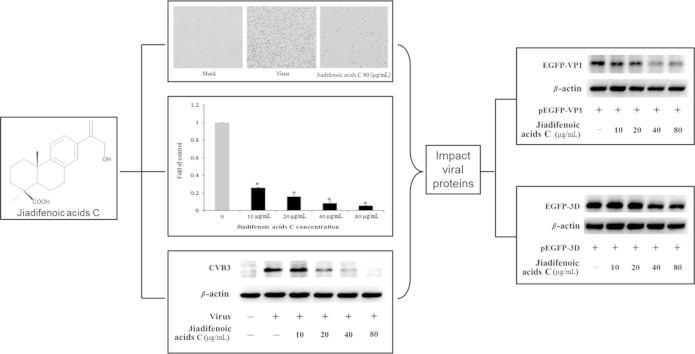
Abstract
Coxsackievirus B type 3 (CVB3) is one of the major causative pathogens associated with viral meningitis and myocarditis, which are widespread in the human population and especially prevalent in neonates and children. These infections can result in dilated cardiomyopathy (DCM) and other severe clinical complications. There are no vaccines or drugs approved for the prevention or therapy of CVB3-induced diseases. During screening for anti-CVB3 candidates in our previous studies, we found that jiadifenoic acids C exhibited strong antiviral activities against CVB3 as well as other strains of Coxsackie B viruses (CVBs). The present studies were carried out to evaluate the antiviral activities of jiadifenoic acids C. Results showed that jiadifenoic acids C could reduce CVB3 RNA and proteins synthesis in a dose-dependent manner. Jiadifenoic acids C also had a similar antiviral effect on the pleconaril-resistant variant of CVB3. We further examined the impact of jiadifenoic acids C on the synthesis of viral structural and non-structural proteins, finding that jiadifenoic acids C could reduce VP1 and 3D protein production. A time-course study with Vero cells showed that jiadifenoic acids C displayed significant antiviral activities at 0–6 h after CVB3 inoculation, indicating that jiadifenoic acids C functioned at an early step of CVB3 replication. However, jiadifenoic acids C had no prophylactic effect against CVB3. Taken together, we show that jiadifenoic acids C exhibit strong antiviral activities against all strains of CVB, including the pleconaril-resistant variant. Our study could provide a significant lead for anti-CVB3 drug development.
KEY WORDS: CVB3, Jiadifenoic acids C, Antiviral activity
Abbreviations: CAR, coxsackievirus and adenovirus receptor; CPE, cytopathic effect; CVB3, coxsackievirus B type 3; CVBs, coxsackie B viruses; DAF, decay accelerating factor; DCM, dilated cardiomyopathy; IC50, 50% inhibitory concentration; IRES, internal ribosomal entry site; MOI, multiplicity of infection; NTR, non-translated region; RBV, ribavirin; RdRp, RNA-dependent RNA polymerase; SI, selectivity index; Vero, African green monkey kidney cells
Graphical abstract
Jiadifenoic acids C exhibit strong antiviral activities against all coxsackievirus B types including pleconaril-resistant CVB3 variant. Results show that it could reduce CVB3 viral RNA and protein synthesis in a dose-dependent manner and the potential targets may be relevant with viral proteins VP1 and 3D.
1. Introduction
Coxsackie B viruses, belonging to the Enterovirus genus of the Picornaviridae family, can cause a broad spectrum of human diseases, and are especially prevalent in neonates and children1. Although the diseases they induce are often mild, such as the common cold, or remain asymptomatic at early stages1, 2, they can develop into severe and fatal diseases like aseptic meningitis, acute and chronic myocarditis, paralytic diseases, rhabdomyolysis, pleurodynia (Bornholm׳s disease), pancreatitis, hepatitis, insulin-dependent diabetes mellitus, and severe septic disease1, 3. Among these, CVB3 is a frequent subtype. It is also one of the major causative agents of chronic, sub-acute, acute, and fulminant myocarditis as well as pancreatitis and aseptic meningitis. It has been reported that more than 50% of human myocarditis is attributable to CVB3 infection. It is estimated that about 10%–20% of people (approximately 20,000–40,000 patients/year in the USA) with symptoms of acute myocarditis induced by CVB3 infection will develop chronic disease, and chronic myocarditis progresses to DCM at a frequency of 3.5–8.5 cases per 100,000 persons (9000–20,000 new cases/year in the USA)1, often requiring heart transplantation4. It can even infect stem cells in the neonatal central nervous system4, and CVB3 may also trigger the onset of type 1 diabetes mellitus5. So it often does great harm to patients׳ hearts, pancreases and central nervous systems, especially in neonates, young children and immunocompromised adult patients6.
CVB3 is a small, non-enveloped, single-stranded and positive-sense RNA virus2, 6, 7. The whole genome of CVB3 is 7.4 kb in length that can encode a large polyprotein. There is a non-translated region (NTR) at both the 5′and 3′ termini of the RNA genome. The 5′-NTR consists of 742 nt and also has the indispensable factors that are required for synthesis and translation of the genome, such as an internal ribosomal entry site (IRES)1, which leads to ribosomal entry and initiates translation of the uncapped RNA genome. The large polyprotein is translated from an open reading frame into a single polypeptide. After translation, the mature viral proteins are released by viral proteinases 2A and 3C as three precursor proteins (P1, P2 and P3) and then processed into the individual proteins. The P1 precursor protein is further processed into the four capsid proteins, VP0 (which is further cleaved to VP4 and VP2), VP3 and VP1. The P2 and P3 precursor proteins are processed into seven non-structural proteins1, 6. Ultimately the CVB virus is composed of four capsid proteins (VP1, VP2, VP3, and VP4)8 and seven non-structural proteins (2A, 2B, 2C, 3A, 3B, 3C, 3D), in which the VP1 protein locates on the surface of the viral capsid, playing an important role in targeting host cells. Inside there is a hydrophobic pocket which is the binding site of the various pocket factors and small antiviral compounds9, 10.
