Abstract
Human enterovirus 71 (EV71) is the main causative pathogen of hand, foot, and mouth disease (HFMD) in children. The epidemic of HFMD has been a public health problem in Asia-Pacific region for decades, and no vaccine and effective antiviral medicine are available. Curcumin has been used as a traditional medicine for centuries to treat a diversity of disorders including viral infections. In this study, we demonstrated that curcumin showed potent antiviral effect again EV71. In Vero cells infected with EV71, the addition of curcumin significantly suppressed the synthesis of viral RNA, the expression of viral protein, and the overall production of viral progeny. Similar with the previous reports, curcumin reduced the production of ROS induced by viral infection. However, the antioxidant property of curcumin did not contribute to its antiviral activity, since N-acetyl-l-cysteine, the potent antioxidant failed to suppress viral replication. This study also showed that extracellular signal-regulated kinase (ERK) was activated by either viral infection or curcumin treatment, but the activated ERK did not interfere with the antiviral effect of curcumin, indicating ERK is not involved in the antiviral mechanism of curcumin. Unlike the previous reports that curcumin inhibited protein degradation through ubiquitin–proteasome system (UPS), we found that curcumin had no impact on UPS in control cells. However, curcumin did reduce the activity of proteasomes which was increased by viral infection. In addition, the accumulation of the short-lived proteins, p53 and p21, was increased by the treatment of curcumin in EV71-infected cells. We further probed the antiviral mechanism of curcumin by examining the expression of GBF1 and PI4KB, both of which are required for the formation of viral replication complex. We found that curcumin significantly reduced the level of both proteins. Moreover, the decreased expression of either GBF1 or PI4KB by the application of siRNAs was sufficient to suppress viral replication. We also demonstrated that curcumin showed anti-apoptotic activity at the early stage of viral infection. The results of this study provide solid evidence that curcumin has potent anti-EV71 activity. Whether or not the down-regulated GBF1 and PI4KB by curcumin contribute to its antiviral effect needs further studies.
KEY WORDS: Curcumin, Enterovirus 71, Viral replication, GBF1, PI4KB, Ubiquitin–proteasome system, Apoptosis
Abbreviations: CVB, coxsackieviurs B; DCFH-DA, dichloro-dihydro-fluorescein diacetate; ERK, extracellular signal-regulated kinase; EV71, enterovirus 71; GBF1, Golgi brefeldin A resistant guanine nucleotide exchange factor 1; GEF, guanine nucleotide exchange factor; HBV, hepatitis B virus; HCV, hepatitis C virus; HFMD, hand, foot, and mouth disease; HIV, human immunodeficiency virus; HPV, human papillomavirus; NAC, N-acetyl-l-cysteine; PARP-1, poly(ADP-ribose) polymerase; PGC-1α, peroxisome proliferator-activated receptor-gamma co-activator 1 alpha; p.i., post-infection; PI4KB, phosphatidylinositol 4-kinase class III catalytic subunit β; PI4P, phosphatidylinositol 4-phosphate; ROS, reactive oxygen species; siRNA, small interfering RNA; SLLVY-AMC, succinyl-Leu-Leu-Val-Tyr-7-amino-4-methylcoumarin; UPS, ubiquitin–proteasome system
Graphical abstract
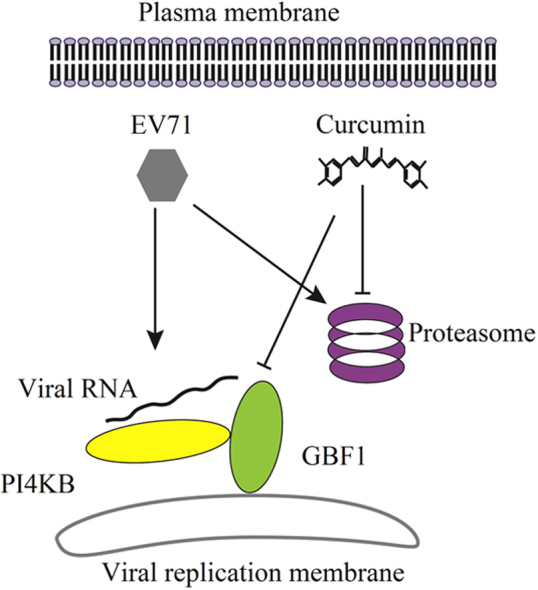
Curcumin shows potent anti EV71 effect through down-regulating GBF1 and PI4KB, both of which may required for the assembly of viral RNA replication complex. The knockdown of both GBF1 and PI4KB is sufficient to reduce EV71 replication. Curvumin׳s inhibition on the activity of proteasomes also contributes to its antiviral activity, while the functional proteasomes are required for EV71 replication.
1. Introduction
Enterovirus 71 (EV71) is a single stranded RNA virus which belongs to genus Enterovirus, family Picornaviridae1. EV71 infection is often asymptomatic or causes mild manifestations such as fever, sore throat and general malaise2. However, it may cause severe and sometimes fatal complications such as encephalitis and pulmonary edema. More importantly, outbreak of EV71 infection has become a public health problem especially in the Asia-Pacific region2, 3, 4. However, since there is no specific anti-EV71 vaccine or medicine available, treatment for the patients with EV71 infection is simply of supportive5. Therefore, to identify the effective anti-EV71 drugs is urgent.
Curcumin is a natural compound extracted from turmeric (Curcuma longa). It has been used widely as a spice and coloring agent in food6. In addition, curcumin is a constituent of many herbal medicines used for diverse medical conditions7, 8, 9. In recent years, extensive studies have shown that curcumin has a variety of pharmacological properties, including anti-inflammation, antitumor and antioxidant10, 11, 12. Accumulating evidence has also demonstrated that curcumin has antiviral activity6, 13, 14, 15, 16, 17. Viruses inhibited by the treatment of curcumin include hepatitis C virus (HCV), hepatitis B virus (HBV), coxsackieviurs B3 (CVB3) and human papillomavirus (HPV)16, 18, 19, 20. It has been reported that curcumin suppresses CVB3 replication via the dysregulation of the ubiquitin-proteasome system (UPS)20. Other studies have shown that the inhibitory effect of curcumin on HCV replication is associated with the suppression of AKT and the inhibition on viral entry13, 18, while curcumin inhibits HBV replication via down-regulation of peroxisome proliferator-activated receptor-gamma co-activator 1 alpha (PGC-1α), a major metabolic co-activator of HBV16. Overall, these findings suggested that curcumin may directly block the replication machinery of viruses or regulate cellular metabolic or signaling pathways which are exploited by viruses.
Although curcumin shows inhibitory effect on a variety of viruses, whether it exerts antiviral effect on EV71 is currently unknown. In the present study, we evaluated potential antiviral activity of curcumin against EV71. To understand the involved mechanisms of the effect of curcumin on viral replication, the influence of curcumin on reactive oxygen species (ROS), apoptosis, extracellular signal-regulated kinase (ERK) pathway and UPS were observed. The expression of phosphatidylinositol 4-kinase class III catalytic subunit β (PI4KB) and Golgi brefeldin A resistant guanine nucleotide exchange factor 1 (GBF1) were also studied.
2. Materials and methods
2.1. Chemical reagents and antibodies
Curcumin, which was dissolved in DMSO before use, and N-acetyl-l-cysteine (NAC) were purchased from Sigma-Aldrich (St. Louis, USA). MG132 and the Reactive Oxygen Species Assay Kit were obtained from Beyotime (China). The fluorimetric substrate succinyl-Leu-Leu-Val-Tyr-7-amino-4-methylcoumarin (SLLVY-AMC) was obtained from MERCK. GBF1 small interfering RNA (siRNA), siRNA of PI4KB, and the scramble control siRNA were synthesized by Takara (Dalian, China).
The monoclonal anti-enterovirus VP1 antibody was purchased from DakoCytomation (clone5-D8/1, Denmark). Anti-PI4KB and anti-GBF1 antibodies were from Becton Dickson. Anti-ERK1/2 and anti-phospho-ERK1/2 antibodies were obtained from Cell Signaling (Danvers, USA). Anti-cleaved caspase 3, antibody against the poly(ADP-ribose) polymerase (PARP-1), and anti-p53 antibody were purchased from Santa Cruz (Dallas, USA). Anti-β-actin and the horseradish peroxidase-conjugated secondary antibodies were obtained from Zhongshan Golden Bridge (Beijing, China).
2.2. Cell culture
Vero cells and RD cells (a human rhabdomyosarcoma cell line) were maintained in the Department of Microbiology, Harbin Medical University. Cells were cultured in complete Dulbecco׳s modified Eagle medium (DMEM, Invitrogen, China) supplemented with 10% fetal bovine serum (FBS) and antibiotics (penicillin and streptomycin) at 37 °C with 5% CO2. Vero cells were used for the studies on EV71 infection and the effect study. RD cells were used for siRNA transfection.
