Abstract
Qi She Pill (QSP) is a traditional Chinese medicine (TCM) prescription that has been used in treating cervical spondylosis radiculopathy for many years. In this study, a simple and sensitive method using ultra-high-performance liquid chromatography–tandem mass spectrometry (UHPLC–MS/MS) on a reverse-phase C18 column was developed for the simultaneous determination of the 19 major components in QSP. We found that the optimum mobile phase for gradient elution was 0.1% formic acid and methanol. The correlation coefficients of all calibration curves were greater than 0.99. Recoveries measured at three concentration levels varied from 95.43% to 102.35%. Relative standard deviations of intra- and inter-day precisions were less than 4.45%. After successfully validating our method, we then applied it to the quantification of 19 components in QSP products to show that this method provides a new standard in quality assessment of TCM prescriptions containing multiple bioactive components.
KEY WORDS: UHPLC–MS/MS, Active components, Traditional Chinese medicine, Qi She Pill (QSP)
Abbreviations: AST-III, astragaloside III; AST-IV, astragaloside IV; BER, berberine; CA, cholic acid; CCS, calycosin; CCSG, calycosin-7-O-β-d-glucoside; CE, collision energy; CSR, cervical spondylosis radiculopathy; DAI, daidzein; FAN, fangchinoline; FOR, formononetin; GA, gallic acid; 5-O-M, 5-O-methylvisammioside; ONO, ononin; PAL, palmatine; QSP, Qi She Pill; RA, rheumatoid arthritis; SEA, senkyunolide A; SEI, senkyunolide I; SIN, sinomenine; SRM, selective reaction monitoring; TCM, traditional Chinese medicine; TET, (+)-tetrandrine; THP, tetrahydropalmatine; THPB, tetrahydroepiberberine
Graphical abstract
An accurate and reliable UPLC–MS/MS method was developed to assess the multiple constituents in QSP. This method provides a new standard in quality control of QSP. Many components in the QSP prescription which are responsible for beneficial effects were detected for the first time.
1. Introduction
Cervical spondylosis radiculopathy (CSR) is a chronic degenerative condition of the cervical spine that affects the vertebral bodies and intervertebral disks of the neck, as well as the components of the spinal canal. People with CSR may experience stiffness and pain or tingling in their necks, shoulders, or arms, leading to an impaired ability to work and a general decrease in their quality of life1, 2, 3, 4. Currently, few drugs can effectively control CSR. However, Qi She Pill (QSP), a traditional Chinese medicine prescription, have been used in the clinical management of CSR for more than 40 years. QSP inhibit inflammation in CSR patients, and published reports have shown their beneficial effects on cervical intervertebral disc degeneration (New drug clinical trial documents number: China State Drug Administration 2003L04416)5, 6, 7.
QSP is composed of six medicinals (Radix Astragali, Rhizoma Chuanxiong, Caulis Sinomenii, Radix Stephaniae Tetrandrae, Artificial Moschus, and Artificial Bovis Calculus). However, little is known about the exact components of QSP; therefore, many potential components may be responsible for their beneficial effects. Our previous quality control study of QSP suggested that the pills are composed of astragaloside, alkaloids and flavonoids, as well as other compounds, that have antiviral and antioxidant actions8, 9, 10, 11, 12, 13, 14, 15 and that have been clinically used in the therapeutic treatment of rheumatoid arthritis (RA) because of their remarkable anti-inflammatory properties.
In Chinese medicines, some active components are regarded as indices of quality control in prescriptions. Currently, only astragaloside IV and sinomenine are used as indices for quality control in the preparation of QSP16. However, it is well known that analysis of just a single or even a few marker compounds is not enough for good quality control of complex herbal products. Thus, an accurate and reliable method that assesses the multiple constituents of QSP is urgently needed to maintain safety by accurately monitoring quality control of QSP, as well as to enhance the efficacy of QSP in CSR control.
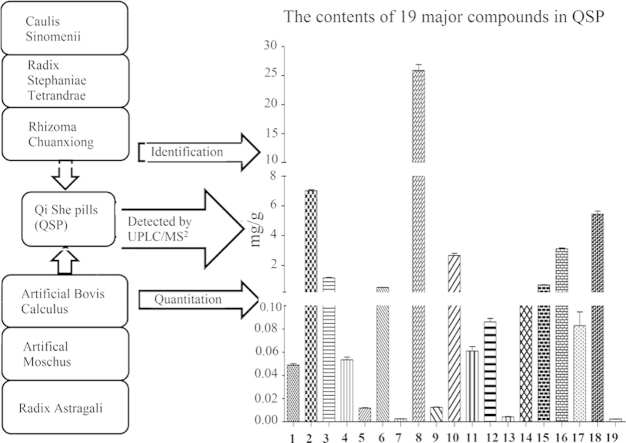
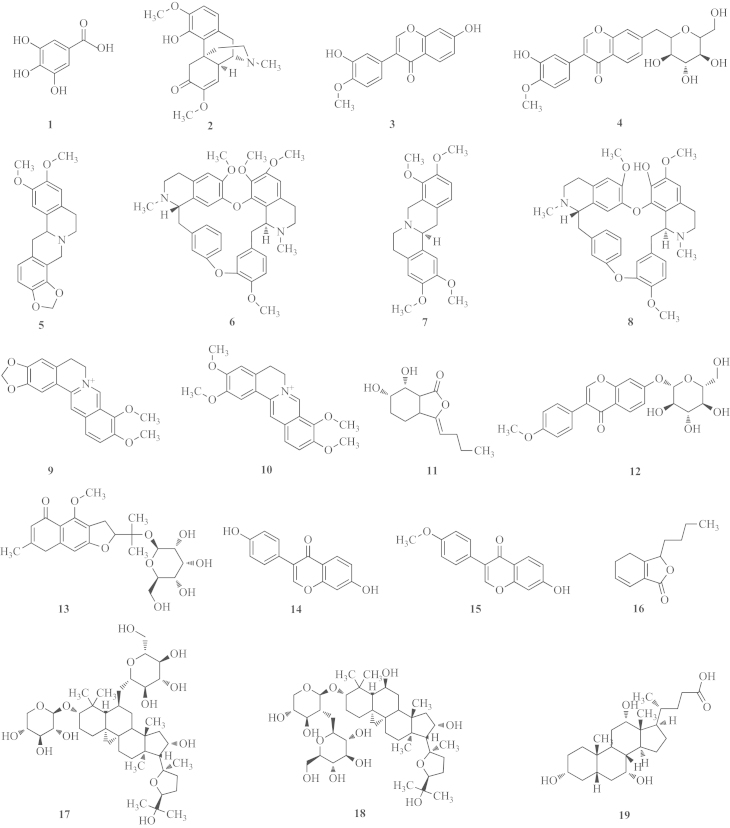
Thus, in this study, a simple, accurate and reliable UHPLC/MS method was developed for the simultaneous determination of the following 19 active components of QSP: (1) gallic acid (GA), (2) sinomenine (SIN), (3) calycosin (CCS), (4) calycosin-7-O-β-d-glucoside (CCSG), (5) tetrahydroepiberberine (THPB), (6) (+)-tetrandrine (TET), (7) tetrahydropalmatine (THP), (8) fangchinoline (FAN), (9) berberine (BER), (10) palmatine (PAL), (11) senkyunolide I (SEI), (12) ononin (ONO), (13) 5-O-methylvisammioside (5-O-M), (14) daidzein (DAI), (15) formononetin (FOR), (16) senkyunolide A (SEA), (17) astragaloside III (AST-III), (18) astragaloside IV (AST-IV), and (19) cholic acid (CA) (Fig. 1). Five batches of prescription extracts were used in this study. Our results indicate that the method proposed by us is particularly suitable for the routine analysis and quality control of QSP. Furthermore, we suggest that our established method would be applicable for quality control in the study of other compounds in TCM.
