Abstract
Arsenic trioxide (ATO) has been identified as an effective treatment for acute promyelocytic leukemia (APL) but is much less effective against solid tumors such as hepatocellular carcinoma (HCC). In the search for ways to enhance its therapeutic efficacy against solid tumors, we have examined its use in combination with a novel derivative of β-elemene, N-(β-elemene-13-yl)tryptophan methyl ester (ETME). Here we report the effects of the combination on cell viability, apoptosis, the cell cycle and mitochondria membrane potential (MMP) in HCC SMMC-7721 cells. We found that the two compounds acted synergistically to enhance antiproliferative activity and apoptosis. The combination also decreased the MMP, down-regulated Bcl-2 and pro-proteins of the caspase family, and up-regulated Bax and BID, all of which were reversed by the p53 inhibitor, pifithrin-α. In addition, the combination induced cell cycle arrest at the G2/M phase and reduced tumor volume and weight in an xenograft model of nude mice. Overall, the results suggest that ETME in combination with ATO may be useful in the treatment of HCC patients particularly those unresponsive to ATO alone.
Key words: Hepatocarcinoma, β-Elemene derivative, As2O3, Apoptosis, p53
Graphical abstract
This paper demonstrates that the combination of the β-elemene derivative, ETME, and arsenic trioxide (ATO) synergistically induces apoptosis and cell cycle arrest of hepatocellular carcinoma cells and reduces tumor volume and weight in an xenograft model in nude mice by a mechanism involving p53.
1. Introduction
On a worldwide basis, hepatocellular carcinoma (HCC) is the sixth most common cancer in terms of incidence but the third most common in causing death. Therefore there is a distinct need for novel chemotherapeutic agents with greater efficacy against HCC1. Arsenic trioxide (ATO) has been shown to successfully treat acute promyelocytic leukemia (APL) but is much less effective against solid tumors such as HCC. In fact in a phase II trial, ATO alone was inefficient in the treatment of HCC, suggesting that strategies to enhance and expand its therapeutic efficacy are needed2.
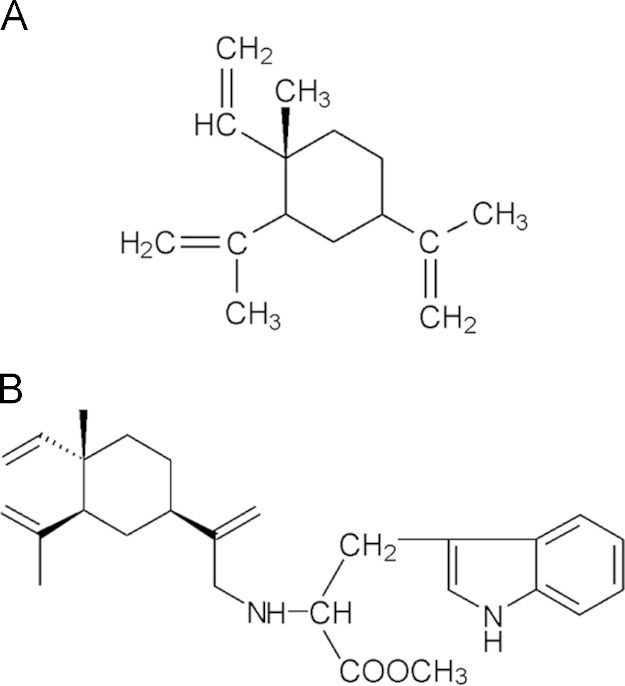
Although β-elemene (Fig. 1A) has been approved for the treatment of advanced cancer in China, its clinical application has been limited by poor antiproliferative efficacy, low water solubility and instability. This has led to the search for new β-elemene derivatives (Fig. 1A) with improved physicochemical and pharmacokinetic properties and enhanced antiproliferative effects. We previously reported that the novel β-elemene derivative, N-(β-elemene-13-yl)tryptophan methyl ester (ETME, Fig. 1B), exerts potent antiproliferative activity against human leukemia cells both alone and in combination with ATO3, 4. However, the efficacy of ETME alone and in combination with ATO in treating HCC is unknown. In this study, we show that the two compounds act synergistically to induce apoptosis and cell cycle arrest at the G2/M phase in HCC SMMC-7721 cells via a p53-dependent pathway and reduce tumor volume and weight in vivo.
