Abstract
Reversed-phase liquid chromatography coupled with electrospray ionization tandem mass spectrometry (ESI-MS/MS) was used to characterize impurities in cefpodoxime proxetil, an ester-modified prodrug. Based on the mechanisms by which cephalosporins are degraded, stress tests were designed and performed. The bulk material and capsule were eluted through a C18 column with formic acid–methanol–water as the mobile phase. In total, 15 impurities were characterized in commercial samples, including 7 known impurities and 8 new impurities. The structures of these unknown compounds were deduced via comparison with the fragmentation patterns of cefpodoxime proxetil. Data from this systematic study will help improve the safety and quality of cefpodoxime proxetil.
Key words: Cefpodoxime proxetil, Cephalosporins, Impurities, LC–MS, Structure identification
Graphical abstract
The impurity profiles of cefpodoxime proxetil are systematically studied by LC–MSn. As a result, total 15 impurities were characterized in commercial samples, including 8 new impurities. We also discussed the cause and mass fragmentation pathways of those new impurities.
1. Introduction
Cefpodoxime proxetil (Fig. 1), an ester-modified prodrug, is an oral, broad-spectrum third generation cephalosporin antibiotic1. It has in vitro activity against many common Gram-positive and Gram-negative pathogens associated with common pediatric infections, so it is a useful option for empirical therapy2. It is listed in the United States Pharmacopeia 36th Edition3, the European Pharmacopeia 7.0 Edition4 and the Japanese Pharmacopeia 15th Edition5. Different analysis methods have been developed6, 7 and compared8 to measure impurities and degradation products. Fukutsu et al.9 identified three degradation products of cefpodoxime proxetil by high-performance liquid chromatography-hyphenated techniques. However, ICH guidelines Q3A require that all impurities (from processing and degradation) be identified above a certain threshold10. Yet, at this time, a systematic study for identifying cefpodoxime proxetil impurities is not available. Hence, we focused on identifying unknown process impurities and degradation products in cefpodoxime proxetil using a chromatographic system from the European Pharmacopeia 7.0 Edition using liquid chromatography with diode array detection (LC-DAD), multiple stage mass spectrometry (MSn), and liquid chromatography–high-resolution mass spectrometry (LC–HRMS) methods along with stress degradation tests, degradation11, 12, 13 and mass fragmentation mechanisms of cephalosporins14, 15, 16, and related synthesis processes17, 18. These studies will inform future quality control and safety of cefpodoxime proxetil products. We characterized 15 impurities including 4 new degradation products and 4 new process impurities, along with 7 known impurities as identified in the European Pharmacopeia. We would discuss these as well as the most likely cause of their appearance and the mass fragmentation pathways of the impurities.
Figure 1.
Proposed chemical structures of 15 impurities and cefpodoxime proxetil.
2. Materials and methods
2.1. Reagents and samples
HPLC-grade acetonitrile was purchased from Thermo Fisher Scientific (Fair Lawn, NJ). Formic acid (98.0%) was supplied by Sigma-Aldrich Co. Ltd. (St. Louis, MO, US) and analytical-grade hydrochloric acid (HCl), sodium hydroxide (NaOH) and hydrogen peroxide (H2O2, 30%) were obtained from Beijing Chemical Works (Beijing, China). A milli-Q water purification system (Millipore, Billerica, MA) was used to further purify glass-distilled water.
Cefpodoxime proxetil RS (Batch No. 130517-200802, containing 95.9% of cefpodoxime) was provided by National Institutes for Food and Drug Control, China. Cefpodoxime proxetil capsules (Batch No. 130401) and bulk material (Batch No. 12072-01) were obtained from Zhejiang Yatai Pharmaceutical Company (Zhejiang, China). Cefpodoxime proxetil systematic RS (Batch No. 1.0) and cefpodoxime proxetil impurity H RS (Batch No. 1.0) were purchased from European Directorate for Quality Medicines (EDQM).
2.2. Reference standard and sample solutions preparation
Approximately 3 mg of cefpodoxime proxetil systematic RS was transferred to a 10 mL volumetric flask, and dissolved in 10 mL of a mixture of water, acetonitrile and acetic acid (99:99:1, v/v/v). This was the systematic RS solution.
