Abstract
Clausenamide (clau) is one of seven novel compounds isolated from Clausena lansium (Lour) skeels. Clau is unusual in that it contains 4 chiral centers yielding 8 pairs of enantiomers. After identification of the configuration of these enantiomers, the synthesis of 16 enantiomers, including optically active clau and (+) and (–)clau was carried out. During this study, many stereochemical and synthetic difficulties were solved and the Baldwin principle was updated. Production scale is now sufficient to meet the needs of clinical practice. In a pharmacological study numerous models and indicators showed that (–)clau is the active enantiomer, while (+)clau is inactive and elicits greater toxicity than (–)clau. The principal pharmacological effects of (–)clau are to increase cognition, demonstrated in ten models of memory impairment, as well as to inhibit β-amyloid (Aβ) toxicity, blocking neurofibrillary tangle formation by inhibiting the phosphorylation of tau protein. This anti-dementia effect is characterized by increased synaptic plasticity both in efficacy and in structure and provides new support for the theory that synaptic loss is the main cause of dementia. (–)Clau is considered to be a promising drug candidate for treatment of Alzheimer׳s disease and other neurodegenerative disorders.
KEY WORDS: (–)Clausenamide, Enantiomers, Cognition, Alzheimer׳s disease pathology, Tau, High phosphorylation, Synaptic plasticity
Graphical abstract
This review summarizes the latest research advance of synthetic (–)clausenamide, one of the optical isomers of clausenamide isolated from Clausena lansium. It is a new type anti-dementia agent, characteristic by improved learning and memory in normal and memory impairment rodents, increased synaptic plasticity in both efficacy and structure, and decreased apoptosis with high phosphorylation of tau protein. Owing to this multi-target effect, the low toxicity and unique mechanism of action suggests that it is a promising drug for treatment of neurodegenerative diseases and many kinds of memory impairment
.
1. Introduction
Alzheimer׳s disease (AD) is an age-related neurodegenerative disease characterized by progressive decline of cognitive function, appearance of senile plaques, neurofibrillary tangles, and loss of neurons and synapses. Few drugs have been found to be effective and safe for the treatment of AD.
