Abstract
Alcoholic liver disease (ALD) is one of the major causes of liver morbidity and mortality worldwide. Chronic alcohol consumption leads to development of liver pathogenesis encompassing steatosis, inflammation, fibrosis, cirrhosis, and in extreme cases, hepatocellular carcinoma. Moreover, ALD may also associate with cholestasis. Emerging evidence now suggests that farnesoid X receptor (FXR) and bile acids also play important roles in ALD. In this review, we discuss the effects of alcohol consumption on FXR, bile acids and gut microbiome as well as their impacts on ALD. Moreover, we summarize the findings on FXR, FoxO3a (forkhead box-containing protein class O3a) and PPARα (peroxisome proliferator-activated receptor alpha) in regulation of autophagy-related gene transcription program and liver injury in response to alcohol exposure.
KEY WORDS: Farnesoid X receptor, Bile acids, Autophagy, Alcoholic liver disease, FoxO3
Abbreviations: ADH, alcohol dehydrogenase; AF, activation function; AKT, protein kinase B; ALD, alcoholic liver disease; ALT, alanine aminotransferase; ASBT, apical sodium dependent bile acid transporter; Atg, autophagy-related; BAAT, bile acid CoA:amino acid N-acyltransferase; BACS, bile acid CoA synthetase; BSEP, bile salt export pump; CA, cholic acid; CB1R, cannabinoid receptor type 1; CDCA, chenodeoxycholic acid; CREB, cAMP response element-binding protein; CREBH, cAMP response element-binding protein, hepatocyte specific; CRTC2, CREB regulated transcription coactivator 2; CYP, cytochrome P450; DCA, deoxycholic acid; DR1, direct repeat 1; 6ECDCA, 6α-ethyl-chenodeoxycholic acid; FGF15/19, fibroblast growth factor 15/19; FGFR4, fibroblast growth factor receptor 4; FoxO3a, forkhead box-containing protein class O3a; FXR, farnesoid X receptor; GGT, gamma-glutamyltranspeptidase; HCC, hepatocellular carcinoma; IR-1, inverted repeat-1; KO, knockout; LC3, light chain 3; LRH-1, liver receptor homolog 1; LXR, liver X receptor; MRP4, multidrug resistance protein 4; NAD+, nicotinamide adenine dinucleotide; NTCP, sodium taurocholate cotransporting polypeptide; OSTα/β, organic solute transporter α/β; PE, phosphatidylethanolamine; PPARα, peroxisome proliferator-activated receptor alpha; ROS, reactive oxygen species; RXRα, retinoid X receptor-alpha; SHP, small heterodimer partner; Sirt1, sirtuin 1; SQSTM, sequestome-1; SREBP1, sterol regulatory element-binding protein 1; TCA, taurocholic acid; TFEB, transcription factor EB; TLR4, toll-like receptor 4; TUDCA, tauro-ursodeoxycholic acid; UDCA, ursodeoxycholic acid; WAY, WAY-362450; WT, wild type
Graphical abstract
This review discusses the effects of alcohol consumption on farnesoid X receptor (FXR), bile acids and gut microbiome as well as their impacts on alcoholic liver disease. Moreover, we summarize the findings on FXR, forkhead box-containing protein class O3a and peroxisome proliferator-activated receptor alpha in regulation of autophagy-related gene transcription program and liver injury in response to alcohol exposure.
1. Introduction
Alcohol consumption is ubiquitous in the United States and worldwide. In moderate amount, alcohol can be beneficial; however, excessive alcohol consumption may result in pathogenesis known as alcoholic liver disease (ALD)1, 2, 3. ALD encompasses a wide spectrum of morbidity initiated by simple steatosis, which may progress to more severe pathologies such as fibrosis, alcoholic hepatitis, cirrhosis, and in extreme cases, hepatocellular carcinoma (HCC)2, 3, 4. It is known that majority of alcoholics develop simple steatosis, but only a small cohort of the patients progress to more severe pathologies2. This is likely because ALD is usually associated with other risk factors including sex, obesity, genetics, and viral hepatitis2, 5, 6, 7. Moreover, cells may adapt to the alcohol exposure and activate cellular protective mechanisms against alcohol-induced detrimental effects.
Interestingly, ALD patients also exhibit manifestations of cholestasis, a liver pathology defined by impaired flow of bile acids resulting in accumulation of hepatic bile acids8. Bile acids are amphipathic detergent-like molecules that are end-products of hepatic cholesterol catabolism9. Due to its detergent-like properties, bile acids are critical for solubilization and absorption of cholesterol, dietary lipids, and fat-soluble vitamins in the intestines10. Moreover, bile acids promote bile flow and cholesterol secretion from the liver11. Intriguingly, bile acids also function as nutrient signaling molecules through activation of farnesoid X receptor (FXR), a bile acid-sensing nuclear receptor that regulates lipid, cholesterol, and glucose metabolism12. FXR is highly expressed in the liver and the intestines, and is also found in kidney and adrenal glands. FXR is a key regulator for bile acid homeostasis13, 14, 15, 16, 17. FXR contains a ligand-independent transcription activation function (AF-1) region, a DNA-binding domain with two highly conserved zinc finger motifs, and a hinge region that mediates simultaneous receptor dimerization and DNA binding in the N-terminus18. A ligand binding domain, a dimerization interface, and a ligand-dependent AF-2 are found in the C-terminus of FXR18. FXR dimerizes with retinoid X receptor-alpha (RXRα), another nuclear receptor, which enables FXR to bind to an inverted repeat-1 response element (IR-1), an inverted AGGTCA sequence separated by one base pair, to initiate transcription of target genes19, 20. FXR is critical for the transcriptional regulation of bile acid synthesis and transport genes in the liver and intestines21, 22, 23. Bile acids, in particular the unconjugated forms, are endogenous ligands for FXR. Moreover, synthetic ligands such as GW4064 and WAY-362450 (WAY) have been identified to be potent FXR agonists24, 25.
FXR regulates bile acid synthesis through two distinct mechanisms. In the liver, FXR up-regulates expression of small heterodimer partner (SHP), a unique nuclear receptor. SHP then interacts with liver receptor homolog-1 (LRH-1) to repress the transcription of bile acid synthesis enzymes, cytochrome P450 7A1 and 8B1 (CYP7A1 and CYP8B1), resulting in decreased bile acid synthesis26, 27, 28. In the intestines, bile acids activate FXR to induce the transcription and secretion of fibroblast growth factor 15/19 (FGF15/19) from the intestines into the portal vein. FGF15/19 then travels and binds to FGF receptor 4 (FGFR4) in the liver to suppress the transcription of Cyp7a1 and in turn inhibits bile acid synthesis21, 22, 23. Indeed, whole body Fxr deficiency in mice results in increased hepatic bile acid levels and liver injury including hepatic steatosis, inflammation, and fibrosis21, 29. Here we reviewed the emerging evidence that FXR may act as a protective factor in ALD by regulating multiple cellular and molecular pathways.
2. Alcohol consumption disrupts bile acid synthesis and enterohepatic circulation
Alcohol consumption induces hepatic metabolic changes, increases oxidative stress and alters lipid metabolism that leads to hepatotoxicity2, 4. Interestingly, alcohol consumption has also been reported to induce cholestasis in all stages of ALD8, 30. Dr. Lieber׳s group31, 32 first observed that chronic alcohol consumption results in increased bile acid pool and decreased excretion of bile acids, suggesting that alcohol consumption may affect the enterohepatic circulation. Currently, it is not clear how alcohol induces cholestasis. However, emerging evidence suggests that alcohol may down-regulate FXR, which results in increased bile acid synthesis and hepatic bile acid pool33, 34.
Taurine conjugation of bile acids can result in reduced hydrophobicity and toxicity35. Taurine and glycine conjugations also promote the transport of bile acids out of the hepatocytes36. Chronic alcohol consumption reduced levels of taurine-conjugated bile acids and increased levels of more toxic unconjugated and glycine-conjugated bile acids in rat liver, duodenum and ileum34. Conversely, taurine supplementation attenuated chronic alcohol-induced steatosis and lipid peroxidation possibly due to inhibition of CYP2E1 activity in rats37. However, it is not clear if taurine supplementation increased taurocholic acid (TCA) level. The reduced levels of taurine-conjugated and increased levels of glycine-conjugated bile acids were due to chronic alcohol-induced perturbation in expression of bile acid metabolism enzymes34.
