Abstract
microRNAs (miRNAs or miRs) are small non-coding RNAs that are involved in post-transcriptional regulation of their target genes in a sequence-specific manner. Emerging evidence demonstrates that miRNAs are critical regulators of lipid synthesis, fatty acid oxidation and lipoprotein formation and secretion. Dysregulation of miRNAs disrupts gene regulatory network, leading to metabolic syndrome and its related diseases. In this review, we introduced epigenetic and transcriptional regulation of miRNAs expression. We emphasized on several representative miRNAs that are functionally involved into lipid metabolism, including miR-33/33⁎, miR122, miR27a/b, miR378/378⁎, miR-34a and miR-21. Understanding the function of miRNAs in lipid homeostasis may provide potential therapeutic strategies for fatty liver disease.
KEY WORDS: Lipid metabolism, microRNAs, Nuclear receptors
Abbreviations: ABCA1, adenosine triphosphate-binding cassette transporter A1; ABCG1, adenosine triphosphate-binding cassette transporter G1; Ago2, argonaute 2; AMPKα, AMP-activated protein kinase α; ApoA1, apolipoprotein A1; ATP8B1, aminophospholipid transporter, class I, type 8B, member 1; BDL, bile-duct ligation; CPT1A, carnitine palmitoyltransferase 1A; CRAT, carnitine O-acetyltransferase; CYP26, cytochrome P450 family 26; CYP3A4, cytochrome P450 family 3 subfamily A polypeptide 4; ERRγ, estrogen-related receptor gamma; FABP7, fatty acid-binding protein 7; FASN, fatty acid synthase; FGF21, fibroblast growth factor 21; FGFR1, fibroblast growth factor receptor 1; FXR, farnesoid X receptor; GABPA, GA binding protein transcription factor alpha subunit; GPC6, glypican 6; HADHB, hydroxyacyl-CoA dehydrogenase/3-ketoacyl-CoA thiolase/enoyl-CoA hydratase trifunctional protein, beta subunit; HCC, hepatocellular carcinoma; HCV, hepatitis C virus; HMGCR, 3-hydroxy-3-methylglutaryl-coenzyme A reductase; HMGCS1, 3-hydroxy-3-methylglutaryl-coenzyme A synthase 1; HNE, 4-hydroxynonenal; IGF1R, insulin-like growth factor 1 receptor; IGFBP3, insulin-like growth factor binding protein 3; INSIG1, insulin induced gene 1; LIPE, lipase hormone-sensitive; LNA, locked nucleic acids; LNPs, lipid-based nanoparticles; LPS, lipopolysaccharide; MED13, mediator complex subunit 13; MHV68, murine γ-herpesvirus 68; miRNAs or miRs, microRNAs; MTTP, microsomal TG transfer protein; NR1D1/REV-ERBα, transcriptional repressor nuclear receptor subfamily 1 group D member 1; NRs, nuclear receptors; PCK1, phosphoenolpyruvate carboxykinase 1; PDCD4, programmed cell death 4; PGC-1, peroxisone proliferator-activated receptor gamma coactivator; PLIN1, perilipin 1; PNA, peptide nucleic acid; PNPLA2, patatin-like phospholipase domain containing 2; PPARγ, peroxisone proliferator-activated receptor gamma; pre-miRNAs, precursor-miRNAs; pri-miRNAs, primary-miRNAs; RTL1, retrotransposon-like 1; RXRα, retinoid X receptor alpha; SHP, small heterodimer partner; SIRT1, sirtuin 1; SIRT6, sirtuin 6; TG, triglyceride; TLR4, toll-like receptor 4
Graphical abstract
This review introduced epigenetic and transcriptional regulation of miRNAs expression. It emphasized on several representative miRNAs that are functionally involved into lipid metabolism, including miR-33/33⁎, miR122, miR27a/b, miR378/378⁎, miR-34a and miR-21. Understanding the function of miRNAs in lipid homeostasis may provide potential therapeutic strategies for fatty liver disease.
1. Introduction
Gene regulation networks are the representation of multiple levels within a cell1. They provide a global view intended to help understand how relationships between molecules dictate cellular behavior. The recent advances in molecular biology and cutting-edge technologies reveal the emerging role of microRNAs in major physiological and pathological processes. microRNAs are small non-coding RNAs involving in post-transcriptional regulation of gene expression by binding to the 3′-UTRs of the target mRNAs. Many microRNA targets are key regulators of lipid metabolism and disease in the liver, suggesting the functional involvement of microRNAs in this process. In this review, we provide new insight into the specific role of microRNAs in lipid metabolism.
2. microRNA regulation network
microRNA genes are normally transcribed by Polymerase II or III2 to generate primary-miRNAs (pri-miRNAs). These large RNA transcripts often contain several hundreds of base pairs in length and encode sequences for multiple miRNA genes. Before exported out of the nucleus by expertin-5, pri-miRNAs, which are capped and polyadenylated, need to be cleaved by Drosha to form hairpin-shaped stemloop structures of approximately 60–70 nucleotides (nt), namely precursor-miRNAs (pre-miRNAs). In the cytoplasm, the pre-miRNAs are further processed by a second RNase III enzyme, named Dicer, into a miRNA-miRNA⁎ duplex. One strand of the duplex, serving as the “guide” strand, is loaded into miRNP, a large multi-protein miRNA ribonucleoprotein complex, and is used to modulate target gene expression. The other strand of the duplex, namely miRNA⁎, is excluded and degraded1. When the two strands of the pre-miRNA are more equal in their distribution, the opposite stand is named miRNA-3p instead of miRNA⁎. In turn, the guide stand is referred to as miRNA-5p, depending on the location to either 5′ or 3′ of the target miRNA molecule. Both strands could potentially have biological effects3.