As to the infection of a permissive host cell, CVB3 mainly binds to two specific cell surface receptors, the coxsackievirus and adenovirus receptor (CAR) or the membrane protein decay accelerating factor (DAF). It is generally accepted that CAR acts as the main entry route for CVB3 internalization since some CVB3 strains do not use DAF as a co-receptor4, 11. Receptors interacting with the virus induce the release of the pocket factor and conformational changes in the capsid, which allows the viral RNA to enter host cells. This step can be blocked by capsid inhibitors, such as the ‘WIN’ compounds12, 13. They may replace the pocket factor and occupy the hydrophobic pocket to prevent virus attachment to cell receptors and prevent conformational changes in the viral capsid, thus preventing the release of the viral RNA from the capsid14, 15. However, replacement of Ile-1092 with Leu or Met in the hydrophobic pocket of VP1 results in drug resistance16, 17. So far, in spite of extensive efforts, there is no approved vaccine or antiviral drug for prevention or therapy of CVB3-induced diseases1. Moreover, no single enterovirus serotype is exclusively associated with a particular disease, and enterovirus serotypes are numerous17. Thus, searching for drugs with a broad antiviral spectrum especially effective on the pleconaril-resistant CVB3 strain is important and necessary.
In a large-scale screening for novel drug candidates against CVB3, we found jiadifenoic acids C (Fig. 1) were able to prevent CVB3 replication. Jiadifenoic acid C, an abietane-type diterpene, was first isolated from the roots of Illicium jiadifengpi which is used as a traditional Chinese medicine for the treatment of rheumatism18.
Figure 1.
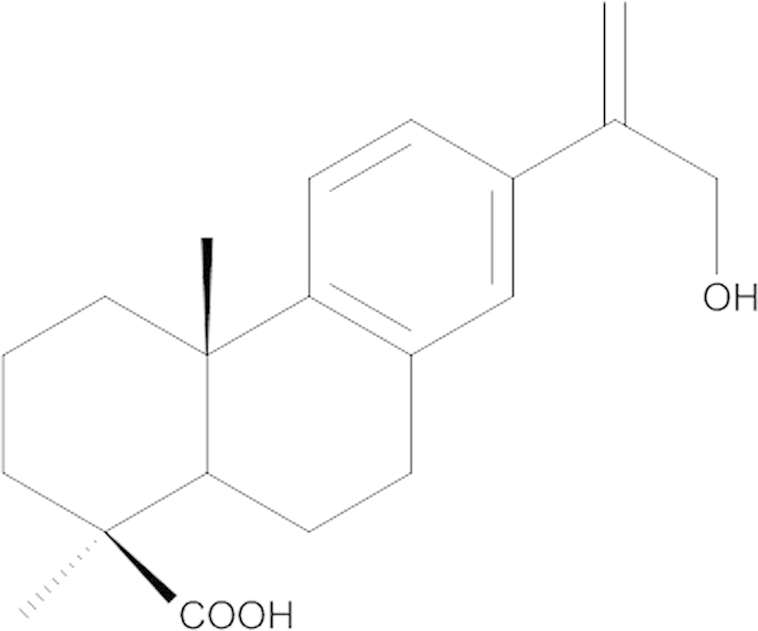
The chemical structure of jiadifenoic acids C.
In the present study, we have demonstrated the antiviral effect of jiadifenoic acids C against the wild-type strain and the pleconaril-resistant variant of CVB3 as well as other strains of CVBs. We also evaluated its effect on reducing CVB3 RNA and protein synthesis as well as its action phase of inhibiting CVB3 replication. Finally, we examined the effect of jiadifenoic acids C on the structural and non-structural viral proteins.
2. Materials and methods
2.1. Cells and virus
African green monkey kidney cells (Vero) were purchased from the American Type Culture Collection and cultured in MEM supplemented with 10% FBS and antibiotics (100 U/mL penicillin G, 100 μg/mL streptomycin) at 37 °C in the presence of 5% CO2.
CVB3 (strain Nancy) was obtained from the American Type Culture Collection and propagated in Vero cells. CVB1 (strain Conn-5), CVB2 (strain Ohio-1), CVB4 (strain J.V.B.), CVB5 (strain Faulkner) and CVB6 (strain Schmitt) were all obtained from the American Type Culture Collection and propagated in Vero cells.
The pleconaril-resistant variant of CVB3 (strain Nancy) was produced by our lab. Briefly, Vero cells (3×105 cells/well) were plated into 24-well culture plates and incubated for 16 h. Cells infected with 200 µL CVB3 (Nancy) at 1000 TCID50 and 100 TCID50, respectively, for 1 h were supplemented with suitable concentrations of jiadifenoic acids C during incubation. When the CPE of cells treated with jiadifenoic acids C reached 4, the virus was collected by centrifugation at 5000 rpm for 15 min at 4 °C after freezing and thawing in −80 °C for three times. The pleconaril-resistant variant of CVB3 was isolated after repeating these steps thirteen-fold19.