Next, 3 mg of cefpodoxime proxetil impurity H RS was added to a 10 mL volumetric flask and dissolved in 10 mL of a mixture of water, acetonitrile and acetic acid (99:99:1, v/v/v). This was the impurity H RS solution.
Then, cefpodoxime proxetil capsule contents (50 mg) were dissolved in 50 mL of a mixture of water, acetonitrile and acetic acid (100%) (99:99:1, v/v/v). This was the sample solution. Cefpodoxime proxetil bulk material (50 mg) was dissolved in 50 mL of a mixture of water, acetonitrile and acetic acid (99:99:1, v/v/v). This was the forced degradation stock solution.
2.3. Instrumentation
The LC/MS system consisted of a 3201 S1-2 binary pump, a 3202 S1-2 vacuum degasser, a 3014 S1-2 column heater, a 3012 S1-2 column switch system, a 3133 S1-2 sampler from SHISEIDO (Tokyo, Japan), an Accela PDA detector (Thermo Fisher Scientific Inc., Waltham, MA) and a 3200Q TRAP mass detector (Applied Biosystem Inc., California), controlled by Analyst® software (version 1.5.1).
2.4. Forced degradation study
The forced degradation stock solution was transferred and degraded under acidic, basic, 60 °C water bath, oxidative, UV and high-temperature conditions, separately.
Acid degradation solution: About 5 mL of stock solution was transferred into a 25 mL volumetric flask. Then, 2 mL of 0.1 mol/L hydrochloric acid was added. This mixture was allowed to stand for 2 h, and then the acidic solution was neutralized with 2 mL of 0.1 mol/L sodium hydroxide.
Base degradation solution: About 5 mL of stock solution was transferred into a 25 mL volumetric flask and 2 mL of 0.1 mol/L sodium hydroxide was added and maintained for 2 h. The basic solution was then neutralized with 2 mL of 0.1 mol/L hydrochloric acid.
Oxidative degradation solution: About 5 mL of stock solution was transferred into a 25 mL volumetric flask. Then, 1.0 mL of 10% hydrogen peroxide solution was added. This mixture was maintained for 2 h.
Water bath degradation solution: About 5 mL of stock solution was transferred into a 25 mL volumetric flask. This solution was kept in a 60 °C water bath for 45 min, and the solution was allowed to cool to room temperature.
UV degradation solution: About 10 mL of stock solution was placed under UV light (254 nm) for 12 h.
High-temperature degradation solution: About 100 mg of cefpodoxime proxetil bulk material was placed in an oven at 60 °C for 2 h. Then 10 mg of the above sample was transferred into a 10 mL volumetric flask. The sample was dissolved and diluted with a mixture of water, acetonitrile and acetic acid (99:99:1, v/v/v).
2.5. Chromatographic conditions
The analysis was carried out on a Kromasil 100-5 C18 column (4.6 mm×150 mm, 5 μm-particle diameter). Mobile phase A contained formic acid–methanol–water (1:400:600, v/v/v). Mobile phase B contained formic acid–methanol–water (1:50:950, v/v/v). UV detection was at 254 nm and the flow rate was kept at 0.6 mL/min. Column oven temperature was 25 °C and the data acquisition time was 165 min. The pump mode was gradient and the program was as follows, time (min)/A (v/v):B (v/v); T0.01/95:5, T65.0/95:5, T145.0/15:85, T155.0/15:85, T155.1/95:5, and T165.0/95:5.
2.6. Mass spectrometry
Tuning and MSn investigation of cefpodoxime proxetil and impurities was carried out using the following optimized MS conditions: electrospray ionization (EPI) positive ionization mode, decluster potential (DP) 50 V, entrance potential (EP) 10 V, collision energy (CE) 40 V, curtain gas: 20.0 L/h, ion source gas 1: 65.0 L/h, ion source gas 2: 60.0 L/h, ion spray voltage (IS): 5500 V, temperature (TEM): 500.0 °C, and Interface heater. Enhanced MS (EMS) and enhanced product ion (EPI) spectra were acquired from m/z 50 to m/z 1200 in 0.1 amu steps with dwell time of 2.0 s. Analyst software (version 1.5.1) was used for data acquisition and processing. Molecular weights of each component were deduced using protonated molecular ions ([M+H]+) and were confirmed using minor adduct ions of [M+Na]+ and [M+K]+ peaks.