Accumulation of hepatic bile acids is one manifestation of ALD pathogenesis, which could be due to alcohol-induced bile acid synthesis. Acute alcohol exposure has been reported to induce bile acid biosynthesis in man and primary cultured human hepatocytes38, 39. Chronic alcohol consumption also induced the transcription of Cyp7a1 and Cyp8b1 and reduced expression of FGFR4, a transcription inhibitor of CYP7A133, 34. Moreover, another study demonstrated that alcohol induced transcription of bile acid synthesis enzymes including Cyp7a1, Cyp7b1, Cyp8b1, and Cyp27a1 by activating cAMP responsive element-binding protein (CREBH), a liver specific transcription factor and a key metabolic regulator, through alcohol-mediated stimulation of the hepatic cannabinoid receptor type 1 (CB1R)40. Taken together, accumulating evidence supports that alcohol consumption alters bile acid synthesis by up-regulating the expression of bile acid synthesis genes, although more studies are needed to further elucidate the mechanisms by which alcohol induces bile acid synthesis.
Chronic alcohol consumption also alters metabolic enzymes that facilitate bile acid conjugation prior to the transport of bile acids into bile canaliculi. Upon alcohol exposure, the enzyme responsible for taurine conjugation, bile acid CoA:amino acid N-acyltransferase (BAAT), was down-regulated. However, the enzyme responsible for glycine conjugation, bile acid CoA synthetase (BACS), was increased upon alcohol exposure34. As a result, chronic alcohol exposure alters bile acid synthesis and conjugation by up-regulating the classic pathway and decreasing BAAT-mediated taurine conjugation.
Chronic alcohol exposure also alters the enterohepatic circulation of bile acids. Alcohol exposure increases the expression of bile acid efflux transporters including the bile salt export pump (BSEP), multidrug resistance protein 4 (MRP4) and organic solute transporter α/β (OSTα/β) and decreases the expression of bile acid uptake transporter, sodium taurocholate cotransporting polypeptide (NTCP) in the liver33, 34. In the ileum, alcohol consumption increases the expression of bile acid transporters including OSTβ and apical sodium dependent bile acid transporter (ASBT)34. Furthermore, alcohol exposure decreases the expression of FGF1534. The transcriptional changes in the ileum may result in increased absorption of bile acids into the portal circulation. Altogether, chronic alcohol consumption increases hepatic and serum bile acid levels. Alcohol-induced accumulation of bile acids may be attributed to increased bile acid synthesis, increased bile acid absorption in the intestines, and increased bile acid efflux in the liver.
3. Role of FXR in alcohol-induced liver injury
Alcohol-induced disruption of the enterohepatic circulation has been attributed to decreased FXR activity. FXR negatively regulates the expression of CYP7A1 and CYP8B1 but positively regulates the expression of BSEP and FGF1526, 29, 41. Chronic alcohol consumption disrupted the interaction of FXR with RXRα by increasing acetylation of FXR, resulting in FXR inactivation33. Decreased acetylation of FXR may be due to alcohol-mediated inhibition of sirtuin 1 (SIRT1), a nicotinamide adenine dinucleotide (NAD+)-dependent histone deacetylase, and activation of acetyltransferase p30033, 42, 43. Alcohol metabolism increased [NADH]:[NAD+] ratio44, which may reduce NAD+-dependent SIRT1 enzymatic activity45. These results suggest that FXR and SIRT1 may be potential pharmacological targets for alleviating alcohol-induced cholestasis and liver injury.
WAY and 6α-ethyl-chenodeoxycholic acid (6ECDCA) are potent FXR-specific agonists25, 46. Intriguingly, pharmacological activation of FXR by WAY and 6ECDCA attenuated chronic alcohol-induced liver injury and steatosis33, 47. FXR regulates sterol regulatory element-binding protein 1 (SREBP1) through the SHP-liver X receptor (LXR) axis, in which SHP inhibits LXR activity resulting in decreased expression of SREBP148. Indeed, FXR activation by 6ECDCA attenuated alcohol-induced steatosis by ablating SREBP1-mediated lipogenesis47. Furthermore, FXR activation also decreased alcohol-mediated reactive oxygen species (ROS) production33, 47. The mechanism of how FXR activation protects against alcohol-induced oxidative stress is currently not well elucidated. However, WAY treatment decreased alcohol-mediated induction of CYP2E1, which may play a role in attenuation of alcohol-induced oxidative stress33. Interestingly, in human hepatocyte-derived cell lines, the proximal promoter sites of human alcohol dehydrogenase (ADH) isomers, ADH1A and ADH1B, have functional IR1. It has been found that FXR binds to the response elements and induces expression of ADH1A and ADH1B, resulting in increased ADH1 enzymatic activity. However, FXR did not induce ADH expression in rodent livers and hepatocytes, indicating that FXR-mediated induction of ADH may be species specific49. FXR may play a protective role against human ALD by inducing ADH-mediated metabolism of alcohol.
Conversely, ablation of Fxr exacerbated alcohol-induced liver injury in an acute alcohol model and the recent established chronic plus binge model (also called Gao-binge model)3, 4, 50, 51. It was reported that Gao-binge treatment suppressed expression of lipid oxidation genes in Fxr knockout (KO) mice, which may contribute to exacerbated hepatic steatosis. Furthermore, Gao-binge treatment induced expression of CD14, the receptor for LPS (lipopolysaccharide), in Fxr KO mice with a higher degree in comparison to WT (wild type) mice. Increased CD14 expression may exacerbate alcohol-induced liver injury by increasing the sensitivity to inflammation51. We also observed a much higher induction of hepatic CYP2E1 after acute alcohol treatment in Fxr KO than that of WT mice, which may contribute to exacerbated alcohol-induced liver injury in Fxr KO mice50. Altogether, these findings suggest that FXR plays a role in protecting the liver from alcohol-induced hepatotoxicity likely by regulating lipid metabolism, sensitivity to inflammation and CYP2E1-mediated oxidative stress.
4. Bile acid modulates alcohol-induced liver injury
Bile acid accumulation in cholestatic conditions can result in hepatotoxicity. As discussed before, alcohol exposure increased bile acid hydrophobicity via accumulation of more toxic unconjugated bile acids. Indeed, rats feed with chronic alcohol together with chenodeoxycholic acid (CDCA), a toxic hydrophobic bile acid, increased the hydrophobicity of pooled bile acids and exacerbated alcohol-induced liver injury52. However, the mechanism by which CDCA increased alcohol-induced liver injury is not elucidated.
Another group demonstrated that infusion of relatively hydrophobic TCA in bile duct obstructed rats or rats with choledochocaval fistula resulted in decreased hepatic ADH and catalase activity. However, the serum ADH activity as well as microsomal alcohol oxidizing system and aldehyde dehydrogenase was greatly increased under these conditions. In contrast, hydrophilic tauro-ursodeoxycholic acid (TUDCA) had no effects on alcohol-metabolizing enzymes53. These data suggest that hydrophobic bile acids may induce leakage of cytosolic alcohol metabolizing enzymes into the serum, resulting in altered alcohol metabolism during cholestasis.
Ursodeoxycholic acid (UDCA) is a cytoprotective therapeutic hydrophilic bile acid approved to treat cholestasis. UDCA protects cholangiocytes against toxicity exerted by hydrophobic bile acids, stimulates hepatobiliary secretion, and inhibits bile acid-induced apoptosis in hepatocytes54. UDCA and its conjugated form, TUDCA both protected against alcohol-induced toxicity in human hepatoblastoma HepG2 cells55. However these studies are questionable since HepG2 cells may not be suitable to study alcohol hepatotoxicity due to the lack of expression of alcohol-metabolizing enzymes such as ADH and CYP2E156, 57. UDCA or TUDCA co-treatment with alcohol protected against alcohol-induced toxicity in ADH-containing human hepatoma cells (SK-Hep-1)58. Moreover, UDCA treatment reduced alcohol-induced liver injury and steatosis in rat livers59. UDCA and TUDCA attenuated alcohol-induced hepatotoxicity through various possible mechanisms including preservation of mitochondrial integrity, improvement of ATP synthesis, and decrease of alcohol-induced oxidative damage independent of CYP2E1 and glutathione60, 61, 62. Moreover, chronic alcohol consumption has been shown to inhibit production of liver prostaglandins, and UDCA treatment restored expression of prostaglandin E and increased linoleoyl-CoA desaturase activity in alcohol-treated rat livers possibly due to enhanced membrane fluidity63. Therefore, UDCA protects against alcohol-induced hepatotoxicity by improving mitochondrial function and attenuating oxidative stress in vivo although its protective effect against alcohol-induced toxicity in vitro is less clear.