In China, in recent years increasing emphasis has been placed on research on natural products and traditional Chinese medicine (TCM). About 150 new drugs have been developed with more than 20 that affect the nervous system. (–)Clausenamide (clau) is the first chiral anti-dementia compound isolated from Clausena lansium, an evergreen plant distributed mainly in Asia and Pacific region. The percolate of the leaves of C. lansium was described in ancient TCM books (Ben Cao Qiu Yuan) for the treatment of edema with yellow tinge of skin, and the edible fruit of C. lansium is quite popular in southern China. Therefore, we believed that it is safe and may have some therapeutic effects. This paper highlights recent advances in the chemistry, pharmacology and pharmacokinetics of (–)clau in anti-dementia research, some of which are very new.
2. Chemistry of clau
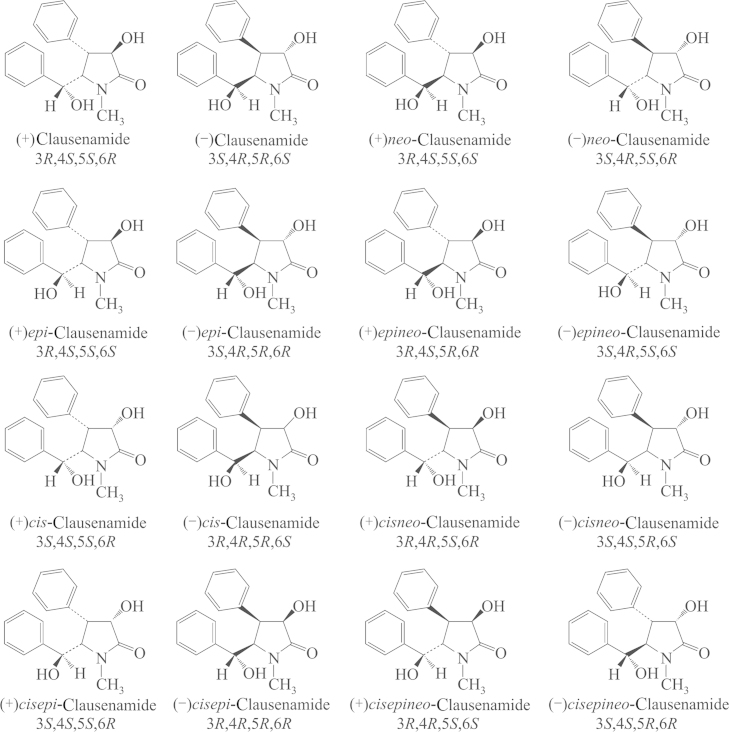
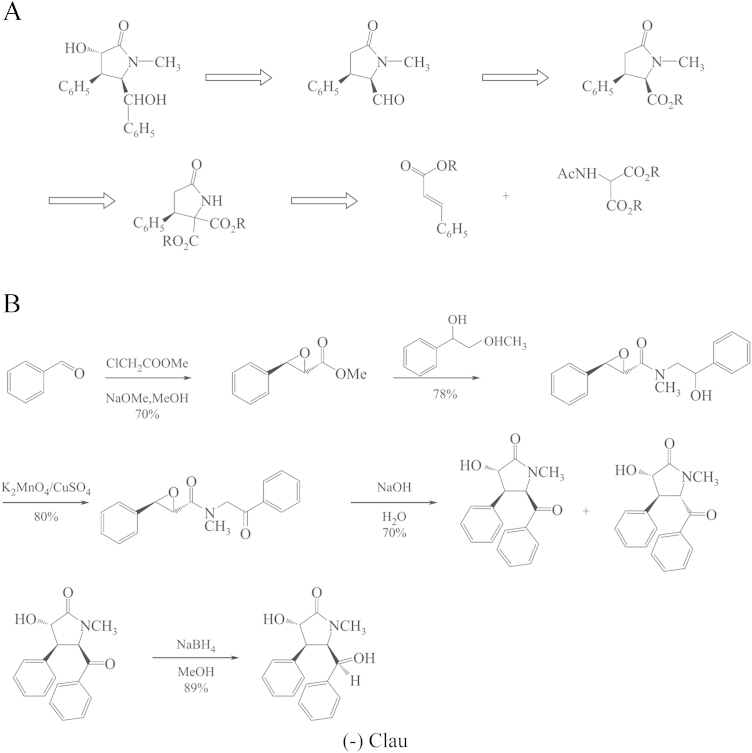
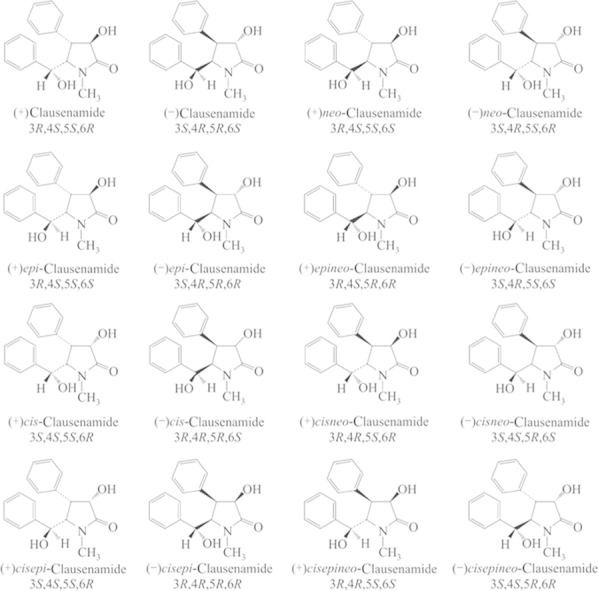
From the aqueous extract of the leaves of C. lansium, chemists isolated seven amides, one of which, clau, is a γ-lactam with four asymmetric carbons yielding 16 enantiomers. The pharmacological evaluation, synthesis and identification of the 16 enantiomers were completed in Prof. Liang Huang׳s laboratory (Institute of Materia Medica, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, China). The structures and configurations of the 16 enantiomers are shown in Fig. 1. A pharmacological study showed that racemic clau had nootropic effects as demonstrated by behavioral and electrophysiological experiments. Then, a question was advanced as to which one, (+) or (–)clau, is the bioactive isomer. Bayer company and our institute attempted to synthesize racemic clau and resolve the (–) and (+)enantiomers. Dr. Hartwig of Bayer Co. reported a 10-step synthesis of racemic clau by building the skeleton first through NC O and C4/C5-linking followed by stepwise stereoselective introduction of substituents. The overall yield was 5.7%–6.4%. Liang Huang et al.1 reported a biomimetic synthetic route based upon the biogenetic relationship perceived among the seven amides isolated, and the total synthesis was reduced to 5 steps with an overall yield of 15%–17%. The two synthetic schemes are shown in Scheme 1.