Figure 1.
The chemical structures of the 19 selected major compounds of QSP. (1) Gallic acid (GA), (2) sinomenine (SIN), (3) calycosin (CCS), (4) calycosin-7-O-β-d-glucoside (CCSG), (5) tetrahydroepiberberine (THPB), (6) (+)-tetrandrine (TET), (7) tetrahydropalmatine (THP), (8) fangchinoline (FAN), (9) berberine (BER), (10) palmatine (PAL), (11) senkyunolide I (SEI), (12) ononin (ONO), (13) 5-O-methylvisammioside (5-O-M), (14) daidzein (DAI), (15) formononetin (FOR), (16) senkyunolide A (SEA), (17) astragaloside III (AST-III), (18) astragaloside IV (AST-IV), and (19) cholic acid (CA).
2. Materials and methods
2.1. Materials and reagents
The herbal portions of QSP, including, Radix Astragali, Rhizoma Chuanxiong, Caulis Sinomenii, Radix Stephaniae Tetrandrae, Artificial Moschus, and Artificial Bovis Calculus were purchased from Shanghai Kangqiao Pharmaceutical Co., Ltd. Standards of GA (110831-201003, >98%), SIN (110774-200507, >98%), TET (110711-200708, >98%), FAN (110793-200605, >98%), BER-HCl (110713-200911, >98%), PAL-HCl (110732-200907, >86%), AST-IV (110781-200613, >98%), and CA (100078-200414, >98%) were provided by the Chinese National Institute for the Control of Pharmaceutical and Biological Products (Beijing, China). Standards of CCS (120628, >98%), CCSG (121219, >97%), SEI (120728, >96%), ONO (120531, >98%), 5-O-M (120620, >98%), DAI (120829, >98%), FOR (120227, >98%), SEA (120828, >96%), and AST-III (120615, >98%) were purchased from Sichuan Weikeqi Biotech Co., Ltd. (Sichuan, China). Standards for THPB (10421-201207, >98%), TET (110711-200708, >98%), and THP (20302-201204, >98%) were purchased from Nanchang Beta Biotech Co., Ltd. (Nan chang, China).
HPLC-grade methanol was obtained from Dikma Technologies Inc. (USA). Formic acid (HPLC-grade) was purchased from CNW Technologies GmbH (Düsseldorf, Germany). Deionized water was purified using the Milli-Q Reagent Water System (Millipore, Bedford, MA, USA). All other reagents were of analytical grade.
2.2. Chromatographic and mass spectrometric conditions
The Dionex 3000 UHPLC system equipped with Thermo TSQ quantum Access MAX triple quadruple mass spectrometry was used for the chromatographic analysis. All separations were carried out on a Waters Acquity UPLC BEH C18 column (50 mm×2.1 mm, 1.7 μm). The mobile phase consisted of (A) methanol and (B) aqueous formic acid (0.1%, v/v) using the following gradient elution: 20% A at 0–1 min, 20%–100% A at 1–8 min, 100% A at 8–12 min, 20% A at 12.1–16 min. The re-equilibration time was 4 min. The temperature of the column oven was 40 °C. The solvent flow rate was 0.3 mL/min. Nitrogen was used as the sheath and auxiliary gas, and Argon was used as the damping and collision gas. Ion spray voltage was set at 3.5 kV(+) and 2.5 kV(−), tube lens offset at 184 V(+) and 141 V(−), sheath gas rate at 35 psi, and auxiliary gas flow rate at 1.5 L/min.
2.3. Standard preparation
Nineteen stock solutions were prepared by dissolving 1.0 mg of each standard in 10 mL of methanol. Appropriate volumes of each stock solution were mixed together. Then, the mixture was diluted serially to prepare the reference working solutions.
2.4. Sample solution preparation
Twenty QS pills of each batch (five batches, 701, 702, 703, 704, 801, are from the same Shanghai Huanghai Pharmaceutical Co., Ltd.) were ground into powder by using a pestle and mortar, and 0.10 g of the powder was ultrasonically extracted with 50 mL of 100% (v/v) methanol for 20 min at 30 °C, and cooled at room temperature. The supernatant was subsequently filtered through a 0.22 μm membrane. An aliquot of 2 μL of the solution was injected into the UHPLC–MS/MS system for analysis.
2.5. Method validation
2.5.1. Linearity and sensitivity
A series of working solutions for all the standard substances were prepared by serial dilutions with methanol. Linearity was assessed by assaying calibration curves in three replicates with standard working solutions containing a defined concentration range (GA, SIN, CCS, CCSG, THPB, TET, THP, FAN, BER-HCl, PAL-HCl, SEI, ONO, 5-O-M, DAI, FOR, SEA, AST-III, AST-IV, and CA).
The sensitivity of the method was evaluated by determining the limit of detection (LOD) and the limit of quantification (LOQ). The LOD was determined as the sample concentration (S) resulting in a peak height greater than three times the baseline noise level (N) (S/N=3). The LOQ was estimated as a signal 10 times greater than that of the baseline noise.
2.5.2. Precision
Intra-day precision and accuracy were assessed by assaying the mixed standard solution samples for three concentrations (n=6) on the same day. The same procedure was performed once a day for 3 consecutive days to determine inter-day precision.
2.5.3. Repeatability
In order to confirm repeatability, six independent sample solutions of QSPs were treated and analyzed as described above. The relative standard deviation (RSD) was taken as a measure of repeatability.
2.5.4. Recovery
A recovery test was conducted to evaluate the accuracy of our method. Accurate amounts of 19 standards were added to a QSPs sample, which was then processed and analyzed as described in the Section 2.2. The total amount of each analyte was calculated from the corresponding calibration curve, and the recovery of each analyte was calculated by using the following equation:
where Amountdetermined is the total amount of each analyte determined, Amountoriginal is the original amount of each analyte determined in samples, and Amountspiked is the spiked amount of each analyte.
