Abstract
Ischemic stroke and ischemia/reperfusion (I/R) injury induced by thrombolytic therapy are conditions with high mortality and serious long-term physical and cognitive disabilities. They have a major impact on global public health. These disorders are associated with multiple insults to the cerebral microcirculation, including reactive oxygen species (ROS) overproduction, leukocyte adhesion and infiltration, brain blood barrier (BBB) disruption, and capillary hypoperfusion, ultimately resulting in tissue edema, hemorrhage, brain injury and delayed neuron damage. Traditional Chinese medicine (TCM) has been used in China, Korea, Japan and other Asian countries for treatment of a wide range of diseases. In China, the usage of compound TCM preparation to treat cerebrovascular diseases dates back to the Han Dynasty. Even thousands of years earlier, the medical formulary recorded many classical prescriptions for treating cerebral I/R-related diseases. This review summarizes current information and underlying mechanisms regarding the ameliorating effects of compound TCM preparation, Chinese materia medica, and active components on I/R-induced cerebral microcirculatory disturbances, brain injury and neuron damage.
KEY WORDS: Ischemia/reperfusion, Antioxidant, Leukocyte adhesion, Hyperpermeability, Brain blood barrier, Neuron
Abbreviations: AIF, apoptosis inducing factor; AMPA, α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid; AP-1, activator protein-1; Asp, aspartate; BBB, brain blood barrier; bFGF, basic fibroblast growth factor; BMEC, brain microvascular endothelial cell; BNDF, brain-derived neurotrophic factor; cAMP, cyclic adenosine monophosphate; Cav-1, caveolin-1; CAT, catalase; CBF, cerebral blood flow; COX-2, cyclooxygenase-2; DHR, dihydrorhodamine 123; DPPH, 1,1-diphenyl-2-picrylhydrazyl radical 2,2-diphenyl-1-(2,4,6-trinitrophenyl) hydrazyl; ERK, extracellular signal-regulated kinase; GABA, γ-aminobutyric acid; Glu, glutamate; Gly, glysine; GRK2, G protein-coupled receptor kinase 2; GSH, glutathione; GSH-Px, glutathione peroxidase; GSSH, glutathione disulfide; HE, hematoxylin and eosin; HIF, hypoxia-inducible factor; HPLC, high performance liquid chromatography; hs-CRP, high-sensitivity C-reactive protein; I/R, ischemia-reperfusion; I-κBα, Inhibitory κBα; ICAM-1, intercellular adhesion molecule-1; IL-1β, interleukin-1β; IL-8, interleukin-8; IL-10, interleukin-10; iNOS, inducible nitric oxide synthase; JAM-1, junctional adhesion molecule-1; JNK, Jun N-terminal kinase; LDH, lactate dehydrogenase; MAPK, mitogen activated protein kinase; MCAO, middle cerebral artery occlusion; MDA, malondialdehyde; MMPs, matrix metalloproteinases; MPO, myeloperoxidase; MRI, magnetic resonance imaging; NADPH, nicotinamide adenine dinucleotide phosphate; NGF, nerve growth factor; NMDA, N-methyl-d-aspartic acid; NF-κB, nuclear factor κ-B; NO, nitric oxide; NSC, neural stem cells; OGD, oxygen-glucose deprivation; 8-OHdG, 8-hydroxydeoxyguanosine; PARP, poly-ADP-ribose polymerase; PMN, polymorphonuclear; RANTES, regulated upon activation normal T-cell expressed and secreted; ROS, reactive oxygen species; rtPA, recombinant tissue plasminogen activator; SFDA, state food and drug administration; SOD, superoxide dismutase; TBARS, thiobarbituric acid reactive substance; TCM, traditional Chinese medicine; TGF-β1, transforming growth factor β1; TIMP-1, tissue inhibitor of metalloproteinase-1; TNF-α, tissue necrosis factor-α; TTC, 2,3,5-triphenyltetrazolium chloride; Tuj-1, class III β-tublin; TUNEL, terminal-deoxynucleoitidyl transferase mediated nick end labeling; VCAM-1, vascular adhesion molecule-1; VEGF, vascular endothelial growth factor; ZO-1, zonula occludens-1
Graphical abstract
TCM preparation, Chinese materia medica and their active compounds show potential to ameliorate I/R-induced cerebral microcirculatory disturbances, brain injury and neuron damage with anti-inflammation, anti-oxidation, anti-apoptosis, anti-excitoxicity and pro-neurogenesis as the major underlying mechanisms.
1. Introduction
Stroke is the second leading cause of mortality in the world, resulting in 6,671,000 deaths (11.9% of all deaths) in 20121. Pathogenically, ischemic stroke caused by vessel occlusions accounts for 85% of all conditions. Since the core of brain tissue undergoes necrotic cell death within a few minutes of the onset of cerebral ischemia, early restoration of blood flow by thrombolytic therapy decreases morbidity and mortality in these patients2. Paradoxically, reperfusion itself evokes additional injury to ischemic penumbra, a region bordering the infarct core, causing the so-called ischemia-reperfusion (I/R) injury. Such injury exacerbates brain damage, leading to increases in severe morbidity in surviving victims. I/R imposes multiple insults to the cerebral microcirculation, including reactive oxygen species (ROS) outburst, inflammatory mediator overproduction, leukocyte infiltration, microvessel hyperpermeabilty, brain blood barrier (BBB) disruption, capillary hypoperfusion, etc. Many of these factors are thought to play significant roles in the pathogenesis of post-ischemic injury in stroke patients3. Much effort has been made to attenuate the microcirculatory disturbances by ablating a single factor in the pathogenesis, including introduction of recombinant tissue plasminogen activator (rtPA)4, 5, antioxidants6, anti-intercellular adhesion molecule-1 (ICAM-1) antibody7, calcium-stabilizing agents8 and anti-excitotoxic agents9. However, clinical trials have failed to show positive effects in patients with ischemic stroke. Other therapeutic approaches, such as anti-inflammatory10 and anti-apoptotic11 agents, are being evaluated, but no successful clinical trials have thus far been reported. These results suggest that such microcirculatory disturbances are part of a complicated pathological process involving multiple, coordinated events. Once initiated, the process may only be interrupted by a remedy consisting of multiple compositions that target the underlying insults.
For more than two thousand years, traditional Chinese medicine (TCM) has been used in China, Korea, Japan and other Asian countries for the clinical treatment of cerebrovascular diseases including stroke, encephalitis, dizziness, insomnia, amnesia, and dementia. In China, the use of compound TCM preparations to treat cerebrovascular diseases dates back to the Han Dynasty. “Treatise on Cold Damage (Shang Han Lun)” and “Synopsis of the Golden Chamber (Jin Kui Yao Lue)” appeared at that time which recorded several classical formulas, including Chaihu Jia Longgu Muli Tang, Guizhi Fuling Wan, Gualou Guizhi Tang, and others, which were devoted to cope with cerebrovascular diseases. In the Tang dynasty, medical formulas were further developed, as shown by “Important Prescriptions Worth a Thousand Gold for Emergency (Qian Jin Fang)” and “Arcane Essentials from the Imperial Library (Wai Tai Mi Yao)”; these documented the use of Xiaoxuming Tang, Dihuang Yinzi and Huanglian Jiedu Tang for the treatment of cerebrovascular diseases.
In the dynasties that followed, more compound TCM preparations were used in clinic. In the Song dynasty, treatments included Sijunzi Tang and Longdan Xiegan Tang, documented from “Formulary of the Bureau Taiping People׳s Welfare Pharmacy (Tai Ping Hui Min He Ji Ju Fang)”. In the Yuan dynasty, medicines included Shengmai San, as reported in “Revelation of Medicine (Yi Xue Qi Yuan)”. In the Jin dynasty, the use of Chaihu Shugan San was mentioned in “Jing-Yue׳s Collected Works (Jing Yue Quan Shu)”. Finally, in the Qing dynasty, Buyang Huanwu Tang and Taohong Siwu Tang were used according to “Correction on Errors in Medical Works (Yi Lin Gai Cuo)” and “Golden Mirror of the Medical Ancestors (Yi Zong Jin Jian)”, respectively.
Nowadays in China, several new compound TCM preparations have been formulated for the treatment of cerebrovascular diseases. Based on classical formulas and approved by the State Food and Drug Administration (SFDA), these include Cerebralcare Granule® (Yangxue Qingnao granule), Tongxinluo capsule, Shenfu injection, Danhong injection, Huatuo Zaizao extractum. The name, composition and origin of the compound TCM preparations that are derived from TCM literatures or approved by the SFDA and cited in the present review are listed in Table 1. In addition, Chinese materia medica as well as active ingredients and components included in compound TCM preparations are listed in Table 2. These represent substances of recent research interest as related to their possible roles in the pathogenesis of I/R-induced brain injury, neuron damage and the underlying mechanisms. The present review is based on 139 references published from 1995 to 2014, mainly focusing on the ameliorating effects and underlying mechanisms of TCM preparations, Chinese materia medica, and active compounds on I/R-induced cerebral microcirculatory disturbances, brain and neuron damage.
Table 1.
The name, composition and origin of compound TCM preparations.
| Compound TCM preparation | Composition | Origin |
|---|---|---|
| Buyang Huanwu decoction | Huangqi (Radix Astragali seu Hedysari), Danggui (Radix Angelicae Sinensis), Chishao (Radix Paeoniae Rubra), Dilong (Lumbricus), Chuanxiong (Rhizoma Ligustici Chuanxiong), Honghua (Flos Carthami), Taoren (Semen Persicae) | “Correction on Errors in Medical Works” (Qing dynasty) |
| Chaihu Jia Longgu Muli Tang | Chaihu (Radix Bupleuri), Longgu (Os Draconis), Huangqin (Radix Scutellariae), Shengjiang (Rhizoma Zingiberis), Qiandan (Minium), Renshen (Radix Ginseng), Guizhi (Ramulus Cinnamomi), Fuling (Poria), Banxia (Rhizoma Pinelliae), Dahuang (Radix et Rhizoma Rhei), Muli (Concha Ostreae), Dazao (Fructus Jujubae) | “Treatise on Cold Damage” (Han dynasty) |
| Danhong injection | Danshen (Radix Salviae Miltiorrhizae), Honghua (Flos Carthami) | Approved by SFDA (Z20026866) |
| Dihuang Yinzi | Shengdihuang (Radix Rehmanniae Recens), Lugen (Rhizoma Phragmitis), Maidong (Radix Ophiopogonis), Renshen (Radix Ginseng), Baimi (Mel), Chenpi (Pericarpium Citri Reticulatae), Shengjiang (Rhizoma Zingiberis) | “Arcane Essentials from the Imperial Library” (Tang dynasty) |
| Fufang Danggui injection | Danggui (Radix Angelicae Sinensis), Chuanxiong (Rhizoma Ligustici Chuanxiong), Honghua (Flos Carthami) | Approved by SFDA (Z42021410) |
| Gualou Guizhi Tang | Gualou (Fructus Trichosanthis), Guizhi (Ramulus Cinnamomi), Baishao (Radix Paeoniae Alba), Gancao (Radix Glycyrrhizae), Shengjiang (Rhizoma Zingiberis), Dazao (Fructus Jujubae) | “Synopsis of the Golden Chamber” (Han dynasty) |
| Guizhi Fuling Wan | Guizhi (Ramulus Cinnamomi), Fuling (Poria), Gancao (Radix Glycyrrhizae), Mudanpi (Cortex Moutan Radicis), Chishao (Radix Paeoniae Rubra), Taoren (Semen Persicae) | “Synopsis of the Golden Chamber” (Han dynasty) |
| Huatuo Zaizao extractum | Danggui (Radix Angelicae Sinensis), Chuanxiong (Rhizoma Ligustici Chuanxiong), Bingpian (Bomeolum Syntheticum), Baishao (Radix Paeoniae Alba), Renshen (Radix Ginseng), Wuweizi (Fructus Schisandrae Chinensis), Maqianzi (Semen Strychni), Honghua (Flos Carthami), Tiannanxing (Rhizoma Arisaematis) | Approved by SFDA (Z44020748) |
| Huanshaodan decoction | Shudihuang (Radix Rehmanniae Preparata), Shanzhuyu (Fructus Corni), Shanyao (Rhizoma Dioscoreae), Gouqizi (Fructus Lycii), Duzhong (Cortex Eucommiae), Bajitian (Radix Morindae Officinalis), Roucongrong (Herba Cistanches), Wuweizi (Fructus Schisandrae Chinensis), Xiaohuixiang (Fructus Foeniculi), Chushizi (Fructus Broussonetiae), Niuxi (Radix Cyathulae), Fuling (Poria) | Approved by SFDA (Z50020189) |
| Huanglian Jiedu Tang | Huangqin (Radix Scutellariae), Huanglian (Rhizoma Coptidis), Huangbai (Cortex Phellodendri), Zhizi (Fructus Gardenia) | “Arcane Essentials from the Imperial Library” (Tang dynasty) |
| Naoshuantong capsule | Puhuang (Pollen Typhae), Chishao (Radix Paeoniae Rubra), Yujin (Radix Curcumae), Tianma (Rhizoma Gastrodiae), Loulu (Radix Rhapontici) | Approved by SFDA (Z20040093) |
| Shenfu injection | Renshen (Radix Ginseng), Fuzi (Radix Aconiti Lateralis Preparata) | Approved by SFDA (Z51020664) |
| Shenqi Fuzheng injection | Dangshen (Radix Codonopsis), Huangqi (Radix Astragali seu Hedysari) | Approved by SFDA (Z19990065) |
| Shengmai San | Renshen (Radix Ginseng), Maidong (Radix Ophiopogonis), Wuweizi (Fructus Schisandrae Chinensis) | “Revelation of Medicine” (Jin dynasty) |
| Taohong Siwu Tang | Shudihuang (Radix Rehmanniae Preparata), Danggui (Radix Angelicae Sinensis), Baishao (Radix Paeoniae Alba), Chuanxiong (Rhizoma Ligustici Chuanxiong), Taoren (Semen Persicae), Honghua (Flos Carthami) | “Golden Mirror of the Medical Ancestors” (Qing dynasty) |
| Tianma Gouteng granule | Tianma (Rhizoma Gastrodiae), Gouteng (Ramulus Uncariae cum Uncis), Shijueming (Concha Haliotidis), Zhizi (Fructus Gardeniae), Huangqin (Radix Scutellariae), Niuxi (Radix Cyathulae), Duzhong (Cortex Eucommiae),Yimucao (Herba Leonuri), Sangjisheng (Herba Taxilli), Shouwuteng (Caulis Polygoni Multiflori), Fuling (Poria) | Approved by SFDA (Z51021084) |
| Tongxinluo capsule | Renshen (Radix Ginseng), Shuizhi (Hirudo), Quanxie (Scorpio), Chishao (Radix Paeoniae Rubra), Chantui (Periostracum Cicadae), Tubiechong (Eupolyphaga seu Steleophaga), Wugong (Scolopendra), Tanxiang (Lignum Santali Albi), Jiangxiang (Lignum Dalbergiae Odoriferae), Ruxiang (Olibanum), Suanzaoren (Semen Ziziphi Spinosae), Bingpian (Bomeolum Syntheticum) | Approved by SFDA (Z19980015) |
| Xiaoxuming decoction | Mahuang (Herba Ephedrae), Guizhi (Ramulus Cinnamomi), Fangfeng (Radix Saposhnikoviae), Fangji (Radix Stephaniae Tetrandrae), Xingren (Semen Armeniacae Amarum), Huangqin (Radix Scutellariae), Renshen (Radix Ginseng), Gancao (Radix Glycyrrhizae), Dazao (Fructus Jujubae), Chuanxiong (Rhizoma Ligustici Chuanxiong), Baishao (Radix Paeoniae Alba), Fuzi (Radix Aconiti Lateralis Preparata), Shengjiang (Rhizoma Zingiberis) | “Important Prescriptions Worth a Thousand Gold for Emergency” (Tang dynasty) |
| Xingnaojing injection | Shexiang (Moschus), Yujin (Radix Curcumae), Bingpian (Bomeolum Syntheticum), Zhizi (Fructus Gardeniae) | Approved by SFDA (Z53021638) |
| Cerebralcare Granule®(Yangxue Qingnao granule) | Danggui (Radix Angelicae Sinensis), Chuanxiong (Rhizoma Ligustici Chuanxiong), Baishao (Radix Paeoniae Alba), Shudihuang (Radix Rehmanniae Preparata), Gouteng (Ramulus Uncariae cum Uncis), Jixueteng (Caulis Spatholobi), Xiakucao (Spica Prunellae), Juemingzi (Semen Cassiae), Zhenzhumu (Concha Margaritifera), Yanhusuo (Rhizoma Corydalis), Xixin (Herba Asari) | Approved by SFDA (Z10960082) |
Table 2.
