Abstract
Bile acids (BAs) are not only digestive surfactants but also important cell signaling molecules, which stimulate several signaling pathways to regulate some important biological processes. The bile-acid-activated nuclear receptor, farnesoid X receptor (FXR), plays a pivotal role in regulating bile acid, lipid and glucose homeostasis as well as in regulating the inflammatory responses, barrier function and prevention of bacterial translocation in the intestinal tract. As expected, FXR is involved in the pathophysiology of a wide range of diseases of gastrointestinal tract, including inflammatory bowel disease, colorectal cancer and type 2 diabetes. In this review, we discuss current knowledge of the roles of FXR in physiology of the digestive system and the related diseases. Better understanding of the roles of FXR in digestive system will accelerate the development of FXR ligands/modulators for the treatment of digestive system diseases.
KEY WORDS: Bile acids, Farnesoid X receptor, Gastrointestinal tract, Inflammatory bowel disease, Colorectal cancer, Type 2 diabetes
Abbreviations: 6-ECDCA, 6α-ethyl-chenodeoxycholic acid; AF2, activation domain; ANGTPL3, angiopoietin-like protein 3; AOM, azoxymethane; AP-1, activator protein-1; Apo, apolipoprotein; ASBT, apical sodium-dependent bile salt transporter; BAAT, bile acid-CoA amino acid N-acetyltransferase; BACS, bile acid-CoA synthetase; BAs, bile acids; BMI, body mass index; BSEP, bile salt export pump; CA, cholic acid; CD, Crohn׳s disease; CDCA, chenodeoxycholic acid; CREB, cAMP regulatory element-binding protein; CYP7A1, cholesterol 7α-hydroxylase; db/db, diabetic mice; DBD, DNA binding domain; DCA, deoxycholic acid; DSS, dextrane sodium sulfate; ERK, extracellular signal-regulated kinase; FABP6, fatty acid-binding protein subclass 6; FFAs, free fatty acids; FGF19, fibroblast growth factor 19; FGFR4, fibroblast growth factor receptor 4; FXR, farnesoid X receptor; FXRE, farnesoid X receptor response element; G6Pase, glucose-6-phosphatase; GLP-1, glucagon-like peptide 1; GLUT2, glucose transporter type 2; GPBAR, G protein-coupled BA receptor; GPCRs, G protein-coupled receptors; GSK3, glycogen synthase kinase 3; HDL-C, high density lipoprotein cholesterol; HNF4α, hepatic nuclear factor 4α; I-BABP, intestinal bile acid-binding protein; IBD, inflammatory bowel disease; IL-1, interleukin 1; KLF11, Krüppel-like factor 11; KRAS, Kirsten rat sarcoma viral oncogene homolog; LBD, ligand binding domain; LCA, lithocholic acid; LRH-1, liver receptor homolog-1; LPL, lipoprotein lipase; MCA, muricholicacid; MRP2, multidrug resistance-associated protein 2; NF-κB, nuclear factor-kappa B; NOD, non-obese diabetic; NRs, nuclear receptors; OSTα, organic solute transporter alpha; OSTβ, organic solute transporter beta; PEPCK, phosphoenol pyruvate carboxykinase; PGC-1α, peroxisome proliferators-activated receptor γ coactivator protein-1α; SHP, small heterodimer partner; SREBP-1c, sterol regulatory element-binding protein 1c; STAT3, signal transducers and activators of transcription 3; T2D, type 2 diabetes; TLCA, taurolithocholic acid; TNBS, trinitrobenzensulfonic acid; TNFα, tumor necrosis factors α; UC, ulcerative colitis; UDCA, ursodeoxycholic acid; VSG, vertical sleeve gastrectomy
Graphical abstract
The bile-acid-activated nuclear receptor, farnesoid X receptor (FXR), is involved in the pathophysiology of a wide range of diseases of gastrointestinal tract, including inflammatory bowel disease, colorectal cancer and type 2 diabetes. In this review, authors discuss current knowledge of the roles of FXR in physiology of the digestive system and the related diseases.
1. Introduction
Bile acids (BAs) are amphipathic molecules synthesized from cholesterol in the liver. They are physiological detergents that play important roles in facilitating hepatobiliary secretion of endobiotic and xenobiotic metabolites. In the intestines, BAs help intestinal absorption of dietary fats, fat-soluble vitamins, and other nutritions1. Over the past decade, BAs change beyond digestive surfactants to signaling molecules in a wide range of biological functions, including glucose and lipid metabolism, energy homeostasis, and the modulation of immune response1, 2, 3. The regulatory functions of BAs are mainly the result of activation of intracellular ligand-activated nuclear receptors (NRs), such as the farnesoid X receptor (FXR, NR1H4)4, 5, 6 and cell surface G protein-coupled receptors (GPCRs), specifically the G protein-coupled BA receptor (TGR5 or GPBAR-1)7, 8. Chenodeoxycholic acid (CDCA) is the most potent BA for FXR9, 10, 11. In contrast, lithocholic acid (LCA) and taurolithocholic acid (TLCA) are most potent endogenous ligands for TGR5 with an EC50 of ~600 and 300 nmol/L, respectively12, 13, 14. FXR has been considered as a master regulator of BA synthesis and secretion, lipid and glucose metabolism in the liver and intestine15, 16. In contrast, activation of TGR5 by BAs stimulated adenylate cyclase, rapid intracellular cAMP production, and protein kinase A activation. Such regulatory function of TGR5 plays important roles in regulating energy metabolism in brown adipose tissue, relaxing and refilling gallbladder, secreting glucagon-like peptide 1 (GLP-1) in intestinal endocrine cells and controlling gastrointestinal motility to help maintain BA, lipid and glucose homeostasis2, 17.
Abnormal BA metabolism has been associated with liver injury, metabolic disorders, cardiovascular diseases and digestive system diseases such as inflammation bowel disease (IBD) and colorectal cancer18, 19, 20, 21. FXR has been suggested to counteract pro-inflammatory and pro-atherogenic responses in cardiovascular diseases22. Moreover, FXR plays a pivotal role in regulating liver inflammation and regeneration as well as in regulating the extent of inflammatory responses, barrier function and prevention of bacterial translocation in the intestinal tract23, 24, 25. Direct modulation of BA receptor activities by synthetic and natural FXR agonists or antagonists has shown promise in treating human diseases related to metabolic perturbations and inflammation23, 26, 27, 28, 29. Here, we will focus on the current understanding of the functions of BAs and FXR in enterohepatic circulation, with special emphasis on their roles in pathophysiology of the gastrointestinal tract.
2. Bile acid nuclear receptor FXR
FXR belongs to a subclass of metabolic receptors within the NR-family and is identified as an NR for BAs4, 5, 6. It is expressed in several tissues, including liver, intestine, adipose tissue, the vascular wall, pancreas and kidney30. Four FXR splice variants have been identified, i.e. FXRα1–4. These isoforms show difference in spatial and temporal expression patterns as well as in transcriptional activities31. The general structure of FXR consists of an N-terminal DNA binding domain (DBD), a unique ligand binding domain (LBD) allowing receptor dimerization, and a C-terminal activation domain (AF2) for co-regulator interactions32. FXR binds to an FXR response element (FXRE) as a heterodimer with RXR or as monomer to regulate gene expression32. A large number of publications have shown that FXR regulates a network of genes in hepatic BA synthesis, biliary BA secretion, intestinal BA absorption, and hepatic BA uptake, thereby playing a key role in the regulation of BA homeostasis33, 34, 35.
BAs were identified as endogenous FXR ligands with high affinity. FXR can be activated by both free and conjugated BAs. The hydrophobic CDCA is the most efficacious ligand of FXR (EC50=approximately 10 µmol/L). The order of potency of BAs is CDCA>LCA=deoxycholic acid (DCA)>cholic acid (CA), whereas hydrophilic BAs, such as ursodeoxycholic acid (UDCA) and muricholicacid (MCA), cannot activate FXR36. These studies have suggested for the first time that BAs are also endocrine hormones16, 32, 37. A number of compounds unrelated to BAs were also found to act as FXR ligands with varying degrees of affinity, including androsterone38 and the exogenous natural products such as forskolin39, epigallocatechin-3-gallate40 and cafestol41. In addition, a series of synthetic BA derivatives have been developed as FXR ligands, such as 6α-ethylchenodeoxycholic acid (6-ECDCA) and bile alcohols, showing a higher affinity with FXR than the original BAs42. Along with the regulation of BA metabolism, accumulated data have demonstrated that FXR is a multipurpose NR that plays an essential role in maintaining lipid and glucose homeostasis32, 37, 43. Thus, activation or repression of FXR can have significant influences on metabolic homeostasis. FXR ligands have been proposed for potential treatment of cholestasis44, liver fibrosis27, inflammatory bowel disease23, atherosclerosis45 and erectile dysfunction46. Detailed analysis of the ChIP-seq data indicates that the global binding patterns of FXR in primary human hepatocytes are similar to those in mouse livers. Therefore, in a major extent, mouse model is suitable for studying human FXR functions47. Since numerous excellent review articles on FXR are already available, we will focus on the roles of FXR in digestive system diseases and type 2 diabetes (T2D).