In addition to the canonical miRNA biogenesis pathway, alternative pathways are emerging, including Drosha/DGCR8-independent or Dicer-independent pathway4. In the Drosha/DGCR8-independent pathway, three groups of miRNAs can generate pre-miRNA-like hairpins that serve directly as Dicer substrates without Drosha/DGCR8 processing, such as Mirtrons, endo-shRNAs and murine γ-herpesvirus 68 (MHV68) tRNA-shRNA fusions2. In the Dicer-independent pathway, pri-miR-451 is processed by Drosha/DGCR8 to generate pre-miR-451 to be incorporated into argonaute 2 (Ago2), the latter cleaves the 3′ arm of pre-miR-451. However, how the mature miR-451 is generated remains unclear5, 6.
2.1. Epigenetic control of microRNA expression
Although much effort has been focused on elucidating the mechanism of miRNA biogenesis and target gene regulation, relatively little is known and published about the regulation of miRNA genes themselves. Based on the location in the genome, miRNA promoters are classified into three different conditions: 1) if miRNAs are embedded within introns or exons of host genes, they can share the same transcriptional control; 2) the embedded miRNAs also can have their own promoters; 3) if miRNAs are located in the intergenic regions, they will only be regulated by their own promoters1, 7, 8. miR-127 is located in the intron of retrotransposon-like 1 (Rtl1), an imprinted mouse retrotransposon-like gene9. It is co-regulated by the Rtl1 promoter, but is also under the control of its own promoter. The miR-127 gene overlaps with the miR-433 gene in a compact genomic space10. Their expression regulation involves epigenetic modification via DNA methylation and histone modification11. The miR-127 promoter represents an example of the complicated miRNAs transcriptional and epigenetic regulation10, 12, 13, 14, 15. The nature of miRNA promoter elements remains one of the most interesting, open problems in the study of miRNA biogenesis, since their identification would aid the understanding of regulatory networks in which miRNAs play a crucial role1.
2.2. Transcriptional control of microRNA expression by nuclear receptors
Nuclear receptors (NRs) are ligand-activated transcription factors that regulate the expression of target genes by binding to the promoters14, 15, 16. Members of the nuclear receptor superfamily have been proved as dominant regulators in lipid metabolism. Numerous NRs regulate miRNAs transcription, including farnesoid X receptor (FXR) and small heterodimer partner (SHP)1. miR-29a promoter activity was significantly increased by FXR through a likely FXR-responsive element17. FXR plays a key role in homeostasis of cholesterol and bile acids, indicating the involvement of miR-29a in such processes. A recent study by Roderburg et al.18 showed that all three members of the miR-29a family (a, b and c) were significantly down-regulated in mouse liver in both CCl4 and common bile-duct ligation (BDL) models. miR-199a-3p was also reported to be repressed by FXR19.
SHP is another important nuclear receptor to maintain cholesterol and bile acid homeostasis by inhibiting the conversion of cholesterol to bile acids. A microarray profiling revealed 21 upregulated miRNAs clustered on chromosome 12, including miR-433 and miR-127, in Shp−/− mice10, 12, 13, 20, 21, 22. Further study identified CREB, which controls hepatic lipid metabolism by repression of peroxisone proliferator-activated receptor gamma (PPARγ) and induction of peroxisone proliferator-activated receptor gamma coactivator (PGC-1), is one of the targets of miR-43320, 23. In addition, miR-433 expression is altered in the adipose tissue of patients with non-alcoholic fatty liver disease24.
3. miRNA regulation of lipid metabolism
Lipid metabolism was reviewed in great detail elsewhere25. Dyslipidemia is strongly associated with metabolic syndrome, obesity, diabetes and fatty liver disease26. Recent studies identified miRNAs in the regulation of plasma levels of lipoproteins and intercellular signaling molecules27, 28. Several representative miRNAs have been shown to have a significant impact on lipid homeostasis29, 30.
3.1. miR-122
miR-122 is not only well known as the first identified miRNA to regulate lipid metabolism, but also the liver-enriched and liver-specific miRNA. miR-122-deletion in whole body or specifically in the liver showed a marked decrease in total serum cholesterol and triglyceride (TG) levels. Anti-miR-122 therapy resulted in a significant reduction (25%–30%) of circulating cholesterol levels27, 29, 31. A set of cholesterol biosynthesis genes was downregulated by an indirect mechanism32, including 3-hydroxy-3-methylglutaryl-coenzyme A synthase 1 (HMGCS1), 3-hydroxy-3-methylglutaryl-coenzyme A reductase (HMGCR) and microsomal TG transfer protein (MTTP)29. The identification of SWI/SNF related matrix associated actin dependent regulator of chromatin subfamily d member 1 (Smarcd1/Baf60a) as novel miR-122 targets suggests the direct regulation of these genes33. Interestingly, the miR-122 locus is transcribed in a circadian fashion, involving the transcriptional repressor nuclear receptor subfamily 1 group D member 1 (NR1D1/REV-ERBα). In liver, most metabolic pathways are under the circadian control. Both lipid and cholesterol metabolism are well known for their daytime-dependent regulation, similar to many other hepatic functions that require coordination of food intake with nutrient-procession and energy homeostasis. The molecular mechanism of miR-122 could represent a novel way of regulation of the circadian rhythm and hepatic metabolism33.
