Abstract
Lipid-based formulations have been an attractive choice among novel drug delivery systems for enhancing the solubility and bioavailability of poorly soluble drugs due to their ability to keep the drug in solubilized state in the gastrointestinal tract. These formulations offer multiple advantages such as reduction in food effect and inter-individual variability, ease of preparation, and the possibility of manufacturing using common excipients available in the market. Despite these advantages, very few products are available in the present market, perhaps due to limited knowledge in the in vitro tests (for prediction of in vivo fate) and lack of understanding of the mechanisms behind pharmacokinetic and biopharmaceutical aspects of lipid formulations after oral administration. The current review aims to provide a detailed understanding of the in vivo processing steps involved after oral administration of lipid formulations, their pharmacokinetic aspects and in vitro in vivo correlation (IVIVC) perspectives. Various pharmacokinetic and biopharmaceutical aspects such as formulation dispersion and lipid digestion, bioavailability enhancement mechanisms, impact of excipients on efflux transporters, and lymphatic transport are discussed with examples. In addition, various IVIVC approaches towards predicting in vivo data from in vitro dispersion/precipitation, in vitro lipolysis and ex vivo permeation studies are also discussed in detail with help of case studies.
KEY WORDS: Pharmacokinetics, Lipolysis, IVIVC, Efflux transporters, Lymphatic delivery, Food effect
Abbreviations: ADME, absorption/distribution/metabolism/elimination; AUC, area under the curve; BCS, biopharmaceutics classification system; BDDCS, biopharmaceutics drug disposition classification system; CACO, human epithelial colorectal adenocarcinoma cells; Cmax, maximum plasma concentration; CMC, critical micellar concentration; CYP, cytochrome; DDS, drug delivery systems; FaSSGF, fasted-state simulated gastric fluid; FaSSIF, fasted-state simulated intestinal fluid; FeSSIF, fed-state simulated intestinal fluid; GIT, gastrointestinal tract; IVIVC, in vitro in vivo correlation; LCT, long chain triglyceride; LFCS, lipid formulation classification system; log P, n-octanol/water partition coefficient; MCT, medium chain triglyceride; MDCK, Madin–Darby canine kidney cells; NCE, new chemical entity; P-app, apparent permeability; P-gp, permeability glycoprotein; SCT, short chain triglyceride; SEDDS, self-emulsifying drug delivery system; SIF, simulated intestinal fluid; SMEDDS, self-microemulsifying drug delivery system; SNEDDS, self-nanoemulsifying drug delivery system; Vit E, vitamin E
Graphical abstract
Despite many advantages of lipid based formulations, very few products are available in the present market perhaps due to limited knowledge in the in vitro tests for prediction of in vivo fate and lack of understanding in the mechanisms behind pharmacokinetic and biopharmaceutical aspects of lipid formulations after oral administration. The current review aims to provide a detailed understanding of the in vivo processing steps involved after oral administration of lipid formulations, their pharmacokinetic aspects and IVIVC perspectives.
1. Introduction
Approximately 40% of the currently marketed formulations and more than 70% of pipeline molecules from top pharmaceutical companies today contain drugs that are poorly soluble1, 2. However, the superior therapeutic efficacy of these poorly soluble molecules (BCS-II and IV) may be the reason that they cannot be always avoided in drug development, and optimal formulation strategies are required to handle them so as to enhance their availability in systemic circulation. Even though there are conventional approaches available for handling poor aqueous solubility, very often advanced drug delivery systems (DDS) are required for developing a stable and acceptable dosage form. The most important category in advanced DDS is lipid-based formulations such as lipid solutions, lipid suspensions and self-emulsifying lipid formulations3, 4. The lipid-based formulations in general are well recognized as a frontline formulation technology to handle the poorly water-soluble compounds. These systems can be designed to present and keep the drug substance in a solubilized state thereby preventing the solubilization and subsequent dissolution step of a poorly water-soluble compound. The extensive research work done by Pouton and Porter5, 6 in the area of lipid formulation development has resulted in increased awareness and understanding about lipid formulations in both industry and academia.
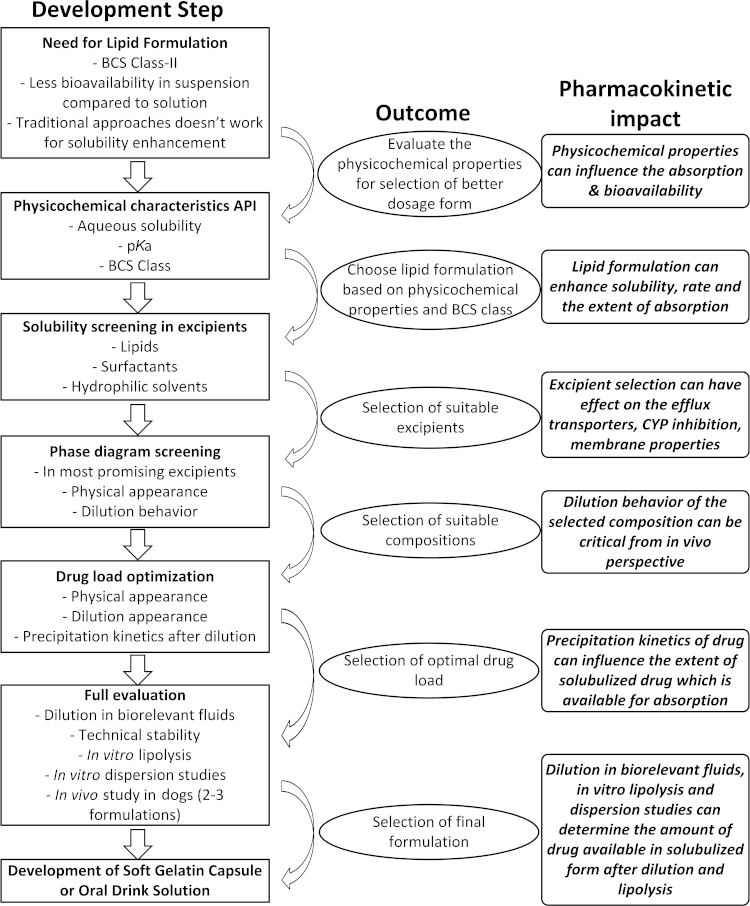
The preparation of lipid-based formulations is considered an easy process when compared with other solid oral dosage forms such as tablets and capsules. The excipients used in lipid formulations include lipids (natural/synthetic origin), surfactants (hydrophilic/hydrophobic), hydrophilic solvents and co-solvents. Once prepared, the lipid-based systems can be administered as solutions after dilution with suitable juices or dietary fluids or in the form of liquid-encapsulated soft gelatin capsules or liquid-filled hard gelatin capsules7. The general process for development of lipid formulations along with the pharmacokinetic importance of each step is presented in Fig. 1. Due to the wide variety of excipients available for preparing lipid-based formulations, Pouton et al.8 introduced a lipid formulation classification system (LFCS) in order to harmonize the understanding about these formulations. As per LFCS, the lipid-based formulations can be classified into four different categories: Types-I, II, III (A and B) and IV. The compositions of these formulation types along with their characteristics, advantages, disadvantages and pharmacokinetic aspects are presented in Table 1. Out of these four systems, Type-II formulations are named as self-emulsifying drug delivery systems (SEDDS, coarse emulsions) and Type-III formulations are named as self-microemulsifying drug delivery systems (SMEDDS, microemulsions) due to their ability to form instantaneous emulsions with minimal energy input.
Figure 1.
General process flow for development of microemulsions indicating the pharmacokinetic importance of each step.
Table 1.
Lipid formulation classification system overview: pharmacokinetic aspects.
| Characteristics | Type-I | Type-II | Type-IIIA | Type-IIIB | Type-IV |
|---|---|---|---|---|---|
| Materials | Oil: 100% | Oil: 40%–80% | Oil: 40%–80% | Oil: <20% | Water soluble |
| Surfactants (water in-soluble, HLB<12): | Surfactants (water soluble or in-soluble): 20%–40% | Surfactants (water soluble, HLB>12): 20%–50% | Surfactants: 80%–100% | ||
| 20%–60% | Co-solvents: 0%–40% | Co-solvents: 20%–50% | Co-solvents: 0%–20% | ||
| Dispersion behavior | No or limited dispersion due to bile salts in GIT | SEDDS | SMEDDS/SEDDS | SMEDDS | Micellar solution |
| Particle size after dispersion | Very coarse | 100–250 nm | 100–250 nm | 50–100 nm | <100 nm |
| Significance of aqueous dilution | Not important due to lack of surfactants | Retains solvent capacity due to the absence of water soluble components | Some loss of solvent capacity leading to drug precipitation | Significant loss of solvent capacity due to the presence of higher quantities of water soluble components | Significant loss of solvent capacity due to surfactant dilution |
| Significance of lipid digestion | Highly important since it is the only mechanism to release drug | Likely to occur but not very important | Not important, but may be inhibited due to own digestion products | Not required and not likely to occur | Not required |
| Advantages | Simple system, good compatibility with capsules | Good solvent capacity, prevents drug precipitation after dilution | Clear dispersion with lesser droplet size, no requirement for digestion | Clear dispersion with lesser droplet size, no requirement for digestion | Good and excellent solvent capacity for many drugs |
| Disadvantages | Poor solvent capacity unless drug is highly lipophilic | Turbid emulsions and in vivo fate depends on digestion | Precipitation of drug likely to occur after dispersion and digestion | Extensive precipitation of drug after dispersion | Extensive precipitation of drug after dispersion |
| Pharmacokinetic behavior | May enhance bioavailability but can result in high interindividual variability due to lack of dispersion | May greatly enhance bioavailability but can result in high interindividual variability due to formation of coarse emulsion | Bioavailability may be enhanced (depending on the extent of precipitation after dispersion and digestion), less interindividual variability due to less particle size formed after dispersion | Bioavailability may be enhanced (depending on the extent of precipitation after dispersion and digestion), less interindividual variability due to less particle size formed after dispersion | May not yield higher bioavailability due to extensive drug precipitation under in vivo conditions |
| Marketed products | Calcitrol (Rocaltrol®), Roche | Cyrlosporin A (Sandimmune®), Novartis | Cyrlosporin A (Neoral®), Novartis | Tipranavir (Aptivus®), Boehringer Ingelheim | Ritonavir (Norvir®), Abbott, Amprenavir (Agenerase®), Glaxosmithkline |
Out of the lipid-based formulations available in the present market, Neoral® and Sandimmun Neoral® are considered to be the first commercial successes9. The complete list of all commercially available lipid formulations is outlined by Strickley10. The data clearly indicate that despite the multiple advantages and extensive research work in academia and industries, there are very few commercially successful products available in the market today. From one side of the coin, this problem can be attributed to scale-up and stability challenges, marketability concerns, lack of in-house soft gelatin manufacturing capabilities, and non-acceptability of soft gelatin capsules in a few countries. From the other side of the coin, critical problems arise due to the lack of availability of in vitro tests that can describe the in vivo behavior of the lipid formulations. There are numerous in vivo ADME processes involved after intake of lipid-based formulations which make the concepts more complex for designing the in vitro tests. Additionally no clear IVIVC relationship has been established for lipid-based formulations, indicating the difficulties in correlating in vitro results with in vivo behavior. This clearly indicates the need for understanding the pharmacokinetic aspects and other related systemic processes for lipid-based formulations. An extensive literature review revealed that a few articles have described the pharmacokinetic aspects from different perspectives but no single article described all pharmacokinetic and IVIVC aspects of lipid-based formulations in detail. In this context, the objective of the present article is to outline the pharmacokinetic aspects and in vivo processing steps occurring after administration of lipid formulations so as to provide a better understanding of the lipid-based formulations from a pharmacokinetic point of view. In addition, multiple IVIVC concepts and methodologies are covered which can further aid in development of successful in vitro prediction tests.
2. Pharmacokinetic aspects of lipid-based formulations
2.1. In vivo drug solubilization and processing
Even though in the present context lipids are described as core excipients in the lipid-based formulations, they are an essential group of constituents in the food we take everyday. The processing of lipids containing drug is essentially similar to that of the dietary lipids present in food or in any other related source. Ingestion of lipid formulations results in increase in the total amount of lipids available in GI tract. These larger quantities of lipid (>2 g, equivalent to two soft gelatin capsules) are capable of stimulating secretion of additional bile through gallbladder contraction thereby increasing the luminal concentration of bile salts11. The increased levels of endogenous bile salts, phospholipids and cholesterol in the presence of lipid and surfactants provide a lipidic microenvironment to form emulsion droplets which will further transform into various components such as vesicular and micellar phases. The poorly water-soluble drug initially dissolved in the formulation will partition into these vesicular and micellar phases. The drug then will partition into the micelles formed due to the combination of bile salts, phospholipids and cholesterol which in turn are called “mixed micelles”12. The formation of mixed micelles is an important step for solubilization and absorption of a poorly water-soluble drug. The partitioning of drug into the oil core of micellar systems creates a concentration gradient across the luminal wall required to drive the absorption process. The inherent bile salts can also act as surfactants along with formulation-derived surfactants and help the drug in solubilization by improving wetting. This process provides intestinal environment with a high solubilization capacity for poorly water-soluble drug thereby enhancing the bioavailability of the compound. Various studies in the literature attempted to study the drug solubilization behavior in different phases generated during lipid digestion using a combination of phase studies coupled with solubility studies13, 14. In addition, the distribution of drug across the colloidal species produced during in vivo processing was monitored with help of electron paramagnetic resonance spectroscopy15. In in vitro experiments the phases occurring during luminal processing can be studied by addition of bile salts, phospholipids and cholesterol to the intestinal fluid. It was also observed that at very high lipid levels, various liquid crystalline structures such as unilamellar, multilamellar and cubic crystalline structures were present during in vivo processing.