3. FXR and enterohepatic circulation
FXR plays a key role in regulation of BA levels in enterohepatic circulation. BAs are synthesized in hepatocytes by cholesterol 7α-hydroxylase (CYP7A1), conjugated with taurine or glycine via bile acid-CoA synthetase (BACS) and bile acid-CoA amino acid N-acetyltransferase (BAAT), and secreted through the bile canalicular membrane by two ABC transporters (bile salt export pump (BSEP) and multidrug resistance-associated protein 2 (MRP2)) into the canalicular lumen1, 33, 37. They are stored in the gallbladder before being excreted into the intestinal lumen, where they function to emulsify dietary lipids and vitamines. In the liver, BAs bind to FXR, which transcriptionally upregulates a protein called small heterodimer partner (SHP; NR0B2) to inhibit trans-activity of hepatic nuclear factor 4α (HNF4α) and liver receptor homolog-1 (LRH-1; NR5A2) that bind to the BA response element in the Cypza1 and Cyp8b1 gene promoters48.
Roughly 95% of the BAs re-absorption occurs at the terminal ileum through the apical sodium-dependent bile salt transporter (ASBT; SLC10A2)49, 50. After transporting inside ileal enterocytes by ASBT, BAs are reversibly bound by the intestinal bile acid-binding protein (I-BABP) (also known as fatty acid-binding protein subclass 6 (FABP6)) expressed in the ileum49, 51. I-BABP has an important role in enterohepatic circulation by regulating BA trafficking. It shuttles BAs from the apical to basolateral membrane in the enterocytes52. Finally, organic solute transporter alpha and beta (OSTα and OSTβ) move bile salts to blood vessels, in accordance with its location at the basolateral membrane53. Mechanistic studies reveal that BAs generate a negative feedback on ASBT expression by FXR-mediated induction of SHP, which binds to and represses the transcriptional activities of LRH-1 for the Cyp7a1 gene54. The negative regulation of ASBT expression was observed in mice. But it was not found in rats due to the absence of an LRH-1 responsive element within the rat Asbt promoter54. Similar to the effect of FXR activation in the hepatocytes, activation of intestine FXR by BAs limits BA uptake and promotes basolateral BA secretion to decrease intracellular BA concentrations. BAs in the enterocytes bind FXR and increase the expression of IBABP and two transporters, OSTα and OSTβ, that are responsible for the transport of BAs from the intestine to the portal vein55, 56. Thus, FXR controls the entire transport of BAs from the intestinal lumen to the enterocytes, within the enterocytes and ultimately to the blood vessel for transportation to the liver.
Interestingly, intestinal FXR activation also generates an endocrine feedback regulation. Fibroblast growth factor 19 (FGF19) in humans, and its mouse homolog FGF15 (sometimes referred to as FGF15/19) are activated by FXR in the ileum57, 58. FGF15/19 is secreted from the ileum to the bloodstream where it circulates to the liver and suppresses BA synthesis through binding and activation of the FGF receptor 4 (FGFR4) complexed with β-Klotho located on the surface of hepatocytes and other epithelial cells59, 60. These effects were not observed in Shp−/− mice, thus suggesting that SHP is required for the suppressive effects of FGF15/1936. Binding of FGF15/19 to the FGFR4/β-Klotho complex strongly suppresses the expression of CYP7A1 through MAPK-dependent pathways, specifically, ERK and JNK pathways61. Extracellular signal-regulated kinase (ERK) was markedly elevated by FGF15 administration to mice and deficiency of both JNK and ERK pathways prevented FGF15-mediated suppression of CYP7A1 and CYP8B1 expression. However, deficiency of either pathway alone had minimal effect on FGF15-mediated suppression of these genes61. Therefore, intestinal FXR activated by BAs downregulates CYP7A1 expression indirectly through the intestinal FGF15/19 synthesis and secretion. In addition, FGF15/19 was reported to work as a hormone to facilitate gallbladder filling by binding to FGFR3, a receptor that is highly expressed in the gallbladder62. These studies indicate that FXR-FGF15-19 signaling contributes to the control of intestinal BA levels. Furthermore, FGF15/19 is also recently reported to activate hepatic glycogen synthesis through elevating the activities of glycogen synthase kinase 3 (GSK3)63 and to inhibit hepatic gluconeogenesis by inhibiting the cAMP regulatory element-binding protein (CREB)–peroxisome proliferators-activated receptor γ coactivator protein-1α (PGC-1α) pathway64.
4. FXR and inflammatory bowel disease
IBD, which primarily includes ulcerative colitis (UC) and Crohn׳s disease (CD), represents a group of chronic disorders characterized by gastrointestinal tract inflammation65. Although many details of IBD have been explored, the exact pathogenetic mechanisms of IBD have not been fully elucidated. At present, IBD is generally believed to result from imbalance of gut microbiota, epithelial dysfunction, and aberrant mucosal immune response66.
Recently, FXR has been implicated to participate in immune modulation and barrier function in the intestine. FXR alleviates inflammation and preserves the integrity of the intestinal epithelial barrier in many ways by regulating the extent of the inflammatory response, maintaining the integrity and function of the intestinal barrier, and preventing bacterial translocation in the intestinal tract67.
First, FXR plays an important role in the mucosal immune response, thereby exerting strong influence on immunoregulation68. Vavassori et al.69 notice that Fxr−/− mice display significantly elevated pro-inflammatory cytokine mRNA expression in the colon. In two complementary murine models (intra-rectal administration of trinitrobenzensulfonic acid (TNBS) and oral administration of dextrane sodium sulfate (DSS)), concurrent administration of the potent synthetic FXR ligand 6-ECDCA represses the expression of various proinflammatory cytokines, chemokines and their receptors in wild type, but not Fxr−/− mice. In addition, Raybould et al.73 show that FXR activation by INT-747 prevents DSS- and TNBS-induced intestinal inflammation, with improvement of colitis symptoms, inhibition of epithelial permeability, and reduced goblet cell loss. Furthermore, FXR activation inhibits proinflammatory cytokine production in vivo in the mouse colonic mucosa, and ex vivo in different immune cell populations23. These results provide strong support for the involvement of FXR in IBD due to counter-regulatory effects on cells of innate immunity23, 69. FXR ligands exert anti-inflammatory activities by antagonizing other signaling pathways, in part through the interaction with other transcription factors, including activator protein-1 (AP-1), and signal transducers and activators of transcription 3 (STAT3)70. Several of the intestinal macrophage genes inhibited by FXR agonists are established targets for nuclear factor-kappa B (NF-κB) (tumor necrosis factors α (TNFα), interleukin 1 (IL-1), IL-6, cyclooxygenase-1, cyclooxygenase-2) and AP-1, two most important transcriptional regulators of innate and adaptive immunity in cells71 (Fig. 1).
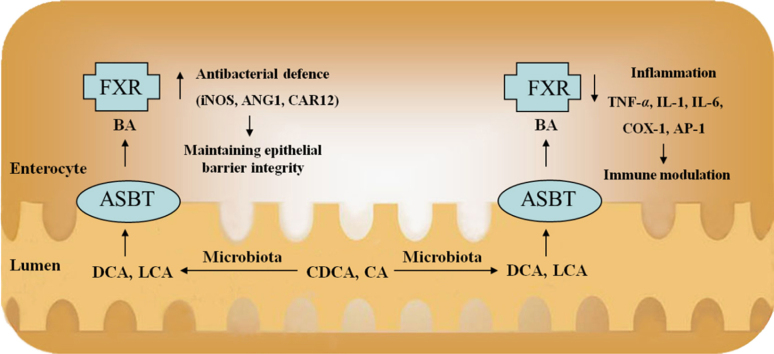
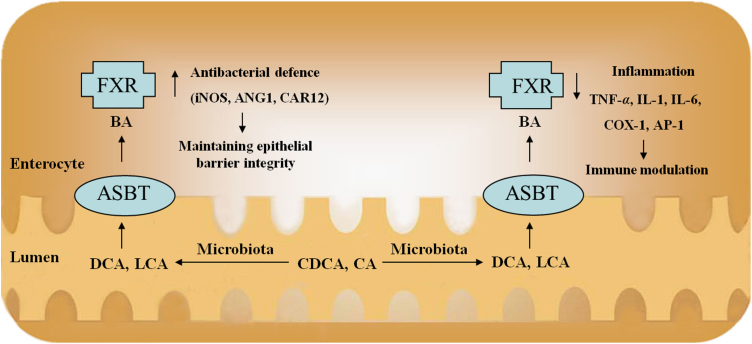
Figure 1.
The roles of FXR in the IBD. FXR activation increases mRNA expression of iNOs, ANG1 and CAR12, which are involved in antibacterial defense by producing antimicrobial peptides (iNOs and ANG1) or maintaining appropriate intestinal pH (CAR12). This is important for the homeostasis of intestinal luminal contents and epithelial barrier integrity. Moreover, FXR activation induces the repression of inflammatory genes (IL-1, IL-6 and MCP-1) and promotes antimicrobial actions.
Second, FXR has been implicated in barrier function by regulating intestinal antibacterial growth. Gut microbiota play important roles in pathogen defense, immunity, and nutrient harvest. Recent evidence suggests that there is a regulatory relationship between the development of IBD and altered gut microbiota72, 73, 74. It has been demonstrated that BAs and gut microbiota are closely related to each other. Gut microbiota are involved in the biotransformation of BAs through deconjugation, dehydroxylation, and reconjugation of BAs75. BAs have antimicrobial activities by damaging the bacterial cell membrane, thus inhibiting bacterial outgrowth76.