The beneficial effects of UDCA against ALD in animal models have led to clinical trials for UDCA therapy in alcoholic cirrhosis although so far these trials yielded mixed results. In a placebo-controlled cross-over trial, the patients were administered with UDCA (15 mg/kg/d) or placebo for 4 weeks. UDCA treatment resulted in a significant decrease in bilirubin, gamma-glutamyltranspeptidase (GGT), and alanine aminotransferase (ALT) levels64. These clinical data thus suggest that UDCA treatment may alleviate alcohol-induced hepatotoxicity despite continued alcohol consumption. In another study using a randomized controlled trial, UDCA (13–15 mg/kg/d) or placebo was administered for 6 months. Patients that received UDCA had decreased levels of GGT and alkaline phosphatase compared to the patients that were received placebo65. However, UDCA treatment also resulted in lower survival rates and increased complications65. It should be noted that the pilot study by Plevris et al. only included patients with initial bilirubin levels below 50 µmol/L and lower Child-Pugh score (A–B with one patient being C) in comparison to the clinical study by Pelletier et al., which consisted of patients displaying higher initial bilirubin levels (all above 50 µmol/L) and the majority of patients were Child-Pugh C. The recruitment of patients with different initial bilirubin levels may be partially contributed to the different outcomes from these two different trials. Moreover, the dose of UDCA may be inappropriate for more severe form of alcohol cirrhosis because the patients in the UDCA groups displayed dramatic increase in total bile acid levels and some patients even displayed serum bile acid levels as high as 1000 µmol/L. Therefore, the dispute in efficiency of UDCA treatment in alcoholic cirrhosis may be attributed to the severity of hepatic damage prior to the initiation of UDCA treatment. More clinical trials are needed to further determine the effects of UDCA on different stages of ALD patients.
5. Gut microbiome and ALD
Gut microbiome is one of the key players in ALD. Bacterial growth and dysbiosis are hallmarks of various liver diseases including ALD. Several groups have extensively reviewed the role of gut microbiome in ALD recently66, 67, 68. ALD patients exhibit bacterial overgrow along the gastrointestinal tract, which affects alcohol metabolism resulting in increased concentration of acetaldehyde69, 70, 71, 72, 73, 74, 75, 76. Endotoxemia is well documented in patients with ALD. Endotoxemia increased hepatic inflammation due to activation of Kupffer cells and subsequent toll-like receptor 4 (TLR4)-mediated cytokine and chemokine production66, 77. Alcohol exposure induces bacterial translocation and increases gut permeability that promote endotoxemia and facilitate the development of ALD66.
Intestinal dysbiosis occurs when the composition of intestinal bacteria is altered, and is a hallmark of ALD. Patients with alcoholic cirrhosis have higher amount of Proteobacteria, Prevotellaceae, and Veillonellaceae, and lower amount of Bacteroidetes in the colon and feces compared to non-cirrhotic alcoholic patients or healthy people78, 79, 80. Moreover, in several animal studies, alcohol-fed animals had higher proportions of Verrucomircobia, Proteobacteria, and Actinobacteria, and lower proportions of Firmicutes including Lactobacillus, Pediococcus, Leuconostoc and Lactococcus72, 73, 81. One study has shown that Bacteroidetes was elevated in chronic alcohol-fed rats, whereas, another study has demonstrated that chronic alcohol feeding decreased the proportion of Bacteroidetes72, 73. Currently, it is not clear how enteric dysbiosis influences the progress of ALD. Interestingly, probiotics and prebiotics including Lactobacillius feedings alleviated alcohol-induced liver injury and restored the gut microbiota in both animal models and alcoholic patients66, 73, 82, 83, 84, 85, 86, 87, 88. Restoring gut microbiota by dietary approach using prebiotics and probiotics emerges as a promising therapy for ALD.
Gut microbiota plays an essential role in bile acid metabolism, in which the intestinal bacteria are involved in biotransformation of bile acids through deconjugation, dehydroxylation, and reconjugation89. Chronic alcohol consumption increased the concentration of unconjugated bile acids along the gastrointestinal tract, especially in the small intestines (duodenum and ileum). Alcohol consumption decreased the concentration of taurine-conjugated bile acids and increased the amount of unconjugated bile acids in the intestinal tract, liver and serum34. The perturbed bile acid profile may be attributed to gut bacterial overgrowth, resulting in increased deconjugation of bile acids and taurine metabolism66. It is known that gut bacteria metabolize majority of taurine into inorganic sulfate, which results in decreased taurine bioavailability90. Interestingly, alcohol feeding increased the formation and excretion of a taurine metabolite, N-acetyltaurine91. Therefore, alcohol-induced taurine metabolism by gut bacteria may decrease taurine available for bile acid conjugation in the liver and alter the systematic bile acid profile.
Conversely, bile acids also regulate the gut flora via their antimicrobial activity92. Rats fed with a cholic acid (CA)-containing diet had increased gut Firmicutes to Bacteroidetes ratio. Moreover, these rats displayed increased level of a toxic bile acid, deoxycholic acid (DCA), in the cecum due to bacterial-mediated 7α dehydroxylation of CA. DCA is extremely toxic and selectively inhibits growth of gut bacteria including Bacteroidetes and Lactobacillus, which results in altered gut microbiota93. Moreover, increased abundance of Firmicutes promotes the growth of DCA-producing bacteria94. Alcohol consumption also increased DCA concentration in the gastrointestinal tract34. Patients with or without alcoholic cirrhosis that are active drinkers exhibited increased secondary bile acids including DCA along with decreased fecal Bacteroidetes80. Therefore, toxic DCA may play a role in alcohol-induced gut bacteria dysbiosis.
FXR activation by bile acids induced expression of genes involved in enteroprotection and inhibited bacterial overgrowth and mucosal injury95. Conversely, Fxr KO mice displayed more severe bacteria overgrowth and epithelial barrier deterioration95. These results suggest that FXR also has antimicrobial activity. As discussed above, alcohol consumption inhibits FXR activation, and it will be interesting to determine the role of FXR in alcohol-induced bacteria dysbiosis and gut permeability in the future.
6. Autophagy in ALD
Macroautophagy (hereafter referred to as autophagy) is an evolutionarily conserved catabolic process responsible for disposing and recycling cellular proteins and damaged/excess organelles in response to starvation and cellular stresses. The autophagy process initiates with formation of isolation membranes, which are elongated and fused to become double membrane autophagosomes96. Autophagosomes then fuse with the lysosomes to complete the degradation process. More than 30 autophagy-related (Atg) genes have been identified in yeast and most of them have mammalian homolog counterparts that participate in the autophagic process97. One of the key steps in the formation of a double membrane autophagosome is the conjugation of the microtubule associated protein 1 light chain 3 (LC3) with phosphatidylethanolamine (PE). LC3-PE translocates from the cytosol to the isolation membrane to promote the formation of autophagosomes98, 99, 100, 101. Sequestome-1 (SQSTM-1)/p62 is an autophagy receptor protein with a LC3 interacting region, which enables p62 to recruit ubiquitinated protein aggregates and deliver them to the autophagosomes for degradation102. p62 is also an autophagic substrate that is normally degraded under starvation-induced autophagy, and is accumulated in autophagy-deficient conditions103, 104, 105. Therefore, monitoring the levels of p62 has been widely used as an autophagic flux marker100.
Autophagic degradation can be either non-selective or selective depending on the cellular conditions. Non-selective autophagy occurs in energy deficient condition such as starvation, and degrades cellular components in order to provide nutrient and energy. Selective autophagy degrades protein aggregates and excessive or damaged organelles as a protective mechanism in either nutrient-rich or poor conditions3, 106, 107.
Autophagy has been shown to play an essential role in liver physiology, and deregulation of hepatic autophagy has been implicated in pathogenesis of various liver diseases including ALD108, 109, 110. We have demonstrated that acute alcohol treatment induced hepatic autophagy to selectively degrade damaged mitochondria and excess lipid droplets111, 112. Acute alcohol-mediated autophagy induction required alcohol-metabolizing enzymes, e.g., CYP2E1 and ADH3, 112. More importantly, pharmacological activation of autophagy protected against alcohol-induced hepatotoxicity and steatosis. Conversely, pharmacological inhibition of autophagy exacerbated alcohol-induced hepatotoxicity112, 113. In contrast to the acute alcohol exposure, the status of autophagy in chronic alcohol exposure is less clear and controversial, which could be reflected by the lack of reliable autophagic flux assay in vivo and the dynamic nature of autophagy during the long time chronic feeding conditions3. Nevertheless, similar to the findings from the acute alcohol exposure, pharmacological activation of autophagy also showed beneficial effects against chronic alcohol feeding-induced liver injury in mice113. Therefore, induction of autophagy may be a promising therapeutic option for ALD.