The structures and sources of active components of Chinese materia medica.
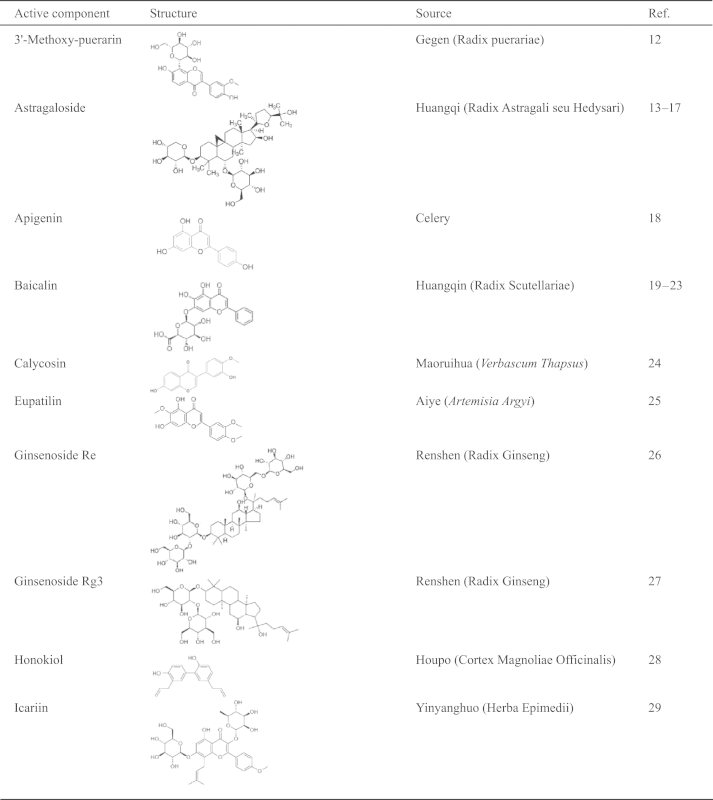 |
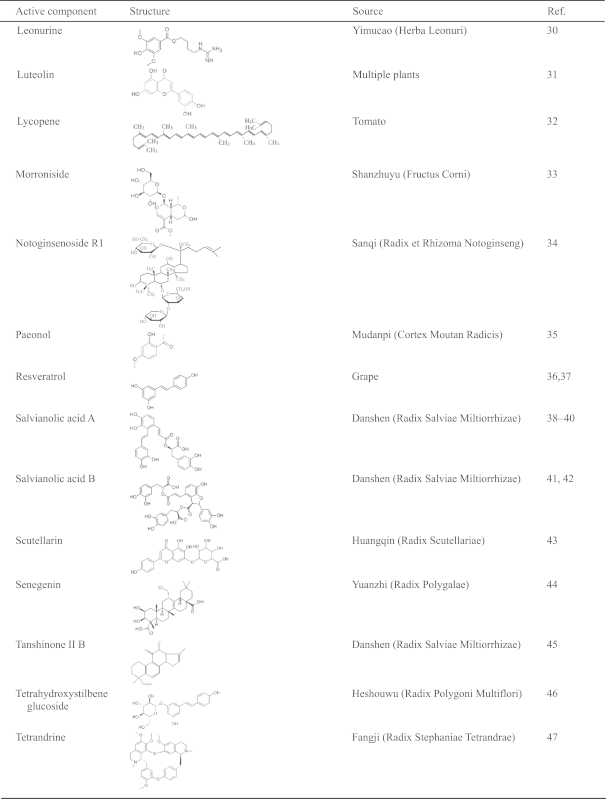 |
2. Effects of TCM preparation, Chinese materia medica, and active compounds on pathogenesis of cerebral microcirculatory disturbances induced by I/R
2.1. Oxidative stress
Significant amounts of ROS are generated during cerebral I/R, which are widely regarded as the initial step in brain damage after stroke. Numerous clinical and experimental observations have shown increased ROS formation during all forms of stroke injury. ROS are highly active and able to react with DNA, protein, and lipid directly, causing damage and dysfunction of the molecules to various degree48. The primary source of ROS during I/R injury is the mitochondria, which produce superoxide anion radicals during the electron transport process. Other potentially important sources of ROS include xanthine oxidase, cyclooxygenase, lipooxygenase, and others, depending on cell types. Oxygen free radicals can also be generated by activated microglia and infiltrating leukocytes via the nicotinamide adenine dinucleotide phosphate (NADPH) oxidase pathway following reperfusion49. In recent studies, researchers found that some compound TCM preparations, Chinese materia medica and active components produce positive effects on cerebrovascular diseases partially due to their antioxidant properties. Using dihydrorhodamine 123 (DHR), a hydrogen peroxide-sensitive mitochondrial probe, researchers demonstrated in vivo that compound TCM preparations, such as Cerebralcare Granule® attenuates DHR fluorescence intensity in gerbil cerebral microvessels, in either the early phase (60 min) or late phase (5 days) of reperfusion after global ischemia50, 51. The single active component notoginsenoside R1 was found to prevent oxidative stress by suppressing both mitochondrial and NADPH oxidase-dependent superoxide generation and inhibiting production of malondialdehyde (MDA), protein carbonyl, and 8-hydroxydeoxyguanosine (8-OHdG) in rat with middle cerebral artery occlusion (MCAO) and reperfusion in vivo34. This compound also had antioxidant activity in primary cortical neurons stimulated by oxygen-glucose deprivation (OGD) followed by reoxygenation in vitro34. Other compound TCM preparations, such as Yiqi Tongluo Jiedu capsule52, as well as several active components from Chinese materia medica (including total glycoside from Chishao, Radix Paeoniae Rubra53, astragaloside13, 14, and tetrahydroxystilbene glucoside46) alleviated ROS production in cerebral tissue via inhibiting inducible nitric oxide synthase (iNOS) activation and nitric oxide (NO) overproduction. Other experiments reported that Chinese materia medica Danshen (Radix Salviae Miltiorrhizae)54 and active component baicalin19 exert their antioxidant effect by lowering adenosine metabolites hypoxanthine and inhibiting cyclooxygenase, respectively. In addition to ROS source regulation, TCM preparation, Chinese materia medica and active compounds ameliorate I/R-induced oxidative stress by scavenging free radicals directly or by modulating tissue anti-oxidant potency. In a recently published study, three classical formulas (Huanglian Jiedu Tang, Chaihu Jia Longgu Muli Tang, and Guizhi Fuling Wan) showed scavenging activity for free-radical superoxide anion radicals, hydroxyl radicals and 1,1-diphenyl-2-picrylhydrazyl radical 2,2-diphenyl-1-(2,4,6-trinitrophenyl) hydrazyl (DPPH), respectively, while suppressing lipid peroxidation55. Another study reported that the single active component salvianolic acid A reduces the MDA contents in the cortex, hippocampus and corpus striatum in I/R rat brain, which may also attribute to its scavenging effect on free hydroxyl radicals in vitro38. Shengmai San, another classical formula, improved oxidative damage manifested as suppression of MDA and thiobarbituric acid reactive substance (TBARS) formation and increased superoxide dismutase (SOD) and glutathione peroxidase (GSH-Px) activities in brain tissues from rodent global or MCAO models. This treatment was active whether given before ischemia or after re-perfusion56, 57, 58. In parallel, aqueous or ethanolic extract from Baihuasuantengguo (Embelia ribes Burm) increased the glutathione (GSH), GSH-Px, glutathione reductase and glutathione-S-transferase levels in both hippocampus and frontal cortex with decreases in lactate dehydrogenase (LDH) level in serum and TBARS levels in hippocampus and frontal cortex in rat after MCAO59, 60. Lavender oil was also found to reduce the levels of mitochondria-generated ROS, MDA and carbonyl, and to upregulate the ratio of GSH/glutathione disulfide (GSSG), the activities of SOD, catalase (CAT) and GSH-Px in brain tissue after focal ischemia61. Similar antioxidant effects have been reported for some other TCM preparation, such as Tongxinluo capsule62, Yimucao (Herba Leonuri) injection63, and active components, such as Yinxing (Folium Ginkgo) extract64, paeonol35 and 3′-methoxy-puerarin12.
2.2. Inflammatory mediators
Inflammation is increasingly recognized to be the key element in the pathological progression of ischemic stroke, as early inflammatory responses may potentiate reperfusion injury in post-ischemic brain tissue. There are several resident cell populations within brain tissue that are able to secrete proinflammatory mediators after an ischemic insult, including endothelial cells, leukocytes, astrocytes, microglia and neurons. Activation of transcription factors via nuclear factor κ-B (NF-κB), mitogen activated protein kinase (MAPK), and activator protein-1 (AP-1) inflammatory signaling pathway causes an increased production of cytokines, chemokines, adhesion molecules and other proinflammatory mediators65. Several agents that can regulate inflammatory mediators or transcription factors reduce infarct size and neurological deficits following focal stroke in rodents; such treatments are considered to be an alternative therapeutic approach in stroke patients66. In this regard, Guizhi Fuling capsules, a classical formula, were reported to down-regulate the expression of pro-inflammatory cytokines [including interleukin-1β (IL-1β) and tissue necrosis factor-α (TNF-α)] and markedly up-regulate the expression of anti-inflammatory cytokines interleukin-10 (IL-10) and IL-10 receptor at both mRNA and protein levels in rats with focal cerebral I/R. The serum levels of these inflammatory cytokines were regulated in the same way67. In other in vivo MCAO experiments, TCM Naomaitong preparation68 and the single active component tetrandrine47 have proven to decrease the expression and mRNA level of TNF-α, vascular adhesion molecule-1 (VCAM-1), ICAM-1 in brain tissue 1 or 3 days after reperfusion, partly due to suppressing NF-κB activation. Similar results were found for TCM preparations, such as Taohong Siwu Tang69, FBD formula [a herbal formula composed of Fuling (Poria), Baizhu (Rhizoma Atractylodis Macrocephalae) and Danggui (Radix Angelicae Sinensis)]70, as well as Chinese materia medica Danshen (Radix Salviae miltiorrhizae) aqueous extract71. A broad spectrum of cytokines and chemokines were abrogated by treatment with these TCM, including high-sensitivity C-reactive protein (hs-CRP), hypoxia-inducible factor (HIF)-1α, IL-1β, interleukin-8 (IL-8), TNF-α, iNOS levels in serum or brain, and TNF-α mRNA and transforming growth factor β1 (TGF-β1) expression in cerebral tissue; these effects were partly due to down-regulation of cerebral Inhibitory-κB-α (I-κBα) and NF-κB phosphrylation70. In a global cerebral ischemia model, treatment with the single active component resveratrol before insult reduced astroglial and microglial activation as well as cyclooxygenase-2 (COX-2) and iNOS expression 7 days after I/R. These effects were attributed to suppression of NF-κB and Jun N-terminal kinase (JNK) activation36. In addition to in vivo results, in vitro experiments demonstrated that another active component honokiol reduced TNF-α and NO level in the primary cultured microglia medium and in the microglia and astrocytes co-culture medium. Also, honokiol was shown to decrease the level of RANTES (regulated upon activation normal T-cell expressed and secreted) protein in medium of microglia or astrocytes, which was related to its effect on microglia NF-κB p65 nuclear translocation28.