The administration of bile or conjugated BAs to ascitic cirrhotic rats or obstructive jaundice rats eliminates intestinal bacterial overgrowth, and decreases bacterial translocation and endotoxemia77, 78. Inagaki et al.79 provide an explanation for this protective effect of BAs by demonstrating that intestinal FXR has a crucial role in limiting bacterial overgrowth and thus protecting the intestine from bacterial-induced damage. They show that mice lacking FXR experience bacterial overgrowth, increase intestinal permeability and contain large amounts of bacteria in mesenteric lymph nodes, as well as inflammation of the intestinal walls. However, activation of intestinal FXR by GW4064 leads to the identification of several novel intestinal FXR target genes, including those encoding angiogenin, carbonic anhydrase 12 and inducible nitric oxide synthase, which have been reported to have antibacterial properties79. The cytokine IL-18 is also induced by FXR stimulation. IL-18 stimulates resistance to an array of pathogens, including intracellular and extracellular bacteria and mycobacteria, and appears to have a protective role during the early, acute phase of mucosal immune response79, 80. These results are consistent with the idea that FXR is critical for controlling intestinal bacterial growth, which has significant implications for maintaining a competent barrier, thereby contributing to the prevention of intestinal inflammation.
5. FXR and colorectal cancer
Colorectal cancer is considered as the third most common form of cancer and the second most common cause of cancer-related death worldwide, leading to an incidence of 1.36 million cases estimated in 2012 (19.2% of total cancer cases) as attending to incidence and mortality statistics81. In addition to inherited mutations, lifestyle, diet and nutritional habits are closely related to the development of colorectal cancer. Recently, there is increasing evidence that a fat-rich diet is positively associated with colon cancer incidence82, 83, 84. Consumption of high-fat diet has been correlated with elevated levels of BAs in the colonic lumen as a consequence of increased fecal excretion of BAs, which at last promote elevated incidence of colorectal cancer85, 86. In addition, population-based studies have shown that subjects who consume a western diet display elevated levels of fecal secondary BAs, mostly DCA and LCA, as do patients diagnosed with colonic carcinomas87, 88. Elevated secondary BA concentrations have detrimental effects on colonic epithelium architecture and function through multiple mechanisms, such as DNA oxidative damage, inflammation, NF-κB activation and enhanced cell proliferation89. Therefore, BAs can be considered as tumor-promoting factors in colorectal cancer development82, 90, 91.
So far, there is considerable evidence for a role of FXR in modulating intestinal tumorigenesis. Given the crucial roles of FXR in maintaining BA concentrations within a physiological range, thereby preventing BA-induced cytotoxicity, the loss of FXR would contribute to tumorigenesis of colorectal cancer. De Gottardi et al.92 first suggest that FXR mRNA expression is decreased in colonic polyps, and even more pronounced, in colonic adenocarcinoma. These results indicate that FXR expression levels may positively correlated to the degree of malignancy of colon cancer and there is a causal link between FXR and colon carcinogenesis in humans. It is indeed further demonstrated by that FXR deficiency leads to significantly increased sizes and numbers of the tumors in two common murine intestine tumorigenesis models: APCmin mice and azoxymethane (AOM)-induced colon cancer93. Modica et al.94 consider that FXR activity is relevant to the pathogenesis of intestinal cancer. On one hand, when FXR is absent, there is an upregulation of Wnt signaling via increased infiltrating neutrophils and TNFα production with expansion of the basal proliferative compartment both in the ileum and in the colon, and a concomitant reduction in the apical, differentiated apoptosis competent compartment. This scenario leads to increased tumor progression and early mortality in mice. On the other hand, when FXR is activated in the differentiated normal enterocytes and in colon cancer cells, there is an induction of apoptosis and removal of genetically altered cells, which may otherwise progress to complete transformation. Thus, up-regulation of FXR expression in colon tumors might be useful in the treatment of colon cancer. Further studies on a larger collection of human frozen colon carcinomas tissues and human cell lines show that FXR expression may be linked to the development of colorectal carcinomas and indicate that altered FXR signaling in neoplastic cells offers novel pathogenetic, prognostic and, in particular, therapeutic insights and perspectives95. Bailey et al.96 investigate the regulation of FXR during the development of human colon cancer. The results showed that FXR is downregulated very early in human colon cancer development, which is partly due to DNA methylation of the FXR promoter and increased V-Kiras2 Kirsten rat sarcoma viral oncogene homolog (KRAS) signaling. Silencing of FXR alone is not sufficient to initiate colon cancer development, but activation of remnant FXR in healthy tissues may prevent and inhibit the promotion of colon cancer93, 94, 97. Restoration of basal FXR expression through inhibition of DNA methylation or KRAS signaling, or through activation of residual FXR, might slow or prevent the progression of colorectal cancer96.
6. FXR, obesity and T2D
The global prevalence of diabetes in 2010 was 280 million people worldwide (around 6.2% of the world׳s total population), and it has been predicted that in 2030 the prevalence will reach more than 7.5% of the world׳s total population, paralleling the aging and body mass index (BMI) of the population. Obesity is a leading risk factor for impaired glucose tolerance and T2D. Overweight and obesity lead to adverse metabolic effects on blood pressure, cholesterol levels, triglycerides levels and insulin resistance98.
In recent years, a body of evidence has surfaced indicating that FXR plays an important role not only in BA but also in lipid and glucose homeostasis99, 100, 101. Specific targeting of FXR may be an effective way to treat obesity-induced metabolic diseases.
Studies in mice with FXR gene ablation or administering FXR agonists provided key information demonstrating a central role of FXR in lipid homeostasis. Fxr−/− mice display elevated serum cholesterol and triglyceride levels and excessive accumulation of fat in the liver99, 100. A more detailed study reveals an increased hepatic synthesis of apolipoprotein B-containing lipoproteins (mainly VLDL) and a reduced clearance rate of HDL cholesteryl esters, both of which have theoretically pro-atherogenic effects in Fxr−/− mice98. Activation of FXR by BAs or synthetic agonists lowers plasma triglyceride levels101, 102 by a mechanism that involves the repression of hepatic transcription factor sterol regulatory element-binding protein 1c (SREBP-1c) expression and its lipogenic target genes in mouse primary hepatocytes and liver103, 104. The suppression effect of FXR on SREBP-1c expression is thought to be mediated by a signaling cascade that involves SHP104. In addition, activation of FXR facilitates the clearance of VLDL and chylomicrons via repressing the expression of microsomal triglyceride transfer protein and apolipoprotein B103. FXR activation also results in the induction of peroxisome proliferator-activated receptor α, which promotes fatty acid β-oxidation105 (Fig. 2). Yet, different FXR isoforms display differential effects. For example, FXRα2 is more effective in reducing elevated HDL levels and transrepressing hepatic expression of CYP8B1, which is the regulator of cholate synthesis. In contrast, FXRα4 is involved in a switch to regulate the hydrophobicity of the bile salt pool31.
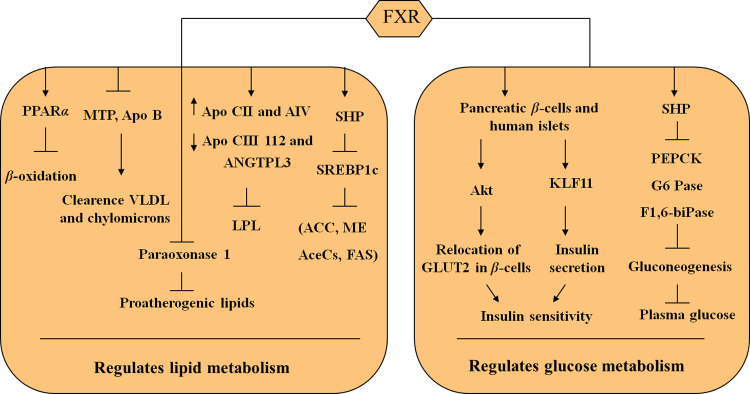
Figure 2.
The roles of FXR in regulating lipid and glucose metabolism. On one hand, FXR plays an important role in regulating lipid metabolism. Activation of FXR by BAs or synthetic agonists lowers plasma triglyceride levels by a mechanism that involves the repression of hepatic transcription factor SREBP-1c expression and its lipogenic target genes in mouse primary hepatocytes and liver. FXR activation also increases the expression of apolipoprotein Apo CII and AIV and decreases the expression of both Apo CIII 112 and ANGTPL3 to stimulate LPL activity. In addition, FXR mediates the repression of paraoxonase 1 to inactivate pro-atherogenic lipids produced by oxidative modification of low-density lipoprotein. Furthermore, FXR activation promotes fatty acid β-oxidation by inducing the expression of PPARα. Finally, activation of FXR facilitates the clearance of very low-density lipoproteins and chylomicrons via repressing the expression of microsomal triglyceride transfer protein and apolipoprotein B (Apo B). On the other hand, FXR exerts a critical role in regulating glucose homeostasis. Activation of FXR in βTC6 cells increases Akt phosphorylation and translocation of the glucose transporter GLUT2 at plasma membrane, increasing the glucose uptake by these cells. FXR-KLF11 regulated pathway has an essential role in the regulation of insulin transcription and secretion induced by glucose. Furthermore, FXR-SHP negative regulatory cascade can regulate gluconeogenesis in the liver.
In addition, FXR activation also directly increases the expression of apolipoprotein Apo CII and AIV102, 106, 107, which are activators of lipoprotein lipase (LPL) activity, and decreases the expression of both ApoCIII108 and ANGTPL3104, which are LPL inhibitors. However, FXR appears to suppress apolipoprotein A-I expression100, 109, 110, the primary protein constituent of high-density lipoprotein defining its size and shape. FXR also regulates the expression of phospholipid transfer protein111 that is responsible for the transfer of phospholipids and cholesterol from low to high-density lipoprotein and suppresses 3-hydroxy-3-methyl-glutaryl-CoA reductase, likely involving sterol regulatory element-binding protein 2112. Another target of FXR is paraoxonase 1, a protein produced in the liver with phospholipase A2 activity that may be important for inactivation of proatherogenic lipids produced by oxidative modification of low-density lipoprotein. FXR mediated repression of paraoxonase 1 involves the induction of fibroblast growth factor 19, its subsequent binding to the fibroblast growth factor receptor 4, and activation of the c-Jun N-terminal kinase pathway113, 114. Finally, FXR represses proprotein convertase subtilisin/kexin 9115, a protein that promotes the intracellular degradation of the low-density lipoprotein receptor by interfering with its recycling to the plasma membrane. Collectively, these findings support the concept that FXR activation decreases plasma lipid levels by suppressing hepatic lipogenesis and lipid secretion and increasing the clearance of lipoproteins from blood (Fig. 2).