Figure 1.
Chemical structures of (A) β-elemene and (B) N-(β-elemene-13-yl)tryptophan methyl ester (ETME).
2. Materials and methods
2.1. Reagents
MTT was obtained from the Ameresco Co., Inc. (Ameresco, CA, USA). Ethidium bromide (EB), acridine orange (AO), propidium iodide (PI), rhodamine 123 (Rh123), pifithrin-α (PFT-α), caspase inhibitor (Z-VAD-FMK), the annexin V-FITC apoptosis detection kit, the BCA protein determination kit and specific secondary antibodies were purchased from Beyotime Biotech. (Haimen, Jiangsu, China). Hoechst 33342 was obtained from Sigma (St. Louis, MO, USA). Antibodies to pro-caspase-3, -8 and -9, Bcl-2, Bax, BID, p53, p27, cyclinD1, cyclinB1 and CDK1 were purchased from Santa Cruz Biotechnology Inc. (Santa Cruz, CA, USA).
2.2. Cell culture
Human HCC SMMC-7721 cells and HepG2 cells were incubated in RPMI 1640 medium supplemented with 10% fetal bovine serum, 100 U/mL penicillin and 100 μg/mL streptomycin. The cultures were maintained at 37 °C in an incubator under 5% CO2.
2.3. Cell proliferative evaluation
Cells were seeded in 96-well plates at a density of 5×103 cells/well and incubated for 24 h before adding ETME, ATO or their combination. After incubation for 48 h, 50 µL MTT reagent (1 mg/mL) was added and the plates incubated at 37 °C for a further 4 h in a humidified incubator under 5% CO2. Supernatants were removed from the wells, the crystalline product dissolved in 100 µL DMSO and the plates incubated at room temperature for 10 min. The absorbance was then measured with a microplate reader (Bio-Rad) at a wavelength of 570 nm. The combination index (CI) was calculated as EA+B/(EA+(1−EA)×EB), where EA, EB and EA+B are the inhibition ratios of A, B and (A+B) respectively. Antagonism, enhancement and synergism are indicated by CI<0.85, 0.85≤CI≤1.15 and CI>1.15, respectively5.
2.4. Cellular apoptotic evaluation
Based on CI values, we selected concentrations of ETME and ATO of 20 and 5 μmol/L respectively and SMMC-7721 cells for further study. Cell apoptosis was evaluated using the AnnexinV-FITC apoptosis detection kit. Briefly, the cells were seeded in 6-well plates and exposed to the indicated concentrations of ETME, ATO and their combination. After treatment for 48 h, cells were harvested, stained according to the manufacturer׳s protocol and analyzed using HCS. Cell apoptosis was also detected using AO and EB staining. In brief, cells treated with ETME, ATO or the combination for 48 h were incubated with AO (10 μg/mL) and EB (10 μg/mL) for 30 min in dark and then analyzed using HCS. EB-positive cells with nuclear shrinkage, blabbing and apoptotic bodies were counted as apoptotic cells and the apoptotic ratio was calculated after observing 300 cells. For caspase inhibitor analysis, cells were pretreated for 2 h with 50 μmol/L Z-VAD-FMK 1.
2.5. Cell cycle analysis
Cells were seeded in 25 cm2 flasks and pre-incubated in RPMI1640 medium supplemented with 0.2% fetal calf serum for 24 h to induce cell cycle synchronization. The synchronous cells were then treated with ETME, ATO or the combination and incubated for 48 h. Cells were washed twice with ice-cold phosphate buffered saline (PBS), fixed, and permeabilized with ice-cold 70% ethanol at −20 °C overnight. Cells were subsequently treated with 50 µg/mL RNase A at room temperature for 30 min, washed with ice-cold PBS and finally stained with 50 µg/mL PI in dark at 4 °C for 30 min. The distribution of cell cycle phases with different DNA contents was analyzed using HCS on the basis of acquiring 10,000 events for each sample1.