2.3. Leukocyte infiltration
A growing body of evidence indicates that recruitment of leukocytes contributes to the initiation and evolution of brain injury after ischemic stroke. The adhesion and migration of neutrophils is evident in cerebral venules from several minutes to a few hours following reperfusion. The population of recruited cells shifts from polymorphonuclear (PMN) to mononuclear leukocytes and lymphocytes, and leukocyte recruitment persists for days to weeks following ischemia72, 73. Rodents with reduced PMN or T-lymphocyte accumulation show reduced infarct volumes and improved neurological outcomes. Prevention of leukocyte–endothelial cell adhesion with adhesion molecule antibodies also protects against stroke injury74. In a rat MCAO model, leukocyte adhesion was observed continuously in cerebral microvessels by infusion of rhodamine 6G, and the number of adherent leukocytes after I/R increased immediately and remained increased during 60 min after reperfusion. Pretreatment with TCM preparation Cerebralcare Granule® attenuated this I/R-elicited enhancement of leukocyte adhesion; the effects of the higher dose (0.8 g/kg) were more significant than those following the lower dose (0.4 g/kg)75. Consistently, Cerebralcare Granule® inhibited leukocyte adhesion either during the early phase (60 min) or in the late phase (5 days) of reperfusion after global ischemia in gerbils50, 51. Using 51Cr-labeled neutrophil, researchers also demonstrated that tetrandrine decreased neutrophils recruitment in brain tissue 24 h after reperfusion in rat with MCAO47. The classic formula Huanglian Jiedu Tang and its constituents inhibited myeloperoxidase (MPO) activity, an indication of neutrophil infiltration, in ischemic brain tissue by 30% after focal I/R76. The same result was observed when using other TCM preparations, such as Shengmai San56, Tongxinluo capsule77, and Chinese materia medica Zhimu (Rhizoma Anemarrhenae)78, either in rodent global or focal I/R injury. FBD formula inhibited PMNs infiltration in ICR mouse brain subjected to repetitive 10 min of common carotid arteries occlusion followed 24 h reperfusion, and in vitro results showed that FBD formula could inhibit TNF-α-triggered PMNs adhesion to ECV304 endothelial cells70. In another in vitro experiment, the single active compound salvianolic acid A was proven to inhibit the adherence of granulocytes on brain microvascular endothelial cells (BMEC); the effect was attributed to decreasing the expression of ICAM-1 on BMEC at the gene and protein levels39.
2.4. Brain blood barrier (BBB) disruption
BBB consists of microvascular endothelial cells, basal lamina, pericytes and astrocyte endfeet. Microvessel hyperpermeability disrupts the normal BBB function during cerebral I/R injury. Microvessel permeability is regulated by both paracellular and transcellular pathways79. Paracellular pathways are mainly governed by tight junctions; the loss of tight junction integrity occurs and directly contributes to cerebral BBB disruption under ischemic stroke conditions80. An alternative mechanism for BBB opening involves upregulation of caveolae, including the expression and phosphorylation of the structural component caveolin-1 (Cav-1), as demonstrated by transmission electron microscopy in endothelial cells in several stroke models81. In a rat MCAO model, recent in vivo studies found that both pre- and post-treatment with the TCM preparation Cerebralcare Granule® reduces FITC-labeled albumin leakage from cerebral venules evoked by I/R, in either the early or late phase after reperfusion75, 82; the same results were found in a gerbil global I/R model50, 51. Further study using confocal microscopy revealed that the continuous distributions of tight junction proteins including claudin-5, occludin, junctional adhesion molecule-1 (JAM-1) and zonula occludens-1 (ZO-1) were disrupted after reperfusion for 3 h and 6 days, concomitant with reduced immune staining. Western blotting indicated the degradation of tight junction proteins in response to I/R. Interestingly, these losses in structural integrity were reversed by Cerebralcare Granule® treatment. In addition, I/R-induced increases in the cytoplasmic caveolae of capillary endothelial cells and cerebral Cav-1 expression were both down-regulated by Cerebralcare Granule®, as observed by electron microscopy and Western blotting, respectively. Taken together, these findings suggest involvement of both the paracellular and transcellular pathways in the beneficial effects of Cerebralcare Granule® on BBB disruption following cerebral I/R injury82. Similarly, treatment with the TCM preparation Tongxinluo capsule increased ZO-1 and occludin expression in cerebral microvessels 24 h after MCAO in mice77. In addition, enzymatic degradation of the extracellular matrix by matrix metalloproteinases (MMPs), secretion of vascular endothelial growth factor (VEGF), ROS production, leukocyte infiltration, and inflammatory mediator release within the ischemic core or peri-infarct area have all been postulated to trigger BBB disruption directly or indirectly during I/R process. To this end, TCM preparations such as naomaitong preparation83, and Panax notoginseng saponins combined with astragaloside15, were reported to protect against cerebral microvessel basement membrane injury via modulating gelatinase system, inhibiting MMP-2 and MMP-9 expression and improving tissue inhibitor of metalloproteinase-1 (TIMP-1) protein level in rodent brain tissue after I/R. Using immunohistochemistry, the single active component salvianolic acid B was shown to alleviate the extravasation of immunoglobulin and attenuate MMP-9 expression induced by cerebral I/R, which was related to the inhibition on p38MAPK activation and extracellular signal-regulated kinase (ERK) 1/2 phosphorylation41. Other TCM preparations, such as Weinaokang preparation84 and Huatuo Zaizao extractum85, may also effectively recover BBB ultrastructure injury induced by I/R via inhibiting expressions of MMP-2 and MMP-9, which might be associated with reduction of G protein-coupled receptor kinase 2 (GRK2) in membrane translocation and activation. Another experiment showed that aromatic resuscitation drugs have the protection effect on BBB by decreasing the level of VEGF in addition to MMP-986. Besides, the effects of TCM preparation, Chinese materia medica and active compounds on ROS production, leukocyte infiltration and inflammation all contribute to ameliorating BBB disruption to some extent, as discussed above.
2.5. Capillary hypoperfusion
It has been known for some time that loss of microvascular patency impairs cerebral vascular re-perfusion following global or focal cerebral ischemia87. After reperfusion, adhered leukocytes, entrapped erythrocytes, and fibrin-platelet deposits obstruct capillary lumens. Moreover, swollen astrocyte endfeet, pericyte contraction and microvessel hyperpermeability all contribute to capillary occlusion after reperfusion88, 89. A recent clinical study showed that perfusion status, rather than successful recanalization, has a significant impact on the outcome of stroke patients90, suggesting that improvement of microcirculatory reperfusion seems to be a promising strategy in stroke patients after thrombolysis. With the use of the intravital microscopy equipped with a high-speed video camera in a rat MCAO model, researchers demonstrated that the number of open capillaries reduced considerably 60 min after reperfusion, and pre-treatment with TCM preparation Cerebralcare Granule® attenuated this alteration75. In addition, cerebral blood flow (CBF) in cortex decreased 3 h after I/R, and this reduction remained for 6 days. Cerebralcare Granule® post-treatment after reperfusion attenuated the I/R-evoked decrease in CBF82. Consistent with the result observed by intravital microscopy and laser Doppler, transmission and scanning electron microscopy clearly identified that pre- or post-treatment with Cerebralcare Granule® ameliorated cerebral microvasculature changes, including narrowed lumen, rough inner surface, swelling endothelial cells and perivascular astrocyte end feet. The treatment also restored the decrease in the number of open capillaries 24 h or 6 days after reperfusion75, 82. Similar results were found when using Cerebralcare Granule® in global I/R injury50. Assessment of hemorheology by a full-automatic hemorheolometer revealed that acupoint-injection of compound Angelica-root injection (Fufang Danggui injection) downregulated blood viscosity including high, medium and low shearing rates, erythrocyte aggregation index, and rigidity index; deformity index was up-regulated, facilitating cerebral blood circulation91. Other TCM preparations, such as Erigeron injection (Dengzhanhuasu injection)92, Astragalus injection (Huangqi injection)93, Naosaitong preparation94, and icariin combined with P. notoginseng saponins29 were also reported to improve blood rheology and CBF after cerebral I/R injury. In addition, Gualou Guizhi decoction reduced cerebral ischemic spasticity, improved the screen test and Hoffman׳s reflex scores, which might be related to modulation of glutamate (Glu) levels and α-amino3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptor expression95.
3. Effects of TCM on brain injury and neuron damage induced by I/R
Within a few minutes of the onset of cerebral ischemia in stroke patients, the core of brain tissue exposed to the most dramatic blood flow reduction is mortally injured, and subsequently undergoes necrotic cell death. The reduction of oxygen and nutrient supply induces a series of metabolic dysfunctions such as reduction in ATP formation and energy failure, loss of cell ion homeostasis, acidosis, membrane depolarization, Ca2+ influx, excessive release of excitatory amino acids, free radical-mediated toxicity, all resulting in brain and neuron necrosis96, 97. Thrombolysis strategies have proven to be the most effective therapies for stroke treatment. However, early reperfusion of ischemic brain tissue can result in harmful consequences, including ROS outburst, overproduction of inflammatory mediators, leukocyte infiltration, microvessel hyperpermeabilty, all of which lead to BBB disruption and capillary hypoperfusion. Ultimately, activation of these cascades culminate in cerebral edema and/or brain hemorrhage and exacerbate brain injury3. On the other hand, cerebral microcirculatory disturbances, together with other neurotoxicity mediators such as Na+, Ca2+ and Glu, are detrimental to neuronal survival in the ischemic penumbra or peri-infarct zone; apoptotic neuron death is a common outcome98.
3.1. Effects of TCM on brain injury induced by I/R
As discussed above, TCM has multiple beneficial roles in cerebral I/R-induced microcirculatory disturbances. Among these are positive effects on brain injury, such as brain infarction, peri-vascular edema and hemorrhage after I/R. In this aspect, HE staining demonstrated a histopathological damage after global or focal cerebral I/R, which could be inhibited by some TCM preparations, such as Shengmai San56, Naomaitong preparation99 and active component baicalin19, 20. By virtue of magnetic resonance imaging (MRI), rat brain edema was observed 3 h after MCAO and remained unchanged 6 days after reperfusion. Interestingly, TCM preparation Cerebralcare Granule® reduced cerebral edema even when administered after the initiation of edema82. Likewise, scanning electron microscopy revealed swollen glia and edema around cerebral microvessels which were abrogated by Cerebralcare Granule®50, 82 and the active component apigenin18, after global or focal I/R injury. By means of high performance liquid chromatography (HPLC) and atomic absorption spectrophotometry, the TCM preparation Shenfu injection was found to decrease glutamate and Ca2+ in brain tissue and reduce excitatory amino acid toxicity, effects which can alleviate tissue edema100. Using Evan׳s blue dye extravasation and/or brain water content assessment, large doses of TCM preparation, Chinese materia medica, and active compounds were demonstrated to restrain BBB disruption and brain tissue edema after I/R, although the mechanisms for the effects of each TCM may differ. For example, combinations of total alkaloids from Gouteng (Ramulus Uncariae cum Uncis) and Xiatianwu (Rhizoma Corydalis Decumbentis)101 and the active compound sodium tanshinone B45 were thought to act through anti-oxidation, whereas the TCM preparation Naomaitong preparation68 and Guizhi Fuling capsules67 may produce beneficial effects by anti-inflammatory mechanisms. In contrast, FBD formula70 and Chinese materia medica Zhimu (Rhizoma Anemarrhenae)78 may act through inhibition of leukocyte infiltration, whereas the TCM preparation Shexiang Xingnaoning preparation102 as suggested may act by anti-thrombotic mechanisms. Finally, Cerebralcare Granule®82 and Tongxinluo capsule77 are thought to maintain the BBB. Consistent with anti-edema effects, the beneficial effects of TCM preparations, Chinese materia medica and active compounds on cerebral infarction after I/R injury have also been reported. For example, 2,3,5-triphenyltetrazolium chloride (TTC) staining showed that the TCM preparation Xuezhikang capsule103, total flavones of Chinese materia medica Huangshukui (Abelmoschus manihot)104, and active compounds, such as calycosin24 and astrogaloside16, inhibited brain infarction mainly by anti-oxidative effect. On the other hand, the classical formula Buyang Huanwu Tang105, and TCM preparations, such as Danhong injection106, Astragalus injection (Huangqi injection)93, and Naosaitong preparation94 were shown to inhibit cerebral tissue necrosis via promoting blood vessel repair105, anticoagulant and antifibrinolytic activity106, and improvement of capillary perfusion. In contrast studies on edema and infarction, few studies have been published showing the ameliorating effect of TCM preparations, Chinese materia medica, and active compounds on cerebral hemorrhage induced by I/R injury. However, these medicinals can alleviate intracerebral hemorrhage in some situations, such as surgery107, traumatic intracranial hematoma108, and artificial cerebral hemorrhage in animal models109.