In addition to its pleiotropic effects on lipid metabolism, FXR plays a critical role in glucose homeostasis. The generation and phenotypic characterization of Fxr−/− mice confirm this vital role in the regulation of lipid metabolism and glucose homeostasis. Fxr−/− mice not only display elevated serum levels of free fatty acids (FFAs), triglycerides and high density lipoprotein cholesterol (HDL-C)99, 100, but also develop signs of insulin resistance as shown by hyperglycemia, impaired glucose tolerance, and severely blunted insulin signaling in both liver and muscle28, 116. High glucose concentrations increased FXR O-GlcNAcylation, thereby enhancing its protein stability and transcriptional activity. The fasting–refeeding experiments show that FXR undergoes O-GlcNAcylation in fed conditions, which is accompanied with increased FXR target gene expression and decreased liver bile acid content117. Activation of FXR by synthetic agonists or hepatic overexpression of a constitutively active FXR by adenovirus-mediated gene transfer reduces blood glucose levels in obese fa/fa rats, diabetic, leptin deficient, diabetic (db/db) mice and wild type mice28, 118. This decrease in plasma glucose levels in db/db mice was associated with decreased glucose-6-phosphatase expression, increased glycogen levels and synthesis in the liver, providing evidence that activation of FXR lowers plasma glucose levels by sensitizing to insulin action26, 116. Pharmacological treatments with BAs or GW4064, both in vitro in human hepatoma cell lines and in vivo in mice, decrease the expression of the gene encoding phosphoenol pyruvate carboxykinase (Pepck) and other gluconeogenic genes such as those encoding glucose-6-phosphatase (G6Pase) and fructose 1,6-bis-phosphatase119, 120. Consistent with these results, CA treatment for five days decreased Pepck and G6Pase mRNA levels in wild-type, but not in Fxr−/− mice121. This was associated with a decrease in levels of fasting blood glucose only in wild-type mice, indicating that FXR negatively regulates gluconeogenesis121 (Fig. 2). Moreover, in vivo GW4064 treatment reduces PEPCK and G6Pase expression in db/db mice28. Paradoxically, some studies28, 116, but not all122, report that FXR activation by GW4064 induces PEPCK expression, leading to an increased glucose output in rodent primary hepatocytes in vitro116.
Recently, there are independent studies identifying a role for FXR in the regulation of insulin sensitivity29, 104, 121. Fxr−/− mice are associated with impaired glucose tolerance and insulin resistance. Moreover, whole-body glucose disposal during a hyperinsulinemic euglycemic clamp is decreased in Fxr−/− mice. Consistent with these observations, insulin signaling is impaired in peripheral insulin-sensitive tissues, including skeletal muscle and white adipose tissue29, 122. Interestingly, treatment with GW4064 significantly improved insulin sensitivity in both db/db28 and ob/ob29 mice. Similar results are obtained when a constitutively active FXR is over-expressed in db/db mice28. In contrast, FXR deficiency was also shown to be associated with normal hepatic insulin sensitivity and signaling121, 122. The reason for this discrepancy is unclear, but may be linked to different genetic backgrounds (C57Bl6/J28, 121 vs. C75Bl6/N121, 122) of the mice and/or the insulin dose used during the clamp. The molecular mechanisms behind the insulin-sensitizing effect of FXR remain poorly defined. Since FXR is not expressed in skeletal muscle, it is conceivable that FXR deficiency alters indirectly insulin signaling in this tissue. Recent studies indicated that FXR is expressed by pancreatic β-cells and human islets and regulates the insulin signaling by genomic and non-genomic effects. Genomic effects include Krüppel-like factor 11 (KLF11)-mediated stimulation of insulin gene expression. Non-genomic effects include an Akt-mediated stimulation of glucose induced relocation of glucose transporter type 2 (GLUT2) in β-cells. Finally, these effects are reproduced in vivo in a rodent model of insulin-deficient diabetes developing in non-obese diabetic (NOD) mice123.
In humans, pharmacological approaches to induce persistent weight loss and improve glucose level of obesity-induced T2D have so far shown limited effectiveness. However, bariatric surgery has become an effective therapeutic option for morbid obesity, surpassing drug therapies and lifestyle interventions124. Vertical sleeve gastrectomy (VSG) is a bariatric procedure that involves the removal of up to 80% of the stomach along the greater curvature, creating a gastric “sleeve” in continuity with the esophagus and pylorus125. VSG induces loss of body weight and fat mass and improves glucose tolerance in humans and in rodent models126, 127, 128, 129, 130, 131. The study of Ryan et al.132 suggests that FXR is required for the sustained maintenance of weight loss and improved glycemic control after VSG. Furthermore, by identifying a model resistant to the effects of bariatric surgery, the authors were able to identify dissect further a role of the microbiota on the positive effects of bariatric surgery. However, the specific mechanisms by which FXR contributes to glucose control by VSG are still unknown. Further investigating the roles of FXR in mediating the anti-obesity and anti-diabetes effect of VSG and other surgeries will be of great interest.
7. Conclusions and future perspectives
Research in BA and FXR signaling during the last 20 years has unraveled its important role in regulation of BA, lipid, glucose, and energy metabolism. In this review, we have summarized the roles of FXR in pathophysiology of the digestive system and the related diseases, including IBD, colorectal cancer and T2D. Recent studies have shown that FXR activation by its ligands affects both immune cells and intestinal epithelium, contributing to intestinal immunomodulation at various levels, thus providing a rationale to extend the clinical trials of FXR ligands to patients with IBD. The critical role of FXR in modulating intestinal tumorigenesis is probably due to its regulation of BA metabolism and detoxification, and its activation may confer protection from BA-induced tumor promoting activities. Activation of FXR improves obesity-induced T2D by regulating lipid metabolism and glucose homeostasis. FXR is required for the positive metabolic effect of VSG surgery. Targeting FXR therefore offers an exciting new perspective for the treatment of these digestive system diseases. However, the therapeutic benefits or risks of synthetic FXR ligands require further consideration in light of differences between mice and humans. One particular challenge in designing FXR agonists is to separate the desired therapeutic effects from the unwanted side effects. The design of organ- or gene-specific FXR modulators may improve their specificity and reduce side effects. A better understanding of the cellular and physiological signaling of FXR and its cofactors will help develop more selective modulators and the development of more efficient therapeutics for digestive system diseases.
Acknowledgments
We are in debt for those authors whose work cannot be cited in this review due to the limited space. This work was supported by National Cancer Institute of United States (No. 1R01-CA139158, to Wendong Huang), National Natural Science Foundation of China (Nos. 81303186 and ZYX-NSFC-016) and China Postdoctoral Science Foundation (No. 2013M531202).
Footnotes
Peer review under responsibility of Institute of Materia Medica, Chinese Academy of Medical Sciences and Chinese Pharmaceutical Association.
