Abstract
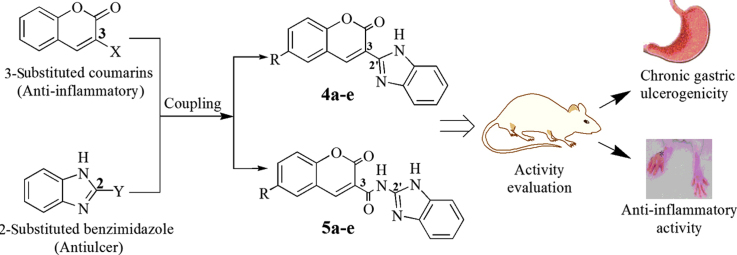
Inspired from occurrence of anti-inflammatory activity of 3-substituted coumarins and antiulcer activity of various 2-substituted benzimidazoles, novel compounds have been designed by coupling coumarin derivatives at 3-position directly or through amide linkage with benzimidazole nucleus at 2-position. The resultant compounds are expected to exhibit both anti-inflammatory and antioxidant activities along with less gastric toxicity profile. Two series of coumarin–benzimidazole derivatives (4a–e and 5a–e) were synthesized and evaluated for anti-inflammatory activity and antioxidant activity. Compounds 4c, 4d and 5a displayed good anti-inflammatory (45.45%, 46.75% and 42.85% inhibition, respectively, versus 54.54% inhibition by indomethacin) and antioxidant (IC50 of 19.7, 13.9 and 1.2 µmol/L, respectively, versus 23.4 µmol/L for butylatedhydroxytoluene) activities. Evaluation of ulcer index and in vivo biochemical estimations for oxidative stress revealed that compounds 4d and 5a remain safe on gastric mucosa and did not induce oxidative stress in tissues. Calculation of various molecular properties suggests the compounds to be sufficiently bioavailable.
KEY WORDS: Anti-inflammatory, Benzimidazoles, Coumarins, DPPH, Gastric toxicity
Graphical abstract
Coumarin–benzimidazole hybrids were designed, synthesized and evaluated for anti-inflammatory, antioxidant and chronic gastric ulceration potentials. Compounds 4c, 4d and 5a displayed the best anti-inflammatory and antioxidant activities, and proved to be gastric safe.
1. Introduction
Inflammation is an important indication in many pathological conditions such as rheumatoid arthritis, osteoarthritis, gout, Alzheimer׳s disease and obesity related diseases1. Chronic inflammatory states lead to a vicious cycle of inflammation and the accompanying pathological states, like obesity can lead to inflammation and the chronic inflammation can promote obesity associated diabetes by inducing insulin resistance2. Therefore, control of inflammation becomes more important in all pathological conditions. The well-known non-steroidal anti-inflammatory drugs (NSAIDs) viz. indomethacin, ibuprofen and naproxen are commonly employed drugs in first line treatment of various chronic inflammatory disease states3.
The major limitation of use of NSAIDs is gastric intolerance, which is manifested by dyspepsia, bleeding and ulcers. It occurs due to prostaglandin synthesis blockade as a result of cyclooxygenase (COX) inhibition by NSAIDs as well as due to acidic character of the NSAIDs themselves4. Users of NSAIDs are found to be at 3 times greater risk of developing serious gastro-intestinal (GIT) adverse effects than the non-users5. Another statistical analysis displays that 23%–31% of patients develop gastric lesions when prescribed NSAIDs for arthritis6. All these facts have arose the need to improve the safety profile of existing NSAIDs or to discover better alternatives. Various COX-2 selective inhibitors have exhibited marked anti-inflammatory effect with reduced GIT toxicity. MK-0966, rofecoxib and celecoxib are selective COX-2 inhibitors with significant anti-inflammatory activity but induce less GIT side-effects in comparison to those of aspirin and ibuprofen7, 8. Coupling of NSAIDs with an antioxidant cysteamine has produced compounds having good activity with less GIT intolerance. Coupling of nitric oxide, a cellular antioxidant with NSAIDs has also been explored successfully to design anti-inflammatory agents with markedly reduced ulcerogenic potential4, 9.
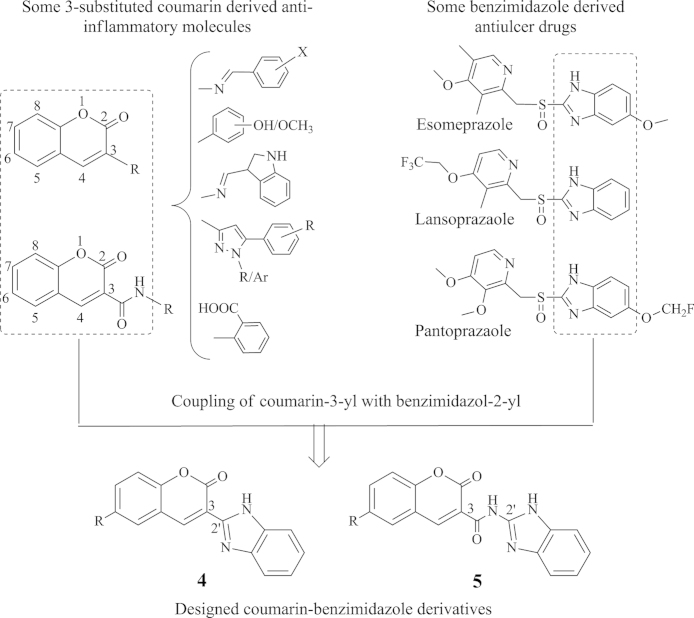
Coumarins form an elite class of compounds, which exhibit a variety of therapeutic activities including antioxidant, anti-inflammatory, antitumor, antiviral, antituberculosis and antimicrobial10, 11, 12, 13. Anti-inflammatory activity of coumarin derived compounds has been reviewed extensively and a structure activity relationship (SAR) has been established wherein it is found that an aromatic group when directly fused or linked through amide linkage at 3-position of coumarin nucleus incurs anti-inflammatory activity (Fig. 1)14, 15, 16. Many such derivatives also possess antioxidant activity through scavenging mechanisms17, 18.
Figure 1.
Design strategy for the target compounds.