2.6. Western blot analysis
After treatment, SMMC-7721 cells were harvested and washed with PBS. Total cellular protein was isolated using the protein extraction buffer and determined using the BCS protein assay kit. Cell lysates in 5× SDS sample buffer were boiled for 5 min after which equal amounts of protein (50 μg/lane) were fractionated using 8% or 12% SDS-PAGE and transferred to PVDF membranes. After blocking with PBST containing 5% dried skimmed milk, the membranes were cultured with primary antibodies at 4 °C overnight. After washing with PBS, the blots were incubated with the corresponding peroxidase-conjugated goat anti-mouse or anti-rabbit secondary antibody followed by detection using ECL plus reagents1.
2.7. Animal treatment
A group of male BALB/c nude mice (n=40) was purchased from the Animal Center, Vital River (Beijing, China). The mice were provided with food and water ad libitum on a 12:12 day/night cycle maintained at around 26 °C. Suspensions of 2×106 SMMC-7721 cells were injected subcutaneously into the armpits of mice and tumor growth monitored every 3 days. When the average tumor volume reached 100 mm3, the tumor-bearing mice were randomly divided into four groups and treated with either saline, 50 mg/kg/d ETME, 3 mg/kg/d ATO or the combination for 2 weeks. The volume of the tumor was calculated from the formula V=1/2 (A×B2), where A is the longest diameter and B the perpendicular diameter measured using calipers. At the end of treatment, mice were sacrificed and the tumors excised for analysis6.
2.8. Data analysis
All data are presented as mean±SD and analyzed using Student׳s t-test or analysis of variance (ANOVA) followed by a q test.
3. Results
3.1. Inhibition of cell growth
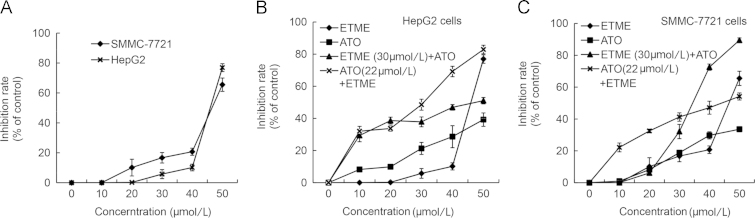
The results of cell growth inhibition studies show that ETME exerts potent antiproliferative effects on both SMMC-7721 and HepG2 cells in a dose-dependent manner with SMMC-7721 cells being more sensitive (Fig. 2A). In terms of the effect of the combination of ETME and ATO, we found that treatment of SMMC-7721 cells with 20 μmol/L ETME and 5 μmol/L ATO for 48 h gave the maximum CI value of 7.29 (Fig. 2B and C). We therefore used this system for further studies.
Figure 2.