3.2. Effects of TCM on neuron damage induced by I/R
A variety of tests are available for evaluation of neurobehavioral function, such as Long׳s test33, 43, 93, Morris water maze test17, 19, 110, 111, 112, eight-arm radical maze test29, 113, step down and step through test40, 53, beam-walking test42, forced swimming test and tail suspension test103. Using these tests, many TCM preparations, Chinese materia medica and active compounds have been shown to alleviate neurological deficits and improve learning and memory after I/R injury. These include TCM preparations such as Xiaoxuming decoction112 and Yizhi capsule110, 111, and Chinese materia medica such as Sanqi (Radix et Rhizoma Notoginseng)113, and Gegen (Radix puerariae) ethanol extract114. The list also includes active compounds, such as astrogaloside17, salvialonic acid A40, salvianolic acid B42, morroniside33 and scutellarin43. The underlying mechanism for neuroprotection in each case is distinct. Candidate mechanisms include anti-apoptosis, anti-excitoxicity and pro-neurogenesis. The following sections will discuss each of these separately.
3.2.1. Effects of TCM on apoptosis
Nissl staining and DNA fragmentation assays demonstrated that the TCM preparation Naoshuantong capsule reduced neuronal apoptosis in ischemic cortex and in the hippocampal CA1 region after rat MCAO; neurological functional deficits were also attenuated. Naoshuantong capsule also suppressed the overexpression of Bax and activation of caspases-3, -8 and -9, inhibited the reduction of Bcl-2 expression and depressed the Bax/Bcl-2 ratio115. Likewise, Nissl and terminal-deoxynucleoitidyl transferase mediated nick end labeling (TUNEL) staining, along with electron microscopic observations, showed that the TCM preparation Cerebralcare Granule® attenuated neuron death in cortex after focal I/R and in hippocampal CA1 region after global I/R. These beneficial effects were partly attributed to improving cerebral microcirculatory disturbances, balancing Bcl-2 superfamily protein expression, and modulation of the PUMA-p53 proapoptotic pathway51, 75. Staining with hematoxylin and eosin (HE), TUNEL and Hoechst 33258 was used to show that the classical formula Dihuang Yinzi116, the TCM preparation Astragalus injection (Huangqi injection)93, and active compounds, such as astragalosides17 and tetrahydroxystilbene glucoside46 attenuated neuronal apoptosis. Mechanisms for these actions include ERK activation, inhibition of JNK phosphorylation, and up-regulation of Akt phosphorylation; this last mechanism may also be important in the neuroprotective effect of eupatilin25 and resveratrol37. Other active compounds, such as Leonurine30 and ginsenoside Re26 were reported to attenuate I/R-induced mitochondrial swelling, reverse changes in mitochondrial membrane potential, and restore cytochrome c levels in mitochondria. The anti-apoptotic actions of these preparations were suggested to contribute to these effects. Another experiment found that the active compound scutellarin alleviated mitochondrial dysfunction through inhibition of poly-ADP-ribose polymerase (PARP) overactivation and the subsequent translocation of apoptosis inducing factor (AIF) from mitochondria to nuclei following cerebral I/R43. Other TCM preparations, such as Buyang Huanwu decoction117, Xingnaojing plus Xuesaitong injection118, Naoshuantong capsule119, Yinxing (Folium Ginkgo) extract120, and active compounds, such as ginsenoside Rg327 and lycopene32 displayed similar neuroprotective actions via anti-apoptotic effects.
3.2.2. Effects of TCM on excitoxicity
Excitatory amino acids accumulate in the extracellular space following ischemia and activate their receptors, leading to intracellular Ca2+ overload followed by neuronal damage. HPLC results demonstrated that the classical formula Buyang Huanwu decoction121, Tianma Gouteng Fang122, and Chinese materia medica Yinxing (Folium Ginkgo) extract123 decreased brain levels of the excitatory amino acids Glu and aspartate (Asp), and increased levels of the inhibitory amino acids γ-aminobutyric acid (GABA), taurine and glycine (Gly). Such a pattern seems to maintain the balance of inhibitory and excitatory amino acids in brain after I/R injury. TCM preparations, Chinese materia medica and active compounds have also been reported to influence the excitatory amino acid receptors. For example, TCM preparations such as Huanshaodan decoction124 and Tianma Cuzhi granules125 were found to reduce the N-methyl-d-aspartic acid (NMDA) receptor activity in both cerebral cortex and hippocampus. Single active components, such as sodium tanshione B45 and senegenin44 decreased NMDA receptor protein expression and mRNA levels of its NR2B subunit in hippocampus. The classical formula Buyang Huanwu decoction126 decreased neurological deficit scores via down-regulating AMPA receptors, glutamate receptor-1 RNA and glutamate receptor-2 protein expression. In primary hippocampal neuron cultures, an active component flavonoid extract from Shishu (Diospyros kaki) leaves127 and Yinxing (Folium Ginkgo) extract128 protected neurons from Glu- or NMDA receptor-induced excitotoxicity. Also, microfluorometry showed that the Chinese materia medica Yinxing (Folium Ginkgo) extract decreased intracellular Ca2+ concentrations in primary cultured hippocampal neurons treated with Glu123. In addition, injections of the TCM preparation Shenqi Fuzheng improved neuronal deficits, an effect which was suggested to be related to inhibition of Ca2+ aggregation in brain129.
3.2.3. Effects of TCM on neurogenesis
Given that the proliferation of endogenous neuron stem cells and neurogenesis occurs in rodents130, as well as in primate131 and patients132 after ischemic stroke, pharmacological interventions related to neurogenesis are believed to be a key strategy to improve the neuron functions after cerebral I/R injury. TCM finds its role in this field as well. It was reported that the TCM preparation Tongxinluo capsule increased the number of nestin-positive neurons and VEGF mRNA expression in the rat subventricular zone and hippocampal subdentate gyrus zone of the ischemic hemisphere after MCAO, indicating a capacity of promoting differentiation and proliferation of the neural stem cells133, 134. In addition to VEGF, brain-derived neurotrophic factor (BNDF)21, 22, 113, basic fibroblast growth factor (bFGF)135 and nerve growth factor (NGF)13 may also be targets for the pro-neurogenic potential of some TCM preparations (e.g., Naoluo Xintong recipe135), Chinese materia medica (e.g., Sanqi (Radix et Rhizoma Notoginseng)113), and active components (baicalin21, 22 and astragaloside13). In an in vitro study, P. notoginseng saponins, active components extract from Sanqi (Radix et Rhizoma Notoginseng), was shown to promote rat hippocampal neural stem cells (NSC) proliferation and the expression of nestin/BrdU. mRNA levels of class III β-tublin (Tuj-1), vimentin, and nestin were also enhanced. Also, P. notoginseng saponins increased area density, optical density and numbers of nestin/BrdU, nestin/vimentin, and nestin/Tuj-1 positive NSC following OGD136. The TCM preparation Weinaokang preparation137 and the single active component salvianolic acid B42, among others, were reported to improve neurogenesis and to promote the repair of ischemic areas.
3.2.4. Other neuroprotective mechanisms of TCM preparation, Chinese materia medica and active compounds
In addition to aforementioned mechanisms, TCM preparations, Chinese materia medica and active compounds are known to alleviate neuron damage after cerebral I/R indirectly via anti-oxidant, anti-inflammatory roles and promotion of capillary perfusion around neurons. For example, the classical formula oren-gedoku-to (Huanglian Jiedu Tang) lowered neuronal death by increasing the expression of Cu/Zn-SOD in the hippocampus of ischemic mice138. The TCM preparation Breviscapine injection (Dengzhanhuasu injection)139, and active compounds, such as total glycoside from Chishao (Radix Paeoniae Rubra)53 and luteolin31, ameliorated neurological deficit and improved neuronal function through anti-oxidant actions as well. In addition, electron microscopic studies found that TCM preparations, such as Cerebralcare Granule®51, 75, Shexiang Xingnaonin preparation102 and the single active compound apigenin18, exhibited a potential to decrease neurological scores and improve pathomorphology and neuron ultrastructure of ischemic cortex and hippocampal CA1 region after I/R. Suggested mechanisms for these effects include inhibition of cytokine production and platelet aggregation102, or promotion of capillary opening51, 75. Similarly, HE staining was used to show that the active compound baicalin increased neurons with pycnotic shape and condensed nuclei in cortex and hippocampus, effects which were related to suppression of NF-κB p65 activation23.
4. Summary
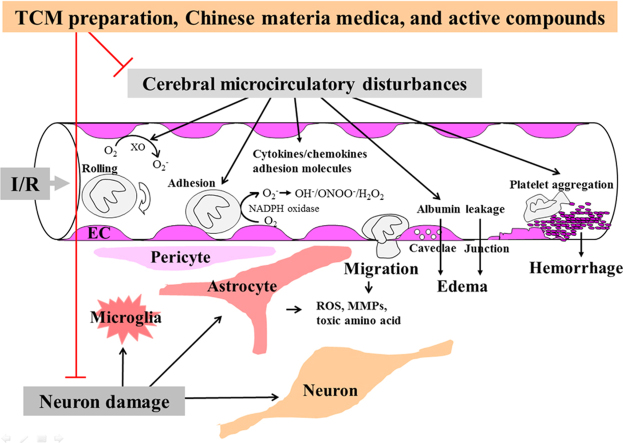
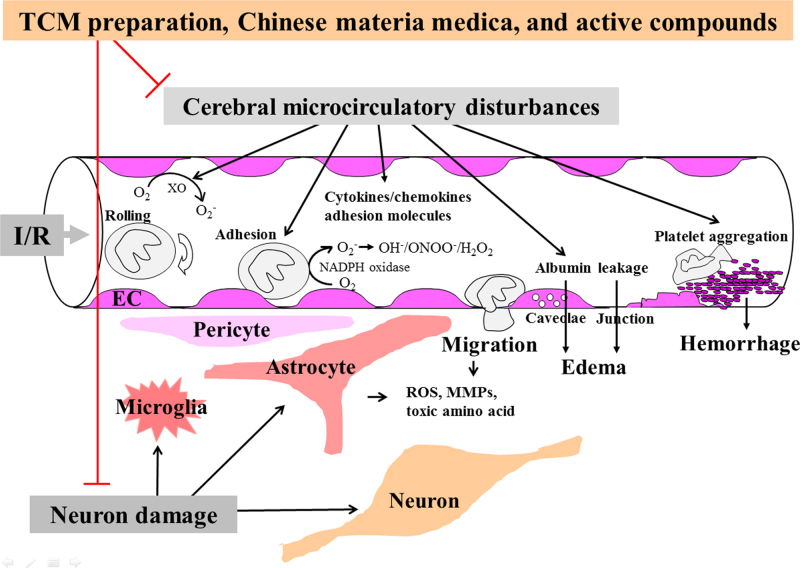
The present paper provides an overview of the ameliorating effects of compound TCM preparations, Chinese materia medica and active components on I/R-induced cerebral microcirculatory disturbances, brain injury and neuron damage, as summarized in Fig. 1.
-
(1)
Many studies demonstrated that compound TCM preparations, Chinese materia medica and their active components can ameliorate I/R-induced cerebral microcirculatory disturbances, including enhanced ROS production, release of cytokines/chemokines/adhesion molecules, leukocyte recruitment, microvessel hyperpermeability, BBB disruption and capillary hypoperfusion (Table 3).
-
(2)
Compound TCM preparations, Chinese materia medica and their active components are capable of ameliorating I/R-induced brain injury, such as brain infarction and perivascular edema. Such effects are attributable to beneficial actions on cerebral microcirculatory disturbances, together with attenuation of excitoxicity (Table 4).
-
(3)
Compound TCM preparations, Chinese materia medica and their active components have the potential to alleviate neurological deficits and improve learning and memory capacity after I/R injury. Underlying mechanisms for these effects include inhibition of apoptosis, excitoxicity, oxidation and inflammation, along with stimulation of pro-neurogenesis (Table 5).
-
(4)
In spite of a number of clinical studies illustrating the ameliorating effects of TCM preparations, Chinese materia medica and active compounds on cerebral I/R-related diseases, strictly randomized, double-blind, placebo-controlled, and multicenter clinical trials remain to be conducted with large samples. More evidence-based data are required to confirm the effects of TCM preparation, Chinese materia medica and active compounds, especially Chinese classic formulas, on ischemic stroke in clinical studies.
-
(5)
Majority of the studies concerning the protective effects of TCM preparation, Chinese materia medica and active compounds on I/R-induced cerebral injury and neuron damage focus on ROS production, cytokine release, leukocyte recruitment and microvessel hyperpermeability. More attention should be paid to other microcirculatory insults, including platelet aggregation, mast cell degranulation, thrombus formation and lymphocyte activation. Furthermore, future studies should place more emphasis on mechanistic exploration in depth rather than merely descriptive observation. For instance, what is the relationship between brain injury (especially cerebral microcirculatory disturbances) and delayed neuron damage? What are the initiating effector/receptor and specific signaling molecules for TCM preparations, Chinese materia medica and active compounds to regulate inflammatory responses, such as ROS production, cytokine release and leukocyte activation? Moreover, additional mechanisms that participate in ischemic brain injury and neuron damage should be addressed further in studying the role of TCM preparation, Chinese materia medica and active compounds in cerebral I/R injury. These energize metabolism, microglia activation, aquaporin molecules, and cyclic adenosine monophosphate (cAMP).
