Abstract
One of the early pathological hallmarks of Alzheimer׳s disease (AD) is the deposition of amyloid-β (Aβ) plaques in the brain. There has been a tremendous interest in the development of Aβ plaques imaging probes for early diagnosis of AD in the past decades. Optical imaging, particularly near-infrared fluorescence (NIRF) imaging, has emerged as a safe, low cost, real-time, and widely available technique, providing an attractive approach for in vivo detection of Aβ plaques among many different imaging techniques. In this review, we provide a brief overview of the state-of-the-art development of NIRF Aβ probes and their in vitro and in vivo applications with special focus on design strategies and optical, binding, and brain-kinetic properties.
KEY WORDS: Alzheimer׳s disease, Blood-brain barrier, Fluorescence probe, Near-infrared fluorescence, Optical imaging, Amyloid-β plagues
Abbreviations: Aβ, amyloid-β; Ach, acetylcholine; AD, Alzheimer’s disease; APP, amyloid peptide precursor; BAP, BODIPY-based Ab imaging probe; BBB, blood-brain barrier; Cy, cyanine dyes; ICG, indocyanine green dyes; MRI, magnetic resonance imaging; NIR, near-infrared; NIRF, near-infrared fluorescence; PET, positron emission tomography; ROS, reactive oxygen species; SPECT, single photon emission computed tomography
Graphical Abstract
The review highlights the recent development of near-infrared (NIR) fluorescent probes for the detection of amyloid plaques in Alzheimer’s disease (AD), an emerging area in molecular imaging and disease diagnosis. This mini review focuses on the probe-design strategy, as well as binding- and brain- kinetic properties of 6 classes of NIR imaging probes. Their pros and cons are also briefly discussed.
1. Introduction
Alzheimer׳s disease (AD) is the most common type of dementia among older people, affecting approximately 35 million people worldwide, with 5 million new cases every year1. Clinical symptoms of AD include progressive cognitive decline, irreversible memory loss, disorientation, language impairment, and emotional instability1. The dilemma places significant mental, social and economic burdens on patients, families, and communities1. Unfortunately, there are no currently effective treatments available to reverse or stop the progress of this devastating disease, primarily due to difficulties in identification of disease etiology2, 3, 4.
Several pathological hallmarks of this disease have been identified, namely, the deposition of amyloid-β (Aβ) plaques and neurofibrillary tangles, elevated reactive oxygen species (ROS), imbalanced metal ion (e.g., Cu, Fe, and Zn) homeostasis, and decreased brain acetylcholine (Ach) levels. Three major theories have been proposed to explain these pathological hallmarks: amyloid cascade3, 5, 6, oxidative stress7, 8, and the metal ion hypotheses7. The amyloid cascade hypothesis is currently the prevailing one. It is believed that the formation Aβ plaques arises from aggregation of peptides Aβ40 and Aβ42, and is the initial event in the pathogenesis of the AD. Aβ40 and Aβ42 are degradation products of amyloid peptide precursor (APP), generated from cleavage by β- and γ-secretases. These cleaved peptides have a tendency to aggregate into different Aβ species such as dimers, oligomers, fibrils, and plaques, and may also interact with metal ions and produce ROS, with subsequent neuronal atrophy and death4. Regardless of the nature of the intertwined toxicological pathways induced by Aβ aggregates, it is widely accepted that the formation of Aβ plaques precedes the clinical symptoms of AD. Therefore, they are excellent diagnostic and predictive biomarkers for the early detection of AD5, 6, 9. Moreover, the current clinical diagnosis of AD is primarily based upon family and patient׳s medical history as well as neurological and neuropsychological observations. Thus, the diagnosis is often inaccurate. Confirmative AD diagnosis can only be made through postmortem histopathological examination of brain Aβ plaques. There exists a great and urgent need to develop non-invasive and accurate probes for Aβ plaques to improve the current diagnosis of AD. Such probes will also be useful in monitoring disease progression and effectiveness of new AD treatments.
In the past decade, significant advances have been made in the design of molecular probes for specific labeling, detection, imaging of Aβ plaques both in vitro and in vivo. A number of different imaging modalities and approaches have been applied, including magnetic resonance imaging (MRI)10, 11, 12, 13, 14, positron emission tomography (PET)15, 16, 17, 18, 19, single photon emission computed tomography (SPECT)20, 21, 22, 23, 24, and optical imaging techniques25. MRI based approaches suffer from low resolution since the size of Aβ plaques typically range from 20 to 60 μm, while only larger plaques (>50 μm) are detectable26. Compared with MRI, radio-labeled PET and SPECT probes are more sensitive methods. Many probes, such as [11C]PIB27, 28, [11C]SB-1329, 30, [11C]AZD218431, [18F]FPIB32, [18F]AZD469433, 34, [18F]FDDNP35, 36, [18F]AV-137, 38, 39, [18F]AV-4540, 41, 42 and [123I]IMPY20, have advanced in clinical trials. PET-based probes are more promising in terms of their translational applications. Three PET probes [18F]FPIB (VizamylTM), [18F]AV-45 (AmyvidTM) and [18F]AV-1 (NeuraceqTM) were recently approved by the FDA. The clinical diagnostic utility of these PET imaging agents is limited: they cannot be used to confirmatively diagnose AD, only to support other diagnostic criteria43. Furthermore, the use of PET probes is limited by high cost and narrow availability, since generation of these probes needs specialized facilities that have a cyclotron for the generation of short-lived radionuclides (e.g., [11C], t1/2=20 min and [18F], t1/2=110 min) and an automated synthetic unit to produce radiolabelled probes. Compared with PET, SPECT has broader availability and lower cost as a routine diagnosis method due to the use of easily-generated radionuclides with longer half-lives (e.g., [125I], t1/2=60.1 d, [123I], t1/2=13.2 h, and [99mTc], t1/2=6.0 h). Current SPECT-based probes either have relatively high background for the radioiodinated probes due to high lipophilicity and nonspecific binding or have poor blood-brain barrier (BBB) penetration in the case of 99mTc-labeled SPECT probes. Only one SPECT probe, [123I]IMPY, has advanced in clinical trials. In general, radionuclear-based imaging modalities PET and SPECT are limited by high cost, radiation exposure, and single signal readout.
In contrast to the radionuclear-based imaging techniques, optical imaging modalities are rather inexpensive; important merits include nonradioactive, real-time imaging with the option of multi-targets tracing in vitro and in vivo, wide availability, and high-resolution imaging depending on the specific technique used44, 45, 46, 47. For in vivo applications, in order to avoid absorption and background autofluorescence and scattering of biological molecules, probe fluorescence emission wavelength in the near-infrared (NIR) region between 650 and 900 nm is advantageous so that one can achieve an optimal penetration depth and high sensitivity. Therefore, NIR fluorescence imaging has emerged as an attractive alternative to PET/SPECT and MRI techniques, and may provide a solution for the early diagnosis of AD. In the following sections, we discuss challenges and design strategies associated with the development of NIRF Aβ probes for in vivo applications, followed by a list of current reported probes and their optical, binding and brain-kinetic properties, as well as in vitro and/or in vivo studies (Table 1).
Table 1.
Summary of NIR imaging probes for Aβ plaques.