It has also been observed that many formulation-related factors can also contribute to lipid processing. Selection of lipid excipients in the formulation is considered an important factor since the structures formed during lipid processing can vary with the types of lipids ingested (such as long chain triglycerides and medium chain triglycerides). Long chain triglycerides are found to be digested slowly compared with medium chain triglycerides, indicating that lipase activity is a function of lipid chain length16. Hence, depending on the lipid chosen, the rate of digestion will vary and thereby contributing to the enhancement of bioavailability. Another related important factor from excipient point of view is the selection of surfactants due to their ability to inhibit the activity of lipases17. This clearly indicates that improving the dispersibility of a formulation by addition of surfactants does not necessarily improve the in vivo performance of the formulation. From other side, the efficiency of lipases on the exposed surface area was studied, which was further dictated by the number of droplets and their size in the emulsion indicating the importance of the dispersibility of the formulation18.
2.2. In vivo dispersibility and impact on pharmacokinetics
The in vivo dispersibility and dilution behavior are two important characteristics of the lipid-based formulation which can have significant impact on the in vivo bioavailability19. The in vivo dispersibility is a measure of the size and polydispersity of the droplets formed during the self-emulsification process aided by gastric agitation. This can be measured in vitro after diluting the formulation in various biorelevant fluids such as FaSSGF (pH 1.6), FaSSIF (pH 6.5) and FeSSIF (pH 5.0) and measuring the droplet size using techniques such as Dynamic Light Scattering (such as Malvern Zetasizer)20. This would provide a clear understanding about the dilution behavior of the formulation under the in vivo conditions in stomach and intestine. In general, the smaller the droplet size, the greater the surface area and the more the lipid digestion. It has been proved that formulations producing lesser droplet size will produce more bioavailability with reduced inter-individual pharmacokinetic variability. The influence of droplet size on bioavailability has been observed for various formulations in the literature. The self-emulsifying formulation of vitamin E has resulted in approximately a 3-fold increase in absorption when compared with vitamin E solubilized in soybean oil, and it was attributed to the finer dispersion size in self-emulsifying formulation21. Similarly for cyclosporine, enhanced bioavailability (about 6.5 times) was observed for a microemulsion formulation when compared with a conventional formulation. This was mainly attributed to the smaller droplet size in the self-microemulsifying formulation of cyclosporine22. However, there were some differences with these findings in the literature with halofantrine. The size of the formulation did not have significant impact on the bioavailability when both the formulations (SEDDS and SMEDDS) were prepared using medium chain triglycerides23. Similarly for atorvaquone the droplet size of the formulation was described as a poor indicator of in vivo performance since various lipid formulations containing different surfactants resulted in different droplet sizes but there was no significant difference in the in vivo results24. Similar findings regarding the droplet size were observed for danazol and ontazolast25, 26. It can be concluded that droplet size is not an important indicator of the in vivo performance and fate of the poorly water-soluble drug depends mainly on the dilution and digestion as they determine solubilization or precipitation of the drug4. This clearly indicates the importance of maintaining drug in the solubilized state for enhanced absorption rather than precipitating after dilution in the larger quantities of biological fluids in the stomach and intestine.
2.3. In vivo dilution and impact on pharmacokinetics
In vitro dilution in biological fluids followed by optical microscopic examination during the formulation development can provide an understanding about the precipitation of a drug after dilution in GI tract. In addition, the saturation solubility in water added to formulations can be studied which would provide an understanding about drug precipitation due to water intake. Increasing the solubilization capacity of the formulation significantly over the desired drug loading can also help to avoid precipitation under in vivo conditions. The amount of drug precipitated depends on the formulation factors as well as physiological factors of the individual under medication. While formulation factors can be controlled, physiological factors cannot be controlled indicating the importance of ensuring the solubilized state using various in vitro tools19. The physiologically related drug precipitation would lead to inconsistent pharmacokinetic profiles leading to higher inter-individual variability20. Dai et al.27 provided an excellent approach for studying the precipitation behavior of poorly water-soluble drug in lipid-based formulations at development stage using only milligrams of NCEs. The authors have suggested an in vitro precipitation determination using a 96-well-plate method with varying dilution factors and durations in biological fluids. Based on this approach, the authors have categorized formulations as no-precipitation, fast-precipitation and slow-precipitation. The in vivo administration of these formulations to dogs resulted in plasma profiles which are in agreement with in vitro precipitation profiles. In another similar study, the ability of Type-II, III-A, III-B and IV systems to maintain the model drug fenofibrate in the solubilized state after dispersion in aqueous solution was examined28. The authors concluded that even though Type-II systems produced turbid emulsions after dilution, they are able to maintain the drug in the solubilized state with very slow precipitation. The Type-IIIA systems maintained fenofibrate in a metastable state after dilution in water for several hours to days. The Type-IIIB systems led to rapid precipitation due to the presence of higher quantities of water-soluble surfactants. Type-IV systems totally failed to maintain the drug in the solubilized state resulting in extensive rapid precipitation. The results indicate the importance of selection of suitable lipid-based systems for maintaining a drug in solubilized state and also indicate that the formulation scientists should not only consider the physical appearance as ultimate aspect but should also consider precipitation of poorly water-soluble drug in the system. While formulating the drug in a Type-II system seems to be an attractive choice for preventing drug precipitation, various other aspects are also studied for maintaining the drug in a supersaturation state without in vivo precipitation. The most interesting approach for formation of supersaturated drug solution in vivo is by incorporation of hydrophilic polymeric excipients in the formulation that can act as precipitation inhibitors29. This approach is very useful for drugs exhibiting lower solubility in lipid excipients and thereby requiring higher clinical doses. The precipitation inhibitors can significantly delay the precipitation times and if the precipitation times are greater than the mean absorption time, the extent of absorption of a poorly water-soluble drug can be increased thereby enhancing the bioavailability30. Most of the commonly used polymer excipients in this category are hydroxypropyl methyl cellulose (HPMC), methyl cellulose (MC), sodium carboxymethyl cellulose (NaCMC) and polyvinyl pyrrolidone (PVP). The use of HPMC as a precipitation inhibitor for paclitaxel in a supersaturable SEDDS resulted in a 9-fold increase in bioavailability in rats31. The use of PVP as precipitation inhibitor for carbamazepine in a supersaturable SMEDDS that enhanced the bioavailability by 5-fold in dogs when compared with a commercially available tablet has also been reported32. The use of polymers as precipitation inhibitors is not only limited to lipid-based formulations but can also be extended to other formulations such as solid dispersions and controlled release tablet formulations. Yamashita et al.33 reported the use of HPMC as precipitation inhibitor for delivery of tacrolimus in the form of solid dispersion wherein it showed 10-fold increase in area under the curve (AUC) and Cmax when compared with crystalline powder.
2.4. Absorption and bioavailability enhancement
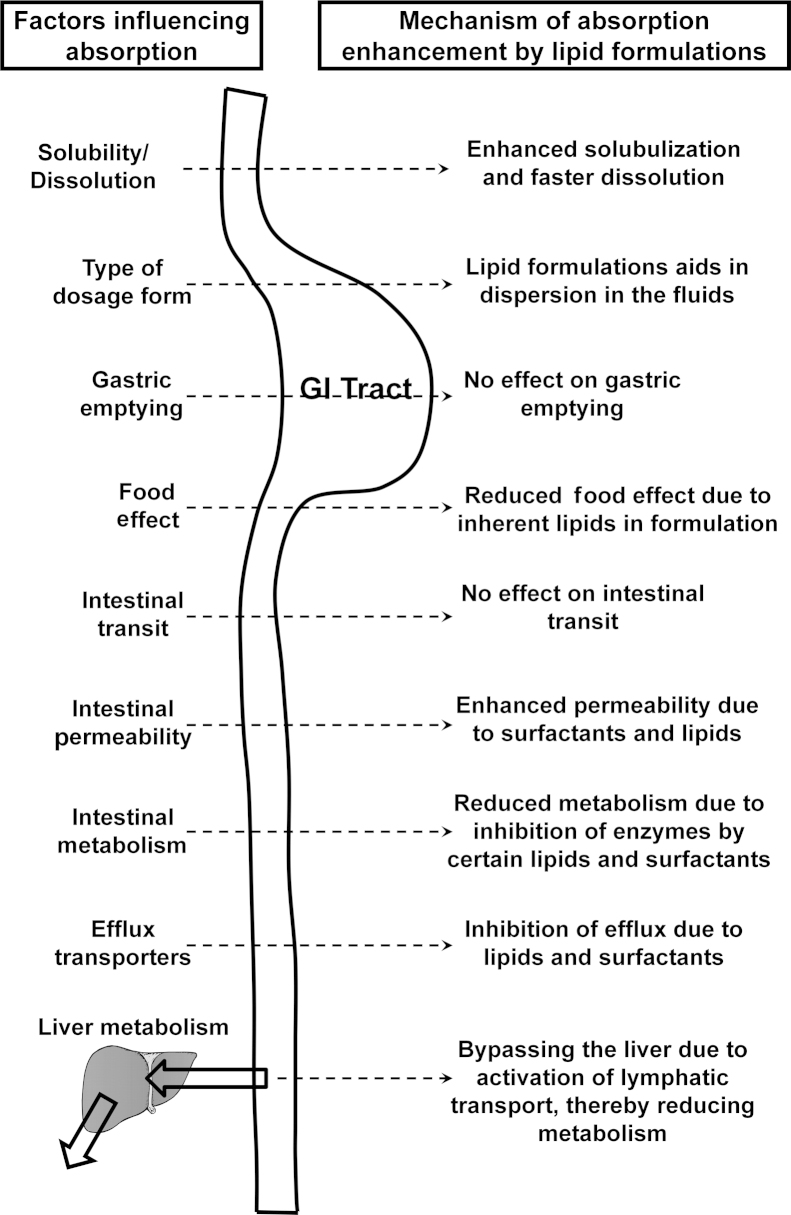
The basic property by which optimized lipid formulations such as SEDDS and SMEDDS enhance the absorption and bioavailability of poorly water-soluble drugs is by presenting the drug in the solubilized form throughout the transit through gastrointestinal tract with minimal precipitation. Various mechanisms have been stated in the literature for bioavailability enhancement such as stimulation of pancreatic and biliary secretions, prolongation of GI residence time to slow down the delivery system to the absorption site and increase the time available for dispersion or dissolution, stimulation of lymphatic transport by overcoming the first pass metabolism, increasing the intestinal wall permeability by membrane fluidization and opening the tight junctions, reduced intestinal metabolism by encapsulating the drug in the form of micelles, and reduced efflux transporter activity by incorporating excipients that can act as efflux inhibitors34. These processes are provided in pictorial representation in Fig. 2. These numerous mechanisms clearly indicate the advantages of using lipid-based formulations for enhancing bioavailability. It is also evident that even if two to three mechanisms from the above stated work for a drug formulated in a lipid system, it would definitely lead to enhanced bioavailability thereby enhancing pharmacological activity. A literature review revealed numerous examples in which the absorption and bioavailability of poorly water-soluble drugs were enhanced by administration in the form of lipid formulations. Selected examples are discussed here and Table 2 summarizes the other cited studies.
Figure 2.
Mechanisms by which lipid formulations can enhance bioavailability.
Table 2.