Figure 1.
The structures and configurations of the 16 enantiomers.
Scheme 1.
Synthesis schemes of racemic clau. A, Hartwig's retrosynthesis scheme of racemic clau; B, Liang Huang's synthesis scheme of racemic clau.
For ensuring (–)clau to be effective, safe, stable and reliable in clinical practice, methods for chiral separation, purification and quality control were developed, which include rapid and reliable method for isomer separation by capillary electrophoresis and chiral column chromatography. Optical purity and the identity of impurities were determined by high performance liquid chromatography (HPLC). The results showed that complete separation of (–)clau and (+)clau was achieved with the optical purity reaching 99%. The single impurity was identified as (–)epi clau with an approximate content of 0.3%.
3. Pharmacological effects
3.1. Effect on memory impairments
Ten animal models were used to evaluate the effect of (–)clau on memory impairment include APP-transgenic mice, aged rats (24–27 months), diabetic mice, cerebral ischemic-reperfusion rats, β-amyloid (Aβ) and other 5 chemically-induced memory impairment models. Oral administration of (–)clau at a dose of 5–10 mg/kg significantly improved learning and memory2, 3. The effective dosage was equal to that of donepezil and much more potent than piracetam.
3.2. Effect on long term potentiation (LTP) of synaptic transmission
Considerable evidence supports the idea that long-lasting activity-dependent plasticity of synaptic transmission is of fundamental importance in the development of neural circuitry and storage of information. As a typical form of this plasticity, LTP in the hippocampus is the neural basis of learning and memory. It is also a good experimental model for investigating cognitive function at cellular and synaptic levels. Surgery and electrophysiological recordings were conducted as described in our publications4, 5, 6, 7.
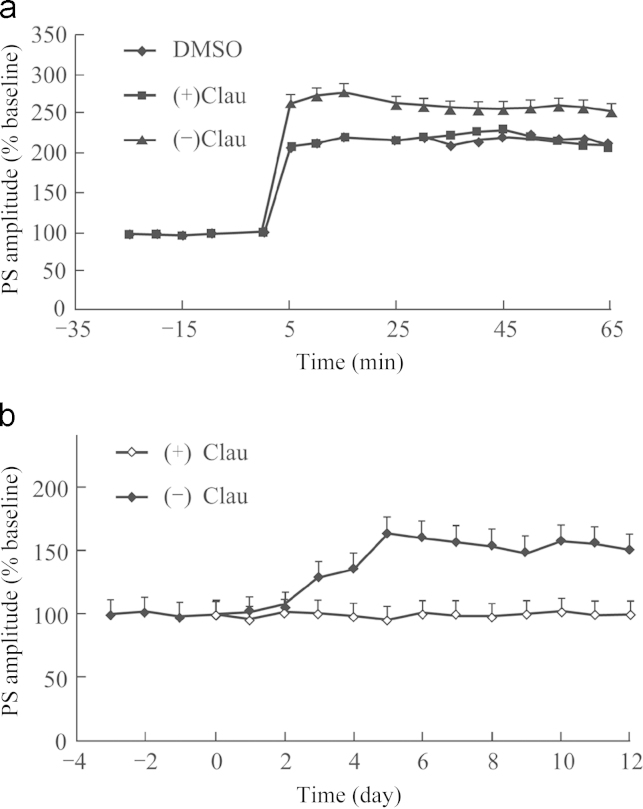
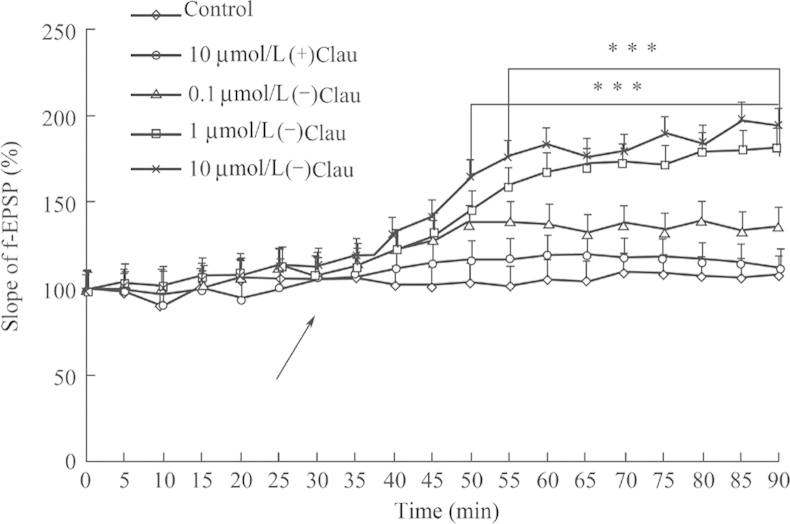
By comparing (–) and (+)clau, the following results were obtained: (–)clau at concentration of 10−7–10−5 mol/L increased basic synaptic transmission and the amplitude of LTP induced by high frequency stimulation in anesthetized or freely moving rats in a concentration-dependent and time-dependent manner (Figure 2, Figure 3). (+)Clau showed no effects on LTP. There are two forms of LTP: one is dependent on the activation of the N-methyl-d-aspartate (NMDA) receptor, and the other is mediated by entry of calcium via a voltage-dependent calcium channel (VDCC). It was demonstrated that (–)clau-induced LTP was inhibited by nimodipine, a specific antagonist of the L-type VDCC. But APV, a specific antagonist of the NMDA receptor, had no inhibitory effect on (–)clau-induced LTP, indicating that VDCCs rather than NMDA receptors are required for an effect of (–)clau to take place. We have also evaluated the 16 enantiomers of clau by testing their effects on synaptic transmission in the hippocampus and found that four had potentiation effects. Nimodipine, piracetam and donepezil showed no effect on LTP8.