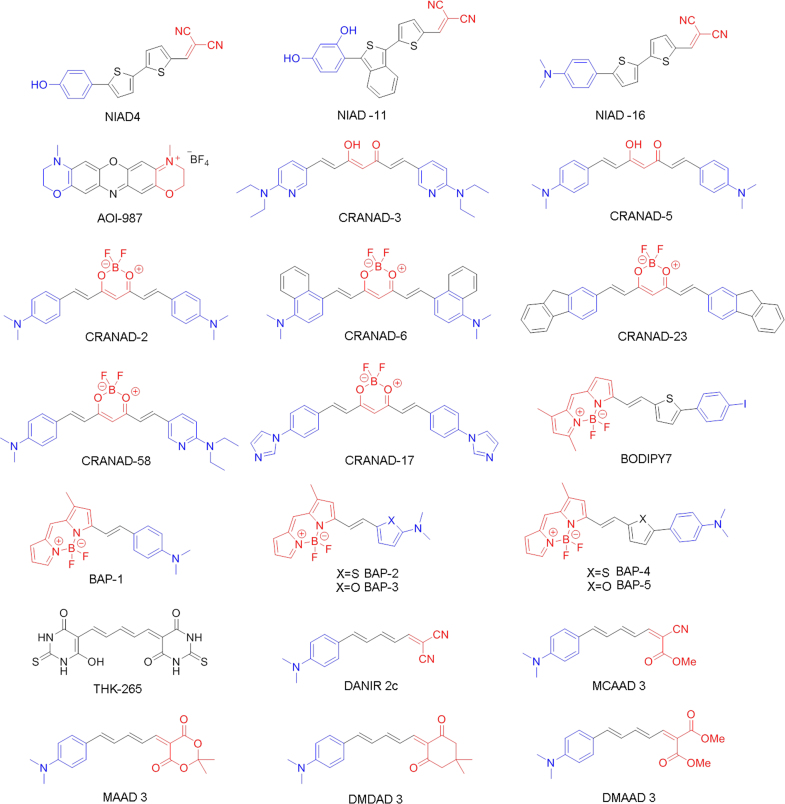
| Name | M. W. | Ki (nmol/L) | Kd (nmol/L) | clogPa (logPb) | ε (M−1 cm−1) | λabs (nm) | λex (nm) | λem (nm) (free) | λem (nm) (binding) | Φ (%) | Intensity incresement (fold) | References |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| NIAD-4 | 334.41 | 10 | – | 4.52 | 35700c | – | 492c | 603c | – | 0.008d, 5e,f | 400f | 48 |
| NIAD-11 | 400.47 | – | – | 4.77 | – | 545g | – | 690g | ~710f | 11e,f | – | 53 |
| NIAD-16 | 361.48 | – | – | 5.33 | – | 470g | – | 720g | – | – | – | 53 |
| AOI-987 | 324.35 | – | 220 | 1.66 | 61930i | 650i | – | 670i | – | 41i | – | 49 |
| THK-265 | 350.37 | – | 97 | −0.29 (1.8) | 96198c | – | 627c | 644c | 650 | 38.5c | 3.6f, 6h | 54 |
| CRANAD-2 | 410.26 | – | 38.7 | 5.56 (3.0) | – | – | 640g | 805g | 715 | 0.6d,40e,f | 70c | 50 |
| CRANAD-3 | 420.55 | – | – | 5.13 | – | – | – | 700g | 640g | – | – | 55 |
| CRANAD-58 | 439.31 | – | – | 5.67 (1.94) | – | – | ~630 | ~750 | ~700f | – | – | 56 |
| CRANAD-17 | 456.25 | – | – | 5.11 | – | – | – | ~600 | ~560f | – | – | 56 |
| BODIPY7 | 530.18 | 108 | – | 9.08 (2.2) | – | 606 | – | 613 | – | 36 | – | 57 |
| BAP-1 | 351.20 | – | 44.1 | 5.47 | – | 604j | 614j | 648j | – | 46.8j | – | 58 |
| BAP-2 | 357.23 | – | 54.6 | 5.24 | – | 651j | 650j | 708j | – | 11.4j | – | 59 |
| BAP-3 | 341.16 | – | 149 | 4.65 | – | 665j | 663j | 705j | – | 4.5j | – | 59 |
| BAP-4 | 433.32 | – | 26.8 | 7.24 | – | 623j | 636j | 704j | – | 9.3j | – | 59 |
| BAP-5 | 417.26 | – | 18.1 | 6.75 | – | 639j | 649j | 723j | – | 4.3j | – | 59 |
| DANIR 2c | 249.31 | 37 | 27 | 2.81 | 50119k | 519k | 597g | 665g | 625h | 4.09k | 12h | 60 |
| MAAD-3 | 327.37 | 354 | – | 4.28 | – | – | – | 704g | 674 g,h | 4.71 d,k 0.048d,g | 15h | 61 |
| DMDAD-3 | 323.43 | 645 | – | 4.36 | – | – | – | 725g | 694 g,h | 2.68 d,k 0.033d,g | 7h | 61 |
| MCAAD-3 | 282.34 | 106 | – | 3.16 | – | – | – | 685g | 654 g,h | 1.23d,k 0.250d,g | 26h | 61 |
| DMMAD-3 | 315.36 | 652 | – | 3.53 | – | – | – | 687g | 642 g,h | 0.10 d,k 0.068d,g | 8h | 61 |
Calculated using ChemBioDraw 12.0 software.
Experimental value.
Measured in methanol.
Quantum yield before binding.
Quantum yield after binding to Aβ fibrils/aggregates.
For Aβ40 fibrils/aggregates.
Measured in PBS.
For Aβ42 fibrils/aggregates.
Measured in serum.
Measured in chloroform.
Measured in dichloromethane.
2. Challenges in developing NIRF Aβ probes
A number of NIR fluorophores such as cyanine dyes (Cy7), indocyanine green dyes (ICG), alexa fluor dyes (660–790 nm), and SRfluor dyes have been developed and employed for in vivo applications; many of them are commercially available47. Nonetheless, these known NIR fluorophores have large molecular weight and intrinsic charges. They are likely to be unsuitable for labeling Aβ plaques in the brain because of their limited BBB permeability. In order to use a fluorophore for in vivo brain Aβ imaging, several criteria are required48, 49, 50: (1) a suitable wavelength of excitation and emission within the NIR range (650−900 nm); (2) high BBB permeability (logP values between 2 and 3.5, or clogP<5.0 are considered optimal51, 52); (3) high affinity for specific labeling of the Aβ plaques in the brain with low nonspecific binding to other proteins; (4) rapid clearance of the unbound dye from the brain; and (5) significant changes in the probe fluorescence properties upon binding to Aβ plaques. It is challenging to design probes meeting all the requirements. First, many NIR fluorophores are often highly-conjugated structures with molecular weight over 600 Da, while a small and compact scaffold with molecular weight less than 600 Da is required for NIRF Aβ probes. Secondly, the probes should have balanced lipophilicity to ensure good BBB penetration and avoid nonspecific binding. Moreover, high affinity and significant fluorescence property changes require fluorophore scaffolds which are challenging to design. Ultimately, it is difficult to predict in vivo properties of a designed NIRF probe before synthesis and testing.
3. NIRF Aβ probes
Compared with a vast number of PET/SPECT probes for Aβ plaques in the literature, there have been relatively few reports on the development of NIRF probes. This is no doubt due to the many challenges discussed in the previous section62. In this section, we cover six different kinds of NIRF Aβ imaging probes in chronological order according to their publication dates. Their structures are shown in Fig. 1. Most of them are highly conjugated molecules containing the donor-acceptor or donor-acceptor-donor architecture. Their structural features are characterized by an electron-donating group linked to an electron-withdrawing group by an highly polarized conjugated π-electron chain, leading to non-linear optical properties, such as fluorescence intensity change in response to environmental change63. Such architecture is particularly useful in the design of NIRF Aβ probes, since the recognition process often involves surrounding environmental changes of the probe48. Moreover, physical, optical, and binding properties can be rationally tailored by varying the conjugated π-chain, the donor, and the acceptor groups48, 60, 61.