References
- 1.Chiang JYL. Bile acids: regulation of synthesis. J Lipid Res. 2009;50:1955–1966. doi: 10.1194/jlr.R900010-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Thomas C, Gioiello A, Noriega L, Strehle A, Oury J, Rizzo G. TGR5-mediated bile acid sensing controls glucose homeostasis. Cell Metab. 2009;10:167–177. doi: 10.1016/j.cmet.2009.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kuipers F, Bloks VW, Groen AK. Beyond intestinal soap-bile acids in metabolic control. Nat Rev Endocrinol. 2014;10:488–498. doi: 10.1038/nrendo.2014.60. [DOI] [PubMed] [Google Scholar]
- 4.Makishima M, Okamoto AY, Repa JJ, Tu H, Learned RM, Luk A. Identification of a nuclear receptor for bile acids. Science. 1999;284:1362–1365. doi: 10.1126/science.284.5418.1362. [DOI] [PubMed] [Google Scholar]
- 5.Parks DJ, Blanchard SG, Bledsoe RK, Chandra G, Consler TG, Kliewer SA. Bile acids: natural ligands for an orphan nuclear receptor. Science. 1999;284:1365–1368. doi: 10.1126/science.284.5418.1365. [DOI] [PubMed] [Google Scholar]
- 6.Wang H, Chen J, Hollister K, Sowers LC, Forman BM. Endogenous bile acids are ligands for the nuclear receptor FXR/BAR. Mol Cell. 1999;3:543–553. doi: 10.1016/s1097-2765(00)80348-2. [DOI] [PubMed] [Google Scholar]
- 7.Kawamata Y, Fujii R, Hosoya M, Harada M, Yoshida H, Miwa M. A G protein-coupled receptor responsive to bile acids. J Biol Chem. 2003;278:9435–9440. doi: 10.1074/jbc.M209706200. [DOI] [PubMed] [Google Scholar]
- 8.Maruyama T, Miyamoto Y, Nakamura T, Tamai Y, Okada H, Sugiyama E. Identification of membrane-type receptor for bile acids (M-BAR) Biochem Biophys Res Commun. 2002;298:714–719. doi: 10.1016/s0006-291x(02)02550-0. [DOI] [PubMed] [Google Scholar]
- 9.Pellicciari R, Costantino G, Fiorucci S. Farnesoid X receptor: from structure to potential clinical applications. J Med Chem. 2005;48:5383–5403. doi: 10.1021/jm0582221. [DOI] [PubMed] [Google Scholar]
- 10.Fiorucci S, Rizzo G, Donini A, Distrutti E, Santucci L. Targeting farnesoid X receptor for liver and metabolic disorders. Trends Mol Med. 2007;13:298–309. doi: 10.1016/j.molmed.2007.06.001. [DOI] [PubMed] [Google Scholar]
- 11.Otte K, Kranz H, Kober I, Thompson P, Hoefer M, Haubold B. Identification of farnesoid X receptor β as a novel mammalian nuclear receptor sensing lanosterol. Mol Cell Biol. 2003;23:864–872. doi: 10.1128/MCB.23.3.864-872.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sato H, Macchiarulo A, Thomas C, Gioiello A, Une M, Hofmann AF. Novel potent and selective bile acid derivatives as TGR5 agonists: biological screening, structure-activity relationships, and molecular modeling studies. J Med Chem. 2008;51:1831–1841. doi: 10.1021/jm7015864. [DOI] [PubMed] [Google Scholar]
- 13.Pellicciari R, Sato H, Gioiello A, Costantino G, Macchiarulo A, Sadeghpour BM. Nongenomic actions of bile acids. Synthesis and preliminary characterization of 23- and 6, 23-alkyl-substituted bile acid derivatives as selective modulators for the G-protein coupled receptor TGR5. J Med Chem. 2007;50:4265–4268. doi: 10.1021/jm070633p. [DOI] [PubMed] [Google Scholar]
- 14.Nguyen A, Bouscarel B. Bile acids and signal transduction: role in glucose homeostasis. Cell Signal. 2008;20:2180–2197. doi: 10.1016/j.cellsig.2008.06.014. [DOI] [PubMed] [Google Scholar]
- 15.Fiorucci S, Mencarelli A, Palladino G, Cipriani S. Bile-acid-activated receptors: targeting TGR5 and farnesoid-X-receptor in lipid and glucose disorders. Trends Pharmacol Sci. 2009;30:570–580. doi: 10.1016/j.tips.2009.08.001. [DOI] [PubMed] [Google Scholar]
- 16.Thomas C, Pellicciari R, Pruzanski M, Auwerx J, Schoonjans K. Targeting bile-acid signalling for metabolic diseases. Nat Rev Drug Discov. 2008;7:678–693. doi: 10.1038/nrd2619. [DOI] [PubMed] [Google Scholar]
- 17.Alemi F, Poole DP, Chiu J, Schoonjans K, Cattaruzza F, Grider JR. The receptor TGR5 mediates the prokinetic actions of intestinal bile acids and is required for normal defecation in mice. Gastroenterology. 2013;44:145–154. doi: 10.1053/j.gastro.2012.09.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Claudel T, Zollner G, Wagner M, Trauner M. Role of nuclear receptors for bile acid metabolism, bile secretion, cholestasis, and gallstone disease. Biochim Biophys Acta. 2011;1812:867–878. doi: 10.1016/j.bbadis.2010.12.021. [DOI] [PubMed] [Google Scholar]
- 19.Porez G, Prawitt J, Gross B, Staels B. Bile acid receptors as targets for the treatment of dyslipidemia and cardiovascular disease: thematic review series: new lipid and lipoprotein targets for the treatment of cardiometabolic diseases. J Lipid Res. 2012;53:1723–1737. doi: 10.1194/jlr.R024794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jones ML, Martoni CJ, Prakash S. Letter to the editor regarding the report of Duboc et al.: connecting dysbiosis, bile-acid dysmetabolism and gut inflammation in inflammatory bowel disease. Gut. 2013;62:654–655. doi: 10.1136/gutjnl-2012-303867. [DOI] [PubMed] [Google Scholar]
- 21.Degirolamo C, Modica S, Palasciano G, Moschetta A. Bile acids and colon cancer: solving the puzzle with nuclear receptors. Trends Mol Med. 2011;17:564–572. doi: 10.1016/j.molmed.2011.05.010. [DOI] [PubMed] [Google Scholar]
- 22.Zhang S, Liu Q, Wang J, Harnish DC. Suppression of interleukin-6-induced C-reactive protein expression by FXR agonists. Biochem Biophys Res Commun. 2009;379:476–479. doi: 10.1016/j.bbrc.2008.12.117. [DOI] [PubMed] [Google Scholar]
- 23.Gadaleta RM, van Erpecum KJ, Oldenburg B, Willemsen EC, Renooij W, Murzilli S. Farnesoid X receptor activation inhibits inflammation and preserves the intestinal barrier in inflammatory bowel disease. Gut. 2011;60:463–472. doi: 10.1136/gut.2010.212159. [DOI] [PubMed] [Google Scholar]
- 24.Meng Z, Wang Y, Wang L, Jin W, Liu N, Pan H. FXR regulates liver repair after CCl4-induced toxic injury. Mol Endocrinol. 2010;24:886–897. doi: 10.1210/me.2009-0286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang L, Wang YD, Chen WD, Wang X, Lou G, Liu N. Promotion of liver regeneration/repair by farnesoid X receptor in both liver and intestine in mice. Hepatology. 2012;56:2336–2343. doi: 10.1002/hep.25905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moschetta A, Bookout AL, Mangelsdorf DJ. Prevention of cholesterol gallstone disease by FXR agonists in a mouse model. Nat Med. 2004;10:1352–1358. doi: 10.1038/nm1138. [DOI] [PubMed] [Google Scholar]
- 27.Fiorucci S, Antonelli E, Rizzo G, Renga B, Mencarelli A, Riccardi L. The nuclear receptor SHP mediates inhibition of hepatic stellate cells by FXR and protects against liver fibrosis. Gastroenterology. 2004;127:1497–1512. doi: 10.1053/j.gastro.2004.08.001. [DOI] [PubMed] [Google Scholar]
- 28.Zhang Y, Lee FY, Barrera G, Lee H, Vales C, Gonzalez FJ. Activation of the nuclear receptor FXR improves hyperglycemia and hyperlipidemia in diabetic mice. Proc Natl Acad Sci USA. 2006;103:1006–1011. doi: 10.1073/pnas.0506982103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cariou B, van Harmelen K, Duran-Sandoval D, van Dijk TH, Grefhorst A, Abdelkarim M. The farnesoid X receptor modulates adiposity and peripheral insulin sensitivity in mice. J Biol Chem. 2006;281:11039–11049. doi: 10.1074/jbc.M510258200. [DOI] [PubMed] [Google Scholar]
- 30.Huber RM, Murphy K, Miao B, Link JR, Cunningham MR, Rupar MJ. Generation of multiple farnesoid-X-receptor isoforms through the use of alternative promoters. Gene. 2002;290:35–43. doi: 10.1016/s0378-1119(02)00557-7. [DOI] [PubMed] [Google Scholar]
- 31.Boesjes M, Bloks VW, Hageman J, Bos T, van Dijk TH, Havinga R. Hepatic farnesoid X-receptor isoforms α2 and α4 differentially modulate bile salt and lipoprotein metabolism in mice. PLoS One. 2014;9:e115028. doi: 10.1371/journal.pone.0115028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang Y, Edwards PA. FXR signaling in metabolic disease. FEBS Lett. 2008;582:10–18. doi: 10.1016/j.febslet.2007.11.015. [DOI] [PubMed] [Google Scholar]
- 33.Matsubara T, Li F, Gonzalez FJ. FXR signaling in the enterohepatic system. Mol Cell Endocrinol. 2013;368:17–29. doi: 10.1016/j.mce.2012.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kuipers F, Claudel T, Sturm E, Staels B. The farnesoid X receptor (FXR) as modulator of bile acid metabolism. Rev Endocr Metab Disord. 2004;5:319–326. doi: 10.1023/B:REMD.0000045103.00467.9a. [DOI] [PubMed] [Google Scholar]
