Abstract
The liver is the central organ involved in lipid metabolism. Dyslipidemia and its related disorders, including non-alcoholic fatty liver disease (NAFLD), obesity and other metabolic diseases, are of increasing public health concern due to their increasing prevalence in the population. Besides their well-characterized functions in cholesterol homoeostasis and nutrient absorption, bile acids are also important metabolic regulators and function as signaling hormones by activating specific nuclear receptors, G-protein coupled receptors, and multiple signaling pathways. Recent studies identified a new signaling pathway by which conjugated bile acids (CBA) activate the extracellular regulated protein kinases (ERK1/2) and protein kinase B (AKT) signaling pathway via sphingosine-1-phosphate receptor 2 (S1PR2). CBA-induced activation of S1PR2 is a key regulator of sphingosine kinase 2 (SphK2) and hepatic gene expression. This review focuses on recent findings related to the role of bile acids/S1PR2-mediated signaling pathways in regulating hepatic lipid metabolism.
Key words: Bile acid, Sphingosine-1 phosphate receptor, Heptic lipid metabolism
Abbreviations: ABC, ATP-binding cassette; AKT/PKB, protein kinase B; BSEP/ABCB11, bile salt export protein; CA, cholic acid; CBA, conjugated bile acids; CDCA, chenodeoxycholic acid; CYP27A1, sterol 27-hydroxylase; CYP7A1, cholesterol 7α-hydroxylase; CYP7B1, oxysterol 7α-hydroxylase; CYP8B1, 12α-hydroxylase; DCA, deoxycholic acid; EGFR, epidermal growth factor receptor; ERK, extracellular regulated protein kinases; FGF15/19, fibroblast growth factor 15/19; FGFR, fibroblast growth factor receptor; FXR, farnesoid X receptor; G-6-Pase, glucose-6-phophatase; GPCR, G-protein coupled receptor; HDL, high density lipoprotein; HNF4α, hepatocyte nuclear factor-4α; IBAT, ileal sodium-dependent bile acid transporter; JNK1/2, c-Jun N-terminal kinase; LCA, lithocholic acid; LDL, low-density lipoprotein; LRH-1, liver-related homolog-1; M1–5, muscarinic receptor 1–5; MMP, matrix metalloproteinase; NAFLD, non-alcoholic fatty liver disease; NK, natural killer cells; NTCP, sodium taurocholate cotransporting polypeptide; PEPCK, PEP carboxykinse; PTX, pertussis toxin; S1P, sphingosine-1-phosphate; S1PR2, sphingosine-1-phosphate receptor 2; SHP, small heterodimer partner; SphK, sphingosine kinase; SPL, S1P lyase; Spns2, spinster homologue 2; SPPs, S1P phosphatases; SRC, proto-oncogene tyrosine-protein kinase; TCA, taurocholate; TGR5, G-protein-coupled bile acid receptor; TNFα, tumor necrosis factor α; VLDL, very-low-density lipoprotein
Graphical abstract
This review focuses on recent findings related to the role of bile acids/S1PR2-mediated signaling pathways in regulating hepatic lipid metabolism.
1. Introduction
Bile acids are synthesized from cholesterol and are known to solubilize cholesterol in the gallbladder and promote the digestion and absorption of dietary fats and fat-soluble vitamins (A, D, E and K) in the intestines. The production of bile acids is also known to be one of the predominant mechanisms for excretion of excess cholesterol from the body1. In addition to their beneficial effects, bile acids also produce toxic effects in the liver. It is becoming increasingly evident that bile acids exert various biological effects by activating different signaling pathways. However, the concept of bile acids acting as signaling molecules is recent. It was not until the past two decades that studies have reported that bile acids act as natural ligands for the farnesoid X receptor (FXRα)2, 3, 4. Following this discovery, bile acids have been shown to activate other nuclear receptors (pregnane X receptor, vitamin D receptor), G protein coupled receptors (GPCRs) (G-protein-coupled bile acid receptor 5 (TGR5), muscarinic receptor 2 (M2), sphingosine-1-phosphate receptor 2 (S1PR2)) and cellular signaling pathways (c-Jun N-terminal kinase (JNK1/2), protein kinase B (AKT), and extracellular regulated protein kinases (ERK1/2))5, 6. Bile acids have also been implicated in the inflammatory response and various liver diseases, as well as the promotion of cancers such as colon cancer and cholangiocarcinoma6, 7, 8. The emerging role of bile acids as hormones and nutrient signaling molecules helped contribute to our understanding of glucose and lipid metabolism. In this review, we will discuss our current understanding of how bile acids and the S1PR2 regulate hepatic lipid metabolism.
2. Enterohepatic circulation of bile acids
Bile acids are synthesized from cholesterol in the hepatocytes and are actively transported into the bile duct system using ATP-binding cassette (ABC) transporter after conjugation with glycine or taurine. Hepatocytes secrete bile acids via bile salt export proteins (BSEP, ABCB11) along with phosphatidylcholine by ABCB4 and cholesterol by ABCG5/ABCG89, 10. As detergent molecules, bile acids keep cholesterol in solution within the gallbladder by forming micelles with cholesterol and phospholipids. The ratio of conjugated bile acids, cholesterol and phospholipids is highly regulated and excess cholesterol has been linked to an increased risk for cholesterol gallstone formation11. Bile is stored in the gallbladder and excreted into the duodenum in response to eating to activate pancreatic lipases and solubilize lipids to promote dietary fat absorption. Approximately 95% of bile acids are reabsorbed through the ileum by ileal sodium-dependent bile acid transporter (IBAT, SLC10A2)12, 13. Bile acids reabsorbed from the intestines travel through the portal blood and return to the liver via the sodium taurocholate cotransporting polypeptide (NTCP, SLC10A1)14. A small portion of primary bile acids are converted into secondary bile acids by anaerobic gut bacteria, which can be either passively absorbed from the large intestine or secreted in the feces. During enterohepatic circulation, bile acids lost through fecal excretion must be replenished by de novo bile acid synthesis.