We recently demonstrated that alcohol also regulates autophagy at the transcriptional level42. Forkhead box-containing protein class O3a (FoxO3a) is a member of FoxO family of evolutionarily conserved DAF-16 like transcription factor114, 115, 116. Multiple post-translation modifications, which include phosphorylation, ubiquitination, acetylation, and methylation, regulate FoxO3a activity114, 116. Protein kinase B (AKT) phosphorylates FoxO3a at serine 253, resulting in sequestration of FoxO3a in the cytoplasm, which inhibits FoxO3a-mediated transcriptional activation117. FoxO3a regulates transcription of genes involved in apoptosis, oxidative stress, cell-cycle transition, and DNA repair114, 117. Moreover, FoxO3a also regulates transcription of Atg genes in skeletal muscles, cardiomyocytes, and liver42, 118, 119, 120, 121. Our group and others have demonstrated that FoxO3a protects against alcohol-induced hepatotoxicity and steatosis by initiating transcription of Atg and antioxidant genes42, 122, 123. Acute alcohol treatment increased expression of Atg genes in mouse livers and primary cultured hepatocytes. Mechanistically, acute alcohol treatment decreased AKT-mediated FoxO3a phosphorylation at serine 253, which resulted in nuclear accumulation of FoxO3a in mouse livers42. Interestingly, induction of SIRT1 activity by resveratrol promoted deacetylation of FoxO3a, which increased FoxO3a-mediated transcription of Atg genes in response to alcohol. Both acute alcohol exposure and chronic alcohol feeding induced more severe steatosis and hepatotoxicity in FoxO3a KO mice compared to WT mice122. These results suggest that FoxO3a protects against alcohol-induced hepatotoxicity likely by inducing expressions of Atg and antioxidant genes.
7. Bile acids and FXR regulate hepatic autophagy in ALD
As discussed above, bile acids are nutrient signaling molecules. Emerging evidence shows that bile acids also regulate autophagy. DCA, a hydrophobic secondary bile acid, induced accumulation of autophagosomes in cultured rodent hepatocytes and human esophageal cells as well as increased LC3-II protein expression in colon epithelial cells124, 125, 126. We demonstrated that bile acids inhibit completion of autophagic process in hepatocytes by decreasing Rab7-mediated fusion of autophagosomes with lysosomes in hepatocytes, a process which is independent of FXR127. Whole body Fxr KO mice had impaired hepatic autophagy as demonstrated by the elevated hepatic p62 and LC3-II levels. This is likely due to the elevated hepatic bile acid levels since hepatocyte-specific Fxr KO mice had normal levels of hepatic bile acids and normal autophagy. Because of the impaired hepatic autophagy in Fxr KO mice, it is not surprising that we further found that alcohol-induced liver injury is exacerbated in Fxr KO mice50. Intriguingly, we further found that alcohol-induced expression of Atg genes (Atg5, Becn-1 and Map1lc3b) was abolished in Fxr KO mouse livers. Furthermore, alcohol-induced hepatic expression of FoxO3a target genes (MnSod, p21, and FoxO3a) in WT mice was also suppressed in Fxr KO mice50. These results suggest that FXR is associated with acute alcohol-induced FoxO3a activation and autophagy in mouse livers. The decreased FoxO3a-mediated expression of Atg genes in acute alcohol-treated mouse livers was likely due to the secondary effects such as increased hepatic AKT activation in Fxr KO mice, but not due to the direct FXR-FoxO3a interaction since we failed to detect such an interaction in either FXR and FoxO3a over-expressed cultured cells or in alcohol-treated mouse livers50.
FXR is a nutrient-sensing nuclear receptor that is activated in fed state by bile acids returning to the liver, whereas peroxisome proliferator-activated receptor alpha (PPARα) is activated by fatty acids during fasting128, 129, 130, 131, 132, 133. Therefore, FXR may also be involved in regulating autophagy in response to nutrient abundance especially in postprandial period. Indeed, two recent studies from Moore׳s group and Kemper׳s group134, 135 independently demonstrated that activation of FXR ablated nutrient starvation-induced autophagy in vitro and in vivo. Pharmacological activation of FXR by GW4064 attenuated the expression of Atg genes in fasted, but not fed mouse livers134, 135. Mechanistically, FXR inhibited transcription of Atg genes by two distinct but complementary mechanisms. First, FXR and PPARα compete for binding to the shared direct repeat 1 (DR1) sites, with opposite transcriptional outcomes on the expression of autophagy genes135. Second, FXR disrupts the interaction of cAMP response element binding protein (CREB) with its co-activator, CREB-regulated transcription coactivator 2 (CRTC2), resulting in decreased expression of Atg genes in fed state134. These findings suggest that FXR negatively regulates autophagy by nutrient status. These observations seem to conflict with our findings that Fxr KO mice had impaired hepatic autophagy. However, it should be noted that Fxr KO mice had increased hepatic bile acids, inflammation and cell death as well as altered various signaling pathways such as AKT that may regulate autophagy29, 136, 137. The impaired hepatic autophagy in Fxr KO mice could be due to the secondary factors as a result of the loss of FXR. Indeed, Fxr KO mice have increased hepatic accumulation of p62, inflammation and developed spontaneous liver tumors, which are very similar to the autophagy-deficient mouse livers127, 136, 137, 138, 139, 140. Therefore, while FXR may inhibit autophagy in response to the nutrient status, FXR is not the only regulator of hepatic autophagy. It is also possible that the chronic loss (or inhibition) of FXR or acute activation of FXR may have different impacts on hepatic autophagy. As discussed above, chronic alcohol consumption has been shown to inhibit FXR activity. Currently, the effect of chronic alcohol exposure on FXR-mediated repression of autophagy gene transcription has not been elucidated. However, one would assume that chronic alcohol exposure might increase the expression of Atg genes due to inhibition of FXR and subsequent impair FXR-mediated repression of Atg gene transcription. Liver-specific Fxr KO mice fed with alcohol will be a very useful model to further test this hypothesis, because these mice would avoid the problems such as increased bile acids and liver injury in whole body Fxr KO mice.
Chronic alcohol-treated Pparα KO mice exhibited hepatomegaly and increased hepatocyte proliferation, enhanced mitochondrial damage, liver injury and inflammation141. Chronic alcohol feeding decreased the DNA-binding affinity of PPARα, which was reversed by treatment with WY14643, a PPARα agonist142. Pharmacological activation of PPARα by WY14643 protected against chronic alcohol-induced hepatotoxicity and steatosis in mice142, 143. These results are consistent with the notion that PPARα promotes autophagy although the expression of autophagy genes and autophagy activity were not determined in these studies.
As discussed above, PPARα and FXR compete for DR1 binding to regulate the expression of Atg genes and FXR negatively regulates transcription factor EB (TFEB), a regulator of lysosomal biogenesis134. PPARα is an important downstream mediator of TFEB in response to nutrient starvation144. Chronic alcohol feeding did not affect CREB phosphorylation or nuclear translocation, but chronic alcohol feeding (four weeks) plus a single alcohol binge increased phosphorylated CREB and decreased nuclear CREB levels in rat livers145. Whether and how TFEB, FXR, CREB and PPARα are integrated or independently participate in the regulation of Atg genes expression after alcohol exposure are currently unknown. It seems that the regulation of autophagy at the transcriptional level after alcohol exposure could be very complex and different from starvation conditions. Nevertheless, future studies on deciphering these complex transcriptional factor-mediated regulations on autophagy after alcohol exposure may offer some novel promising approaches for treating ALD.
8. Conclusions and perspectives
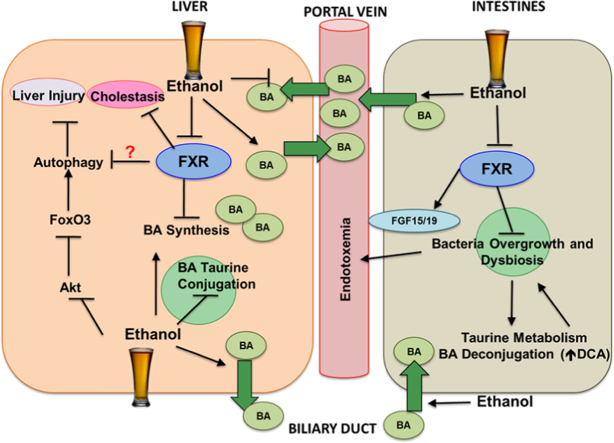
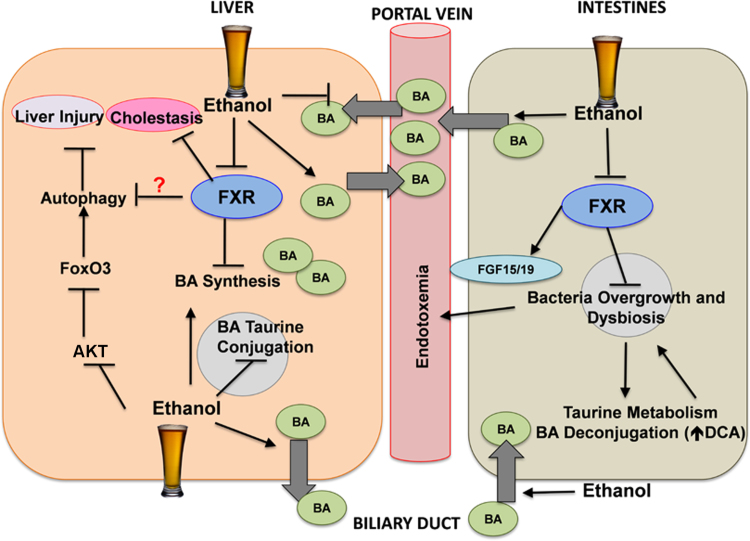
ALD is one of the major causes of liver morbidity and mortality worldwide. Currently, there is no effective treatment for ALD. The last treatment proposed for ALD was in the 1970s, in which corticosteroids were used to treat alcoholic steatohepatitis. Therefore, developing novel pathophysiological-targeted therapies is urgently needed. FXR has emerged as a novel possible therapeutic target in ALD. Recent evidence demonstrated that alcohol exposure impairs FXR activation, which results in increased bile acid synthesis and pool. Moreover, FXR protects against alcohol-induced gut bacteria dysbiosis and overgrowth as well as the accumulation of toxic bile acids such as DCA. Increased bile acids may inhibit the completion of autophagic degradation in hepatocytes. Therefore, restoring bile acid homeostasis is important for the autophagy function, which is an important protective mechanism against ALD. Paradoxically, FXR may inhibit the induction of autophagy in response to nutrient starvation. However, the effect of alcohol exposure on FXR-mediated repression of autophagy needs to be further deciphered. Moreover, other transcription factors including FoxO3a, PPARα, CREB and TFEB have been implicated in the regulation of Atg gene expression. Therefore, elucidating the mechanism of how transcriptional factor-mediated regulation on autophagy after alcohol exposure may lead to generate promising therapeutic target for ALD in the future. In summary, the FXR-bile acid axis may be a promising therapeutic target for ALD. More studies are needed to further examine the role of FXR and bile acids in alcohol-induced hepatotoxicity and steatosis. The molecular and cellular events of alcohol on FXR, bile acids, gut microbiome and autophagy are summarized in Fig. 1.