2.3. Viral infection
EV71 BrCr strain was kindly offered by Professor Mingli Wang, Department of Microbiology, Anhui Medical University, China. Virus was propagated in Vero cells cultured in DMEM supplemented with 10% FBS and antibiotics (50 U/mL penicillin and 50 mg/mL streptomycin). Cells were cultured to 80% confluence, and then infected with EV71 at a multiplicity of infection (MOI) of 10 or sham-infected with serum-free DMEM for 1 h. The media were removed and cells were cultured in complete DMEM till harvest. To determine the effect of curcumin on viral infection, cells were cultured in DMEM containing curcumin at various concentrations after EV71 infection.
2.4. Virus titration
Virus titer was assessed by 50% tissue culture infectivity dose (TCID50) method according to the procedure described previously21, 22. Briefly, virus was propagated in Vero cells till 80% CPE was reached. Cells were harvested and viruses were released by freezing and thawing for 3 times. Cell debris was removed by centrifugation and the supernatant was stored at −80 °C. To titer the viruses, Vero cells were grown in 96-well plates at the density of 1×104 cells/well. Virus was prepared in a series of 10-fold dilution with serum-free DMEM. 100 μL of virus preparation at each dilution was added to 3 repeated wells, respectively. After 1 h of absorption, the culture medium was added and cells were cultured for 24 h. Then TCID50 was calculated.
2.5. Reverse transcription-quantitative polymerase chain reaction
Viral RNA was determined by reverse transcription-quantitative polymerase chain reaction (RT-qPCR) as described previously23. In brief, total RNA was extracted with TRIzol (Takara, China). RT-PCR was performed with forward primer 5′-CCCCTGAATGCGGCTAAT-3′ and reverse primer 5′-CAATTGTCACCATAAGCAGCCA-3′. The RNA of GAPDH was amplified as internal control with the forward primer 5′-CTCTGCTCCTCCTGTTCGAC-3′ and reverse primer 5′-TTAAAAGCAGCCCTGGTGAC-3′. The reverse transcription was performed at 42 °C for 30 min and followed by 40 cycles of thermal cycling at 95 °C for 3 s and 60 °C for 30 s. The fluorescence emission of SYBR green I was monitored and analyzed by using a LightCycler 2.0 (Roche, Basel, Switzerland).
2.6. siRNA transfection
RD cells were grown in 24-well plates to 50% confluence. Cells were transfected with siRNA of GBF1 or PI4KB (designated as si-GBF1, and si-PI4KB, respectively) by Lipofectamine 2000 (Invitrogen). The sequence of si-GBF1 and si-PI4KB were 3′-CAACACACCUACUAUCUCU-5′ and 3′-GGGAUGACCUUCGGCAAGAUU-5′, respectively. 48 h after transfection, cells were incubated in serum-free DMEM without antibiotics for 1 h and then cultured in DMEM with 10% FBS.
2.7. Western blotting
Vero cells were washed with PBS and incubated with RIPA lysis buffer (Thermo, Rockford, USA) containing protease inhibitor PMSF (Beyotime, Beijing, China) for 15 min on ice. Cell homogenate was centrifuged at 12,000g for 10 min at 4 °C. Protein concentration of the cellular homogenate was determined by Bradford assay (Bio-Rad, Hercules, USA). Equal amount of proteins was subjected to SDS–PAGE and then transferred to PVDF membrane. The membrane was blocked by 5% skim milk for 4 h at 37 °C and then incubated with primary antibody at 4 °C overnight. Membrane was washed and then incubated with secondary antibody conjugated with horseradish peroxidase (HRP) for 1 h at 37 °C. Immunoreactive bands were visualized by staining the membrane with Super Signal West Pico (Thermo, USA).
2.8. Reactive oxygen species assay
ROS was detected by the fluorimetric probe dichloro-dihydro-fluorescein diacetate (DCFH-DA) according to the protocol provided by the manufacturer. Briefly, Vero cells were cultured to 80% confluence in 24-well plates with the density of 5×104 cell/well. The culture media were removed and the cells were incubated in 500 μL serum-free DMEM containing DCFH-DA at 10 μmol/L for 1 h. Fluorescence was observed in microscope.
2.9. Proteasome activity
The chymotrypsin-like activity of the 20S proteasome was determined by using the fluorogenic substrate SLLVY-AMC as described previously20. Briefly, cell lysates were prepared as described above without treatment of protease inhibitor. Fresh cytoplasmic proteins were extracted from Vero cells and the concentrations of the proteins were determined. 10 μL of cytoplasmic protein was incubated with 75 μmol/L fluorogenic substrate SLLVY-AMC in final volume of 100 μL assay buffer (20 mmol/L Tris–HCl, pH 8.0, 1 mmol/L ATP and 2 mmol/L MgCl2) for 1 h at 30 °C in a 96-well microplate. The fluorescence product AMC was determined by a microplate reader at an emission wavelength of 465 nm. The relative activity of the proteasome was normalized to the concentration of the cytoplasmic protein.
2.10. Statistical analysis
The results of experiments are shown as average with standard deviation. Paired t-tests were performed. P values less than 0.05 were considered significant differences and are indicated by asterisks in the figures.
3. Results
3.1. Curcumin inhibits EV71 replication
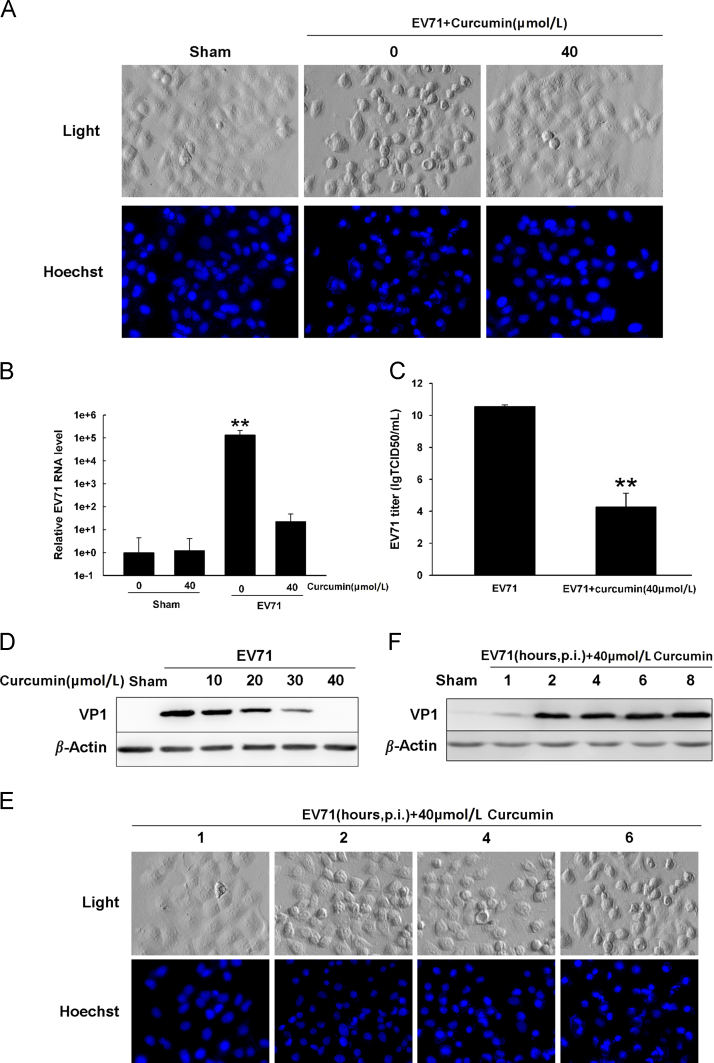
Previous studies have shown that curcumin has antiviral activities against human immunodeficiency virus (HIV), herpes simplex virus, HCV and CVB319, 20, 24, 25. In this study, we evaluated the effect of curcumin on the replication of EV71 in vitro. Vero cells were infected with EV71 at MOI=10 and cultured for 8 h in the medium containing 40 μmol/L curcumin which was added at 1 h after post-infection (p.i.). We observed that EV71 infection induced a typical cytopathic effect of rounded cell shape and condensed nuclei. However, EV71-infected cells treated with curcumin had no significant morphological alterations compared with the uninfected cells (Fig. 1A). Quantitative real-time PCR and Western blot analyses showed that curcumin significantly decreased the production of EV71 RNA and almost completely abolished the synthesis of viral proteins (Fig. 1B and D). Virus yield declined about 106-fold in curcumin-treated cells compared with that in untreated cells (Fig. 1C).
Figure 1.