The plasmids, including pEGFP-VP1, pEGFP-VP4-VP2-VP3 (pEGFP-VP4-3), pEGFP-2C, pEGFP-3A, pEGFP-3B, pEGFP-3C and pEGFP-3D, were all kindly provided by Professor Zhaohua Zhong, Department of Microbiology, Harbin Medical University.
2.2. Compounds
Jiadifenoic acids C were kindly provided by Professor Shishan Yu, State Key Laboratory of Bioactive Substance and Function of Natural Medicines, Institute of Materia Medica, Chinese Academy of Medical Sciences and Peking Union Medical College. Pleconaril was kindly provided by Professor Xianjin Luo, School of Chemistry and Chemical Engineering, Shanghai Jiaotong University. Ribavirin (RBV) injection (100 mg/mL) was provided by Hangzhou Minsheng Pharmaceutical Co., Ltd.
2.3. Cytotoxicity assay
The cytotoxic effect of jiadifenoic acids C on Vero cells was assayed by CPE. Briefly, cells (3×104 cells/well) were seeded into 96-well culture plates and incubated overnight at 37 °C under 5% CO2. The medium was removed and different concentrations (0.82, 2.47, 7.41, 22.22, 66.67 and 200 µg/mL) of jiadifenoic acids C were applied. The result was measured with three repeats and the experiment performed in triplicate. After 3 days׳ incubation, the cytotoxicity of jiadifenoic acids C was determined by CPE assay. TC50 was defined as the concentration that inhibits 50% cellular growth in comparison with untreated controls, and calculated by the Reed and Muench method.
2.4. CPE inhibition assay for anti-CVB3
The anti-CVB3 activity of jiadifenoic acids C was also assayed by the CPE method. Briefly, cells (3×104 cells/well) were plated into 96-well culture plates and incubated for 16 h. The medium was removed and cells were infected with 100 µL CVB3 of 100 TCID50 for 1 h, after which various concentrations of jiadifenoic acids C were added immediately for incubation until the CPE of the control group cells reached 4. Each experiment was tested in triplicate and performed at least thrice separately. The 50% inhibitory concentration (IC50) was determined by the Reed and Muench method. The selectivity index (SI) was calculated as the ratio of TC50/IC50.
2.5. RT-PCR quantification of CVB3 RNA
Vero cells (9×105 cells/well) were plated into 6-well culture plates and incubated for 16 h. The medium was removed and cells were infected with 600 µL CVB3 (Nancy) of 100 TCID50 for 1 h, after which various concentrations of jiadifenoic acids C were added immediately for incubation for another 24 h. Cellular and total viral RNA were extracted from infected cells using the RNeasy Mini kit (QIAGEN) according to the manufacturer׳s instructions.
One-step quantitative real-time polymerase chain reaction (qRT-PCR) was carried out using the ABI 7500 Fast Real-Time PCR system (Applied Biosystems) with SuperScript III Platinum SYBR Green One-step RT PCR Kit (invitrogen) and CVB3 forward primer (5′-TGCTCCGCAGTTAGGATTAGC-3′) and reverse primer (5′-ACATGGTGCGAAGAGTCTATTGAG-3′) targeting a conserved region of the VP1 gene20, using β-actin as internal control with the forward primer (5′-TGACGGGGTCACCCACACTGTGCCCATCTA-3′) and reverse primer (5′-CTAGAAGCATTTGCGGTGGACGATG-3′)21.
The PCR assay was carried out in a volume of 25 μL consisting of 12.5 μL of 2×SYBR Green Reaction Mix, 0.5 μL of 10 μmol/L of each oligonucleotide primer, 0.5 μL of SuperScript III RT/Platinum Tap Mix, 1 μL ROX Reference and 1 μL of RNA extracted from the samples. The target fragment amplification was carried out as follows: reverse transcription at 50 °C for 3 min; initial activation of HotStar Taq DNA Polymerase at 95 °C for 5 min; 40 cycles in two steps: 95 °C for 15 s, 60 °C for 30 s. At the end of the amplification cycles melting temperature analysis was carried out by a slow increase in temperature (0.1 °C/s) up to 95 °C.
2.6. Western blot analysis of CVB3 protein
Vero cells (9×105 cells/well) were plated into 6-well culture plates for incubation for 16 h. The medium was removed and the cells were infected with 600 µL CVB3 (Nancy) of 100 TCID50 for 1 h, after which various concentrations of jiadifenoic acids C were added immediately for incubation for 36 h. After incubation, the cells were rapidly washed with ice-cold PBS and ice-cold M-PER mammalian protein extraction reagent (Thermo) containing halt protease inhibitor single-use cocktail (Thermo) was added. The cells were scraped, collected and lysed for 30 min. The cell lysates were centrifuged at 12,000×g for 15 min at 4 °C to yield the whole cell extract. The protein concentration was determined by the BCA assay (Thermo) according to the instructions of the manufacturer. Samples from these supernatant fractions (10 μg protein) were denatured and subjected to SDS–PAGE using a 10% (w/v) running gel. Proteins were transferred to a PVDF membrane and the membrane was incubated successively at room temperature with 5% (w/v) non-fat milk in TBST for 1 h. Membranes were then incubated at room temperature using an mouse anti-coxsackie virus B3 monoclonal antibody (Millipore) and an anti-β-actin (Cell Signaling) antibody used at a dilution of 1:1000 and 1:3000 in 3% (w/v) non-fat milk for 2 h21. The membranes were washed with TBST 3 times for 10 min each and incubated with a 1:5000 dilution of anti-mouse horseradish peroxidase antibody (Cell Signaling) for 1 h. Following each incubation, the membrane was washed extensively with TBST.