Benzimidazole is another multifacet nucleus possessing a wide range of biological activities19. This nucleus bearing at its 2-position a heterocycle through linker has been found in many clinically available antiulcer drugs. It reveals that benzimidazole substituted with an appropriate group at 2-position is an important structural feature for gastric safety of the molecule20. Therefore, the present study is undertaken to design novel molecules through coupling of 2-position of benzimidazole nucleus with 3-position of 6-substituted coumarin nucleus (Fig. 1), which can be exploited as viable alternatives to the existing NSAIDs. The resultant molecules are expected to exhibit both anti-inflammatory and antioxidant activities but still being less gastro-toxic.
2. Results and discussion
2.1. Chemistry
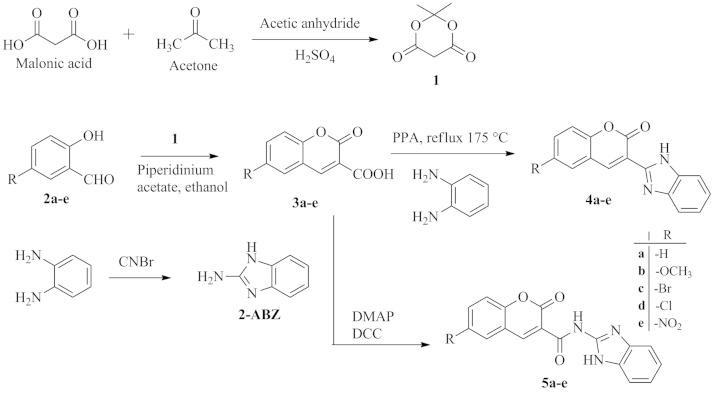
The target compounds were synthesized as shown in Scheme 1. Initially, Meldrum׳s acid (1) was prepared by treating malonic acid with acetone in the presence of catalytic amounts of sulphuric acid and acetic anhydride. Different salicylaldehydes (2a–e) were reacted with 1 in the presence of piperidinium acetate to obtain 6-substituted coumarin-3-carboxylic acids (3a–e). These intermediates were then used to synthesize target compounds 4a–e by refluxing them with o-phenylenediamine under inert environment (nitrogen) in the presence of catalytic amounts of polyphosphoric acid (PPA). The target compounds 5a–e were synthesized by coupling these intermediates with 2-aminobenzimidazole in the presence of dicyclohexylcarbodiimide (DCC) and 4-dimethylaminopyridine (DMAP). Many literature reports reveal the use of both orthophosphoric acid (OPA) and PPA for the formation of benzimidazole nucleus21, 22. In the present study, PPA was used as catalyst as OPA produced more by products and took longer reaction times. All target compounds were obtained in good yields and were found to be pure as assured by single spots in thin layer chromatographic plates (TLC). Structures of the synthesized compounds were confirmed by IR, NMR and high resolution mass (HRMS) spectral techniques. Formation of benzimidazole nucleus in compounds 4 and 5 was ascertained by the disappearance of –COOH band, due to –COOH group of compounds 2, in their IR spectra. In addition, IR spectra of 5 showed an amide band in the range of 1640–1690 cm−1. Presence of benzimidazole nucleus was confirmed by appearance of signals due to –NH and four aromatic protons of the nucleus in their 1H NMR spectra. The labile –NH protons were detected in the range of δ 8.9–9.2, which was confirmed by deuterium exchange experiments. The 13C NMR spectra showed distinct resonances in agreement with the proposed structure. The methoxy derivatives 4b and 5b showed distinct peaks due to methoxyl carbon at around δ 55. The benzimidazole carbons were detected at δ 116.21–125.51, and the coumarinyl carbonyl carbon was found at δ 155.62–164.05. Carbon atoms of the benzene ring of coumarin nucleus showed downfield or upfield shifts in consonant with the type of substituent present on the ring. Finally, the HRMS data, recorded with electrospray ionization in positive polarity (+ESI), of each compound showed that the mass of [MH+] ion was in close agreement with its accurate theoretical mass.
Scheme 1.
Synthesis of test compounds 4 and 5.
2.2. Anti-inflammatory activity
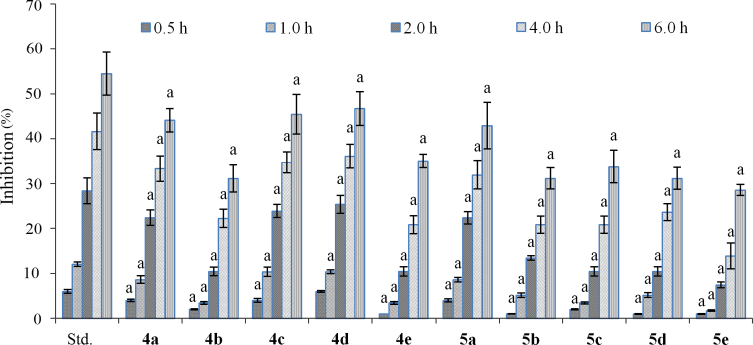
It was evaluated in terms of percent (%) inhibition of formalin induced oedema in rat paw. The activity was monitored at 0.5, 1, 2, 4 and 6 h after administration. It was found that the activity continuously increased with time. All test compounds exhibited good to moderate anti-inflammatory activity, which was comparable to indomethacin at each time period (Fig. 2). Further, the inhibition profile of each compound was similar to that of indomethacin at each time slab, which suggested that the mechanism of action of the compounds might be similar to that of indomethacin. Compounds 4a–e showed anti-inflammatory effects better than those of compounds 5a–e. Compounds 4d and 4c were maximally potent with 46.75% and 45.45% inhibition of paw oedema, respectively. From the other series, compound 5a was the most potent with 42.85% inhibition. These results suggest that an electron withdrawing group (–Cl or –Br) increases the anti-inflammatory potency whereas electron releasing group (–OCH3) decreases the potency. Further, it was found that an amide linkage in the molecule decreased the activity.
Figure 2.
Anti-inflammatory effect of compounds of series 4 and 5. Values are represented as mean±SD (n=6). aValues are statistically different from standard at each time interval, P<0.05.