3. Bile acid synthesis
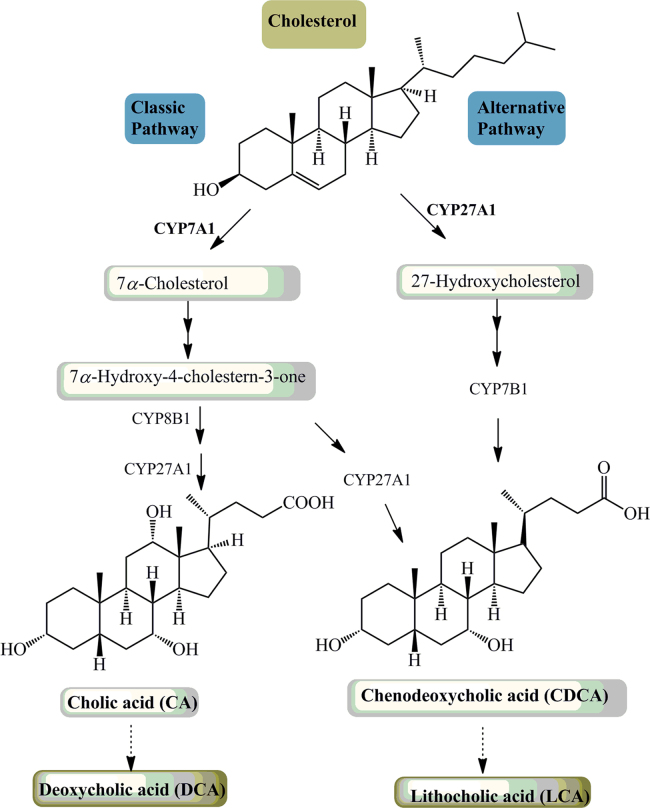
Bile acids are direct end-products of cholesterol catabolism. In humans, two primary bile acids, CA (3α, 7α, 12α-trihydroxy-cholanoic acid or cholic acid) and CDCA (3α, 7α-dihydroxy-cholanoic acid or chenodeoxycholic acid), are formed in the liver through two synthetic pathways, the neutral pathway and the acidic pathway (Fig. 1). The neutral pathway, also called the classic pathway, is the major pathway of generating bile acids for humans under physiological conditions and produces both CA and CDCA. The initiation of bile acid synthesis involves the enzyme cholesterol 7α-hydroxylase (CYP7A1) to catalyze the 7α-hydroxylation of cholesterol. In this rate-limiting step, CYP7A1 gene expression is tightly regulated at the transcriptional level and by a negative feedback mechanism involving bile acids, glucagon, tumor necrosis factor α (TNFα) and fibroblast growth factor 15/19 (FGF15/19). In ileocytes, bile acids stimulate the production of FGF15/19 which can bind to the fibroblast growth factor receptor 4 (FGFR4)/β-Klotho complex on the cell membrane of hepatocytes and regulate bile acids and carbohydrate metabolism via activating several signaling cascades including JNK1/2 and ERK1/215, 16, 17. Activation of the JNK1/2 pathway has been shown to repress Cyp7a1 gene expression in hepatocytes18. Fgfr4 and β-Klotho null mice have been shown to contain increased Cyp7a1 mRNA levels and bile acid levels. These results demonstrate the critical role FGF15/19, an FXR target gene, plays in the regulation of CYP7A1 and bile acid synthesis. In addition, FXRα can induce the expression of an atypical orphan nuclear receptor, small heterodimer partner (SHP). SHP has no DNA-binding domain and functions as a common transcriptional repressor of nuclear receptors. SHP can form a heterodimer with several transcription factors, including hepatocyte nuclear factor 4α (HNF-4α) and liver-related homolog-1 (LRH-1), to inhibit their transactivation activities, which results in inhibiting Cyp7a1 and sterol 12α-hydroxylase (Cyp8b1) transcription19, 20.
Figure 1.
Bile acid synthesis and metabolism. Two major pathways are involved in bile acid synthesis. The neutral (or classic) pathway is controlled by CYP7A1 in the endoplasmic reticulum. The acidic (or alternative) pathway is initiated by sterol CYP27A1 in mitochondria. CYP8B1 is required to synthesize CA. Oxysterol 7α-hydroxylase (CYP7B1) is involved in the formation of CDCA in the acidic pathway. The neutral pathway is also able to form CDCA by CYP27A1. Primary bile acids are metabolized by gut bacteria to form the secondary bile acids, DCA and LCA.
The acidic pathway is initiated by sterol 27-hydroxylase (CYP27A1) in the mitochondrial inner membrane and has been shown to be more active in cirrhosis and various liver diseases21, 22. Since cholesterol concentration is very low in the inner mitochondrial membrane, the rate limiting step in acidic pathway may be the transport of cholesterol into the mitochondrion. The acidic pathway generates mostly CDCA. In addition to the liver, CYP27A1 is ubiquitously expressed in most tissues including the macrophages. CYP27A1 can catalyze cholesterol to form oxysterols by introducing a hydroxyl group to the carbon at either the 27 or 25 position in cholesterol23, 24, 25. The products, 27-hydroxycholesterol and 25-hydroxycholesterol, are known to be regulatory oxysterols that are important in maintaining cholesterol and fat levels in the liver26.
The primary bile acids CA and CDCA are converted into deoxycholic acid (DCA) and lithocholic acid (LCA) respectively by a small population of intestinal anaerobic bacteria.