Figure 1.
Schematic summary of how the TCM preparation, Chinese materia medica and active compounds ameliorate I/R-induced cerebral microcirculatory disturbances, brain injury and neuron damage. TCM preparation, Chinese materia medica and active compounds inhibit I/R-induced multiple insults in cerebral microcirculation, including ROS outburst, inflammatory mediators overproduction, leukocyte infiltration, microvessel hyperpermeabilty, platelet aggregation, etc., leading to BBB disruption and capillary hypoperfusion, which culminate in ameliorating cerebral edema and/or brain hemorrhage, and brain injury. On the other hand, by improving cerebral microcirculation, together with anti-apoptosis, anti-excitoxicity and pro-neurogenesis effects, TCM preparation, Chinese materia medica and active compounds have potential to alleviate neurological deficits after I/R. BBB, blood brain barrier; EC, endothelial cell; H2O2, hydrogen peroxide; I/R, ischemia/reperfusion; MMPs, matrix metalloproteinases; NADPH, nicotinamide adenine dinucleotide phosphate; O2−, superoxide anion; OH−, hydroxyl radicals; ONOO−, peroxynitrite anion; ROS, reactive oxygen species, TCM, traditional Chinese medicine; XO, xanthine oxidase; ⊥ denotes inhibition.
Table 3.
Effect of TCM preparation, Chinese materia medica and active compounds on pathogenesis of cerebral microcirculatory disturbances induced by I/R.
| Effect | Target | TCM | Refs. |
|---|---|---|---|
| Amelioration of oxidative stress | ROS source | Cerebralcare Granule® | 50, 51 |
| Yiqi Tongluo Jiedu capsule | 52 | ||
| Chishao total glycoside | 53 | ||
| Danshen | 54 | ||
| Astragaloside | 13, 14 | ||
| Baicalin | 19 | ||
| Notoginsenoside R1 | 34 | ||
| Tetrahydroxystilbene glucoside | 46 | ||
| Free-radical scavenger | Chaihu Jia Longgu Muli Tang | 55 | |
| Guizhi Fuling Wan | 55 | ||
| Huanglian Jiedu Tang | 55 | ||
| Salvianolic acid A | 38 | ||
| Anti-oxidase activity | Shengmai San | 56, 57, 58 | |
| Tongxinluo capsule | 62 | ||
| Baihuasuantenggou extract | 59, 60 | ||
| Lavender oil | 61 | ||
| Yimucao injection | 63 | ||
| Yinxing extract | 64 | ||
| 3′-Methoxy-puerarin | 12 | ||
| Paeonol | 35 | ||
| Amelioration of inflammation | Cytokines/chemokines | FBD formula | 71 |
| Guizhi Fuling capsule | 67 | ||
| Naomaitong preparation | 68 | ||
| Taohong Siwu Tang | 69 | ||
| Danshen | 71 | ||
| Honokiol | 28 | ||
| Tetrandrine | 47 | ||
| Adhesion molecules | Naomaitong preparation | 68 | |
| Tetrandrine | 47 | ||
| Other mediators (TGF-β1, iNOS, Cox-2) | Taohong Siwu Tang | 69 | |
| Danshen | 71 | ||
| Honokiol | 28 | ||
| Resveratrol | 36 | ||
| Amelioration of leukocyte infiltration | Leukocyte-endothelial interaction | Cerebralcare Granule® | 50, 51, 75 |
| FBD formula | 70 | ||
| Salvianolic acid A | 39 | ||
| Tetrandrine | 47 | ||
| MPO increase | Huanglian Jiedu Tang | 76 | |
| Shengmai San | 56 | ||
| Tongxinluo capsule | 77 | ||
| Zhimu | 78 | ||
| Amelioration of BBB disruption | Endothelial cell junction | Tongxinluo capsule | 77 |
| Cerebralcare Granule® | 82 | ||
| MMP/TIMP balance | Huatuo Zaizao extractum | 85 | |
| Naomaitong preparation | 83 | ||
| Weinaokang preparation | 84 | ||
| Aromatic resuscitation drugs | 86 | ||
| Panax notoginseng saponins plus astragaloside | 15 | ||
| Salvianolic acid B | 41 | ||
| Amelioration of capillary hypoperfusion | CBF | Naosaitong preparation | 94 |
| Cerebralcare Granule® | 50, 75, 82 | ||
| Astragalus injection | 93 | ||
| Erigeron injection | 92 | ||
| Hemorheology | Compond Angelica-root injection | 91 | |
| Icariin plus Panax notoginseng saponins | 29 | ||
| Cerebral spasticity | Gualou Guizhi decoctiom | 95 | |
Table 4.
Effect of TCM preparation, Chinese materia medica and active compounds on brain injury induced by I/R.
| Effect | Mechanism | TCM | Refs. |
|---|---|---|---|
| Amelioration of brain edema | Inhibiting excitoxicity | Shenfu injection | 100 |
| Anti-oxidation | Shengmai San | 56 | |
| Cerebralcare Granule® | 50 | ||
| Gouteng total alkaloids | 101 | ||
| Xiatianwu total alkaloids | 101 | ||
| Sodium tanshinone B | 45 | ||
| Anti-inflammation | Guizhi Fuling capsules | 67 | |
| Naomaitong preparation | 68 | ||
| Shengmai San | 56 | ||
| Baicalin | 20 | ||
| Inhibiting leukocyte infiltration | FBD formula | 70 | |
| Cerebralcare Granule® | 50, 75 | ||
| Zhimu | 78 | ||
| Anti-thrombosis | Shexiang Xingnaoning preparation | 102 | |
| Maintaining BBB | Tongxinluo capsule | 77 | |
| Cerebralcare Granule® | 82 | ||
| Amelioration of brain infarction | Anti-oxidation | Xuezhikang capsule | 103 |
| Huangshukui total flavones | 104 | ||
| Astragaloside | 16 | ||
| Calycosin | 24 | ||
| Anti-thrombosis | Danhong injection | 106 | |
| Improve capillary perfusion | Naosaitong preparation | 94 | |
| Astragalus injection | 93 | ||
| Promote blood vessel repair | Buyang Huanwu Tang | 105 | |
Table 5.
Effect of TCM preparation, Chinese materia medica and active compounds on neuron damage induced by I/R.
| Effect | Mechanism | TCM | Refs. |
|---|---|---|---|
| Anti-neuronal apoptosis | Down-regulating caspases | Buyang Huanwu Tang | 117 |
| Ginsenoside Rg3 | 27 | ||
| Morroniside | 33 | ||
| To balance Bcl-2/Bax | Naoshuantong capsule | 115 | |
| Xiaoxuming decoction | 112 | ||
| Cerebralcare Granule® | 51 | ||
| Lycopene | 32 | ||
| Restoring mitochondrial membrane potential | Leonurine | 30 | |
| Ginsenoside Re | 26 | ||
| Regulating p53-PUMA pathway | Cerebralcare Granule® | 75 | |
| Regulating MAPK/Akt pathway | Dihuang Yinzi | 116 | |
| Astragalus injection | 93 | ||
| Astragaloside | 17 | ||
| Eupatilin | 25 | ||
| Resveratrol | 37 | ||
| Tetrahydroxystilbene glucoside | 46 | ||
| Regulating PARP-AIF pathway | Scutellarin | 43 | |
| Anti-neurotoxicity | Decreasing excitoxicity amino acids | Buyang Huanwu Tang | 121 |
| Tianma Gouteng Fang | 122 | ||
| Gegen ethonal extract | 114 | ||
| Yinxing extract | 120, 123 | ||
| Decreasing excitoxicity amino acid receptor | Buyang Huanwu Tang | 126 | |
| Huanshaodan decoction | 124 | ||
| Tianma Cuozhi granules | 125 | ||
| Shishu leaves flavonoids extract | 127 | ||
| Yinxing extract | 128 | ||
| Senegenin | 44 | ||
| Sodium tanshinone B | 45 | ||
| Decreasing [Ca2+]i | Shenqi Fuzheng injection | 129 | |
| Yinxing extract | 123 | ||
| Neurogenesis | Promoting NSC proliferation | Tongxinluo capsule | 133, 134 |
| Panax notoginseng saponins | 136 | ||
| Increasing neurotrophic factors (VEGF, BNDF, bFGF, NGF) | Naoluo Xintong recipe | 135 | |
| Sanqi | 113 | ||
| Astragaloside | 13 | ||
| Baicalin | 21, 22 | ||
| Promoting neuronal self-repair | Weinaokang preparation | 137 | |
| Salvianolic acid B | 42 | ||
| Amelioration of microcirculatory disturbances | Anti-oxidation | Huanglian Jiedu Tang | 138 |
| Xingnaojing plus Xuesaitong injection | 118 | ||
| Breviscapine injection | 139 | ||
| Chishao total glycoside | 80 | ||
| Yinxing extract | 120 | ||
| Icariin plus Panax notoginseng saponins | 29 | ||
| Luteolin | 31 | ||
| Lycopene | 32 | ||
| Salvianolic acid A | 40 | ||
| Anti-inflammation | Shexiang Xingnaonin preparation | 102 | |
| Yinxing extract | 120 | ||
| Apigenin | 18 | ||
| Baicalin | 19, 23 | ||
| Lycopene | 32 | ||
| Promoting capillary perfusion | Cerebralcare Granule® | 51, 75 | |
Ischemic stroke, post I/R-induced cerebral microcirculatory disturbances, subsequent brain injury, and neuron damage are complicated processes, and require interventions at multiple targets for successful treatment. TCM preparations and Chinese materia medica contain multiple active ingredients which been widely used for decades in the clinic as a potential therapeutic strategy for I/R-related cerebral diseases. Further clinical and experimental research is needed to understand their actions.
Footnotes
Peer review under responsibility of Institute of Materia Medica, Chinese Academy of Medical Sciences and Chinese Pharmaceutical Association.
References
- 1.WHO infobase. Available from: 〈http://www.who.int/healthinfo/global_burden_disease/〉.