Figure 2.
Effect of (–) or (+)clau on LTP in the DG of (a) anesthetized and (b) freely moving rats induced by high frequency stimuli (HFS). (a): LTP was induced using an HFS protocol of 100 Hz (10 bursts of 5 stimuli, 0.2 ms duration, 200 μs interburst interval). HFS was applied 15 min after drug administration. (b): The stimulation and recording electrodes were fixed to the skull using acrylic dental cement and then all rats were housed individually on a 12-h light/dark cycle with food and water available ad libitum, and tested during the light cycle. All rats were injected with 16 U penicillin to prevent infection, allowed to recover from surgery for 7 days, and evaluated to ensure normal mobility. (–)Clau and (+) clau at a dose of 8 mg/kg was administrated orally. From day 8, the population spike were evoked by stimulation at low frequency (0.33 Hz, duration 200 μs), 10 evoked responses were averaged as the efficacy of synaptic transmission.
Figure 3.
Effect of (–)clau on LTP with different doses. (–)Clau was administered by intracerebroventricular injection and the population spike amplitude was recorded every 5 min. ***P<0.001 vs control animals. f-EPSP: field-excitatory postsynaptic potential.
4. Nootropic mechanism of (–)clau
As mentioned above, (–)clau improved learning and memory in 10 models of memory impairment and increased synaptic plasticity in anesthetized or freely moving rats. For elucidation of the nootropic mechanism of (–)clau, many studies were conducted and revealed that a moderate increase of [Ca2+]i, stimulation of central cholinergic neurons, and enhancement of synaptogenesis are important factors in inducing the nootropic effects of (–)clau.
4.1. (–)Clau increased [Ca2+]i at a moderate level
Calcium ions are ubiquitous intracellular second messengers that act as key regulators of innumerable processes, such as the control of cell growth and differentiation, maintenance of cytoskeletal integrity, learning, memory and synaptic activity.
There are many mechanisms by which calcium levels are modulated, for example, calcium influx and accumulation, Ca2+ clearance, cytoplasmic Ca2+ buffering, and controlling the temporal and special distribution of Ca2+ in the neurons. These mechanisms maintain [Ca2+]i at an extremely low level, so that a relatively small or localized change in [Ca2+]i can be used by the cell as a signal to trigger distinct physiological processes. For a drug, elevation of [Ca2+]i to a moderate level is an important effect that might activate neuroprotective pathway. The well-known ability of KCl to promote survival of neurons in cell culture may be explained by the moderate elevation of [Ca2+]i. Several neurotrophic factors such as nerve growth factor (NGF) and brain derived nootropic factor (BDNF) are also known to induce small elevations of [Ca2+]i in neurons in which they promote long term survival and protect against excitotoxic insults9, 10.