2.3. In vitro antioxidant activity
The antioxidant potential was evaluated as radical scavenging capacity using 2,2-diphenyl-1-picrylhydrazyl (DPPH) method. DPPH being composed of unstable free radical traps any radicals produced by the test compound and bring about a visible colour change ranging from violet to pale yellow to colourless. The degree of change in absorbance with respect to control is calculated as antioxidant potential. Due to the non-linear relationship in the DPPH method, probit regression was applied and EC50 values were calculated using BLeSq software23. Series 5a–e is found to be more potent than series 4a–e as depicted in Table 1. Compounds 4d and 5a were found to be most potent with EC50 value 13.92±1.4 μmol/L and 1.2±0.1 μmol/L, respectively. The study reveals that electron withdrawing groups increase the antioxidant potential which may be due to the intensification of positive charge on –NH of amide associated with negative inductive effect of these groups. The positive charge intensification may lead to free radical quenching. Moreover, electron withdrawing groups are themselves good free radical quenchers. On the contrary, substitution with electron releasing groups was found to decrease the radical scavenging potential possibly due to their positive inductive effect.
Table 1.
In vitro antioxidant activity by DPPH method.
| Compound | Inhibition (%) |
EC50a (µmol/L) | |||||
|---|---|---|---|---|---|---|---|
| 1 µmol/L | 2 µmol/L | 5 µmol/L | 10 µmol/L | 20 µmol/L | 50 µmol/L | ||
| 4a | 2.2±0.1 | 7.5±0.4 | 25.2±1.6 | 47.1±3.3 | 62.8±6.4 | 69.5±4.4 | 15.5±1.5 |
| 4b | 15.1±0.5 | 17.2±0.6 | 22.9±1.2 | 25.5±1.8 | 28.7±1.3 | 35.3±1.5 | >50.0 |
| 4c | 1.6±0.1 | 6.1±0.2 | 19.8±1.8 | 37.8±3.1 | 56.2±3.0 | 65.4±4.3 | 19.7±1.7 |
| 4d | 0.7±0.1 | 4.1±0.3 | 22.0±0.8 | 32.6±2.7 | 63.8±3.6 | 70.1±4.3 | 13.92±1.4 |
| 4e | 0.4±0.01 | 3.8±0.2 | 18.8±1.8 | 37.2±2.5 | 50.9±4.7 | 59.5±3.0 | 22.5±1.7 |
| 5a | 45.3±3.8 | 55.8±2.2 | 64.1±4.0 | 69.0±4.8 | 71.6±2.8 | 75.8±3.7 | 1.2±0.1 |
| 5b | 0.7±0.04 | 2.7±0.1 | 22.19±0.4 | 30.0±4.0 | 39.0±1.9 | 56.3±3.9 | 28.5±1.8 |
| 5c | 36.0±0.9 | 55.8±3.0 | 64.5±2.4 | 68.0±2.5 | 71.1±4.9 | 75.9±4.4 | 1.9±0.1 |
| 5d | 17.2±0.6 | 41.3±3.0 | 60.1±3.6 | 71.8±3.4 | 76.3±5.4 | 90.5±4.9 | 4.0±0.4 |
| 5e | 25.4±0.6 | 42.7±1.5 | 55.0±2.3 | 59.7±3.8 | 64.1±3.4 | 75.9±4.9 | 5.0±0.5 |
| BHTb | 5.6±0.7 | 19.2±1.8 | 28.0±1.7 | 37.5±2.8 | 48.9±3.7 | 60.4±2.9 | 23.4±3.1 |
EC50, concentration which possesses 50% radicle scavenging ability.
Butylatedhydroxytoluene.
2.4. Gastric safety and in vivo oxidative stress
Based on anti-inflammatory and in vitro antioxidant activities, the compounds 4c, 4d and 5a were selected for evaluation of gastric safety as well as in vivo oxidative stress. The compounds 4d and 5a were found to be safe on gastric mucosa as indicated by their low ulcer index (0.67 and 0.75, respectively) in comparison to indomethacin which has scored an ulcer index of 3.17 (Table 2). The increased catalase and glutathione levels and decreased thiobarbituric acid reactive substances (TBARS) levels in animals treated with standard drug with respect to the control group indicated that indomethacin had induced oxidative stress in the tissues. In consonant with the gastric safety profile, the compounds 4d and 5a have been found to exert no oxidative stress on the tissue as indicated by the catalase, glutathione and TBARS levels being almost equal to those of control group.
Table 2.
Biochemical estimations and ulcer index of 4c, 4d and 5a.
| Compound | Catalase (µmol/L/mg) | TBARS (nmol/L/mg) | Glutathione (µmol/L/100 mg) | Ulcer index |
|---|---|---|---|---|
| Control | 22.14±0.98 | 0.63±0.09 | 184.10±5.03 | 0.17±0.25 |
| Standard (ID) | 7.79±0.19 | 5.32±0.11 | 85.20±3.10 | 3.17±1.03 |
| 4c | 15.83±3.21a, b | 1.67±0.15a, b | 87.09±4.04a | 2.25±0.31a, b |
| 4d | 20.46±2.02b | 0.98±0.02a, b | 169.84±5.09a, b | 0.67±0.25a, b |
| 5a | 21.96±1.70b | 0.89±0.07a, b | 176.41±3.19a, b | 0.75±0.25a, b |
Values are statistically different from control, P<0.05.
Values are statistically different from standard drug, P<0.05.
These results indicated the compounds 4d and 5a to be maximally safe on gastric mucosa as well as inducing negligible oxidative stress. The lower gastric safety and relatively poor oxidative stress parameters of compound 4c may be attributed to the presence of bromo group in the molecule.
2.5. Molecular properties calculation
“Lipinski׳s rule of 5” predicts oral bioavailability, intestinal absorption and blood brain barrier permeability of a new molecule through its molecular properties that include lipophilicity (log P), total polar surface area (TPSA), nON (number of H-bond acceptors), nOHNH (number of H-bond donors) and molecular weight. These properties are calculated using molinspiration calculations software24, 25. Log P and TPSA are the two most important properties for this prediction. TPSA is closely related to hydrogen bonding potential of a compound. Molecules with TPSA of about 160 Å or more are expected to have poor intestinal absorption26.
All compounds have been found to have nON and nOHNH in the ranges of 4–9 and 1–2, respectively, molecular weights less than 400, Log P in the range of 2.5–4.3, and TPSA in the range of 58–133 (Table 3). Moreover, all the compounds have zero violation of this rule. Hence, these parameters suggest that the compounds are expected to exhibit good oral bioavailability and intestinal absorption.