2.7. Time course assay
The antiviral activity of jiadifenoic acids C was also examined at different time periods before and after viral infection. Briefly, cells (3×104 cells/well) were seeded and incubated for 16 h as previously described. The cell monolayer was then infected with 100 µL CVB3 (Nancy) at a multiplicity of infection (MOI) of 1 for 1 h. 80 μg/mL jiadifenoic acids C in 100 μL were added following the protocol. The cells were incubated until the CPE of the control group cells reached 4 and then assayed with the CPE assay.
2.8. Western blot analysis of CVB3׳s structural and non-structural proteins
Vero cells (9×105 cells/well) were plated into 6-well culture plates for incubation for 16 h. The medium was removed and cells were transfected with 4 μg of plasmids expressing the EGFP-tagged VP1, VP4-3, 2A, 2B, 2C, 3A, 3B, 3C, and 3D, respectively (pEGFP-VP1, pEGFP-VP4-VP2-VP3 (pEGFP-VP4-3), pEGFP-2C, pEGFP-3A, pEGFP-3B, pEGFP-3C and pEGFP-3D), using Lipofectamine 2000 Reagent (invitrogen) and opti-MEM (1×) Reduced Serum Medium (Gibco) according to the instructions of the manufacturer. After 48 h, the medium was removed and the cells were treated with various concentrations (0, 10, 20, 40 and 80 μg/mL) of jiadifenoic acids C for incubation for another 36 h. The cells were harvested for western blot assay as described above.
2.9. CPE inhibition assay for anti-coxsackievirus B type
The anti-coxsackievirus activity of jiadifenoic acids C also was assayed by the CPE method. Briefly, cells (3×104 cells/well) were plated into 96-well culture plates and incubated for 16 h. The medium was removed and the cells were infected with 100 µL coxsackievirus at 100 TCID50 for 1 h, after which various concentrations of jiadifenoic acids C were added immediately for incubation until the CPE of the control group cells reached 4. Each experiment was measured in triplicate and performed at least thrice separately. The IC50 was determined by the Reed and Muench method. The SI was calculated as the ratio of TC50/IC50.
3. Results
3.1. Inhibition of CVB3 replication by jiadifenoic acids C in vitro
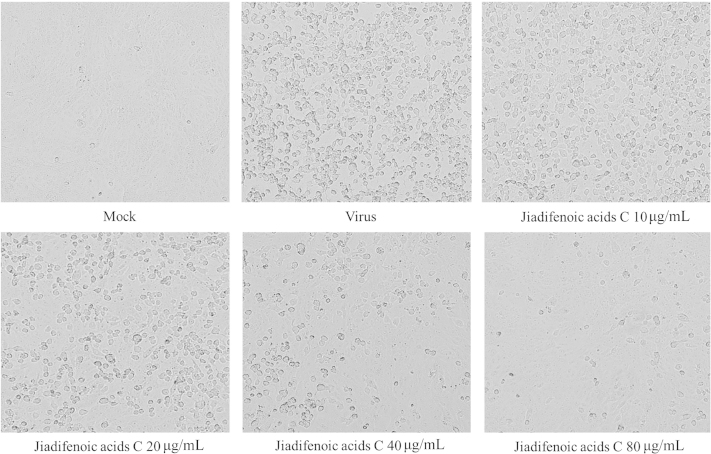
The cytotoxic effect of jiadifenoic acids C on Vero cells was measured with a conventional CPE method, and the TC50 was 115.47 µg/mL. We used a CPE reduction assay to study the anti-CVB3 activities of jiadifenoic acids C. As shown in Fig. 2, jiadifenoic acids C significantly reduced CVB3-induced CPE in a dose-dependent manner. The IC50 and selectivity index of jiadifenoic acids C for CVB3 infection in Vero cells are shown in Table 1.
Figure 2.
Inhibition of CVB3-induced CPE by jiadifenoic acids C. Confluent Vero cultures were inoculated with 100 TCID50 CVB3 (Nancy). After 1 h of adsorption at 37 °C, the monolayers were washed with PBS and incubated at 37 °C in the maintenance medium (MEM plus 2% FBS) with or without various concentrations of jiadifenoic acids C. The morphological changes in Vero cells were observed until the CPE of the control group cells reached 4.
Table 1.
Antiviral activities of jiadifenoic acids C against CVB3 in vitro.