4. Sphingosine-1-phosphate and sphingosine-1-phosphate receptors
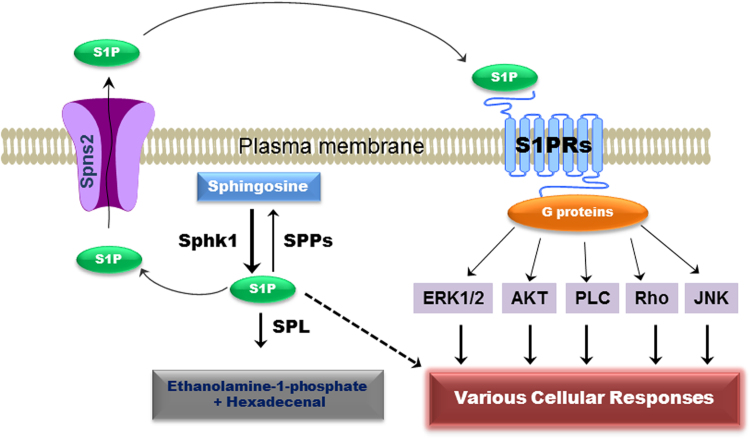
Sphingosine-1-phosphate (S1P) is a simple, but potent bioactive sphingolipid. It is involved in a variety of cellular processes, including cell proliferation, differentiation, motility, angiogenesis, inflammation and malignant transformation27. S1P is synthesized by sphingosine kinase 1 (SphK1) or sphingosine kinase 2 (SphK2), which transfer the phosphate from ATP to the 1-hydroxyl group of sphingosine. SphK1 and SphK2 reside in different subcellular compartments and regulate the production of S1P. SphK1 is located in the cytoplasm, and upon an external signal, it translocates to the plasma membrane to convert sphingosine to S1P. Intracellular S1P can act as a signaling molecule, but its target has not been well-characterized. S1P can be converted to sphingosine by cytosolic S1P phosphatases or degraded by S1P lyase (SPL) to ethanolamine phosphate and hexadecanal. In order to activate the cell surface GPCRs, S1P must be transported to the cell׳s exterior, but S1P is a small polar phospholipid and cannot easily pass the membrane lipid bilayer without transporters. ABC transporters, including ABCA1 and ABCC1, have been identified as active transporters of S1P28, 29. In addition, spinster homologue 2 (Spns2), a member of the major facilitator superfamily, has been shown to mediate S1P secretion30, 31. Extracellular S1P exerts its function via activating five different G protein coupled receptors (S1PR1–5) on the cell membrane to induce various cellular responses32, 33, 34 (Fig. 2). In the nucleus, pERK1/2 phosphorylates and activates SphK2 to synthesize S1P. Nuclear S1P has been identified as a potent histone deacetylase 1 and 2 inhibitor34.
Figure 2.
Sphingosine-1-phosphate-mediated signaling pathways. Intracellular S1P is synthesized from sphingosine by SphK1. S1P can be converted back to sphingosine by S1P phosphatases (SPPs) or degraded to ethanolamine-1-phosphate and hexadecenal by SPL. Intracellular S1P can directly activate various cellular signaling pathways or be exported out of cells by specific transporters in the cell membrane. Extracellular S1P exerts its biological functions through activation of five G-protein coupled receptors, which are coupled to different G proteins and activate different cellular responses.
S1PRs are differentially expressed in various tissues, and the individual S1PR expression level varies under different physiological and pathological conditions35. Each S1PR is coupled to specific G proteins, which mediate unique functions. S1PR1 is ubiquitously expressed and plays a key role in angiogenesis, vascular maturation and immune cell trafficking36, 37. Deletion of S1PR1 affects maturation and is embryonically lethal38. S1PR1 has been reported only to be linked with Gαi39. S1PR2 is highly expressed in vascular smooth muscle cells, heart, liver, kidney, spleen, lung and brain40, 41. Unlike S1PR1, mice deficient in S1PR2 exhibit no phenotypic defects, but develop spontaneous and sporadic seizures42. S1pr2−/− mouse studies have also shown S1PR2 to be responsible for proper development of the auditory and vestibular systems43. In addition, S1PR2 is coupled to different G proteins. Each of these G proteins activates different pathways involved in various biological processes. S1PR2 has been reported to be required for mast cell degranulation and chemotaxis towards the site of inflammation44. S1PR3 is highly expressed in the brain, heart, lung, spleen, kidney, liver, intestine and skeletal muscle45. Similar to S1PR2, S1PR3 is also coupled to different G proteins. S1PR3 plays an important role in pulmonary epithelial and endothelial barriers46. S1PR4 is primarily expressed in leukocytes and has been suggested to be a regulator of T cell cytokine production47. S1PR5 expression is largely localized in the white matter of the central nervous system with oligodendrocytes having the highest expression. S1pr5 knockout mice did not exhibit any changes in myelination48. The functional role of S1PR5 is unclear. However, recent studies have suggested a role in natural killer cells (NK) mobilization in the immune system41, 49.
5. Bile acids, S1PR2 and lipid metabolism
The role of bile acids regulating lipid metabolism in humans has been well established. Cholesterol gallstone patients treated with CDCA experience a decrease in hepatic very-low-density lipoprotein (VLDL) production and plasma triglyceride levels. Patients with hypercholesterolemia received bile acid binding resins, which increased serum levels of VLDL-triglycerides and high density lipoprotein (HDL)-cholesterol while reducing low-density lipoprotein (LDL)-cholesterol levels50, 51, 52. It has been proposed that bile acids regulate hepatic lipid homeostasis through the coordinate regulation of Fxr, Shp, Lxr and Srebp1c genes53. In this model, FXR-induced activation of SHP interacts with LXR to suppress the transcriptional activity of SREBP-1c, a transcription factor known to induce genes involved in fatty acid, triglyceride and VLDL biosynthesis53, 54, 55. In addition, FXR induces the expression of genes that regulate lipoprotein and triglyceride metabolism, including ApoA-V, ApoC-II, ApoC-III, ApoE, PPARα and syndecan-156, 57, 58. Fxr−/− mice have been shown to accumulate hepatic lipids, cholesterol and triglycerides, whereas wild type mice exhibit decreased plasma cholesterol and triglycerides upon treatment with bile acids or FXR agonists59. The FXR inducible gene, FGF19, has been shown to repress lipogenesis and increase metabolism. Fgf19 transgenic mice exhibited resistance to diet-induced obesity and insulin resistance60, 61, 62, 63, 64. Interestingly, overexpressing Cyp7a1 in mice prevents fat-induced obesity and insulin resistance65.