Bioavailability enhancement by lipid based formulations.
| Drug/BCS class | Formulation (Excipients) | Test system | Results | Mechanism |
|---|---|---|---|---|
| Acyclovir (III)35 | Microemulsion (Labrafac, Labrasol, Plurol oleique, water) | Sprague-Dawley rats | Relative bioavailability enhanced by 12.78 times when compared with tablet | Enhanced solubilization in microemulsion |
| Atorvastatin (II)36 | SMEDDS | Beagle dogs | Significant increase in relative bioavailability (about 1.5 times) for all three formulations when compared with tablet | Enhanced intestinal solubility and mucosal permeability |
| (a) Labrafil, Cremophor RH40, propylene glycol | ||||
| (b) Estol, Cremophor RH40, propylene glycol | ||||
| (c) Labrafac, Cremophor RH40, propylene glycol | ||||
| Carvedilol (II)37 | SEDDS (Labrfil M1944CS, Tween 80, Transcutol) | Beagle dogs | Relative bioavailability enhanced by 4.1 times when compared with tablet | Enhanced solubility and dissolution rate |
| Coenzyme Q10 (II)38 | SEDDS (Myvacet 9-45, Labrafac CM-10, Lauroglycol) | Coonhound dogs | Relative bioavailability enhanced by 2 times when compared with powder | Enhanced aqueous solubilization |
| Cyclosporin (II)22 | SEDDS-Sandimmune (corn oil, ethanol) | Humans | Increased relative bioavailability and Cmax in humans. Neoral superior to Sandimmune in terms of reduced food effect, dose linearity and reduced interindividual variability | Due to formation of microemulsion after aqueous dilution for Neoral when compared with Sandimmune |
| SMEDDS-Neoral (corm oil glycerides, Cremophor RH40, ethanol) | ||||
| Danazol (II)25 | LCT solution (soyabean oil) | Beagle dogs | Relative bioavailability in the order of LCT solution>LCT-SMEDDS>MCT-SMEDDS>micronized powder. | Enhanced intestinal solubilization resulted in increased relative bioavailability. Significant drug precipitation in MCT-SMEDDS |
| LCT-SMEDDS (soyabean oil, Maisine 35-1, Cremophor EL, ethanol) | ||||
| MCT-SMEDDS (Captex 355, Capmul MCM, Cremophor EL) | ||||
| Griseofluvin (II)39 | Corn oil emulsion, corn oil suspension | Rats | Relative bioavailability in the order of corn oil emulsion>corn oil suspension>aqueous suspension | Enhanced solubilization in emulsion resulted in more relative bioavailability |
| Halofantrine (II)23 | MCT-SEDDS (Captex 355, Capmul MCM, Cremophor EL, ethanol) | Beagle dogs | Higher relative bioavailability from LCT SMEDDS compared with other formulations. All formulations enhanced bioavailability by 6–8 times when compared with solid tablet formulation | Enhanced solubilization and prevention of precipitation after aqueous dispersion |
| MCT-SMEDDS (Captex 355, Capmul MCM, Cremophor EL, ethanol) | ||||
| LCT-SMEDDS (soyabean oil, Maisine 35-1, Cremophor EL, ethanol) | ||||
| Ibuprofen (II)40 | Microemulsion (MCT oil, DGMO-C, HCO-40) | Rats | Bioavailability in microemulsion comparable with organic solution and higher than aqueous suspension | Enhanced solubility of compound in oil |
| Indomethacin (II)41 | SEDDS (Tween 85, ethyl oleate) | Sprague-Dawley rats | Relative bioavailability of SEDDS 1.57 times higher when compared with aqueous suspension | Improvement in the solubility and dissolution |
| Itraconazole (II)42 | SEDDS (Transcutol, Pluronic L64, tocopherol acetate) | Sprague-Dawley rats | Relative bioavailability of SEDDS was significantly higher than marketed capsule and reduced food effect when administered in SEDDS | Enhanced dissolution by incorporation in SEDDS |
| Ontazolast (II)26 | SEDDS (Gelucire 44/14, Peceol) | Charles River CD rats | Absolute bioavailability increased atleast by 10 times from SEDDS formulations. SEDDS formulations enhanced lymphatic transport | SEDDS formulation improved dissolution and solubility, and also enhanced bioavailability through lymphatic absorption thereby bypassing extensive hepatic metabolism |
| Penclomedine (Not available)43 | LCT (soyabean oil, Triolein), MCT (Trioctanoin), SCT (Tributyrin), liquid paraffin emulsion | Rats | Bioavailability is in the order of MCT>LCT>paraffin emulsion>SCT>aqueous suspension | Higher bioavailability in MCT is due to reduced drug precipitation during lipid digestion |
| Phenytoin (II)44 | Corn oil emulsion, corn oil suspension | Rats | Relative bioavailability in rats is in the order of emulsion>oil suspension>aqueous suspension | Enhanced solubility in lipid emulsion |
| Progesterone (IV)45 | SEDDS (mono-di-glycerides, Polysorbate 80) | Beagle dogs | Relative bioavailability of SEDDS is 9 times higher than the aqueous suspension | Enhancement of solubility and permeability when administered in SEDDS |
| Seocalcitol (Not available)46 | LCT-SMEDDS (sesame oil, Peceol, Cremophor RH40) | Sprague-Dawley rats | Absolute bioavailability LCT-SMEDDS is equal to MCT-SMEDDS | Similar solubility values of drug in SIF may have resulted in similar bioavailability |
| MCT-SMEDDS (Vicscoleo, Akoline, Cremophor RH40) | ||||
| Silymarin (Not available)47 | SMEDDS (Tween 80, ethyl alcohol, ethyl linoleate) | Rabbits | Relative bioavailability of SMEDDS is 1.88 and 48.82 times higher than PEG solution and suspension respectively | Alternative pathways such as lymphatic transport contribution to enhanced bioavailability |
| Simvastatin (II)48 | SMEDDS (Caproyl 90, Cremophor EL, Carbitol) | Beagle dogs | Relative bioavailability is 1.5 times higher in SMEDDS compared with conventional tablet | Enhanced solubility in lipid excipients |
| Tocotrienols (II)49 | Two SEDDS (Tween 80 and Labrasol) | Humans | Relative bioavailability enhanced by 2–3 times when compared with non-self-emulsifying formulation | Enhanced solubility and finer dispersion properties |
| Vitamin E (II)21 | SEDDS (Tween 80, Span 80 and palm oil) | Humans | Relative bioavailability of SEDDS is 2-folds higher than oil solution | Presence of surfactant in SEDDS led to higher bioavailability when compared with oil solution |
The most important and classical example in the bioavailability enhancement by lipid formulations is Sandimmune® and Sandimmune Neoral® formulations of cyclosporine A by Novartis50. Initially Sandimmune® was introduced into European market in 1981 as a SEEDS formulation containing Labrafil M1944CS, olive oil and ethanol. When dispersed in water, this formulation forms an oil-in-water coarse macroemulsion with high polydispersity values. In 1994 again cyclosporine was introduced as a SMEDDS formulation containing Cremophor RH40, corn oil glycerides, propylene glycol and ethanol under the brand name of Sandimmune Neoral®. This formulation forms a spontaneous microemulsion with droplet size less than 100 nm. Compared with Sandimmune®, Sandimmun Neoral® provided more extent in oral absorption and bioavailability. The improved dispersion characteristic of Neoral® has been attributed to the enhanced bioavailability of cyclosporine A.
The oral bioavailability of BCS-II anticancer drug exemestane is enhanced and dose is reduced by formulating the drug in SMEDDS formulation51. SMEDDS formulation for exemestane was developed using castor oil, Transcutol P and Labrasol. This formulation provided higher solubilization capacity for exemestane with mean droplet size less than 28.5 nm after dilution in aqueous fluids. This formulation filled into hard gelatin capsule showed faster dissolution rates compared with the conventional tablet formulation. The optimized formulation showed higher Cmax (about 1.53 times) and AUC (about 2.87 times) when compared with conventional suspension formulation in rats. The enhancement in bioavailability is attributed to increased solubilization, rapid emulsification with low droplet size from SMEDDS formulation.
The oral bioavailability of a BCS-IV drug furosemide is enhanced by incorporating the drug into SMEDDS formulation containing Captex 500 and Cremophor EL52. The optimized formulation showed a pH-independent increase in dissolution rate in SGF and phosphate buffer when compared with a marketed tablet formulation. The results showed that SMEDDS formulation improved the oral bioavailability with reduced pharmacokinetic variability which was mainly attributed to improved pH-independent solubility and dissolution rate.
SMEDDS formulation was used as a strategy to improve the oral absorption of poorly water-soluble drug pranlukast hemihydrate53. The optimized formulation consisted of triethylcitrate, Tween 20, Span 20, triethanolamine and benzyl alcohol. The SMEDDS formulation demonstrated higher saturation solubility values (>150 times) and dissolution rates for pranlukast. In rats, the absorption of pranlukast improved by about 3-fold when dosed in SMEDDS, compared with a suspension formulation and it was attributed to increased dissolution rates in the form of SMEDDS.
2.5. Role of excipient selection on bioavailability
In general, there is a wide choice of excipients available for formulating poorly water-soluble drugs in the lipid formulations. The typical excipients present in lipid-based formulations are: (a) lipids: synthetic or natural lipids; (b) surfactants: non-ionic lipophilic or hydrophilic surfactants; (c) hydrophilic solvents: for better dispersion and to increase solvent capacity; (d) cosolvents: for reducing the viscosity of a formulation and to facilitate dispersion4. From a formulation perspective, the excipient selection will have an impact on the drug load, dispersion characteristics, solubilization of drug and, importantly, on stability. However, it is also important to consider excipients from pharmacokinetic aspects which can ultimately affect the bioavailability.
2.5.1. Lipids
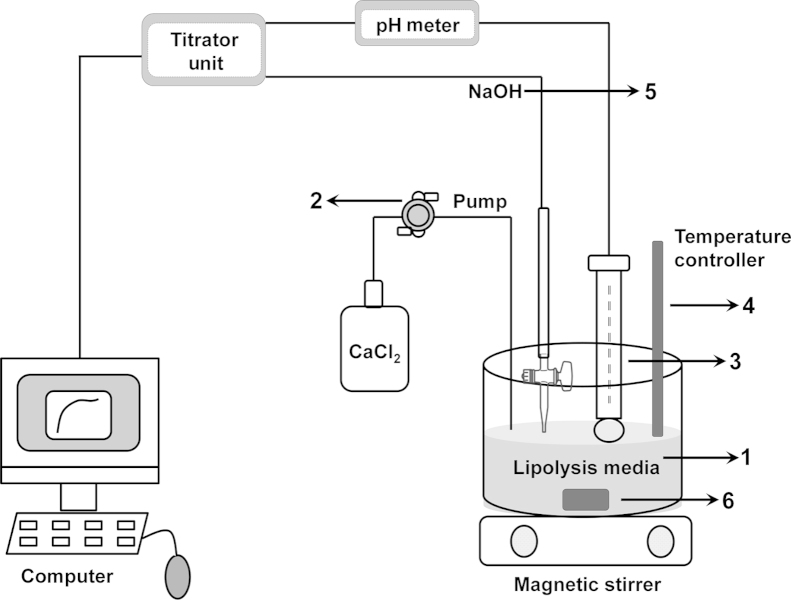
The basic function of lipids is to solubilize the drug and keep it in a dissolved state throughout the transit in the GI tract. If lipid digestion leads to drug precipitation, it needs to be resolubilized in intestinal fluids to get absorbed. This is especially important for BCS class-II and IV54. In vitro, the lipolysis can be simulated using a pH stat titration apparatus and the in vivo conditions are simulated by adding the lipid formulation into the biorelevant buffers (fasted or fed condition simulating) and subsequently adding lipases for digestion55. Many factors can contribute to the results obtained from in vitro lipolysis experiment and these factors along with their importance in predicting in vivo data are represented schematically in Fig. 356, 57. For example, calcium chloride is used to react with the free fatty acids generated during lipolysis and it forms insoluble complexes with fatty acids. If calcium chloride is added at a rapid rate, it will lead to more lipolysis thereby leading to the faster precipitation generating amorphous drug. This clearly indicates the importance of optimization of parameters during the lipolysis experiment for the accurate prediction of drug state after lipolysis in vivo. In addition, lipolysis can be carried out in fasted and fed media for predicting the food effect of drugs prepared in lipid formulations.
Figure 3.
In vitro lipid digestion experiment indicating the critical process parameters that can influence the prediction of in vivo data. (1) Composition of lipid digestion media-pancreatin source, bile salts concentration, pH and volume of media and amount of lipid in the formulation. (2) Rate of calcium chloride addition-faster the rate faster the digestion. (3) pH of the media – optimize between 5.5 and 7.5. (4) Temperature – representing biological conditions at 37 °C. (5) Molarity of NaOH – low molarity leads to higher end volume of media and higher molarity leads to titrator overshooting and experimental error. (6) Stirring speed – higher speed to mix the components thoroughly.
Various types of lipid excipients can be used to formulate lipid-based preparations from long, medium and short chain triglycerides (LCTs, MCTs, and SCTs)6. In general, LCTs are digested slowly compared with MCTs and at the end of digestion process MCTs are digested completely. In addition, drug is maintained in a solubilized state to a greater extent in MCTs due to higher solvent capacity. Due to its less solvent capacity, SCT results in extensive precipitation of the drug that settles at the bottom of the lipolysis vessel. Observations for progesterone in in vitro lipolysis experiments provided a rank order correlation MCTs>LCTs>SCTs and in vivo results in rats were in agreement with in vitro data58. Similar observations were found for drugs such as griseofulvin and penclomedine59. However, for few drugs such as vitamin D3 and halofantrine contradictory results were obtained and these results were attributed to lymphatic transport of these drugs when administered in LCTs (discussed in detail in below)60, 61. There are few other cases (e.g., dexamethasone) where in no significant differences in bioavailability were noted with respect to administration in different chain lengths62. In addition to the above mentioned aspects of lipid digestion capabilities, the absorption profiles of drug can be changed depending on the type of lipid selected. For example, MCT promotes portal vein absorption through liver and LCT promotes lymphatic absorption thereby bypassing the first pass metabolism (discussed in detail in later sections).