In addition, miR-122 is involved in several hepatic disorders, as down-regulation of miR-122 is often associated with hepatocellular carcinoma (HCC), and miR-122 is found to bind to two sites in the 5′-UTR of the hepatitis C virus (HCV) genome and enhance its translation and replication34, 35.
3.2. miR-33/33⁎
miR-33 is another extensively investigated microRNA that modulates genes involved in lipid metabolism36. The family of miR-33 includes two members, miR-33a and miR-33b. miR-33a and miR-33b are located in intronic regions within sterol regulatory element-binding proteins SREBP-2 and SREBP-1, respectively37. As key transcriptional regulators of lipid metabolism, SREBPs control many genes involved in cholesterol and fatty acid biosynthesis and uptake, along with TG and phospholipids production38. Both miRNAs are co-transcribed with their host genes and regulate similar physiological processes. Silencing of miR-33 in vivo increased plasma HDL levels by targeting adenosine triphosphate-binding cassette transporter A1 (ABCA1) and adenosine triphosphate-binding cassette transporter G1 (ABCG1), thereby attenuating cholesterol efflux to apolipoprotein A1 (ApoA1) or reducing cholesterol efflux to nascent HDL39, 40. Moreover, miR-33-deficient mice had significantly higher serum HDL cholesterol levels41. Interestingly, in addition to other targets of miR-33, including AMP-activated protein kinase α (AMPKα), ATPase, aminophospholipid transporter, class I, type 8B, member 1 (ATP8B1), hydroxyacyl-CoA dehydrogenase/3-ketoacyl-CoA thiolase/enoyl-CoA hydratase (trifunctional protein), beta subunit (HADHB), carnitine palmitoyltransferase 1A (CPT1A), sirtuin 6 (SIRT6), glypican 6 (GPC6) and phosphoenolpyruvate carboxykinase 1 (PCK1), the host gene SREBP-1 is one of the targets of miR-33. This auto-feedback regulation fine-tuned the cholesterol homeostasis to a delicate balance42.
Intriguingly, as the opposite stand to miR-33, miR-33⁎ shares similar lipid metabolic network. miR-33⁎ also represses key regulators involved in cholesterol homeostasis and specifically targets cholesterol efflux (NPC1), fatty acid metabolism (CROT), insulin signaling (IRS2) and lipid metabolism (SRC1, SRC3 and NFYC)43. Although antagonizing miR-33 may develop as an attractive strategy for raising plasma HDL and are against atherosclerosis, caution should be taken because long-term therapeutic silencing of miR-33 may increase circulating triglyceride levels44, 45, 46.
3.3. miR-27a/27b
Retinoid X receptor alpha (RXRα) plays a central role in adipogenesis through forming a heterodimer with PPARγ and other nuclear receptors. Both miR-27a and miR-27b are demonstrated to target RXRα and regulate fat metabolism47. Furthermore, PPARγ is a target of miR-27b48 and ABCA1 a target of miR-27a. Due to their sharing of the same seed region between miR-27a and miR-27b, it is reasonable to predict that both miRNAs could target PPARγ and ABCA1. Overexpression of miR-27a accelerates adipolysis by releasing more glycerol and free fatty acids in the adipocytes49 and represses lipid storage in cells. In addition, miR-27a inhibits the expression of many lipid metabolic genes, including fatty acid synthase (FASN), SREBP-1, SREBP-2, PPARα and PPARγ, as well as ApoA1, ApoB100 and ApoE350. Thus, miR-27 may regulate lipid metabolism by reducing lipid synthesis and increasing lipid secretion from cells.
3.4. miR-378/378⁎
miR-378 and miR-378⁎ are embedded in the PGC-1β gene and counterbalance the metabolic actions of PGC-1β. A reduction of adipocyte size in miR-378/378⁎ knockout mice indicates that both miRNAs are required for efficient hypertrophy and lipid uptake in white adipocytes. Several targets of miR-378/378⁎ are found, including carnitine O-acetyltransferase (CRAT), mediator complex subunit 13 (MED13)51, estrogen-related receptor gamma (ERRγ), GA binding protein transcription factor alpha subunit (GABPA)52, insulin-like growth factor 1 receptor (IGF1R)53, and ABCG154. miR-378 is also downregulated in 4-hydroxynonenal (HNE), one of several lipid oxidation products, treated cells55. In addition, C/EBPα and C/EBPβ positively increase miR-378/378⁎ transcriptional activity56, 57. Furthermore, inhibition of miR-378 attenuates lipolysis and reduces the expression of lipase hormone-sensitive (LIPE), perilipin 1 (PLIN1) and patatin-like phospholipase domain containing 2 (PNPLA2), a set of genes encoding key lipolytic regulators58.