(A) The antiproliferative activity of ETME in HepG2 cells and SMMC-7721 cells tested after 48 h treatment; (B) the anti-proliferative activity of ETME, ATO and their combination in HepG2 cells after 48 h treatment; (C) the anti-proliferative activity of ETME, ATO and their combination in SMMC-7721 cells after 48 h treatment. Data are expressed as mean±SD, n=3.
3.2. SMMC-7721 cell apoptosis
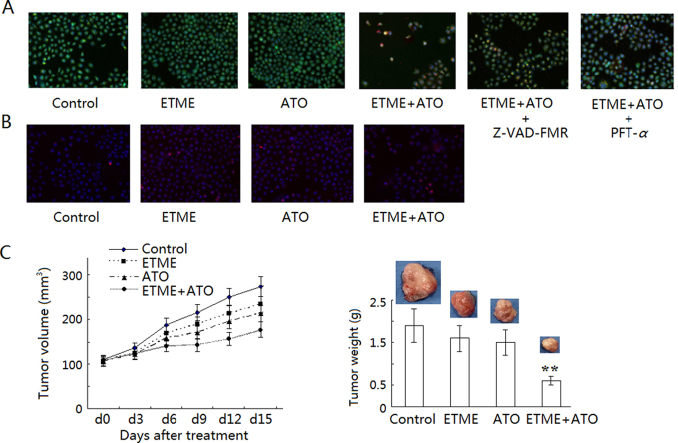
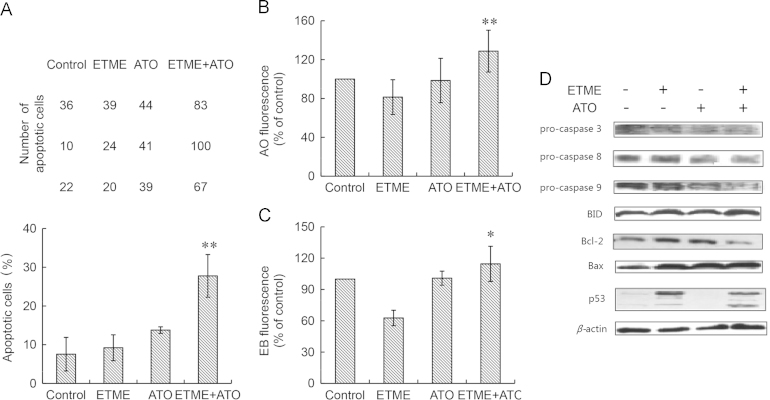
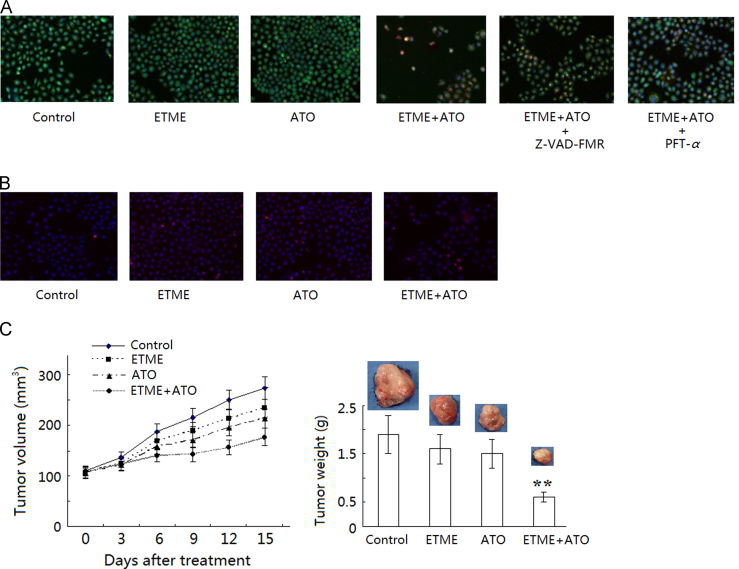
Using Annexin-FITC staining, treatment with ETME and ATO alone caused no significant differences in the rate of apoptosis compared with the control group (P>0.05) but treatment with the combination led to a significantly greater rate of apoptosis (P<0.05) compared with the control group (Fig. 3A). Furthermore, using AO/EB staining (Fig. 4A), treatment with the combination caused significant increases in AO (Fig. 3B) and EB (Fig. 3C) fluorescence compared with the control group (P<0.05). These results suggest the synergistic antiproliferative effect is at least partly mediated through apoptosis.
Figure 3.
Effect of the combination of ETME (20 μmol/L) and ATO (5 μmol/L) on apoptosis of SMMC-7721 cells after 48 h using β-actin as loading control. (A) Top, original data, Bottom, bar chart showing statistical results; (B) fluorescence intensity of AO after treatment; (C) fluorescence intensity of EB after treatment; (D) protein expression detected by Western blot after treatment. Data are expressed as mean±SD, n=3. *P<0.05, **P<0.01 vs control.
Figure 4.
Synergistic effect of the combination of ETME (20 μmol/L) and ATO (5 μmol/L) on apoptosis, the cell cycle and tumor growth of SMMC-7721 cells after 48 hs. (A) Apoptosis detected by AO/EB staining (100×); (B) cell cycle progress detected by PI staining (100×); (C) tumor volume (left) and weight (right) of SMMC-7721 cell xenografts in nude mice after treatment with ETME, ATO or their combination for 14 d. Data are expressed as mean±SD, n=10. **P<0.01 vs control.
In order to further investigate the mechanism of the synergistic effect, Western blot analysis was used to determine the protein levels of apoptotic related proteins. The results show that the levels of p53, Bax and BID proteins were not significantly changed after treatment with ETME alone (Fig. 3D) but treatment with the combination promoted the activation of caspase-3, caspase-8 and caspase-9, up-regulated the protein levels of p53, Bax and BID and down-regulated the protein expression of Bcl-2 compared with the control (untreated) group (Fig. 3D).