2.5.5. Stability
Stability was tested by assaying the samples; the same sample solution was stored at 4 °C and analyzed every 12 h for 2 days. In addition, the 19 standard stock solutions were examined by ultra-high-performance liquid chromatography–tandem mass spectrometry every three days to investigate whether obvious degradation had occurred under the preservation condition.
3. Results and discussion
3.1. Optimization of extraction conditions
For efficient extraction of the active components in QSP, important factors such as methanol concentration (50%, 75%, and 100%, v/v), ultrasonic time (20, 30 and 40 min) and sample-solvent ratio (1:250, 1:500 and 1:750, w/v) were investigated by using an orthogonal L9 (34) design. The results revealed that 500 times of 100% (v/v) methanol for 20 min was the optimum sample extraction condition.
3.2. Optimization of UHPLC–ESI/MS conditions
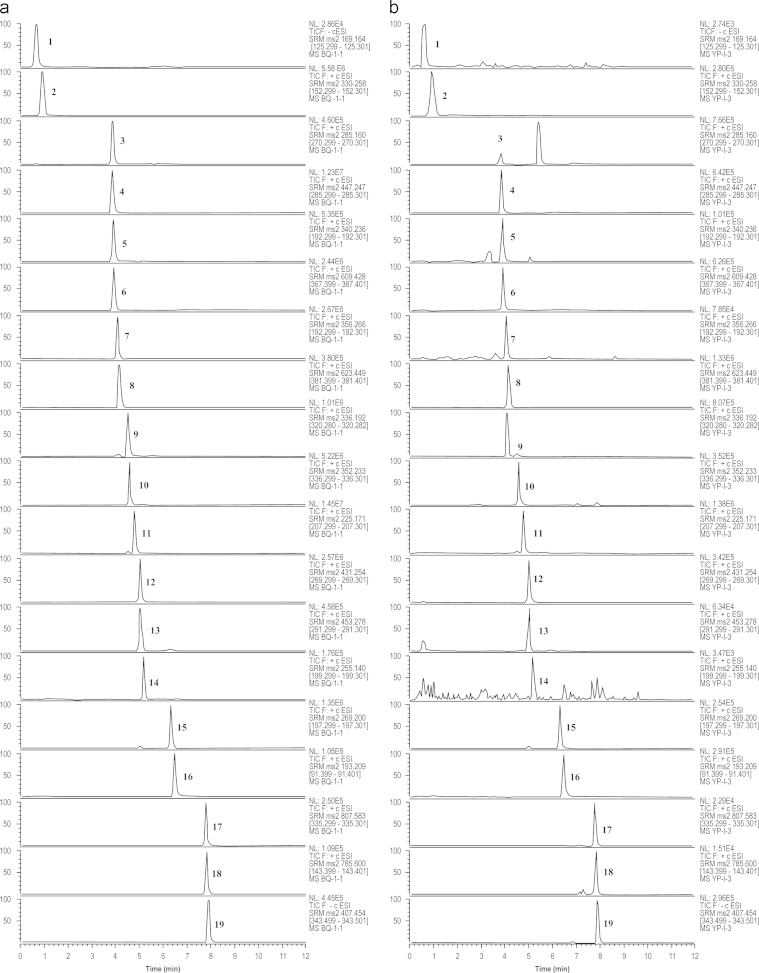
The 19 marker compounds were selected to obtain the optimum elution conditions because they belong to different chemical classes and cover a wide range of polarities. Thus, alterations in the various UHPLC parameters, including the mobile phase modifier, flow rate, and column temperature, were investigated. Based on sensitivity, we found that the optimum UHPLC conditions were obtained when the mobile phase modifier was 0.1% formic acid. Separation was better when the column temperature was kept at 40 °C and flow rate was 0.3 mL/min (Fig. 2).
Figure 2.
Typical chromatograms of UHPLC–MS/MS for (a) mixed standard substance and (b) sample.
The detection model of MS was selected in the experiment, the organic acids (CA, GA) in the negative ion mode are excellent to produce quasi-molecular ion [M-H]−. However, the alkaloids (SIN, PAL, BER, THPB, TET, THP, FAN), saponins (AST-IV), lactones (SEI, SEA) and flavonoids (CCS, CCSG, ONO, 5-O-M, DAI, FOR) are optimal to produce quasi-molecular ion [M+H]+ in positive ion mode. Nevertheless, in this mode, the adduct ion peak [M+Na]+ is the best for AST-III.
Quantitative analysis was performed by monitoring [M+H]+, [M+Na]+ or [M−H]− for analytes in selective reaction monitoring (SRM) mode. The 19 components were identified by comparing their retention times, the [M+H]+ [M+Na]+ or [M−H]−, and MS/MS fragments with those of reference standards. The results showed that the highest sensitivity was obtained at a certain value of fragment collision energy (CE) (Table 1).
Table 1.
Mass spectrometric parameters and fragment spectrum of analytical compounds.
| Compound | Abbreviations | Ionization mode | Monitoring mode | Parent (m/z) | Product (m/z) | Tube lens (V) | Collision energy (eV) | RT (min) |
|---|---|---|---|---|---|---|---|---|
| Gallic acid | GA | ESI− | [M−H]− | 169.16 | 125.30 | 75 | 16 | 0.61 |
| Sinomenine | SIN | ESI+ | [M+H]+ | 330.25 | 152.30 | 95 | 58 | 0.96 |
| Calycosin | CCS | ESI+ | [M+H]+ | 285.16 | 270.30 | 99 | 23 | 3.84 |
| Calycosin-7-O-β-d- glucoside | CCSG | ESI+ | [M+H]+ | 447.24 | 285.30 | 98 | 20 | 3.86 |
| Tetrahydroepiberberine | THPB | ESI+ | [M+H]+ | 340.23 | 192.30 | 99 | 27 | 3.90 |
| (+)-Tetrandrine | TET | ESI+ | [M+H]+ | 609.42 | 367.40 | 103 | 39 | 3.92 |
| Tetrahydropalmatine | THP | ESI+ | [M+H]+ | 356.26 | 192.30 | 103 | 27 | 4.08 |
| Fangchinoline | FAN | ESI+ | [M+H]+ | 623.44 | 381.40 | 130 | 40 | 4.14 |
| Palmatine | PAL | ESI+ | [M+H]+ | 352.23 | 336.30 | 89 | 29 | 4.57 |
| Senkyunolide I | SEI | ESI+ | [M+H]+ | 225.17 | 207.30 | 86 | 8 | 4.78 |
| 5-O-Methylvisamminol | 5-O-M | ESI+ | [M+H]+ | 453.27 | 291.30 | 108 | 22 | 5.03 |
| Ononin | ONO | ESI+ | [M+H]+ | 431.25 | 269.30 | 97 | 17 | 4.98 |
| Daidzein | DAI | ESI+ | [M+H]+ | 255.14 | 199.30 | 105 | 23 | 5.17 |
| Formononetin | FOR | ESI+ | [M+H]+ | 269.20 | 197.30 | 97 | 34 | 6.34 |
| Astragaloside IV | AST-IV | ESI+ | [M+H]+ | 785.60 | 143.40 | 143 | 14 | 7.74 |
| Cholic acid | CA | ESI− | [M−H]− | 407.45 | 343.50 | 141 | 35 | 7.88 |
| Astragaloside III | AST-III | ESI+ | [M+Na]+ | 807.53 | 335.30 | 184 | 57 | 7.81 |
| Senkyunolide A | SEA | ESI+ | [M+H]+ | 193.20 | 91.40 | 86 | 24 | 6.47 |
| Berberine | BER | ESI+ | [M+H]+ | 336.19 | 320.28 | 94 | 30 | 4.51 |
3.3. Method validation
Full calibration curves of the 19 compounds were obtained with relatively wide concentration ranges, and the correlation coefficients of all the calibration curves were greater than 0.9990. For different components, the LOD ranged from 0.2 ng/mL to 8 ng/mL, and the LOQ ranged from 0.4 ng/mL to 50 ng/mL. The recoveries were 95.4%–104% and the RSD values were less than 3.35% (Table 2). The intra-day and inter-day accuracies for the 19 compounds were 96.5–105% and 94.8–105%, respectively. The RSD values of the intra-day and inter-days precisions were 0.21–4.45% and 0.29–4.23%, respectively. The mixed standard solution samples were found to be stable from 0 to 24 h and their RSD values was less than 2.91%. RSD values for the repeatability of the analysis ranged from 0.71% to 2.60% (Table 3).