High resolution-mass spectrum (HR-MS) investigation was accomplished with a dual gradient UltiMate 3000 HPLC system (Dionex Inc., Sunnyvale, CA) equipped with a LTQ Orbit trap XL high resolution mass spectrometer (Thermo Fisher Scientific Inc.) with the following MS conditions: positive ionization mode, FT cell recording window from m/z 100 to 1200, and resolution 60,000. Data processing was performed using Perl script (Quant Merge) software.
3. Results and discussion
3.1. Impurity analysis by HPLC
Cefpodoxime proxetil bulk material and capsule solutions were analyzed using the HPLC-DAD (Fig. 2a and b) and 15 impurity peaks were detected from the two samples. All 15 capsule impurities originated from cefpodoxime proxetil bulk material. To determine the source of impurities, cefpodoxime proxetil bulk material was degraded under alkaline, acidic, 60 °C water bath, oxidation, high-temperature, and UV irradiation conditions, based on published cephalosporin degradation conditions19, 20. Typical chromatograms for the different forced degradation conditions are shown in Fig. 2c–g.
Figure 2.
Typical chromatograms of cefpodoxime proxetil capsule and bulk material and degradation products under different forced degradation conditions: (a, capsule (Batch No. 130401); b, bulk material (Batch No. ECP12072-01); c, 60 °C water bath degradation; d, high-temperature degradation; e, oxidative degradation; f, UV degradation; and g, UV+oxidative degradation).
Stress tests suggested that IMP-S1, IMP-S3, IMP-S4, and IMP-S6 originated from degradation and that IMP-S7–17 were introduced during synthesis. IMP-S3 increased in the 60 °C water bath and under high temperatures. IMP-S4 and IMP-S6 increased under UV degradative conditions. One potential impurity, IMP-S1a, was detected after exposure to the 60 °C water bath and UV degradation conditions, and four other potential impurities were detected under oxidative conditions (IMP-S1b–e). We have characterized all these degradation products.
3.2. Mass fragmentation pathway of cefpodoxime proxetil
Understanding cefpodoxime proxetil fragmentation pathways can help identify impurity structures. Cefpodoxime proxetil was analyzed in positive ion mode firstly by direct flow injection using a methanol/water (1:1) mixture as the solvent through MSn. Mass spectra and the cefpodoxime proxetil mass fragmentation pathway are shown in Fig. 3a–c.
Figure 3.
Mass spectra and mass fragmentation pathway of cefpodoxime proxetil (a, +EMS; b, EPI@558.1 [M+H]; and c, fragmentation pathway).
3.3. Analysis of cefpodoxime proxetil systematic RS and impurity H RS
To rapidly identify the known impurities of cefpodoxime proxetil, cefpodoxime proxetil systematic RS and impurity H RS obtained from EDQM were analyzed. Compared to standard chromatograms provided by EDQM, the cefpodoxime proxetil impurity B (I, II), C, D (I, II) peaks and impurity H (I, II) peaks were detected and marked by the retention time and UV spectra, and identified via LC/MS analysis. Typical chromatograms and mass spectra data for impurities are shown in Fig. 4a and b and Table 1.
Figure 4.
Typical chromatograms of impurities in cefpodoxime proxetil systematic RS (a) and impurity H RS (b).
Table 1.
Mass spectra data of impurities in cefpodoxime proxetil.