Curcumin inhibits EV71 replication. (A) Cells were infected with EV71 and cultured in the medium containing 40 μmol/L curcumin for 7 h, after which were stained with Hoechst33342 to view the nuclei. (B) Cells were treated as described in (A). EV71 RNA level was determined by RT-qPCR and normalized to the RNA level of GAPDH. **P<0.01 compared with uninfected cells. (C) Cells were treated as described in (A) and virus titer was determined by TCID50. **P<0.01 compared with EV71-infected cells without curcumin treatment. (D) EV71-infected cells were treated with curcumin at various concentrations. Proteins were extracted and western blot analysis was performed with anti-VP1 antibody. (E) Cells were infected with EV71 for 8 h and curcumin was added to the culture medium at various time points after p.i. Cells were stained with Hoechst33342 to view the nuclei. (F) Cells were infected with EV71 for 8 h and curcumin was added to the culture medium at various time points after p.i. Proteins were extracted and VP1 was analyzed by western blotting. Error bars show standard deviations. n=4. Results are representative of three independent infection experiments.
To determine the stage of viral replication that was affected by curcumin, cells were infected with EV71 (MOI=10) and cultured in the medium containing curcumin. We found that CPE was suppressed when curcumin was added to the medium shortly after viral infection (at 1 h after p.i.) (Fig. 1E). Correspondingly, the expression of VP1 was also significantly inhibited when cells were treated with curcumin at 1 h after p.i.. These data indicate that curcumin could effectively inhibit EV71 replication when it was applied at the early stage of EV71 replication.
3.2. The anti-EV71 effect of curcumin does not depend on its antioxidant activity
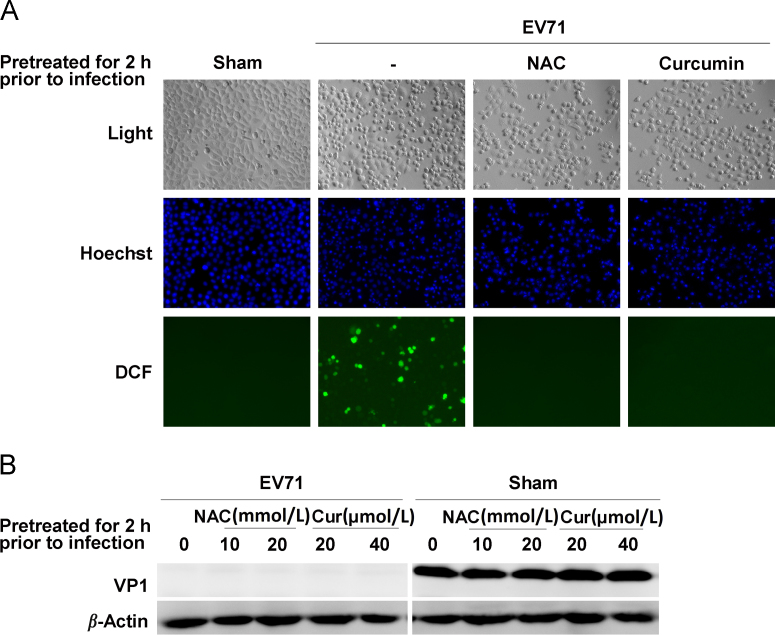
Accumulating evidence suggests that oxidative stress affects the interaction between viruses and cells by providing a favorite cellular environment for the replication of viral pathogens26, 27. Since curcumin is a natural phenolic compound with impressive antioxidant property27, 28, 29, 30, we investigated whether the antioxidant property of curcumin is involved in its antiviral activity. Cells were pretreated with curcumin for 2 h and then infected with EV71 (MOI=10) for 8 h in the media with no curcumin supplement. The production of ROS was measured by staining the cells with DCFH-DA. As shown in Fig. 2, EV71 infection induced a significant increase in the production of ROS, while ROS production was blocked by the pretreatment of curcumin (Fig. 2A). Pretreatment with curcumin or the potent antioxidant NAC did not block CPE in EV71-infected cells (Fig. 2A). Western blot analysis showed that pretreatment with either curcumin or NAC did not inhibit the synthesis of VP1 (Fig. 2B). These data show that curcumin pretreatment did not protect the cells against EV71 infection, even thought the production of ROS was inhibited. Thus, the antioxidant property of curcumin did not endow the anti-EV71 capability to the cells. These results indicate that the antiviral effect of curcumin does not depend on its antioxidant property.
Figure 2.
The antiviral effect of curcumin does not depend on its antioxidant activity. (A) Cells were pretreated with curcumin or NAC for 2 h, and then were infected with EV71 and cultured in the media with no curcumin or NAC supplement for 8 h. Cells were stained with DCFH-DA to view the production of ROS. Cell nuclei were stained with Hoechst33342. (B) Cells were pretreated with curcumin or NAC at various concentrations for 2 h and then infected with EV71 for 8 h in the media with no curcumin or NAC supplement. VP1 was analyzed by western blotting. Results are representative of three independent infection experiments.
3.3. The antiviral effect of curcumin is not related with the activation of ERK
It has been reported that MAPK-ERK signaling pathway is required for the replication of EV71. EV71 replication was significantly decreased when mitogen activated protein kinase (MAPK) pathway was inhibited by siRNA or specific inhibitory drug31, 32, 33. In addition, previous studies suggested that curcumin could also affect MAPK pathway34, 35, 36. To investigate whether the antiviral mechanism of curcumin is related with its influence on ERK, we analyzed the alteration of ERK1/2 in EV71-infected cells treated with curcumin. Cells were infected with EV71 and curcumin at various concentrations was added to the media at 1 h after p.i. As shown in Fig. 3, curcumin treatment alone significantly increased ERK1/2 phosphorylation, while EV71 infection further elevated the levels of the phosphorylated ERK1/2. In the meantime, VP1 expression was significantly decreased by the treatment of curcumin at 20 and 40 μmol/L. These results show that both curcumin treatment and EV71 infection promotes the activation of ERK1/2. These data also indicate that the anti-EV71 activity of curcumin overcomes the effect of ERK activation, which might be beneficial to the replication of EV71. In other words, ERK activation was not involved in the antiviral mechanism of curcumin.
Figure 3.
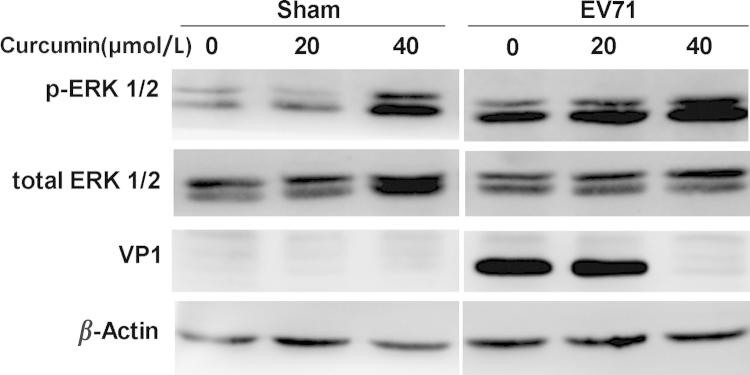
The antiviral effect of curcumin was not related with the activated ERK. Cells were infected with EV71 for 8 h and curcumin was added to the culture medium at 1 h after p.i. Cell lysates were probed by the antibodies against VP1, ERK1/2 and phosphorylated ERK1/2 (p-ERK1/2) in western blot analysis. Results are representative of three independent infection experiments.