3.3. Apoptosis and the p53 pathway
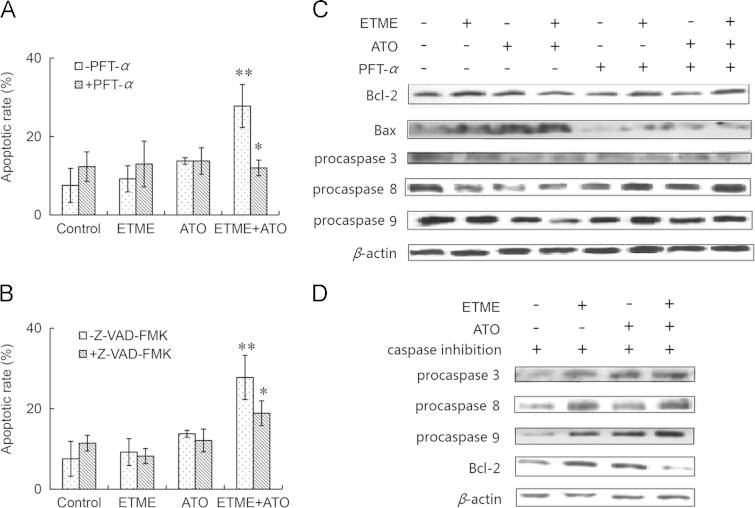
Activation of p53 can induce apoptosis and bring about cell cycle arrest and senescence7. To determine the role of p53 in the synergistic apoptotic effect of ETME and ATO, we evaluated the effect of the p53-specific inhibitor, PFT-α. As shown in Fig. 5A, pre-treatment of cells with PFT-α markedly reduced apoptosis compared with the untreated group. Furthermore, Western blot analysis showed that the impact of the two agents either alone or in combination on Bcl-2, Bax, caspase-3, caspase-8 and caspase-9 was reversed by PFT-α pre-treatment (Fig. 5B). In addition, pre-treatment of cells with the caspase inhibitor, Z-VAD-FMK, attenuated apoptosis (Fig. 5C), caspase activation and Bcl-2 down-regulation (Fig. 5D). These results suggest that the apoptotic effect of the combination of ETME and ATO is due to the activation of the p53 signaling pathway and members of the caspase family.
Figure 5.
Effect of pre-treatment with PFT-α and Z-VAD-FMK on apoptosis of SMMC-7721 cells caused by treatment with ETME (20 μmol/L), ATO (5 μmol/L) or their combination for 48 h. (A) Effect of pre-treatment with PFT-α (10 μg/mL) for 1 h on apoptosis; (B) effect of pre-treatment with PFT-α (10 μg/mL) for 1 h on protein expression detected by Western blot; (C) effect of pre-treatment with Z-VAD-FMK (10 μg/mL) for 1 h on apoptosis; (D) effect of pre-treatment with Z-VAD-FMK (10 μg/mL) for 1 h on protein expression detected by Western blot. Data are expressed as mean±SD, n=3. *P<0.05, **P<0.01 vs control.
3.4. Effect on cell cycle progression
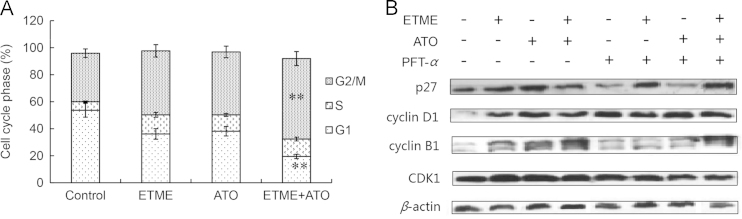
p53 is also linked with control of cell cycle progression7, 8. After treatment with ETME, ATO or their combination for 48 h, the number of cells in the G2/M phase was much greater than in the untreated group and the highest in the group treated with the combination (Figs. 6A and 4B). Furthermore, Western blot analysis showed that the levels of cell cycle-related proteins such as p27, cyclin D1, cyclin B1 and CDK1 were much increased compared with the untreated group and could be partly attenuated by PFT-α (Fig. 6B).
Figure 6.