Table 2.
Method validation for the determination of 19 selected markers.
| Compound | Linearity | R2 | Linear range (μg/mL) | LOQ (ng/mL) | LOD (ng/mL) | Repeatability RSD (%) | Recovery |
|
|---|---|---|---|---|---|---|---|---|
| Mean (%) | RSD (%) | |||||||
| GA | y=282812x+24.416 | 0.9995 | 0.005–0.751 | 5.00 | 1.00 | 2.35 | 98.73 | 0.02 |
| SIN | y=2926.5x+222087 | 0.9999 | 0.125–50.074 | 1.00 | 0.20 | 1.07 | 99.32 | 1.01 |
| CCS | y=71.833x+15326 | 0.9996 | 0.050–37.500 | 50.00 | 10.00 | 1.62 | 97.92 | 1.98 |
| CCSG | y=28401x+505043 | 0.9994 | 0.015–2.250 | 1.00 | 0.20 | 1.59 | 97.33 | 2.71 |
| THPB | y=21441x+28069 | 0.9998 | 0.001–0.201 | 1.00 | 0.20 | 2.60 | 100.37 | 1.65 |
| TET | y=3569.2x+20912 | 0.9999 | 0.025–3.750 | 2.50 | 0.50 | 1.83 | 100.05 | 1.29 |
| THP | y=65754x+52637 | 0.9998 | 0.001–0.188 | 0.40 | 0.08 | 1.37 | 97.79 | 2.32 |
| FAN | y=140.33x+1012.8 | 0.9997 | 0.101–20.020- | 10.00 | 2.00 | 0.82 | 97.67 | 1.39 |
| PAL | y=59845x+168187 | 0.9998 | 0.001–0.502 | 1.00 | 0.20 | 1.52 | 95.43 | 3.35 |
| SEI | y=2006.1x+518976 | 0.9998 | 0.050–25.005 | 15.00 | 3.00 | 1.37 | 98.28 | 1.40 |
| 5-O-M | y=2468.9x+7624 | 0.9999 | 0.002–1.125 | 1.00 | 0.20 | 2.31 | 99.52 | 0.19 |
| ONO | y=8931.9x+48181 | 0.9996 | 0.005–1.125 | 5.00 | 1.00 | 0.82 | 99.25 | 2.95 |
| DAI | y=1910.2x+2638.8 | 0.9999 | 0.003–0.500 | 2.50 | 0.50 | 1.16 | 100.99 | 0.60 |
| FOR | y=3897.8x+33528 | 0.9999 | 0.005–1.500 | 5.00 | 1.00 | 0.71 | 101.34 | 2.16 |
| AST-IV | y=84.323x+2717.1 | 0.9998 | 0.040–6.001 | 40.00 | 8.00 | 1.99 | 95.87 | 0.63 |
| CA | y=255.55x+12167 | 0.9999 | 0.015–22.500 | 15.00 | 3.00 | 2.01 | 101.37 | 0.73 |
| AST-III | y=567.31x−4102.5 | 0.9994 | 0.015–2.503 | 12.50 | 2.50 | 1.10 | 99.87 | 1.34 |
| SEA | y=135.55x+15755 | 0.9999 | 0.010–37.500 | 10.00 | 2.00 | 1.68 | 98.98 | 0.54 |
| BER | y=72211x+19097 | 0.9998 | 0.001–0.100 | 0.50 | 0.10 | 1.47 | 102.35 | 0.24 |
LOD is limit of detection; LOQ is limit of quantification; RSD is relative standard deviation; see Table 1 for abbreviation definitions of the 19 compounds.
Table 3.
Intra-and inter-day variability and stability of the 19 components.