Table 3.
TPSA and molecular properties of test compounds.
| Compound | TPSAa | Log Pb | MWc | nONd | nOHNHe | n violf | n rotbg |
|---|---|---|---|---|---|---|---|
| 4a | 58.894 | 3.549 | 262.268 | 4 | 1 | 0 | 1 |
| 4b | 68.128 | 3.582 | 292.294 | 5 | 1 | 0 | 2 |
| 4c | 58.894 | 4.334 | 341.164 | 4 | 1 | 0 | 1 |
| 4d | 58.894 | 4.203 | 296.713 | 4 | 1 | 0 | 1 |
| 4e | 104.718 | 3.484 | 307.265 | 7 | 1 | 0 | 2 |
| 5a | 87.992 | 2.645 | 305.293 | 6 | 2 | 0 | 2 |
| 5b | 97.226 | 2.677 | 335.319 | 7 | 2 | 0 | 3 |
| 5c | 87.992 | 3.43 | 384.189 | 6 | 2 | 0 | 2 |
| 5d | 87.992 | 3.299 | 339.738 | 6 | 2 | 0 | 2 |
| 5e | 133.816 | 2.58 | 350.29 | 9 | 2 | 0 | 3 |
Total polar surface area.
Lipophilicity.
Molecular weight.
Hydrogen bond aceeptors.
Hydrogen bond donors.
Number of violations.
Number of rotatable bonds.
3. Conclusions
Two series of compounds were designed and synthesized by conjugating coumarin at 3-position with benzimidazole at 2-position through a single bond (series 4) as well as an amide linkage (series 5). The series 4 was found to possess anti-inflammatory activity better than the series 5. Compounds 4c, 4d and 5a exhibited maximum inhibition of inflammation. On the contrary, series 5 exhibited very potent antioxidant activities with 5a being the most potent and even more active than the standard compound BHT. SAR was established on the basis of the results obtained. Maximally potent compounds were found to be safe on gastric mucosa with least ability to induce oxidative stress in tissues except compound 4c. All compounds possessed sufficiently good oral bioavailability and hence, compounds 4d and 5a could be taken as lead for development of potent anti-inflammatory and antioxidant activities yet being safe to gastric mucosa.
4. Experimental
The reagents and solvents were of laboratory grade and were procured from different suppliers (LobaChemie, Mumbai; SD Fine, Mumbai; Merck, Mumbai). Melting points were determined in open capillaries using Digital Auto Melting Point Apparatus (Labtronics) and are uncorrected. Purity of the compounds was ascertained by TLC using precoated aluminium TLC plates visualized in a UV/Iodine chamber. Infrared spectra were recorded on an Alpha-E FTIR spectrophotometer (BrukerOptik, Germany) using potassium bromide optics. 1H and 13C NMR spectra were recorded on a BrukerAvance II spectrometer (400 MHz) using tetramethylsilane (TMS) as internal standard and chemical shifts are given in ppm. Mass spectra were recorded using a Q-TOF micromass spectrometer (Waters, MA, USA).
Wistar rats (150–250 g) of either sex were employed for the study. They were exposed to 12 h light/dark cycle and the animals had free access to food and water. The animals were given standard laboratory pellet chow diet and water ad libitum, both being withdrawn 12 h prior to experiment. The experimental protocol was duly approved by Institutional Animal Ethical Committee and the care of animals was done as per guidelines of Committee for the Purpose of Control and Supervision of Experiments on Animals, Government of India. The animals were administered orally with 20 mg/kg of indomethacin and the test compounds at equimolar doses to standard drug.
4.1. Chemistry
Synthesis of Meldrum׳s acid (1) and intermediate compound 3a (2-oxo-2H-chromen-3-carboxylic acid) has been reported in literature27, 28. Other derivatives of series 3 are novel compounds whose spectral data are as follows:
6-Methoxy-2-oxo-2H-chromen-3-carboxylic acid (3b): Yield: 88%; pale yellow crystals; mp: 210–212 °C; 1H NMR: δ 11.32 (s, 1H), 8.69 (s, 1H), 7.39 (d, 1H, J=2.88 Hz), 7.32–7.34 (m, 1H), 7.28 (dd, 1H, JA=2.88 Hz, JB=6.2 Hz), 3.83 (s, 3H); 13C NMR: δ 162.78, 158.53, 155.62, 148.09, 124.26, 122.35, 119.02, 118.81, 118.06, 111.67, 55.83; IR (KBr): 3048, 2990, 2763–3048, 1758, 1671, 1494, 1405, 1237 cm−1; HRMS (ESI+) m/z: calcd. for : 221.0444, found 221.0378.
6-Bromo-2-oxo-2H-chromen-3-carboxylic acid (3c): Yield: 70%; mp: 194–196 °C; white crystals; 1H NMR: δ 9.59 (s, 1H), 8.57 (s, 1H), 7.94 (d, 1H, J=2.32 Hz), 7.74 (dd, 1H, JA=2.36 Hz, JB=6.48 Hz), 7.28 (d, 1H, J=8.84 Hz); 13C NMR: δ 165.57, 157.23, 154.78, 136.72, 129.66, 127.87, 122.35, 120.59, 119.61, 119.29; IR (KBr): 3099, 2740–3099, 1759, 1678, 1556, 1204, 1029 cm−1; HRMS (ESI+) m/z: calcd. for : 268.9444, found 268.9377.
6-Chloro-2-oxo-2H-chromen-3-carboxylic acid (3d): Yield: 69%; white crystals; mp: 120–122 °C; 1H NMR: δ 9.29 (s, 1H), 8.51 (s, 1H), 7.87 (d, 1H, J=2.52 Hz), 7.61 (dd, 1H, JA=2.92 Hz, JB=6.32 Hz), 7.37 (d, 1H, J=8.88 Hz); 13C NMR: δ 160.73, 159.89, 150.81, 134.99, 130.48, 127.62, 125.76, 121.83, 120.52, 120.08; IR (KBr): 3047, 2625–3047, 1755, 1682, 1562, 1237, 1079 cm−1; HRMS (ESI+) m/z: calcd. for : 224.9949, found 224.9880.