ETME synergized with As2O3 to induce cell cycle arrest in SMMC-7721 cells. (A) The cell cycle progress of SMMC-7721 cells was detected after treatment with ETME, As2O3 or their combination for 48 h. Data are expressed as mean±SD, n=3. *P<0.05, **P<0.01 vs control. (B) The protein expression of SMMC-7721 cells was detected after treatment with ETME, As2O3 or their combination for 48 h. β-actin is used as a loading control.
3.5. Tumor growth in nude mice
After treatment with ETME or ATO alone, tumor volume and weight in the xenograft model degraded but not significantly compared with the control group (P>0.05). In contrast, treatment with the combination significantly reduced (P<0.05) the tumor volume and weight compared with the control group (Fig. 4C).
4. Discussion
β-Elemene displays potent antitumor activity both in vitro and in vivo and has been identified as a complementary drug for cancer treatment in China9, 10, 11, 12, 13. Previous research has revealed that the novel derivative of β-elemene, ETME, has higher water-solubility and exhibits potent antiproliferative activity against human leukemia cells3. In the present study, we found that ETME displayed similar activity against SMMC-7721 and HepG2 cells and, in combination with ATO, produced a greater antiproliferative effect than either agent alone both in vitro and in vivo. This synergistic effect was previously observed in human leukemia cells and suggests the combination may be useful in the treatment of HCC patients unresponsive to ATO alone.
Previous research in our laboratory data demonstrated that ETME and other β-elemene derivatives such as DX1, alone or in combination with ATO, could induce apoptosis in human leukemia cells3, 4. We have now shown this to also be the case in SMMC-7721 cells where the apoptotic effect was accompanied by up-regulation of p53, BID and Bax, down-regulation of Bcl-2 and activation of members of the caspase family. The latter changes are in contrast to the effects of the combination on human leukemia cells where apoptosis was accompanied by the activation of caspase-3 but no changes in the expression of Bcl-2 and Bax3.
To detect whether p53 is the key mediator of apoptosis produced by the combination, SMMC-7721 cells were pretreated with the p53 inhibitor PFT-α. The results indicate that PFT-α not only reversed the synergistic apoptotic effect of the combination of ETME and ATO but also blocked the accompanying changes in p53, BID, Bax, Bcl-2 and members of the caspase family. This clearly indicates that p53 plays an important role in the synergistic apoptotic effect. In addition, given that the caspase inhibitor, Z-VAD-FMK, also attenuated apoptosis, it appears that members of the caspase family also play a key role.
Since p53 also regulates the cell cycle14, 15, we examined the effects of treatment with ETME and ATO on it. The results show that the cell cycle was arrested at the G2/M phase and that the expression of p27, cyclin D1, cyclin B1 and CDK1 was induced but not in the presence of PFT-α. This suggests that p53 exerts a vital role in the ability of the combination of ETME and ATO to induce cell cycle arrest.
5. Conclusions
The present study shows that the combination of ETME and ATO induces apoptosis and cell cycle arrest of hepatocellular carcinoma cells in a p53-dependent manner and reduced tumor volume and weight in an xenograft model of nude mice. The results suggest that the combination may be useful in the treatment of patients with HCC.
Acknowledgment
This work was supported by the Guangdong Natural Science Foundation (Grant No. S2011040000529).
Footnotes
Peer review under responsibility of Institute of Materia Medica, Chinese Academy of Medical Sciences and Chinese Pharmaceutical Association.
Contributor Information
Chaojie Wang, Email: wcjsxq@henu.edu.cn.
Song-qiang Xie, Email: xiesq@henu.edu.cn.