| Compound | Concentration (μg/mL) | Intra-day (n=6) |
Inter-day (n=6) |
Stability (n=5) |
||
|---|---|---|---|---|---|---|
| Observed | RSD% | Observed | RSD% | RSD% | ||
| GA | 0.201 | 0.199±0.001 | 2.22 | 0.197±0.001 | 1.19 | 2.59 |
| 1.015 | 1.017±0.016 | 1.61 | 1.009±0.009 | 0.89 | ||
| 5.075 | 5.067±0.121 | 2.34 | 5.050±0.044 | 0.87 | ||
| SIN | 0.490 | 0.492±0.004 | 0.72 | 0.497±0.021 | 4.23 | 2.52 |
| 2.450 | 2.441±0.022 | 0.91 | 2.432±0.030 | 1.23 | ||
| 11.250 | 11.230±0.091 | 0.81 | 11.274±0.218 | 1.92 | ||
| CCS | 1.009 | 1.001±0.045 | 4.45 | 1.003±0.008 | 0.83 | 2.63 |
| 5.045 | 5.039±0.192 | 1.71 | 5.021±0.031 | 0.61 | ||
| 25.225 | 25.195±0.976 | 3.86 | 25.178±0.424 | 1.69 | ||
| CCSG | 0.060 | 0.058±0.000 | 0.21 | 0.059±0.001 | 1.31 | 0.74 |
| 0.301 | 0.294±0.009 | 3.06 | 0.299±0.011 | 3.68 | ||
| 1.506 | 1.491±0.020 | 1.36 | 1.497±0.007 | 0.45 | ||
| THPB | 0.394 | 0.389±0.018 | 4.12 | 0.392±0.012 | 2.93 | 1.81 |
| 1.970 | 1.943±0.024 | 1.24 | 1.944±0.009 | 0.46 | ||
| 9.850 | 9.689±0.010 | 0.10 | 9.689±0.310 | 3.21 | ||
| TET | 0.997 | 0.989±0.022 | 2.22 | 0.987±0.005 | 0.53 | 1.96 |
| 4.985 | 4.892±0.021 | 0.44 | 4.882±0.084 | 1.72 | ||
| 24.925 | 24.907±0.828 | 3.34 | 24.914±0.712 | 2.86 | ||
| THP | 0.049 | 0.049±0.000 | 2.05 | 0.049±0.000 | 2.66 | 1.36 |
| 0.245 | 0.246±0.006 | 2.43 | 0.244±0.008 | 3.24 | ||
| 12.560 | 12.454±0.316 | 2.53 | 12.495±0.132 | 1.03 | ||
| FAN | 0.499 | 0.491±0.002 | 0.41 | 0.436±0.005 | 1.25 | 1.23 |
| 2.495 | 2.499±0.025 | 1.00 | 2.474±0.019 | 0.77 | ||
| 12.475 | 12.464±0.411 | 3.29 | 12.482±0.325 | 2.60 | ||
| PAL | 0.100 | 0.997±0.010 | 1.07 | 0.987±0.011 | 1.11 | 1.44 |
| 0.501 | 0.493±0.004 | 0.81 | 0.481±0.017 | 3.53 | ||
| 2.506 | 2.486±0.029 | 1.16 | 2.490±0.061 | 2.60 | ||
| SEI | 1.001 | 0.999±0.013 | 1.30 | 0.997±0.005 | 0.50 | 2.91 |
| 5.005 | 5.013±0.174 | 3.43 | 5.011±0.097 | 1.98 | ||
| 25.251 | 25.188±0.405 | 1.61 | 25.224±0.219 | 0.86 | ||
| 5-O-M | 0.030 | 0.030±0.000 | 2.19 | 0.030±0.000 | 1.45 | 1.33 |
| 0.150 | 0.149±0.002 | 1.34 | 0.148±0.000 | 0.36 | ||
| 0.750 | 0.737±0.003 | 0.48 | 0.728±0.004 | 0.82 | ||
| ONO | 0.031 | 0.030±0.001 | 3.33 | 0.030±0.000 | 0.52 | 2.45 |
| 0.155 | 0.156±0.001 | 0.64 | 0.153±0.000 | 0.74 | ||
| 0.775 | 0.763±0.019 | 2.49 | 0.766±0.015 | 1.95 | ||
| DAI | 0.009 | 0.009±0.000 | 1.71 | 0.009±0.000 | 4.21 | 2.83 |
| 0.045 | 0.046±0.001 | 2.15 | 0.046±0.001 | 2.78 | ||
| 0.226 | 0.222±0.003 | 1.35 | 0.223±0.003 | 1.99 | ||
| FOR | 0.041 | 0.041±0.000 | 1.52 | 0.041±0.008 | 0.29 | 0.97 |
| 0.205 | 0.202±0.005 | 2.47 | 0.201±0.003 | 1.49 | ||
| 1.025 | 1.017±0.030 | 2.94 | 1.009±0.024 | 2.37 | ||
| AST-IV | 0.161 | 0.158±0.006 | 3.79 | 0.163±0.007 | 4.29 | 1.89 |
| 0.805 | 0.799±0.010 | 1.25 | 0.780±0.009 | 1.15 | ||
| 4.025 | 4.011±0.113 | 2.82 | 4.021±0.026 | 0.64 | ||
| CA | 0.299 | 0.273±0.009 | 3.29 | 0.232±0.003 | 0.83 | 2.42 |
| 1.495 | 1.475±0.024 | 1.62 | 1.475±0.029 | 1.96 | ||
| 7.475 | 7.500±0.031 | 0.41 | 7.529±0.059 | 0.78 | ||
| AST-III | 0.499 | 0.474±0.007 | 1.47 | 0.475±0.012 | 2.53 | 0.97 |
| 2.475 | 2.466±0.022 | 0.89 | 2.444±0.031 | 1.27 | ||
| 12.375 | 12.280±0.115 | 0.93 | 12.248±0.202 | 1.64 | ||
| SEA | 1.001 | 0.998±0.020 | 2.01 | 0.998±0.028 | 2.81 | 2.86 |
| 5.005 | 5.011±0.099 | 1.97 | 5.006±0.017 | 0.34 | ||
| 25.025 | 24.974±0.804 | 3.21 | 24.930±0.614 | 2.46 | ||
| BER | 0.002 | 0.002±0.000 | 0.82 | 0.002±0.000 | 1.01 | 0.20 |
| 0.010 | 0.010±0.000 | 0.35 | 0.010±0.000 | 1.32 | ||
| 0.050 | 0.498±0.003 | 0.61 | 0.476±0.005 | 1.05 | ||
3.4. Sample analysis
QSP is selected as the object of this study is based on its excellent clinical efficacy. As it is a famous traditional Chinese prescription, QSP has long been investigated. Reference standards are the keys to quality control of its bioactivities. The major bioactive components in QSP include lactones, saponins, alkaloids, flavonoids, organic acids. The newly established analytical method was subsequently applied to determine the 19 compounds: 2 lactones (11, 16), 2 organic acids (1, 19), 2 saponins (17, 18), 6 flavonoids (3, 4, 12, 13, 14, 15), 7 alkaloids (2, 5, 6, 7, 8, 9, 10). The target compounds were identified based on comparison of retention time and mass information obtained from LC–MS analysis of the standard compounds. Quantitative analyses were performed by means of external standard methods.
In accordance with the Pharmacopoeia of China, astragaloside IV (18) is a commonly used chemical marker, and it is used to represent saponins in most cases when preparations containing Radix Astragali are evaluated. Indeed, astragaloside IV has long been known to show antivirus, and antioxidant17, 18, 19, 20, 21, pharmacological actions in immune and circulatory systems, supplemental calcium on bone mineral density and bone metabolism, etc. However, the curative effects of TCMs are due to a number of bioactive compounds, not just one22, flavonoids are another important family of bioactive chemicals due to their hepatoprotective, antioxidative, antiviral and antihypertensive effects, their immunostimulant properties, their ability to strengthen general resistance, etc.23. Therefore, two saponins and six flavonoids derived from Radix Astragali were used as chemical markers in QSP.