3.4. Curcumin suppresses the activity of ubiquitin-proteasome during EV71 infection
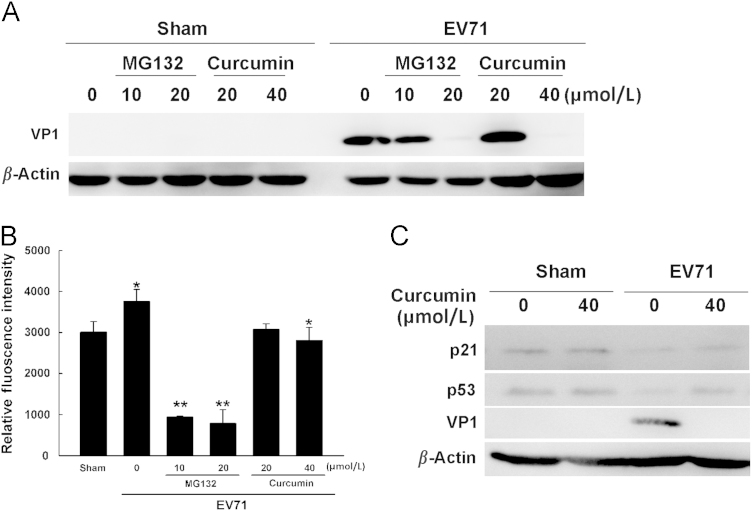
Studies in the recent years have demonstrated that UPS can also be re-directed to favor viral replication37, 38, 39, 40, 41. In addition, curcumin has been reported to inhibit the production of Japanese encephalitis virus and dengue virus by dysregulating UPS42, 43. To study whether UPS is involved in the antiviral effect of curcumin, cells were infected with EV71 for 8 h and 40 μmol/L curcumin was added to the culture medium at 1 h after p.i. The activity of proteasomes was determined at 5 h of p.i. The levels of p53 and p21 were also determined since both proteins are degraded through UPS. As shown in Fig. 4, VP1 expression was significantly inhibited by the treatment of curcumin or MG132 (Fig. 4A). Since MG132 directly suppresses the activity of proteasomes, these data indicate that functional proteasome is required for EV71 replication, and inhibiting the activity of proteasomes is sufficient to block viral replication. We further measured the in vitro activity of proteasomes in virus-infected cells. We observed that the activity of proteasomes was increased by EV71 infection, while it was reduced by the treatment of curcumin in virus-infected cells (Fig. 4B). Correspondingly, viral infection promoted the degradation of p53 and p21, while the levels of both proteins were increased by the treatment of curcumin in virus-infected cells (Fig. 4C). However, curcumin per se did not alter the level of p53 and p21 (Fig. 4C) in sham-infected cells, indicating that curcumin has no impact on UPS in normal cells. These data imply that the inhibitory effect of curcumin on UPS during EV71 infection might be the result of the suppressed viral replication.
Figure 4.
Curcumin suppresses the activity of ubiquitin-proteasome during EV71 infection. (A) Cells were infected with EV71 for 8 h. Curcumin or MG132 was added to the culture medium at 1 h after p.i. VP1 was analyzed by western blotting. (B) Cells were treated as described in (A). At 5 h after p.i., the activity of the proteasomes was determined. *P<0.05 compared with sham-infected cells or the cells infected with EV71 without treatment; **P<0.01 compared with the cells infected with viruses without treatment. (C) Cells were infected with EV71 for 8 h and curcumin was added to the culture medium at 1 h after p.i. The levels of p53, p21 and VP1 were determined by western blotting. Error bars represent standard deviations. Results are representative of three independent infection experiments.
3.5. Curcumin down-regulates GBF1 and PI4KB during EV71 infection
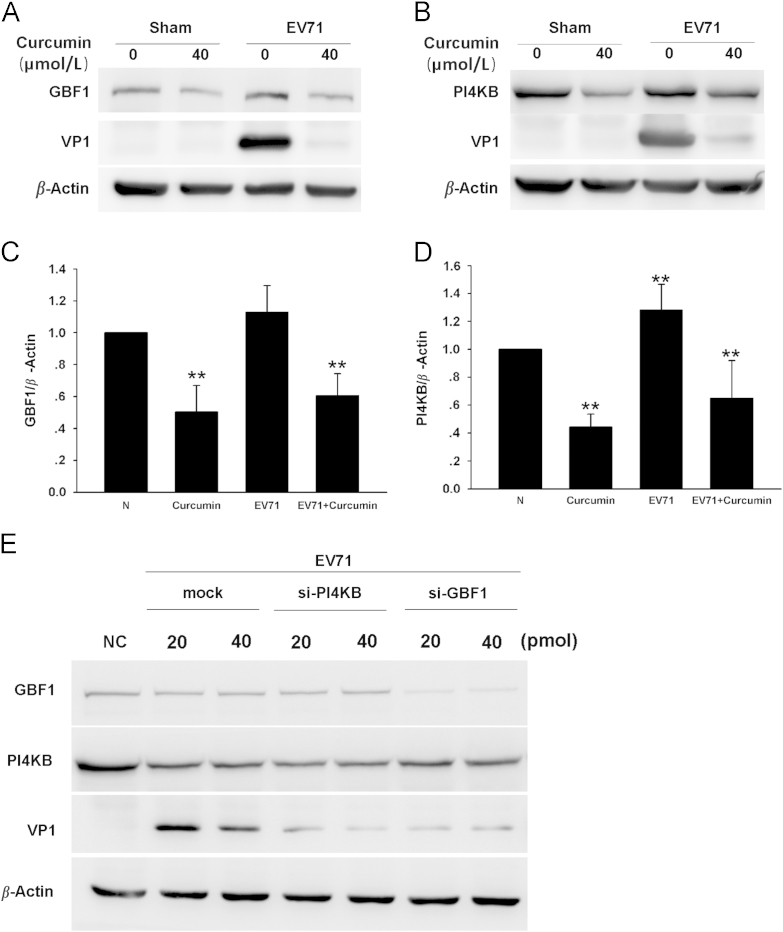
Studies have demonstrated that CVB3 uses its non-structural protein 3A to form replication complex. The assembly of the viral replication complex is facilitated by a guanine nucleotide exchange factor, GBF144, 45, 46. Moreover, to form the replication complex, cytoplasmic membrane containing specific phospholipid, phosphatidylinositol 4-phosphate (PI4P), is also indispensible for the recruitment of viral RNA-dependent RNA polymerase47, 48, 49. Since PI4KB is the enzyme that catalyzes the formation of PI4P, it is also required for the replication of EV7148, 50. In this study, we studied whether curcumin achieves its anti-EV71 effect by influencing GBF1 or PI4KB. RD cells were infected with EV71 and cultured in the medium containing 40 μmol/L curcumin for 8 h. Western blot analysis showed that curcumin treatment significantly decreased the expression of both GBF1 and PI4KB in the cells infected with EV71 as well as in control cells (Fig. 5A–D). The reduced level of both GBF1 and PI4KB might be the results of decreased synthesis or increased degradation of these proteins. Our results also show that EV71 infection significantly increased the level of PI4KB, while the level of GBF1 was not altered (Fig. 5C and D). Since EV71 promotes the activity of proteasomes (Fig. 4), the increased PI4KB level was likely due to promoted protein synthesis rather than its inhibited degradation. The treatment of curcumin significantly decreased the levels of GBF1 and PI4KB in EV71-infected cells (Fig. 5A–D). These data indicate that GBF1 and PI4KB are among the target proteins that are influenced by curcumin, indicating its down-regulatory effect on GBF1 and PI4KB.
Figure 5.
Curcumin down-regulates GBF1 and PI4KB during EV71 infection. (A) Cells were infected with EV71 for 8 h and curcumin was added to the culture medium at 1 h after p.i. Cell lysates were probed with antibody against GBF1 and VP1. (B) Cells were treated as described in (A). Cell lysates were probed by antibodies against PI4KB and VP1. (C) and (D) The relative quantity of GBF1 and PI4KB in the electrophoresis bands in A and B. ** P<0.01 compared with normal cell control (NC). (E) Cells were transfected with siRNA of PI4KB or GBF1 for 48 h and then infected with EV71 for 8 h. The levels of PI4KB, GBF1 and VP1 were determined by western blotting. Error bars show standard deviations. n=4. Results are representative of three independent experiments.
To further study the involvement of GBF1 and PI4KB in the replication of EV71, RD cells were treated with the siRNA of either GBF1 or PI4KB for 48 h and then infected with EV71 for 8 h. Western blot analysis showed that the synthesis of VP1 was significantly declined when the expression of GBF1 or PI4KB was inhibited (Fig. 5E). Taken together, these results indicate that both GBF1 and PI4KB are required for EV71 replication, and the inhibition of either of them would significantly impact viral replication. These data also suggest that GBF1 and PI4KB might be involved in the anti-EV71 mechanism of curcumin.
3.6. Cucumin suppresses apoptosis induced by EV71 infection
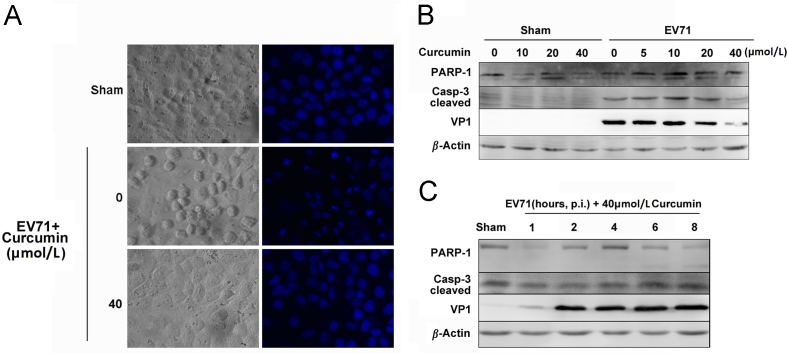
Apoptosis is often targeted by viruses to benefit their replication51, 52, 53, 54. To investigate how curcumin influences virus-induced apoptosis, Vero cells were infected with EV71 and grown in the medium containing curcumin for 8 h. The nuclear morphological alteration was observed by staining cells with Hoechst33342. The activated caspase 3 and PARP-1 were analyzed by western blotting. As shown in Fig. 6, cells infected with EV71 predominantly contained condensed nuclei, while the virus-infected cells treated with 40 μmol/L curcumin showed no significant abnormality in morphology. Western blot analysis showed that the levels of cleaved caspase-3 and PARP-1 were increased by viral infection, indicating that EV71 infection induced apoptosis (Fig. 6B). The levels of both PARP-1 and cleaved caspase-3 were declined by the treatment of 40 μmol/L curcumin (Fig. 6B), indicating that curcumin suppressed the apoptosis induced by viral infection. However, curcumin treatment did not alter the levels of both proteins in sham-infected cells (Fig. 6B), indicating that curcumin has no impact on apoptosis when it is applied to normal cells. We also demonstrated that decreased PARP-1 and cleaved caspase-3 occurred only when curcumin was added to the medium shortly after EV71 infection (at 1 h after p.i.), corresponding to the inhibited expression of VP1 (Fig. 6C). These data collectively indicate that curcumin could suppress the apoptosis induced by EV71 when it is applied at the early stage of viral infection.