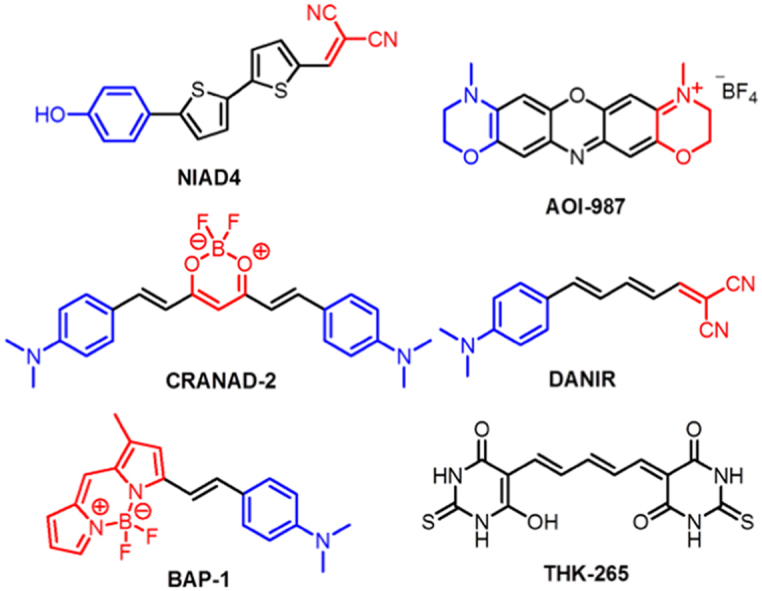
Figure 1.
Structures of NIR fluorescence probes covered in this review (donor and acceptor groups were labeled in blue and red, respectively). (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
3.1. NIAD-4 and its analogs (NIAD-4, NIAD-11, and NIAD-16)
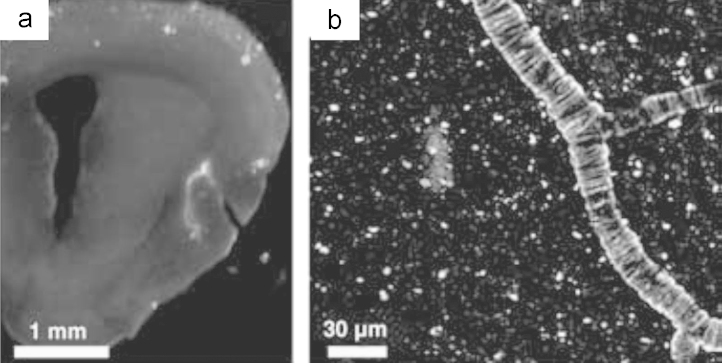
In 2005, Swager et al.48 rationally designed the fluorescent probe NIAD-4 based upon the donor-π-bridge-acceptor architecture, which utilizes a highly polarizable bisthiophene to link the donor group (p-hydroxyphenyl) and the acceptor group (dicyanomethlyene). NIAD-4 showed excellent binding affinity (Ki=10 nmol/L) for Aβ aggregates and a dramatic enhancement of the fluorescence intensity (about 400-fold) when mixing with Aβ aggregates. The increased fluorescence intensity was caused by reduced free rotation of aromatic rings in the excited state. In vivo two-photon imaging experiments in transgenic mice demonstrated NIAD-4 readily crossed the BBB after intravenous injection and labeled Aβ plaques in brain and cerebrovascular amyloid angiopathy on blood vessels (Fig. 2)48. In a separate study, NIAD-4 showed a broader pH tolerance than Thioflavin T in monitoring amyloid formation process, especially under acidic condition64. NIAD-4 presented the first example of the rational design of Aβ specific probes to achieve emission wavelength over 600 nm upon binding with Aβ aggregates. However, the maximum emission wavelength of the probe NIAD-4 is only 603 nm, not in the optimal range of 650−900 nm. Studies with this probe required the use of the invasive cranial window technique to perform in vivo fluorescence imaging, which is impractical for clinical diagnosis48. To achieve longer emissions, the same group subsequently developed a series of NIAD-4 analogs, including NIAD-11 and NIAD-1653, 65. NIAD-16 could distinguish vascular and nonvascular Aβ plaques from background signal through fluorescence lifetime imaging53.
Figure 2.
(a) In vitro staining of Aβ deposits with NIAD-4 in a coronal section of a transgenic mouse brain. Scale bar, 1 mm. (b) In vivo two-photon fluorescent image of Aβ plaques and cerebrovascular amyloid angiopathy. Scale bar, 30 μm. (Adapted with permission from Ref.48. Copyright 2005 Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim.)
3.2. AOI-987
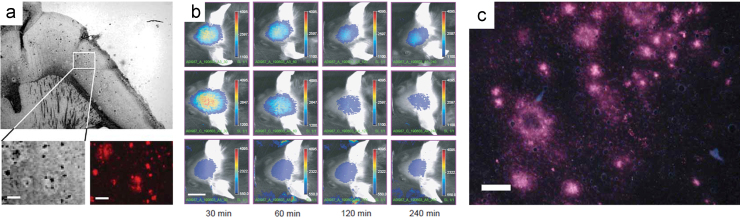
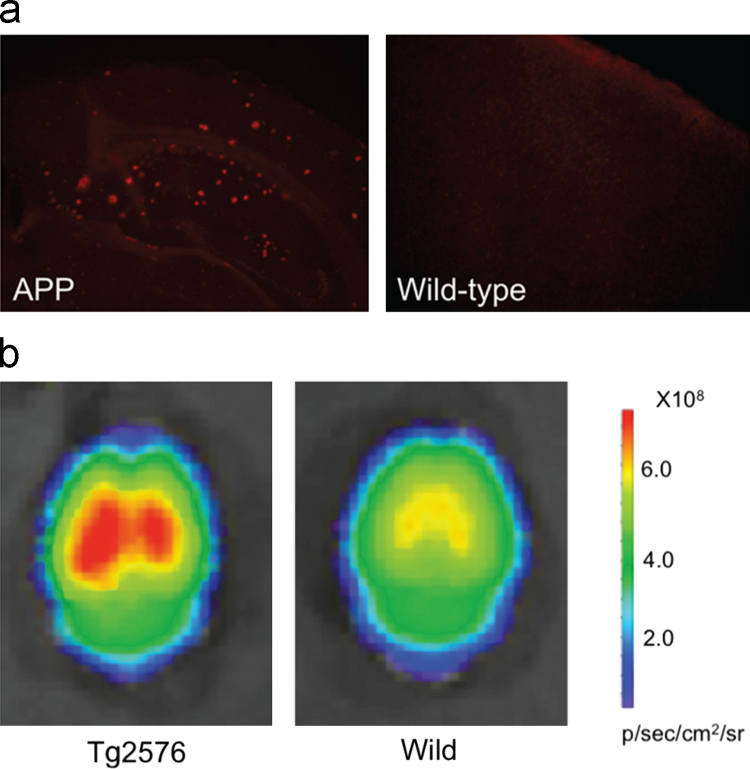
In the same year, Gremlich et al.49 at Novartis designed and synthesized longer wavelength benzophenoxazine dyes, as NIRF probes for Aβ plaques. They could monitor the progression of Aβ deposition in APP23 transgenic mice, an animal model of AD. Among them, AOI-987 offered the best in vivo results. Although AOI-987 is a charged molecule and has only moderate affinity for Aβ aggregates (Kd=220 nmol/L), the probe was able to penetrate the BBB and specifically label Aβ plaques as identified by ex vivo fluorescence imaging examination of brain slides (Fig. 3c). In addition, AOI-987 has an absorption and emission wavelength within the NIR range (650 and 670 nm, respectively) and a high quantum yield of 0.41. This fulfills the prerequisites for high sensitivity in vivo applications. In vivo time-dependent NIRF imaging experiments shown in Fig. 3b differentiated APP23 mice from wild type mice in as early as 9 months old. However, AOI-987 has the unfavorable properties of a small Stokes shift (25 nm) and marginal fluorescence changes upon mixing with Aβ aggregates. In addition, the clearance rate of AOI-987 in the brain is low and extended washout time (4 h) is needed to clearly differentiate specific and nonspecific binding in vivo49.