- 2.Murray V., Norrving B., Sandercock P.A.G., Terént A., Waldlaw J.M., Wester P. The molecular basis of thrombolysis and its clinical application in stroke. J Intern Med. 2010;267:191–208. doi: 10.1111/j.1365-2796.2009.02205.x. [DOI] [PubMed] [Google Scholar]
- 3.Zheng Z., Yenari M.A. Post-ischemic inflammation: molecular mechanisms and therapeutic implications. Neurol Res. 2004;26:884–892. doi: 10.1179/016164104X2357. [DOI] [PubMed] [Google Scholar]
- 4.Frey J.L. Recombinant tissue plasminogen activator (rtPA) for stroke. The perspective at 8 years. Neurologist. 2005;11:123–133. doi: 10.1097/01.nrl.0000156205.66116.84. [DOI] [PubMed] [Google Scholar]
- 5.Tsirka S.E. Clinical implications of the involvement of tPA in neuronal cell death. J Mol Med (Berl) 1997;75:341–347. doi: 10.1007/s001090050119. [DOI] [PubMed] [Google Scholar]
- 6.Shuaib A., Lees K.R., Lyden P., Grotta J., Davalos A., Davis S.M. NXY-059 for the treatment of acute ischemic stroke. N Engl J Med. 2007;357:562–571. doi: 10.1056/NEJMoa070240. [DOI] [PubMed] [Google Scholar]
- 7.Furuya K., Takeda H., Azhar S., McCarron R.M., Chen Y., Ruetzler C.A. Examination of several potential mechanisms for the negative outcome in a clinical stroke trial of enlimomab, a murine anti-human intercellular adhesion molecule-1 antibody a bedside-to-bench study. Stroke. 2001;32:2665–2674. doi: 10.1161/hs3211.098535. [DOI] [PubMed] [Google Scholar]
- 8.Cohan S.L. Pharmacology of calcium antagonists: clinical relevance in neurology. Eur Neurol. 1990;30 Suppl 2:28–30. doi: 10.1159/000117188. [DOI] [PubMed] [Google Scholar]
- 9.Danton G.H., Dietrich W.D. The search for neuroprotective strategies in stroke. AJNR Am J Neuroradiol. 2004;25:181–194. [PMC free article] [PubMed] [Google Scholar]
- 10.Beech J.S., Reckless J., Mosedale D.E., Grainger D.J., Williams S.C., Menon D.K. Neuroprotection in ischemia-reperfusion injury: an antiinflammatory approach using a novel broad-spectrum chemokine inhibitor. J Cereb Blood Flow Metab. 2001;21:683–689. doi: 10.1097/00004647-200106000-00006. [DOI] [PubMed] [Google Scholar]
- 11.Robertson G.S., Crocker S.J., Nicholson D.W., Schulz J.B. Neuroprotection by the inhibition of apoptosis. Brain Pathol. 2000;10:283–292. doi: 10.1111/j.1750-3639.2000.tb00262.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang Y.B., Du G.Y., Xiong Y.L., Zhao Y., Cui H.F., Cao C.Y. Protective effects of 3′-methoxy-puerarin on rat brain suffering from ischemia. Chin Mater Med. 2008;33:537–540. [PubMed] [Google Scholar]
- 13.Yin Y.Y., Li W.P., Gong H.L., Zhu F.F., Li W.Z., Wu G.C. Protective effect of astragaloside on focal cerebral ischemia/reperfusion injury in rats. Am J Chin Med. 2010;38:517–527. doi: 10.1142/S0192415X10008020. [DOI] [PubMed] [Google Scholar]
- 14.Yang J.H., Li J.H., Lu J., Zhang Y.Y., Zhu Z.H., Wan H.T. Synergistic protective effect of astragaloside IV-tetramethylpyrazine against cerebral ischemic-reperfusion injury induced by transient focal ischemia. J Ethnopharmacol. 2012;140:64–72. doi: 10.1016/j.jep.2011.12.023. [DOI] [PubMed] [Google Scholar]
- 15.Huang X., Tan H., Chen B., Deng C. Influence of astragalosides and Panax notoginseng saponins compatibility on MMP-9 and TIMP-1 after cerebral ischemia-reperfusion in mice. Chin Mater Med. 2010;35:2187–2191. [PubMed] [Google Scholar]
- 16.Luo Y., Qin Z., Hong Z., Zhang X., Ding D., Fu J.H. Astragaloside IV protects against ischemic brain injury in a murine model of transient focal ischemia. Neurosci Lett. 2004;363:218–223. doi: 10.1016/j.neulet.2004.03.036. [DOI] [PubMed] [Google Scholar]
- 17.Wu Y.Y., Wu W.Y., Gong H.L., Li W.Z., Yin Y.Y. Astragalosides attenuate learning and memory impairment in rats following ischemia-reperfusion injury. Mol Med Rep. 2014;9:1319–1324. doi: 10.3892/mmr.2014.1969. [DOI] [PubMed] [Google Scholar]
- 18.Liu C., Tu F.X., Chen X. Neuroprotective effects of apigenin on acute transient focal cerebral ischemia-reperfusion injury in rats. J Chin Med Mater. 2008;31:870–873. [PubMed] [Google Scholar]
- 19.Cheng O.M., Li Z.H., Han Y., Jiang Q.S., Yan Y., Cheng K. Baicalin improved the spatial learning ability of global ischemia/reperfusion rats by reducing hippocampal apoptosis. Brain Res. 2012;1470:111–118. doi: 10.1016/j.brainres.2012.06.026. [DOI] [PubMed] [Google Scholar]
- 20.Liu P., Wang J.Y., Li Q., Xu F.Y., Wang Z.Y., Xu H.Y. Effect of baicalin on HSP70 expression of hippocampal neurons in focal brain ischemia-reperfusion injury rats. Acta Pharmacol Sin. 2006;41:619–624. [PubMed] [Google Scholar]
- 21.Zhang Z.J., Li P., Wang Z., Li P.T., Zhang W.S., Sun Z.H. A comparative study on the individual and combined effects of baicalin and jasminoidin on focal cerebral ischemia-reperfusion injury. Brain Res. 2006;1123:188–195. doi: 10.1016/j.brainres.2006.09.063. [DOI] [PubMed] [Google Scholar]
- 22.Cao Y.G., Mao X.Y., Sun C.Y., Zheng P., Gao J.Q., Wang X.R. Baicalin attenuates global cerebral ischemia/reperfusion injury in gerbils via anti-oxidative and anti-apoptotic pathways. Brain Res Bull. 2011;85:396–402. doi: 10.1016/j.brainresbull.2011.05.002. [DOI] [PubMed] [Google Scholar]
- 23.Xue X., Qu X.J., Yang Y., Sheng X.H., Cheng F., Jiang E.N. Baicalin attenuates focal cerebral ischemic reperfusion injury through inhibition of nuclear factor κB p65 activation. Biochem Biophys Res Commun. 2010;403:398–404. doi: 10.1016/j.bbrc.2010.11.042. [DOI] [PubMed] [Google Scholar]
- 24.Guo C., Tong L., Xi M.M., Yang H.F., Dong H.L., Wen A.D. Neuroprotective effect of calycosin on cerebral ischemia and reperfusion injury in rats. J Ethnopharmacol. 2012;144:768–774. doi: 10.1016/j.jep.2012.09.056. [DOI] [PubMed] [Google Scholar]
- 25.Cai M.D., Phan P.T., Hong J.G., Kim D.H., Kim J.M., Park S.J. The neuroprotective effect of eupatilin against ischemia/reperfusion-induced delayed neuronal damage in mice. Eur J Pharmacol. 2012;689:104–110. doi: 10.1016/j.ejphar.2012.05.042. [DOI] [PubMed] [Google Scholar]
- 26.Chen L.M., Zhou X.M., Cao Y.L., Hu W.X. Neuroprotection of ginsenoside Re in cerebral ischemia-reperfusion injury in rats. J Asian Nat Prod Res. 2008;10:439–445. doi: 10.1080/10286020801892292. [DOI] [PubMed] [Google Scholar]
- 27.He B., Chen P., Yang J.Y., Yun Y., Zhang X.C., Yang R.H. Neuroprotective effect of 20(R)-ginsenoside Rg3 against transient focal cerebral ischemia in rats. Neurosci Lett. 2012;526:106–111. doi: 10.1016/j.neulet.2012.08.022. [DOI] [PubMed] [Google Scholar]
- 28.Zhang P., Liu X.Y., Zhu Y.J., Chen S.Z., Zhou D.M., Wang Y.Y. Honokiol inhibits the inflammatory reaction during cerebral ischemia reperfusion by suppressing NF-κB activation and cytokine production of glial cells. Neurosci Lett. 2013;534:123–127. doi: 10.1016/j.neulet.2012.11.052. [DOI] [PubMed] [Google Scholar]
- 29.Zheng M., Qu L., Lou Y. Effects of icariin combined with Panax notoginseng saponins on ischemia reperfusion-induced cognitive impairments related with oxidative stress and CA1 of hippocampal neurons in rat. Phytother Res. 2008;22:597–604. doi: 10.1002/ptr.2276. [DOI] [PubMed] [Google Scholar]
- 30.Qi J., Hong Z.Y., Xin H., Zhu Y.Z. Neuroprotective effects of leonurine on ischemia/reperfusion-induced mitochondrial dysfunctions in rat cerebral cortex. Biol Pharm Bull. 2010;33:1958–1964. doi: 10.1248/bpb.33.1958. [DOI] [PubMed] [Google Scholar]
- 31.Zhang Y.C., Gan F.F., Shelar S.B., Ng K.Y., Chew E.H. Antioxidant and Nrf2 inducing activities of luteolin, a flavonoid constituent in Ixeris sonchifolia Hance, provide neuroprotective effects against ischemia-induced cellular injury. Food Chem Toxicol. 2013;59:272–280. doi: 10.1016/j.fct.2013.05.058. [DOI] [PubMed] [Google Scholar]
- 32.Wei Y., Shen X.N., Mai J.Y., Shen H., Wang R.Z., Wu M. The effects of lycopene on reactive oxygen species and anoxic damage in ischemia reperfusion injury in rats. J Prev Med. 2010;44:34–38. [PubMed] [Google Scholar]
- 33.Wang W., Xu J.D., Li L., Wang P.C., Ji X.M., Ai H.X. Neuroprotective effect of morroniside on focal cerebral ischemia in rats. Brain Res Bull. 2010;83:196–201. doi: 10.1016/j.brainresbull.2010.07.003. [DOI] [PubMed] [Google Scholar]
- 34.Meng X., Wang M., Wang X., Sun G., Ye J., Xu H. Suppression of NADPH oxidase- and mitochondrion-derived superoxide by Notoginsenoside R1 protects against cerebral ischemia-reperfusion injury through estrogen receptor-dependent activation of Akt/Nrf2 pathways. Free Radic Res. 2014;48:823–838. doi: 10.3109/10715762.2014.911853. [DOI] [PubMed] [Google Scholar]
- 35.Zhang G., Yu Z., Zhao H. Protective effect of paeonol on repeated cerebral ischemia in rats. J Chin Med Mater. 1997;20:626–628. [PubMed] [Google Scholar]
- 36.Simão F., Matté A., Pagnussat A.S., Netto C.A., Salbego C.G. Resveratrol preconditioning modulates inflammatory response in the rat hippocampus following global cerebral ischemia. Neurochem Int. 2012;61:659–665. doi: 10.1016/j.neuint.2012.06.009. [DOI] [PubMed] [Google Scholar]
- 37.Simão F., Matté A., Pagnussat A.S., Netto C.A., Salbego C.G. Resveratrol prevents CA1 neurons against ischemic injury by parallel modulation of both GSK-3β and CREB through PI3-K/Akt pathways. Eur J Neurosci. 2012;36:2899–2905. doi: 10.1111/j.1460-9568.2012.08229.x. [DOI] [PubMed] [Google Scholar]
- 38.Du G.H., Zhang J.T. Protective effects of salvianolic acid A against impairment of memory induced by cerebral ischemia-reperfusion in mice. Acta Pharmacol Sin. 1997;110:65–68. [PubMed] [Google Scholar]
- 39.Jiang M., Wang X.Y., Zhou W.Y., Li J., Wang J., Gou L.P. Cerebral protection of salvianolic acid A by the inhibition of granulocyte adherence. Am J Chin Med. 2011;39:111–120. doi: 10.1142/S0192415X11008683. [DOI] [PubMed] [Google Scholar]
- 40.Du G., Zhang J. Protective effects of salvianolic acid A against impairment of memory induced by cerebral ischemia-reperfusion in mice. Chin Med J (Engl) 1997;110:65–68. [PubMed] [Google Scholar]
- 41.Li Q., Han L.P., Li Z.H., Zhang J.T., Tang M.K. Salvianolic acid B alleviate the disruption of blood-brain barrier in rats after cerebral ischemia-reperfusion by inhibiting MAPK pathway. Acta Pharmacol Sin. 2010;45:1485–1490. [PubMed] [Google Scholar]
- 42.Zhong J., Tang M.K., Zhang Y., Xu Q.P., Zhang J.T. Effect of salvianolic acid B on neural cells damage and neurogenesis after brain ischemia-reperfusion in rats. Acta Pharmacol Sin. 2007;42:716–721. [PubMed] [Google Scholar]
- 43.Zhang H.F., Hu X.M., Wang L.X., Xu S.Q., Zeng F.D. Protective effects of scutellarin against cerebral ischemia in rats: evidence for inhibition of the apoptosis-inducing factor pathway. Planta Med. 2009;75:121–126. doi: 10.1055/s-0028-1088368. [DOI] [PubMed] [Google Scholar]
- 44.Xie W., Yang Y., Gu X., Zheng Y., Sun Y.E., Liang Y. Senegenin attenuates hepatic ischemia-reperfusion induced cognitive dysfunction by increasing hippocampal NR2B expression in rats. PLoS One. 2012;7:e45575. doi: 10.1371/journal.pone.0045575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cai Q., Wang H.W., Hua S.Y., Tan J.Z., Zhou T., Li C.S. Neutroprotective efficacy of sodium tanshinone B on hippocampus neuron in a rat model of focal cerebral ischemia. Chin J Integr Med. 2012;18:837–845. doi: 10.1007/s11655-012-1266-9. [DOI] [PubMed] [Google Scholar]
- 46.Wang T., Gu J., Wu P.F., Wang F., Xiong Z., Yang Y.J. Protection by tetrahydroxystilbene glucoside against cerebral ischemia: involvement of JNK, SIRT1, and NF-κB pathways and inhibition of intracellular ROS/RNS generation. Free Radic Biol Med. 2009;47:229–240. doi: 10.1016/j.freeradbiomed.2009.02.027. [DOI] [PubMed] [Google Scholar]