2.5.2. Surfactants
Cremophor EL and Cremophor RH40 are the most common and most studied excipients in the category of nonionic surfactants. Even though Cremophor EL and Cremophor RH40 are from the same family of surfactants, Cremophor EL has lower degree of ethoxylation compared with Cremophor RH40 and it is unsaturated. However, similar to lipid excipients, most important differences from the in vivo digestion point of view need to be considered for their use in the formulation. With regard to both the surfactants, Cremophor RH40 is less readily digested when compared with Cremophor EL63. The reasons for the differences in digestion are not clear but it have been attributed to the differences in the reactivities of the saturated castor oil glyceride backbone in Cremophor RH40 leading to different reaction products when compared with Cremophor EL. Due to its lesser digestion properties, Cremophor RH40 seems to be an attractive choice for keeping the drug in the solubilized state for longer time when compared with Cremophor EL. As observed with danazol, SMEDDS containing Cremophor RH40 maintained more drug in the aqueous phase throughout digestion process when compared Cremophor EL, resulting in a 3-fold increase in bioavailability with Cremophor RH4064. Even though the above theories look convincing, under in vivo conditions, the relative affinity of lipases towards lipid and surfactant will ultimately decide the degrees of digestion and drug solubilization, indicating the importance of formulation optimization using in vitro digestion experiments. From another perspective, the reduction in bioavailability of danazol in dogs when the proportional content of Cremophor EL is increased in the formulation has been reported65. This was primarily attributed to the fact that reduction of lipid increased the chances of drug precipitation due to dilution of Cremophor EL under the in vivo conditions, which ultimately failed to maintain the drug in the solubilized state. This clearly indicates the necessity to optimize the ratio of lipid to surfactant for enhanced bioavailability. In addition, excess of surfactant can lead to micelle formation and it can also hinder the absorption process due to the dramatic increase in the molecular weight of the whole complex. This can be an important aspect from an in vitro studies point of view (Caco-2, MDCK cell line studies for estimation of permeability) and may not be relevant in vivo due to dilution of surfactants in the larger gastric fluids66.
On the other side, long term use of surfactants is not recommended due to their ability to disrupt the cell membrane and their toxic and adverse effects (e.g., Cremophor EL). Several antiviral protease inhibitors such as ritonavir are available in market as oral solution and soft gelatin capsules containing huge amounts of surfactants. These formulations are taken in large numbers on a daily basis due to the dosage requirements. Experience from these formulations suggests that these dosage form regimen are well tolerated but long term effects need to be evaluated. Even though there are no official guidelines for recommended daily permissible intake of these excipients in humans and special populations such as pediatrics, it should be ensured that these excipients should be used as little as possible in the formulation so as to avoid adverse/toxic effects until regulatory limits are available67.
2.5.3. Hydrophilic solvents
The hydrophilic solvents such as triethyl citrate, polyethylene glycol 400 (PEG400) and propylene glycol are used in smaller quantities in lipid-based Type-III formulations to aid in self-emulsification through a phenomenon called “dispersion and stranding”68. Keeping excipient related toxicities aside, only few of the hydrophilic solvents have significant pharmacokinetic concerns. One excipient in this category is PEG400, due to its influence on gastric motility and subsequent effect on drug absorption when ingested in larger quantities (≥2.5 g). The decreased intestinal transit time is due to poor absorption of this excipient from intestine that results in PEG400-induced osmotic activity due to water retention, thereby stimulating intestinal motility and transit. Since the small intestine is the major site for absorption of many compounds, reduction in transit time will decrease the time available for absorption for many of the poorly soluble and slowly dissolving drug compounds such as ranitidine69. However, at lower concentrations such as 1 g, PEG400 was shown to significantly enhance the absorption of ranitidine probably due to modulation of intestinal permeability70. Even though the amounts of hydrophilic solvents used in the lipid-based formulations are minimal, they should be selected with caution due to the above mentioned impact on the pharmacokinetics.
2.5.4. Cosolvents
In general, cosolvents such as ethanol are used at very low concentration to reduce the viscosity and to aid in the dispersion process. At these lower concentrations the cosolvents do not possess any pharmacokinetic importance.
2.6. Lymphatic transport
Lymphatic transport is an attractive absorptive pathway for enhancing bioavailability of lipophilic drugs. The main advantage of lymphatic absorption is bypassing hepatic metabolism and thereby increasing blood concentrations and therapeutic efficacy for extensively metabolized drugs71. Among the various animal models available for study of the lymphatic transport of small molecules, a rat model with a cannulated mesenteric lymph duct is widely used. Other models such as sheep and dog are widely used for studying lymphatic transport after parenteral and oral administrations, respectively72.
While lymphatic transport is directly proportional to lipophilicity, a drug molecule with a partition coefficient (log P)>5 and triglyceride solubility >50 mg/mL can be forced to be absorbed via lymphatic transport using lipid-based formulations rather than through portal vein and liver73. It has been shown that drugs solubilized in MCT and SCT (number of carbons C<12) are primarily absorbed into the portal blood and encounter hepatic enzymes, and drugs which are solubilized in LCT (C>12) are transported via intestinal lymphatic system and subsequently are absorbed to systemic circulation. During lipolysis in the GI tract, digestion products such as fatty acids and monoglycerides with C>12 are combined with phospholipids and cholesterol present in enterocyte to form triglycerides. The formed triglycerides along with entrapped drug are packed into chylomicrons and subsequently enter the lymphatic system thereby circumventing the liver and preventing hepatic metabolism. Fatty acids and monoglycerides with C<12 are not combined with chylomicrons and hence are absorbed into the portal vein. Triglyceride solubility of the drug is a driving factor for partitioning into lipid-enriched chylomicrons and hence the more the triglyceride solubility, the more the partitioning. This is the fundamental underlying mechanism for lymphatic absorption mediated by formulating the drug with LCT rather than with MCT and SCT74. The degree of saturation and amount of lipid are also found to have an effect on the lymphatic transport of drug molecules.
Charman and Stella75 studied the lymphatic transport of DDT (log P, 6.19) and hexachlorobenzene (log P, 6.53) in an in situ rat model system. When administered with oleic acid, the lymphatic transport of DDT and hexachlorobenzene was 33.5% and 2.3%, respectively, in the rat in situ model. Even though the log P values for both compounds are similar, the difference in the extent of lymphatic transport was attributed to 13-fold difference in the triglyceride solubility values. In another study by Dahan and Hoffman60 the in vivo performance of vitamin D3 was evaluated in formulations consisting of LCT, MCT and SCT. Even though an in vitro lipolysis experiment estimated rank order of MCT>LCT>SCT, based on the drug retained in aqueous part after lipolysis, in vivo results have provided a different rank order LCT>MCT>SCT. This was primarily attributed to the fact that lymphatic transport of vitamin D3 is enhanced in LCT thereby resulting in more in vivo extent in absorption. This is also clear from the fact that when lymphatic transport is blocked by pre-administration of cycloheximide, the in vivo data correlated with in vitro lipolysis data60. This clearly indicates the limitation of in vitro lipolysis that it cannot predict the in vivo performance for the compounds which are absorbed via lymphatic route. In another study, a good linear correlation between ex vivo uptake of lipophilic drugs by chylomicrons with reported intestinal lymphatic bioavailability in rats was reported76. In contrast, the most recommended log P and solubility in triglycerides provided only moderate correlation with lymphatic bioavailability. Based on these results, they have also developed a simple screening model which considers the degree of association of lipophilic drugs with isolated chylomicrons that can be used to predict intestinal lymphatic transport. Similarly Gershkovich and Hoffman76 have also observed that log P and triglyceride solubility values do not necessarily guarantee lymphatic transport. Similar contradicting results were reported where poor lymphatic transport (3% of dose transported into intestinal lymph) of penclomedine was observed even though it has log P 5.48 and triglyceride solubility of 175 mg/mL43. Despite these contradictory results, intestinal lymphatic transport still serves as an excellent opportunity to enhance the oral bioavailability of lipophilic drugs.
2.7. Food effect reduction
Food effect can be described as an increase or decrease in the rate and extent of drug absorption in presence of food. The food effect is most studied over the past few decades and it depends on a number of factors arising from physiology, dosage form and, importantly, physicochemical properties77. Various aspects such as enhanced solubility in the presence of fat components of food, stimulation of bile secretion, surfactant effects by food components, delay in gastric emptying to enhance the absorption time, and inhibition of efflux transporters by food components are recognized as potential mechanisms for bioavailability enhancement of poorly soluble drugs in the presence of food78.
The lipid components present in the food can perform the same function as lipid components present in the lipid-based formulations. The dietary lipids present in the food can act as solubilizers for drug and subsequently undergo lipid digestion by gastric and pancreatic lipases similar to the process described for lipid-based formulations so as to produce various micellar species. It has also been shown that a few lipid components in the food can enhance chylomicron production and can stimulate the lymphatic transport to enhance the bioavailability79. Hence it is evident that if proper lipids and surfactants are chosen for formulating a poorly water-soluble drug, they can also act as surrogates for food components and thereby nullifying the food effect and increasing patient compliance by avoiding the high fat meal consumption with dosage form.
The reduction in food effect for itraconazole using SMEDDS formulation was reported in healthy volunteers80. The SMEDDS formulation exhibited enhanced Cmax and AUC values under both fasted and fed conditions when compared with conventional capsule formulation and demonstrated similar plasma concentration time profiles under both fasted and fed conditions, indicating an insignificant food effect. Similarly, the reduced food effect for the highly lipophilic compound torcetrapib after administration in lipid-based formulations was demonstrated. The optimized formulations showed enhanced fasted state exposure and reduced food effect from 5-fold to 3-fold in dogs at dose levels of 90 mg81. The optimized formulations were also found to show reduced inter-individual pharmacokinetic variability.
2.8. Bioavailability enhancement by transporter inhibition
The efflux transporters present in the intestine are known to reduce the bioavailability of many drugs. The Biopharmaceutics Drug Disposition Classification System (BDDCS) demonstrated that drugs with poor solubility (class-II) will not be able to saturate the efflux transporters such as P-glycoprotein present in the intestine, hence limiting their bioavailability78. It was also reported that the bioavailability and pharmacological activity of many anticancer agents have been reduced drastically due to the action of drug efflux transporters such as P-glycoprotein, breast cancer resistant protein and multidrug resistance protein present in the intestine that flushes out the absorbed drug from enterocyte back into the intestine. This might also lead to the problem of multidrug resistance thereby reducing potency of a drug with repeated administration82.
It has been reported in the literature that formulating drugs in lipids (e.g., LCT and MCT) and surfactants (e.g., Cremophor EL, Cremophor RH40, Tween 80, d-α-tocopheryl polyethylene glycol 1000 succinate (Vit E TPGS)) can enhance the bioavailability by inhibiting the efflux transporters such as P-glycoprotein (P-gp)83, 84. It has been reported that inhibition of efflux will lead to increase in concentration and residence time of the drug in the enterocyte leading to increased drug available for lymphatic transport. In addition, many of the synthetic and semi-synthetic lipids can inhibit the drug efflux transporters similar to the effects shown by lipid components present in fat enriched food. Various mechanisms have been proposed for inhibition of transporters by excipients, but the main concept behind inhibition is membrane fluidization-induced conformational changes and cholesterol depletion. In addition, the micelles formed during emulsification can mask the active drug thereby not exposing it to the efflux transporters. A few hydrophilic solvents, such as PEG400, are also known to inhibit certain efflux transporters so as to enhance the bioavailability85. Hence, when the above mentioned excipients such as lipids, surfactants and hydrophilic solvents are used in lipid-based formulations they may enhance the bioavailability not only due to their solubilization capabilities, but also due to the inhibition of efflux transporters.
Paclitaxel is one of the most important chemotherapeutic agents and it is a substrate for P-gp and CYP3A4. The bioavailability of paclitaxel is very limited and hence it is formulated in a 1:1 ratio of Cremophor EL and dehydrated ethanol for intravenous administration (Taxol®). Various efforts have been undertaken to enhance the oral bioavailability of paclitaxel through formulating in nanoparticles and supersaturated SEDDS containing HPMC as a precipitation inhibitor along with P-gp inhibitors such as Vit E TPGS and Cremophor EL30, 86. This formulation resulted in 5-fold higher bioavailability when compared with the traditional Taxol® formulation. Even though bioavailability has increased with this formulation it did not yield sufficient therapeutic levels, indicating that either the SEDDS formulation was not able to circumvent the P-gp effect or due to metabolism by CYP3A4. To evaluate this possibility, the SEDDS formulations were dosed along with cyclosporine that further enhanced the bioavailability of paclitaxel when compared with Taxol®. This clearly indicates that pure SEDDS formulation enhanced the bioavailability of paclitaxel either due to solubilization or due to P-gp inhibition or due to combination of both.