3.5. miR-34a
miR-34a has multiple roles in the regulation of cell cycle, apoptosis, differentiation and migration because of its expressional control by p5359, 60. However, its induction by FXR raised the possibility of a new role of miR-34a in lipid metabolism61. Sirtuin1 (SIRT1) was identified as one of the targets of miR-34a. Hepatic overexpression of miR-34a increased acetylation of PGC-1α62. On the other hand, miR-34a negatively regulates RXRα through binding to its 3′-UTR and decrease the induction of cytochrome P450 family 26 (CYP26) and cytochrome P450 family 3 subfamily A polypeptide 4 (CYP3A4)63. Moreover, through targeting fibroblast growth factor receptor 1 (FGFR1), miR-34a contributes to attenuating fibroblast growth factor 21 (FGF21) signaling in obese mice. The expression of miR-34a was elevated in obesity, indicating its potential role in inhibiting fat browning and weight loss64.
3.6. miR-21
The expression of miR-21 was decreased in liver from high-fat diet-fed mice compared to chow fed mice. Overexpression of miR-21 markedly blocked stearic acid (SA) induced intracellular lipid accumulation by targeting fatty acid-binding protein 7 (FABP7)65. In miR-21 knockout mice, a group of lipid metabolic genes was changed compared with wild-type mice. PPARα and insulin-like growth factor binding protein 3 (IGFBP3) were also targeted directly by miR-2166, 67, 68. Further studies showed that miR-21 targeted programmed cell death 4 (PDCD4) to regulate lipid accumulation through the toll-like receptor 4 (TLR4) and NF-ĸB pathway in lipopolysaccharide (LPS)-stimulated macrophages68.
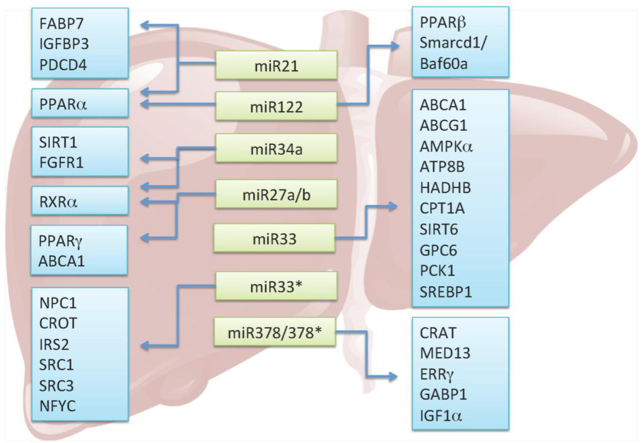
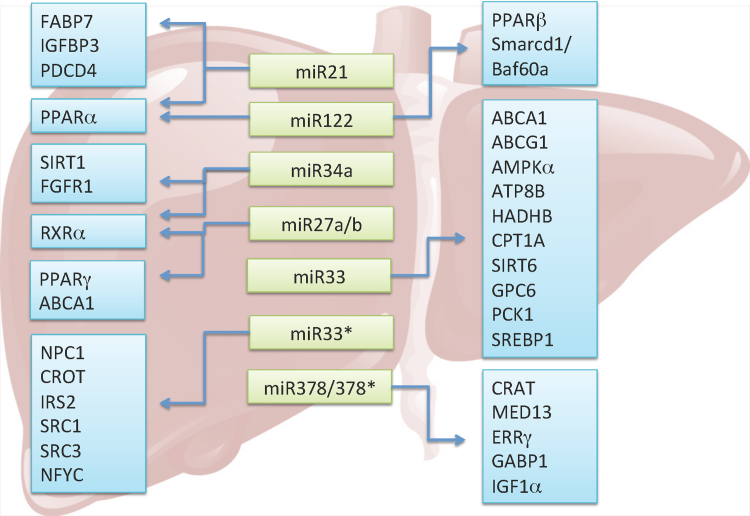
Additional miRNAs were also shown to regulate lipid metabolism. miR-758, miR-26, and miR-106b all targeted ABCA1. miR-370 targeted CPT1A and miR-24 targeted insulin induced gene 1 (INSIG1). Altogether these findings suggest that miRNAs play important roles in regulating lipid and lipoprotein homeostasis, which is summarized in Fig. 1.
Figure 1.
Regulatory role of miRNAs in lipid metabolism. Light green boxes highlight miRNAs involved in lipid metabolism. Blue boxes represent target genes of miRNAs. The miRNAs and their respective targets are linked by arrows.
4. Therapeutic potential of microRNAs
Although miRNAs represent a new class of potential targets for therapeutic intervention, there are obstacles to their clinical application, such as poor efficiency for cellular uptake. Lipofection of an antisense oligonucleotide based on a locked nucleic acids (LNA)/2′-O-methylene mixmer or electroporation of a peptide nucleic acid (PNA) oligomer is effective at blocking miR-122 activity, highlighting the use of miRNA in future therapeutic application69. A non-covalent peptide-based strategy was used for efficient delivery of miR-122 mimic or inhibitor, which appeared to be effective to alter cholesterol levels without cytotoxicity70. The recently emerged lipid-based nanoparticles (LNPs) and LNP-DP1 provide more alternate ways for delivering siRNA and miRNA in vitro and in vivo71.
5. Conclusion and perspectives
A key role of miRNAs in lipoprotein and lipid metabolism is emerging. Because multiple genes can be targeted by one miRNA and one gene may be targeted by a group of miRNAs, the mRNA and miRNA regulatory network remains complicated. Recent evidence suggests that circulating extracellular miRNAs can also be biologically active in intercellular communication72. Future research works that integrate proteomics, systems biology and high-through technologies will help to develop better therapeutic strategies that target miRNAs.