Figure 1.
Schematic diagram of the cellular and molecular events of alcohol exposure on FXR, enterohepatic circulation, gut microbiome and autophagy. Alcohol treatment inhibits FXR in the liver, which results in increased bile acid synthesis. Moreover, alcohol exposure decreases taurine conjugation of bile acids and increases efflux of bile acids out of the hepatocytes into the portal vein and bile duct. Acute alcohol exposure also induces autophagy by inhibiting AKT, which results in FoxO3a activation and FoxO3a-mediated up-regulation of Atg genes. Alcohol increases the uptake of bile acids into the enterocytes and promotes efflux of bile acids into the portal circulation from the intestines. Alcohol exposure also inhibits intestinal FXR activation, which leads to decreased FGF15/19 expression and promotes bacteria overgrowth and dysbiosis. Increased abundance of intestinal bacteria promotes taurine metabolism and bile acid deconjugation. Finally, increased levels of unconjugated bile acids including DCA exacerbate alcohol-induced dysbiosis. FXR may negatively regulate autophagy and cholestasis, and autophagy protects against ALD.
Acknowledgments
The research work in Wenxing Ding׳s lab was supported in part by the National Institutes of Health (Nos. R01 AA020518 and R01 DK102142), National Center for Research Resources (No. 5P20RR021940), the National Institute of General Medical Sciences (Nos. 8P20 GM103549 and T32 ES007079), and an Institutional Development Award (IDeA) from the National Institute of General Medical Sciences of the National Institutes of Health (No. P20 GM103418).
Footnotes
Peer review under responsibility of Institute of Materia Medica, Chinese Academy of Medical Sciences and Chinese Pharmaceutical Association.
References
- 1.Ding WX, Manley S, Ni HM. The emerging role of autophagy in alcoholic liver disease. Exp Biol Med. 2011;236:546–556. doi: 10.1258/ebm.2011.010360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gao B, Bataller R. Alcoholic liver disease: pathogenesis and new therapeutic targets. Gastroenterology. 2011;141:1572–1585. doi: 10.1053/j.gastro.2011.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Williams JA, Manley S, Ding WX. New advances in molecular mechanisms and emerging therapeutic targets in alcoholic liver diseases. World J Gastroenterol. 2014;20:12908–12933. doi: 10.3748/wjg.v20.i36.12908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li Y, Wang S, Ni HM, Huang HQ, Ding WX. Autophagy in alcohol-induced multiorgan injury: mechanisms and potential therapeutic targets. BioMed Res Int. 2014;2014:498491. doi: 10.1155/2014/498491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wilfred de Alwis NM, Day CP. Genetics of alcoholic liver disease and nonalcoholic fatty liver disease. Semin Liver Dis. 2007;27:44–54. doi: 10.1055/s-2006-960170. [DOI] [PubMed] [Google Scholar]
- 6.Tsukamoto H, Machida K, Dynnyk A, Mkrtchyan H. “Second hit” models of alcoholic liver disease. Semin Liver Dis. 2009;29:178–187. doi: 10.1055/s-0029-1214373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.O׳Shea RS, Dasarathy S, McCullough AJ. Alcoholic liver disease. Hepatology. 2010;51:307–328. doi: 10.1002/hep.23258. [DOI] [PubMed] [Google Scholar]
- 8.Tung BY, Carithers R.L. Jr. Cholestasis and alcoholic liver disease. Clin Liver Dis. 1999;3:585–601. doi: 10.1016/s1089-3261(05)70086-6. [DOI] [PubMed] [Google Scholar]
- 9.Modica S, Gadaleta RM, Moschetta A. Deciphering the nuclear bile acid receptor FXR paradigm. Nucl Recept Signal. 2010;8:e005. doi: 10.1621/nrs.08005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhou H, Hylemon PB. Bile acids are nutrient signaling hormones. Steroids. 2014;86:62–68. doi: 10.1016/j.steroids.2014.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li TG, Chiang JYL. Bile acid signaling in liver metabolism and diseases. J Lipids. 2012;2012:754067. doi: 10.1155/2012/754067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li TG, Chiang JYL. Bile acid signaling in metabolic disease and drug therapy. Pharmacol Rev. 2014;66:948–983. doi: 10.1124/pr.113.008201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Forman BM, Goode E, Chen J, Oro AE, Bradley DJ, Perlmann T. Identification of a nuclear receptor that is activated by farnesol metabolites. Cell. 1995;81:687–693. doi: 10.1016/0092-8674(95)90530-8. [DOI] [PubMed] [Google Scholar]
- 14.Seol W, Choi HS, Moore DD. Isolation of proteins that interact specifically with the retinoid X receptor: two novel orphan receptors. Mol Endocrinol. 1995;9:72–85. doi: 10.1210/mend.9.1.7760852. [DOI] [PubMed] [Google Scholar]
- 15.Makishima M, Okamoto AY, Repa JJ, Tu H, Learned RM, Luk A. Identification of a nuclear receptor for bile acids. Science. 1999;284:1362–1365. doi: 10.1126/science.284.5418.1362. [DOI] [PubMed] [Google Scholar]
- 16.Parks DJ, Blanchard SG, Bledsoe RK, Chandra G, Consler TG, Kliewer SA. Bile acids: natural ligands for an orphan nuclear receptor. Science. 1999;284:1365–1368. doi: 10.1126/science.284.5418.1365. [DOI] [PubMed] [Google Scholar]
- 17.Wang H, Chen J, Hollister K, Sowers LC, Forman BM. Endogenous bile acids are ligands for the nuclear receptor FXR/BAR. Mol Cell. 1999;3:543–553. doi: 10.1016/s1097-2765(00)80348-2. [DOI] [PubMed] [Google Scholar]
- 18.Chawla A, Repa JJ, Evans RM, Mangelsdorf DJ. Nuclear receptors and lipid physiology: opening the X-files. Science. 2001;294:1866–1870. doi: 10.1126/science.294.5548.1866. [DOI] [PubMed] [Google Scholar]
- 19.Lefebvre P, Cariou B, Lien F, Kuipers F, Staels B. Role of bile acids and bile acid receptors in metabolic regulation. Physiol Rev. 2009;89:147–191. doi: 10.1152/physrev.00010.2008. [DOI] [PubMed] [Google Scholar]
- 20.Laffitte BA, Kast HR, Nguyen CM, Zavacki AM, Moore DD, Edwards PA. Identification of the DNA binding specificity and potential target genes for the farnesoid X-activated receptor. J Biol Chem. 2000;275:10638–10647. doi: 10.1074/jbc.275.14.10638. [DOI] [PubMed] [Google Scholar]
- 21.Kim I, Ahn SH, Inagaki T, Choi M, Ito S, Guo GL. Differential regulation of bile acid homeostasis by the farnesoid X receptor in liver and intestine. J Lipid Res. 2007;48:2664–2672. doi: 10.1194/jlr.M700330-JLR200. [DOI] [PubMed] [Google Scholar]
- 22.Inagaki T, Choi M, Moschetta A, Peng L, Cummins CL, McDonald JG. Fibroblast growth factor 15 functions as an enterohepatic signal to regulate bile acid homeostasis. Cell Metab. 2005;2:217–225. doi: 10.1016/j.cmet.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 23.Song KH, Li T, Owsley E, Strom S, Chiang JY. Bile acids activate fibroblast growth factor 19 signaling in human hepatocytes to inhibit cholesterol 7α-hydroxylase gene expression. Hepatology. 2009;49:297–305. doi: 10.1002/hep.22627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Maloney PR, Parks DJ, Haffner CD, Fivush AM, Chandra G, Plunket KD. Identification of a chemical tool for the orphan nuclear receptor FXR. J Med Chem. 2000;43:2971–2974. doi: 10.1021/jm0002127. [DOI] [PubMed] [Google Scholar]
- 25.Flatt B, Martin R, Wang TL, Mahaney P, Murphy B, Gu XH. Discovery of XL335 (WAY-362450), a highly potent, selective, and orally active agonist of the farnesoid X receptor (FXR) J Med Chem. 2009;52:904–907. doi: 10.1021/jm8014124. [DOI] [PubMed] [Google Scholar]