In our investigation of (–)clau-induced Ca2+ signaling in primary cultures of cortical neurons by using laser confocal microscopy, it was demonstrated that (–)clau induced only a small [Ca2+]i change after application of 20 mmol/L Mg2+ extracellularly11. In this condition, (–)clau could improve learning and memory, increase synaptic plasticity in both efficacy and structure, increase acetylcholine release and activate nootropic signal transduction pathways.
4.2. Stimulation of central cholinergic neurons
The deficits in cognition and memory storage observed in age-associated memory impairment (AAMI) and AD are associated with a deficiency of the central cholinergic system. Thus, cholinergic stimulants may be therapeutically used in the treatment of AD patients. It was demonstrated that a single administration of (–)clau (10, 20, 50 ng/kg) significantly ameliorated the reduction of acetylcholine induced by anisodine (a M-cholinergic receptor antagonist, 10 mg/kg i.p.) in a dose-dependent manner. But (+)clau had no effect on acetylcholine levels when administered in similar doses. Further study showed that (–)clau increased choline acetyltransferase (ChAT) activity in cortex, hippocampus and striatum significantly. As ChAT is a key enzyme for synthesis of Ach, the protective effect of (–)clau on Ach reduction by anisodine is likely the result of increased Ach synthesis and release12, 13. Furthermore, (–)clau exhibited neurotrophic action, i.e., stimulated the proliferation of cholinergic neurons and supported survival and neurite outgrowth of cholinergic neurons. (–)Clau and NGF had a similar neurotrophic action but were mediated by different mechanisms.
4.3. (–)Clau increased synaptogenesis
Basic and clinical studies in recent years have shown that senile plaque and neurofibrillary tangle are not related to the degree of cognitive decline. Synaptic loss occurs in all kinds of dementias and other neurodegenerative diseases. Synaptic loss suspended signal deliver and transduction, damaged the receptors, ion channels and proteins contained in front and posterior synaptic membranes, leading to neuropsychological dysfunction, memory deficit and dementia. Therefore, synaptic loss was considered as a main cause of dementia, and even some scientists proposed that ‘synaptic loss=AD’. For this reason, the new strategy for treatment of AD is limiting synaptic loss and increasing synaptogenesis14, 15.
In order to ascertain the basis of the morphology of nootropic action of (–)clau, the effect of (–)clau on brain development was studied. (–)Clau at dosages of 5 and 10 mg/kg by gavage to mice once a day for 4 weeks facilitated learning and memory acquisition in step-down and step-through tests and increased the thickness of cerebral cortex, and increased synapse density significantly in the dentate cells over pyramidal cells in the hippocampal region, based on a quantitative technique of synapse analysis. In another study, (–)clau increased mossy fiber sprouting and the expression of growth-associate protein (GAP-43). Synapses are the essential structure in the central nervous system (CNS) through which signals are transmitted, processed and integrated among neurons. (–)Clau increased synaptic plasticity in both efficacy and structure which provide morphological and physiological evidence to support nootropic effects of (–)clau.
5. Activation of learning and memory signal transduction induced by (–)clau
Study of the learning and memory signal transduction pathway is essential for elucidation of nootropic mechanisms. This study can provide us much information, for example, in finding a target receptor, genes that encode key proteins, crosstalk between different signaling cascades, explanation for long term memory formation at the molecular level, and so on. There are two kinds of nootropic signaling pathways induced by (–)clau.
5.1. Phospholipase C–protein kinase C (PLC–PKC)-mediated signaling pathway
Studies have shown that (–)clau has no specific binding to NMDA receptors, but it could increase NMDA receptor density and glutamate release. Furthermore, (–)clau was shown to be a potassium channel antagonist which blocks potassium efflux and induced membrane depolarization. The latter effect could remove Mg2+ around NMDA receptors, promoting glutamate binding to the NMDA receptor. After ligand-induced receptor activation, the signal transduction was initiated beginning with the activation of PLC via G protein. PLC hydrolyzes phosphatidylinositol 4,5-bisphosphate (PIP2) into inositol 1,4,5-trisphosphate (IP3) and diacyl glycerol (DAG), the latter activates PKC which in turn phosphorylates a series of protein kinases including cAMP-response element binding protein (CREB) in nuclei. Phosphorylated CREB transcribed memory-related genes. In this study, the gene products were zif/268 and BDNF.
