2009–2010. Emerg Infect Dis. 2011;17:1807–1815. doi: 10.3201/eid1710.110117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bright R.A., Medina M.J., Xu X. Incidence of adamantine resistance among influenza A (H3N2) viruses isolated worldwide from 1994 to 2005: a cause for concern. Lancet. 2005;366:1175–1181. doi: 10.1016/S0140-6736(05)67338-2. [DOI] [PubMed] [Google Scholar]
- 4.Deyde V.M., Xu X., Bright R.A. Surveillance of resistance to adamantanes among influenza A (H3N2) and A (H1N1) viruses isolated worldwide. J Infect Dis. 2007;196:249–257. doi: 10.1086/518936. [DOI] [PubMed] [Google Scholar]
- 5.Hurt A.C., Lee R.T., Leang S.K., Cui L., Deng Y.M., Phuah S.P. Increased detection in Australia and Singapore of a novel influenza A(H1N1)2009 variant with reduced oseltamivir and zanamivir sensitivity due to a S247N. Euro Surveill. 2011;16:19884. [PubMed] [Google Scholar]
- 6.Hurt A.C., Holien J.K., Parker M., Kelso A., Barr I.G. Zanamivir-resistant influenza viruses with a novel neuraminidase mutation. J Virol. 2009;83:10366–10373. doi: 10.1128/JVI.01200-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Relman D.A., Choffnes E.R., Mack A. National Academies Press; Washington, DC: 2010. The domestic and international impacts of the 2009-H1N1 influenza a pandemic: global challenges, global solutions, workshop summary. [PubMed] [Google Scholar]
- 8.Workman P. Overview: translating HSP 90 biology into HSP 90 drugs. Curr Cancer Drug Target. 2003;3:297–300. doi: 10.2174/1568009033481868. [DOI] [PubMed] [Google Scholar]
- 9.Whitesell L., Lindquist S.L. HSP 90 and the chaperoning of cancer. Nat Rev Cancer. 2005;5:761–772. doi: 10.1038/nrc1716. [DOI] [PubMed] [Google Scholar]
- 10.Sasaki K., Yasuda H., Onodera K. Growth inhibition of virus transformed cells in vitro and antitumor activity in vivo of geldanamycin and its derivatives. J Antibiot. 1979;32:849–851. doi: 10.7164/antibiotics.32.849. [DOI] [PubMed] [Google Scholar]
- 11.Bedard K.M., Wang M.L., Proll S.C., Loo Y.M., Katze M.G., Gale M. Isoflavone agonists of IRF-3 dependent signaling have antiviral activity against RNA viruses. J Virol. 2012;86:7334–7344. doi: 10.1128/JVI.06867-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Richman D.D. Antiviral drug resistance. Antivir Res. 2006;71:117–121. doi: 10.1016/j.antiviral.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 13.Colman P.M. New antivirals and drug resistance. Annu Rev Biochem. 2009;78:95–118. doi: 10.1146/annurev.biochem.78.082207.084029. [DOI] [PubMed] [Google Scholar]
- 14.Krepstakies M., Luciflra J., Naqel C.H., Zeisel M.B., Holstermann B., Hohenberq H. A new class of synthetic peptide inhibitors blocks attachment and entry of human pathogenic viruses. J Infect Dis. 2012;205:1654–1664. doi: 10.1093/infdis/jis273. [DOI] [PubMed] [Google Scholar]
- 15.ElSawy K.M., Twarock R., Verma C.S., Caves L.S. Peptide inhibitors of viral assembly: a novel route to broad-spectrum antivirals. J Chem Inform Model. 2012;52:770–776. doi: 10.1021/ci200467s. [DOI] [PubMed] [Google Scholar]
- 16.Peng Z.G., Zhao Z.Y., Li Y.P., Wang Y.P., Hao L.H., Fan B. Host APOBEC3G is an innate defensive factor and drug target against hepatitis C virus. Hepatology. 2011;53:1080–1089. doi: 10.1002/hep.24160. [DOI] [PubMed] [Google Scholar]
- 17.Shan G.Z., Peng Z.G., Li Y.H., Li D., Li Y.P., Meng S. A novel class of geldanamycin analogues as HCV replication inhibitors targeting Hsp90: synthesis, structure-activity relationships, and anti-HCV activity in GS4.3 replicon cells. J Antibiot. 2011;64:177–182. doi: 10.1038/ja.2010.161. [DOI] [PubMed] [Google Scholar]
- 18.Li Y.P., Shan G.Z., Peng Z.G., Zhu J.H., Meng S., Zhang T. Synthesis and biological evaluation of HSP90 inhibitors geldanamycin derivatives with broad antiviral activities. Antiviral Chem Chemother. 2010;20:259–268. doi: 10.3851/IMP1631. [DOI] [PubMed] [Google Scholar]
- 19.Mackenzie A.R., Moody C.J., Rees C.W. Synthesis of the bacterial coenzyme methoxatin. Tetrahedron. 1986;42:3259–3268. [Google Scholar]
- 20.Corey E.J., Schmidt G. Useful procedures for the oxidation of alcohols involving pyridinium dichromate in approtic media. Tetrahedron Lett. 1979;20:399–402. [Google Scholar]