| CVB3 (Nancy) | Jiadifenoic acids C |
Pleconaril |
RBV |
||||||
|---|---|---|---|---|---|---|---|---|---|
| TC50 (μg/mL) | IC50 (μg/mL) | SI | TC50 (μg/mL) | IC50 (ng/mL) | SI | TC50 (mg/mL) | IC50 (μg/mL) | SI | |
| Standard strain | 115.47 | 18.63±10 | 6.19 | 15.41 | 0.38±0.1 | 40553 | >10 | 366±74 | >27.3 |
| Resistant strain | 115.47 | 52.58±19.9 | 2.2 | 15.41 | 13.8±3.55 | 1117 | >10 | 515±75 | >19.4 |
3.2. Inhibition of viral RNA synthesis by jiadifenoic acids C
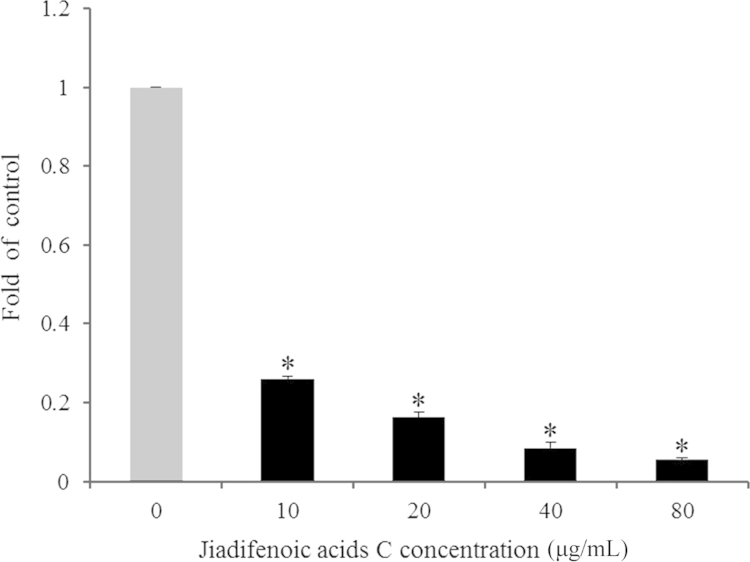
To examine the effect of jiadifenoic acids C on the synthesis of CVB3 RNA, the CVB3 (Nancy)-infected cells were treated with jiadifenoic acids C and the viral RNA in cells was extracted and analyzed using the RT-PCR assay. As shown in Fig. 3, jiadifenoic acids C inhibited viral RNA synthesis in a dose-dependent manner.
Figure 3.
Jiadifenoic acids C reduce CVB3 viral RNA synthesis in Vero cells. Vero cells (9×105 cells/well) were plated into 6-well culture plates for incubation for 16 h. The medium was removed and cells were infected with 600 μL CVB3 of 100 TCID50 for 1 h, after which various concentrations of jiadifenoic acids C were added immediately and the incubation continued for 24 h. The cells were harvested for one-step qRT-PCR assay. *P<0.001 vs control.
3.3. Inhibition of viral protein synthesis by jiadifenoic acids C
To examine the effect of jiadifenoic acids C on the synthesis of CVB3 proteins, the CVB3 (Nancy)-infected cells were treated with jiadifenoic acids C and the viral proteins in the cells were analyzed using western blotting. As shown in Fig. 4, jiadifenoic acids C inhibited the viral protein synthesis in a dose-dependent manner.
Figure 4.
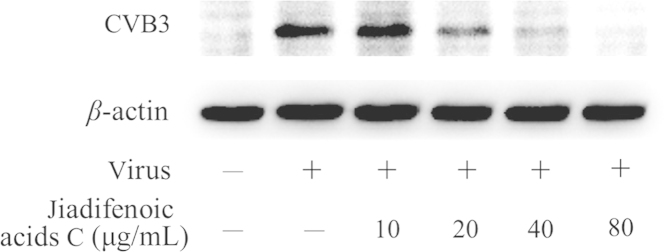
Jiadifenoic acids C reduces CVB3 viral proteins (VP1) synthesis in Vero cells by western blot assay. Vero cells (9×105 cells/well) were plated into 6-well culture plates for incubation for 16 h. The medium was removed and cells were infected with 600 μL CVB3 (Nancy) of 100 TCID50 for 1 h, after which various concentrations of jiadifenoic acids C were added and incubation continued for 36 h. The cells were harvested for western blot assay.
3.4. Time course assay
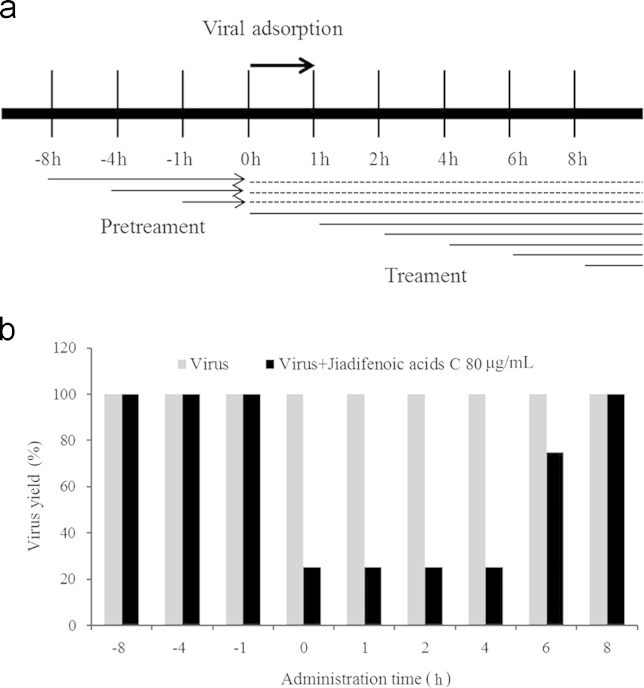
To examine the action phase of jiadifenoic acids C in inhibiting CVB3, the CVB3 (Nancy)-infected cells were treated with 80 µg/mL jiadifenoic acids C and the viral suppression rate in cells was analyzed using CPE. As shown in Fig. 5, treatment with jiadifenoic acids C at 0–6 h after CVB3 infection inhibited viral replication, while they were ineffective at 8 h and −8 to −1 h of CVB3 infection.