6-Nitro-2-oxo-2H-chromen-3-carboxylic acid (3e): Yield: 80%; yellow crystals, mp 234–236 °C; 1H NMR: δ 10.69 (s, 1H), 8.83–8.87 (m, 2H), 8.47 (dd, 1H, JA=2.68 Hz, JB=7.88 Hz), 7.58 (d, 1H, J=9.12 Hz); 13C NMR: δ 160.96, 160.73, 157.89, 149.33, 128.34, 127.41, 122.54, 121.96, 121.62, 118.48; IR (KBr): 3115, 2865–3240, 1738, 1723, 1570, 1535, 1207 cm−1; HRMS (+ESI) m/z: calcd. for : 236.0190, found 236.0123.
4.1.1. Synthesis of test compounds 4a–e
Coumarin carboxylic acid, 3a–e (0.001 mol) was fused with o-phenylenediamine (0.001 mol) in the presence of polyphosphoric acid (PPA) (1 g/mmol) under nitrogen. The reaction mixture was refluxed in an oil bath at 170–180 °C for 0.5–1 h, cooled and poured into ice water. The resulting mixture was basified to pH 8 with 25% ammonium hydroxide solution. The precipitates were filtered, washed with water and dried to get crude product which was recrystallized from aqueous ethanol21. The compounds were found to be pure confirmed by spectral data given below:
3-(1H-Benzo[d]imidazol-2-yl)-2H-chromen-2-one (4a): Yield: 75%; green amorphous solid; mp: 290–292 °C; 1H NMR: δ 9.20 (s, 1H, NH), 8.17 (s, 1H), 7.64–7.76 (m, 4H), 7.40–7.47 (m, 2H), 7.33–7.37 (m, 2H); 13C NMR: δ 162.26, 152.76, 146.12, 140.35, 139.93, 138.11, 130.56, 129.87, 128.61, 125.11, 123.81, 122.99, 119.26, 118.69, 117.68, 117.23; IR (KBr): 3120, 1740, 1600, 1568, 1261, 1226 cm−1; HR-MS (+ESI) m/z: calcd. for : 263.0815, found 263.0824.
3-(1H-Benzo[d]imidazol-2-yl)-6-methoxy-2H-chromen-2-one (4b): Yield: 65%; orange amorphous solid; mp: 192–194 °C; 1H NMR: δ 9.16 (s, 1H, NH), 8.7 (s, 1H), 7.73–7.75 (m, 1H), 7.61–7.62 (m, 1H), 7.50–7.53 (m, 1H), 7.42–7.44 (m, 1H), 7.35–7.38 (m, 1H), 7.28–7.30 (m, 1H), 6.78–6.95 (m, 1H), 3.88 (s, 3H); 13C NMR: δ 164.05, 156.98, 155.72, 148.94, 148.17, 141.00, 141.00, 129.25, 123.10, 122.06, 122.06, 118.53, 118.53, 118.39, 117.27, 111.83, 55.8; IR (KBr): 3048, 2907, 1748, 1492, 1449, 1269, 1232 cm−1; HR-MS (ESI+) m/z: calcd. for : 293.0921, found 293.0925.
3-(1H-Benzo[d]imidazol-2-yl)-6-bromo-2H-chromen-2-one (4c): Yield: 80%; yellow amorphous solid; mp: 240–242 °C; 1H NMR: δ 9.15 (s, 1H), 8.29–8.34 (m, 2H), 7.88–7.91 (m, 1H), 7.71–7.76 (m, 1H), 7.55 (d, 2H, J=8.84 Hz), 7.27–7.32 (m, 2H); 13C NMR: δ 163.87, 153.60, 152.37, 145.45, 141.00, 135.25, 135.25, 132.07, 131.49, 122.83, 122.83, 121.03, 119.95, 118.52, 116.33, 116.33; IR (KBr): 3045, 1741, 1625, 1516, 1242, 1204, 1055 cm−1; HR-MS (ESI+) m/z: calcd. for : 340.9920, found 340.9929.
3-(1H-Benzo[d]imidazol-2-yl)-6-chloro-2H-chromen-2-one (4d): Yield: 63%; green amorphous solid; mp: 258–260 °C; 1H NMR: δ 9.39 (br s, 1H, NH), 7.75–7.78 (m, 2H), 7.62 (dd, each 2H, JA=2.44 Hz, JB=6.44 Hz), 7.40–7.42 (m, 2H), 7.25–7.27 (m, 2H); 13C NMR: δ 158.83, 151.83, 145.34, 140.87, 140.87, 138.39, 132.30, 128.75, 128.75, 128.37, 122.63, 122.63, 120.43, 118.12, 117.72, 117.72; IR (KBr): 3055, 1717, 1655, 1529, 1315, 1224, 1066 cm−1; HR-MS (ESI+) m/z: calcd. for : 297.0425, found 297.1436.
3-(1H-Benzo[d]imidazol-2-yl)-6-nitro-2H-chromen-2-one (4e): Yield: 60%; brown amorphous solid; mp>290 °C; 1H NMR: δ 9.29 (s, 1H), 8.50 (d, 2H, J=9.76 Hz), 7.75–7.85 (m, 2H), 7.54–7.66 (m, 2H), 7.31–7.32 (m, 2H); 13C NMR: δ 162.92, 155.36, 146.68, 144.61, 143.77, 141.12, 140.36, 128.98, 14.58, 123.24, 122.82, 122.55, 122.55, 118.22, 118.22, 116.32; IR (KBr): 3119, 1750, 1646, 1546, 1497, 1330, 1220 cm−1; HR-MS (ESI+) m/z: calcd. for : 308.0666, found 308.0615.