- 47.Liu S.J., Zhou S.W., Xue C.S. Effect of tetrandrine on neutrophilic recruitment response to brain ischemia/reperfusion. Acta Pharmacol Sin. 2001;22:971–975. [PubMed] [Google Scholar]
- 48.Allen C.L., Bayraktutan U. Oxidative stress and its role in the pathogenesis of ischaemic stroke. Int J Stroke. 2009;4:461–470. doi: 10.1111/j.1747-4949.2009.00387.x. [DOI] [PubMed] [Google Scholar]
- 49.Stoll G., Jander S., Schroeter M. Inflammation and glial responses in ischemic brain lesions. Prog Neurobiol. 1998;56:149–171. doi: 10.1016/s0301-0082(98)00034-3. [DOI] [PubMed] [Google Scholar]
- 50.Xu X.S., Ma Z.Z., Wang F., Hu B.H., Wang C.S., Liu Y.Y. The antioxidant Cerebralcare Granule attenuates cerebral microcirculatory disturbance during ischemia-reperfusion injury. Shock. 2009;32:201–209. doi: 10.1097/SHK.0b013e3181996d61. [DOI] [PubMed] [Google Scholar]
- 51.Sun K., Hu Q., Zhou C.M., Xu X.S., Wang F., Hu B.H. Cerebralcare Granule®, a Chinese herb compound preparation, improves cerebral microcirculatory disorder and hippocampal CA1 neuron injury in gerbils after ischemia-reperfusion. J Ethnopharmacol. 2010;130:398–406. doi: 10.1016/j.jep.2010.05.030. [DOI] [PubMed] [Google Scholar]
- 52.Zhang Y.Y., Wan H.T., Lai L.L., Yang J.H., Chen W.Y., Zhou H.F. The effect and mechanism of Yiqi Tongluo Jiedu capsule against cerebral ischemia reperfusion injury. Acta Pharmacol Sin. 2012;47:1153–1158. [PubMed] [Google Scholar]
- 53.Yang J., Wang J., Feng P., Li Y., Ma C., Xu S. Protective effect of total paeony glycoside against cerebral ischemia-reperfusion injury in mice. J Chin Med Mat. 2000;23:95–97. [PubMed] [Google Scholar]
- 54.Kuang P., Tao Y., Shi J. Effect of radix Salviae miltiorrhizae on extracellular adenosine and evaluation of its protective efficacy in ischemic reperfusion rat-microdialysis, HPLC and histopathologic studies. J Tradit Chin Med. 1997;17:140–147. [PubMed] [Google Scholar]
- 55.Fushitani S., Minakuchi K., Tsuchiya K., Takasugi M., Murakami K. Studies on attenuation of post-ischemic brain injury by kampo medicines-inhibitory effects of free radical production II. Yakugaku Zasshi. 1995;115:611–617. doi: 10.1248/yakushi1947.115.8_611. [DOI] [PubMed] [Google Scholar]
- 56.Li L.H., Wang J.S., Kong L.Y. Protective effects of shengmai san and its three fractions on cerebral ischemia-reperfusion injury. Chin J Nat Med. 2013;11:222–230. doi: 10.1016/S1875-5364(13)60020-5. [DOI] [PubMed] [Google Scholar]
- 57.Ichikawa H., Wang L., Konishi T. Prevention of cerebral oxidative injury by post-ischemic intravenous administration of Shengmai San. Am J Chin Med. 2006;34:591–600. doi: 10.1142/S0192415X06004120. [DOI] [PubMed] [Google Scholar]
- 58.Wang X.J., Magara T., Konishi T. Prevention and repair of cerebral ischemia-reperfusion injury by Chinese herbal medicine, shengmai san, in rats. Free Radic Res. 1999;31:449–455. doi: 10.1080/10715769900301011. [DOI] [PubMed] [Google Scholar]
- 59.Bhandari U., Ansari M.N. Protective effect of aqueous extract of Embelia ribes Burm fruits in middle cerebral artery occlusion-induced focal cerebral ischemia in rats. Indian J Pharmacol. 2008;40:215–220. doi: 10.4103/0253-7613.44153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nazam Ansari M., Bhandari U., Islam F., Tripathi C.D. Evaluation of antioxidant and neuroprotective effect of ethanolic extract of Embelia ribes Burm in focal cerebral ischemia/reperfusion-induced oxidative stress in rats. Fundam Clin Pharmacol. 2008;22:305–314. doi: 10.1111/j.1472-8206.2008.00580.x. [DOI] [PubMed] [Google Scholar]
- 61.Wang D., Yuan X., Liu T., Liu L.L., Hu Y.L., Wang Z.H. Neuroprotective activity of lavender oil on transient focal cerebral ischemia in mice. Molecules. 2012;17:9803–9817. doi: 10.3390/molecules17089803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ding S.J., Li J.S. Anti-oxidant effects of Tongxinluo on ATPase in focal brain ischemia-reperfusion rats. J Cent S Univ Med Sci. 2006;31:552–555. [PubMed] [Google Scholar]
- 63.Xie C.X., Yang Y.Q., Lu J.P., Tang M., Zhou W. Protective effect of Yimucao (Herba leonuri) injection against cerebral ischemia: an experimental study in mice and rats. South Med J. 2007;27:1528–1530. [PubMed] [Google Scholar]
- 64.Zhou L., Ming L., Jiang Q. Protective effect of extract of Folium Ginkgo on repeated cerebral ischemia-reperfusion injury. Chinese Western Med. 2000;20:356–358. [PubMed] [Google Scholar]
- 65.Wang Q., Tang X.N., Yenari M.A. The inflammatory response in stroke. J Neuroimmunol. 2007;184:53–68. doi: 10.1016/j.jneuroim.2006.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Barone F.C., Parsons A.A. Therapeutic potential of anti-inflammatory drugs in focal stroke. Expert Opin Investig Drugs. 2000;9:2281–2306. doi: 10.1517/13543784.9.10.2281. [DOI] [PubMed] [Google Scholar]
- 67.Li T.J., Qiu Y., Mao J.Q., Yang P.Y., Rui Y.C., Chen W.S. Protective effects of Guizhi-Fuling-Capsules on rat brain ischemia/reperfusion injury. J Pharmacol Sci. 2007;105:34–40. doi: 10.1254/jphs.fp0070450. [DOI] [PubMed] [Google Scholar]
- 68.Li J.S., Gao J.F., Zhou Y.L., Liu K. Neuro-protective effect of Naomaitong to inflammatory cascade response after focal cerebral ischemia reperfusion in aged rats. Chin Mater Med. 2006;31:1804–1807. [PubMed] [Google Scholar]
- 69.Wu C.J., Chen J.T., Yen T.L., Jayakumar T., Chou D.S., Hsiao G. Neuroprotection by the traditional chinese medicine, Tao-Hong-Si-Wu-Tang, against middle cerebral artery occlusion-induced cerebral ischemia in rats. Evid Based Complement Alternat Med. 2011;2011:803015. doi: 10.1155/2011/803015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lin Z.H., Zhu D.N., Yan Y.Q., Yu B.Y. Herbal formula FBD extracts prevented brain injury and inflammation induced by cerebral ischemia-reperfusion. J Ethnopharmacol. 2008;118:140–147. doi: 10.1016/j.jep.2008.03.023. [DOI] [PubMed] [Google Scholar]
- 71.Liang X.Y., Li H.N., Yang X.Y., Zhou W.Y., Niu J.G., Chen B.D. Effect of Danshen aqueous extract on serum hs-CRP, IL-8, IL-10, TNF-α levels, and IL-10 mRNA, TNF-α mRNA expression levels, cerebral TGF-β1 positive expression level and its neuroprotective mechanisms in CIR rats. Mol Biol Rep. 2013;40:3419–3427. doi: 10.1007/s11033-012-2419-9. [DOI] [PubMed] [Google Scholar]
- 72.Gavins F., Yilmaz G., Granger D.N. The evolving paradigm for blood cell-endothelial cell interactions in the cerebral microcirculation. Microcirculation. 2007;14:667–681. doi: 10.1080/10739680701404903. [DOI] [PubMed] [Google Scholar]
- 73.Yilmaz G., Arumugam T.V., Stokes K.Y., Granger D.N. Role of T lymphocytes and interferon-γ in ischemic stroke. Circulation. 2006;113:2105–2112. doi: 10.1161/CIRCULATIONAHA.105.593046. [DOI] [PubMed] [Google Scholar]
- 74.Ishikawa M., Zhang J.H., Nanda A., Granger D.N. Inflammatory responses to ischemia and reperfusion in the cerebral microcirculation. Front Biosci. 2004;9:1339–1347. doi: 10.2741/1330. [DOI] [PubMed] [Google Scholar]
- 75.Wang F., Hu Q., Chen C.H., Xu X.S., Zhou C.M., Zhao Y.F. The protective effect of Cerebralcare Granule® on brain edema, cerebral microcirculatory disturbance, and neuron injury in a focal cerebral ischemia rat model. Microcirculation. 2012;19:260–272. doi: 10.1111/j.1549-8719.2011.00155.x. [DOI] [PubMed] [Google Scholar]
- 76.Hwang Y.S., Shin C.Y., Huh Y., Ryu J.H. Hwangryun-Hae-Dok-tang (Huanglian-Jie-Du-Tang) extract and its constituents reduce ischemia-reperfusion brain injury and neutrophil infiltration in rats. Life Sci. 2002;71:2105–2117. doi: 10.1016/s0024-3205(02)01920-3. [DOI] [PubMed] [Google Scholar]
- 77.Liu Y., Tang G.H., Sun Y.H., Lin X.J., Wei C., Yang G.Y. The protective role of Tongxinluo on blood–brain barrier after ischemia-reperfusion brain injury. J Ethnopharmacol. 2013;148:632–639. doi: 10.1016/j.jep.2013.05.018. [DOI] [PubMed] [Google Scholar]
- 78.Oh J.K., Hyun S.Y., Oh H.R., Jung J.W., Park C., Lee S.Y. Effects of Anemarrhena asphodeloides on focal ischemic brain injury induced by middle cerebral artery occlusion in rats. Biol Pharm Bull. 2007;30:38–43. doi: 10.1248/bpb.30.38. [DOI] [PubMed] [Google Scholar]
- 79.Knowland D., Arac A., Sekiguchi K.J., Hsu M., Lutz S.E., Perrino J. Stepwise recruitment of transcellular and paracellular pathways underlies blood–brain barrier breakdown in stroke. Neuron. 2014;82:603–617. doi: 10.1016/j.neuron.2014.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sandoval K.E., Witt K.A. Blood–brain barrier tight junction permeability and ischemic stroke. Neurobiol Dis. 2008;32:200–219. doi: 10.1016/j.nbd.2008.08.005. [DOI] [PubMed] [Google Scholar]
- 81.Cipolla M.J., Crete R., Vitullo L., Rix R.D. Transcellular transport as a mechanism of blood–brain barrier disruption during stroke. Front Biosci. 2004;9:777–785. doi: 10.2741/1282. [DOI] [PubMed] [Google Scholar]
- 82.Huang P., Zhou C.M., Hu Q., Liu Y.Y., Hu B.H., Chang X. Cerebralcare Granule® attenuates blood-brain barrier disruption after middle cerebral artery occlusion in rats. Exp Neurol. 2012;237:453–463. doi: 10.1016/j.expneurol.2012.07.017. [DOI] [PubMed] [Google Scholar]
- 83.Li J.S., Liu K., Wang M.H. Modulation effect of naomaitong on gelatinase system after cerebral ischemia/reperfusion in rats LI. Chin Western Med. 2006;26:14–17. [PubMed] [Google Scholar]
- 84.Zheng Y.Q., Liu J.X., Li X.Z., Xu L. Effects and mechanism of Weinaokang on reperfusion-induced vascular injury to cerebral microvessels after global cerebral ischemia. Chin J Integr Med. 2010;16:145–150. doi: 10.1007/s11655-010-0145-5. [DOI] [PubMed] [Google Scholar]
- 85.Zheng Y.Q., Yao M.J., Liu J.X., Song W.T., Li L., Liu S.B. Effect and mechanism of huatuo zaizao extractum on focal cerebral ischemia/reperfusion-induced blood–brain barrier injury in rats. Chin Mater Med. 2013;38:585–590. [PubMed] [Google Scholar]
- 86.Ni C., Zeng N., Xu F., Guo L., Liu J., Wang J. Effects of aromatic resuscitation drugs on blood brain barrier in cerebral ischemia-reperfusion injury model rats. Chin Mater Med. 2011;36:2562–2566. [PubMed] [Google Scholar]
- 87.Little J.R., Kerr F.W., Sundt T.M., Jr. Microcirculatory obstruction in focal cerebral ischemia. Relationship to neuronal alterations. Mayo Clin Proc. 1975;50:264–270. [PubMed] [Google Scholar]
- 88.Garcia J.H., Liu K.F., Yoshida Y., Chen S., Lian J. Brain microvessels: factors altering their patency after the occlusion of a middle cerebral artery (Wistar rat) Am J Pathol. 1994;145:728–740. [PMC free article] [PubMed] [Google Scholar]
- 89.Yemisci M., Gursoy-Ozdemir Y., Vural A., Can A., Topalkara K., Dalkara T. Pericyte contraction induced by oxidative-nitrative stress impairs capillary reflow despite successful opening of an occluded cerebral artery. Nat Med. 2009;15:1031–1037. doi: 10.1038/nm.2022. [DOI] [PubMed] [Google Scholar]
- 90.De Silva D.A., Fink J.N., Christensen S., Ebinger M., Bladin C., Levi C.R. Assessing reperfusion and recanalization as markers of clinical outcomes after intravenous thrombolysis in the echoplanar imaging thrombolytic evaluation trial (EPITHET) Stroke. 2009;40:2872–2874. doi: 10.1161/STROKEAHA.108.543595. [DOI] [PubMed] [Google Scholar]
- 91.Chen F., Yan Z.K., Yang B. Effects of acupoint-injection of compound Angelica-root Injectio on cerebral Bcl-2 and Bax immunoactivity and hemorheology in rats with cerebral ischemia-reperfusion injury. Acupuncture. 2011;36:85–89. [PubMed] [Google Scholar]
- 92.Chen K., Dong W. Study on effect of Erigeron injection in prevention and treatment of cerebral ischemic injury. Chin Western Med. 1998;18:684–686. [PubMed] [Google Scholar]