Figure 5.
Time course assay of jiadifenoic acids C against CVB3. (a) An illustration of the treatment with jiadifenoic acids C. Confluent Vero cells were inoculated with CVB3 (Nancy) at a multiplicity of infection (MOI) of 1 for 1 h. Jiadifenoic acids C (80 µg/mL) were added at the designated times. (b) The morphological changes in Vero cells were observed until the CPE of the control group cells reached 4. The results show CPE induced by CVB3 infection with or without jiadifenoic acids C treatment, and the virus control group is set as 100%.
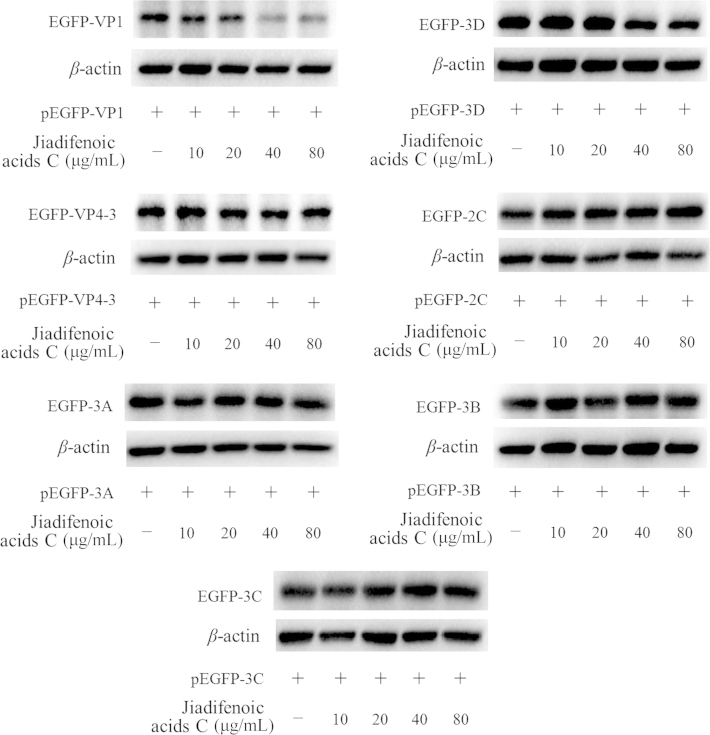
3.5. Inhibition of the synthesis of various viral structural proteins and non-structural proteins by jiadifenoic acids C
To examine which viral components are affected by jiadifenoic acids C, Vero cells were transfected with plasmids expressing the EGFP-tagged VP1, VP4-3, 2A, 2B, 2C, 3A, 3B, 3C and 3D, respectively. After 48 h the medium was removed and cells were treated with various concentrations of jiadifenoic acids C for incubation for another 36 h, and the cells were harvested for western blot assay. As shown in Fig. 6, jiadifenoic acids C could reduce the EGFP-VP1 and EGFP-3D production in a dose-dependent manner.
Figure 6.
Jiadifenoic acids C reduce CVB3 viral proteins (VP1 and 3D) synthesis in Vero cells by western blot assay. Vero cells (9×105 cells/well) were plated into 6-well culture plates for incubation for 16 h. The medium was removed and cells were transfected with 4 μg plasmids pEGFP-VP1, pEGFP-VP4-VP2-VP3 (pEGFP-VP4-3), pEGFP-2C, pEGFP-3A, pEGFP-3B, pEGFP-3C and pEGFP-3D. After 48 h cells were treated with various concentrations of jiadifenoic acids C and incubation continued for 36 h. The cells were harvested for western blot assay.
3.6. Inhibition of coxsackievirus B-type replication by jiadifenoic acids C in vitro
The CPE reduction assay was used to study anti-coxsackievirus B-type activities of jiadifenoic acids C. Indeed, jiadifenoic acids C exhibited activities against CVB1, B2, B3, B4, B5 and B6 in Vero cells. The IC50 and selectivity index of jiadifenoic acids C for CVBs infections are shown in Table 2.
Table 2.
Antiviral activities of jiadifenoic acids C against coxsackievirus in vitro.
| Virus | Jiadifenoic acids C |
Pleconaril |
RBV |
||||||
|---|---|---|---|---|---|---|---|---|---|
| TC50 (μg/mL) | IC50 (μg/mL) | SI | TC50 (μg/mL) | IC50 (ng/mL) | SI | TC50 (mg/mL) | IC50 (μg/mL) | SI | |
| CVB1 | 115.47 | 5.05±1.46 | 22.87 | 15.41 | 0.885±0.095 | 17,412 | >10 | 515±75 | >19.42 |
| CVB2 | 115.47 | 10.9±4.3 | 10.59 | 15.41 | 0.5±0.16 (μg/mL) | 30.82 | >10 | 554±37 | >18.05 |
| CVB4 | 115.47 | 25.16±15.8 | 4.59 | 15.41 | 2.81±1.5 | 5484 | >10 | 331±109 | >30.21 |
| CVB5 | 115.47 | 6.03±1.9 | 19.15 | 15.41 | 1.13±0.75 | 13,637 | >10 | 605±15 | >16.53 |
| CVB6 | 115.47 | 13.9±1.5 | 8.31 | 15.41 | 6.46±1 | 2385 | >10 | 366±74 | >29.76 |
4. Discussion
Myocarditis is inflammation of the myocardium which most frequently follows enterovirus (picornavirus family) infection8. CVBs represent the most commonly identified infectious agents associated with acute and chronic myocarditis. CVB3 is one of the most common viral etiologic variants3. It can induce severe infections towards heart, pancreas and brain. In recent years the incidence rate appears to be rising in China. However, its specific pathogenesis has not been fully understood yet. And to date there is no approved vaccine or antiviral drug for prevention or therapy of CVB3-induced diseases1. Moreover, there are large numbers of enterovirus serotypes and no single enterovirus serotype is exclusively associated with a particular disease. Thus, the search for drugs with a broad antiviral spectrum to prevent and treat myocarditis is timely.