Table 4 shows the contents determined for each compound. FAN (8) exhibited the highest concentrations, followed by SIN (2). These two components are known to have immunosuppressive, anti-inflammatory, antinociceptive effects24, 25, 26, 27. The volatile oil, however, has also demonstrated lots of bioactive effects, such as vasorelaxation28, anti-inflammatory29, and so on. Therefore, we chose two major compounds from the volatile oil of Rhizoma Chuanxiong, as chemical markers in the study. Based on their biological functions, the compounds we selected—nineteen in all—were reasonable and suitable as chemical markers that could be used to evaluate the bioactivity of this formula.
Table 4.
Determination of the 19 active compounds in QSP.
| Compound | Content (μg/g) |
||||
|---|---|---|---|---|---|
| Sample 1 | Sample 2 | Sample 3 | Sample 4 | Sample 5 | |
| GA | 43.76±1.92 | 47.38±4.55 | 43.25±7.75 | 43.17±3.72 | 48.9±1.26 |
| SIN | 6935.29±68.69 | 6735.97±23.05 | 6358.01±90.97 | 5798.44±139.38 | 7014.12±75.45 |
| CCS | 969.37±0.53 | 931.99±10.03 | 932.62±32.25 | 807.34±88.41 | 1150.36±47.43 |
| CCSG | 46.65±2.05 | 37.67±0.58 | 38.73±1.68 | 31.81±1.28 | 53.43±2.41 |
| THPB | 8.57±0.06 | 10.79±0.24 | 11.02±0.18 | 10.66±0.07 | 11.84±0.32 |
| TET | 407.14±8.88 | 623.01±7.01 | 532.93±9.66 | 499.42±0.84 | 515.91±8.99 |
| THP | 1.84±0.03 | 2.35±0.06 | 2.38±0.07 | 2.16±0.08 | 2.58±0.10 |
| FAN | 20080.48±201.73 | 29477.52±206.5 | 26732.32±195.98 | 24011.75±924.08 | 25849.01±1020.28 |
| PAL | 9.85±0.01 | 11.58±0.27 | 11.62±0.2 | 10.52±0.08 | 12.53±0.35 |
| SEI | 3831.75±21.89 | 2158.99±104 | 2109.92±83.71 | 1765.67±143.07 | 2673.94±140.79 |
| 5-O-M | 48.27±0.11 | 44.32±4.42 | 42.08±1.66 | 37.33±2.72 | 61.05±3.76 |
| ONO | 67.6±0.53 | 62.76±0.99 | 60.71±0.83 | 49.79±1.88 | 86.12±2.99 |
| DAI | 3.70±0.02 | 4.83±0.16 | 4.97±0.27 | 3.75±0.2 | 4.28±0.30 |
| FOR | 115.85±0.83 | 146.59±2.65 | 144.07±5.23 | 129.86±5.35 | 140.35±1.72 |
| AST-IV | 531.71±7.08 | 539.47±5.01 | 572.01±6.08 | 592.19±19.64 | 590.2±21.40 |
| CA | 2948.31±20.12 | 2871.39±44.34 | 2976.32±64.78 | 2725.37±44.74 | 3109.66±71.34 |
| AST-III | 77.62±0.77 | 99.23±1.58 | 81.12±5.84 | 76.5±0.43 | 82.86±11.83 |
| SEA | 4499.6±0.62 | 4183.84±24.24 | 3999.85±98.35 | 4183.43±27.72 | 5447.95±200.42 |
| BER | 1.8±0.03 | 2.32±0.03 | 2.26±0.10 | 1.86±0.07 | 2.37±0.05 |
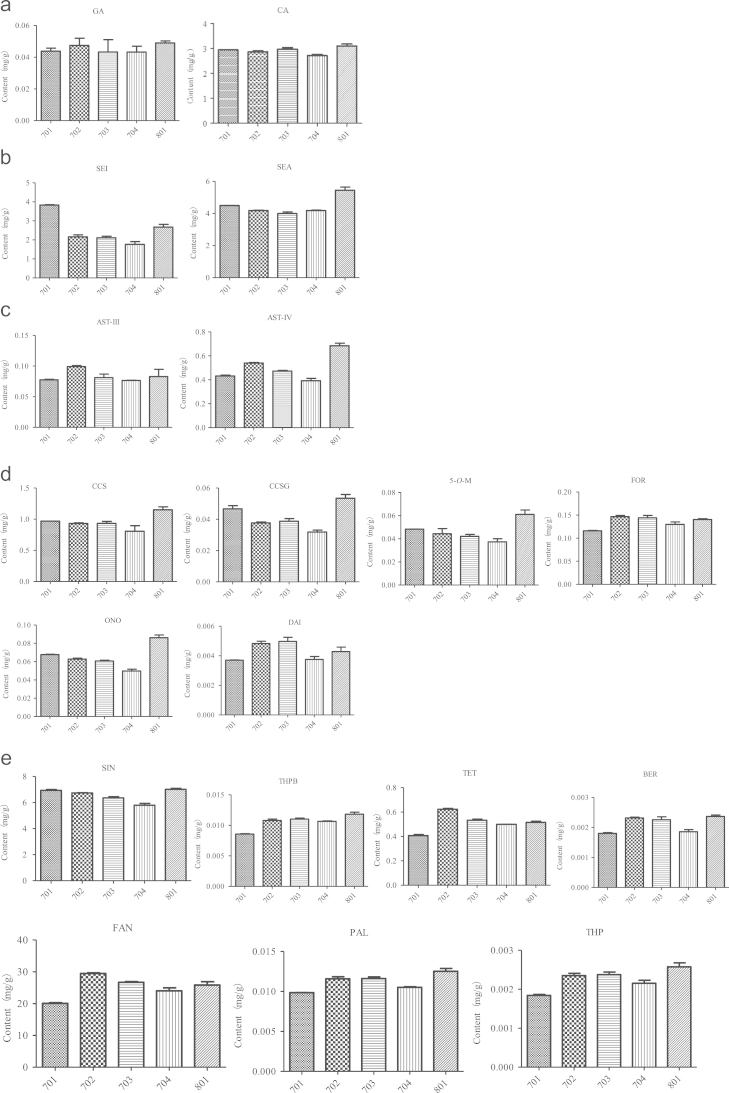
Most study for TCM quality evaluation was focus on different pharmaceutical companies on the same products rather than one pharmaceutical company on different batches30, 31, 32. We convinced that the determination of various ingredients in different batches made by the same pharmaceutical company can truly reflect the preparation quality. Therefore, in this study, five batches of prescription extracts of the same pharmaceutical company were used in this study. The results indicated that the amount of most components determined is similar in different batches. However, the content of some components in five batches, such as CCSG, TET, FAN, SEI, SEA, ONO, DAI, 5-O-M, was variable (Fig. 3). This phenomenon may occur for several reasons, the quality of herbal medicine, including seasonal changes, harvesting time, cultivation sites, post-harvesting processing, which may influence the content of some components determined in QSP. In addition, the intra-batch variability of determination may also contribute to the variation of contents.