| Peak No. | Peak name | Component name | Retention time (min) | MW | Formula | [M+H], [M+Na] | Typical fragmental ion peak |
|---|---|---|---|---|---|---|---|
| 1 | IMP-S1a | Imp B diast Ia | 46.3 | 527 | C20H25N5O8S2 | 528, 550 | 484, 440, 424, 398, 380, 241, 210, 197, 126 |
| 2 | API-1 | Cefpodoxime proxetil diast I | 50.1 | 557 | C21H27N5O9S2 | 558, 580 | 526, 428, 410, 382, 324, 306, 285, 241, 225, 211, 167, 156, 126 |
| 3 | IMP-S3 | Imp B diast IIa | 56.9 | 527 | C20H25N5O8S2 | 528, 550 | 484, 440, 424, 398, 380, 241, 210, 197, 126 |
| Imp Ca | 56.9 | 557 | C21H27N5O9S2 | 558, 580 | 526, 498, 482, 438, 428, 396, 378, 350, 322, 241, 210, 142, 126 | ||
| 4 | IMP-S4a | Imp-D diast Ia | 62.7 | 557 | C21H27N5O9S2 | 558, 580 | 526, 428, 410, 320, 241, 211, 156, 126 |
| 5 | API-2 | Cefpodoxime proxetil diast II | 68.6 | 557 | C21H27N5O9S2 | 558, 580 | 526, 428, 410, 382, 324, 306, 285, 241, 225, 211, 167, 156, 126 |
| 6 | IMP-S6a | Imp-D diast IIa | 76.9 | 557 | C21H27N5O9S2 | 558, 80 | 526, 428, 410, 320, 241, 211, 156, 126 |
| 7 | IMP-S7 | Imp-Ib | 87.6 | 687 | C27H37N5O12S2 | 688, 710 | 600, 584, 558,526, 428, 410, 382, 277, 241, 167, 126 |
| 12 | IMP-S12 | Imp-J diast Ib | 113.5 | 643 | C25H33N5O11S2 | 644, 666 | 612, 514, 496, 468, 382, 350, 225, 167, 125 |
| 13 | IMP-S13 | Imp-J-Diast IIb | 116.1 | 643 | C25H33N5O11S2 | 644, 666 | 612, 514, 496, 468, 382, 350, 225, 167, 125 |
| 15 | IMP-S15 | Imp-H diast Ia | 122.2 | 1114 | C42H54N10O18S4 | 1115, 1137 | 1083, 1051, 953, 935, 909, 761, 526, 500, 396, 352 |
| 16 | IMP-S16 | Imp-H diast IIa | 123.9 | 1114 | C42H54N10O18S4 | 1115, 1137 | 1083, 1051, 953, 935, 909, 761, 526, 500, 396, 352, |
| 1a | IMP-S1a | Imp Aa | 2.72 | 427 | C15H17N5O6S2 | 428, 450 | 396, 368, 324, 272, 241, 210, 167, 156, 125 |
| 1b | IMP-S1b | Imp K Diast Ib | 20.27 | 573 | C21H27N5O10S2 | 574, 596 | 556, 524, 426, 408, 394, 301, 274, 225, 156, 125 |
| 1c | IMP-S1c | Imp L Diast Ib | 23.34 | 573 | C21H27N5O10S2 | 574, 596 | 556, 524, 426, 394, 350, 274, 225, 125 |
| 1d | IMP-S1d | Imp K Diast IIb | 27.61 | 573 | C21H27N5O10S2 | 574, 596 | 556, 524, 426, 408, 394, 301, 274, 225, 156, 125 |
| 1e | IMP-S1e | Imp L Diast IIb | 30.15 | 573 | C21H27N5O10S2 | 574, 596 | 556, 524, 426, 394, 350, 274, 225, 125 |
Note: these impurities are reported in European Pharmacopeia 7.0.
These unknown impurities are characterized for the first time.
3.4. Investigation of known impurities in cefpodoxime proxetil capsules
A total of five impurities including IMP-S1, S3 (two components S3a and S3b), S4 and S6 were identified as known impurities by comparing the retention times, UV spectra and MS data of the impurities in samples with systematic RS and impurity H RS. The typical TIC of cefpodoxime proxetil capsules is depicted in Fig. 5a. The mass spectra of these five impurities are shown in Fig. 5b–h.
Figure 5.
Typical TIC and mass spectra of cefpodoxime proxetil capsule (a: TIC; b: IMP-S1; c: IMP-S3a; d: IMP-S3b; e: IMP-S4; f: IMP-S6; g: IMP-S16; and h: IMP-S17).
3.4.1. IMP-S1
The ions at m/z 528.4 and m/z 550.3 in the +EMS spectrum were hypothesized to be [M+H]+ and [M+Na]+ ion peaks, respectively (Fig. 5b). Therefore the molecular weight of IMP-S1 was proposed to be 527.4. This IMP-S1was identified as Imp B diast I by comparing fragmental ion peaks at m/z 558.4 as well as the retention time. The chemical structure is shown in Fig. 1.
3.4.2. IMP-S3
There were two co-elutes in the IMP-S3 peak, including the main peak (S3a) and a minor peak (S3b). The molecular weight of the main component (S3a) was deduced to be 557.2 based on the ions at m/z 558.2 [M+H]+ and m/z 580.3 [M+Na]+ (Fig. 5c). The molecular weight of the minor component (S3b) was proposed to be 527.4 due to the ions at 528.4 [M+H]+ and 550.3 [M+Na]+ (Fig. 5d). IMP-S3a and IMP-S3b were identified as Imp C and Imp B diast II, separately (Fig. 1).