- 93.Liu G., Song J., Guo Y., Wang T., Zhou Z. Astragalus injection protects cerebral ischemic injury by inhibiting neuronal apoptosis and the expression of JNK3 after cerebral ischemia reperfusion in rats. Behav Brain Funct. 2013;9:36. doi: 10.1186/1744-9081-9-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Zhang Y.J., Zhang J.J., Yan X.L., Tang Y., Liu Y. Study of cerebral protective effects of naosaitong in animals. Chin Mater Med. 2003;28:856–861. [PubMed] [Google Scholar]
- 95.Huang J., Tao J., Xue X., Yang S., Han P., Lin Z. Gua Lou Gui Zhi decoction exerts neuroprotective effects on post-stroke spasticity via the modulation of glutamate levels and AMPA receptor expression. Int J Mol Med. 2013;31:841–848. doi: 10.3892/ijmm.2013.1262. [DOI] [PubMed] [Google Scholar]
- 96.Dirnagl U., Iadecola C., Moskowitz M.A. Pathobiology of ischaemic stroke: an integrated view. Trends Neurosci. 1999;22:391–397. doi: 10.1016/s0166-2236(99)01401-0. [DOI] [PubMed] [Google Scholar]
- 97.Lipton P. Ischemic cell death in brain neurons. Physiol Rev. 1999;79:1431–1568. doi: 10.1152/physrev.1999.79.4.1431. [DOI] [PubMed] [Google Scholar]
- 98.Won S.J., Kim D.Y., Gwag B.J. Cellular and molecular pathways of ischemic neuronal death. J Biochem Mol Biol. 2002;35:67–86. doi: 10.5483/bmbrep.2002.35.1.067. [DOI] [PubMed] [Google Scholar]
- 99.Ren X.Q., Li J.S., Feng Y.M., Liu Y.Q. Neuro-protective effect of naomaitong to brain damage after focal cerebral ischemia reperfusion (I/R) in the aged rats. Chin Mater Med. 2004;29:66–70. [PubMed] [Google Scholar]
- 100.Wang Z.F., Zhong L., Li Y.S. The protective effects of Shenfu injection on the global cerebral ischemia/reperfusion injury of rats. Chin J Appl Phys. 2012;28:462–465. [PubMed] [Google Scholar]
- 101.Hu X.Y., Sun A.S., Sui Y.X. Effects of combined use of total alkaloids of Uncaria rhynchophylla and Coryadlis ambailis migo on cerebral ischemia-reperfusion injury in rats. Chin Western Med. 2007;27:1007–1009. [PubMed] [Google Scholar]
- 102.Li W.Z., Yin Y.Y., Cao X., Li W.P. Protective effects of Shexiang Xingnaonin on focal cerebral ischemia/reperfusion injury and mechanism. Chin Mater Med. 2008;33:1195–1199. [PubMed] [Google Scholar]
- 103.Zhou F.Y., Zhang J., Song T., Gao F., Wu J.M. Effects of xuezhikang and simvastatin on cerebral ischemia-reperfusion injury in rat. Chin Mater Med. 2006;31:1447–1450. [PubMed] [Google Scholar]
- 104.Wen J.Y., Chen Z.W. Protective effect of pharmacological preconditioning of total flavones of abelmoschl manihot on cerebral ischemic reperfusion injury in rats. Am J Chin Med. 2007;35:653–661. doi: 10.1142/S0192415X07005144. [DOI] [PubMed] [Google Scholar]
- 105.Zhang Y.K., Han X.Y., Che Z.Y. Effects of buyang huanwu tang combined with bone marrow mesenchymal stem cell transplantation on the expression of VEGF and Ki-67 in the brain tissue of the cerebral ischemia-reperfusion model rat. J Tradit Chin Med. 2010;30:278–282. doi: 10.1016/s0254-6272(10)60056-8. [DOI] [PubMed] [Google Scholar]
- 106.He Y., Wan H.T., Du Y.G., Bie X.D., Zhao T., Fu W. Protective effect of Danhong injection on cerebral ischemia-reperfusion injury in rats. J Ethnopharmacol. 2012;144:387–394. doi: 10.1016/j.jep.2012.09.025. [DOI] [PubMed] [Google Scholar]
- 107.Fang Y.J., Zhang Y., Ke Z.H., Zhou Z.G., Zhou F., Bai L.N. Effects of rhubarb powder on serum complement 3, complement 4, and hs-CRP in patients with intracerebral hemorrhage. Chin Western Med. 2013;33:168–171. [PubMed] [Google Scholar]
- 108.Sun M., Zhang J.J., Shan J.Z., Zhang H., Jin C.Y., Xu S. Clinical observation of Danhong Injection (herbal TCM product from Radix Salviae miltiorrhizae and Flos Carthami tinctorii) in the treatment of traumatic intracranial hematoma. Phytomedicine. 2009;16:683–689. doi: 10.1016/j.phymed.2009.03.020. [DOI] [PubMed] [Google Scholar]
- 109.Qin F., Wang C., Jin G., Hua J. Effects of Xijiao Dihuang decoction on the expressions of bcl-2, caspase-3, TNF-alpha and IL-6 after acute intracerebral hemorrhage in rats. Chin Mater Med. 2009;34:1566–1569. [PubMed] [Google Scholar]
- 110.Li L., Wang W., Wu H., Sun L., Xu J. Effects of Capsule Yizhi on the delayed neuronal death in hippocampal CA1 region and memory function after global cerebral ischemia in rats. J Chin Med Mater. 2004;27:506–509. [PubMed] [Google Scholar]
- 111.Ba E.P., Ouyang S., Li L., Xu J.P. Effects of Yizhi capsule, a preparation of traditional Chinese medicines, on delayed neuronal death in hippocampal CA1 region and memory function of rats after ischemia-reperfusion injury. J First Militar Med Univ. 2004;24:749–751. [PubMed] [Google Scholar]
- 112.Zhu X.H., Li S.J., Hu H.H., Sun L.R., Das M., Gao T.M. Neuroprotective effects of Xiao-Xu-Ming decoction against ischemic neuronal injury in vivo and in vitro. J Ethnopharmacol. 2010;127:38–46. doi: 10.1016/j.jep.2009.09.054. [DOI] [PubMed] [Google Scholar]
- 113.Chuang C.M., Hsieh C.L., Lin H.Y., Lin J.G. Panax notoginseng Burk attenuates impairment of learning and memory functions and increases ED1, BDNF and β-secretase immunoreactive cells in chronic stage ischemia-reperfusion injured rats. Am J Chin Med. 2008;36:685–693. doi: 10.1142/S0192415X08006156. [DOI] [PubMed] [Google Scholar]
- 114.Yan B., Wang D.Y., Xing D.M., Ding Y., Wang R.F., Lei F. The antidepressant effect of ethanol extract of radix puerariae in mice exposed to cerebral ischemia reperfusion. Pharmacol Biochem Behav. 2004;78:319–325. doi: 10.1016/j.pbb.2004.04.010. [DOI] [PubMed] [Google Scholar]
- 115.Xiang J., Tang Y.P., Wu P., Gao J.P., Cai D.F. Chinese medicine Nao-Shuan-Tong attenuates cerebral ischemic injury by inhibiting apoptosis in a rat model of stroke. J Ethnopharmacol. 2010;131:174–181. doi: 10.1016/j.jep.2010.06.021. [DOI] [PubMed] [Google Scholar]
- 116.Hu R., Yin C.L., Wu N., Cui G.Y., Meng H., Wu X.G. Traditional Chinese herb Dihuang Yinzi (DY) plays neuroprotective and anti-dementia role in rats of ischemic brain injury. J Ethnopharmacol. 2009;121:444–450. doi: 10.1016/j.jep.2008.09.035. [DOI] [PubMed] [Google Scholar]
- 117.Tang Y.H., Li H., Chen B.Y. Effect of active fraction of buyang huanwu decoction on caspase expression in rats after focal cerebral ischemic reperfusion. Chin Western Med. 2006;26:533–537. [PubMed] [Google Scholar]
- 118.Guo F., Lu X.W., Xu Q.P. Protective effect of Xingnaojing and Xuesaitong injections on cerebral ischemic reperfusion injury in rats. Chin Med. 2010;90:1645–1647. [PubMed] [Google Scholar]
- 119.Shi Q.H., Xiang J., Zhu X.Y., Cai D.F. Protective effects of Chinese herbal medicine Naoshuantong on neurovascular unit in rats with cerebral ischemia/reperfusion injury. Chin Integr Med. 2012;10:1135–1139. doi: 10.3736/jcim20121010. [DOI] [PubMed] [Google Scholar]
- 120.Zhou Q.P., Lu J.F., Wang H.P., Xia Q. Ginkgo biloba extract protects brain from ischemia/reperfusion injuries. J Zhejiang Univ Med Sci. 2010;39:442–447. doi: 10.3785/j.issn.1008-9292.2010.04.019. [DOI] [PubMed] [Google Scholar]
- 121.Wang L.S., Huang Y.W., Wu J.H., Lv G.B., Zhou L.L., Jia J. Effect of Buyang Huanwu decoction on amino acid content in cerebrospinal fluid of rats during ischemic/reperfusion injury. J Pharm Biomed Anal. 2013;86:143–150. doi: 10.1016/j.jpba.2013.07.046. [DOI] [PubMed] [Google Scholar]
- 122.Zhang C.Y., Du G.Y., Wang W., Ye Z.G., Wang D.Q., Sun X.F. Effects of tianma gouteng fang on transmitter amino acids in the hippocampus extracellular liquids in freely moving rats subjected to brain ischemia. Chin Mater Med. 2004;29:1061–1065. [PubMed] [Google Scholar]
- 123.Hu B., Sun S.G., Mei Y.W. Protective effect of Ginkgo biloba extract on cerebral ischemia/reperfusion injury in rats. Chin Western Med. 2003;23:436–440. [PubMed] [Google Scholar]
- 124.Zuo P.P., Liu N., Luo P.Y. Protection mechanism of huanshaodan decoction in the brain. Chin Western Med. 1997;17:420–422. [PubMed] [Google Scholar]
- 125.Cao C.Y., Du G.Y., Zuo P.P., Liu X.F., Wang W.T., Zhao Y. Protective mechanism of tianmacuzhi granules in brain. Chin Mater Med. 2001;26:269–272. [PubMed] [Google Scholar]
- 126.Zhao L.D., Wang J.H., Jin G.R., Zhao Y., Zhang H.J. Neuroprotective effect of Buyang Huanwu decoction against focal cerebral ischemia/reperfusion injury in rats–time window and mechanism. J Ethnopharmacol. 2012;140:339–344. doi: 10.1016/j.jep.2012.01.026. [DOI] [PubMed] [Google Scholar]
- 127.Bei W.J., Zang L.Q., Guo J., Peng W.L., Xu A.L., Good D.A. Neuroprotective effects of a standardized flavonoid extract from Diospyros kaki leaves. J Ethnopharmacol. 2009;126:134–142. doi: 10.1016/j.jep.2009.07.034. [DOI] [PubMed] [Google Scholar]
- 128.Xiao Z.Y., Sun C.K., Xiao X.W., Lin Y.Z., Li S., Ma H. Effects of Ginkgo biloba extract against excitotoxicity induced by NMDA receptors and mechanism thereof. Chin Med. 2006;86:2479–2484. [PubMed] [Google Scholar]
- 129.Cai Y.M., Hu H.T., Ma X.Y. Protective effect of shenqi fuzheng injection on cerebral ischemia/reperfusion injured aged rats. Chin J Integr Tradit Chin West Med. 2006;26:10–14. [PubMed] [Google Scholar]
- 130.Jin K.L., Minami M., Lan J.Q., Mao X.O., Batteur S., Simon R.P. Neurogenesis in dentate subgranular zone and rostral subventricular zone after focal cerebral ischemia in the rat. Proc Natl Acad Sci USA. 2001;98:4710–4715. doi: 10.1073/pnas.081011098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Gould E., Reeves A.J., Graziano M.S., Gross C.G. Neurogenesis in the neocortex of adult primates. Science. 1999;286:548–552. doi: 10.1126/science.286.5439.548. [DOI] [PubMed] [Google Scholar]
- 132.Jin K.L., Wang X.M., Xie L., Mao X.O., Zhu W., Wang Y. Evidence for stroke-induced neurogenesis in the human brain. Proc Natl Acad Sci USA. 2006;103:13198–13202. doi: 10.1073/pnas.0603512103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Wang L.X., Yin R.X., Sun J.B. Effect of Tongxinluo on nestin and vascular endothelial growth factor mRNA expression in rat brain tissue after cerebral ischemia-reperfusion injury. Southern Med J. 2008;28:2131–2135. [PubMed] [Google Scholar]
- 134.Yin R.X., Lu B.X., Wang L.X., Fan J.Z., Lu C.J., Liu Y.X. Nestin activation after rat cerebral ischemia-reperfusion injury and its changes in response to Tongxinluo treatment. South Med J. 2006;26:777–779. [PubMed] [Google Scholar]
- 135.Wang J., Liu X. Effects of Naoluo Xintong Recipe on expression of HSP70 and bFGF in focal ischemia-reperfusion rats. J Chin Integr Med. 2004;2:271–273. doi: 10.3736/jcim20040411. [DOI] [PubMed] [Google Scholar]
- 136.Si Y.C., Zhang J.P., Xie C.E., Zhang L.J., Jiang X.N. Effects of Panax notoginseng saponins on proliferation and differentiation of rat hippocampal neural stem cells. Am J Chin Med. 2011;39:999–1013. doi: 10.1142/S0192415X11009366. [DOI] [PubMed] [Google Scholar]
- 137.Zheng Y.Q., Liu J.X., Xu L., Yao M.J., Song W.T. Study on effect of weinaokang and bilobalide on autophagy and neurogenesis induced by focal cerebral ischemia reperfusion. Chin Mater Med. 2013;38:2182–2186. [PubMed] [Google Scholar]
- 138.Kondo Y., Kondo F., Asanuma M., Tanaka K., Ogawa N. Protective effect of oren-gedoku-to against induction of neuronal death by transient cerebral ischemia in the C57BL/6 mouse. Neurochem Res. 2000;25:205–209. doi: 10.1023/a:1007515318434. [DOI] [PubMed] [Google Scholar]
- 139.Guo C., Zhu Y.R., Weng Y., Wang S.Q., Guan Y., Wei G. Therapeutic time window and underlying therapeutic mechanism of breviscapine injection against cerebral ischemia/reperfusion injury in rats. J Ethnopharmacol. 2014;151:660–666. doi: 10.1016/j.jep.2013.11.026. [DOI] [PubMed] [Google Scholar]