4.1.2. Synthesis of test compounds 5a–e
A suspension of 3a–e (0.01 mol) and DCC (2.3 g, 0.011 mol) in dried dichloromethane (DCM) (100 mL) was vigorously stirred for 30 min under nitrogen. A solution of 2-aminobenzimidazole (0.01 mol) dissolved in dried DCM (30 mL) and freshly distilled pyridine (50 mL) along with DMAP (0.050 g) was added to the reaction mixture at 0 °C in 15 min. The solution was stirred at 0 °C for 2 h followed by overnight stirring at room temperature. The solution was filtered to remove dicyclohexylurea and the filtrate was evaporated in vacuum to yield dry solid which was dissolved in dried ethyl acetate with heating in a water bath. The residue was filtered and the filtrate was washed with distilled water. The ethyl acetate layer was dried with magnesium sulphate and evaporated in vacuum. The resulting crude solid was recrystallized with methanol to yield corresponding amide22. The spectral data of the compounds of series 5a–e are as follows:
N-(1H-Benzo[d]imidazol-2-yl)-2-oxo-2H-chromen-3-carboxamide (5a): Yield: 54%; yellow amorphous solid; mp: 198–200 °C; 1H NMR: δ 9.04 (s, 1H), 8.30 (s, 1H), 7.74–7.80 (m, 2H), 7.59–7.62 (m, 1H), 7.54 (dd, 1H, JA=1.44 Hz, JB=6.32 Hz), 7.31–7.38 (m, 2H), 7.24–7.26 (m, 2H), 6.45 (br s, 1H); 13C NMR: δ 163.21, 156.58, 153.25, 152.81, 143.57, 142.16, 132.97, 130.59, 125.87, 124.57, 124.57, 122.93, 118.39, 117.29, 116.21, 116.21, 114.81; IR (KBr): 3054, 1707, 1664, 1644, 1531, 1315, 1225 cm−1; HR-MS (ESI+) m/z: calcd. for : 306.2949, found 306.2889.
N-(1H-Benzo[d]imidazol-2-yl)-6-methoxy-2-oxo-2H-chromen-3-carboxamide (5b): Yield: 51%; white amorphous solid; mp: 186–188 °C; 1H NMR: δ 8.96 (s, 1H), 8.17 (s, 1H), 8.0 (s, 1H), 7.84 (d, 1H, J=7.96 Hz), 7.29–7.31 (m, 2H), 7.22–7.23 (m, 2H), 7.18–7.20 (m, 1H), 5.50 (br s, 1H), 3.81 (s, 3H); 13C NMR: δ 162.17, 159.43, 155.16, 152.83, 147.61, 138.26, 135.36, 124.93, 122.41, 122.41, 120.43,118.30, 117.24, 116.89, 116.53, 114.56, 111.20, 55.75; IR (KBr): 3055, 2889, 1707, 1643, 1640, 1531, 1372, 1339, 1227 cm−1; HR-MS (ESI+) m/z: calcd. for : 336.3209, found 336.2987.
N-(1H-Benzo[d]imidazol-2-yl)-6-bromo-2-oxo-2H-chromen-3-carboxamide (5c): Yield: 50%; pale white amorphous solid; mp: 202–204 °C; 1H NMR: δ 9.09 (s, 1H), 8.3 (s, 1H), 8.22 (s, 1H), 7.91–7.97 (m, 1H), 7.77–7.79 (m, 1H), 7.54–7.59 (m, 2H), 7.37–7.40 (m, 2H), 5.57 (br s, 1H); 13C NMR δ: 162.96, 159.86, 152.78, 151.75, 137.24, 135.81, 134.61, 132.76, 125.51, 124.68, 123.87, 120.36, 119.36, 118.99, 116.37, 115.98, 115.62; IR (KBr): 3054, 1723, 1646, 1641, 1535, 1341, 1256, 1083 cm−1; HR-MS (ESI) m/z: calcd. for : 383.9978, found 383.9989.
N-(1H-Benzo[d]imidazol-2-yl)-6-chloro-2-oxo-2H-chromen-3-carboxamide (5d): Yield: 61%; white amorphous solid; mp: 212–214 °C; 1H NMR: δ 9.12 (s, 1H), 8.36 (s, 1H), 8.10 (s, 1H), 7.50–7.52 (m, 1H), 7.38–7.40 (m, 1H), 7.33–7.36 (m, 2H), 7.21–7.25 (m, 2H), 5.42 (br s, 1H); 13C NMR: δ 163.60, 158.96, 151.75, 150.50, 138.94, 136.69, 131.57, 129.78, 125.98, 123.95, 122.72, 121.21, 119.12, 118.58, 116.09, 115.86, 115.24; IR (KBr): 3054, 1708, 1690, 1645, 1534, 1340, 1266, 1044 cm−1; HR-MS (ESI+) m/z: calcd. for : 340.0483, found 340.0498.
N-(1H-Benzo[d]imidazol-2-yl)-6-nitro-2-oxo-2H-chromen-3-carboxamide (5e): Yield: 56%; yellow amorphous solid; mp>290 °C; 1H NMR: δ 9.15 (s, 1H), 8.84 (d, 1H, J=2.72 Hz), 8.41–8.52 (m, 1H), 8.31–8.34 (m, 1H), 7.61–7.70 (m, 1H), 7.40–7.45 (m, 1H), 7.33–7.35 (m, 1H), 7.20–7.22 (m, 2H), 6.89 (br s, 1H); 13C NMR: δ 163.29, 159.48, 158.65, 151.21, 145.89, 140.27, 139.94, 124.45, 124.45, 124.24, 123.79, 123.50, 122.11, 119.01, 117.12, 116.81, 114.97; IR (KBr): 3050, 1738, 1709, 1680, 1561, 1529, 1268, 1229 cm−1; HR-MS (+ESI) m/z: calcd. for : 351.0724, found 351.0740.
4.2. Anti-inflammatory activity
The inflammation was induced by injecting 0.1 mL of formalin in subplantular region of rat׳s hind paw. Animals were divided into various groups each of six rats. The control group received vehicle (0.5% sodium carboxymethylcellulose (SCMC)) whereas the standard groups received the standard drug indomethacin at a dose of 20 mg/kg orally. Various test groups were administered test compounds at the dose equimolar to the standard drug 1 h prior to the formalin injection. The paw volume was measured by plethysmograph and the change in paw volume was noted periodically over 0–6 h29. The inhibition was calculated by the formula reported by Chu and Kovacs30.