Diterpenes forms a large and structurally diverse class of secondary metabolites isolated from plants and have a wide spectrum of useful biological activities. Jiadifenoic acid C, an abietane-type diterpene, was first isolated from the roots of Illicium jiadifengpi which is used as a traditional Chinese medicine for the treatment of rheumatism18. In our previous study, jiadifenoic acid C was reported to exhibit antiviral activities against CVB2, B3, B4, and B6. In order to evaluate jiadifenoic acids C in a more systematic manner, we carried out a series of experiments.
In this study, the antiviral effects of jiadifenoic acids C against all strains of CVBs have been demonstrated. Jiadifenoic acids C could reduce CVB3 RNA and protein synthesis in a dose-dependent manner. It could also prevent the replication of a pleconaril-resistant CVB3 variant. A time-course study showed that jiadifenoic acids C functions at an early step of CVB3 replication but has no prophylactic effect against CVB3, suggesting that it most likely does not inhibit CVB3 entry and uncoating, but suppresses viral RNA/protein biosynthesis. Moreover, the initial analysis of the effect of jiadifenoic acids C on VP1, VP2-4, 2C, 3A, 3B, 3C, and 3D showed that jiadifenoic acids C could inhibit viral protein VP1 and 3D expression in plasmid-transfected cells, indicating that jiadifenoic acids C׳s antiviral effects may be associated with structural protein VP1 and non-structural protein 3D. However, as the viral proteins׳ expression in the plasmid-transfected cells did not rely on viral replication, but depended on host cellular transcription and translation machinery, it was unclear how the selective reduction of the two viral proteins was accomplished. Perhaps it is because jiadifenoic acids C could promote the degradation of these viral proteins. Since jiadifenoic acids C have a similar effect on both the pleconaril-resistant variant of CVB3 and the wild-type strain, we suppose that it most likely inhibits CVB3 replication in a different way than does pleconaril. The 3D protein is the viral RNA-dependent RNA polymerase (RdRp), which plays an essential role in the replication machinery of RNA viruses22, 23. How jiadifenoic acids C promote viral 3D protein degradation in a dose-dependent manner remains unresolved, and requires further exploration.
Although CVB3 is known to be a potent pathogen that can cause acute or chronic cardiac-related diseases as defined by the Dallas criteria, CVB3-specific prophylactic or therapeutic procedures are lacking. Jiadifenoic acids C can actively inhibit replication of CVBs as well as the pleconaril-resistant CVB3. Our findings may provide a significant lead for anti-CVB3 drug optimization and development as well as DCM therapy. We will continue to clarify the mechanism by which jiadifenoic acids C mediate their antiviral effects.
Acknowledgments
This work was supported by the National Science and Technology Major Project of the Ministry of Science and Technology of China (2012ZX09301002-001-015 and 2012ZX10004501-004-001).
Footnotes
Peer review under responsibility of Institute of Materia Medica, Chinese Academy of Medical Sciences and Chinese Pharmaceutical Association.
References
- 1.Kim D.S., Nam J.H. Characterization of attenuated coxsackievirus B3 strains and prospects of their application as live-attenuated vaccines. Expert Opin Biol Ther. 2010;10:179–190. doi: 10.1517/14712590903379502. [DOI] [PubMed] [Google Scholar]
- 2.Huber M., Selinka H.C., Kandolf R. Tyrosine phosphorylation events during coxsackievirus B3 replication. J Virol. 1997;71:595–600. doi: 10.1128/jvi.71.1.595-600.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fechner H., Pinkert S., Geisler A., Poller W., Kurreck J. Pharmacological and biological antiviral therapeutics for cardiac coxsackievirus infections. Molecules. 2011;16:8475–8503. doi: 10.3390/molecules16108475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Koho T., Koivunen M.R., Oikarinen S., Kummola L., Makinen S., Mähönen A.J. Coxsackievirus B3 VLPs purified by ion exchange chromatography elicit strong immune responses in mice. Antiviral Res. 2014;104:93–101. doi: 10.1016/j.antiviral.2014.01.013. [DOI] [PubMed] [Google Scholar]
- 5.Martin U., Jarasch N., Nestler M., Rassmann A., Munder T., Seitz S. Antiviral effects of pan-caspase inhibitors on the replication of coxsackievirus B3. Apoptosis. 2007;12:525–533. doi: 10.1007/s10495-006-0015-y. [DOI] [PubMed] [Google Scholar]
- 6.Kim D.S., Nam J.H. Application of attenuated coxsackievirus B3 as a viral vector system for vaccines and gene therapy. Human Vaccines. 2011;7:410–416. doi: 10.4161/hv.7.4.14422. [DOI] [PubMed] [Google Scholar]