3.4.3. IMP-S4 and S5
The molecular weights of IMP-S4 and IMP-S5 were both deduced to be 557.4 according to the ions at m/z 558.4 [M+H]+ and m/z 580.3 [M+Na]+ (Fig. 5e and f) compared to mass data from cefpodoxime proxetil systematic RS, IMP-S4 and S5 were confirmed to be Imp-D diast I and Imp-D diast II, respectively (Fig. 1).
3.4.4. IMP-S15 and S16
Similar to IMP-S4 and S5, IMP-S15 and IMP-S16 were of the same molecular weight (1114.2 Da) based on the ions at m/z 1115.3 [M+H]+ and m/z 1137.3 [M+Na]+ (Fig. 5g and h). These impurities were deduced to be cefpodoxime proxetil dimers, Imp-H diast I and Imp-H diast II, respectively (Fig. 1). Thus, 7 impurities were identified via comparison of cefpodoxime proxetil systematic RS and Imp-H RS provided from EDQM.
3.5. Investigation of unknown components in commercial cefpodoxime proxetil samples
The 9 impurity peaks in the cefpodoxime proxetil samples aside from the 7 reported impurities were derived from the commercial synthesis process. A detailed elucidation was conducted on IMP-S7, IMP-S12, and IMP-S13 based on the mass spectral data below.
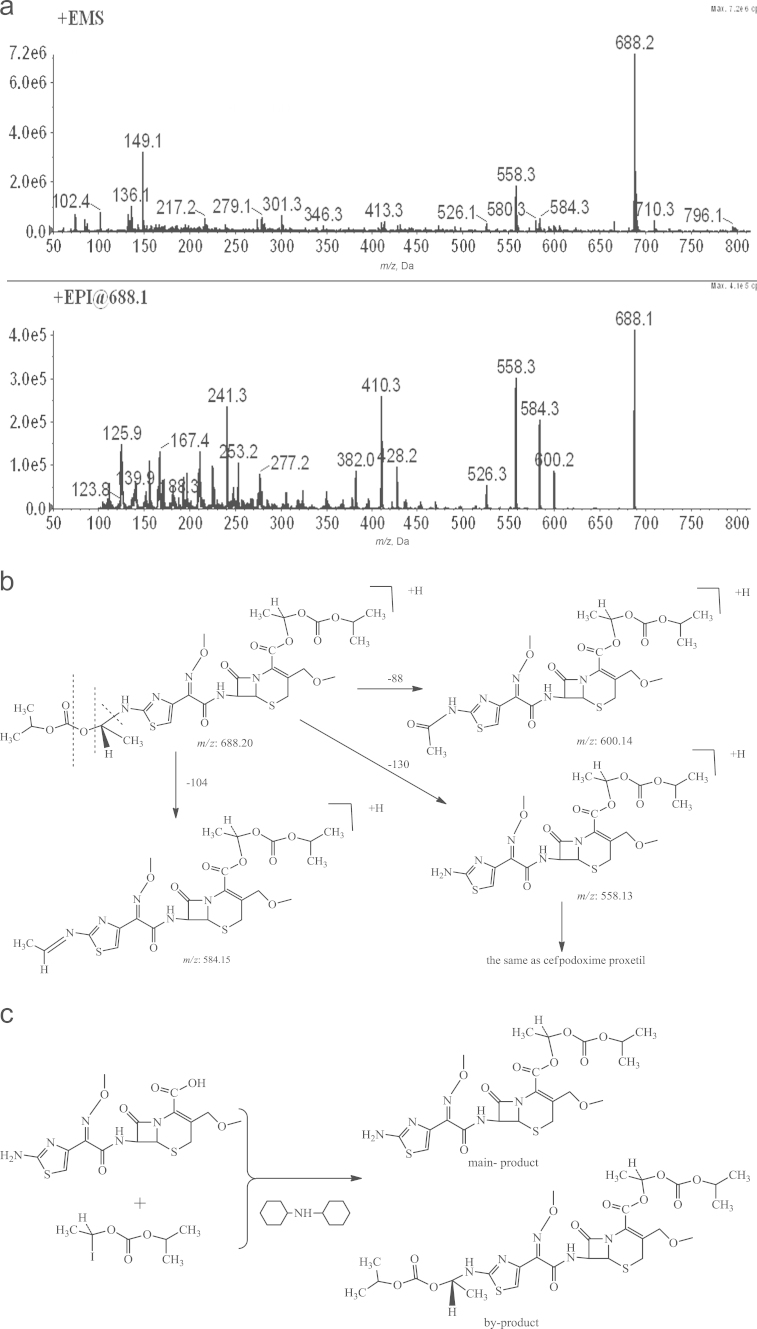
3.5.1. IMP-S7
The +EMS of IMP-S7 presented ions at 688.2 [M+H]+ and 710.4 [M+Na]+ peaks (Fig. 6a) indicated a molecular weight of 687.2. Compared to the molecular weight of cefpodoxime proxetil, the molecular weight of IMP-S7 (130 Da) suggested an ester substituent in IMP-S4. The EPI of IMP-S7 showed that the ion at 688.2[M+H]+ was broken into a fragmental ion m/z 558 due to the loss of a 130-Da ester group, and other fragmental ions less than m/z 688 were all in accordance with those of cefpodoxime proxetil. Therefore, we deduced that another 130-Da ester group was substituted in IMP-S7.