5.2. CaMKII–ERK mediated signaling pathway
Britain in 1997 reported that mitogen-activated protein kinase kinase (MAPKK) played a role in modulating synaptic plasticity. Extracellular-regulated protein kinase (ERK) was regulated by several protein kinases upstream, such as protein kinase A (PKA), PKC, calmodulin-dependent protein kinase (CaMK) II, CaMK IV, etc. Among them, CaMK II is the main protein contained in postsynaptic density (PSD) preparations, reaching 20–40%. (–)Clau (10−5 mol/L) increased the population spike (PS) of the hippocampal dentate gyrus, with peaks at 5 and 30 min. (–)Clau also promoted the transient enhancement of upstream Ca2+ and activated CaMKII at the 2nd minute in vitro. The downstream protein, CREB was phosphorylated at the 9th minute. Pretreatment with KN93, a CaMKII antagonist and PD98059, a ERK inhibitor, could partially block the (–)clau-induced LTP. The final gene products were zif/268 and BDNF, which are responsible for long term memory formation and LTP.
6. Preclinical safety evaluation
For evaluating the safety of (–)clau, acute and chronic toxicity were carried out. In mice, the LD50 of (–)clau by oral administration was 5290 mg/kg. Rats and beagle dogs were used in a chronic study for 7 months. Results with rats showed that there was no apparent toxicity at the dose of 40 and 80 mg/kg. (–)Clau produced mild toxicity at a dose of 160 or 80 mg/kg, as indicated by hepatic steatosis and focal necrosis. These pathological changes could be reversed after drug withdrawal. The ‘no observed effect level’ (NOEL) in rats (80 mg/kg) was equal to 48 times and 7.9 times the recommended clinical dosage, according to a body weight and body surface area conversion chart. In beagle dogs (–)clau showed minor toxicity as indicated by increased serum urea nitrogen and the level of ketone in the urine. The NOEL was 60 mg/kg which was equivalent to 21 times the recommended clinical dosage. All of these studies indicated that (–)clau is a safe candidate for the treatment of dementia.
7. The pharmacokinetics of clau
The pharmacokinetics of clau enantiomers and their metabolites were investigated in Wistar rats and beagle dogs. After intravenous and oral administration of (–) and (+)clau, their major metabolites were determined simultaneously by reverse-phase HPLC and HPLC–MS/MS. As the pharmacologic effects of clau were investigated in rats, the pharmacokinetic profiles of clau in rats were reported here16, 17, 18.
7.1. Main pharmacokinetic parameters
After intravenous and oral administration at dose of 80 and 160 mg/kg of each enantiomer, plasma concentrations of (–) and (+)clau and its major metabolites were determined simultaneously by reverse-phase HPLC with UV detection. The mean plasma levels of (+)clau were higher at all time points than those of (–)clau. (+)Clau exhibited greater Tmax, Cmax, t1/2β, AUC0–12 h, AUC0–∞ and smaller CL (or CL/F) and Vd (or Vd/F) than its antipode, implying that the absorption, distribution and elimination of (–)clau were more rapid than those of (+)clau.
7.2. Tissue distribution
Fifteen min after oral administration, (–) and (+)clau were found in brain, liver, intestine, stomach, ovary, testis, spleen, kidney, heart, fat, muscle, lung and spinal cord. The content of (–)clau in target tissues (hippocampus, cortex, cerebellum) was higher than that of (+)clau. But at 4 h post-dose, the results were reversed.
7.3. Plasma protein binding
When (–)clau at concentrations of 5 and 20 μmol/L were added to rat plasma, the mean percentage of (–)clau in the binding form was 28.5±0.6%, while the mean percentage of (+)clau was 38.05±2.6%, which was significantly higher than that of its antipode. But similar results were not observed in rabbit plasma, in which the bound fractions of both (+) and (–)clau were higher than that in rat plasma. There were significant species difference in the binding of (–)clau between rabbits (47.2±4.9%) and rat (28.5±0.6%).