3. IVIVC potential for lipid-based formulations
The use of in vitro–in vivo correlation for various Biopharmaceutics Classification System (BCS) classes is described in the seminal article of Amidon et al.87 that resulted in the birth of BCS. BCS class-I drugs are proved to be excellent candidates and class-IV drugs are considered to be poor candidates for IVIVC due to their solubility and permeability characteristics. While the application of IVIVC to class-III drugs is limited due to their poor permeability, the solubility/dissolution properties of poorly water-soluble class-II compounds can be greatly enhanced by formulation approaches such as solid dispersion and lipid formulations such as SEDDS and SMEDDS, thereby making them behave as class-I compounds in vivo. The rapid dissolution of class-II compounds will enable the time required for complete dissolution to be significantly less than gastric emptying time, thereby enhancing the possibility of achieving strong IVIVC correlation88. Out of the different lipid formulations available, Type-II and III formulations disperse rapidly in the in vivo fluids thereby reducing the time required for complete solubilization in the in vivo biological fluids, which increases the potential for achieving IVIVC for poorly soluble compounds. The following sections detail the various approaches used for developing the in vitro and in vivo correlations for various lipid-based formulations.
3.1. In vitro dispersion/precipitation test and in vivo data
The in vitro dispersion or precipitation test is an attractive and simple test for determining the amount of the drug in a solubilized state after dispersing the lipid formulation into aqueous fluids. The rationale for using this test to predict the in vivo performance is based on the fact that the completely solubilized drug will be readily absorbed whereas precipitated drug will not be available for absorption. Hence, when using this test to screen different lipid formulations, the percentage of solubilized drug in biological fluids in vitro can be correlated with the amount of the drug absorbed in vivo or with AUC, thereby possibility of achieving an IVIVC or rank ordering formulations. The dispersion and precipitation behavior of a poorly soluble NCE in various lipid-based formulations using 96-well microtiter plate in various biological fluids such as SIF, FaSSIF and FeSSIF was reported27. The authors have short-listed three formulations based on precipitation kinetics (fast, slow and no precipitation) and studied the pharmacokinetics in dogs. There was a good correlation between the precipitation kinetics observed in biological fluids versus AUC values, where the rank ordering was fast<slow<no precipitation. In addition, the in vitro precipitation in FeSSIF correlated well with in vivo performance indicating that lipid excipients can act as surrogates for food components. The IVIVC for BCS class-II drug cyclosporine from SMEDDS formulation was reported89. In this work, a new generic SMEDDS formulation for cyclosporine was developed and compared with two reference SMEDDS formulations. All three formulations were evaluated for in vitro dissolution (in pH 1.2, 4.5 and 6.8) and in vivo in dogs. Strong Level A correlations were achieved for fraction dissolved versus fraction absorbed. The author concluded that BCS class-II compounds will behave as class-I compounds when administered in optimized SMEDDS formulation. Similar Level A IVIVC correlations were established for various poorly water-soluble drugs such as ritonavir and lopinavir administered as lipid formulations in soft gelatin capsules90, 91. Various IVIVC examples from the literature using in vitro dispersion/dissolution/precipitation and in vivo data are presented in Table 3.
Table 3.
Selected IVIVC examples based on in vitro dispersion/precipitation/dissolution and in vivo pharmacokinetic data.
| Drug/Formulation | In vitro dispersion/precipitation/dissolution data | In vivo data | IVIVC |
|---|---|---|---|
| Cyclosporin (Soft gelatin capsule)89 | In vitro dissolution (in pH 1.2, 4.5 and 6.8) for one generic and two reference formulations | In vivo bioavailability in dogs | Level A correlation between in vivo fraction absorbed versus in vitro fraction dissolved for test and reference formulations (R2=0.992) |
| JNJ-25894934 (NCE, Soft gelatin capsule)27 | Formulations categorized based on precipitation kinetics: fast (solubility: 76.37 mg/mL), slow (105.61 mg/mL) and non-precipitating (96.04 mg/mL) formulations in FaSSIF and FeSSIF | Pharmacokinetic results in Mongrel dogs showed lowest bioavailability for fast precipitating and similar bioavailability for slow and non-precipitating formulations in fasted state and fed state | Good agreement between in vitro precipitation and in vivo pharmacokinetic data, good correlation between fed state precipitation kinetics and in vivo data compared with fasted state precipitation kinetics |
| Ritonavir (Soft gelatin capsule)90 | In vitro dissolution data in various media containing water and sodium lauryl sulfate (SLS–0.3%, 0.5%, 0.7%, 1%) | In vivo pharmacokinetic data in humans | Strong Level A correlations were obtained in between percent dissolved versus percent absorbed (R2 values: 0.952, 0.935, 0.993, 0.963 for 0.3%, 0.5%, 0.7%, 1% SLS respectively) |
| Lopinavir (Soft gelatin capsule)91 | In vitro dissolution data using media 2.3% SLS at pH 6.0 and USP Type-I apparatus at 25 rpm | In vivo pharmacokinetic data in humans | Strong Level A correlation was obtained (R=0.997). The equation obtained was Fraction absorbed=−0.0019+1.0075×Fraction dissolved |
| Arundic acid (Soft gelatin capsule)92 | In vitro dissolution data in pH 8.0 dissolution medium or the pH 6.8 dissolution medium containing 2% sodium dodecyl sulfate | In vivo pharmacokinetic data in humans | IVIVC was established by plotting in vitro dissolution time versus in vivo absorption time for both dissolution media. pH 6.8 dissolution media produced stronger correlations |
| Fenofibrate93 | In vitro dissolution of three SMEDDS formulations was performed in FaSSGF pH 2 and FaSSIF-V2(PO4) | In vivo data in human volunteers | In vitro dissolution and in silico simulations were used to predict in vivo human plasma profiles. The point estimates for Cmax and AUC fell within range of 0.8–1.25 indicating the accurate simulation of in vivo profiles from in vitro data |
Even though achieving correlation with this test appears simple, there are many limitations involved. Firstly, if the precipitated drug redissolves at later points in the intestine due to pH change or solid state change of the precipitated drug, the obtained IVIVC correlation or rank ordering will not be valid. Secondly and most importantly it is not only the dispersion but also the lipid digestion by gastric and pancreatic lipases that plays an important role in maintaining the drug in the solubilized state. Hence, even if the drug remains in a solubilized state after dispersion, it can very well precipitate due to lipid digestion. The role of lipid digestion in achieving IVIVC for lipid formulations is described in the following section.
3.2. In vitro lipolysis and in vivo data
Out of all the methods used for establishing IVIVC for lipid-based formulations, in vitro lipolysis plays a major role due to its capability to simulate the most important steps in the absorption of lipid-based formulations such as dispersion and digestion. The limitation of the in vitro dispersion/precipitation test to predict the in vivo performance can be overcome by incorporating in vitro lipolysis. In in vitro lipolysis, the formulation is added to the buffer solution containing various components such as bile salts, thereby simulating the fasted/fed after ingestion of lipid-based formulation. Immediately thereafter enzymes such as pancreatic lipase are added and the lipolysis process is initiated. Samples are taken at various time points and centrifuged and the aqueous phase is analyzed for drug content. Correlation between the amount of drug present in the aqueous phase at different time points and the in vivo plasma concentration time profiles, and percentage of solubilized drug at the end of digestion versus pharmacokinetic parameters such as AUC or Cmax, can be used to develop IVIVC relationships for lipid-based formulations.
Various studies have demonstrated the utility of in vitro lipolysis to predict in vivo data. The relative bioavailability of halofantrine formulated using MCT and LCT-based formulations was reported74. In this study, the authors attempted to correlate in vitro drug solubilization with digestion data to the in vivo data. The authors found strong correlations between the data only when lower lipid masses were used. Out of LCT and MCT, the superior bioavailability was observed with the LCT-based formulation. The authors concluded that the solubilization capacity of the formulations is highly dependent on the nature of the lipid used, and the predictivity of the in vitro lipolysis is dependent on the amount of lipid used in the experiment. This indicates the importance of optimization of various parameters in lipolysis experiments for better prediction of in vivo bioavailability. Reymond et al.94 demonstrated correlations between in vitro and in vivo data for cyclosporine formulated in MCT and LCT. The in vitro lipolysis indicated that higher amounts of solubilized cyclosporine present the aqueous phase in case of MCT compared with LCT. However in in vivo studies, LCT resulted in higher bioavailability compared with MCT. The authors concluded that since LCT is digested slowly compared with MCT, after 12 min of lipolysis more cyclosporine was present in the MCT. However in the in vivo situation, cyclosporine has highest affinity toward the oil phase containing triglycerides and hence affinity towards LCT is higher compared with MCT. This case study clearly explains that not only the design of in vitro lipolysis should be considered, but also the inherent properties of the drug when developing IVIVC.
In another similar study, the poorly water-soluble drug danazol was formulated in three formulations: LCT-solution, LCT-SMEDDS and MCT-SMEDDS25. In vitro lipolysis indicated significantly higher solubilized danazol for LCT-SMEDDS when compared with MCT-SMEDDS and LCT-solution. The in vivo results are in agreement with in vitro lipolysis wherein higher absorption was observed for LCT-SMEDDS formulations when compared with MCT-SMEDDS. The utility of in vitro dynamic lipolysis and neuro-fuzzy networks for establishing IVIVC for SMEDDS formulations was reported for probucol95. In this study, probucol was formulated in an oil solution, two SMEDDS and one nano-emulsifying (SNEDDS) formulation. Higher percentages of solubilized probucol were observed for SMEDDS and SNEDDS formulations when compared with oil solution (rank order SMEDDS>SNEDDS>oil solution). The in vivo results demonstrated good correlation with in vitro data and similar rank order was observed. The developed neuro fuzzy model achieved significantly higher prediction abilities for different formulations.
The in vitro lipolysis experiment can be used to predict the food effect for lipid-based formulations. The fasted and fed state gastrointestinal lipolysis experiment to establish IVIVC for SNEDDS solidified SNEDDS and conventional tablet formulation was reported for cinnarizine96. During the lipolysis experiment the fed state was simulated by addition of 3.5% of whole fat milk to the lipolysis media. In the fasted state lipolysis model the rank order for the percentage solubilized cinnarizine in aqueous phase was SNEDDS>solidified SNEDDS>tablet, which was in good agreement with the in vivo data in dogs. In the fed state model similar amounts of cinnarizine were solubilized for all the formulations. The fed state in vivo dog study indicated similar performance for SNEDDS and solidified SNEDDS whereas the performance of the conventional tablet was improved. This clearly indicates that conventional tablet formulation showed a food effect whereas SNEDDS and solidified SNEDDS showed an absence of a food effect. This example demonstrates the utility of in vitro lipolysis experiments to predict food effects for lipid-based formulations. Various IVIVC examples from the literature using in vitro lipolysis and in vivo data are presented in Table 4.
Table 4.