- 26.Goodwin B, Jones SA, Price RR, Watson MA, McKee DD, Moore LB. A regulatory cascade of the nuclear receptors FXR, SHP-1, and LRH-1 represses bile acid biosynthesis. Mol Cell. 2000;6:517–526. doi: 10.1016/s1097-2765(00)00051-4. [DOI] [PubMed] [Google Scholar]
- 27.Lee FY, Lee H, Hubbert ML, Edwards PA, Zhang Y. FXR, a multipurpose nuclear receptor. Trends Biochem Sci. 2006;31:572–580. doi: 10.1016/j.tibs.2006.08.002. [DOI] [PubMed] [Google Scholar]
- 28.Sanyal S, Båvner A, Haroniti A, Nilsson LM, Lundåsen T, Rehnmark S. Involvement of corepressor complex subunit GPS2 in transcriptional pathways governing human bile acid biosynthesis. Proc Natl Acad Sci USA. 2007;104:15665–15670. doi: 10.1073/pnas.0706736104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sinal CJ, Tohkin M, Miyata M, Ward JM, Lambert G, Gonzalez FJ. Targeted disruption of the nuclear receptor FXR/BAR impairs bile acid and lipid homeostasis. Cell. 2000;102:731–744. doi: 10.1016/s0092-8674(00)00062-3. [DOI] [PubMed] [Google Scholar]
- 30.Glover SC, McPhie JL, Brunt PW. Cholestasis in acute alcoholic liver disease. Lancet. 1977;2:1305–1307. doi: 10.1016/s0140-6736(77)90360-9. [DOI] [PubMed] [Google Scholar]
- 31.Lefevre AF, DeCarli LM, Lieber CS. Effect of ethanol on cholesterol and bile acid metabolism. J Lipid Res. 1972;13:48–55. [PubMed] [Google Scholar]
- 32.Vendemiale G, Jayatilleke E, Shaw S, Lieber CS. Depression of biliary glutathione excretion by chronic ethanol feeding in the rat. Life Sci. 1984;34:1065–1073. doi: 10.1016/0024-3205(84)90020-1. [DOI] [PubMed] [Google Scholar]
- 33.Wu W, Zhu B, Peng X, Zhou M, Jia D, Gu J. Activation of farnesoid X receptor attenuates hepatic injury in a murine model of alcoholic liver disease. Biochem Biophys Res Commun. 2014;443:68–73. doi: 10.1016/j.bbrc.2013.11.057. [DOI] [PubMed] [Google Scholar]
- 34.Xie G, Zhong W, Li H, Li Q, Qiu Y, Zheng X. Alteration of bile acid metabolism in the rat induced by chronic ethanol consumption. FASEB J. 2013;27:3583–3593. doi: 10.1096/fj.13-231860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hofmann AF, Roda A. Physicochemical properties of bile acids and their relationship to biological properties: an overview of the problem. J Lipid Res. 1984;25:1477–1489. [PubMed] [Google Scholar]
- 36.Dawson PA, Lan T, Rao A. Bile acid transporters. J Lipid Res. 2009;50:2340–2357. doi: 10.1194/jlr.R900012-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kerai MD, Waterfield CJ, Kenyon SH, Asker DS, Timbrell JA. Taurine: protective properties against ethanol-induced hepatic steatosis and lipid peroxidation during chronic ethanol consumption in rats. Amino Acids. 1998;15:53–76. doi: 10.1007/BF01345280. [DOI] [PubMed] [Google Scholar]
- 38.Axelson M, Mörk B, Sjövall J. Ethanol has an acute effect on bile acid biosynthesis in man. FEBS Lett. 1991;281:155–159. doi: 10.1016/0014-5793(91)80382-d. [DOI] [PubMed] [Google Scholar]
- 39.Nilsson LM, Sjövall J, Strom S, Bodin K, Nowak G, Einarsson C. Ethanol stimulates bile acid formation in primary human hepatocytes. Biochem Biophys Res Commun. 2007;364:743–747. doi: 10.1016/j.bbrc.2007.10.039. [DOI] [PubMed] [Google Scholar]
- 40.Chanda D, Kim YH, Li T, Misra J, Kim DK, Kim JR. Hepatic cannabinoid receptor type 1 mediates alcohol-induced regulation of bile acid enzyme genes expression via CREBH. PLoS One. 2013;8:e68845. doi: 10.1371/journal.pone.0068845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lu TT, Makishima M, Repa JJ, Schoonjans K, Kerr TA, Auwerx J. Molecular basis for feedback regulation of bile acid synthesis by nuclear receptors. Mol Cell. 2000;6:507–515. doi: 10.1016/s1097-2765(00)00050-2. [DOI] [PubMed] [Google Scholar]
- 42.Ni HM, Du K, You M, Ding WX. Critical role of FoxO3a in alcohol-induced autophagy and hepatotoxicity. Am J Pathol. 2013;183:1815–1825. doi: 10.1016/j.ajpath.2013.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.You M, Liang X, Ajmo JM, Ness GC. Involvement of mammalian sirtuin 1 in the action of ethanol in the liver. Am J Physiol Gastrointest Liver Physiol. 2008;294:G892–G898. doi: 10.1152/ajpgi.00575.2007. [DOI] [PubMed] [Google Scholar]
- 44.Mezey E. Dietary fat and alcoholic liver disease. Hepatology. 1998;28:901–905. doi: 10.1002/hep.510280401. [DOI] [PubMed] [Google Scholar]
- 45.Haigis MC, Guarente LP. Mammalian sirtuins-emerging roles in physiology, aging, and calorie restriction. Genes Dev. 2006;20:2913–2921. doi: 10.1101/gad.1467506. [DOI] [PubMed] [Google Scholar]
- 46.Pellicciari R, Fiorucci S, Camaioni E, Clerici C, Costantino G, Maloney PR. 6α-Ethyl-chenodeoxycholic acid (6-ECDCA), a potent and selective FXR agonist endowed with anticholestatic activity. J Med Chem. 2002;45:3569–3572. doi: 10.1021/jm025529g. [DOI] [PubMed] [Google Scholar]
- 47.Lívero FA, Stolf AM, Dreifuss AA, Bastos-Pereira AL, Chicorski R, de Oliveira LG. The FXR agonist 6ECDCA reduces hepatic steatosis and oxidative stress induced by ethanol and low-protein diet in mice. Chem Biol Interact. 2014;217:19–27. doi: 10.1016/j.cbi.2014.03.014. [DOI] [PubMed] [Google Scholar]
- 48.Watanabe M, Houten SM, Wang L, Moschetta A, Mangelsdorf DJ, Heyman RA. Bile acids lower triglyceride levels via a pathway involving FXR, SHP, and SREBP-1c. J Clin Invest. 2004;113:1408–1418. doi: 10.1172/JCI21025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Langhi C, Pedraz-Cuesta E, Haro D, Marrero PF, Rodríguez JC. Regulation of human class I alcohol dehydrogenases by bile acids. J Lipid Res. 2013;54:2475–2484. doi: 10.1194/jlr.M039404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Manley S, Ni HM, Williams JA, Kong B, DiTacchio L, Guo G. Farnesoid X receptor regulates forkhead Box O3a activation in ethanol-induced autophagy and hepatotoxicity. Redox Biol. 2014;2:991–1002. doi: 10.1016/j.redox.2014.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kong B., Zhan L., Shen J., Forostyan T., Wang L., Ding W.X. Hepatoprotective effects of farnesoid X receptor in a mouse chronic alcohol feeding model. Hepatology. 2014;60:766A–771A. [Google Scholar]
- 52.Montet AM, Oliva L, Beaugé F, Montet JC. Bile salts modulate chronic ethanol-induced hepatotoxicity. Alcohol Alcohol. 2002;37:25–29. doi: 10.1093/alcalc/37.1.25. [DOI] [PubMed] [Google Scholar]
- 53.Kim YH, Shin MJ. Effects of high taurocholate load on activities of hepatic alcohol metabolizing enzymes. Exp Mol Med. 2002;34:123–130. doi: 10.1038/emm.2002.18. [DOI] [PubMed] [Google Scholar]
- 54.Paumgartner G, Beuers U. Ursodeoxycholic acid in cholestatic liver disease: mechanisms of action and therapeutic use revisited. Hepatology. 2002;36:525–531. doi: 10.1053/jhep.2002.36088. [DOI] [PubMed] [Google Scholar]
- 55.Neuman MG, Cameron RG, Shear NH, Bellentani S, Tiribelli C. Effect of tauroursodeoxycholic and ursodeoxycholic acid on ethanol-induced cell injuries in the human Hep G2 cell line. Gastroenterology. 1995;109:555–563. doi: 10.1016/0016-5085(95)90345-3. [DOI] [PubMed] [Google Scholar]
- 56.Clemens DL, Halgard CM, Miles RR, Sorrell MF, Tuma DJ. Establishment of a recombinant hepatic cell line stably expressing alcohol dehydrogenase. Arch Biochem Biophys. 1995;321:311–318. doi: 10.1006/abbi.1995.1400. [DOI] [PubMed] [Google Scholar]