7.4. Metabolites of clau
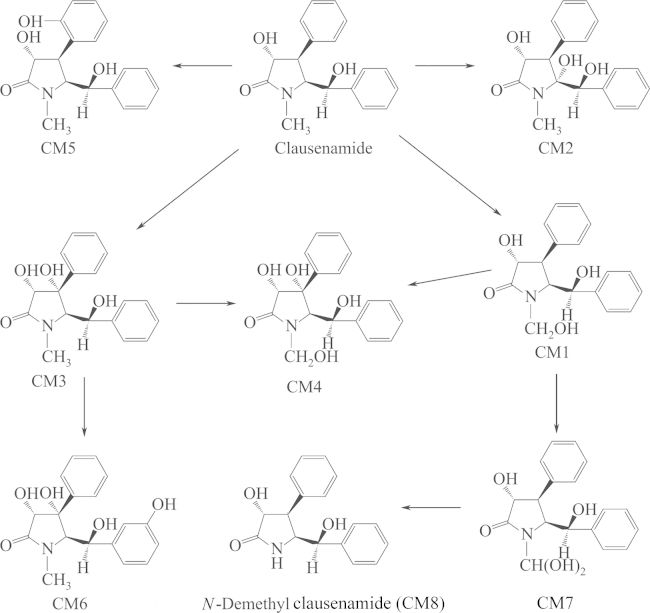
Metabolites of (–) and (+)clau in urine, feces, bile and blood were detected using RP-HPLC–DAD. Eight metabolites, including 6-hydroxyl-clau (CM1), 5-hydroxyl-clau (CM2), 4-hydroxyl-clau (CM3), 4,6-dihydroxyl-clau (CM4), 4-phenyl-m-hydroxyl-clau (CM5), 4,7-phenyl-m-hydroxyl-clau (CM6), 3-dihydroxyl-clau (CM7) and N-demethyl-clau (CM8) were found and identified. The 8 main metabolic routes are shown in Fig. 4. The main metabolite of (–)clau was 7-hydroxyl-clau, while the main metabolite of (+)clau was 4-hydroxyl-clau.
Figure 4.
The main metabolites of (–) and (+)clau.
7.5. Elimination of clau
Sixty hour after administration of single dose of each enantiomer (80 mg/kg), bile, urine and feces of rats were collected and analyzed by HPLC. Results revealed that (–)clau and its metabolites were excreted via gastrointestinal route and (+)clau and its metabolites via biliary route. The total percentage of three excretory pathways was 20% for (–)clau and 25.9% for (+)clau, suggesting that the majority of parent drug may be transferred into metabolites which were expelled from the body or further transferred into other forms of metabolites.
7.6. Metabolism of clau enantiomers
To identify which cytochrome P450 (CYP) isoform(s) are responsible for the metabolism of clau enantiomers in rats, the effect of various CYP isoform inducers and inhibitors on the formation of clau metabolites was investigated with liver microsomes. Induction and inhibition studies suggested that CYP3A was the predominant isoform responsible for the metabolism of clau enantiomers (Fig. 4).
8. Conclusions
In conclusion, synthetic (–)clau, one of the optical isomers of clau isolated from C. lansium, is a new type of anti-dementia agent, characteristic by improved learning and memory in normal and memory impairment rodents, increased synaptic plasticity in both efficacy and structure, and decreased apoptosis with high phosphorylation of tau protein. Owing to this multi-target effect, the low toxicity and unique mechanism of action suggests that it is a promising drug for treatment of neurodegenerative diseases and many kinds of memory impairment.
Footnotes
Peer review under responsibility of Institute of Materia Medica, Chinese Academy of Medical Sciences and Chinese Pharmaceutical Association.