Acknowledgments
This work is supported by National Institutes of Health (Nos. R01 DK080440 and AHA 13GRNT14700043), and VA Merit Award 1I01BX002634 to Li Wang.
Footnotes
Peer review under responsibility of Institute of Materia Medica, Chinese Academy of Medical Sciences and Chinese Pharmaceutical Association.
References
- 1.Yang Z.H., Wang L. Regulation of microRNA expression and function by nuclear receptor signaling. Cell Biosci. 2011;1:31. doi: 10.1186/2045-3701-1-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yang J.S., Lai E.C. Alternative miRNA biogenesis pathways and the interpretation of core miRNA pathway mutants. Mol Cell. 2011;43:892–903. doi: 10.1016/j.molcel.2011.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guo L., Lu Z.H. The fate of miRNA⁎ strand through evolutionary analysis: implication for degradation as merely carrier strand or potential regulatory molecule? PLoS One. 2010;5:e11387. doi: 10.1371/journal.pone.0011387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ha M., Kim V.N. Regulation of microRNA biogenesis. Nat Rev Mol Cell Biol. 2014;15:509–524. doi: 10.1038/nrm3838. [DOI] [PubMed] [Google Scholar]
- 5.Cheloufi S., Dos Santos C.O., Chong M.M., Hannon G.J. A Dicer-independent miRNA biogenesis pathway that requires Ago catalysis. Nature. 2010;465:584–589. doi: 10.1038/nature09092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cifuentes D., Xue H.L., Taylor D.W., Patnode H., Mishima Y., Cheloufi S. A novel miRNA processing pathway independent of Dicer requires Argonaute2 catalytic activity. Science. 2010;328:1694–1698. doi: 10.1126/science.1190809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang H., Smith G.W., Yang Z., Jiang Y., McCloskey M., Greenberg K. Short hairpin RNA-mediated knockdown of Vegfa in Müller cells reduces intravitreal neovascularization in a rat model of retinopathy of prematurity. Am J Pathol. 2013;183:964–974. doi: 10.1016/j.ajpath.2013.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yang Z., Wang H., Jiang Y., Hartnett M.E. VEGFA activates erythropoietin receptor and enhances VEGFR2-mediated pathological angiogenesis. Am J Pathol. 2014;184:1230–1239. doi: 10.1016/j.ajpath.2013.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cui X.S., Zhang D.X., Ko Y.G., Kim N.H. Aberrant epigenetic reprogramming of imprinted microRNA-127 and Rtl1 in cloned mouse embryos. Biochem Biophys Res Commun. 2009;379:390–394. doi: 10.1016/j.bbrc.2008.12.148. [DOI] [PubMed] [Google Scholar]
- 10.Song G., Wang L. Transcriptional mechanism for the paired miR-433 and miR-127 genes by nuclear receptors SHP and ERRγ. Nucleic Acids Res. 2008;36:5727–5735. doi: 10.1093/nar/gkn567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Saito Y., Liang G., Egger G., Friedman J.M., Chuang J.C., Coetzee G.A. Specific activation of microRNA-127 with downregulation of the proto-oncogene BCL6 by chromatin-modifying drugs in human cancer cells. Cancer Cell. 2006;9:435–443. doi: 10.1016/j.ccr.2006.04.020. [DOI] [PubMed] [Google Scholar]
- 12.Song G., Wang L. miR-433 and miR-127 arise from independent overlapping primary transcripts encoded by the miR-433-127 locus. PLoS One. 2008;3:e3574. doi: 10.1371/journal.pone.0003574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Song G., Wang L. A conserved gene structure and expression regulation of miR-433 and miR-127 in mammals. PLoS One. 2009;4:e7829. doi: 10.1371/journal.pone.0007829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Song G., Wang L. Nuclear receptor SHP activates miR-206 expression via a cascade dual inhibitory mechanism. PLoS One. 2009;4:e6880. doi: 10.1371/journal.pone.0006880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Song G., Zhang Y., Wang L. microRNA-206 targets notch3, activates apoptosis, and inhibits tumor cell migration and focus formation. J Biol Chem. 2009;284:31921–31927. doi: 10.1074/jbc.M109.046862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang Y., Hagedorn C.H., Wang L. Role of nuclear receptor SHP in metabolism and cancer. Biochim Biophys Acta. 2011;1812:893–908. doi: 10.1016/j.bbadis.2010.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li J., Zhang Y., Kuruba R., Gao X., Gandhi C.R., Xie W. Roles of microRNA-29a in the antifibrotic effect of farnesoid X receptor in hepatic stellate cells. Mol Pharmacol. 2011;80:191–200. doi: 10.1124/mol.110.068247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Roderburg C., Urban G.W., Bettermann K., Vucur M., Zimmermann H., Schmidt S. micro-RNA profiling reveals a role for miR-29 in human and murine liver fibrosis. Hepatology. 2011;53:209–218. doi: 10.1002/hep.23922. [DOI] [PubMed] [Google Scholar]