Figure 6.
Curcumin suppresses apoptosis induced by EV71 infection. (A) Vero cells were infected with EV71 and grown in the medium containing curcumin for 8 h. Cells were stained with Hoechst33342 to view the nuclei. (B) Cells were infected with EV71 for 8 h and curcumin was added to the culture medium at 1 h after p.i. PARP-1, cleaved caspase 3 and VP1 were determined by western blotting. Cells treated with DMSO were used as controls. (C) Cells were infected with EV71 for 8 h. Curcumin was added to the culture medium at various time points after p.i. PARP-1, cleaved caspase 3 and VP1 were determined by western blotting. Results are representative of three independent infection experiments.
4. Discussion
EV71 is one of the major causative pathogens of hand, foot and mouth disease (HFMD) which may lead to severe and fatal damage of central nervous system, heart failure and pulmonary edema55, 56. Twenty-one fatal cases were reported during the outbreak of HFMD in Guangdong Province of China in 200857. During the outbreaks of HFMD in China, herbal extracts of traditional Chinese medicine have been used as adjuvant treatment and encouraging therapeutic effect against viral infection has been obtained58, 59, 60. The therapeutic antiviral effect of medicinal herbs inspired us to study the anti-EV71 efficacy of curcumin, which has been widely used as an ingredient of traditional medicine around the world. To our knowledge, this is the first study to show that curcumin suppressed EV71 replication in vitro.
As a natural compound, curcumin has been reported to have antiviral activity against influenza virus, Rift Valley Fever virus, CVB and HCV14, 20, 25, 61. In this study, we demonstrated that the application of curcumin at the early stage of EV71 replication significantly suppressed viral RNA synthesis, viral protein expression and the overall viral progeny production. Our data indicate that curcumin has potent anti-EV71 effect in vitro.
Previous evidence suggests that oxidative stress is involved in the pathogenesis of viral infections. Studies showed that increased oxidative stress induced by selenium-deficient diet exacerbated the myocarditis of mice after CVB3 infection62, 63, and disrupting cellular redox balance effectively promoted EV71 replication and cell death64, 65. In addition, attenuated production of ROS reduced the EV71 replication66. In consistent with the reported studies, we found that EV71 infection did induce a significant increase of ROS. However, the inhibited production of ROS in cells treated with curcumin or the ROS scavenger NAC prior to viral infection did not show suppressive effect on both viral replication and cell death. This study indicates that the anitioxidant activity of curcumin is not involved in its antiviral effect. Our study also indicates that targeting ROS alone is not sufficient to suppress EV71 replication. However, further studies are needed to elucidate the role of ROS in EV71 replication.
Previous study showed that the activation of ERK was required for EV71 replication67. On the other hand, curcumin has been reported to target ERK signaling in cancer cells68, 69. Unlike the effect of curcumin on cancer cells, in which p-ERK was decreased by the treatment of curcumin68, 70, the present study demonstrated that ERK was activated by curcumin. Moreover, it seems that curcumin treatment and EV71 infection had synergetic effect on ERK activation. However, EV71 replication was still effectively inhibited by the treatment of curcumin even through ERK was highly active. If the activated ERK is required for EV71 replication as it was previously reported31, 67, our data indicate that ERK activation is not sufficient to support viral replication. We can also infer that ERK pathway is not involved in the anti-EV71 mechanism of curcumin.
Ubiquitin–proteasome system is essential for cells to regulate short-lived proteins involved in critical cellular processes such as DNA replication, cell cycle control and immune reactions71, 72. Previous study showed that curcumin induced the degradation of ErB2 protein by promoting the association of ErB2 with ubiquitin ligase CHIP73, while Ben et al.74 reported that curcumin promoted the degradation of the inducible nitric oxide synthase by up-regulating ubiquitination. The study of Si et al.20 has demonstrated that curcumin suppresses CVB3 replication through inhibiting the activity of proteasomes and the cellular deubiquitinating process. In this study, we found that the addition of proteasome inhibitor MG132 completely blocked EV71 replication. These results indicate that UPS is indispensible for the replication of EV71. However, unlike the previous studies, we found that curcumin showed no impact on UPS in sham-infected cells, but curcumin did suppress the degradation of p53 and p21 in EV71-infected cells. Since we did not study whether or not curcumin could directly influence the activity of proteasomes or the process of unbiquination, here we could not conclude that the accumulation of p53 and p21 is resulted from the direct inhibition of curcumin on UPS. It could also be the consequence of the suppressed viral replication. Therefore, the precise role of UPS in the anti-EV71 mechanism of curcumin remains to be studied further.
The critical role of cytoplasmic membrane in the replication of viruses has been recognized during the past few years48, 75, 76. Studies have shown that RNA viruses such as CVB3, poliovirus and Aichi virus, utilize cytoplasmic membrane to form replication complex48, 75, 76. To form the replication complex, 3A protein of CVB3 is required to bind the cytoplasmic membranes enriched in PI4P, which is converted from phosphatidylinositol by PI4KB. In addition, the binding of CVB3 3A to organelle membranes requires GBF1, a guanine nucleotide exchange factor (GEF). Other studies showed that inhibitor of PI4KB showed significant antiviral effect against poliovirus77, and knockdown of PI4KB could significantly inhibit HCV replication78. Here we found that curcumin showed antiviral effect at the early stage of EV71 infection when viral RNA replication is the major event. Therefore, we proposed that curcumin may suppress EV71 replication by interfering with the formation of viral replication complex. We demonstrated that the expression of both GBF1 and PI4KB were significantly suppressed by curcumin in EV71-infected and in sham-infected cells. More importantly, the decreased expression of GBF1 and PI4KB by siRNAs directly inhibited the replication of EV71, indicating that EV71 replication requires both GBF1 and PI4KB. However, we cannot jump to the conclusion from the present data that the down-regulated GBF1 and PI4KB contribute to the anti-EV71 mechanism of curcumin. The precise role of GBF1 and PI4KB in the antiviral effect of curcumin may be demonstrated by blocking the down-regulatory effect of curcumin on these two proteins. On the other hand, considering the fact that GBF1 and PI4KB are important molecules for the formation of viral replication complex for picornaviruses, it is possible that these two proteins are involved in the anti-EV71 mechanism of curcumin.
Apoptosis is the common target of many types of viruses to promote their replication or viral release. In addition, curcumin has been reported to induce apoptosis in a variety of cancer cell lines48, 50, 69, 79. To evaluate the role of apoptosis in the antiviral mechanism of curcumin, we examined the cellular morphological changes and the activation of enzymes required for apoptosis. We found that apoptosis was induced by EV71 infection. However, unlike the reported promoting effect of curcumin on apoptosis in cancer cells, we found that curcumin had no impact on apoptosis in sham-infected cells, while it significantly suppressed apoptosis in EV71-infected cells. However, whether the inhibited apoptosis is the direct function of curcumin or the consequence of the suppressed viral replication remains unclear. In spite of this, our data still demonstrated that curcumin has antiviral and cellular protective activity in EV71-infected cells.
5. Conclusions
In summary, this study demonstrated that curcumin showed potent antiviral effect against EV71 infection. The antiviral mechanism of curcumin was related with neither its antioxidant activity nor its impact on ERK pathway. The present study also showed that EV71 replication requires GBF1 and PI4KB, and both proteins were down-regulated by the treatment of curcumin in EV71-infected cells. However, the role of GBF1 and PI4KB in the anti-EV71 mechanism of curcumin needs further studies. Furthermore, this study showed that curcumin suppressed UPS and apoptosis in EV71-infected cells. Therefore, curcumin could be a potential anti-EV71 natural compound with both antiviral and cellular protective activities.