References
- 1.Hu J.F., Chu S.F., Ning N., Yuan Y.H., Xue W., Chen N.H., et al. Protective effect of (–)clausenamide against Abeta-induced neurotoxicity in differentiated PC12 cells. Neurosci Lett. 2010;483:78–82. doi: 10.1016/j.neulet.2010.07.067. [DOI] [PubMed] [Google Scholar]
- 2.Zhang J., Cheng Y., Zhang J.T. Protective effect of (–)clausenamide against neurotoxicity induced by okadaic acid and beta-amyloid peptide 25–35. Acta Pharm Sin. 2007;42:935–942. (in Chinese) [PubMed] [Google Scholar]
- 3.Tang K., Zhang J.T. (–)Clausenamide improves long-term potentiation impairment and attenuates apoptosis after transient middle cerebral artery occlusion in rats. Neurol Res. 2003;25:713–717. doi: 10.1179/016164103101202219. [DOI] [PubMed] [Google Scholar]
- 4.Wang X.Y., Zhang J.T. Effect of ginsenoside Rb1 on long-term potentiation in the dentate gyrus of anaesthetized rats. J Asian Nat Prod Res. 2003;5:1–4. doi: 10.1080/10286020290029009. [DOI] [PubMed] [Google Scholar]
- 5.Wang X.Y., Zhang J.T. NO mediates ginsenoside Rg1-induced long-term potentiation in anesthetized rats. Acta Pharmacol Sin. 2001;22:1099–1102. [PubMed] [Google Scholar]
- 6.Xu L., Liu S.L., Zhang J.T. (–)Clausenamide potentiates synaptic transmission in the dentate gyrus of rats. Chirality. 2005;17:239–244. doi: 10.1002/chir.20150. [DOI] [PubMed] [Google Scholar]
- 7.Barritt G.J. Receptor-activated Ca2+ inflow in animal cells: a variety of pathways tailored to meet different intracellular Ca2+ signalling requirements. Biochem J. 1999;337:153–169. [PMC free article] [PubMed] [Google Scholar]
- 8.Berridge M.J. Neuronal calcium signaling. Neuron. 1998;21:13–26. doi: 10.1016/s0896-6273(00)80510-3. [DOI] [PubMed] [Google Scholar]
- 9.Tang K., Zhang J.T. Mechanism of (–)clausenamide induced calcium transient in primary culture of rat cortical neurons. Life Sci. 2004;74:1427–1434. doi: 10.1016/j.lfs.2003.08.009. [DOI] [PubMed] [Google Scholar]
- 10.Duan W.Z., Zhang J.T. Stimulation of central cholinergic neurons by (–)clausenamide in vitro. Acta Pharmacol Sin. 1998;19:332–336. (in Chinese) [PubMed] [Google Scholar]
- 11.Duan W.Z., Zhang J.T. Effects of (–), (+)clausenamide on anisodine-induced acetylcholine decrease and associated memory deficits in the mouse brain. Acta Pharm Sin. 1998;33:259–263. (in Chinese) [PubMed] [Google Scholar]
- 12.Bayer A.J., Bullock R., Jones R.W., Wilkinson D., Paterson K.R., Jenkins L., et al. Evaluation of the safety and immunogenicity of synthetic Aβ42 (AN1792) in patients with AD. Neurology. 2005;64:94–101. doi: 10.1212/01.WNL.0000148604.77591.67. [DOI] [PubMed] [Google Scholar]
- 13.Selkoe D.J. Alzheimer׳s disease is a synaptic failure. Science. 2002;298:789–791. doi: 10.1126/science.1074069. [DOI] [PubMed] [Google Scholar]
- 14.Ning N., Hu J.F., Sun J.D., Han N., Zhang J.T., Chen N.H. (–)Clausenamide facilitates synaptic transmission at hippocampal Schaffer collateral-CA1 synapses. Eur J Pharmacol. 2012;682:50–55. doi: 10.1016/j.ejphar.2012.02.004. [DOI] [PubMed] [Google Scholar]
- 15.Hu J.F., Niu F., Ning N., Duan W.Z., Chu S.F., Xue W., et al. Activation of ERK1/2–CREB pathway during potentiating synaptic transmission of (–)clausenamide in rat dentate gyrus. J Asian Nat Prod Res. 2012;14:256–262. doi: 10.1080/10286020.2011.650885. [DOI] [PubMed] [Google Scholar]
- 16.Zhu C.J., Zhang J.T. Stereoselective plasma protein binding and target tissue distribution of clausenamide enantiomers in rats. Chirality. 2009;21:402–406. doi: 10.1002/chir.20623. [DOI] [PubMed] [Google Scholar]
- 17.Zhu C.J., Zhang J.T. Stereoselective pharmacokinetics of clausenamide enantiomers and their major metabolites after single intravenous and oral administration to rats. Chirality. 2003;15:668–673. doi: 10.1002/chir.10278. [DOI] [PubMed] [Google Scholar]
- 18.Zhu C.J., Zhang J.T. Identification of rat cytochrome P450 forms involved in the metabolism of clausenamide enantiomers. Chirality. 2003;15:448–455. doi: 10.1002/chir.10228. [DOI] [PubMed] [Google Scholar]