- 19.Lee C.G., Kim Y.W., Kim E.H., Meng Z., Huang W., Hwang S.J. Farnesoid X receptor protects hepatocytes from injury by repressing miR-199a-3p, which increases levels of LKB1. Gastroenterology. 2012;142:1206–1217. doi: 10.1053/j.gastro.2012.01.007. (e7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yang Z., Tsuchiya H., Zhang Y., Hartnett M.E., Wang L. microRNA-433 inhibits liver cancer cell migration by repressing the protein expression and function of cAMP response element-binding protein. J Biol Chem. 2013;288:28893–28899. doi: 10.1074/jbc.M113.502682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yang Z., Zhang Y., Wang L. A feedback inhibition between miRNA-127 and TGFβ/c-Jun cascade in HCC cell migration via MMP13. PLoS One. 2013;8:e65256. doi: 10.1371/journal.pone.0065256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang Y., Yang Z., Whitby R., Wang L. Regulation of miR-200c by nuclear receptors PPARα, LRH-1 and SHP. Biochem Biophys Res Commun. 2011;416:135–139. doi: 10.1016/j.bbrc.2011.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Herzig S., Hedrick S., Morantte I., Koo S.H., Galimi F., Montminy M. CREB controls hepatic lipid metabolism through nuclear hormone receptor PPAR-γ. Nature. 2003;426:190–193. doi: 10.1038/nature02110. [DOI] [PubMed] [Google Scholar]
- 24.Estep M., Armistead D., Hossain N., Elarainy H., Goodman Z., Baranova A. Differential expression of miRNAs in the visceral adipose tissue of patients with non-alcoholic fatty liver disease. Aliment Pharmacol Ther. 2010;32:487–497. doi: 10.1111/j.1365-2036.2010.04366.x. [DOI] [PubMed] [Google Scholar]
- 25.Lavoie J.M., Gauthier M.S. Regulation of fat metabolism in the liver: link to non-alcoholic hepatic steatosis and impact of physical exercise. Cell Mol Life Sci. 2006;63:1393–1409. doi: 10.1007/s00018-006-6600-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Beaven S.W., Tontonoz P. Nuclear receptors in lipid metabolism: targeting the heart of dyslipidemia. Annu Rev Med. 2006;57:313–329. doi: 10.1146/annurev.med.57.121304.131428. [DOI] [PubMed] [Google Scholar]
- 27.Esau C., Davis S., Murray S.F., Yu X.X., Pandey S.K., Pear M. miR-122 regulation of lipid metabolism revealed by in vivo antisense targeting. Cell Metab. 2006;3:87–98. doi: 10.1016/j.cmet.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 28.Nikel P.I., Kim J., de Lorenzo V. Metabolic and regulatory rearrangements underlying glycerol metabolism in Pseudomonas putida KT2440. Environ Microbiol. 2014;16:239–254. doi: 10.1111/1462-2920.12224. [DOI] [PubMed] [Google Scholar]
- 29.Tsai W.C., Hsu S.D., Hsu C.S., Lai T.C., Chen S.J., Shen R. microRNA-122 plays a critical role in liver homeostasis and hepatocarcinogenesis. J Clin Invest. 2012;122:2884–2897. doi: 10.1172/JCI63455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Szabo G., Bala S. microRNAs in liver disease. Nat Rev Gastroenterol Hepatol. 2013;10:542–552. doi: 10.1038/nrgastro.2013.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hsu S.H., Wang B., Kota J., Yu J., Costinean S., Kutay H. Essential metabolic, anti-inflammatory, and anti-tumorigenic functions of miR-122 in liver. J Clin Invest. 2012;122:2871–2883. doi: 10.1172/JCI63539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sacco J., Adeli K. microRNAs: emerging roles in lipid and lipoprotein metabolism. Curr Opin Lipidol. 2012;23:220–225. doi: 10.1097/MOL.0b013e3283534c9f. [DOI] [PubMed] [Google Scholar]
- 33.Gatfield D., Le Martelot G., Vejnar C.E., Gerlach D., Schaad O., Fleury-Olela F. Integration of microRNA miR-122 in hepatic circadian gene expression. Genes Dev. 2009;23:1313–1326. doi: 10.1101/gad.1781009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jopling C.L., Yi M., Lancaster A.M., Lemon S.M., Sarnow P. Modulation of hepatitis C virus RNA abundance by a liver-specific microRNA. Science. 2005;309:1577–1581. doi: 10.1126/science.1113329. [DOI] [PubMed] [Google Scholar]
- 35.Thomas M., Deiters A. microRNA miR-122 as a therapeutic target for oligonucleotides and small molecules. Curr Med Chem. 2013;20:3629–3640. doi: 10.2174/0929867311320290009. [DOI] [PubMed] [Google Scholar]