Figure 3.
(a) In vitro staining of Aβ plaques with AOI-987 in a transgenic mouse brain section [scale bars, 1 mm (large panel) and 100 μm (lower panels)]. (b) In vivo images of female 17-month-old APP23 transgenic (top row) and wild-type (middle row) mice at different time points (30, 60, 120, 240 min) after injected i.v. with 0.1 mg/kg AOI-987, and corresponding images of a female 17-month-old transgenic APP23 mouse treated with 0.9% saline (bottom row) (scale bar, 1 cm; color scale bars in arbitrary units). (c) Ex vivo NIRF image of a brain section (20 μm thickness) of 16-month-old female transgenic mouse administrated with 0.1 mg/kg AOI-987 (scale bar, 100 μmol/L). (Adapted with permission from Ref.49. Copyright 2005 Macmillan Publishers Ltd.: Nature Biotechnology.)
3.3. Curcumin derivatives (CRANAD-2, CRANAD-3, CRANAD-58, CRANAD-17, etc.)
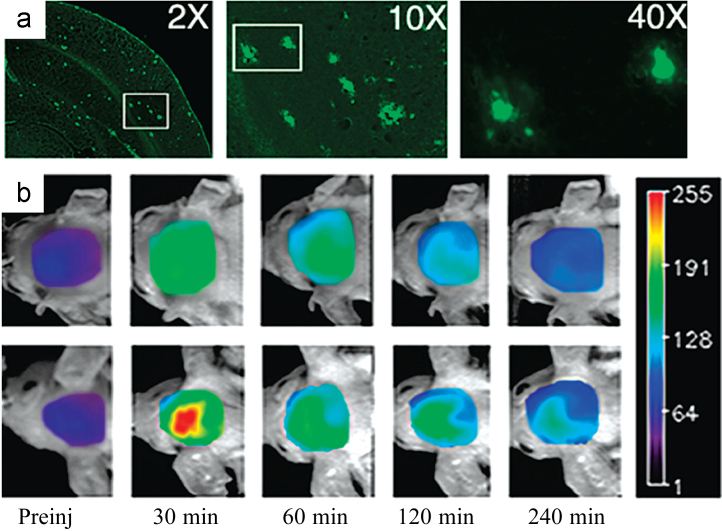
To improve detection signals with noticeable fluorescence intensity alternation and a large Stokes shift, Moore and colleagues50 designed and synthesized a novel class of NIRF probes derived from curcumin structure. In the structure, a difluoroboronate moiety and two p-dimethyamino phenyl groups were integrated into the curcumin scaffold to form a donor-acceptor-donor architecture, significantly increasing the emission wavelength to 805 nm. In this series, CRANAD-2 showed high affinity (Kd=38.7 nmol/L) and drastic fluorescence changes (70-fold fluorescence intensity increase, 90 nm hypochromic shift) upon binding to Aβ aggregates. Since bulky analogs CRANAD-6 and CRANAD-23 (structures not shown) did not show significant fluorescence change, it was assumed that the binding site of CRANAD-2 is stereo-hindered, likely to be the hydrophobic site containing the core fragment (KLVFF)56. In vitro staining experiments revealed that CRANAD-2 was capable of selective detection of Aβ plaques in a brain section from a 12-month old APP-PS1 transgenic mouse (Fig. 4a). Notably, CRANAD-2 could differentiate Tg2576 mice from wild type at an early time point (30 min) after injection by comparison of fluorescence intensities in in vivo studies (Fig. 4b). CRANAD-2 meets most of the requirements as a NIRF Aβ probe in vitro and in vivo. Compared with PIB, a well-studied PET probe for Aβ plaques, CRANAD-2 has lower brain entrance/clearance rates50. In another study reported by the same group, CRANAD-2 was used in combination with CRANAD-5 as a non-conjugated FRET pair for differentiating Aβ monomers from higher aggregated Aβ species including dimers66.
Figure 4.
(a) In vitro staining of Aβ plaques with CRANAD-2 in a twelve old APP-PS1 transgenic mouse brain section (magnification: left, 2×; middle, 10×; right, 40×). (b) In vivo images of female 19-month-old wild type (top row) and Tg2576 (bottom row) mice at different time points (30, 60, 120, 240 min) after injected i.v. with 5.0 mg/kg CRANAD-2. (Adapted with permission from Ref.50. Copyright 2009 American Chemical Society.)
By replacing of benzene with pyridine and dimethylamino with diethylamino groups in CRANAD-2, with subsequent removal of the difluoroboron bridge, the same group reported another probe, CRANAD-3, in 2012. CRANAD-3 displayed significant fluorescence property changes upon binding to Aβ aggregates. What was different from CRANAD-2 was that it also interacted with soluble Aβ monomers and dimers, and displayed fluorescence signal change. In vivo imaging studies using transgenic APP/PS1 mice exhibited that CRANAD-3 could differentiate 2 month-old APP/PS1 mice from wild type mice. Furthermore, notably, CRANAD-3 could separate specific and nonspecific binding fluorescence signal of the probe in spectral unmixing imaging studies55.
More recently, new CRANAD-2 analogs were designed and synthesized aiming for NIRF imaging of soluble and insoluble Aβ species and inhibition of copper-ion induced Aβ aggregation56. Among them, CRANAD-58 showed different fluorescence response towards soluble and insoluble Aβ species. Significant fluorescence intensity increase (91.9-fold for Aβ40, 113.6-fold for Aβ42) and high affinity (Kd=105.8 nmol/L for Aβ40, 45.8 nmol/L for Aβ42) for Aβ monomers was observed. Similar fluorescent intensity changes were also seen with Aβ dimers, but to a lesser extent. In vivo experiments revealed that CRANAD-58 was able to detect soluble Aβ species in transgenic APP/PS1 mice at the age of 4 months. Another analog, CRANAD-17, containing two copper coordinating imidazoles, could compete and interfere with copper induced crosslinking of Aβ. CRANAD-17 induced 68% more of Aβ monomers as compared with non-treated samples in in vitro anti-crosslinking studies, indicating potential usage as theranostic agent56.