Selected IVIVC examples based on in vitro lipolysis and in vivo pharmacokinetic data.
| Drug | In vitro lipolysis data | In vivo data | IVIVC |
|---|---|---|---|
| Halofantrine23 | The in vitro lipid digestion and solubilization data provided rank order of LCT solution>LCT/MCT blend>MCT solution | The relative bioavailability in Beagle dogs was in the order of LCT solution>LCT/MCT blend>MCT solution | No IVIVC was established, but correlation between rank ordering was obtained |
| Griseofluvin39 | The in vitro lipid digestion provided rank order of MCT>LCT>SCT>H2O formulation | The in vivo study in dogs provided rank order of MCT>LCT>SCT>H2O formulation | Excellent regression (R2>0.98) was obtained between dose solubilized in the aqueous phase of the in vitro lipolysis medium and the in vivo AUC values |
| Cinnarizine96 | Fasted state in vitro lipolysis resulted in rank order of HGC-SNEDDS>Tablet-SNEDDS>conventional tablet | Fasted state in vivo study in dogs provided rank order of HGC-SNEDDS>Tablet-SNEDDS>conventional tablet, in fed state only the performance of conventional tablet was improved | No IVIVC was established but rank ordering was obtained |
| No difference observed in fed state in vitro lipolysis | |||
| Dexamethasone62 | The in vitro lipolysis provided similar performance for MCT=LCT=SCT | The in vivo study in rats resulted in rank order of MCT=LCT=SCT | No IVIVC established but correlation between rank ordering was obtained |
| Danazol25 | The in vitro lipolysis resulted in rank order of LC-SMEDDS>MC-SMEDDS | The in vivo study in dogs resulted in rank order of LCT-solution>LC-SMEDDS>MC-SMEDDS | No IVIVC was established, but correlation between rank ordering was obtained |
| Probucol95 | The rate and extent of release of probucol to the aqueous micellar phase was in the rank order of SMEDDS>SNEDDS>pure oil formulation | The bioavailability in mini-pigs was in the order of SMEDDS>SNEDDS>pure oil formulation | IVIVC was established using Adaptive neuro-Fuzzy Modeler (AFM) models. The model achieved significantly high prediction ability (correlation>0.91) for different formulations |
| Vitamin D360 | Dynamic in vitro lipolysis model provided rank order of MCT>LCT>SCT | In vivo data in rats provided rank order of LCT>MCT>SCT; however in bile cannulated rats, the rank order obtained was MCT>LCT>SCT | IVIVC was not established but rank ordering was obtained between in vitro lipolysis data and bile cannulated rats in vivo data |
3.3. Ex vivo intestinal permeability and in vivo data
Ex vivo intestinal permeability study includes the use of an animal intestine to study the transport of drug from the mucosal to the serosal layer. In this study, the permeability characteristics of pure drug (or formulation) can be studied using an experimental set up named as “Ussing chamber system”. This system has been widely used in academics and in pharmaceutical industries for evaluation of the apparent permeability characteristics (Papp) of a NCE. Recently this technique was utilized for lipid-based formulations to predict or rank order for in vivo performance. The ex vivo permeability technique was used to study the transport of adefovir dipivoxil formulated in solid SNEDDS formulation wherein the solid SNEDDS formulation showed superior permeability characteristics compared to drug suspension, thereby enhancing the chances of improving bioavailability97. In another study, the ex vivo permeability characteristics of the poorly water-soluble drug talinolol formulated in SNEDDS was evaluated using porcine small intestine and the developed SNEDDS formulation showed enhanced permeability characteristics compared to suspension98. These results were very well correlated with the in vivo results in Wistar rats. The correlation between ex vivo rat intestinal studies and in vivo bioavailability for fexofenadine formulated in lipid surfactants such as Gelucire 44/14 and Vit E TPGS was reported99. The authors observed increased ex vivo permeability characteristics (A-to-B) for lipid formulations when compared with a pure drug formulation. The authors also reported decreased B-to-A permeability for lipid formulations indicating the inhibition of efflux transporters. The results from ex vivo permeability studies correlated very well with the results obtained from in vivo bioavailability studies in male Wistar rats. The ex vivo permeability characteristics of lipolytic products for lipid formulations of dexamethasone and griseofulvin in LCT, MCT and SCT were reported62. Superior permeability characteristics were found for lipolytic products produced from SCT formulations when compared with LCT and MCT formulations. However, the in vivo bioavailability results correlated well with the in vitro lipolysis results where the rank order of MCT>LCT>SCT was observed for Griseofulvin and LCT=MCT=SCT was observed for dexamethasone respectively. The authors concluded that formulating in SCT might enhance the permeability characteristics but overall intelligent optimization of lipid formulations can only be achieved with in vitro lipolysis, and most importantly, permeability does not correlate with in vivo bioavailability for class-II compounds. Various IVIVC examples from the literature using ex vivo permeability and in vivo data are presented in Table 5.
Table 5.
Selected IVIVC examples based on ex vivo permeability and in vivo pharmacokinetic data.
| Drug | Ex vivo permeability data | In vivo data | IVIVC |
| Fexofenadine99 | Ex vivo permeability in Wistar rats showed enhanced permeability in lipid formulations compared with pure drug and showed rank order of Vit E TPGS formulation>Gelucire 44/14 formulation>pure drug | In vivo study in rats showed rank order for AUC of Vit E TPGS formulation>Gelucire 44/14 formulation>pure drug | No IVIVC was established but good correlation was observed between ex vivo permeability and in vivo data |
| Ginger Oleoresin100 | The proposed SMEDDS formulation showed 2-fold enhancement in the intestinal permeability when compared with pure drug | The in vivo studies revealed 1.6 times enhanced bioavailability when compared with pure drug | |
| Talinolol98 | The proposed SMEDDS formulation showed enhanced permeability when compared with pure drug | The in vivo studies confirmed the enhanced bioavailability for SMEDDS formulation when compared with pure drug | |
Overall, the summary of different ways to achieve IVIVC for lipid-based formulations is represented in Fig. 4.
Figure 4.
Different ways to achieve IVIVC for lipid based formulations.
4. Conclusions and future perspective
Even though lipid-based formulations demonstrate multiple advantages over available formulation technologies today, they only constitute a 2%–4% share in the commercially available formulations in US, UK and Japan10. The reasons for significantly lesser market share can be attributed to multiple reasons but most important, contributing factors are lack of availability of standardized in vitro tests and poor understanding of the pharmacokinetic behavior of lipid formulations after oral ingestion. While the standardization and harmonization of in vitro tools such as lipolysis have already been initiated as a part of LFCS (Lipid Formulations Classification System), the knowledge about the pharmacokinetic aspects and processes involved after ingestion of lipid formulations remains a gray area101. Understanding the pharmacokinetic aspects will not only help in designing the optimum lipid-based formulations, but also will help in designing the in vitro tests that can be used to predict in vivo behavior correctly, thereby establishing IVIVC relationships. From the in vivo processing point of view, dispersion and digestion are found to have major impact on the availability of a drug for absorption. With the in vitro tools such as dissolution/precipitation in biological fluids and in vitro lipolysis, these effects can be predicted to a certain extent but not completely. Out of all the in vitro tests available today for evaluation of lipid formulations, only in vitro lipolysis is found to be more relevant to describe the in vivo pharmacokinetic data, but it still has limitations such as inability to predict in vivo fate of drugs which are transported through lymphatic route and also when drug efflux transporters come into play. Despite these limitations and hurdles, lipid-based formulations still remain an attractive area for enhancing the bioavailability of poorly water-soluble drugs. In addition, with the advent of novel lipid-based formulations such as dried emulsions, spray-dried emulsions, solid SMEDDS, supersaturated SMEDDS and self-emulsifying solid dispersions, the poorly water-soluble drug can be delivered as a solid dosage form by formulating with minimal amounts of lipid and surfactants, which bypasses the limitations of conventional lipid formulations but yet without compromising the bioavailability.
Footnotes
Peer review under responsibility of Institute of Materia Medica, Chinese Academy of Medical Sciences and Chinese Pharmaceutical Association.
References
- 1.Basavaraj S., Betageri G.V. Can formulation and drug delivery reduce attrition during drug discovery and development-review of feasibility, benefits and challenges. Acta Pharm Sin B. 2014;4:3–17. doi: 10.1016/j.apsb.2013.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fahr A., Liu X.L. Drug delivery strategies for poorly water-soluble drugs. Expert Opin Drug Deliv. 2007;4:403–416. doi: 10.1517/17425247.4.4.403. [DOI] [PubMed] [Google Scholar]
- 3.Lakshman J.P. Formulation, bioavailability, and manufacturing process enhancement: novel applications of melt extrusion in enabling product development. In: Michael A. Repka, Nigel Langley, James DiNunzio., editors. Melt extrusion, materials, technology and drug product design. Springer; New York: 2013. pp. 329–362. [Google Scholar]
- 4.Pouton C.W. Formulation of poorly water-soluble drugs for oral administration: physicochemical and physiological issues and the lipid formulation classification system. Eur J Pharm Sci. 2006;29:278–287. doi: 10.1016/j.ejps.2006.04.016. [DOI] [PubMed] [Google Scholar]
- 5.Pouton C.W. Lipid formulations for oral administration of drugs: non-emulsifying, self-emulsifying and ‘self-microemulsifying’ drug delivery systems. Eur J Pharm Sci. 2000;11 Suppl 2:S93–S98. doi: 10.1016/s0928-0987(00)00167-6. [DOI] [PubMed] [Google Scholar]
- 6.Pouton C.W., Porter CJH. Formulation of lipid-based delivery systems for oral administration: materials, methods and strategies. Adv Drug Deliv Rev. 2008;60:625–637. doi: 10.1016/j.addr.2007.10.010. [DOI] [PubMed] [Google Scholar]
- 7.Gullapalli R.P. Soft gelatin capsules (softgels) J Pharm Sci. 2010;99:4107–4148. doi: 10.1002/jps.22151. [DOI] [PubMed] [Google Scholar]
- 8.Porter C.J.H., Pouton C.W., Cuine J.F., Charman W.N. Enhancing intestinal drug solubilisation using lipid-based delivery systems. Adv Drug Deliv Rev. 2008;60:673–691. doi: 10.1016/j.addr.2007.10.014. [DOI] [PubMed] [Google Scholar]
- 9.Li P., Hynes S.R., Haefele T.F., Pudipeddi M., Royce A.E., Serajuddin A.T.M. Development of clinical dosage forms for a poorly water-soluble drug II: formulation and characterization of a novel solid microemulsion preconcentrate system for oral delivery of a poorly water-soluble drug. J Pharm Sci. 2009;98:1750–1764. doi: 10.1002/jps.21547. [DOI] [PubMed] [Google Scholar]
- 10.Strickley R.G. Currently marketed oral lipid-based dosage forms: drug products and excipients. In: David J. Hauss., editor. Oral lipid based formulations. Informa Healthcare; New York: 2007. pp. 1–32. [Google Scholar]
- 11.Kossena G.A., Charman W.N., Wilson C.G., O’Mahony B., Lindsay B., Hempenstall J.M. Low dose lipid formulations: effects on gastric emptying and biliary secretion. Pharm Res. 2007;24:2084–2096. doi: 10.1007/s11095-007-9363-8. [DOI] [PubMed] [Google Scholar]