Acknowledgments
This work was supported by the grants of National Natural Science Foundation of China to Zhaohua Zhong (Grant No. 81271825) and Wenran Zhao (Grant No. 31270198). We are grateful to professor Mingli Wang, Department of Microbiology, Anhui Medical University, China, for providing EV71 BrCr strain. We thank Heilongjiang Provincial Key Laboratory of Pathogens and Immunity, and Heilongjiang Provincial Science and Technology Innovation Team in Higher Education Institutes for Infection and Immunity in Harbin Medical University, for technical support. Northern Translational Medicine Research Center of Harbin Medical University also provided technical support for this work.
Footnotes
Peer review under responsibility of Institute of Materia Medica, Chinese Academy of Medical Sciences and Chinese Pharmaceutical Association.
Contributor Information
Wenran Zhao, Email: zhaowr@ems.hrbmu.edu.cn.
Zhaohua Zhong, Email: zhongzh@hrbmu.com.cn.
References
- 1.Liu L., Zhao H., Zhang Y., Wang J., Che Y., Dong C. Neonatal rhesus monkey is a potential animal model for studying pathogenesis of EV71 infection. Virology. 2011;412:91–100. doi: 10.1016/j.virol.2010.12.058. [DOI] [PubMed] [Google Scholar]
- 2.Yip C.C., Lau S.K., Lo J.Y., Chan K.H., Woo P.C., Yuen K.Y. Genetic characterization of EV71 isolates from 2004 to 2010 reveals predominance and persistent circulation of the newly proposed genotype D and recent emergence of a distinct lineage of subgenotype C2 in Hong Kong. Virol J. 2013;10:222. doi: 10.1186/1743-422X-10-222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liang Z.L., Mao Q.Y., Wang Y.P., Zhu F.C., Li J.X., Yao X. Progress on the research and development of inactivated EV71 whole-virus vaccines. Hum Vaccin Immunother. 2013;9:1701–1705. doi: 10.4161/hv.24949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mao Q., Dong C., Li X., Gao Q., Guo Z., Yao X. Comparative analysis of the immunogenicity and protective effects of inactivated EV71 vaccines in mice. PLoS One. 2012;7:e46043. doi: 10.1371/journal.pone.0046043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Premanand B., Kiener T.K., Meng T., Tan Y.R., Jia Q., Chow V.T. Induction of protective immune responses against EV71 in mice by baculovirus encoding a novel expression cassette for capsid protein VP1. Antiviral Res. 2012;95:311–315. doi: 10.1016/j.antiviral.2012.05.017. [DOI] [PubMed] [Google Scholar]
- 6.Chen T.Y., Chen D.Y., Wen H.W., Ou J.L., Chiou S.S., Chen J.M. Inhibition of enveloped viruses infectivity by curcumin. PLoS One. 2013;8:e62482. doi: 10.1371/journal.pone.0062482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yang C., Wu K., Li S.H., You Q. Protective effect of curcumin against cardiac dysfunction in sepsis rats. Pharm Biol. 2013;51:482–487. doi: 10.3109/13880209.2012.742116. [DOI] [PubMed] [Google Scholar]
- 8.Wang Y., Yin H., Wang L., Shuboy A., Lou J., Han B. Curcumin as a potential treatment for Alzheimer׳s disease: a study of the effects of curcumin on hippocampal expression of glial fibrillary acidic protein. Am J Chin Med. 2013;41:59–70. doi: 10.1142/S0192415X13500055. [DOI] [PubMed] [Google Scholar]
- 9.Kurita T., Makino Y. Novel curcumin oral delivery systems. Anticancer Res. 2013;33:2807–2821. [PubMed] [Google Scholar]
- 10.Shehzad A., Rehman G., Lee Y.S. Curcumin in inflammatory diseases. Biofactors. 2013;39:69–77. doi: 10.1002/biof.1066. [DOI] [PubMed] [Google Scholar]
- 11.Noorafshan A., Ashkani-Esfahani S. A review of therapeutic effects of curcumin. Curr Pharm Des. 2013;19:2032–2046. [PubMed] [Google Scholar]
- 12.Murakami A., Furukawa I., Miyamoto S., Tanaka T., Ohigashi H. Curcumin combined with turmerones, essential oil components of turmeric, abolishes inflammation-associated mouse colon carcinogenesis. Biofactors. 2013;39:221–232. doi: 10.1002/biof.1054. [DOI] [PubMed] [Google Scholar]
- 13.Anggakusuma, Colpitts C.C., Schang L.M., Rachmawati H., Frentzen A., Pfaender S. Turmeric curcumin inhibits entry of all hepatitis C virus genotypes into human liver cells. Gut. 2013;63:1137–1149. doi: 10.1136/gutjnl-2012-304299. [DOI] [PubMed] [Google Scholar]
- 14.Narayanan A., Kehn-Hall K., Senina S., Lundberg L., van Duyne R., Guendel I. Curcumin inhibits Rift Valley fever virus replication in human cells. J Biol Chem. 2012;287:33198–33214. doi: 10.1074/jbc.M112.356535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zandi K., Ramedani E., Mohammadi K., Tajbakhsh S., Deilami I., Rastian Z. Evaluation of antiviral activities of curcumin derivatives against HSV-1 in Vero cell line. Nat Prod Commun. 2010;5:1935–1938. [PubMed] [Google Scholar]
- 16.Rechtman M.M., Har-Noy O., Bar-Yishay I., Fishman S., Adamovich Y., Shaul Y. Curcumin inhibits hepatitis B virus via down-regulation of the metabolic coactivator PGC-1alpha. FEBS Lett. 2010;584:2485–2490. doi: 10.1016/j.febslet.2010.04.067. [DOI] [PubMed] [Google Scholar]
- 17.Vajragupta O., Boonchoong P., Morris G.M., Olson A.J. Active site binding modes of curcumin in HIV-1 protease and integrase. Bioorg Med Chem Lett. 2005;15:3364–3368. doi: 10.1016/j.bmcl.2005.05.032. [DOI] [PubMed] [Google Scholar]
- 18.Chen M.H., Lee M.Y., Chuang J.J., Li Y.Z., Ning S.T., Chen J.C. Curcumin inhibits HCV replication by induction of heme oxygenase-1 and suppression of AKT. Int J Mol Med. 2012;30:1021–1028. doi: 10.3892/ijmm.2012.1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kutluay S.B., Doroghazi J., Roemer M.E., Triezenberg S.J. Curcumin inhibits herpes simplex virus immediate-early gene expression by a mechanism independent of p300/CBP histone acetyltransferase activity. Virology. 2008;373:239–247. doi: 10.1016/j.virol.2007.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Si X., Wang Y., Wong J., Zhang J., McManus B.M., Luo H. Dysregulation of the ubiquitin-proteasome system by curcumin suppresses coxsackievirus B3 replication. J Virol. 2007;81:3142–3150. doi: 10.1128/JVI.02028-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhong Z., Li X., Zhao W., Tong L., Liu J., Wu S. Mutations at nucleotides 573 and 579 within 5′-untranslated region augment the virulence of coxsackievirus B1. Virus Res. 2008;135:255–259. doi: 10.1016/j.virusres.2008.04.012. [DOI] [PubMed] [Google Scholar]
- 22.Bopegamage S., Kovacova J., Vargova A., Motusova J., Petrovicova A., Benkovicova M. Coxsackie B virus infection of mice: inoculation by the oral route protects the pancreas from damage, but not from infection. J Gen Virol. 2005;86:3271–3280. doi: 10.1099/vir.0.81249-0. [DOI] [PubMed] [Google Scholar]
- 23.Tong L., Lin L., Wu S., Guo Z., Wang T., Qin Y. MiR-10a⁎ up-regulates coxsackievirus B3 biosynthesis by targeting the 3D-coding sequence. Nucleic Acids Res. 2013;41:3760–3771. doi: 10.1093/nar/gkt058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang H.S., Ruan Z., Sang W.W. HDAC1/NFkappaB pathway is involved in curcumin inhibiting of Tat-mediated long terminal repeat transactivation. J Cell Physiol. 2011;226:3385–3391. doi: 10.1002/jcp.22691. [DOI] [PubMed] [Google Scholar]
- 25.Kim K., Kim K.H., Kim H.Y., Cho H.K., Sakamoto N., Cheong J. Curcumin inhibits hepatitis C virus replication via suppressing the Akt-SREBP-1 pathway. FEBS Lett. 2010;584:707–712. doi: 10.1016/j.febslet.2009.12.019. [DOI] [PubMed] [Google Scholar]