- 35.Rizzo G, Renga B, Mencarelli A, Pellicciari R, Fiorucci S. Role of FXR in regulating bile acid homeostasis and relevance for human diseases. Curr Drug Targets Immune Endocr Metabol Disord. 2005;5:289–303. doi: 10.2174/1568008054863781. [DOI] [PubMed] [Google Scholar]
- 36.Li Y, Jadhav K, Zhang Y. Bile acid receptors in non-alcoholic fatty liver disease. Biochem Pharmacol. 2013;86:1517–1524. doi: 10.1016/j.bcp.2013.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lee FY, Lee H, Hubbert ML, Edwards PA, Zhang Y. FXR, a multipurpose nuclear receptor. Trends Biochem Sci. 2006;31:572–580. doi: 10.1016/j.tibs.2006.08.002. [DOI] [PubMed] [Google Scholar]
- 38.Wang SG, Lai K, Moy FJ, Bhat A, Hartman HB, Evans MJ. The nuclear hormone receptor farnesoid X receptor (FXR) is activated by androsterone. Endocrinology. 2006;147:4025–4033. doi: 10.1210/en.2005-1485. [DOI] [PubMed] [Google Scholar]
- 39.Howard WR, Pospisil JA, Njolito E, Noonan DJ. Catabolites of cholesterol synthesis pathways and forskolin as activators of the farnesoid X-activated nuclear receptor. Toxicol Appl Pharmacol. 2000;163:195–202. doi: 10.1006/taap.1999.8869. [DOI] [PubMed] [Google Scholar]
- 40.Li G, Lin W, Araya JJ, Chen T, Timmermann BN, Guo GL. A tea catechin, epigallocatechin-3-gallate, is a unique modulator of the farnesoid X receptor. Toxicol Appl Pharmacol. 2012;258:268–274. doi: 10.1016/j.taap.2011.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ricketts ML, Boekschoten MV, Kreeft AJ, Hooiveld GJ, Moen CJ, Müller M. The cholesterol-raising factor from coffee beans, cafestol, as an agonist ligand for the farnesoid and pregnane X receptors. Mol Endocrinol. 2007;21:1603–1616. doi: 10.1210/me.2007-0133. [DOI] [PubMed] [Google Scholar]
- 42.Pellicciari R, Fiorucci S, Camaioni E, Clerici C, Costantino G, Maloney PR. 6α-Ethyl-chenodeoxycholic acid (6-ECDCA), a potent and selective FXR agonist endowed with anticholestatic activity. J Med Chem. 2002;45:3569–3572. doi: 10.1021/jm025529g. [DOI] [PubMed] [Google Scholar]
- 43.Lefebvre P, Cariou B, Lien F, Kuipers F, Staels B. Role of bile acids and bile acid receptors in metabolic regulation. Physiol Rev. 2009;89:147–191. doi: 10.1152/physrev.00010.2008. [DOI] [PubMed] [Google Scholar]
- 44.Liu YP, Binz J, Numerick MJ, Dennis S, Luo GZ, Desai B. Hepatoprotection by the farnesoid X receptor agonist GW4064 in rat models of intra- and extrahepatic cholestasis. J Clin Invest. 2003;112:1678–1687. doi: 10.1172/JCI18945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mencarelli A, Fiorucci S. FXR an emerging therapeutic target for the treatment of atherosclerosis. J Cell Mol Med. 2010;14:79–92. doi: 10.1111/j.1582-4934.2009.00997.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Morelli A, Vignozzi L, Maggi M, Adorini L. Farnesoid X receptor activation improves erectile dysfunction in models of metabolic syndrome and diabetes. Biochim Biophys Acta. 2011;1812:859–866. doi: 10.1016/j.bbadis.2010.10.013. [DOI] [PubMed] [Google Scholar]
- 47.Zhan L, Liu HX, Fang YP, Kong B, He YQ, Zhong XB. Genome-wide binding and transcriptome analysis of human farnesoid X receptor in primary human hepatocytes. PLoS One. 2014;9:e105930. doi: 10.1371/journal.pone.0105930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Goodwin B, Jones SA, Price RR, Watson MA, McKee DD, Moore LB. A regulatory cascade of the nuclear receptors FXR, SHP-1, and LRH-1 represses bile acid biosynthesis. Mol Cell. 2000;6:517–526. doi: 10.1016/s1097-2765(00)00051-4. [DOI] [PubMed] [Google Scholar]
- 49.Wong MH, Oelkers P, Craddock AL, Dawson PA. Expression cloning and characterization of the hamster ileal sodium-dependent bile acid transporter. J Biol Chem. 1994;269:1340–1347. [PubMed] [Google Scholar]
- 50.Shneider BL. Intestinal bile acid transport: biology, physiology, and pathophysiology. J Pediatr Gastroenterol Nutr. 2001;32:407–417. doi: 10.1097/00005176-200104000-00002. [DOI] [PubMed] [Google Scholar]
- 51.Kramer W, Girbig F, Gutjahr U, Kowalewski S, Jouvenal K, Müller G. Intestinal bile acid absorption. Na+-dependent bile acid transport activity in rabbit small intestine correlates with the coexpression of an integral 93-kDa and a peripheral 14-kDa bile acid-binding membrane protein along the duodenum–ileum axis. J Biol Chem. 1993;268:18035–18046. [PubMed] [Google Scholar]
- 52.Coppola CP, Gosche JR, Arrese M, Ancowitz B, Madsen J, Vanderhoof J. Molecular analysis of the adaptive response of intestinal bile acid transport after ileal resection in the rat. Gastroenterology. 1998;115:1172–1178. doi: 10.1016/s0016-5085(98)70088-5. [DOI] [PubMed] [Google Scholar]
- 53.Dawson PA, Hubbert M, Haywood J, Craddock AL, Zerangue N, Christian WV. The heteromeric organic solute transporter α–β, Ostα–Ostβ, is an ileal basolateral bile acid transporter. J Biol Chem. 2005;280:6960–6968. doi: 10.1074/jbc.M412752200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chen F, Ma L, Dawson PA, Sinal CJ, Sehayek E, Gonzalez FJ. Liver receptor homologue-1 mediates species- and cell line-specific bile acid-dependent negative feedback regulation of the apical sodium-dependent bile acid transporter. J Biol Chem. 2003;278:19909–19916. doi: 10.1074/jbc.M207903200. [DOI] [PubMed] [Google Scholar]
- 55.Grober J, Zaghini I, Fujii H, Jones SA, Kliewer SA, Willson TM. Identification of a bile acid-responsive element in the human ileal bile acid-binding protein gene. Involvement of the farnesoid X receptor/9-cis-retinoic acid receptor heterodimer. J Biol Chem. 1999;274:29749–29754. doi: 10.1074/jbc.274.42.29749. [DOI] [PubMed] [Google Scholar]
- 56.Lee H, Zhang YQ, Lee FY, Nelson SF, Gonzalez FJ, Edwards PA. FXR regulates organic solute transporters α and β in the adrenal gland, kidney, and intestine. J Lipid Res. 2006;47:201–214. doi: 10.1194/jlr.M500417-JLR200. [DOI] [PubMed] [Google Scholar]
- 57.Holt JA, Luo GZ, Billin AN, Bisi J, McNeill YY, Kozarsky KF. Definition of a novel growth factor-dependent signal cascade for the suppression of bile acid biosynthesis. Genes Dev. 2003;17:1581–1591. doi: 10.1101/gad.1083503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Inagaki T, Choi M, Moschetta A, Peng L, Cummins CL, McDonald JG. Fibroblast growth factor 15 functions as an enterohepatic signal to regulate bile acid homeostasis. Cell Metab. 2005;2:217–225. doi: 10.1016/j.cmet.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 59.Xie MH, Holcomb I, Deuel B, Dowd P, Huang A, Vagts A. FGF-19, a novel fibroblast growth factor with unique specificity for FGFR4. Cytokine. 1999;11:729–735. doi: 10.1006/cyto.1999.0485. [DOI] [PubMed] [Google Scholar]
- 60.Hughes SE. Differential expression of the fibroblast growth factor receptor (FGFR) multigene family in normal human adult tissues. J Histochem Cytochem. 1997;45:1005–1019. doi: 10.1177/002215549704500710. [DOI] [PubMed] [Google Scholar]
- 61.Kong B, Wang L, Chiang JYL, Zhang Y, Klaassen CD, Guo GL. Mechanism of tissue-specific farnesoid X receptor in suppressing the expression of genes in bile-acid synthesis in mice. Hepatology. 2012;56:1034–1043. doi: 10.1002/hep.25740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Choi M, Moschetta A, Bookout AL, Peng L, Umetani M, Holmstrom SR. Identification of a hormonal basis for gallbladder filling. Nat Med. 2006;12:1253–1255. doi: 10.1038/nm1501. [DOI] [PubMed] [Google Scholar]
- 63.Kir S, Beddow SA, Samuel VT, Miller P, Previs SF, Suino-Powell K. FGF19 as a postprandial, insulin-independent activator of hepatic protein and glycogen synthesis. Science. 2011;331:1621–1624. doi: 10.1126/science.1198363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Potthoff MJ, Boney-Montoya J, Choi M, He T, Sunny NE, Satapati S. FGF15/19 regulates hepatic glucose metabolism by inhibiting the CREB-PGC-1α pathway. Cell Metab. 2011;13:729–738. doi: 10.1016/j.cmet.2011.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sartor RB. Mechanisms of disease: pathogenesis of Crohn׳s disease and ulcerative colitis. Nat Clin Pract Gastroenterol Hepatol. 2006;3:390–407. doi: 10.1038/ncpgasthep0528. [DOI] [PubMed] [Google Scholar]
- 66.Podolsky DK. Inflammatory bowel disease. N Engl J Med. 2002;347:417–429. doi: 10.1056/NEJMra020831. [DOI] [PubMed] [Google Scholar]