- 12.Kossena G.A., Boyd B.J., Porter C.J.H., Charman W.N. Separation and characterization of the colloidal phases produced on digestion of common formulation lipids and assessment of their impact on the apparent solubility of selected poorly water-soluble drugs. J Pharm Sci. 2003;92:634–648. doi: 10.1002/jps.10329. [DOI] [PubMed] [Google Scholar]
- 13.Kaukonen A.M., Boyd B.J., Porter C.J.H., Charman W.N. Drug solubilization behavior during in vitro digestion of simple triglyceride lipid solution formulations. Pharm Res. 2005;21:245–253. doi: 10.1023/b:pham.0000016282.77887.1f. [DOI] [PubMed] [Google Scholar]
- 14.Kossena G.A., Charman W.N., Boyd B.J., Dunstan D.E., Porter C.J.H. Probing drug solubilization patterns in the gastrointestinal tract after administration of lipid-based delivery systems: a phase diagram approach. J Pharm Sci. 2004;93:332–348. doi: 10.1002/jps.10554. [DOI] [PubMed] [Google Scholar]
- 15.Rübe A., Klein S., Mäder K. Monitoring of in vitro fat digestion by electron paramagnetic resonance spectroscopy. Pharm Res. 2006;23:2024–2029. doi: 10.1007/s11095-006-9069-3. [DOI] [PubMed] [Google Scholar]
- 16.Sek L., Porter C.J.H., Kaukonen A.M., Charman W.N. Evaluation of the in-vitro digestion profiles of long and medium chain glycerides and the phase behavior of their lipolytic products. J Pharm Pharmacol. 2002;54:29–41. doi: 10.1211/0022357021771896. [DOI] [PubMed] [Google Scholar]
- 17.Delorme V., Dhouib R., Canaan S., Fotiadu F., Carrière F., Cavalier J.F. Effects of surfactants on lipase structure, activity, and inhibition. Pharm Res. 2011;28:1831–1842. doi: 10.1007/s11095-010-0362-9. [DOI] [PubMed] [Google Scholar]
- 18.Li Y., McClements D.J. New mathematical model for interpreting pH-stat digestion profiles: impact of lipid droplet characteristics on in vitro digestibility. J Agric Food Chem. 2010;58:8085–8092. doi: 10.1021/jf101325m. [DOI] [PubMed] [Google Scholar]
- 19.Porter C.J.H., Charman W.N. In vitro assessment of oral lipid based formulations. Adv Drug Deliv Rev. 2001;50 Suppl 1:S127–S147. doi: 10.1016/s0169-409x(01)00182-x. [DOI] [PubMed] [Google Scholar]
- 20.Narang A.S., Delmarre D., Gao D. Stable drug encapsulation in micelles and microemulsions. Int J Pharm Sci. 2007;345:9–25. doi: 10.1016/j.ijpharm.2007.08.057. [DOI] [PubMed] [Google Scholar]
- 21.Julianto T., Yuen K.H., Noor A.M. Improved bioavailability of vitamin E with a self- emulsifying formulation. Int J Pharm. 2000;200:53–57. doi: 10.1016/s0378-5173(00)00337-9. [DOI] [PubMed] [Google Scholar]
- 22.Trull A.K., Tan K.K., Tan L., Alexander G.J., Jamieson N.V. Absorption of cyclosporin from conventional and new microemulsion oral formulations in liver transplant recipients with external biliary diversion. Br J Clin Pharmacol. 1995;39:627–631. doi: 10.1111/j.1365-2125.1995.tb05722.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Khoo S.M., Humberstone A.J., Porter C.J.H., Edwards G.A., Charman W.N. Formulation design and bioavailability assessment of lipidic self-emulsifying formulations of halofantrine. Int J Pharm. 1998;167:155–164. [Google Scholar]
- 24.Sek L., Boyd B.J., Charman W.N., Porter C.J. Examination of the impact of a range of Pluronic surfactants on the in-vitro solubilization behavior and oral bioavailability of lipidic formulations of atovaquone. J Pharm Pharmacol. 2006;58:809–820. doi: 10.1211/jpp.58.6.0011. [DOI] [PubMed] [Google Scholar]
- 25.Porter C.J., Kaukonen A.M., Boyd B.J., Edwards G.A., Charman W.N. Susceptibility to lipase-mediated digestion reduces the oral bioavailability of danazol after administration as a medium-chain lipid-based microemulsion formulation. Pharm Res. 2004;21:1405–1412. doi: 10.1023/b:pham.0000036914.22132.cc. [DOI] [PubMed] [Google Scholar]
- 26.Hauss D.J., Fogal S.E., Ficorilli J.V., Price C.A., Roy T., Jayaraj A.A. Lipid-based delivery systems for improving the bioavailability and lymphatic transport of a poorly water-soluble LTB4 inhibitor. J Pharm Sci. 1998;87:164–169. doi: 10.1021/js970300n. [DOI] [PubMed] [Google Scholar]
- 27.Dai W.G., Dong L.C., Shi X.F., Nguyen J., Evans J., Xu Y.D. Evaluation of drug precipitation of solubility-enhancing liquid formulations using milligram quantities of a new molecular entity (NME) J Pharm Sci. 2007;96:2957–2969. doi: 10.1002/jps.20886. [DOI] [PubMed] [Google Scholar]
- 28.Mohsin K., Long M.A., Pouton C.W. Design of lipid‐based formulations for oral administration of poorly water‐soluble drugs: precipitation of drug after dispersion of formulations in aqueous solution. J Pharm Sci. 2009;98:3582–3595. doi: 10.1002/jps.21659. [DOI] [PubMed] [Google Scholar]
- 29.Tang J.L., Sun J., He Z.G. Self-emulsifying drug delivery systems: strategy for improving oral delivery of poorly soluble drugs. Curr Drug Ther. 2007;2:85–93. [Google Scholar]
- 30.Warren D.B., Benameur H., Porter C.J.H., Pouton C.W. Using polymeric precipitation inhibitors to improve the absorption of poorly water-soluble drugs: a mechanistic basis for utility. J Drug Target. 2010;18:704–731. doi: 10.3109/1061186X.2010.525652. [DOI] [PubMed] [Google Scholar]
- 31.Gao P., Rush B.D., Pfund W.P., Huang T.H., Bauer J.M., Morozowich W. Development of a supersaturable SEDDS (S-SEDDS) formulation of paclitaxel with improved oral bioavailability. J Pharm Sci. 2003;92:2386–2398. doi: 10.1002/jps.10511. [DOI] [PubMed] [Google Scholar]
- 32.Zhang N., Zhang W.D., Jin Y.H., Quan D.Q. Studies on preparation of carbamazepine (CBZ) supersaturatable self-microemulsifying (S-SMEDDS) formulation and relative bioavailability in beagle dogs. Pharm Dev Technol. 2011;16:415–421. doi: 10.3109/10837451003774419. [DOI] [PubMed] [Google Scholar]
- 33.Yamashita K., Nakate T., Okimoto K., Ohike A., Tokunaga Y., Ibuki R. Establishment of new preparation method for solid dispersion formulation of tacrolimus. Int J Pharm. 2003;267:79–91. doi: 10.1016/j.ijpharm.2003.07.010. [DOI] [PubMed] [Google Scholar]
- 34.Porter C.J.H., Trevaskis N.L., Charman W.N. Lipids and lipid-based formulations: optimizing the oral delivery of lipophilic drugs. Nat Rev Drug Discov. 2007;6:231–248. doi: 10.1038/nrd2197. [DOI] [PubMed] [Google Scholar]
- 35.Ghosh P.K., Majithiya R.J., Umrethia M.L., Murthy RSR. Design and development of microemulsion drug delivery system of acyclovir for improvement of oral bioavailability. AAPS PharmSciTech. 2006;7:E172–E177. doi: 10.1208/pt070377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shen H., Zhong M. Preparation and evaluation of self-microemulsifying drug delivery systems (SMEDDS) containing atorvastatin. J Pharm Pharmacol. 2006;58:1183–1191. doi: 10.1211/jpp.58.9.0004. [DOI] [PubMed] [Google Scholar]
- 37.Wei L.L., Sun P.N., Nie S.F., Pan W.S. Preparation and evaluation of sedds and smedds containing carvedilol. Drug Dev Ind Pharm. 2005;31:785–794. doi: 10.1080/03639040500216428. [DOI] [PubMed] [Google Scholar]
- 38.Kommuru T.R., Gurley B., Khan M.A., Reddy I.K. Self-emulsifying drug delivery systems (SEDDS) of coenzyme Q10: formulation development and bioavailability assessment. Int J Pharm. 2001;212:233–246. doi: 10.1016/s0378-5173(00)00614-1. [DOI] [PubMed] [Google Scholar]
- 39.Bates T.R., Carrigan P.J. Apparent absorption kinetics of micronized griseofulvin after its oral administration on single- and multiple-dose regimens to rats as a corn oil-in-water emulsion and aqueous suspension. J Pharm Sci. 1975;64:1475–1481. doi: 10.1002/jps.2600640910. [DOI] [PubMed] [Google Scholar]
- 40.Araya H., Tomita M., Hayashi M. The novel formulation design of O/W microemulsion for improving the gastrointestinal absorption of poorly water soluble compounds. Int J Pharm. 2005;305:61–74. doi: 10.1016/j.ijpharm.2005.08.022. [DOI] [PubMed] [Google Scholar]
- 41.Kim J.Y., Ku Y.S. Enhanced absorption of indomethacin after oral or rectal administration of a self-emulsifying system containing indomethacin to rats. Int J Pharm. 2000;194:81–89. doi: 10.1016/s0378-5173(99)00367-1. [DOI] [PubMed] [Google Scholar]
- 42.Hong J.Y., Kim J.K., Song Y.K., Park J.S., Kim C.K. A new self-emulsifying formulation of itraconazole with improved dissolution and oral absorption. J Control Release. 2006;110:332–338. doi: 10.1016/j.jconrel.2005.10.002. [DOI] [PubMed] [Google Scholar]
- 43.Myers R.A., Stella V.J. Factors affecting the lymphatic transport of penclomedine (NSC-338720), a lipophilic cytotoxic drug: comparison to DDT and hexachlorobenzene. Int J Pharm. 1992;80:51–62. [Google Scholar]
- 44.Chakrabarti S., Belpaire F.M. Bioavailability of phenytoin in lipid containing dosage forms in rats. J Pharm Pharmacol. 1978;30:330–331. doi: 10.1111/j.2042-7158.1978.tb13247.x. [DOI] [PubMed] [Google Scholar]
- 45.Tuleu C., Newton M., Rose J., Euler D., Saklatvala R., Clarke A. Comparative bioavailability study in dogs of a self-emulsifying formulation of progesterone presented in a pellet and liquid form compared with an aqueous suspension of progesterone. J Pharm Sci. 2004;93:1495–1502. doi: 10.1002/jps.20068. [DOI] [PubMed] [Google Scholar]
- 46.Grove M., Müllertz A., Nielsen J.L., Pedersen G.P. Bioavailability of seocalcitol: II: development and characterisation of self-microemulsifying drug delivery systems (SMEDDS) for oral administration containing medium and long chain triglycerides. Eur J Pharm Sci. 2006;28:233–242. doi: 10.1016/j.ejps.2006.02.005. [DOI] [PubMed] [Google Scholar]
- 47.Wu W., Wang Y., Que L. Enhanced bioavailability of silymarin by self-microemulsifying drug delivery system. Eur J Pharm Biopharm. 2006;63:288–294. doi: 10.1016/j.ejpb.2005.12.005. [DOI] [PubMed] [Google Scholar]
- 48.Kang B.K., Lee J.S., Chon S.K., Jeong S.Y., Yuk S.H., Khang G. Development of self-microemulsifying drug delivery systems (SMEDDS) for oral bioavailability enhancement of simvastatin in beagle dogs. Int J Pharm. 2004;274:65–73. doi: 10.1016/j.ijpharm.2003.12.028. [DOI] [PubMed] [Google Scholar]
- 49.Yap S.P., Yuen K.H. Influence of lipolysis and droplet size on tocotrienol absorption from self-emulsifying formulations. Int J Pharm. 2004;281:67–78. doi: 10.1016/j.ijpharm.2004.05.015. [DOI] [PubMed] [Google Scholar]
- 50.Gao Z.G., Choi H.G., Shin H.J., Park K.M., Lim S.J., Hwang K.J. Physicochemical characterization and evaluation of a microemulsion system for oral delivery of cyclosporin A. Int J Pharm. 1998;161:75–86. [Google Scholar]
- 51.Singh A.K., Chaurasiya A., Awasthi A., Mishra G., Asati D., Khar R.K. Oral bioavailability enhancement of exemestane from self-microemulsifying drug delivery system (SMEDDS) AAPS PharmSciTech. 2009;10:906–916. doi: 10.1208/s12249-009-9281-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Deshmukh A., Nakhat P., Yeole P. Formulation and in-vitro evaluation of self microemulsifying drug delivery system (SMEDDS) of furosemide. Pharm Lett. 2010;2:94–106. [Google Scholar]
- 53.Baek M.K., Lee J.H., Cho Y.K., Kim H.H., Lee G.W. Self-microemulsifying drug-delivery system for improved oral bioavailability of pranlukast hemihydrate: preparation and evaluation. Int J Nanomed. 2013;8:167–176. doi: 10.2147/IJN.S37338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Carlert S., Pålsson A., Hanisch G., von Corswant C., Nilsson C., Lindfors L. Predicting intestinal precipitation—a case example for a basic BCS class II drug. Pharm Res. 2010;27:2119–2130. doi: 10.1007/s11095-010-0213-8. [DOI] [PubMed] [Google Scholar]
- 55.Larsen A.T., Sassene P., Müllertz A. In vitro lipolysis models as a tool for the characterization of oral lipid and surfactant based drug delivery systems. Int J Pharm. 2011;417:245–255. doi: 10.1016/j.ijpharm.2011.03.002. [DOI] [PubMed] [Google Scholar]
- 56.Fatouros D.G., Bergenstahl B., Mullertz A. Morphological observations on a lipid-based drug delivery system during in vitro digestion. Eur J Pharm Sci. 2007;31:85–94. doi: 10.1016/j.ejps.2007.02.009. [DOI] [PubMed] [Google Scholar]
- 57.Sassene P.J., Knopp M.M., Hesselkilde J.Z., Koradia V., Larsen A., Rades T. Precipitation of a poorly soluble model drug during in vitro lipolysis: characterization and dissolution of the precipitate. J Pharm Sci. 2010;99:4982–4991. doi: 10.1002/jps.22226. [DOI] [PubMed] [Google Scholar]