- 26.Baugh M.A. HIV: reactive oxygen species, enveloped viruses and hyperbaric oxygen. Med Hypotheses. 2000;55:232–238. doi: 10.1054/mehy.2000.1048. [DOI] [PubMed] [Google Scholar]
- 27.Zhou B., Zuo Y., Li B., Wang H., Liu H., Wang X. Deubiquitinase inhibition of 19S regulatory particles by 4-arylidene curcumin analog AC17 causes NF-kappaB inhibition and p53 reactivation in human lung cancer cells. Mol Cancer Ther. 2013;12:1381–1392. doi: 10.1158/1535-7163.MCT-12-1057. [DOI] [PubMed] [Google Scholar]
- 28.Tharakan B., Hunter F.A., Smythe W.R., Childs E.W. Curcumin inhibits reactive oxygen species formation and vascular hyperpermeability following haemorrhagic shock. Clin Exp Pharmacol Physiol. 2010;37:939–944. doi: 10.1111/j.1440-1681.2010.05414.x. [DOI] [PubMed] [Google Scholar]
- 29.Derochette S., Franck T., Mouithys-Mickalad A., Ceusters J., Deby-Dupont G., Lejeune J.P. Curcumin and resveratrol act by different ways on NADPH oxidase activity and reactive oxygen species produced by equine neutrophils. Chem Biol Interact. 2013;206:186–193. doi: 10.1016/j.cbi.2013.09.011. [DOI] [PubMed] [Google Scholar]
- 30.Moon H.J., Ko W.K., Han S.W., Kim D.S., Hwang Y.S., Park H.K. Antioxidants, like coenzyme Q10, selenite, and curcumin, inhibited osteoclast differentiation by suppressing reactive oxygen species generation. Biochem Biophys Res Commun. 2012;418:247–253. doi: 10.1016/j.bbrc.2012.01.005. [DOI] [PubMed] [Google Scholar]
- 31.Wong W.R., Chen Y.Y., Yang S.M., Chen Y.L., Horng J.T. Phosphorylation of PI3K/Akt and MAPK/ERK in an early entry step of enterovirus 71. Life Sci. 2005;78:82–90. doi: 10.1016/j.lfs.2005.04.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Semple J.I., Smits V.A., Fernaud J.R., Mamely I., Freire R. Cleavage and degradation of Claspin during apoptosis by caspases and the proteasome. Cell Death Differ. 2007;14:1433–1442. doi: 10.1038/sj.cdd.4402134. [DOI] [PubMed] [Google Scholar]
- 33.Mimnaugh E.G., Xu W., Vos M., Yuan X., Neckers L. Endoplasmic reticulum vacuolization and valosin-containing protein relocalization result from simultaneous hsp90 inhibition by geldanamycin and proteasome inhibition by velcade. Mol Cancer Res. 2006;4:667–681. doi: 10.1158/1541-7786.MCR-06-0019. [DOI] [PubMed] [Google Scholar]
- 34.Arora S., Yang J.M., Hait W.N. Identification of the ubiquitin-proteasome pathway in the regulation of the stability of eukaryotic elongation factor-2 kinase. Cancer Res. 2005;65:3806–3810. doi: 10.1158/0008-5472.CAN-04-4036. [DOI] [PubMed] [Google Scholar]
- 35.Spencer M.L., Theodosiou M., Noonan D.J. NPDC-1, a novel regulator of neuronal proliferation, is degraded by the ubiquitin/proteasome system through a PEST degradation motif. J Biol Chem. 2004;279:37069–37078. doi: 10.1074/jbc.M402507200. [DOI] [PubMed] [Google Scholar]
- 36.Zhang L., Wang C. PAX3-FKHR transformation increases 26S proteasome-dependent degradation of p27Kip1, a potential role for elevated Skp2 expression. J Biol Chem. 2003;278:27–36. doi: 10.1074/jbc.M205424200. [DOI] [PubMed] [Google Scholar]
- 37.Si X., Gao G., Wong J., Wang Y., Zhang J., Luo H. Ubiquitination is required for effective replication of coxsackievirus B3. PLoS One. 2008;3:e2585. doi: 10.1371/journal.pone.0002585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kitagawa Y., Yamaguchi M., Zhou M., Nishio M., Itoh M., Gotoh B. Human parainfluenza virus type 2 V protein inhibits TRAF6-mediated ubiquitination of IRF7 to prevent TLR7- and TLR9-dependent interferon induction. J Virol. 2013;87:7966–7976. doi: 10.1128/JVI.03525-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Maelfait J., Beyaert R. Emerging role of ubiquitination in antiviral RIG-I signaling. Microbiol Mol Biol Rev. 2012;76:33–45. doi: 10.1128/MMBR.05012-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhou Q., Zhu K., Cheng H. Ubiquitination in host immune response to human papillomavirus infection. Arch Dermatol Res. 2011;303:217–230. doi: 10.1007/s00403-011-1141-0. [DOI] [PubMed] [Google Scholar]
- 41.Eguchi H., Herschenhous N., Kuzushita N., Moss S.F. Helicobacter pylori increases proteasome-mediated degradation of p27(kip1) in gastric epithelial cells. Cancer Res. 2003;63:4739–4746. [PubMed] [Google Scholar]
- 42.Padilla S.L., Rodriguez A., Gonzales M.M., Gallego G.J., Castano O.J. Inhibitory effects of curcumin on dengue virus type 2-infected cells in vitro. Arch Virol. 2014;159:573–579. doi: 10.1007/s00705-013-1849-6. [DOI] [PubMed] [Google Scholar]
- 43.Dutta K., Ghosh D., Basu A. Curcumin protects neuronal cells from Japanese encephalitis virus-mediated cell death and also inhibits infective viral particle formation by dysregulation of ubiquitin-proteasome system. J Neuroimmune Pharmacol. 2009;4:328–337. doi: 10.1007/s11481-009-9158-2. [DOI] [PubMed] [Google Scholar]
- 44.van der Linden L., van der Schaar H.M., Lanke K.H., Neyts J., van Kuppeveld F.J. Differential effects of the putative GBF1 inhibitors Golgicide A and AG1478 on enterovirus replication. J Virol. 2010;84:7535–7542. doi: 10.1128/JVI.02684-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yang C.L., Ma Y.G., Xue Y.X., Liu Y.Y., Xie H., Qiu G.R. Curcumin induces small cell lung cancer NCI-H446 cell apoptosis via the reactive oxygen species-mediated mitochondrial pathway and not the cell death receptor pathway. DNA Cell Biol. 2012;31:139–150. doi: 10.1089/dna.2011.1300. [DOI] [PubMed] [Google Scholar]
- 46.Yadav B., Taurin S., Larsen L., Rosengren R.J. RL71, a second-generation curcumin analog, induces apoptosis and downregulates Akt in ER-negative breast cancer cells. Int J Oncol. 2012;41:1119–1127. doi: 10.3892/ijo.2012.1521. [DOI] [PubMed] [Google Scholar]
- 47.van der Schaar H.M., van der Linden L., Lanke K.H., Strating J.R., Purstinger G., de Vries E. Coxsackievirus mutants that can bypass host factor PI4KIIIbeta and the need for high levels of PI4P lipids for replication. Cell Res. 2012;22:1576–1592. doi: 10.1038/cr.2012.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Xu M.X., Zhao L., Deng C., Yang L., Wang Y., Guo T. Curcumin suppresses proliferation and induces apoptosis of human hepatocellular carcinoma cells via the wnt signaling pathway. Int J Oncol. 2013;43:1951–1959. doi: 10.3892/ijo.2013.2107. [DOI] [PubMed] [Google Scholar]
- 49.Berger K.L., Cooper J.D., Heaton N.S., Yoon R., Oakland T.E., Jordan T.X. Roles for endocytic trafficking and phosphatidylinositol 4-kinase III alpha in hepatitis C virus replication. Proc Natl Acad Sci USA. 2009;106:7577–7582. doi: 10.1073/pnas.0902693106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chang Z., Xing J., Yu X. Curcumin induces osteosarcoma MG63 cells apoptosis via ROS/Cyto-C/Caspase-3 pathway. Tumour Biol. 2014;35:753–758. doi: 10.1007/s13277-013-1102-7. [DOI] [PubMed] [Google Scholar]