S1PR2 is highly expressed in the liver and plays a unique and critical role in the pathophysiology of the liver. The role of S1PR2 in bile acid-mediated hepatic lipid metabolism was identified in recent studies. In primary rodent hepatocytes, conjugated bile acids activate S1PR2, which further activates the downstream ERK1/2 and AKT signaling pathways. Bile acid-mediated activation of ERK1/2 and AKT signaling pathway plays an important role in the regulation of hepatic glucose and lipid metabolism33, 66, 67. In primary rat hepatocytes, insulin and bile acids both activated glycogen synthase activity to a similar extent. Infusion of taurocholate (TCA) into the chronic bile fistula rat rapidly activated the AKT and ERK1/2 signaling pathway and glycogen synthase activity68. In addition, TCA induced a rapid down-regulation of the gluconeogenic genes, PEP carboxykinase (Pepck) and glucose-6-phophatase (G-6Pase) and a marked up-regulation of Shp mRNA in the livers67. These results suggest that TCA has insulin-like activity to regulate hepatic glucose metabolism both in vitro and in vivo.
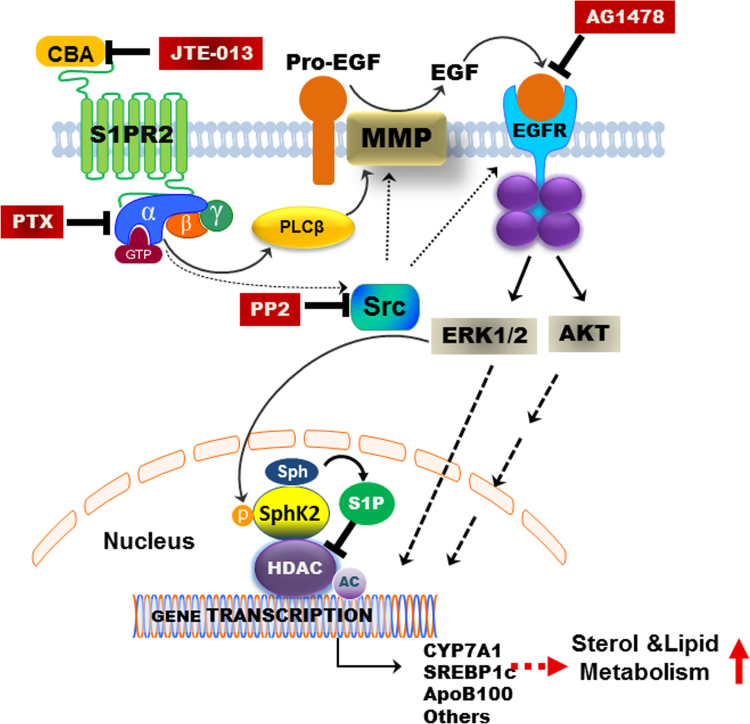
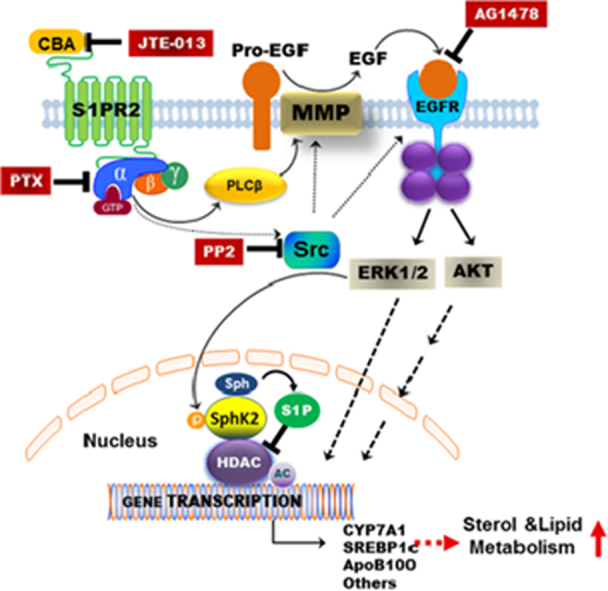
A recent study reported S1pr2 null mice rapidly develop overt fatty livers on a high fat diet compared to wild-type mice, suggesting that S1PR2 is an important regulator of hepatic lipid metabolism69. Infusion of taurocholate into the chronic bile fistula rat or overexpression of the gene encoding S1PR2 in mouse hepatocytes significantly up-regulated hepatic SphK2, but not SphK1. Interestingly, key genes encoding nuclear receptors/enzymes involved in nutrient metabolism were significantly down-regulated in livers of S1pr2−/− and Sphk2−/− mice. In contrast, overexpression of the gene encoding S1PR2 in primary mouse hepatocytes increased mRNA levels of key genes involved in nutrient metabolism. Previous studies have identified nuclear S1P as an endogenous inhibitor of HDAC1/234. In the primary hepatocytes of S1pr2−/− and Sphk2−/− mice, nuclear S1P levels and the acetylation of H3K9, H4K5 and H2BK12 were significantly decreased. Both S1pr2−/− and Sphk2−/− mice rapidly developed fatty livers on a high fat diet suggesting the importance of conjugated bile acids, S1PR2 and SphK2 in regulating hepatic lipid metabolism. Our previous studies in rodent hepatocytes and human hepatocytes also reported that bile acid-induced ERK1/2 activation can be blocked not only by pertussis toxin (PTX), but also by the epidermal growth factor receptor (EGFR; ErbB-1) antagonist (AG1478) and the proto-oncogene tyrosine-protein kinase (SRC) inhibitor PP270. As illustrated in Fig. 3, inhibition of S1PR2 activation either by chemical antagonist (JTE-013) or PTX will block ERK1/2 activation in hepatocytes, suggesting that S1PR2-mediated signaling is the upstream of EGFR-mediated signaling.
Figure 3.
Conjugated bile acids regulate sterol and lipid metabolism via activating S1PR2. In hepatocytes, CBA activates the S1PR2 on the cell membrane, which induces the activation of ERK1/2 through activation of Gi protein. Activation of Gi will further induce activation of SRC, which can activate the matrix metalloproteinase (MMP) and EGFR. EGFR-induced ERK1/2 activation can directly activate gene transcription in the nucleus or further induce the activation of nuclear SPHK2 and the increase of nuclear S1P levels. Nuclear S1P is a strong inhibitor of nuclear HDAC1/2. Inhibition of HDAC will increase the acetylation and transcription of a lot of genes involved in nutrient and lipid metabolism such as CYP7A1, SREBP1c and ApoB-100.