Figure 6.
Typical mass spectra, synthetic route and mass fragmentation pathway of IMP-S7 (a: mass spectra; b: synthetic route; and c: mass fragmentation pathway).
When an esterification reaction occurred at the cefpodoxime acid group, the same reaction might occur at the cefotaxime group to form by-products, according to the possible side reaction principle of cefpodoxime proxetil; the reaction principle is shown in Fig. 6b. The mass fragmentation pathways of IMP-S7 are shown in Fig. 6c. IMP-S7 is defined as Imp I, and its chemical structure is shown in Fig. 1.
3.5.2. IMP-S12 and S13
The EMS of IMP-S12 revealed the presence of a m/z 644.3 [M+H]+ peak and a m/z 666.2 [M+Na]+ peak (Fig. 7a), indicating a molecular weight of 643.4. Compared to cefpodoxime proxetil, the molecular weight of IMP-S12 was 86 Da. The EPI at 644.3 [M+H]+ spectrum showed that the ion at m/z 644 was broken into an ion at m/z 612 with the loss of a fragmental ion at m/z 32, suggesting that they shared the same side group at the 3rd position, and was broken into an ion at m/z 514 with the loss of a fragmental ion at m/z130, indicating the same ester group was substituted in the carboxyl group. Furthermore, the fragmental ion at m/z 241 from fragmentation pathway A was present in the EPI spectrum, and the ion at m/z 327 was 86 Da larger than the ion at m/z 241. Thus, the fragmental ion at m/z 76 was substituted in the side group at 7th-position.
Figure 7.
Typical mass spectra, HR-mass spectra, proposed synthetic route and mass fragmentation pathway of IMP-S12 and S13 (a: mass spectra; b: HR-MS spectra; c: synthetic route; and d: mass fragmentation pathway).
To obtain a precise molecular composition, impurities were analyzed by HRMS (Fig. 7b). The molecular composition of the impurity was calculated to be C25H33N5O11S2, suggesting an additional C4H6O2 group was present in IMP-S12 compared to molecular cefpodoxime proxetil.
Based on cefpodoxime proxetil synthesis, we speculated that the cefotaxime group at the 7th position reacted with an isopropanol oxy chloride group and produced this by-product (Fig. 7c for reaction). From the above analysis, the chemical structure of IMP-S12 was elucidated (Fig. 1). The mass spectral fragmentation pathway of IMP-S12 is depicted in Fig. 7d.
Based on a similar elucidation step, the IMP-S13 was deduced to be the isomer of IMP-S12 with a chiral center at the ester group, a finding that is in accordance with the presence of two main cefpodoxime proxetil peaks.
As a result, IMP-S12 and IMP-S13 were defined as Imp J Diast I and Imp J II, repectively; their chemical structures are shown in Fig. 1.
3.6. Potential degradation impurities
The stress test indicated that cefpodoxime proxetil was degraded under UV light, oxidative conditions, high temperatures and 60 °C water bath. Degradation impurities increased during storage and transportation, affecting product quality. Therefore, characterizing these impurities using an LC/MS method was the next step. Five degradation impurities were identified as described below.