- 57.Dai Y, Rashba-Step J, Cederbaum AI. Stable expression of human cytochrome P4502E1 in HepG2 cells: characterization of catalytic activities and production of reactive oxygen intermediates. Biochemistry. 1993;32:6928–6937. doi: 10.1021/bi00078a017. [DOI] [PubMed] [Google Scholar]
- 58.Henzel K, Thorborg C, Hofmann M, Zimmer G, Leuschner U. Toxicity of ethanol and acetaldehyde in hepatocytes treated with ursodeoxycholic or tauroursodeoxycholic acid. Biochim Biophys Acta. 2004;1644:37–45. doi: 10.1016/j.bbamcr.2003.10.017. [DOI] [PubMed] [Google Scholar]
- 59.Oliva L, Beaugé F, Choquart D, Montet AM, Guitaoui M, Montet JC. Ursodeoxycholate alleviates alcoholic fatty liver damage in rats. Alcohol Clin Exp Res. 1998;22:1538–1543. [PubMed] [Google Scholar]
- 60.Tabouy L, Zamora AJ, Oliva L, Montet AM, Beaugé F, Montet JC. Ursodeoxycholate protects against ethanol-induced liver mitochondrial injury. Life Sci. 1998;63:2259–2270. doi: 10.1016/s0024-3205(98)00511-6. [DOI] [PubMed] [Google Scholar]
- 61.Nguyen TD, Oliva L, Villard PH, Puyoou F, Sauze C, Montet AM. Cyp2e1 and Cyp3a1/2 gene expression is not associated with the ursodeoxycholate effect on ethanol-induced lipoperoxidation. Life Sci. 1999;65:1103–1113. doi: 10.1016/s0024-3205(99)00344-6. [DOI] [PubMed] [Google Scholar]
- 62.Vendemiale G, Grattagliano I, Signorile A, Altomare E. Ethanol-induced changes of intracellular thiol compartmentation and protein redox status in the rat liver: effect of tauroursodeoxycholate. J Hepatol. 1998;28:46–53. doi: 10.1016/s0168-8278(98)80201-8. [DOI] [PubMed] [Google Scholar]
- 63.Lukivskaya OY, Maskevich AA, Buko VU. Effect of ursodeoxycholic acid on prostaglandin metabolism and microsomal membranes in alcoholic fatty liver. Alcohol. 2001;25:99–105. doi: 10.1016/s0741-8329(01)00171-9. [DOI] [PubMed] [Google Scholar]
- 64.Plevris JN, Hayes PC, Bouchier IAD. Ursodeoxycholic acid in the treatment of alcoholic liver disease. Eur J Gastroenterol Hepatol. 1991;3:653–656. [Google Scholar]
- 65.Pelletier G, Roulot D, Davion T, Masliah C, Causse X, Oberti F. A randomized controlled trial of ursodeoxycholic acid in patients with alcohol-induced cirrhosis and jaundice. Hepatology. 2003;37:887–892. doi: 10.1053/jhep.2003.50118. [DOI] [PubMed] [Google Scholar]
- 66.Zhong W, Zhou ZX. Alterations of the gut microbiome and metabolome in alcoholic liver disease. World J Gastrointest Pathophysiol. 2014;5:514–522. doi: 10.4291/wjgp.v5.i4.514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Schnabl B, Brenner DA. Interactions between the intestinal microbiome and liver diseases. Gastroenterology. 2014;146:1513–1524. doi: 10.1053/j.gastro.2014.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chen P, Schnabl B. Host-microbiome interactions in alcoholic liver disease. Gut Liver. 2014;8:237–241. doi: 10.5009/gnl.2014.8.3.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gabbard SL, Lacy BE, Levine GM, Crowell MD. The impact of alcohol consumption and cholecystectomy on small intestinal bacterial overgrowth. Dig Dis Sci. 2014;59:638–644. doi: 10.1007/s10620-013-2960-y. [DOI] [PubMed] [Google Scholar]
- 70.Bode JC, Bode C, Heidelbach R, Dürr HK, Martíni GA. Jejunal microflora in patients with chronic alcohol abuse. Hepatogastroenterology. 1984;31:30–34. [PubMed] [Google Scholar]
- 71.Casafont Morencos F, de las Heras Castaño G, Martín Ramos L, López Arias MJ, Ledesma F, Pons Romero F. Small bowel bacterial overgrowth in patients with alcoholic cirrhosis. Dig Dis Sci. 1996;41:552–556. doi: 10.1007/BF02282340. [DOI] [PubMed] [Google Scholar]
- 72.Yan AW, Fouts DE, Brandl J, Stärkel P, Torralba M, Schott E. Enteric dysbiosis associated with a mouse model of alcoholic liver disease. Hepatology. 2011;53:96–105. doi: 10.1002/hep.24018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bull-Otterson L, Feng W, Kirpich I, Wang Y, Qin X, Liu Y. Metagenomic analyses of alcohol induced pathogenic alterations in the intestinal microbiome and the effect of Lactobacillus rhamnosus GG treatment. PloS One. 2013;8:e53028. doi: 10.1371/journal.pone.0053028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Väkeväinen S, Tillonen J, Salaspuro M, Jousimies-Somer H, Nuutinen H, Färkkilä M. Hypochlorhydria induced by a proton pump inhibitor leads to intragastric microbial production of acetaldehyde from ethanol. Aliment Pharmacol Ther. 2000;14:1511–1518. doi: 10.1046/j.1365-2036.2000.00858.x. [DOI] [PubMed] [Google Scholar]
- 75.Visapää JP, Tillonen J, Salaspuro M. Microbes and mucosa in the regulation of intracolonic acetaldehyde concentration during ethanol challenge. Alcohol Alcohol. 2002;37:322–326. doi: 10.1093/alcalc/37.4.322. [DOI] [PubMed] [Google Scholar]
- 76.Baraona E, Julkunen R, Tannenbaum L, Lieber CS. Role of intestinal bacterial overgrowth in ethanol production and metabolism in rats. Gastroenterology. 1986;90:103–110. doi: 10.1016/0016-5085(86)90081-8. [DOI] [PubMed] [Google Scholar]
- 77.Rao R. Endotoxemia and gut barrier dysfunction in alcoholic liver disease. Hepatology. 2009;50:638–644. doi: 10.1002/hep.23009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Mutlu EA, Gillevet PM, Rangwala H, Sikaroodi M, Naqvi A, Engen P.A. Colonic microbiome is altered in alcoholism. Am J Physiol Gastrointest Liver Physiol. 2012;302:G966–G978. doi: 10.1152/ajpgi.00380.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Chen Y, Yang F, Lu H, Wang B, Chen Y, Lei D. Characterization of fecal microbial communities in patients with liver cirrhosis. Hepatology. 2011;54:562–572. doi: 10.1002/hep.24423. [DOI] [PubMed] [Google Scholar]
- 80.Kakiyama G, Hylemon PB, Zhou H, Pandak WM, Heuman DM, Kang DJ. Colonic inflammation and secondary bile acids in alcoholic cirrhosis. Am J Physiol Gastrointest Liver Physiol. 2014;306:G929–G937. doi: 10.1152/ajpgi.00315.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Mutlu E, Keshavarzian A, Engen P, Forsyth CB, Sikaroodi M, Gillevet P. Intestinal dysbiosis: a possible mechanism of alcohol-induced endotoxemia and alcoholic steatohepatitis in rats. Alcohol Clin Exp Res. 2009;33:1836–1846. doi: 10.1111/j.1530-0277.2009.01022.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Forsyth CB, Farhadi A, Jakate SM, Tang Y, Shaikh M, Keshavarzian A. Lactobacillus GG treatment ameliorates alcohol-induced intestinal oxidative stress, gut leakiness, and liver injury in a rat model of alcoholic steatohepatitis. Alcohol. 2009;43:163–172. doi: 10.1016/j.alcohol.2008.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Nanji AA, Khettry U, Sadrzadeh SM. Lactobacillus feeding reduces endotoxemia and severity of experimental alcoholic liver (disease) Proc Soc Exp Biol Med. 1994;205:243–247. doi: 10.3181/00379727-205-43703. [DOI] [PubMed] [Google Scholar]
- 84.Komatsuzaki N, Shima J. Effects of live Lactobacillus paracasei on plasma lipid concentration in rats fed an ethanol-containing diet. Biosci Biotechnol Biochem. 2012;76:232–237. doi: 10.1271/bbb.110390. [DOI] [PubMed] [Google Scholar]