4.3. Antioxidant activity
4.3.1. DPPH assay
The antioxidant activity of the test compounds was evaluated in terms of hydrogen donating or radical scavenging ability with the DPPH method taking BHT as standard drug31. The activity was evaluated by taking different concentrations of each test and standard compound. 700 µL of each concentration of the standard solution of ascorbic acid and test compounds in methanol was mixed with the same volume of methanolic solution of 700 µmol/L DPPH. The mixed solution was shaken vigorously, allowed to stand in dark at room temperature for 30 min and its absorbance was read at 515 nm using a UV–Vis spectrophotometer (Beckman, USA). The standard/test solution was replaced with methanol to serve as control. The antiradical activity was calculated in terms of inhibition using the following equation: [(AbsControl−AbsTest)/AbsControl]×100%. Different sample concentrations of each test compound and standard were used in order to obtain a calibration curve for each test compound as well as BHT was constructed by taking inhibition(%) as abscissa and concentration as coordinate to calculate the EC50 values (the concentration required to obtain a 50% radical scavenging activity). Data analysis was performed as reported by Locatelli et al.23 to compute EC50 on the basis of probit regression. The antioxidant activity of each test compound was performed in triplicate and EC50 values are reported as mean±standard deviation (SD).
4.3.2. In vivo biochemical estimations
Glandular parts of the extracted stomachs were homogenized in cold phosphate buffer (pH 7.4) for 2 min. The homogenized contents were centrifuged at 800×g for 10 min followed by centrifugation at 12,000×g for 15 min. The resulting supernatant was used for catalase32, lipid peroxidation (LPO)33 and glutathione reductase assays34.
4.4. Chronic ulcerogenicity test
Albino rats were used to perform this test. The control group received vehicle whereas the test group and the standard group received the test compounds (4 and 5, respectively) and the standard drug (indomethacin) at the therapeutic dose for anti-inflammatory effects for a period of 28 days orally. The rats were then sacrificed and the stomach was removed and opened along the greater curvature. The inner surface was washed slowly with normal saline and was examined for the severity of ulceration according to the following scale: 0=normal grey coloured stomach, 0.5=pink to red colouration of stomach, 1=spot ulcer, 1.5=haemorrhagic ulcer, 2=ulcer<5, 3=ulcer>5, 4=ulcers with bleeding. Mean ulcer score for the control, standard and each test compound was calculated and reported as ulcer index35.
4.5. Statistical analysis
All results were expressed as mean±SD. The statistical significance was determined by one-way analysis of variance (ANOVA) followed by Dunnet׳s test and the results were found significant at P<0.05.
Acknowledgement
The authors thank the Sophisticated Analytical Instrumentation Facility (SAIF), Panjab University for NMR, Mass and HR-MS spectral analyses. The authors are also thankful to the AICTE, New Delhi (India) for providing fellowship to two of the authors (Radha Krishan Arora and Navneet Kaur).
Footnotes
Peer review under responsibility of Institute of Materia Medica, Chinese Academy of Medical Sciences and Chinese Pharmaceutical Association.
References
- 1.Medzhitov R. Inflammation: new adventures of an old flame. Cell. 2010;140:771–776. doi: 10.1016/j.cell.2010.03.006. [DOI] [PubMed] [Google Scholar]
- 2.Grivennikov SI, Greten FR, Karin M. Immunity, inflammation and cancer. Cell. 2010;140:883–899. doi: 10.1016/j.cell.2010.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kankala S, Kankala RK, Gundepaka P, Thota N, Nerella S, Gangula MR. Regioselective synthesis of isoxazole–mercaptobenzimidazole hybrids and there in vivo analgesic and anti-inflammatory activity studies. Bioorg Med Chem Lett. 2013;23:1306–1309. doi: 10.1016/j.bmcl.2012.12.101. [DOI] [PubMed] [Google Scholar]
- 4.El-Nezhawy AO, Biuomy AR, Hassan FS, Ismaiel AK, Omar HA. Design, synthesis and pharmacological evaluation of omeprazole-like agents with anti-inflammatory activity. Bioorg Med Chem. 2013;21:1661. doi: 10.1016/j.bmc.2013.01.070. [DOI] [PubMed] [Google Scholar]
- 5.Gabriel SE, Jaakkimainen L, Bombardier C. Risk for serious gastrointestinal complications related to use of nonsteroidal anti-inflammatory drugs a meta-analysis. Ann Intern Med. 1991;115:787–796. doi: 10.7326/0003-4819-115-10-787. [DOI] [PubMed] [Google Scholar]
- 6.Caruso I, Porro GB. Gastroscopic evaluation of anti-inflammatory agents. Br Med J. 1980;280:75. doi: 10.1136/bmj.280.6207.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lanza F, Rack M, Simon T, Quan H, Bolognese J, Hoover M. Specific inhibition of cyclooxygenase‐2 with MK‐0966 is associated with less gastroduodenal damage than either aspirin or ibuprofen. Aliment Pharmacol Ther. 1999;13:761–767. doi: 10.1046/j.1365-2036.1999.00529.x. [DOI] [PubMed] [Google Scholar]
- 8.Ray WA, Stein CM, Daugherty JR, Hall K, Arbogast PG, Griffin MR. COX-2 selective non-steroidal anti-inflammatory drugs and risk of serious coronary heart disease. J Lancet. 2002;360:1071–1073. doi: 10.1016/S0140-6736(02)11131-7. [DOI] [PubMed] [Google Scholar]
- 9.Wallace JL, Soldato PD. The therapeutic potential of NO‐NSAIDs. Fundam Clin Pharmacol. 2003;17:11–20. doi: 10.1046/j.1472-8206.2003.00125.x. [DOI] [PubMed] [Google Scholar]