Figure 3.
Contents of tested compounds in the five batches of (a) organic acids; (b) lactones; (c) saponins; (d) flavonoids; (e) alkaloids.
The discrepancy of some ingredients in difference batches confirm that just a few marker compounds (astragaloside IV, sinomenine)and one batch was not enough for good quality control of complex herbal products. Thus, in the present study 19 determined compounds could be considered as quality control markers for the continuous research on QSP quality. The simultaneous determination of the major components of QSP provides a basis for quality control and material base investigation of the pharmacological effects of QSP.
4. Conclusions
A variety of analytical methods were established for analyzing chemical constituents of QSP16, 33, but these methods only focused on the determination of a single or a few marker compounds, which, might not accurately reflect the quality of the complex herbal products34.
Therefore, it was necessary to develop a relatively simple and efficient method for the quality control of QSP. Thus, we have developed and validated a simple and rapid UHPLC–MS/MS method for the simultaneous determination of 19 active compounds in QSP. High repeatability, intra-day and inter-day precision, accuracy, and recovery were achieved with this method in our validation procedure. Thus, our proposed method could improve quality control for QSP. Moreover, the results provide a basis for further research of the prescription in vivo.
This is the latest report where the evaluation of commercial products with as many as 19 bioactive constituents is considered. This method of determining many bioactive compounds simultaneously will play an important role in the further development of TCM compound preparations and their products.
Acknowledgments
This research was supported by the Key Discipline of Shanghai City Board of Education (No. J50302), Higher Specialized Research Fund for the Doctoral Program (No. 20123107110007), Alliance Program-problem of bidding project (No. 2012033), MOST “significant Drug Discovery” major science and technology “Twelve Five” Implementation Plan (2011ZX09302-006-04), Ministry of Education, “Innovative Research Team” (1RT1270), Ministry of Education Key Laboratory of Ministry (Education Technology (2009) 98), Shanghai “most important” Clinical Center construction project, Shanghai University of Traditional Chinese Medicine “085 Project” class TCM Discipline Project “Boot Innovation Program” (085ZY1204), Shanghai Science and Technology Commission of modern medicine special (09dZ1977200), Shanghai university innovation team plans (Shanghai Branch Board of Education (2009) No. 6).
Footnotes
Peer review under responsibility of Institute of Materia Medica, Chinese Academy of Medical Sciences and Chinese Pharmaceutical Association.
Contributor Information
Yongjun Wang, Email: yjwang88@hotmail.com.
Ning Zhang, Email: ningzh18@126.com.
References
- 1.Radpasand M. Use of a multimodal conservative management protocol for the treatment of a patient with cervical radiculopathy. J Chiropr Med. 2011;10:36–46. doi: 10.1016/j.jcm.2010.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hangai M., Kaneoka K., Kuno S., Hinotsu S., Sakane M., Mamizuka N. Factors associated with lumbar intervertebral disc degeneration in the elderly. Spine J. 2008;8:732–740. doi: 10.1016/j.spinee.2007.07.392. [DOI] [PubMed] [Google Scholar]
- 3.Tampin B., Slater H., Hall T., Lee G. Quantitative sensory testing somatosensory profiles in patients with cervical radiculopathy are distinct from those in patients with nonspecific neck–arm pain. Pain J. 2012;153:2403–2414. doi: 10.1016/j.pain.2012.08.007. [DOI] [PubMed] [Google Scholar]
- 4.Reyes-Sanchez A., Miramontes V., Olivarez L.M.R. Initial clinical experience with a next-generation artificial disc for the treatment of symptomatic degenerative cervical radiculopathy. SAS J. 2010;4:9–15. doi: 10.1016/j.esas.2010.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liang Q.Q., Xi Z.J., Bian Q., Cui X.J., Li C.G., Hou W. Herb formula Fufangqishe-Pill prevents upright posture-induced intervertebral disc degeneration at the lumbar in rats. J Pharm Sci. 2010;113:23–31. doi: 10.1254/jphs.09231fp. [DOI] [PubMed] [Google Scholar]
- 6.Zhou Q., Wang Y.J., Shi Q., Sun P., Zhou C.J., Hu Z.J. Effects of yiqi huayu recipe and its decomposed formulas on apoptosis-related factors of anulus fibrosus cells in rats. Chin J Integr Med. 2005;3:466–469. doi: 10.3736/jcim20050612. [DOI] [PubMed] [Google Scholar]
- 7.Shi Q., Wang Y.J., Li C.G. Study on Yiqi Huayu Bushen Recipe and its disassembled recipes in regulating mRNA expression of collagens and metabolic enzymes in extracellular matrix of cervical disc. Chin J Integr Med. 2007;21:141–146. [PubMed] [Google Scholar]
- 8.Liu H., Wei W., Sun W.Y., Li X. Protective effects of astragaloside IV on porcine-serum-induced hepatic fibrosis in rats and in vitro effects on hepatic stellate cells. J Ethnopharmacol. 2009;122:502–508. doi: 10.1016/j.jep.2009.01.035. [DOI] [PubMed] [Google Scholar]
- 9.Li M., Qu Z.Y., Zhao Z.W., Wu S.X., Liu Y.Y., Wei X.Y. Astragaloside IV protects against focal cerebral ischemia/reperfusion injury correlating to suppression of neutrophils adhesion-related molecules. Neurochem Int. 2012;60:458–465. doi: 10.1016/j.neuint.2012.01.026. [DOI] [PubMed] [Google Scholar]
- 10.Chen D.P., Wong C.K., Leung P.C., Fung K.P., Lau C.B., Lau C.P. Anti-inflammatory activities of Chinese herbal medicine sinomenine and Liang Miao San on tumor necrosis factor-α-activated human fibroblast-like synoviocytes in rheumatoid arthritis. J Ethnopharmacol. 2011;137:457–468. doi: 10.1016/j.jep.2011.05.048. [DOI] [PubMed] [Google Scholar]
- 11.Cai X.H., Wang S., Chen B.A. Research advances on the pharmacological effects of tetrandrine. Chin J Nat Med. 2011;9:473–480. [Google Scholar]