3.4. BODIPY based probes (BODIPY7, BAP-1 to BAP-5)
The high quantum yield, biocompatibility, and high lipophilicity of the BODIPY fluorophore render it attractive for the design of NIRF probes. In this regard, inspired by NIAD-4, Ono and his team57 reported their first BODIPY-derived fluorescence/SPECT dual probe BODIPY7. It contains a conjugated thiophene-phenyl chain similar to NIAD-4. BODIPY7 has modest affinity (Ki=108 nmol/L) for Aβ aggregates and is able to detect Aβ plaques in in vitro staining of brain slides from an animal model of AD. The low BBB permeability, the short absorption/emission wavelength (606/613 nm), and the narrow Stokes shift restricts in vivo imaging applications57. Two years later, the same group developed a new BODIPY-based Aβ imaging probe (BAP-1) with a "privileged” p-dimethylamino phenyl group to improve in vivo properties. BAP-1 showed high affinity (Kd=44.1 nmol/L) and a significant fluorescence intensity increase upon binding to Aβ aggregates. It also has exceptional brain kinetic profiles and demonstrated specific labeling of Aβ plaques based on in vitro and ex vivo staining studies (Fig. 5). Nonetheless, it failed in in vivo imaging experiments using Tg2576 mice as the disease model, mainly due to unfavorable nonspecific binding in the scalp. Furthermore, the emission wavelength of BAP-1 (648 nm) was still short for in vivo imaging58. In 2013, several BAP-1 analogs (BAP-2, BAP-3, BAP-4, and BAP-5) with emission wavelength over 700 nm were disclosed. Similar to BAP-1, they were able to selectively label Aβ plaques in vitro and ex vivo. The probe, BAP-2, was selected for in vivo imaging but failed due to the same problem of higher accumulation in the scalp than in the brain, potentially related to high lipophilicity of the BODIPY group59. These studies reveal the issues associated with BODIPY-based probes, including narrow Stokes shifts causing potential interference from Raman and Rayleigh scattering, and high lipophilicity leading to nonspecific binding and high background in lipid membranes.
Figure 5.
(a) In vitro staining of Aβ plaques with BAP-1 in a Tg2576 mouse brain section versus a wild-type mouse brain section. (b) Comparison of the ex vivo fluorescence intensity in the brain of a 25-month-old Tg2576 and age-matched wild-type mice 1 h after intravenous administration of BAP-1. (Adapted with permission from Ref.58. Copyright 2012 American Chemical Society.)
3.5. THK-265
Inspired by the reported studies, Okamura and colleagues54 screened a collection of simple conjugated compounds, which led to the new NIRF Aβ probe, THK-265. This probe possess an emission wavelength around 650 nm combined with favorable physical properties such as high quantum yield, high molar absorption coefficients, and moderate logP value. A high binding affinity (Kd=97 nmol/L) and 6-fold fluorescence intensity increase upon mixing with Aβ42 fibrils were observed, albeit no significant change in emission wavelength. THK-265 was further evaluated for its in vivo imaging performance using AβPP transgenic mice. Such studies demonstrated that this probe crossed the BBB and selectively labeled Aβ plaques in the brain following intravenous administration. Compared with AOI-987 under the same experimental conditions, THK-265 showed an earlier differentiation time and better imaging contrast between transgenic mice and wild type and higher sensitivity for plaque detection in vivo. Most importantly, the fluorescence intensity of THK-265 correlated well with Aβ plaque burden, indicating its potential in monitoring progression of the Aβ aggregation in AD54. Subsequently, Schmidt and Pahnke67 demonstrated that indeed THK-265 could be used for direct monitoring and evaluating different cerebral Aβ aggregation levels in different stage of AD progression in an animal AD model.
3.6. DANIR 2c and its analogs (MAAD-3, DMDAD-3, MCAAD-3,and DMMAD-3)
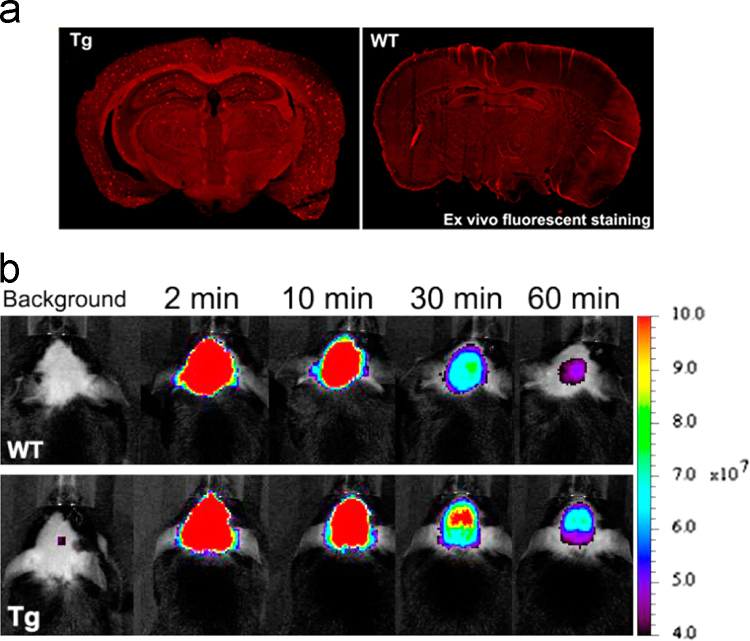
Earlier experiences from previous studies of the BODIPY series of NIRF Aβ probes led to the design of improved ones. By replacement of the undesired BODIPY with simple conjugated systems, Ono and Cui et al.60 designed and synthesized a new series of structurally simplified Aβ fluorescent probes DANIRs. The p-dimethylamino phenyl moiety was used as the donor group on one side of polymethine, with dicyanomethylene as the acceptor on the other end. This design significantly reduced molecular weights of the probes and improved brain kinetics. The best probe in the series, DANIR 2c, was able to efficiently cross the BBB and label Aβ plaques (Fig. 6a) with a fast washout rate of the unbound probe. This probe differentiated between Tg mice and wild type as early as 30 min after in vivo administration of the probe (Fig. 6b), a significantly shorter time as compared with that from AOI-987. DANIR 2c also has favorable optical properties (emission wavelength at 665 nm), a 12-fold increase in intensity upon mixing with Aβ aggregates, and excellent affinity for Aβ aggregates (Ki=37 nmol/L, Kd=27 nmol/L). DANIR 2c meets most of the requirements as an optimal probe for in vivo imaging of Aβ plaques60. One shortcoming is the blue-shift of this probe’s emission wavelength to only 625 nm (shorter than 650 nm) following binding to Aβ plaques.
Figure 6.
(a) Ex vivo image of Aβ plaques in a Tg mouse brain section treated with intravenous administration of 0.4 mg/kg DANIR 2c. (b) In vivo images of female 22-month-old wild type (top row) and APPsw/PSEN1 transgenic (bottom row) mice at different time points (2, 10, 30, 60 min) after injected with 0.4 mg/kg DANIR 2c. (Adapted with permission from Ref.60. Copyright 2014 American Chemical Society.)
Encouraged by the excellent performance of DANIR 2c, Cui et al.61 then turned to its analogs for better NIRF probes with longer emission wavelength. Four analogs MAAD-3, DMDAD-3, MCAAD-3, and DMMAD-3 were synthesized, by differing in the donor group. These analogs showed extended emission wavelength and significant reduced binding affinity to Aβ aggregates compared with DANIR 2c. Docking simulations suggested that these probes likely bind to the same binding site as IMPY, which was a thin hydrophobic groove parallel to the fibrillar axis formed by VAL 18 and PHE 20. Increased bulkiness of the acceptor group within these analogs caused a reduced binding efficiency. One analog, MCAAD-3, which had the highest affinity of 106 nmol/L among the series, was selected for in vivo imaging studies. Similar to DANIR 2c, MCAAD-3 exhibited good brain kinetics, including rapid initial uptake and fast egress. Furthermore, the latter could differentiate Tg from wild type mice at the earliest point of 30 min after dosing as well. Overall, MCAAD-3 may be a better NIRF probe for in vivo imaging than DANIR 2c, as the emission wavelength was at 654 nm when bound to Aβ aggregates.