References
- 1.Xie SQ, Zhang YH, Li Q, Xu FH, Miao JW, Zhao J. 3-Nitro-naphthalimide and nitrogen mustard conjugate NNM-25 induces hepatocellular carcinoma apoptosis via PARP-1/p53 pathway. Apoptosis. 2012;17:725–734. doi: 10.1007/s10495-012-0712-7. [DOI] [PubMed] [Google Scholar]
- 2.Chen G, Wang K, Yang BY, Tang B, Chen JX, Hua ZC. Synergistic antitumor activity of oridonin and arsenic trioxide on hepatocellular carcinoma cells. Int J Oncol. 2012;40:139–147. doi: 10.3892/ijo.2011.1210. [DOI] [PubMed] [Google Scholar]
- 3.Yu ZY, Wang R, Xu LY, Dong JH, Jing YK. N-(β-elemene-13-yl)tryptophan methyl ester induces apoptosis in human leukemia cells and synergizes with arsenic trioxide through a hydrogen peroxide dependent pathway. Cancer Lett. 2008;269:165–173. doi: 10.1016/j.canlet.2008.04.034. [DOI] [PubMed] [Google Scholar]
- 4.Yu ZY, Wang R, Xu LY, Xie SW, Dong JH, Jing YK. β-elemene piperazine derivatives induce apoptosis in human leukemia cells through downregulation of c-FLIP and generation of ROS. PLoS One. 2011;6:e15843. doi: 10.1371/journal.pone.0015843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang R, Li C, Song DD, Zhao GS, Zhao LX, Jing YK. Ethacrynic acid butyl-ester induces apoptosis in leukemia cells through a hydrogen peroxide-mediated pathway independent of glutathione S-transferase P1-1 inhibition. Cancer Res. 2007;67:7856–7864. doi: 10.1158/0008-5472.CAN-07-0151. [DOI] [PubMed] [Google Scholar]
- 6.Zhao XY, Yang S, Chen YR, Li PC, Dou MM, Zhang J. Resveratrol and arsenic trioxide act synergistically to kill tumor cells in vitro and in vivo. PLoS One. 2014;9:e98925. doi: 10.1371/journal.pone.0098925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Levine AJ, Oren M. Timeline: the first 30 years of p53: growing ever more complex. Nat Rev Cancer. 2009;9:749–758. doi: 10.1038/nrc2723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Freed-Pastor WA, Prives C. Mutant p53: one name, many proteins. Genes Dev. 2012;26:1268–1286. doi: 10.1101/gad.190678.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang G, Li X, Huang F, Zhao J, Ding H, Cunningham C. Antitumor effect of β-elemene in non-small-cell lung cancer cells is mediated via induction of cell cycle arrest and apoptotic cell death. Cell Mol Life Sci. 2005;62:881–893. doi: 10.1007/s00018-005-5017-3. [DOI] [PubMed] [Google Scholar]
- 10.Li X, Wang G, Zhao J, Ding H, Cunningham C, Chen F. Antiproliferative effect of β-elemene in chemoresistant ovarian carcinoma cells is mediated through arrest of the cell cycle at the G2-M phase. Cell Mol Life Sci. 2005;62:894–904. doi: 10.1007/s00018-005-5027-1. [DOI] [PubMed] [Google Scholar]
- 11.Wang YZ, Deng YH, Mao SR, Jin SX, Wang J, Bi DZ. Characterization and body distribution of β-elemene solid lipid nanoparticles (SLN) Drug Dev Ind Pharm. 2005;31:769–778. doi: 10.1080/03639040500216329. [DOI] [PubMed] [Google Scholar]
- 12.Li QQ, Wang GD, Huang FR, Banda M, Reed E. Antineoplastic effect of β-elemene on prostate cancer cells and other types of solid tumour cells. J Pharm Pharmacol. 2010;62:1018–1027. doi: 10.1111/j.2042-7158.2010.01135.x. [DOI] [PubMed] [Google Scholar]
- 13.Xu LY, Tao SJ, Wang XM, Yu ZY, Wang MW, Chen D. The synthesis and anti-proliferative effects of β-elemene derivatives with mTOR inhibition activity. Bioorg Med Chem. 2006;14:5351–5356. doi: 10.1016/j.bmc.2006.03.041. [DOI] [PubMed] [Google Scholar]
- 14.Chen YC, Chang HY, Deng JS, Chen JJ, Huang SS, IH Lin. Hispolon from Phellinus linteus induces G0/G1 cell cycle arrest and apoptosis in NB4 human leukaemia cells. Am J Chin Med. 2013;41:1439–1457. doi: 10.1142/S0192415X13500961. [DOI] [PubMed] [Google Scholar]
- 15.Zhou YF, Bi YY, Yang CH, Yang JB, Jiang Y, Meng FM. Magnolol induces apoptosis in MCF-7 human breast cancer cells through G2/M phase arrest and caspase-independent pathway. Pharmazie. 2013;68:755–762. [PubMed] [Google Scholar]