- 10.Fylaktakidou KC, Hadjipavlou-Litina DJ, Litinas KE, Nicolaides DN. Natural and synthetic coumarin derivatives with anti-inflammatory/antioxidant activities. Curr Pharm Des. 2004;10:3813–3833. doi: 10.2174/1381612043382710. [DOI] [PubMed] [Google Scholar]
- 11.Rosskopf F, Kraus J, Franz G. Imunological and antitumor effects of coumarin and some derivatives. Pharmazie. 1992;47:139. [PubMed] [Google Scholar]
- 12.Hwu JR, Singha R, Hong SC, Chang YH, Das AR, Vliegen I. Synthesis of new benzimidazole–coumarin conjugates as anti-hepatitis C virus agents. Antiviral Res. 2008;77:157–162. doi: 10.1016/j.antiviral.2007.09.003. [DOI] [PubMed] [Google Scholar]
- 13.Patel RV, Kumari P, Rajani DP, Chikhalia KH. Synthesis of coumarin-based 1, 3, 4-oxadiazol-2ylthio-N-phenyl/benzothiazolylacetamides as antimicrobial and antituberculosis agents. Med Chem Res. 2013;22:195–210. [Google Scholar]
- 14.Bansal Y, Sethi P, Bansal G. Coumarin: a potential nucleus for anti-inflammatory molecules. Med Chem Res. 2013;22:3049–3060. [Google Scholar]
- 15.Roussaki M, Kontogiorgis CA, Hadjipavlou-Litina D, Hamilakis S, Detsi A. A novel synthesis of 3-aryl coumarins and evaluation of their antioxidant and lipoxygenase inhibitory activity. Bioorg Med Chem Lett. 2010;20:3889–3892. doi: 10.1016/j.bmcl.2010.05.022. [DOI] [PubMed] [Google Scholar]
- 16.Sreeja S, Mathan S, Kumaran J. Design, synthesis and pharmacological evaluation of new coumarin derivatives. Int J Adv Pharm Biol Sci. 2012;2:80–91. [Google Scholar]
- 17.Rodríguez SA, Nazareno MA, Baumgartner MT. Effect of different C3-aryl substituents on the antioxidant activity of 4-hydroxycoumarin derivatives. Bioorg Med Chem. 2011;19:6233–6238. doi: 10.1016/j.bmc.2011.09.012. [DOI] [PubMed] [Google Scholar]
- 18.Stefani HA, Ueogjan KG, Manarin F, Farsky SH, Zukerman-Schpector J, Caracelli I. Synthesis, biological evaluation and molecular docking studies of 3-(triazolyl)-coumarin derivatives: effect on inducible nitric oxide synthase. Eur J Med Chem. 2012;58:117. doi: 10.1016/j.ejmech.2012.10.010. [DOI] [PubMed] [Google Scholar]
- 19.Bansal Y, Silakari O. The therapeutic journey of benzimidazoles: a review. Bioorg Med Chem. 2012;20:6208. doi: 10.1016/j.bmc.2012.09.013. [DOI] [PubMed] [Google Scholar]
- 20.Patil A, Ganguly S, Surana S. A systematic review of benzimidazole derivatives as an antiulcer agent. Rasayan J Chem. 2008;3:447–460. [Google Scholar]
- 21.Li YF, Wang GF, He PL, Huang WG, Zhu FH, Gao HY. Synthesis and anti-hepatitis B virus activity of novel benzimidazole derivatives. J Med Chem. 2006;49:4790–4794. doi: 10.1021/jm060330f. [DOI] [PubMed] [Google Scholar]
- 22.Ma Y, Luo W, Quinn PJ, Liu Z, Hider RC. Design, synthesis, physicochemical properties, and evaluation of novel iron chelators with fluorescent sensors. J Med Chem. 2004;47:6349–6362. doi: 10.1021/jm049751s. [DOI] [PubMed] [Google Scholar]
- 23.Locatelli M, Gindro R, Travaglia F, Coïsson JD, Rinaldi M, Arlorio M. Study of the DPPH-scavenging activity: development of a free software for the correct interpretation of data. Food chem. 2009;114:889–897. [Google Scholar]
- 24.Ertl P, Rohde B, Selzer P. Fast calculation of molecular polar surface area as a sum of fragment-based contributions and its application to the prediction of drug transport properties. J Med Chem. 2000;43:3714–3717. doi: 10.1021/jm000942e. [DOI] [PubMed] [Google Scholar]
- 25.Lipinski CA, Lombardo F, Dominy BW, Feeney PJ. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv Drug Delivery Rev. 1997;23:3–25. doi: 10.1016/s0169-409x(00)00129-0. [DOI] [PubMed] [Google Scholar]
- 26.Alafeefy AM, Alqasoumi SI, Ashour AE, Masand V, Al-Jaber NA, Ben Hadda T. Quinazoline–tyrphostin as a new class of antitumor agents, molecular properties prediction, synthesis and biological testing. Eur J Med Chem. 2012;53:133–140. doi: 10.1016/j.ejmech.2012.03.044. [DOI] [PubMed] [Google Scholar]
- 27.Davidson D, Bernhard SA. The structure of Meldrum׳s supposed β-lactonic acid. J Am Chem Soc. 1948;70:3426–3428. doi: 10.1021/ja01190a060. [DOI] [PubMed] [Google Scholar]
- 28.Song A, Wang X, Lam KS. A convenient synthesis of coumarin-3-carboxylic acids via Knoevenagel condensation of Meldrum׳s acid with ortho-hydroxyaryl aldehydes or ketones. Tetrahedron Lett. 2003;44:1755–1758. [Google Scholar]
- 29.Fereidoni M, Ahmadiani A, Semnanian S, Javan M. An accurate and simple method for measurement of paw edema. J Pharmacol Toxicol Methods. 2000;43:11–14. doi: 10.1016/s1056-8719(00)00089-7. [DOI] [PubMed] [Google Scholar]
- 30.Chu D, Kovacs B. Anti-inflammatory activity in oak gall extracts. Arch Int Pharmacodyn Ther. 1977;230:166–176. [PubMed] [Google Scholar]
- 31.Xua Z, Lua B, Xiang Q, Lia Y, Lia S, Lina Y. Radical-scavenging activities of marine-derived xyloketals and related chromanes. Acta Pharm Sin B. 2013;3:322–327. [Google Scholar]
- 32.Aebi H. Catalase in vitro. Methods Enzymol. 1974;2:673–684. doi: 10.1016/s0076-6879(84)05016-3. [DOI] [PubMed] [Google Scholar]
- 33.Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979;95:351–358. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- 34.Beutler E, Duron O, Kelly BM. Improved method for the determination of blood glutathione. J Labor Clin Med. 1963;61:882. [PubMed] [Google Scholar]
- 35.Boligona AA, Freitasa RB, Bruma TF, Waczukc EP, Klimaczewskic CV, Ávilac, Margareth DS. Antiulcerogenic activity of Scutia buxifolia on gastric ulcers induced by ethanol in rats. Acta Pharm Sin B. 2014 doi: 10.1016/j.apsb.2014.05.001. Available from: http://dx.doi.org/10.1016/j.apsb.2014.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]