Inflammation is believed to be an important factor in the development of type 2 diabetes and fatty liver disease. A Western diet is correlated with low grade chronic inflammation and insulin resistance. Inhibition of the insulin signaling pathway may decrease the ability of bile acids to activate FXRα and induce SHP and other FXR target genes, leading to an increased risk of fatty liver and non-alcoholic fatty liver disease (NAFLD). It has been reported that S1P inhibits macrophage migration to the inflammation site via S1PR2 activation71. Hepatocyte proliferation and matrix remodeling occur during liver injury, and S1PR2 has been suggested to play a role in promoting the wound healing response72.
6. Conclusions and future directions
Bile acids mediate complex biological activities. Current data supports the extensive interplay between bile acid-mediated signaling and insulin signaling in the regulation of lipid and nutrient metabolism in the liver. Bile acids not only activate nuclear receptors, but also activate GPCRs. The emerging roles of bile acids and S1PR2 in hepatic lipid metabolism have paved the way for the future direction of bile acid signaling research. Currently, it is unclear what physiological role of ERK1/2 activation by bile acids and S1PR2 has on hepatic lipid metabolism. Future studies aimed at elucidating the downstream target genes of ERK1/2 and the mechanisms by which these genes are activated are important. Since the identification of GPCR, a lot of drugs have been developed to treat various diseases. GPCRs are the most successful category of drug targets to date73. Understanding the molecular basis of bile acids and S1PR2 in signaling pathways may provide an avenue for drug targets and therapeutic discoveries in various hepatic lipid disorders.
Acknowledgements
The work was supported by A.D. Williams Award (to Huiping Zhou), National Institutes of Health (NIH, No. R01 DK-057543 to Phillip B. Hylemon and Huiping Zhou). This study is also partially supported by VA Merit Awards (No. 1BX0013828-01 to Phillip B. Hylemon; No. 1I01BX001390 to Huiping Zhou).
Footnotes
Peer review under responsibility of Institute of Materia Medica, Chinese Academy of Medical Sciences and Chinese Pharmaceutical Association.
References
- 1.Zakim D, Boyer TD. Hepatology: A Textbook of Liver Disease. 3rd ed. Philadelphia: W.B. Saunders 1996.
- 2.Parks DJ, Blanchard SG, Bledsoe RK, Chandra G, Consler TG, Kliewer SA. Bile acids: natural ligands for an orphan nuclear receptor. Science. 1999;284:1365–1368. doi: 10.1126/science.284.5418.1365. [DOI] [PubMed] [Google Scholar]
- 3.Makishima M, Okamoto AY, Repa1 JJ, Tu H, Marc Learned R, Luk A. Identification of a nuclear receptor for bile acids. Science. 1999;284:1362–1365. doi: 10.1126/science.284.5418.1362. [DOI] [PubMed] [Google Scholar]
- 4.Wang H, Chen J, Hollister K, Sowers LC, Forman BM. Endogenous bile acids are ligands for the nuclear receptor FXR/BAR. Mol Cell. 1999;3:543–553. doi: 10.1016/s1097-2765(00)80348-2. [DOI] [PubMed] [Google Scholar]
- 5.Schaap FG, Trauner M, Jansen PL. Bile acid receptors as targets for drug development. Nat Rev Gastroenterol Hepatol. 2014;11:55–67. doi: 10.1038/nrgastro.2013.151. [DOI] [PubMed] [Google Scholar]
- 6.de Aguiar Vallim TQ, Tarling EJ, Edwards PA. Pleiotropic roles of bile acids in metabolism. Cell Metab. 2013;17:657–669. doi: 10.1016/j.cmet.2013.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lefebvre P, Cariou B, Lien F, Kuipers F, Staels B. Role of bile acids and bile acid receptors in metabolic regulation. Physiol Rev. 2009;89:147–191. doi: 10.1152/physrev.00010.2008. [DOI] [PubMed] [Google Scholar]
- 8.Liu R, Zhao R, Zhou X, Liang X, Campbell DJ, Zhang X. Conjugated bile acids promote cholangiocarcinoma cell invasive growth through activation of sphingosine 1-phosphate receptor 2. Hepatology. 2014;60:908–918. doi: 10.1002/hep.27085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Trauner M, Boyer JL. Bile salt transporters: molecular characterization, function, and regulation. Physiol Rev. 2003;83:633–671. doi: 10.1152/physrev.00027.2002. [DOI] [PubMed] [Google Scholar]
- 10.Quazi F, Molday RS. Lipid transport by mammalian ABC proteins. Essays Biochem. 2011;50:265–290. doi: 10.1042/bse0500265. [DOI] [PubMed] [Google Scholar]
- 11.LaRusso NF, Hoffman NE, Hofmann AF, Northfield TC, Thistle JL. Effect of primary bile acid ingestion on bile acid metabolism and biliary lipid secretion in gallstone patients. Gastroenterology. 1975;69:1301–1314. [PubMed] [Google Scholar]
- 12.Craddock AL, Love MW, Daniel RW, Kirby LC, Walters HC, Wong MH. Expression and transport properties of the human ileal and renal sodium-dependent bile acid transporter. Am J Physiol. 1998;274:G157–G169. doi: 10.1152/ajpgi.1998.274.1.G157. [DOI] [PubMed] [Google Scholar]
- 13.Hofmann AF. The continuing importance of bile acids in liver and intestinal disease. Arch Intern Med. 1999;159:2647–2658. doi: 10.1001/archinte.159.22.2647. [DOI] [PubMed] [Google Scholar]