4. Conclusions
This review highlights the development of NIRF imaging probes for in vivo detection of Aβ plaques in the past ten years. Six structurally distinct NIRF fluorophore scaffolds of Aβ probes have been developed. Most of these probes present high affinity for Aβ in vitro. As for in vivo imaging applications, pharmacokinetics-related properties are as vital as optical properties. Such pharmacokinetics considerations include in vivo stability, low-affinity for serum albumin, and reasonable lipophilicity, all of which are required for fast initial uptake into brain and fast washout to reduce nonspecific binding. In addition, significant fluorescence signal changes upon binding to Aβ are required. Other considerations regarding optical properties include absorption/excitation/emission in NIR region, high molar absorption coefficient, high quantum yield, and longer Stokes shifts. The currently reported probes fell short on one or several aspects of these required properties. NIAD-4 and DANIR 2c display a short emission wavelength, but charged AOI-987 is difficult to penetrate the BBB. CRANAD-2 has a slow egress, and BODIPYs suffer from a short Stokes shift and nonspecific binding in the scalp. Marginal fluorescent signal changes are observed with THK-265 upon binding to Aβ aggregates. MCAAD-3 has a relatively lower binding affinity than that of NIAD-4 or DANIR 2c.
Despite these concerns, development of these probes demonstrate the feasibility of NIRF imaging using Aβ specific fluorescence probes as a low-cost, convenient, readily available, and real-time approach for early diagnosis of AD in mice AD models. We believe that, in the future, the NIRF Aβ probes with enhanced pharmacokinetics and optical properties will be great benefits to human health through improved early AD diagnosis, evaluation of disease progression and clinical therapeutic outcomes.
Acknowledgments
The work was supported by the Fundamental Research Funds for the Central Universities and East China University of Science and Technology (start-up funds, Wei Wang), and the China 111 Project (Grant B07023, Wei Wang) is gratefully acknowledged.
Footnotes
Peer review under responsibility of Institute of Materia Medica, Chinese Academy of Medical Sciences and Chinese Pharmaceutical Association.
Contributor Information
Kaiyan Lou, Email: kylou@ecust.edu.cn.
Wei Wang, Email: wwang@unm.edu.
References
- 1.Thies W., Bleiler L., Alzheimer׳s Association Alzheimer׳s disease facts and figures. Alzheimers Dement. 2013;9:208–245. doi: 10.1016/j.jalz.2013.02.003. [DOI] [PubMed] [Google Scholar]
- 2.Selkoe D.J. The origins of Alzheimer disease—a is for amyloid. J Am Med Assoc. 2000;283:1615–1617. doi: 10.1001/jama.283.12.1615. [DOI] [PubMed] [Google Scholar]
- 3.Hamley I.W. The amyloid beta peptide: a chemist׳s perspective. Role in Alzheimer׳s and fibrillization. Chem Rev. 2012;112:5147–5192. doi: 10.1021/cr3000994. [DOI] [PubMed] [Google Scholar]
- 4.Savelieff M.G., DeToma A.S., Derrick J.S., Lim M.H. The ongoing search for small molecules to study metal-associated amyloid-β species in Alzheimer׳s disease. Acc Chem Res. 2014;47:2475–2482. doi: 10.1021/ar500152x. [DOI] [PubMed] [Google Scholar]
- 5.Hardy J.A., Higgins G.A. Alzheimer׳s disease: the amyloid cascade hypothesis. Science. 1992;256:184–185. doi: 10.1126/science.1566067. [DOI] [PubMed] [Google Scholar]
- 6.Hardy J., Selkoe D.J. The amyloid hypothesis of Alzheimer׳s disease: progress and problems on the road to therapeutics. Science. 2002;297:353–356. doi: 10.1126/science.1072994. [DOI] [PubMed] [Google Scholar]
- 7.Kepp K.P. Bioinorganic chemistry of Alzheimer׳s disease. Chem Rev. 2012;112:5193–5239. doi: 10.1021/cr300009x. [DOI] [PubMed] [Google Scholar]
- 8.Rauk A. The chemistry of Alzheimer׳s disease. Chem Soc Rev. 2009;38:2698–2715. doi: 10.1039/b807980n. [DOI] [PubMed] [Google Scholar]
- 9.Kung H.F. The β-amyloid hypothesis in Alzheimer׳s disease: seeing is believing. ACS Med Chem Lett. 2012;3:265–267. doi: 10.1021/ml300058m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Poduslo J.F., Wengenack T.M., Curran G.L., Wisniewski T., Sigurdsson E.M., Macura S.I. Molecular targeting of Alzheimer׳s amyloid plaques for contrast-enhanced magnetic resonance imaging. Neurobiol Dis. 2002;11:315–329. doi: 10.1006/nbdi.2002.0550. [DOI] [PubMed] [Google Scholar]
- 11.Flaherty D.P., Walsh S.M., Kiyota T., Dong Y., Ikezu T., Vennerstrom J.L. Polyfluorinated bis-styrylbenzene β-amyloid plaque binding ligands. J Med Chem. 2007;50:4986–4992. doi: 10.1021/jm070085f. [DOI] [PubMed] [Google Scholar]
- 12.Li S., He H., Cui W., Gu B., Li J., Qi Z. Detection of Aβ plaques by a novel specific MRI probe precursor CR-BSA-(Gd-DTPA)n in APP/PS1 transgenic mice. Anat Rec. 2010;293:2136–2143. doi: 10.1002/ar.21209. [DOI] [PubMed] [Google Scholar]
- 13.Martins A.F., Morfin J.F., Kubíčková A., Kubíček V., Buron F., Suzenet F. PiB-conjugated, metal-based imaging probes: multimodal approaches for the visualization of β-amyloid plaques. ACS Med Chem Lett. 2013;4:436–440. doi: 10.1021/ml400042w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sun W.G., Li H.H. Application of MRI molecular probes in the diagnosis of Alzheimer׳s disease. Prog Anat Sci. 2013;19:464–466. [Google Scholar]
- 15.Rowe C.C., Villemagne V.L. Brain amyloid imaging. J Nucl Med Technol. 2013;41:11–18. doi: 10.2967/jnumed.110.076315. [DOI] [PubMed] [Google Scholar]
- 16.Mathis C.A., Mason N.S., Lopresti B.J., Klunk W.E. Development of positron emission tomography β-amyloid plaque imaging agents. Semin Nucl Med. 2012;42:423–432. doi: 10.1053/j.semnuclmed.2012.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Koo J.H., Byun Y. Current status of PET-imaging probes of β-amyloid plaques. Arch Pharm Res. 2013;36:1178–1184. doi: 10.1007/s12272-013-0193-4. [DOI] [PubMed] [Google Scholar]
- 18.Zhu L., Ploessl K., Kung H.F. PET/SPECT imaging agents for neurodegenerative diseases. Chem Soc Rev. 2014;43:6683–6691. doi: 10.1039/c3cs60430f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Henriksen G., Yousefi B.H., Drzezga A., Wester H.J. Development and evaluation of compounds for imaging of β-amyloid plaque by means of positron emission tomography. Eur J Nucl Med Mol Imag. 2008;35 Suppl 1:S75–S81. doi: 10.1007/s00259-007-0705-x. [DOI] [PubMed] [Google Scholar]