- 36.Sun X., Feinberg M.W. microRNA-management of lipoprotein homeostasis. Circ Res. 2014;115:2–6. doi: 10.1161/CIRCRESAHA.114.304228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Najafi-Shoushtari S.H., Kristo F., Li Y., Shioda T., Cohen D.E., Gerszten R.E. microRNA-33 and the SREBP host genes cooperate to control cholesterol homeostasis. Science. 2010;328:1566–1569. doi: 10.1126/science.1189123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jeon T.I., Osborne T.F. SREBPs: metabolic integrators in physiology and metabolism. Trends Endocrinol Metab. 2012;23:65–72. doi: 10.1016/j.tem.2011.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rayner K.J., Suárez Y., Dávalos A., Parathath S., Fitzgerald M.L., Tamehiro N. miR-33 contributes to the regulation of cholesterol homeostasis. Science. 2010;328:1570–1573. doi: 10.1126/science.1189862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Marquart T.J., Allen R.M., Ory D.S., Baldan A. miR-33 links SREBP-2 induction to repression of sterol transporters. Proc Natl Acad Sci USA. 2010;107:12228–12232. doi: 10.1073/pnas.1005191107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Horie T., Ono K., Horiguchi M., Nishi H., Nakamura T., Nagao K. microRNA-33 encoded by an intron of sterol regulatory element-binding protein 2 (SREBP2) regulates HDL in vivo. Proc Natl Acad Sci USA. 2010;107:17321–17326. doi: 10.1073/pnas.1008499107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Horie T., Nishino T., Baba O., Kuwabara Y., Nakao T., Nishiga M. microRNA-33 regulates sterol regulatory element-binding protein 1 expression in mice. Nat Commun. 2013;4:2883. doi: 10.1038/ncomms3883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Goedeke L., Vales-Lara F.M., Fenstermaker M., Cirera-Salinas D., Chamorro-Jorganes A., Ramírez C.M. A regulatory role for microRNA 33⁎ in controlling lipid metabolism gene expression. Mol Cell Biol. 2013;33:2339–2352. doi: 10.1128/MCB.01714-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Goedeke L., Salerno A., Ramírez C.M., Guo L., Allen R.M., Yin X. Long-term therapeutic silencing of miR-33 increases circulating triglyceride levels and hepatic lipid accumulation in mice. EMBO Mol Med. 2014;6:1133–1141. doi: 10.15252/emmm.201404046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Allen R.M., Marquart T.J., Jesse J.J., Baldán A. Control of very low-density lipoprotein secretion by N-ethylmaleimide-sensitive factor and miR-33. Circ Res. 2014;115:10–22. doi: 10.1161/CIRCRESAHA.115.303100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rayner K.J., Esau C.C., Hussain F.N., McDaniel A.L., Marshall S.M., van Gils J.M. Inhibition of miR-33a/b in non-human primates raises plasma HDL and lowers VLDL triglycerides. Nature. 2011;478:404–407. doi: 10.1038/nature10486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ji J., Zhang J., Huang G., Qian J., Wang X., Mei S. Over-expressed microRNA-27a and 27b influence fat accumulation and cell proliferation during rat hepatic stellate cell activation. FEBS Lett. 2009;583:759–766. doi: 10.1016/j.febslet.2009.01.034. [DOI] [PubMed] [Google Scholar]
- 48.Jennewein C., von Knethen A., Schmid T., Brune B. microRNA-27b contributes to lipopolysaccharide-mediated peroxisome proliferator-activated receptor γ (PPARγ) mRNA destabilization. J Biol Chem. 2010;285:11846–11853. doi: 10.1074/jbc.M109.066399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang T., Li M., Guan J., Li P., Wang H., Guo Y. microRNAs miR-27a and miR-143 regulate porcine adipocyte lipid metabolism. Int J Mol Sci. 2011;12:7950–7959. doi: 10.3390/ijms12117950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shirasaki T., Honda M., Shimakami T., Horii R., Yamashita T., Sakai Y. microRNA-27a regulates lipid metabolism and inhibits hepatitis C virus replication in human hepatoma cells. J Virol. 2013;87:5270–5286. doi: 10.1128/JVI.03022-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Carrer M., Liu N., Grueter C.E., Williams A.H., Frisard M.I., Hulver M.W. Control of mitochondrial metabolism and systemic energy homeostasis by microRNAs 378 and 378⁎. Proc Natl Acad Sci USA. 2012;109:15330–15335. doi: 10.1073/pnas.1207605109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Eichner L.J., Perry M.C., Dufour C.R., Bertos N., Park M., St-Pierre J. miR-378(⁎) mediates metabolic shift in breast cancer cells via the PGC-1β/ERRγ transcriptional pathway. Cell Metab. 2010;12:352–361. doi: 10.1016/j.cmet.2010.09.002. [DOI] [PubMed] [Google Scholar]
- 53.Knezevic I., Patel A., Sundaresan N.R., Gupta M.P., Solaro R.J., Nagalingam R.S. A novel cardiomyocyte-enriched microRNA, miR-378, targets insulin-like growth factor 1 receptor: implications in postnatal cardiac remodeling and cell survival. J Biol Chem. 2012;287:12913–12926. doi: 10.1074/jbc.M111.331751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang D., Yan X., Xia M., Yang Y., Li D., Li X. Coenzyme Q10 promotes macrophage cholesterol efflux by regulation of the activator protein-1/miR-378/ATP-binding cassette transporter G1-signaling pathway. Arterioscler Thromb Vasc Biol. 2014;34:1860–1870. doi: 10.1161/ATVBAHA.113.302879. [DOI] [PubMed] [Google Scholar]