- 14.Döring B, Lütteke T, Geyer J, Petzinger E. The SLC10 carrier family: transport functions and molecular structure. Curr Top Membr. 2012;70:105–168. doi: 10.1016/B978-0-12-394316-3.00004-1. [DOI] [PubMed] [Google Scholar]
- 15.Inagaki T, Choi M, Moschetta A, Peng L, Cummins CL, McDonald JG. Fibroblast growth factor 15 functions as an enterohepatic signal to regulate bile acid homeostasis. Cell Metab. 2005;2:217–225. doi: 10.1016/j.cmet.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 16.Potthoff MJ, Kliewer SA, Mangelsdorf DJ. Endocrine fibroblast growth factors 15/19 and 21: from feast to famine. Genes Dev. 2012;26:312–324. doi: 10.1101/gad.184788.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cicione C, Degirolamo C, Moschetta A. Emerging role of fibroblast growth factors 15/19 and 21 as metabolic integrators in the liver. Hepatology. 2012;56:2404–2411. doi: 10.1002/hep.25929. [DOI] [PubMed] [Google Scholar]
- 18.Gupta S, Stravitz RT, Dent P, Hylemon PB. Down-regulation of cholesterol 7α-hydroxylase (Cyp7a1) gene expression by bile acids in primary rat hepatocytes is mediated by the c-Jun N-terminal kinase pathway. J Biol Chem. 2001;276:15816–15822. doi: 10.1074/jbc.M010878200. [DOI] [PubMed] [Google Scholar]
- 19.Lee YK, Schmidt DR, Cummins CL, Choi M, Peng L, Zhang Y. Liver receptor homolog-1 regulates bile acid homeostasis but is not essential for feedback regulation of bile acid synthesis. Mol Endocrinol. 2008;22:1345–1356. doi: 10.1210/me.2007-0565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mataki C, Magnier BC, Houten SM, Annicotte JS, Argmann C, Thomas C. Compromised intestinal lipid absorption in mice with a liver-specific deficiency of liver receptor homolog 1. Mol Cell Biol. 2007;27:8330–8339. doi: 10.1128/MCB.00852-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Axelson M, Sjövall J. Potential bile acid precursors in plasma--possible indicators of biosynthetic pathways to cholic and chenodeoxycholic acids in man. J Steroid Biochem. 1990;36:631–640. doi: 10.1016/0022-4731(90)90182-r. [DOI] [PubMed] [Google Scholar]
- 22.Crosignani A, Del Puppo M, Longo M, De Fabiani E, Caruso D, Zuin M. Changes in classic and alternative pathways of bile acid synthesis in chronic liver disease. Clin Chim Acta. 2007;382:82–88. doi: 10.1016/j.cca.2007.03.025. [DOI] [PubMed] [Google Scholar]
- 23.Li X, Hylemon P, Pandak WM, Ren S. Enzyme activity assay for cholesterol 27-hydroxylase in mitochondria. J Lipid Res. 2006;47:1507–1512. doi: 10.1194/jlr.M600117-JLR200. [DOI] [PubMed] [Google Scholar]
- 24.Li X, Pandak WM, Erickson SK, Ma Y, Yin L, Hylemon P. Biosynthesis of the regulatory oxysterol, 5-cholesten-3β,25-diol 3-sulfate, in hepatocytes. J Lipid Res. 2007;48:2587–2596. doi: 10.1194/jlr.M700301-JLR200. [DOI] [PubMed] [Google Scholar]
- 25.Cali JJ, Russell DW. Characterization of human sterol 27-hydroxylase. A mitochondrial cytochrome P-450 that catalyzes multiple oxidation reaction in bile acid biosynthesis. J Biol Chem. 1991;266:7774–7778. [PubMed] [Google Scholar]
- 26.Björkhem I. Cerebrotendinous xanthomatosis. Curr Opin Lipidol. 2013;24:283–287. doi: 10.1097/MOL.0b013e328362df13. [DOI] [PubMed] [Google Scholar]
- 27.Maceyka M, Harikumar KB, Milstien S, Spiegel S. Sphingosine-1-phosphate signaling and its role in disease. Trends Cell Biol. 2012;22:50–60. doi: 10.1016/j.tcb.2011.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mitra P, Oskeritzian CA, Payne SG, Beaven MA, Milstien S, Spiegel S. Role of ABCC1 in export of sphingosine-1-phosphate from mast cells. Proc Natl Acad Sci USA. 2006;103:16394–16399. doi: 10.1073/pnas.0603734103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.English D, Welch Z, Kovala AT, Harvey K, Volpert OV, Brindley DN. Sphingosine 1-phosphate released from platelets during clotting accounts for the potent endothelial cell chemotactic activity of blood serum and provides a novel link between hemostasis and angiogenesis. FASEB J. 2000;14:2255–2265. doi: 10.1096/fj.00-0134com. [DOI] [PubMed] [Google Scholar]
- 30.Kawahara A, Nishi T, Hisano Y, Fukui H, Yamaguchi A, Mochizuki N. The sphingolipid transporter Spns2 functions in migration of zebrafish myocardial precursors. Science. 2009;323:524–527. doi: 10.1126/science.1167449. [DOI] [PubMed] [Google Scholar]
- 31.Fukuhara S, Simmons S, Kawamura S, Inoue A, Orba Y, Tokudome T. The sphingosine-1-phosphate transporter Spns2 expressed on endothelial cells regulates lymphocyte trafficking in mice. J Clin Invest. 2012;122:1416–1426. doi: 10.1172/JCI60746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Karimian G, Buist-Homan M, Schmidt M, Tietge UJ, de Boer JF, Klappe K. Sphingosine kinase-1 inhibition protects primary rat hepatocytes against bile salt-induced apoptosis. Biochim Biophys Acta. 2013;1832:1922–1929. doi: 10.1016/j.bbadis.2013.06.011. [DOI] [PubMed] [Google Scholar]