- 20.Zhuang Z.P., Kung M.P., Wilson A., Lee C.W., Plössl K., Hou C. Structure–activity relationship of imidazo[1,2-a]pyridines as ligands for detecting β-amyloid plaques in the brain. J Med Chem. 2003;46:237–243. doi: 10.1021/jm020351j. [DOI] [PubMed] [Google Scholar]
- 21.Bois F., Baldwin R.M., Amici L., Al-Tikriti M.S., Kula N., Baldessarini R. Synthesis, radiolabeling, and baboon SPECT imaging of 2β-carbomethoxy-3β-(3׳-[123I]iodophenyl)tropane ([123I]YP256) as a serotonin transporter radiotracer ([123I]YP256) a potential serotonin transporter radiotracer) Nucl Med Biol. 2008;35:53–59. doi: 10.1016/j.nucmedbio.2007.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Watanabe H., Ono M., Kimura H., Kagawa S., Nishii R., Fuchigami T. A dual fluorinated and iodinated radiotracer for PET and SPECT imaging of β-amyloid plaques in the brain. Bioorg Med Chem Lett. 2011;21:6519–6522. doi: 10.1016/j.bmcl.2011.08.063. [DOI] [PubMed] [Google Scholar]
- 23.Cheng Y., Ono M., Kimura H., Ueda M., Saji H. Technetium-99 m labeled pyridyl benzofuran derivatives as single photon emission computed tomography imaging probes for β-amyloid plaques in Alzheimer׳s brains. J Med Chem. 2012;55:2279–2286. doi: 10.1021/jm201513c. [DOI] [PubMed] [Google Scholar]
- 24.Yang Y., Cui M., Jin B., Wang X.D., Li Z.J., Yu P.R. 99mTc-labeled dibenzylideneacetone derivatives as potential SPECT probes for in vivo imaging of β-amyloid plaque. Eur J Med Chem. 2013;64:90–98. doi: 10.1016/j.ejmech.2013.03.057. [DOI] [PubMed] [Google Scholar]
- 25.Zhang X.L., Ran C.Z. Dual functional small molecule probes as fluorophore and ligand for misfolding proteins. Curr Org Chem. 2013;17:580–593. doi: 10.2174/1385272811317060004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Golde T.E., Bacskai B.J. Bringing amyloid into focus. Nat Biotechnol. 2005;23:552–554. doi: 10.1038/nbt0505-552. [DOI] [PubMed] [Google Scholar]
- 27.Mathis C.A., Wang Y.M., Holt D.P., Huang G.F., Debnath M.L., Klunk W.E. Synthesis and evaluation of 11C-labeled 6-substituted 2-arylbenzothiazoles as amyloid imaging agents. J Med Chem. 2003;46:2740–2754. doi: 10.1021/jm030026b. [DOI] [PubMed] [Google Scholar]
- 28.Klunk W.E., Engler H., Nordberg A., Wang Y., Blomqvist G., Holt D.P. Imaging brain amyloid in Alzheimer׳s disease with Pittsburgh Compound-B. Ann Neurol. 2004;55:306–319. doi: 10.1002/ana.20009. [DOI] [PubMed] [Google Scholar]
- 29.Ono M., Wilson A., Nobrega J., Westaway D., Verhoeff P., Zhuang Z.P. 11C-labeled stilbene derivatives as Aβ-aggregate-specific PET imaging agents for Alzheimer׳s disease. Nucl Med Biol. 2003;30:565–571. doi: 10.1016/s0969-8051(03)00049-0. [DOI] [PubMed] [Google Scholar]
- 30.Verhoeff N., Wilson A.A., Takeshita S., Trop L., Hussey D., Singh K. In-vivo imaging of Alzheimer disease β-amyloid with [11C]SB-13 PET. Am J Geriatr Psychiatry. 2004;12:584–595. doi: 10.1176/appi.ajgp.12.6.584. [DOI] [PubMed] [Google Scholar]
- 31.Johnson A.E., Jeppsson F., Sandell J., Wensbo D., Neelissen J.A., Juréus A. AZD2184: a radioligand for sensitive detection of β-amyloid deposits. J Neurochem. 2009;108:1177–1186. doi: 10.1111/j.1471-4159.2008.05861.x. [DOI] [PubMed] [Google Scholar]
- 32.Vandenberghe R., van Laere K., Ivanoiu A., Salmon E., Bastin C., Triau E. 18F-flutemetamol amyloid imaging in Alzheimer disease and mild cognitive impairment: a phase 2 trial. Ann Neurol. 2010;68:319–329. doi: 10.1002/ana.22068. [DOI] [PubMed] [Google Scholar]
- 33.Juréus A., Swahn B.M., Sandell J., Jeppsson F., Johnson A.E., Johnström P. Characterization of AZD4694, a novel fluorinated Aβ plaque neuroimaging PET radioligand. J Neurochem. 2010;114:784–794. doi: 10.1111/j.1471-4159.2010.06812.x. [DOI] [PubMed] [Google Scholar]
- 34.Cselényi Z., Jönhagen M.E., Forsberg A., Halldin C., Julin P., Schou M. Clinical validation of 18F-AZD4694, an amyloid-β-specific PET radioligand. J Nucl Med. 2012;53:415–424. doi: 10.2967/jnumed.111.094029. [DOI] [PubMed] [Google Scholar]
- 35.Agdeppa E.D., Kepe V., Liu J., Flores-Torres S., Satyamurthy N., Petric A. Binding characteristics of radiofluorinated 6-dialkylamino-2-naphthylethylidene derivatives as positron emission tomography imaging probes for β-amyloid plaques in Alzheimer׳s disease. J Neurosci. 2001;21:RC189. doi: 10.1523/JNEUROSCI.21-24-j0004.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shoghi-Jadid K., Small G.W., Agdeppa E.D., Kepe V., Ercoli L.M., Siddarth P. Localization of neurofibrillary tangles and β-amyloid plaques in the brains of living patients with Alzheimer disease. Am J Geriatr Psychiatry. 2002;10:24–35. [PubMed] [Google Scholar]
- 37.Zhang W., Oya S., Kung M.P., Hou C., Maier D.L., Kung H.F. F-18 polyethyleneglycol stilbenes as PET imaging agents targeting Aβ aggregates in the brain. Nucl Med Biol. 2005;32:799–809. doi: 10.1016/j.nucmedbio.2005.06.001. [DOI] [PubMed] [Google Scholar]
- 38.Rowe C.C., Ackerman U., Browne W., Mulligan R., Pike K.L., O׳Keefe G. Imaging of amyloid β in Alzheimer׳s disease with 18F-BAY94-9172, a novel PET tracer: proof of mechanism. Lancet Neurol. 2008;7:129–135. doi: 10.1016/S1474-4422(08)70001-2. [DOI] [PubMed] [Google Scholar]
- 39.Barthel H., Gertz H.J., Dresel S., Peters O., Bartenstein P., Buerger K. Cerebral amyloid-β PET with florbetaben (18F) in patients with Alzheimer׳s disease and healthy controls: a multicentre phase 2 diagnostic study. Lancet Neurol. 2011;10:424–435. doi: 10.1016/S1474-4422(11)70077-1. [DOI] [PubMed] [Google Scholar]
- 40.Zhang W., Kung M.P., Oya S., Hou C., Kung H.F. 18F-labeled styrylpyridines as PET agents for amyloid plaque imaging. Nucl Med Biol. 2007;34:89–97. doi: 10.1016/j.nucmedbio.2006.10.003. [DOI] [PubMed] [Google Scholar]
- 41.Choi S.R., Golding G., Zhuang Z., Zhang W., Lim N., Hefti F. Preclinical properties of 18F-AV-45: a PET agent for Aβ plaques in the brain. J Nucl Med. 2009;50:1887–1894. doi: 10.2967/jnumed.109.065284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wong D.F., Rosenberg P.B., Zhou Y. In vivo imaging of amyloid deposition in Alzheimer disease using the radioligand 18F-AV-45 (florbetapir [corrected] F 18) J Nucl Med. 2010;51:913–920. doi: 10.2967/jnumed.109.069088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Williams S.C.P. Alzheimer׳s imaging agents struggle to find a market outside trials. Nat Med. 2013;19:1551. doi: 10.1038/nm1213-1551. [DOI] [PubMed] [Google Scholar]