- 12.Cao F.L., Shang G.W., Wang Y., Yang F., Li C.L., Chen J. Antinociceptive effects of intragastric dl-tetrahydropalmatine on visceral and somatic persistent nociception and pain hypersensitivity in rats. Pharmacol Biochem Behav. 2011;100:199–204. doi: 10.1016/j.pbb.2011.08.016. [DOI] [PubMed] [Google Scholar]
- 13.Lu Y., Jiang F., Jiang H., Wu K., Zheng X., Cai Y. Gallic acid suppresses cell viability, proliferation, invasion and angiogenesis in human glioma cells. Eur J Pharm Sci. 2010;641:102–107. doi: 10.1016/j.ejphar.2010.05.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.He C.Y., Wang S., Feng Y., Liang S., Lin X., Xu D.S. Pharmacokinetics, tissue distribution and metabolism of senkyunolide I, a major bioactive component in Ligusticum chuanxiong Hort. (Umbelliferae) J Ethnopharmacol. 2012;142:706–713. doi: 10.1016/j.jep.2012.05.047. [DOI] [PubMed] [Google Scholar]
- 15.Chen L.Y., Li Z.X., Tang Y., Cui X., Luo R., Guo S. Isolation, identification and antiviral activities of metabolites of calycosin-7-O-β-d-glucopyranoside. J Pharm Biomed Anal. 2011;56:382–389. doi: 10.1016/j.jpba.2011.05.033. [DOI] [PubMed] [Google Scholar]
- 16.Liu M., Zhang N., Wang Y.J., Shi Q., Shen P.Z., Xu N. Determination of Sinomenine in Qishejingkang Pills by HPLC. Lishizhen Med Mater Med Res. 2003;14:67–68. [Google Scholar]
- 17.Zhang W.J., Hufnag P., Binder B.R., Wojta J. Antiinflammatory activity of astragaloside IV is mediated by inhibition of NF-kappaB activation and adhesion molecule expression. J Thromb Haemost. 2003;90:904–914. doi: 10.1160/TH03-03-0136. [DOI] [PubMed] [Google Scholar]
- 18.Wang S., Li J., Huang H., Gao W., Zhuang C., Li B. Anti-hepatitis B virus activities of astragaloside IV isolated from Radix Astragali. Biol Pharm Bull. 2009;32:132–135. doi: 10.1248/bpb.32.132. [DOI] [PubMed] [Google Scholar]
- 19.Zhang Z.C., Li S.J., Yang Y.Z., Chen R.Z., Ge J.B., Chen H.Z. Effect of astragaloside on cardiomyocyte apoptosis in murine coxsackievirus B3 myocarditis. J Asian Nat Prod Res. 2007;9:145–151. doi: 10.1080/10286020412331286506. [DOI] [PubMed] [Google Scholar]
- 20.Lu S., Zhang J., Yang D. Effects of Astragaloside in treating myocardial injury and myocardial Sarco/Endoplasmic Ca2+-ATPase of viral myocarditis mice. Chin J Integr Med. 1999;19:672–674. [PubMed] [Google Scholar]
- 21.Yin X., Zhang Y., Yu J., Zhang P., Shen J., Qiu J. Theantioxidative efects of astragalus saponin I protect against developmentof early diabetic nephropathy. J Pharmacol Sci. 2006;101:166–173. doi: 10.1254/jphs.fp0050041. [DOI] [PubMed] [Google Scholar]
- 22.Meng D., Chen X.J., Bian Y.Y., Li P., Zhang J.N. Effect of Astragalosides on intracellular calcium overload in cultured cardiac myocytes of neonatal rats. Am J Chin Med. 2005;33:11–20. doi: 10.1142/S0192415X05002618. [DOI] [PubMed] [Google Scholar]
- 23.Xie Z.F., Lou Z.C., Huang X.K. New World Press; Beijing: 1994. Classified dictionary of traditional Chinese medicine; p. 374. [Google Scholar]
- 24.Choi H.S., Kim H.S., Min K.R., Kim Y., Lim H.K., Chang Y.K. Anti-inflammatory effects of fangchinoline and tetrandrine. J Ethnopharmacol. 2000;69:173–179. doi: 10.1016/s0378-8741(99)00141-5. [DOI] [PubMed] [Google Scholar]
- 25.Wu C.J., Wang Y.H., Lin C.J., Chen H.H., Chen Y.J. Tetrandrine down-regulates ERK/NF-κB signaling and inhibits activation of mesangial cells. Toxicology in Vitro. 2011;25:1834–1840. doi: 10.1016/j.tiv.2011.09.024. [DOI] [PubMed] [Google Scholar]
- 26.Zhao Y., Li J., Yu K.Q., Liu Y., Chen X.G. Sinomenine inhibits maturation of monocyte-derived dendritic cellsthrough blocking activation of NF-kappa B. Int Immunopharmacol. 2007;7:637–645. doi: 10.1016/j.intimp.2007.01.007. [DOI] [PubMed] [Google Scholar]
- 27.Wang Q.X., Li X.K. Immunosuppressive and anti-in ammatory activities of sinomenine. Int Immunopharmacol. 2011;11:373–376. doi: 10.1016/j.intimp.2010.11.018. [DOI] [PubMed] [Google Scholar]
- 28.Chan S.S.K., Cheng T.Y., Lin G. Relaxation effects of ligustilide and senkyunolide A, two main constituents of Ligusticum chuanxiong, in rat isolated aorta. J Ethnopharmacol. 2007;3:677–680. doi: 10.1016/j.jep.2006.12.018. [DOI] [PubMed] [Google Scholar]
- 29.Or T.C., Yang C.L., Law A.H., Li J.C., Lau A.S. Isolation and identification of anti-inflammatory constituents from Ligusticum chuanxiong and their underlying mechanisms of action on microglia. Neuropharmacology. 2011;6:823–831. doi: 10.1016/j.neuropharm.2010.12.002. [DOI] [PubMed] [Google Scholar]
- 30.Yi L., Qi L.W., Li P., Ma Y.H. Simultaneous determination of bioactive constituents in Danggui Buxue Tang for quality control by HPLC coupled with a diode array detector, an evaporative light scattering detector and mass spectrometry. Anal Bioanal Chem. 2007;389:571–580. doi: 10.1007/s00216-007-1431-8. [DOI] [PubMed] [Google Scholar]
- 31.Wang Z.J., Wo S.K., Wang L., Lau C., Lee V.H., Chow M.S. Simultaneous quantification of active components in the herbs and products of Si-Wu-Tang by high performance liquid chromatography–mass spectrometry. J Pharm Biomed Anal. 2009;50:232–244. doi: 10.1016/j.jpba.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 32.Peng J.B., Jia H.M., Liu Y.T., Zhang H.W., Dong S., Zou Z.M. Qualitative and quantitative characterization of chemical constituents in Xin-Ke-Shu preparations by liquid chromatography coupled with a LTQ Orbitrap mass spectrometer. J Pharm Biomed Anal. 2011;55:984–995. doi: 10.1016/j.jpba.2011.03.045. [DOI] [PubMed] [Google Scholar]
- 33.Du S.M., Pan Y.F., Zhang N. Simultaneous determination of nine constituents in Qishe Pill by HPLC internal standard method. Chin Tradit Patent Med. 2012;34:1271–1276. [Google Scholar]
- 34.Xue T., Roy R. Studying traditional Chinese medicine. Science. 2003;300:740–741. doi: 10.1126/science.300.5620.740. [DOI] [PubMed] [Google Scholar]