- 33.Strub GM, Maceyka M, Hait NC, Milstien S, Spiegel S. Extracellular and intracellular actions of sphingosine-1-phosphate. Adv Exp Med Biol. 2010;688:141–155. doi: 10.1007/978-1-4419-6741-1_10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hait NC, Allegood J, Maceyka M, Strub GM, Harikumar KB, Singh SK. Regulation of histone acetylation in the nucleus by sphingosine-1-phosphate. Science. 2009;325:1254–1257. doi: 10.1126/science.1176709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Takuwa Y, Okamoto Y, Yoshioka K, Takuwa N. Sphingosine-1-phosphate signaling in physiology and diseases. Biofactors. 2012;38:329–337. doi: 10.1002/biof.1030. [DOI] [PubMed] [Google Scholar]
- 36.Liu Y, Wada R, Yamashita T, Mi Y, Deng CX, Hobson JP. Edg-1, the G protein-coupled receptor for sphingosine-1-phosphate, is essential for vascular maturation. J Clin Invest. 2000;106:951–961. doi: 10.1172/JCI10905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Matloubian M, Lo CG, Cinamon G, Lesneski MJ, Xu Y, Brinkmann V. Lymphocyte egress from thymus and peripheral lymphoid organs is dependent on S1P receptor 1. Nature. 2004;427:355–360. doi: 10.1038/nature02284. [DOI] [PubMed] [Google Scholar]
- 38.Allende ML, Proia RL. Sphingosine-1-phosphate receptors and the development of the vascular system. Biochim Biophys Acta. 2002;1582:222–227. doi: 10.1016/s1388-1981(02)00175-0. [DOI] [PubMed] [Google Scholar]
- 39.Adada M, Canals D, Hannun YA, Obeid LM. Sphingosine-1-phosphate receptor 2. FEBS J. 2013;280:6354–6366. doi: 10.1111/febs.12446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Waeber C, Blondeau N, Salomone S. Vascular sphingosine-1-phosphate S1P1 and S1P3 receptors. Drug News Perspect. 2004;17:365–382. doi: 10.1358/dnp.2004.17.6.829028. [DOI] [PubMed] [Google Scholar]
- 41.Rosen H, Gonzalez-Cabrera PJ, Sanna MG, Brown S. Sphingosine 1-phosphate receptor signaling. Annu Rev Biochem. 2009;78:743–768. doi: 10.1146/annurev.biochem.78.072407.103733. [DOI] [PubMed] [Google Scholar]
- 42.MacLennan AJ, Carney PR, Zhu WJ, Chaves AH, Garcia J, Grimes JR. An essential role for the H218/AGR16/Edg-5/LP(B2) sphingosine 1-phosphate receptor in neuronal excitability. Eur J Neurosci. 2001;14:203–209. doi: 10.1046/j.0953-816x.2001.01634.x. [DOI] [PubMed] [Google Scholar]
- 43.MacLennan AJ, Benner SJ, Andringa A, Chaves AH, Rosing JL, Vesey R. The S1P2 sphingosine 1-phosphate receptor is essential for auditory and vestibular function. Hear Res. 2006;220:38–48. doi: 10.1016/j.heares.2006.06.016. [DOI] [PubMed] [Google Scholar]
- 44.Jolly PS, Bektas M, Olivera A, Gonzalez-Espinosa C, Proia RL, Rivera J. Transactivation of sphingosine-1-phosphate receptors by FcepsilonRI triggering is required for normal mast cell degranulation and chemotaxis. J Exp Med. 2004;199:959–970. doi: 10.1084/jem.20030680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ishii I, Friedman B, Ye X, Kawamura S, McGiffert C, Contos JJ. Selective loss of sphingosine 1-phosphate signaling with no obvious phenotypic abnormality in mice lacking its G protein-coupled receptor, LP(B3)/EDG-3. J Biol Chem. 2001;276:33697–33704. doi: 10.1074/jbc.M104441200. [DOI] [PubMed] [Google Scholar]
- 46.Gon Y, Wood MR, Kiosses WB, Jo E, Sanna MG, Chun J. S1P3 receptor-induced reorganization of epithelial tight junctions compromises lung barrier integrity and is potentiated by TNF. Proc Natl Acad Sci USA. 2005;102:9270–9275. doi: 10.1073/pnas.0501997102. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 47.Wang W, Graeler MH, Goetzl EJ. Type 4 sphingosine 1-phosphate G protein-coupled receptor (S1P4) transduces S1P effects on T cell proliferation and cytokine secretion without signaling migration. FASEB J. 2005;19:1731–1733. doi: 10.1096/fj.05-3730fje. [DOI] [PubMed] [Google Scholar]
- 48.Terai K, Soga T, Takahashi M, Kamohara M, Ohno K, Yatsugi S. Edg-8 receptors are preferentially expressed in oligodendrocyte lineage cells of the rat CNS. Neuroscience. 2003;116:1053–1062. doi: 10.1016/s0306-4522(02)00791-1. [DOI] [PubMed] [Google Scholar]
- 49.Walzer T, Chiossone L, Chaix J, Calver A, Carozzo C, Garrigue-Antar L. Natural killer cell trafficking in vivo requires a dedicated sphingosine 1-phosphate receptor. Nat Immunol. 2007;8:1337–1344. doi: 10.1038/ni1523. [DOI] [PubMed] [Google Scholar]
- 50.Grundy SM, Ahrens EH, Jr, Salen G. Interruption of the enterohepatic circulation of bile acids in man: comparative effects of cholestyramine and ileal exclusion on cholesterol metabolism. J Lab Clin Med. 1971;78:94–121. [PubMed] [Google Scholar]
- 51.Nestel PJ, Grundy SM. Changes in plasma triglyceride metabolism during withdrawal of bile. Metabolism. 1976;25:1259–1268. doi: 10.1016/s0026-0495(76)80009-1. [DOI] [PubMed] [Google Scholar]
- 52.Angelin B, Einarsson K, Hellström K, Leijd B. Effects of cholestyramine and chenodeoxycholic acid on the metabolism of endogenous triglyceride in hyperlipoproteinemia. J Lipid Res. 1978;19:1017–1024. [PubMed] [Google Scholar]