- 51.Giri M.S., Nebozyhn M., Raymond A., Gekonge B., Hancock A., Creer S. Circulating monocytes in HIV-1-infected viremic subjects exhibit an antiapoptosis gene signature and virus- and host-mediated apoptosis resistance. J Immunol. 2009;182:4459–4470. doi: 10.4049/jimmunol.0801450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Perng G.C., Maguen B., Jin L., Mott K.R., Osorio N., Slanina S.M. A gene capable of blocking apoptosis can substitute for the herpes simplex virus type 1 latency-associated transcript gene and restore wild-type reactivation levels. J Virol. 2002;76:1224–1235. doi: 10.1128/JVI.76.3.1224-1235.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lomonosova E., Subramanian T., Chinnadurai G. Requirement of BAX for efficient adenovirus-induced apoptosis. J Virol. 2002;76:11283–11290. doi: 10.1128/JVI.76.22.11283-11290.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chen W., Sulcove J., Frank I., Jaffer S., Ozdener H., Kolson D.L. Development of a human neuronal cell model for human immunodeficiency virus (HIV)-infected macrophage-induced neurotoxicity: apoptosis induced by HIV type 1 primary isolates and evidence for involvement of the Bcl-2/Bcl-xL-sensitive intrinsic apoptosis pathway. J Virol. 2002;76:9407–9419. doi: 10.1128/JVI.76.18.9407-9419.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chen F., Li J., Liu T., Wang L., Li Y. MRI characteristics of brainstem encephalitis in hand-foot-mouth disease induced by enterovirus type 71-will different MRI manifestations be helpful for prognosis? Eur J Paediatr Neurol. 2013;17:486–491. doi: 10.1016/j.ejpn.2013.03.004. [DOI] [PubMed] [Google Scholar]
- 56.Gaaloul I., Riabi S., Harrath R., Evans M., Salem N.H., Mlayeh S. Sudden unexpected death related to enterovirus myocarditis: histopathology, immunohistochemistry and molecular pathology diagnosis at post-mortem. BMC Infect Dis. 2012;12:212. doi: 10.1186/1471-2334-12-212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sun L.M., Zheng H.Y., Zheng H.Z., Guo X., He J.F., Guan D.W. An enterovirus 71 epidemic in Guangdong Province of China, 2008: epidemiological, clinical, and virogenic manifestations. Jpn J Infect Dis. 2011;64:13–18. [PubMed] [Google Scholar]
- 58.Lin Y.J., Lai C.C., Lai C.H., Sue S.C., Lin C.W., Hung C.H. Inhibition of enterovirus 71 infections and viral IRES activity by Fructus gardeniae and geniposide. Eur J Med Chem. 2013;62:206–213. doi: 10.1016/j.ejmech.2012.12.038. [DOI] [PubMed] [Google Scholar]
- 59.Lin T.Y., Liu Y.C., Jheng J.R., Tsai H.P., Jan J.T., Wong W.R. Anti-enterovirus 71 activity screening of chinese herbs with anti-infection and inflammation activities. Am J Chin Med. 2009;37:143–158. doi: 10.1142/S0192415X09006734. [DOI] [PubMed] [Google Scholar]
- 60.Wu B.W., Pan T.L., Leu Y.L., Chang Y.K., Tai P.J., Lin K.H. Antiviral effects of Salvia miltiorrhiza (Danshen) against enterovirus 71. Am J Chin Med. 2007;35:153–168. doi: 10.1142/S0192415X07004709. [DOI] [PubMed] [Google Scholar]
- 61.Ou J.L., Mizushina Y., Wang S.Y., Chuang D.Y., Nadar M., Hsu W.L. Structure-activity relationship analysis of curcumin analogues on anti-influenza virus activity. FEBS J. 2013;280:5829–5840. doi: 10.1111/febs.12503. [DOI] [PubMed] [Google Scholar]
- 62.Beck M.A., Levander O.A., Handy J. Selenium deficiency and viral infection. J Nutr. 2003;133:1463S–1467SS. doi: 10.1093/jn/133.5.1463S. [DOI] [PubMed] [Google Scholar]
- 63.Gomez R.M., Berria M.I., Levander O.A. Host selenium status selectively influences susceptibility to experimental viral myocarditis. Biol Trace Elem Res. 2001;80:23–31. doi: 10.1385/BTER:80:1:23. [DOI] [PubMed] [Google Scholar]
- 64.Ho H.Y., Cheng M.L., Weng S.F., Leu Y.L., Chiu D.T. Antiviral effect of epigallocatechin gallate on enterovirus 71. J Agric Food Chem. 2009;57:6140–6147. doi: 10.1021/jf901128u. [DOI] [PubMed] [Google Scholar]
- 65.Ho H.Y., Cheng M.L., Weng S.F., Chang L., Yeh T.T., Shih S.R. Glucose-6-phosphate dehydrogenase deficiency enhances enterovirus 71 infection. J Gen Virol. 2008;89:2080–2089. doi: 10.1099/vir.0.2008/001404-0. [DOI] [PubMed] [Google Scholar]
- 66.Tung W.H., Hsieh H.L., Lee I.T., Yang C.M. Enterovirus 71 induces integrin beta1/EGFR-Rac1-dependent oxidative stress in SK-N-SH cells: role of HO-1/CO in viral replication. J Cell Physiol. 2011;226:3316–3329. doi: 10.1002/jcp.22677. [DOI] [PubMed] [Google Scholar]
- 67.Wang B., Zhang H., Zhu M., Luo Z., Peng Y. MEK1-ERKs signal cascade is required for the replication of enterovirus 71 (EV71) Antiviral Res. 2012;93:110–117. doi: 10.1016/j.antiviral.2011.11.001. [DOI] [PubMed] [Google Scholar]
- 68.Zhang D.D., Lo S.C., Sun Z., Habib G.M., Lieberman M.W., Hannink M. Ubiquitination of Keap1, a BTB-Kelch substrate adaptor protein for Cul3, targets Keap1 for degradation by a proteasome-independent pathway. J Biol Chem. 2005;280:30091–30099. doi: 10.1074/jbc.M501279200. [DOI] [PubMed] [Google Scholar]
- 69.Guo Y., Shan Q., Gong Y., Lin J., Shi F., Shi R. Curcumin induces apoptosis via simultaneously targeting AKT/mTOR and RAF/MEK/ERK survival signaling pathways in human leukemia THP-1 cells. Pharmazie. 2014;69:229–233. [PubMed] [Google Scholar]
- 70.Chi Y., Hong Y., Zong H., Wang Y., Zou W., Yang J. CDK11p58 represses vitamin D receptor-mediated transcriptional activation through promoting its ubiquitin–proteasome degradation. Biochem Biophys Res Commun. 2009;386:493–498. doi: 10.1016/j.bbrc.2009.06.061. [DOI] [PubMed] [Google Scholar]
- 71.Guan K., Zheng Z., Song T., He X., Xu C., Zhang Y. MAVS regulates apoptotic cell death by decreasing K48-linked ubiquitination of voltage-dependent anion channel 1. Mol Cell Biol. 2013;33:3137–3149. doi: 10.1128/MCB.00030-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Chen Z., Luo X., Lu Y., Zhu T., Wang J., Tsun A. Ubiquitination signals critical to regulatory T cell development and function. Int Immunopharmacol. 2013;16:348–352. doi: 10.1016/j.intimp.2013.01.023. [DOI] [PubMed] [Google Scholar]
- 73.Gandhy S.U., Kim K., Larsen L., Rosengren R.J., Safe S. Curcumin and synthetic analogs induce reactive oxygen species and decreases specificity protein (Sp) transcription factors by targeting microRNAs. BMC Cancer. 2012;12:564. doi: 10.1186/1471-2407-12-564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Feng J.Y., Liu Z.Q. Feruloylacetone as the model compound of half-curcumin: synthesis and antioxidant properties. Eur J Med Chem. 2011;46:1198–1206. doi: 10.1016/j.ejmech.2011.01.039. [DOI] [PubMed] [Google Scholar]
- 75.Lanke K.H., van der Schaar H.M., Belov G.A., Feng Q., Duijsings D., Jackson C.L. GBF1, a guanine nucleotide exchange factor for Arf, is crucial for coxsackievirus B3 RNA replication. J Virol. 2009;83:11940–11949. doi: 10.1128/JVI.01244-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wessels E., Duijsings D., Lanke K.H., Melchers W.J., Jackson C.L., van Kuppeveld F.J. Molecular determinants of the interaction between coxsackievirus protein 3A and guanine nucleotide exchange factor GBF1. J Virol. 2007;81:5238–5245. doi: 10.1128/JVI.02680-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Arita M., Kojima H., Nagano T., Okabe T., Wakita T., Shimizu H. Phosphatidylinositol 4-kinase III beta is a target of enviroxime-like compounds for antipoliovirus activity. J Virol. 2011;85:2364–2372. doi: 10.1128/JVI.02249-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Borawski J., Troke P., Puyang X., Gibaja V., Zhao S., Mickanin C. Class III phosphatidylinositol 4-kinase alpha and beta are novel host factor regulators of hepatitis C virus replication. J Virol. 2009;83:10058–10074. doi: 10.1128/JVI.02418-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Berger K.L., Randall G. Potential roles for cellular cofactors in hepatitis C virus replication complex formation. Commun Integr Biol. 2009;2:471–473. doi: 10.4161/cib.2.6.9261. [DOI] [PMC free article] [PubMed] [Google Scholar]