3.6.1. IMP-S1a
Impurity IMP-S1a was a typical degradation impurity discovered after exposure to the 60 °C water bath (Fig. 2c, possible degradation process). The mass spectra of impurity IMP-S1a displayed a molecular ion peak at m/z 428.0 [M+H]+, and at m/z 450.0 [M+Na]+ (Fig. 8a). The molecular weight was estimated to be 427.2, consistent with the molecular weight of cefpodoxime acid. The EPI at m/z 428.0 of IMP-S1a showed the same fragmental ions as cefpodoxime acid. Hence impurity IMP-S1a is identified as cefpodoxime acid (Imp A) and its chemical structure is shown in Fig. 1.
Figure 8.
Typical mass spectra of IMP-1a, IMP-1b, IMP-1d, and IMP-1c, IMP-1e and TIC of oxidation degradation (a: IMP-1a; b: TIC of oxidative degradation; c: IMP-1b and IMP-1d; and d: IMP-1c and IMP-1e).
3.6.2. IMP-S1b–e
IMP-S1b, S1c, S1d, S1e peaks were four typical degradation impurities derived from oxidation, and these were present in the systematic RS obtained from EDQM but without structural elucidation. The TIC of these four impurities is shown in Fig. 8b.
The EMS of these four impurities (IMP-S1b–e) all had ion peaks at m/z 574.3 [M+H]+ and m/z 596.1 [M+Na]+ (Fig. 8c and d), indicating that each was an isomer with a molecular weight of 573.2. Compared to cefpodoxime proxetil, these four oxidation impurities were 16 Da larger, suggesting that an additional oxygen atom was substituted in these impurities.
According to the oxidation reaction principle, the oxidation position was deduced to locate the sulfur atom in the matrix and a sulfoxide group was formed. The EPI spectra indicated that the fragmentation ions of IMP-S1b and IMP-S1d were similar, suggesting that they were a pair of oxidative products of cefpodoxime proxetil. IMP-S1c and IMP-S1e presented similar fragmental ions as well, differing only with ion peak intensity compared to the former two isomers. IMP-S1c and IMP-S1e were likely formed due to a further isomerization of IMP-S1b and IMP-S1d at the cefotaxime group. The possible isomerization of delta 3 was excluded because there was no obvious difference in the mass spectra among these four impurities.
To validate this deduction, a further oxidative degradation was conducted on the UV degraded solution. The ratio of the peak area of IMP-S1c and IMP-S1e compared to that of IMP-S1c and IMP-S1e obtained from the product only via oxidative degradation increased by about 30% (Fig. 2e and g), and confirmed the structural elucidation. Impurities IMP-S1c and IMP-S1e were deduced to be the E-isomers of IMP-S1b and IMP-S1d, respectively. As a result, IMP-S1b and IMP-S1d were defined as Imp K Diast I and Imp KII, and IMP-S1c and IMP-S1e were defined as Imp L Diast I and Imp L II, respectively. The chemical structures of these four impurities are shown in Fig. 1.
4. Conclusion
The impurities in commercial cefpodoxime proxetil samples were characterized based on MS/MS fragmentation pathways and chromatographic behaviors. In total, 15 impurities were detected in the sample. Based on published cephalosporin degradation mechanisms, stress tests were designed and performed. The data showed that four impurities were degradation products and 11 impurities originated from the synthesis process. In addition, five impurities were potential degradation products. Seven known impurities were accurately elucidated by comparison with the mass spectra of the systematic RS and impurity H RS. Eight new impurities were elucidated for the first time based on the synthesis process and the mass fragmentation pathway of cefpodoxime proxetil. The remaining five synthetic impurities were not characterized by MS/MS methods because they were in very low concentrations. This systematic study to identify impurities in cefpodoxime proxetil samples will assist us to improve its quality and safety.
Acknowledgment
Financial support by the Twelfth Five-year National Science and Technology Support Program “The research and development of new material for separation and integration demonstration” (No. 2012BAK25B02) is gratefully acknowledged.
Footnotes
Peer review under responsibility of Institute of Materia Medica, Chinese Academy of Medical Sciences and Chinese Pharmaceutical Association.