- 53.Watanabe M, Houten SM, Wang L, Moschetta A, Mangelsdorf DJ, Heyman RA. Bile acids lower triglyceride levels via a pathway involving FXR, SHP, and SREBP-1c. J Clin Invest. 2004;113:1408–1418. doi: 10.1172/JCI21025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhang Y, Castellani LW, Sinal CJ, Gonzalez FJ, Edwards PA. Peroxisome proliferator-activated receptor-γ coactivator 1α (PGC-1α) regulates triglyceride metabolism by activation of the nuclear receptor FXR. Genes Dev. 2004;18:157–169. doi: 10.1101/gad.1138104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Horton JD, Goldstein JL, Brown MS. SREBPs: activators of the complete program of cholesterol and fatty acid synthesis in the liver. J Clin Invest. 2002;109:1125–1131. doi: 10.1172/JCI15593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kast HR, Nguyen CM, Sinal CJ, Jones SA, Laffitte BA, Reue K. Farnesoid X-activated receptor induces apolipoprotein C-II transcription: a molecular mechanism linking plasma triglyceride levels to bile acids. Mol Endocrinol. 2001;15:1720–1728. doi: 10.1210/mend.15.10.0712. [DOI] [PubMed] [Google Scholar]
- 57.Houten SM, Watanabe M, Auwerx J. Endocrine functions of bile acids. EMBO J. 2006;25:1419–1425. doi: 10.1038/sj.emboj.7601049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Claudel T, Staels B, Kuipers F. The farnesoid X receptor: a molecular link between bile acid and lipid and glucose metabolism. Arterioscler Thromb Vasc Biol. 2005;25:2020–2030. doi: 10.1161/01.ATV.0000178994.21828.a7. [DOI] [PubMed] [Google Scholar]
- 59.Zhang Y, Lee FY, Barrera G, Lee H, Vales C, Gonzalez FJ. Activation of the nuclear receptor FXR improves hyperglycemia and hyperlipidemia in diabetic mice. Proc Natl Acad Sci USA. 2006;103:1006–1011. doi: 10.1073/pnas.0506982103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lundåsen T, Gälman C, Angelin B, Rudling M. Circulating intestinal fibroblast growth factor 19 has a pronounced diurnal variation and modulates hepatic bile acid synthesis in man. J Intern Med. 2006;260:530–536. doi: 10.1111/j.1365-2796.2006.01731.x. [DOI] [PubMed] [Google Scholar]
- 61.Tomlinson E, Fu L, John L, Hultgren B, Huang X, Renz M. Transgenic mice expressing human fibroblast growth factor-19 display increased metabolic rate and decreased adiposity. Endocrinology. 2002;143:1741–1747. doi: 10.1210/endo.143.5.8850. [DOI] [PubMed] [Google Scholar]
- 62.Fu L, John LM, Adams SH, Yu XX, Tomlinson E, Renz M. Fibroblast growth factor 19 increases metabolic rate and reverses dietary and leptin-deficient diabetes. Endocrinology. 2004;145:2594–2603. doi: 10.1210/en.2003-1671. [DOI] [PubMed] [Google Scholar]
- 63.Bhatnagar S, Damron HA, Hillgartner FB. Fibroblast growth factor-19, a novel factor that inhibits hepatic fatty acid synthesis. J Biol Chem. 2009;284:10023–10033. doi: 10.1074/jbc.M808818200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Miyata M, Sakaida Y, Matsuzawa H, Yoshinari K, Yamazoe Y. Fibroblast growth factor 19 treatment ameliorates disruption of hepatic lipid metabolism in farnesoid X receptor (Fxr)-null mice. Biol Pharm Bull. 2011;34:1885–1889. doi: 10.1248/bpb.34.1885. [DOI] [PubMed] [Google Scholar]
- 65.Li T, Owsley E, Matozel M, Hsu P, Novak CM, Chiang JY. Transgenic expression of cholesterol 7α-hydroxylase in the liver prevents high-fat diet-induced obesity and insulin resistance in mice. Hepatology. 2010;52:678–690. doi: 10.1002/hep.23721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Studer E, Zhou X, Zhao R, Wang Y, Takabe K, Nagahashi M. Conjugated bile acids activate the sphingosine-1-phosphate receptor 2 in primary rodent hepatocytes. Hepatology. 2012;55:267–276. doi: 10.1002/hep.24681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Cao R, Cronk ZX, Zha W, Sun L, Wang X, Fang Y. Bile acids regulate hepatic gluconeogenic genes and farnesoid X receptor via G(α)i-protein-coupled receptors and the AKT pathway. J Lipid Res. 2010;51:2234–2244. doi: 10.1194/jlr.M004929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Fang Y, Studer E, Mitchell C, Grant S, Pandak WM, Hylemon PB. Conjugated bile acids regulate hepatocyte glycogen synthase activity in vitro and in vivovia Gαi signaling. Mol Pharmacol. 2007;71:1122–1128. doi: 10.1124/mol.106.032060. [DOI] [PubMed] [Google Scholar]
- 69.Nagahashi M, Takabe K, Liu R, Peng K, Wang X, Wang Y. Conjugated bile acid activated S1P receptor 2 is a key regulator of sphingosine kinase 2 and hepatic gene expression. Hepatology. 2014 doi: 10.1002/hep.27592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Dent P, Fang Y, Gupta S, Studer E, Mitchell C, Spiegel S. Conjugated bile acids promote ERK1/2 and AKT activation via a pertussis toxin-sensitive mechanism in murine and human hepatocytes. Hepatology. 2005;42:1291–1299. doi: 10.1002/hep.20942. [DOI] [PubMed] [Google Scholar]
- 71.Michaud J, Im DS, Hla T. Inhibitory role of sphingosine 1-phosphate receptor 2 in macrophage recruitment during inflammation. J Immunol. 2010;184:1475–1483. doi: 10.4049/jimmunol.0901586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Serriere-Lanneau V, Teixeira-Clerc F, Li L, Schippers M, de Wries W, Julien B. The sphingosine 1-phosphate receptor S1P2 triggers hepatic wound healing. FASEB J. 2007;21:2005–2013. doi: 10.1096/fj.06-6889com. [DOI] [PubMed] [Google Scholar]
- 73.Guo D, Hillger JM, IJzerman AP, Heitman LH. Drug-target residence time—a case for G protein-coupled receptors. Med Res Rev. 2014;34:856–892. doi: 10.1002/med.21307. [DOI] [PubMed] [Google Scholar]