- 7.Hwang H.Y., Kim J.Y., Lim J.Y., Chung S.K., Nam J.H., Park S.I. Coxsackievirus B3 modulates cell death by downregulating activating transcription factor 3 in HeLa cells. Virus Res. 2007;130:10–17. doi: 10.1016/j.virusres.2007.05.007. [DOI] [PubMed] [Google Scholar]
- 8.Huber S.A. Autoimmunity in coxsackievirus B3 induced myocarditis. Autoimmunity. 2006;39:55–61. doi: 10.1080/08916930500484906. [DOI] [PubMed] [Google Scholar]
- 9.Wang T., Yu B., Lin L., Zhai X., Han Y., Qin Y. A functional nuclear localization sequence in the VP1 capsid protein of coxsackievirus B3. Virology. 2012;433:513–521. doi: 10.1016/j.virol.2012.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Makarov V.A., Riabova O.B., Granik V.G., Wutzler P., Schmidtke M. Novel [(biphenyloxy)propyl]isoxazole derivatives for inhibition of human rhinovirus 2 and coxsackievirus B3 replication. J Antimicrob Chemother. 2005;55:483–488. doi: 10.1093/jac/dki055. [DOI] [PubMed] [Google Scholar]
- 11.Zhang Q.M., Song W.Q., Li Y.J., Qian J., Zhai A.X., Wu J. Over-expression of mitochondrial antiviral signaling protein inhibits coxsackievirus B3 infection by enhancing type-I interferons production. Virology J. 2012;9:312. doi: 10.1186/1743-422X-9-312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Romero J.R. Pleconaril: a novel antipicornaviral drug. Expert Opin Investig Drugs. 2001;10:369–379. doi: 10.1517/13543784.10.2.369. [DOI] [PubMed] [Google Scholar]
- 13.Rotbart H.A., Webster A.D. Treatment of potentially life-threatening enterovirus infections with pleconaril. Clin Infect Dis. 2001;32:228–235. doi: 10.1086/318452. [DOI] [PubMed] [Google Scholar]
- 14.Groarke J.M., Pevear D.C. Attenuated virulence of pleconaril-resistant coxsackievirus B3 variants. J Infect Dis. 1999;179:1538–1541. doi: 10.1086/314758. [DOI] [PubMed] [Google Scholar]
- 15.Desmond R.A., Accortt N.A., Talley L., Villano S.A., Soong S.J., Whitley R.J. Enteroviral meningitis: natural history and outcome of pleconaril therapy. Antimicrob Agents Chemother. 2006;50:2409–2414. doi: 10.1128/AAC.00227-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schmidtke M., Wutzler P., Zieger R., Riabova O.B., Makarov V.A. New pleconaril and [(biphenyloxy)propyl]isoxazole derivatives with substitutions in the central ring exhibit antiviral activity against pleconaril-resistant coxsackievirus B3. Antiviral Res. 2009;81:56–63. doi: 10.1016/j.antiviral.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 17.Schmidtke M., Hammerschmidt E., Schüler S., Zell R., Birch-Hirschfeld E., Makarov V.A. Susceptibility of coxsackievirus B3 laboratory strains and clinical isolates to the capsid function inhibitor pleconaril: antiviral studies with virus chimeras demonstrate the crucial role of amino acid 1092 in treatment. J Antimicrob Chemother. 2005;56:648–656. doi: 10.1093/jac/dki263. [DOI] [PubMed] [Google Scholar]
- 18.Zhang G.J., Li Y.H., Jiang J.D., Yu S.S., Qu J., Ma S.G. Anti-coxsackie virus B diterpenes from the roots of Illicium jiadifengpi. Tetrahedron. 2013;69:1017–1023. [Google Scholar]
- 19.Sarisky R.T., Quail M.R., Clark P.E., Nguyen T.T., Halsey W.S., Wittrock R.J. Characterization of herpes simplex viruses selected in culture for resistance to penciclovir or acyclovir. J Virology. 2001;75:1761–1769. doi: 10.1128/JVI.75.4.1761-1769.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ahn J., Ko A., Jun E.J., Won M., Kim Y.K., Ju E.S. Antiviral effects of small interfering RNA simultaneously inducing RNA interference and type 1 interferon in coxsackievirus myocarditis. Antimicrob Agents Chemother. 2012;56:3516–3523. doi: 10.1128/AAC.06050-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang H.Q., Meng S., Li Z.R., Peng Z.G., Han Y.X., Guo S.S. The antiviral effect of 7-hydroxyisoflavone against Enterovirus 71 in vitro. J Asian Nat Prod Res. 2013;15:382–389. doi: 10.1080/10286020.2013.770737. [DOI] [PubMed] [Google Scholar]
- 22.Gazina E.V., Smidansky E.D., Holien J.K., Harrison D.N., Cromer B.A., Arnold J.J. Amiloride is a competitive inhibitor of coxsackievirus B3 RNA polymerase. J Virol. 2011;85:10364–10374. doi: 10.1128/JVI.05022-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gruez A., Selisko B., Roberts M., Bricogne G., Bussetta C., Jabafi I. The crystal structure of coxsackievirus B3 RNA-dependent RNA polymerase in complex with its protein primer VPg confirms the existence of a second VPg binding site on Picornaviridae polymerases. J Virol. 2008;82:9577–9590. doi: 10.1128/JVI.00631-08. [DOI] [PMC free article] [PubMed] [Google Scholar]