- 67.Gadaleta RM, van Mil SW, Oldenburg B, Siersema PD, Klomp LW, van Erpecum KJ. Bile acids and their nuclear receptor FXR: relevance for hepatobiliary and gastrointestinal disease. Biochim Biophys Acta. 2010;1801:683–692. doi: 10.1016/j.bbalip.2010.04.006. [DOI] [PubMed] [Google Scholar]
- 68.Schote AB, Turner JD, Schiltz J, Muller CP. Nuclear receptors in human immune cells: expression and correlations. Mol Immunol. 2007;44:1436–1445. doi: 10.1016/j.molimm.2006.04.021. [DOI] [PubMed] [Google Scholar]
- 69.Vavassori P, Mencarelli A, Renga B, Distrutti E, Fiorucci S. The bile acid receptor FXR is a modulator of intestinal innate immunity. J Immunol. 2009;183:6251–6261. doi: 10.4049/jimmunol.0803978. [DOI] [PubMed] [Google Scholar]
- 70.Fiorucci S, Cipriani S, Mencarelli A, Renga B, Distrutti E, Baldelli F. Counter-regulatory role of bile acid activated receptors in immunity and inflammation. Curr Mol Med. 2010;10:579–595. doi: 10.2174/1566524011009060579. [DOI] [PubMed] [Google Scholar]
- 71.Wagner EF, Eferl R. Fos/AP-1 proteins in bone and the immune system. Immunol Rev. 2005;208:126–140. doi: 10.1111/j.0105-2896.2005.00332.x. [DOI] [PubMed] [Google Scholar]
- 72.Comito D, Romano C. Dysbiosis in the pathogenesis of pediatric inflammatory bowel diseases. Int J Inflamm. 2012;2012:687143. doi: 10.1155/2012/687143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Raybould HE. Gut microbiota, epithelial function and derangements in obesity. J Physiol. 2012;590:441–446. doi: 10.1113/jphysiol.2011.222133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Elson CO, Cong Y. Host-microbiota interactions in inflammatory bowel disease. Gut Microbes. 2012;3:332–344. doi: 10.4161/gmic.20228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ridlon JM, Kang DJ, Hylemon PB. Bile salt biotransformations by human intestinal bacteria. J Lipid Res. 2006;47:241–259. doi: 10.1194/jlr.R500013-JLR200. [DOI] [PubMed] [Google Scholar]
- 76.Kurdi P, Kawanishi K, Mizutani K, Yokota A. Mechanism of growth inhibition by free bile acids in lactobacilli and bifidobacteria. J Bacteriol. 2006;188:1979–1986. doi: 10.1128/JB.188.5.1979-1986.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ding JW, Andersson R, Soltesz V, Willén R, Bengmark S. The role of bile and bile acids in bacterial translocation in obstructive jaundice in rats. Eur Surg Res. 1993;25:11–19. doi: 10.1159/000129252. [DOI] [PubMed] [Google Scholar]
- 78.Lorenzo-Zúñiga V, Bartolí R, Planas R, Hofmann AF, Viñado B, Hagey LR. Oral bile acids reduce bacterial overgrowth, bacterial translocation, and endotoxemia in cirrhotic rats. Hepatology. 2003;37:551–557. doi: 10.1053/jhep.2003.50116. [DOI] [PubMed] [Google Scholar]
- 79.Inagaki T, Moschetta A, Lee YK, Peng L, Zhao GX, Downes M. Regulation of antibacterial defense in the small intestine by the nuclear bile acid receptor. Proc Natl Acad Sci USA. 2006;103:3920–3925. doi: 10.1073/pnas.0509592103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Biet F, Locht C, Kremer L. Immunoregulatory functions of interleukin 18 and its role in defense against bacterial pathogens. J Mol Med. 2002;80:147–162. doi: 10.1007/s00109-001-0307-1. [DOI] [PubMed] [Google Scholar]
- 81.Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359–E386. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 82.Bernstein C, Holubec H, Bhattacharyya AK, Nguyen H, Payne CM, Zaitlin B. Carcinogenicity of deoxycholate, a secondary bile acid. Arch Toxicol. 2011;85:863–871. doi: 10.1007/s00204-011-0648-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Butler LM, Wang R, Koh WP, Stern MC, Yuan JM, Yu MC. Marine n-3 and saturated fatty acids in relation to risk of colorectal cancer in Singapore Chinese: a prospective study. Int J Cancer. 2009;124:678–686. doi: 10.1002/ijc.23950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Carroll KK. Dietary fats and cancer. Am J Clin Nutr. 1991;53 Suppl:1064S–1067S. doi: 10.1093/ajcn/53.4.1064S. [DOI] [PubMed] [Google Scholar]
- 85.Hill MJ. Colon cancer: a disease of fibre depletion or of dietary excess? Digestion. 1974;11:289–306. doi: 10.1159/000197593. [DOI] [PubMed] [Google Scholar]
- 86.Stamp DH. Three hypotheses linking bile to carcinogenesis in the gastrointestinal tract: certain bile salts have properties that may be used to complement chemotherapy. Med Hypotheses. 2002;59:398–405. doi: 10.1016/s0306-9877(02)00125-1. [DOI] [PubMed] [Google Scholar]
- 87.Bajor A, Gillberg PG, Abrahamsson H. Bile acids: short and long term effects in the intestine. Scand J Gastroenterol. 2010;45:645–664. doi: 10.3109/00365521003702734. [DOI] [PubMed] [Google Scholar]
- 88.McGarr SE, Ridlon JM, Hylemon PB. Diet, anaerobic bacterial metabolism, and colon cancer: a review of the literature. J Clin Gastroenterol. 2005;39:98–109. [PubMed] [Google Scholar]
- 89.Pearson JR, Gill CI, Rowland IR. Diet, fecal water, and colon cancer-development of a biomarker. Nutr Rev. 2009;67:509–526. doi: 10.1111/j.1753-4887.2009.00224.x. [DOI] [PubMed] [Google Scholar]
- 90.Bernstein H, Bernstein C, Payne CM, Dvorakova K, Garewal H. Bile acids as carcinogens in human gastrointestinal cancers. Mutat Res. 2005;589:47–65. doi: 10.1016/j.mrrev.2004.08.001. [DOI] [PubMed] [Google Scholar]
- 91.Debruyne PR, Bruyneel EA, Li X, Zimber A, Gespach C, Mareel MM. The role of bile acids in carcinogenesis. Mutat Res. 2001;480–481:359–369. doi: 10.1016/s0027-5107(01)00195-6. [DOI] [PubMed] [Google Scholar]
- 92.De Gottardi A, Touri F, Maurer CA, Perez A, Maurhofer O, Ventre G. The bile acid nuclear receptor FXR and the bile acid binding protein IBABP are differently expressed in colon cancer. Dig Dis Sci. 2004;49:982–989. doi: 10.1023/b:ddas.0000034558.78747.98. [DOI] [PubMed] [Google Scholar]
- 93.Maran RR, Thomas A, Roth M, Sheng Z, Esterly N, Pinson D. Farnesoid X receptor deficiency in mice leads to increased intestinal epithelial cell proliferation and tumor development. J Pharmacol Exp Ther. 2009;328:469–477. doi: 10.1124/jpet.108.145409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Modica S, Murzilli S, Salvatore L, Schmidt DR, Moschetta A. Nuclear bile acid receptor FXR protects against intestinal tumorigenesis. Cancer Res. 2008;68:9589–9594. doi: 10.1158/0008-5472.CAN-08-1791. [DOI] [PubMed] [Google Scholar]
- 95.Lax S, Schauer G, Prein K, Kapitan M, Silbert D, Berghold A. Expression of the nuclear bile acid receptor/farnesoid X receptor is reduced in human colon carcinoma compared to nonneoplastic mucosa independent from site and may be associated with adverse prognosis. Int J Cancer. 2012;130:2232–2239. doi: 10.1002/ijc.26293. [DOI] [PubMed] [Google Scholar]
- 96.Bailey AM, Zhan L, Maru D, Shureiqi I, Pickering CR, Kiriakova G. FXR silencing in human colon cancer by DNA methylation and KRAS signaling. Am J Physiol Gastrointest Liver Physiol. 2014;306:G48–G58. doi: 10.1152/ajpgi.00234.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Vaquero J, Briz O, Herraez E, Muntané J, Marin JJ. Activation of the nuclear receptor FXR enhances hepatocyte chemoprotection and liver tumor chemoresistance against genotoxic compounds. Biochim Biophys Acta. 2013;1833:2212–2219. doi: 10.1016/j.bbamcr.2013.05.006. [DOI] [PubMed] [Google Scholar]
- 98.Riobó Serván P. Obesity and diabetes. Nutr Hosp. 2013;28 Suppl 5:S138–S143. doi: 10.3305/nh.2013.28.sup5.6929. [DOI] [PubMed] [Google Scholar]
- 99.Sinal CJ, Tohkin M, Miyata M, Ward JM, Lambert G, Gonzalez FJ. Targeted disruption of the nuclear receptor FXR/BAR impairs bile acid and lipid homeostasis. Cell. 2000;102:731–744. doi: 10.1016/s0092-8674(00)00062-3. [DOI] [PubMed] [Google Scholar]
- 100.Lambert G, Amar MJ, Guo G, Brewer HB, Jr, Gonzalez FJ, Sinal CJ. The farnesoid X-receptor is an essential regulator of cholesterol homeostasis. J Biol Chem. 2003;278:2563–2570. doi: 10.1074/jbc.M209525200. [DOI] [PubMed] [Google Scholar]
- 101.Maloney PR, Parks DJ, Haffner CD, Fivush AM, Chandra G, Plunket KD. Identification of a chemical tool for the orphan nuclear receptor FXR. J Med Chem. 2000;43:2971–2974. doi: 10.1021/jm0002127. [DOI] [PubMed] [Google Scholar]
- 102.Kast HR, Nguyen CM, Sinal CJ, Jones SA, Laffitte BA, Reue K. Farnesoid X-activated receptor induces apolipoprotein C-II transcription: a molecular mechanism linking plasma triglyceride levels to bile acids. Mol Endocrinol. 2001;15:1720–1728. doi: 10.1210/mend.15.10.0712. [DOI] [PubMed] [Google Scholar]