- 44.Ntziachristos V., Bremer C., Weissleder R. Fluorescence imaging with near-infrared light: new technological advances that enable in vivo molecular imaging. Eur Radiol. 2003;13:195–208. doi: 10.1007/s00330-002-1524-x. [DOI] [PubMed] [Google Scholar]
- 45.Ntziachristos V. Fluorescence molecular imaging. Annu Rev Biomed Eng. 2006;8:1–33. doi: 10.1146/annurev.bioeng.8.061505.095831. [DOI] [PubMed] [Google Scholar]
- 46.Frangioni J.V. In vivo near-infrared fluorescence imaging. Curr Opin Chem Biol. 2003;7:626–634. doi: 10.1016/j.cbpa.2003.08.007. [DOI] [PubMed] [Google Scholar]
- 47.Licha K., Olbrich C. Optical imaging in drug discovery and diagnostic applications. Adv Drug Deliv Rev. 2005;57:1087–1108. doi: 10.1016/j.addr.2005.01.021. [DOI] [PubMed] [Google Scholar]
- 48.Nesterov E.E., Skoch J., Hyman B.T., Klunk W.E., Bacskai B.J., Swager T.M. In vivo optical imaging of amyloid aggregates in brain: design of fluorescent markers. Angew Chem Int Ed Engl. 2005;44:5452–5456. doi: 10.1002/anie.200500845. [DOI] [PubMed] [Google Scholar]
- 49.Hintersteiner M., Enz A., Frey P., Jaton A.L., Kinzy W., Kneuer R. In vivo detection of amyloid-β deposits by near-infrared imaging using an oxazine-derivative probe. Nat Biotechnol. 2005;23:577–583. doi: 10.1038/nbt1085. [DOI] [PubMed] [Google Scholar]
- 50.Ran C., Xu X., Raymond S.B., Ferrara B.J., Neal K., Bacskai B.J. Design, synthesis, and testing of difluoroboron-derivatized curcumins as near-infrared probes for in vivo detection of amyloid-β deposits. J Am Chem Soc. 2009;131:15257–15261. doi: 10.1021/ja9047043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ametamey S.M., Honer M., Schubiger P.A. Molecular imaging with PET. Chem Rev. 2008;108:1501–1516. doi: 10.1021/cr0782426. [DOI] [PubMed] [Google Scholar]
- 52.Clark DE. Computational prediction of blood-brain barrier permeation. In Doherty A, editor. Annual reports in medicinal chemistry, San Diego: Elsevier Academic Press; 2005. p, 403–15
- 53.Raymond S.B., Skoch J., Hills I.D., Nesterov E.E., Swager T.M., Bacskai B.J. Smart optical probes for near-infrared fluorescence imaging of Alzheimer׳s disease pathology. Eur J Nucl Med Mol Imaging. 2008;35 Suppl 1:S93–S98. doi: 10.1007/s00259-007-0708-7. [DOI] [PubMed] [Google Scholar]
- 54.Okamura N., Mori M., Furumoto S., Yoshikawa T., Harada R., Ito S. In vivo detection of amyloid plaques in the mouse brain using the near-infrared fluorescence probe THK-265. J Alzheimers Dis. 2011;23:37–48. doi: 10.3233/JAD-2010-100270. [DOI] [PubMed] [Google Scholar]
- 55.Ran C., Moore A. Spectral unmixing imaging of wavelength-responsive fluorescent probes: an application for the real-time report of amyloid β species in Alzheimer׳s disease. Mol Imaging Biol. 2012;14:293–300. doi: 10.1007/s11307-011-0501-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhang X.L., Tian Y.L., Li Z., Tian X.Y., Sun H.B., Liu H. Design and synthesis of curcumin analogues for in vivo fluorescence imaging and inhibiting copper-induced cross-linking of amyloid β species in Alzheimer׳s disease. J Am Chem Soc. 2013;135:16397–16409. doi: 10.1021/ja405239v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ono M., Ishikawa M., Kimura H., Hayashi S., Matsumura K., Watanabe H. Development of dual functional SPECT/fluorescent probes for imaging cerebral β-amyloid plaques. Bioorg Med Chem Lett. 2010;20:3885–3888. doi: 10.1016/j.bmcl.2010.05.027. [DOI] [PubMed] [Google Scholar]
- 58.Ono M., Watanabe H., Kimura H., Saji H. BODIPY-based molecular probe for imaging of cerebral β-amyloid plaques. ACS Chem Neurosci. 2012;3:319–324. doi: 10.1021/cn3000058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Watanabe H., Ono M., Matsumura K., Yoshimura M., Kimura H., Saji H. Molecular imaging of β-amyloid plaques with near-infrared boron dipyrromethane (BODIPY)-based fluorescent probes. Mol Imaging. 2013;12:338–347. [PubMed] [Google Scholar]
- 60.Cui M.C., Ono M., Watanabe H., Kimura H., Liu B.L., Saji H. Smart near-infrared fluorescence probes with donor-acceptor structure for in vivo detection of β-amyloid deposits. J Am Chem Soc. 2014;136:3388–3394. doi: 10.1021/ja4052922. [DOI] [PubMed] [Google Scholar]
- 61.Fu H., Cui M., Tu P., Pan Z., Liu B. Evaluation of molecules based on the electron donor–acceptor architecture as near-infrared β-amyloidal-targeting probes. Chem Commun. 2014;50:11875–11878. doi: 10.1039/c4cc04907a. [DOI] [PubMed] [Google Scholar]
- 62.Cui M.C. Past and recent progress of molecular imaging probes for β-amyloid plaques in the brain. Curr Med Chem. 2014;21:82–112. doi: 10.2174/09298673113209990216. [DOI] [PubMed] [Google Scholar]
- 63.Meyers F., Marder S.R., Pierce B.M., Bredas J.L. Electric-field modulated nonlinear-optical properties of donor-acceptor polyenes: sum-over-states investigation of the relationship between molecular polarizabilities (α, β and γ) and bond-length alternation. J Am Chem Soc. 1994;116:10703–10714. [Google Scholar]
- 64.Brandenburg E., von Berlepsch H., Koksch B. Specific in situ discrimination of amyloid fibrils versus α-helical fibres by the fluorophore NIAD-4. Mol BioSyst. 2012;8:557–564. doi: 10.1039/c1mb05370a. [DOI] [PubMed] [Google Scholar]
- 65.Meek S.T., Nesterov E.E., Swager T.M. Near-infrared fluorophores containing benzo[c]heterocycle subunits. Org Lett. 2008;10:2991–2993. doi: 10.1021/ol800988w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ran C.Z., Zhao W., Moir R.D., Moore A. Non-conjugated small molecule FRET for differentiating monomers from higher molecular weight amyloid β species. PLoS One. 2011;6:e19362. doi: 10.1371/journal.pone.0019362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Schmidt A., Pahnke J. Efficient near-infrared in vivo imaging of amyoid-β deposits in Alzheimer׳s disease mouse models. J Alzheimers Dis. 2012;30:651–664. doi: 10.3233/JAD-2012-112168. [DOI] [PubMed] [Google Scholar]