- 55.Pizzimenti S., Ferracin M., Sabbioni S., Toaldo C., Pettazzoni P., Dianzani M.U. microRNA expression changes during human leukemic HL-60 cell differentiation induced by 4-hydroxynonenal, a product of lipid peroxidation. Free Radic Biol Med. 2009;46:282–288. doi: 10.1016/j.freeradbiomed.2008.10.035. [DOI] [PubMed] [Google Scholar]
- 56.Gerin I., Bommer G.T., McCoin C.S., Sousa K.M., Krishnan V., MacDougald O.A. Roles for miRNA-378/378⁎ in adipocyte gene expression and lipogenesis. Am J Physiol Endocrinol Metab. 2010;299:E198–E206. doi: 10.1152/ajpendo.00179.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.John E., Wienecke-Baldacchino A., Liivrand M., Heinäniemi M., Carlberg C., Sinkkonen L. Dataset integration identifies transcriptional regulation of microRNA genes by PPARγ in differentiating mouse 3T3-L1 adipocytes. Nucleic Acids Res. 2012;40:4446–4460. doi: 10.1093/nar/gks025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kulyté A., Lorente-Cebrián S., Gao H., Mejhert N., Agustsson T., Arner P. microRNA profiling links miR-378 to enhanced adipocyte lipolysis in human cancer cachexia. Am J Physiol Endocrinol Metab. 2014;306:E267–E274. doi: 10.1152/ajpendo.00249.2013. [DOI] [PubMed] [Google Scholar]
- 59.Raver-Shapira N., Marciano E., Meiri E., Spector Y., Rosenfeld N., Moskovits N. Transcriptional activation of miR-34a contributes to p53-mediated apoptosis. Mol Cell. 2007;26:731–743. doi: 10.1016/j.molcel.2007.05.017. [DOI] [PubMed] [Google Scholar]
- 60.Chen F., Hu S.J. Effect of microRNA-34a in cell cycle, differentiation, and apoptosis: a review. J Biochem Mol Toxicol. 2012;26:79–86. doi: 10.1002/jbt.20412. [DOI] [PubMed] [Google Scholar]
- 61.Lee J., Padhye A., Sharma A., Song G., Miao J., Mo Y.Y. A pathway involving farnesoid X receptor and small heterodimer partner positively regulates hepatic sirtuin 1 levels via microRNA-34a inhibition. J Biol Chem. 2010;285:12604–12611. doi: 10.1074/jbc.M109.094524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Choi S.E., Fu T., Seok S., Kim D.H., Yu E., Lee K.W. Elevated microRNA-34a in obesity reduces NAD+ levels and SIRT1 activity by directly targeting NAMPT. Aging Cell. 2013;12:1062–1072. doi: 10.1111/acel.12135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Oda Y., Nakajima M., Tsuneyama K., Takamiya M., Aoki Y., Fukami T. Retinoid X receptor α in human liver is regulated by miR-34a. Biochem Pharmacol. 2014;90:179–187. doi: 10.1016/j.bcp.2014.05.002. [DOI] [PubMed] [Google Scholar]
- 64.Fu T., Seok S., Choi S., Huang Z., Suino-Powell K., Xu H.E. microRNA 34a inhibits beige and brown fat formation in obesity in part by suppressing adipocyte fibroblast growth factor 21 signaling and SIRT1 function. Mol Cell Biol. 2014;34:4130–4142. doi: 10.1128/MCB.00596-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ahn J., Lee H., Jung C.H., Ha T. Lycopene inhibits hepatic steatosis via microRNA-21-induced downregulation of fatty acid-binding protein 7 in mice fed a high-fat diet. Mol Nutr Food Res. 2012;56:1665–1674. doi: 10.1002/mnfr.201200182. [DOI] [PubMed] [Google Scholar]
- 66.Chau B.N., Xin C., Hartner J., Ren S., Castano A.P., Linn G. microRNA-21 promotes fibrosis of the kidney by silencing metabolic pathways. Sci Transl Med. 2012;4:121ra18. doi: 10.1126/scitranslmed.3003205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kida K., Nakajima M., Mohri T., Oda Y., Takagi S., Fukami T. PPARα is regulated by miR-21 and miR-27b in human liver. Pharm Res. 2011;28:2467–2476. doi: 10.1007/s11095-011-0473-y. [DOI] [PubMed] [Google Scholar]
- 68.Feng J., Li A.T., Deng J.Y., Yang Y.H., Dang L.L., Ye Y.P. miR-21 attenuates lipopolysaccharide-induced lipid accumulation and inflammatory response: potential role in cerebrovascular disease. Lipids Health Dis. 2014;13:27. doi: 10.1186/1476-511X-13-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Fabani M.M., Gait M.J. miR-122 targeting with LNA/2′-O-methyl oligonucleotide mixmers, peptide nucleic acids (PNA), and PNA-peptide conjugates. RNA. 2008;14:336–346. doi: 10.1261/rna.844108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wang L., Tang W., Yan S., Zhou L., Shen T., Huang X. Efficient delivery of miR-122 to regulate cholesterol metabolism using a non-covalent peptide-based strategy. Mol Med Rep. 2013;8:1472–1478. doi: 10.3892/mmr.2013.1691. [DOI] [PubMed] [Google Scholar]
- 71.Wang X., Yu B., Ren W., Mo X., Zhou C., He H. Enhanced hepatic delivery of siRNA and microRNA using oleic acid based lipid nanoparticle formulations. J Control Release. 2013;172:690–698. doi: 10.1016/j.jconrel.2013.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Boon R.A., Vickers K.C. Intercellular transport of microRNAs. Arterioscler Thromb Vasc Biol. 2013;33:186–192. doi: 10.1161/ATVBAHA.112.300139. [DOI] [PMC free article] [PubMed] [Google Scholar]