- 58.MacGregor K.J., Embleton J.K., Lacey J.E., Perry E.A., Solomon L.J., Seager H. Influence of lipolysis on drug absorption from the gastro-intestinal tract. Adv Drug Deliv Rev. 1997;25:33–46. [Google Scholar]
- 59.Kaukonen A.M., Boyd B.J., Charman W.N., Porter C.J.H. Drug solubilization behavior during in vitro digestion of suspension formulations of poorly water-soluble drugs in triglyceride lipids. Pharm Res. 2004;21:254–260. doi: 10.1023/b:pham.0000016283.87709.a9. [DOI] [PubMed] [Google Scholar]
- 60.Dahan A., Hoffman A. Evaluation of a chylomicron flow blocking approach to investigate the intestinal lymphatic transport of lipophilic drugs. Eur J Pharm Sci. 2005;24:381–388. doi: 10.1016/j.ejps.2004.12.006. [DOI] [PubMed] [Google Scholar]
- 61.Khoo S.M., Shackleford D.M., Porter C.J.H., Edwards G.A., Charman W.N. Intestinal lymphatic transport of halofantrine occurs after oral administration of a unit-dose lipid-based formulation to fasted dogs. Pharm Res. 2003;20:1460–1465. doi: 10.1023/a:1025718513246. [DOI] [PubMed] [Google Scholar]
- 62.Dahan A., Hoffman A. The effect of different lipid based formulations on the oral absorption of lipophilic drugs: the ability of in vitro lipolysis and consecutive ex vivo intestinal permeability data to predict in vivo bioavailability in rats. Eur J Pharm Biopharm. 2007;67:96–105. doi: 10.1016/j.ejpb.2007.01.017. [DOI] [PubMed] [Google Scholar]
- 63.Rahman M.A., Hussain A., Hussain M.S., Mirza M.A., Iqbal Z. Role of excipients in successful development of self-emulsifying/microemulsifying drug delivery system (SEDDS/SMEDDS) Drug Dev Ind Pharm. 2013;39:1–19. doi: 10.3109/03639045.2012.660949. [DOI] [PubMed] [Google Scholar]
- 64.Cuiné J.F., McEvoy C.L., Charman W.N., Pouton C.W., Edwards G.A., Benameur H. Evaluation of the impact of surfactant digestion on the bioavailability of danazol after oral administration of lipidic self-emulsifying formulations to dogs. J Pharm Sci. 2008;97:995–1012. doi: 10.1002/jps.21246. [DOI] [PubMed] [Google Scholar]
- 65.Cuiné J.F., Charman W.N., Pouton C.W., Edwards G.A., Porter C.J.H. Increasing the proportional content of surfactant (Cremophor EL) relative to lipid in self-emulsifying lipid-based formulations of danazol reduces oral bioavailability in beagle dogs. Pharm Res. 2007;24:748–757. doi: 10.1007/s11095-006-9194-z. [DOI] [PubMed] [Google Scholar]
- 66.Markopoulos C., Thoenen F., Preisig D., Symillides M., Vertzoni M., Parrott N. Biorelevant media for transport experiments in the Caco-2 model to evaluate drug absorption in the fasted and the fed state and their usefulness. Eur J Pharm Biopharm. 2014;86:438–448. doi: 10.1016/j.ejpb.2013.10.017. [DOI] [PubMed] [Google Scholar]
- 67.Pawar S., Kumar A. Issues in the formulation of drugs for oral use in children. Pediatr Drugs. 2002;4:371–379. doi: 10.2165/00128072-200204060-00004. [DOI] [PubMed] [Google Scholar]
- 68.López-Montilla J.C., Herrera-Morales P.E., Pandey S., Shah D.O. Spontaneous emulsification: mechanisms, physicochemical aspects, modeling, and applications. J Dispers Sci Technol. 2002;23:219–268. [Google Scholar]
- 69.Basit A.W., Podczeck F., Newton J.M., Waddington W.A., Ell P.J., Lacey L.F. Influence of polyethylene glycol 400 on the gastrointestinal absorption of ranitidine. Pharm Res. 2002;19:1368–1374. doi: 10.1023/a:1020315228237. [DOI] [PubMed] [Google Scholar]
- 70.Schulze J.D.R., Waddington W.A., Ell P.J., Parsons G.E., Coffin M.D., Basit A.W. Concentration-dependent effects of polyethylene glycol 400 on gastrointestinal transit and drug absorption. Pharm Res. 2003;20:1984–1988. doi: 10.1023/b:pham.0000008046.64409.bd. [DOI] [PubMed] [Google Scholar]
- 71.Yáñez J.A., Wang S.W., Knemeyer I.W., Wirth M.A., Alton K.B. Intestinal lymphatic transport for drug delivery. Adv Drug Deliv Rev. 2011;63:923–942. doi: 10.1016/j.addr.2011.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Edwards G.A., Porter C.J.H., Caliph S.M., Khoo S.M., Charman W.N. Animal models for the study of intestinal lymphatic drug transport. Adv Drug Deliv Rev. 2001;50:45–60. doi: 10.1016/s0169-409x(01)00148-x. [DOI] [PubMed] [Google Scholar]
- 73.O’Driscoll C.M. Lipid-based formulations for intestinal lymphatic delivery. Eur J Pharm Sci. 2002;15:405–415. doi: 10.1016/s0928-0987(02)00051-9. [DOI] [PubMed] [Google Scholar]
- 74.Caliph S.M., Charman W.N., Porter C.J.H. Effect of short-, medium-, and long-chain fatty acid-based vehicles on the absolute oral bioavailability and intestinal lymphatic transport of halofantrine and assessment of mass balance in lymph-cannulated and non-cannulated rats. J Pharm Sci. 2002;89:1073–1084. doi: 10.1002/1520-6017(200008)89:8<1073::aid-jps12>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 75.Charman W.N.A., Stella V.J. Estimating the maximal potential for intestinal lymphatic transport of lipophilic drug molecules. Int J Pharm. 1986;34:175–178. [Google Scholar]
- 76.Gershkovich P., Hoffman A. Uptake of lipophilic drugs by plasma derived isolated chylomicrons: linear correlation with intestinal lymphatic bioavailability. Eur J Pharm Sci. 2005;26:394–404. doi: 10.1016/j.ejps.2005.07.011. [DOI] [PubMed] [Google Scholar]
- 77.Singh B.N. Effects of food on clinical pharmacokinetics. Clin Pharmacokinet. 1999;37:213–255. doi: 10.2165/00003088-199937030-00003. [DOI] [PubMed] [Google Scholar]
- 78.Custodio J.M., Wu C.Y., Benet L.Z. Predicting drug disposition, absorption/elimination/transporter interplay and the role of food on drug absorption. Adv Drug Deliv Rev. 2008;60:717–733. doi: 10.1016/j.addr.2007.08.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hauss D.J. Oral lipid-based formulations. Adv Drug Deliv Rev. 2007;59:667–676. doi: 10.1016/j.addr.2007.05.006. [DOI] [PubMed] [Google Scholar]
- 80.Woo J.S., Song Y.K., Hong J.Y., Lim S.J., Kim C.K. Reduced food-effect and enhanced bioavailability of a self-microemulsifying formulation of itraconazole in healthy volunteers. Eur J Pharm Sci. 2008;33:159–165. doi: 10.1016/j.ejps.2007.11.001. [DOI] [PubMed] [Google Scholar]
- 81.Perlman M.E., Murdande S.B., Gumkowski M.J., Shah T.S., Rodricks C.M., Thornton-Manning J. Development of a self-emulsifying formulation that reduces the food effect for torcetrapib. Int J Pharm. 2008;351:15–22. doi: 10.1016/j.ijpharm.2007.09.015. [DOI] [PubMed] [Google Scholar]
- 82.Gottesman M.M., Fojo T., Bates S.E. Multidrug resistance in cancer: role of ATP–dependent transporters. Nat Rev Cancer. 2002;2:48–58. doi: 10.1038/nrc706. [DOI] [PubMed] [Google Scholar]
- 83.Constantinides P.P., Wasan K.M. Lipid formulation strategies for enhancing intestinal transport and absorption of P-glycoprotein (P-gp) substrate drugs: in vitro/in vivo case studies. J Pharm Sci. 2007;96:235–248. doi: 10.1002/jps.20780. [DOI] [PubMed] [Google Scholar]
- 84.Rege B.D., Kao J.P.Y., Polli J.E. Effects of nonionic surfactants on membrane transporters in Caco-2 cell monolayers. Eur J Pharm Sci. 2002;16:237–246. doi: 10.1016/s0928-0987(02)00055-6. [DOI] [PubMed] [Google Scholar]
- 85.Johnson B.M., Charman W.N., Porter C.J.H. An in vitro examination of the impact of polyethylene glycol 400, Pluronic P85, and vitamin E D-α-tocopheryl polyethylene glycol 1000 succinate on P-glycoprotein efflux and enterocyte-based metabolism in excised rat intestine. AAPS PharmSci. 2002;4:193–205. doi: 10.1208/ps040440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kollipara S., Bende G., Movva S., Saha R. Application of rotatable central composite design in the preparation and optimization of poly(lactic-co-glycolic acid) nanoparticles for controlled delivery of paclitaxel. Drug Dev Ind Pharm. 2010;36:1377–1387. doi: 10.3109/03639045.2010.487263. [DOI] [PubMed] [Google Scholar]
- 87.Amidon G.L., Lennernäs H., Shah V.P., Crison J.R. A theoretical basis for a biopharmaceutic drug classification: the correlation of in vitro drug product dissolution and in vivo bioavailability. Pharm Res. 1995;12:413–420. doi: 10.1023/a:1016212804288. [DOI] [PubMed] [Google Scholar]
- 88.Dressman J.B., Reppas C. In vitro–in vivo correlations for lipophilic, poorly water-soluble drugs. Eur J Pharm Sci. 2000;11 Suppl 2:S73–S80. doi: 10.1016/s0928-0987(00)00181-0. [DOI] [PubMed] [Google Scholar]
- 89.Yang S.G. Biowaiver extension potential and IVIVC for BCS class II drugs by formulation design: case study for cyclosporine self-microemulsifying formulation. Arch Pharm Res. 2010;33:1835–1842. doi: 10.1007/s12272-010-1116-2. [DOI] [PubMed] [Google Scholar]
- 90.Rossi R.C., Dias C.L., Donato E.M., Martins L.A., Bergold A.M., Fröehlich P.E. Development and validation of dissolution test for ritonavir soft gelatin capsules based on in vivo data. Int J Pharm. 2007;338:119–124. doi: 10.1016/j.ijpharm.2007.01.036. [DOI] [PubMed] [Google Scholar]
- 91.Donato E.M., Martins L.A., Fröehlich E.E., Bergold A.M. Development and validation of dissolution test for lopinavir, a poorly water-soluble drug, in soft gel capsules, based on in vivo data. J Pharm Biomed Anal. 2008;47:547–552. doi: 10.1016/j.jpba.2008.02.014. [DOI] [PubMed] [Google Scholar]
- 92.Nishimura H., Hayashi C., Aiba T., Okamoto I., Miyamoto Y., Nakade S. Application of the correlation of in vitro dissolution behavior and in vivo plasma concentration profile (IVIVC) for soft-gel capsules-a pointless pursuit? Biol Pharm Bull. 2007;30:2221–2225. doi: 10.1248/bpb.30.2221. [DOI] [PubMed] [Google Scholar]
- 93.Fei Y., Kostewicz E.S., Sheu M.T., Dressman J.B. Analysis of the enhanced oral bioavailability of fenofibrate lipid formulations in fasted humans using an in vitro-in silico-in vivo approach. Eur J Pharm Biopharm. 2013;85:1274–1284. doi: 10.1016/j.ejpb.2013.03.001. [DOI] [PubMed] [Google Scholar]
- 94.Reymond J.P., Sucker H. In vitro model for ciclosporin intestinal absorption in lipid vehicles. Pharm Res. 1988;5:673–676. doi: 10.1023/a:1015987223407. [DOI] [PubMed] [Google Scholar]
- 95.Fatouros D.G., Nielsen F.S., Douroumis D., Hadjileontiadis L.J., Mullertz A. In vitro–in vivo correlations of self-emulsifying drug delivery systems combining the dynamic lipolysis model and neuro-fuzzy networks. Eur J Pharm Biopharm. 2008;69:887–898. doi: 10.1016/j.ejpb.2008.01.022. [DOI] [PubMed] [Google Scholar]
- 96.Christophersen P.C., Christiansen M.L., Holma R., Kristensen J., Jacobsen J., Abrahamsson B. Fed and fasted state gastro-intestinal in vitro lipolysis: in vitro–in vivo relations of a conventional tablet, a SNEDDS and a solidified SNEDDS. Eur J Pharm Sci. 2014;57:232–239. doi: 10.1016/j.ejps.2013.09.007. [DOI] [PubMed] [Google Scholar]
- 97.Gupta S., Chavhan S., Sawant K.K. Self-nanoemulsifying drug delivery system for adefovir dipivoxil: design, characterization, in vitro and ex vivo evaluation. Colloids Surf A: Physicochem Eng Asp. 2011;392:145–155. [Google Scholar]
- 98.Ghai G., Sinha V.R. Nanoemulsions as self-emulsified drug delivery carriers for enhanced permeability of the poorly water-soluble selective β1-adrenoreceptor blocker Talinolol. Nanomedicine. 2012;8:618–626. doi: 10.1016/j.nano.2011.08.015. [DOI] [PubMed] [Google Scholar]
- 99.Eedara B.B., Veerareddy P.R., Jukanti R., Bandari S. Improved oral bioavailability of fexofenadine hydrochloride using lipid surfactants: ex vivo, in situ and in vivo studies. Drug Dev Ind Pharm. 2014;40:1030–1043. doi: 10.3109/03639045.2013.801984. [DOI] [PubMed] [Google Scholar]
- 100.Garg A., Kumar A., Kohli K. Dilution phenomenon in mixed surfactant based self-microemulsifying formulations of ginger oleoresin: ex vivo and in vivo performances. Int J Drug Deliv. 2013;5:270–283. [Google Scholar]
- 101.Williams H.D., Sassene P., Kleberg K., Bakala-N’Goma J.C., Calerone M., Janniv V. Toward the establishment of standardized in vitro tests for lipid-based formulations, part 1: method parameterization and comparison of in vitro digestion profiles across a range of representative formulations. J Pharm Sci. 2012;101:3360–3380. doi: 10.1002/jps.23205. [DOI] [PubMed] [Google Scholar]