References
- 1.Todd WM. Cefpodoxime proxetil: a comprehensive review. Int J Antimicrob Agent. 1994;4:37–62. doi: 10.1016/0924-8579(94)90062-0. [DOI] [PubMed] [Google Scholar]
- 2.Fulton B, Perry CM. Cefpodoxime proxetil: a review of its use in the management of bacterial infections in paediatric patients. Paediatr Drug. 2001;3:137–158. doi: 10.2165/00128072-200103020-00006. [DOI] [PubMed] [Google Scholar]
- 3.United States Pharmacopeia. 36th ed. Rockville, MD: USP Convention; 2012.
- 4.European Pharmacopeia. 7th ed. Strasbourg: Council of Europe; 2013.
- 5.Japanese Pharmacopeia. XV. The Society of Japanese Pharmacopeia; 2012.
- 6.Xue J, Hu CQ, Jin SH. Development and validation of an HPLC method for the determ ination of cefpodoxime proxetil and its related substances in dry syrups. Chin J Antibiot. 2003;28:633–637. [Google Scholar]
- 7.Wang J, Wang CF. Determination of related substances in cefpodoxime proxetil by HPLC. Chin J Pharm. 2002;33:450–451. [Google Scholar]
- 8.Wang MJ, Zou WB, Xue J, Hu CQ. Comparison of three RP-HPLC methods for analysis of cefpodoxime proxetil and related substances. Chromatographia. 2007;65:69–75. [Google Scholar]
- 9.Fukutsu N, Kawasaki T, Saito K, Nakazawa H. Application of high-performance liquid chromatography hyphenated techniques for identification of degradation products of cefpodoxime proxetil. J Chromatogr A. 2006;1129:153–159. doi: 10.1016/j.chroma.2006.06.102. [DOI] [PubMed] [Google Scholar]
- 10.ICH, Q1A(R2), Stability testing of new drug substances and products. In: Proceedings of the international conference on harmonization, IFPMA, Geneva; 2012.
- 11.Deshpande AD, Baheti KG, Chatterjee NR. Degradation of β-lactam antibiotics. Curr sci (Bangalore) 2004;87:1684–1695. [Google Scholar]
- 12.Yamana T, Tsuji A. Comparative stability of cephalosporins in aqueous solution: kinetics and mechanisms of degradation. J Pharm Sci. 1976;65:1563–1574. doi: 10.1002/jps.2600651104. [DOI] [PubMed] [Google Scholar]
- 13.Vilanova B, Donoso J, Muñoz F, García-Blanco F. The degradation mechanism of an oral cephalosporin: cefaclor. Helv Chim Acta. 1996;79:1793–1802. [Google Scholar]
- 14.Li J, Zhang DS, Chong XM, Hu CQ. Influence of substituent groups at the 3-position on the mass spectral fragmentation pathways of cephalosporins. Rapid Commun Mass Spectrom. 2010;24:2143–2150. doi: 10.1002/rcm.4626. [DOI] [PubMed] [Google Scholar]
- 15.Bharathi C, Prasad CS, Bharathi DV, Shankar R, Rao VJ, Dandala R. Structural identification and characterization of impurities in ceftizoxime sodium. J Pharm Biomed. 2007;43:733–740. doi: 10.1016/j.jpba.2006.07.031. [DOI] [PubMed] [Google Scholar]
- 16.Scandola M, Tarzia G, Gaviraghi G, Chiarello D, Traldi P. Mass spectrometric approaches in structural characterization of cephalosporins. Biol Mass Spectrom. 1989;18:851–854. doi: 10.1002/bms.1200181002. [DOI] [PubMed] [Google Scholar]
- 17.Rodriguez JC, Hernández R, González M, Z Rodriguez, Tolón B, Velez H. An improved method for preparation of cefpodoxime proxetil. IL Farmaco. 2003;58:363–369. doi: 10.1016/s0014-827x(03)00051-x. [DOI] [PubMed] [Google Scholar]
- 18.Yao YH, Liu AM, Chen JW. Synthesis of cefpodoxime proxetil. Chin J Pharm. 2008;39:90–91. [Google Scholar]
- 19.Chen ZK, Hu CQ. The degradation mechanism of cephalosporins. World Notes Antibiot. 2004;25:249–265. [Google Scholar]
- 20.Hu M, Hu CQ, Liu WY. Identification of degradation compounds of cephalosporins by LC/MS. Chin Pharm Anal. 2005;25:369–373. [Google Scholar]