- 103.Zhang YQ, Castellani LW, Sinal CJ, Gonzalez FJ, Edwards PA. Peroxisome proliferator-activated receptor-γ coactivator 1α (PGC-1α) regulates triglyceride metabolism by activation of the nuclear receptor FXR. Genes Dev. 2004;18:157–169. doi: 10.1101/gad.1138104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Watanabe M, Houten SM, Wang L, Moschetta A, Mangelsdorf DJ, Heyman RA. Bile acids lower triglyceride levels via a pathway involving FXR, SHP, and SREBP-1c. J Clin Invest. 2004;113:1408–1418. doi: 10.1172/JCI21025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Pineda Torra I, Claudel T, Duval C, Kosykh V, Fruchart JC, Staels B. Bile acids induce the expression of the human peroxisome proliferator-activated receptor α gene via activation of the farnesoid X receptor. Mol Endocrinol. 2003;17:259–272. doi: 10.1210/me.2002-0120. [DOI] [PubMed] [Google Scholar]
- 106.Fruchart-Najib J, Baugé E, Niculescu LS, Pham T, Thomas B, Rommens C. Mechanism of triglyceride lowering in mice expressing human apolipoprotein A5. Biochem Biophys Res Commun. 2004;319:397–404. doi: 10.1016/j.bbrc.2004.05.003. [DOI] [PubMed] [Google Scholar]
- 107.Kardassis D, Roussou A, Papakosta P, Boulias K, Talianidis I, Zannis VI. Synergism between nuclear receptors bound to specific hormone response elements of the hepatic control region-1 and the proximal apolipoprotein C-II promoter mediate apolipoprotein C-II gene regulation by bile acids and retinoids. Biochem J. 2003;372:291–304. doi: 10.1042/BJ20021532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Claudel T, Inoue Y, Barbier O, Duran-Sandoval D, Kosykh V, Fruchart J. Farnesoid X receptor agonists suppress hepatic apolipoprotein CIII expression. Gastroenterology. 2003;125:544–555. doi: 10.1016/s0016-5085(03)00896-5. [DOI] [PubMed] [Google Scholar]
- 109.Fuchs M, Ivandic B, Müller O, Schalla C, Scheibner J, Bartsch P. Biliary cholesterol hypersecretion in gallstone-susceptible mice is associated with hepatic up-regulation of the high-density lipoprotein receptor SRBI. Hepatology. 2001;33:1451–1459. doi: 10.1053/jhep.2001.24373. [DOI] [PubMed] [Google Scholar]
- 110.Claudel T, Sturm E, Duez H, Torra IP, Sirvent A, Kosykh V. Bile acid-activated nuclear receptor FXR suppresses apolipoprotein A–I transcription via a negative FXR response element. J Clin Invest. 2002;109:961–971. doi: 10.1172/JCI14505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Urizar NL, Dowhan DH, Moore DD. The farnesoid X-activated receptor mediates bile acid activation of phospholipid transfer protein gene expression. J Biol Chem. 2000;275:39313–39317. doi: 10.1074/jbc.M007998200. [DOI] [PubMed] [Google Scholar]
- 112.Hubbert ML, Zhang Y, Lee FY, Edwards PA. Regulation of hepatic Insig-2 by the farnesoid X receptor. Mol Endocrinol. 2007;21:1359–1369. doi: 10.1210/me.2007-0089. [DOI] [PubMed] [Google Scholar]
- 113.Gutierrez A, Ratliff EP, Andres AM, Huang X, McKeehan WL, Davis RA. Bile acids decrease hepatic paraoxonase 1 expression and plasma high-density lipoprotein levels via FXR-mediated signaling of FGFR4. Arterioscler Thromb Vasc Biol. 2006;26:301–306. doi: 10.1161/01.ATV.0000195793.73118.b4. [DOI] [PubMed] [Google Scholar]
- 114.Shih DM, Kast-Woelbern HR, Wong J, Xia YR, Edwards PA, Lusis AJ. A role for FXR and human FGF-19 in the repression of paraoxonase-1 gene expression by bile acids. J Lipid Res. 2006;47:384–392. doi: 10.1194/jlr.M500378-JLR200. [DOI] [PubMed] [Google Scholar]
- 115.Langhi C, Le May C, Kourimate S, Caron S, Staels B, Krempf M. Activation of the farnesoid X receptor represses PCSK9 expression in human hepatocytes. FEBS Lett. 2008;582:949–955. doi: 10.1016/j.febslet.2008.02.038. [DOI] [PubMed] [Google Scholar]
- 116.Stayrook KR, Bramlett KS, Savkur RS, Ficorilli J, Cook T, Christe ME. Regulation of carbohydrate metabolism by the farnesoid X receptor. Endocrinology. 2005;146:984–991. doi: 10.1210/en.2004-0965. [DOI] [PubMed] [Google Scholar]
- 117.Berrabah W, Aumercier P, Gheeraert C, Dehondt H, Bouchaert E, Alexandre J. Glucose sensing O-GlcNAcylation pathway regulates the nuclear bile acid receptor farnesoid X receptor (FXR) Hepatology. 2014;59:2022–2033. doi: 10.1002/hep.26710. [DOI] [PubMed] [Google Scholar]
- 118.Cipriani S, Mencarelli A, Palladino G, Fiorucci S. FXR activation reverses insulin resistance and lipid abnormalities and protects against liver steatosis in Zucker (fa/fa) obese rats. J Lipid Res. 2010;51:771–784. doi: 10.1194/jlr.M001602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.De Fabiani E, Mitro N, Gilardi F, Caruso D, Galli G, Crestani M. Coordinated control of cholesterol catabolism to bile acids and of gluconeogenesis via a novel mechanism of transcription regulation linked to the fasted-to-fed cycle. J Biol Chem. 2003;278:39124–39132. doi: 10.1074/jbc.M305079200. [DOI] [PubMed] [Google Scholar]
- 120.Yamagata K, Daitoku H, Shimamoto Y, Matsuzaki H, Hirota K, Ishida J. Bile acids regulate gluconeogenic gene expression via small heterodimer partner-mediated repression of hepatocyte nuclear factor 4 and Foxo1. J Biol Chem. 2004;279:23158–23165. doi: 10.1074/jbc.M314322200. [DOI] [PubMed] [Google Scholar]
- 121.Ma K, Saha PK, Chan L, Moore DD. Farnesoid X receptor is essential for normal glucose homeostasis. J Clin Invest. 2006;116:1102–1109. doi: 10.1172/JCI25604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Cariou B, van Harmelen K, Duran-Sandoval D, van Dijk T, Grefhorst A, Bouchaert E. Transient impairment of the adaptive response to fasting in FXR-deficient mice. FEBS Lett. 2005;579:4076–4080. doi: 10.1016/j.febslet.2005.06.033. [DOI] [PubMed] [Google Scholar]
- 123.Renga B, Mencarelli A, Vavassori P, Brancaleone V, Fiorucci S. The bile acid sensor FXR regulates insulin transcription and secretion. Biochim Biophys Acta. 2010;1802:363–372. doi: 10.1016/j.bbadis.2010.01.002. [DOI] [PubMed] [Google Scholar]
- 124.Pories WJ. Bariatric surgery: risks and rewards. J Clin Endocrinol Metab. 2008;93 Suppl 1:S89–S96. doi: 10.1210/jc.2008-1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Miras AD, le Roux CW. Mechanisms underlying weight loss after bariatric surgery. Nat Rev Gastroenterol Hepatol. 2013;10:575–584. doi: 10.1038/nrgastro.2013.119. [DOI] [PubMed] [Google Scholar]
- 126.Abbatini F, Rizzello M, Casella G, Alessandri G, Capoccia D, Leonetti F. Long-term effects of laparoscopic sleeve gastrectomy, gastric bypass, and adjustable gastric banding on type 2 diabetes. Surg Endosc. 2010;24:1005–1010. doi: 10.1007/s00464-009-0715-9. [DOI] [PubMed] [Google Scholar]
- 127.Karamanakos SN, Vagenas K, Kalfarentzos F, Alexandrides TK. Weight loss, appetite suppression, and changes in fasting and postprandial ghrelin and peptide-YY levels after Roux-en-Y gastric bypass and sleeve gastrectomy: a prospective, double blind study. Ann Surg. 2008;247:401–407. doi: 10.1097/SLA.0b013e318156f012. [DOI] [PubMed] [Google Scholar]
- 128.Pereferrer FS, Gonzàlez MH, Rovira AF, Blasco SB, Rivas AM, del Castillo Déjardin D. Influence of sleeve gastrectomy on several experimental models of obesity: metabolic and hormonal implications. Obes Surg. 2008;18:97–108. doi: 10.1007/s11695-007-9351-4. [DOI] [PubMed] [Google Scholar]
- 129.Peterli R, Wölnerhanssen B, Peters T, Devaux N, Kern B, Christoffel-Courtin C. Improvement in glucose metabolism after bariatric surgery: comparison of laparoscopic Roux-en-Y gastric bypass and laparoscopic sleeve gastrectomy: a prospective randomized trial. Ann Surg. 2009;250:234–241. doi: 10.1097/SLA.0b013e3181ae32e3. [DOI] [PubMed] [Google Scholar]
- 130.Stefater MA, Pérez-Tilve D, Chambers AP, Wilson-Pérez HE, Sandoval DA, Berger J. Sleeve gastrectomy induces loss of weight and fat mass in obese rats, but does not affect leptin sensitivity. Gastroenterology. 2010;138:2426–2436. doi: 10.1053/j.gastro.2010.02.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Stefater MA, Sandoval DA, Chambers AP, Wilson-Pérez HE, Hofmann SM, Jandacek R. Sleeve gastrectomy in rats improves postprandial lipid clearance by reducing intestinal triglyceride secretion. Gastroenterology. 2011;141:939. doi: 10.1053/j.gastro.2011.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Ryan KK, Tremaroli V, Clemmensen C, Kovatcheva-Datchary P, Myronovych A, Karns R. FXR is a molecular target for the effects of vertical sleeve gastrectomy. Nature. 2014;509:183–188. doi: 10.1038/nature13135. [DOI] [PMC free article] [PubMed] [Google Scholar]