- 85.Chang B, Sang LX, Wang Y, Tong J, Zhang D, Wang BY. The protective effect of VSL#3 on intestinal permeability in a rat model of alcoholic intestinal injury. BMC Gastroenterol. 2013;13:151. doi: 10.1186/1471-230X-13-151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Singh AK, Pandey SK, Naresh Kumar G. Pyrroloquinoline quinone-secreting probiotic Escherichia coli Nissle 1917 ameliorates ethanol-induced oxidative damage and hyperlipidemia in rats. Alcohol Clin Exp Res. 2014;38:2127–2137. doi: 10.1111/acer.12456. [DOI] [PubMed] [Google Scholar]
- 87.Kirpich IA, Solovieva NV, Leikhter SN, Shidakova NA, Lebedeva OV, Sidorov PI. Probiotics restore bowel flora and improve liver enzymes in human alcohol-induced liver injury: a pilot study. Alcohol. 2008;42:675–682. doi: 10.1016/j.alcohol.2008.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Stadlbauer V, Mookerjee RP, Hodges S, Wright GA, Davies NA, Jalan R. Effect of probiotic treatment on deranged neutrophil function and cytokine responses in patients with compensated alcoholic cirrhosis. J Hepatol. 2008;48:945–951. doi: 10.1016/j.jhep.2008.02.015. [DOI] [PubMed] [Google Scholar]
- 89.Ridlon JM, Kang DJ, Hylemon PB. Bile salt biotransformations by human intestinal bacteria. J Lipid Res. 2006;47:241–259. doi: 10.1194/jlr.R500013-JLR200. [DOI] [PubMed] [Google Scholar]
- 90.Hepner GW, Sturman JA, Hofmann AF, Thomas PJ. Metabolism of steroid and amino acid moieties of conjugated bile acids in man. 3. Cholyltaurine (taurocholic acid) J Clin Invest. 1973;52:433–440. doi: 10.1172/JCI107200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Shi X, Yao D, Chen C. Identification of N-acetyltaurine as a novel metabolite of ethanol through metabolomics-guided biochemical analysis. J Biol Chem. 2012;287:6336–6349. doi: 10.1074/jbc.M111.312199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kurdi P, Kawanishi K, Mizutani K, Yokota A. Mechanism of growth inhibition by free bile acids in lactobacilli and bifidobacteria. J Bacteriol. 2006;188:1979–1986. doi: 10.1128/JB.188.5.1979-1986.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Islam KB, Fukiya S, Hagio M, Fujii N, Ishizuka S, Ooka T. Bile acid is a host factor that regulates the composition of the cecal microbiota in rats. Gastroenterology. 2011;141:1773–1781. doi: 10.1053/j.gastro.2011.07.046. [DOI] [PubMed] [Google Scholar]
- 94.Ridlon JM, Kang DJ, Hylemon PB, Bajaj JS. Bile acids and the gut microbiome. Curr Opin Gastroenterol. 2014;30:332–338. doi: 10.1097/MOG.0000000000000057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Inagaki T, Moschetta A, Lee YK, Peng L, Zhao G, Downes M. Regulation of antibacterial defense in the small intestine by the nuclear bile acid receptor. Proc Natl Acad Sci U S A. 2006;103:3920–3925. doi: 10.1073/pnas.0509592103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Mizushima N, Yoshimori T, Levine B. Methods in mammalian autophagy research. Cell. 2010;140:313–326. doi: 10.1016/j.cell.2010.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Klionsky DJ, Emr SD. Autophagy as a regulated pathway of cellular degradation. Science. 2000;290:1717–1721. doi: 10.1126/science.290.5497.1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kabeya Y, Mizushima N, Ueno T, Yamamoto A, Kirisako T, Noda T. LC3, a mammalian homologue of yeast Apg8p, is localized in autophagosome membranes after processing. EMBO J. 2000;19:5720–5728. doi: 10.1093/emboj/19.21.5720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ohsumi Y. Molecular dissection of autophagy: two ubiquitin-like systems. Nat Rev Mol Cell Biol. 2001;2:211–216. doi: 10.1038/35056522. [DOI] [PubMed] [Google Scholar]
- 100.Klionsky DJ, Abdalla FC, Abeliovich H, Abraham RT, Acevedo-Arozena A, Adeli K. Guidelines for the use and interpretation of assays for monitoring autophagy. Autophagy. 2012;8:445–544. doi: 10.4161/auto.19496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Yang Z, Klionsky DJ. Mammalian autophagy: core molecular machinery and signaling regulation. Curr Opin Cell Biol. 2010;22:124–131. doi: 10.1016/j.ceb.2009.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Manley S, Williams JA, Ding WX. Role of p62/SQSTM1 in liver physiology and pathogenesis. Exp Biol Med. 2013;238:525–538. doi: 10.1177/1535370213489446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Komatsu M, Waguri S, Koike M, Sou YS, Ueno T, Hara T. Homeostatic levels of p62 control cytoplasmic inclusion body formation in autophagy-deficient mice. Cell. 2007;131:1149–1163. doi: 10.1016/j.cell.2007.10.035. [DOI] [PubMed] [Google Scholar]
- 104.Ni HM, Boggess N, McGill MR, Lebofsky M, Borude P, Apte U. Liver-specific loss of Atg5 causes persistent activation of NRF2 and protects against acetaminophen-induced liver injury. Toxicol Sci. 2012;127:438–450. doi: 10.1093/toxsci/kfs133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Kirkin V, Lamark T, Sou YS, Bjørkøy G, Nunn JL, Bruun JA. A role for NBR1 in autophagosomal degradation of ubiquitinated substrates. Mol Cell. 2009;33:505–516. doi: 10.1016/j.molcel.2009.01.020. [DOI] [PubMed] [Google Scholar]
- 106.Reggiori F, Komatsu M, Finley K, Simonsen A. Selective types of autophagy. Int J Cell Biol. 2012;2012:156272. doi: 10.1155/2012/156272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ding WX, Yin XM. Mitophagy: mechanisms, pathophysiological roles, and analysis. Biol Chem. 2012;393:547–564. doi: 10.1515/hsz-2012-0119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Czaja MJ, Ding WX, Donohue T.M. Jr., Friedman SL, Kim JS, Komatsu M. Functions of autophagy in normal and diseased liver. Autophagy. 2013;9:1131–1158. doi: 10.4161/auto.25063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Yin XM, Ding WX, Gao W. Autophagy in the liver. Hepatology. 2008;47:1773–1785. doi: 10.1002/hep.22146. [DOI] [PubMed] [Google Scholar]
- 110.Ding WX. Role of autophagy in liver physiology and pathophysiology. World J Biol Chem. 2010;1:3–12. doi: 10.4331/wjbc.v1.i1.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Ding WX, Li M, Yin XM. Selective taste of ethanol-induced autophagy for mitochondria and lipid droplets. Autophagy. 2011;7:248–249. doi: 10.4161/auto.7.2.14347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Ding WX, Li M, Chen X, Ni HM, Lin CW, Gao W. Autophagy reduces acute ethanol-induced hepatotoxicity and steatosis in mice. Gastroenterology. 2010;139:1740–1752. doi: 10.1053/j.gastro.2010.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Lin CW, Zhang H, Li M, Xiong X, Chen X, Chen X. Pharmacological promotion of autophagy alleviates steatosis and injury in alcoholic and non-alcoholic fatty liver conditions in mice. J Hepatol. 2013;58:993–999. doi: 10.1016/j.jhep.2013.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Huang H, Tindall DJ. Dynamic FoxO transcription factors. J Cell Sci. 2007;120:2479–2487. doi: 10.1242/jcs.001222. [DOI] [PubMed] [Google Scholar]
- 115.Arden KC. FOXO animal models reveal a variety of diverse roles for FOXO transcription factors. Oncogene. 2008;27:2345–2350. doi: 10.1038/onc.2008.27. [DOI] [PubMed] [Google Scholar]
- 116.Tikhanovich I, Cox J, Weinman SA. Forkhead box class O transcription factors in liver function and disease. J Gastroenterol Hepatol. 2013;28 Suppl 1:S125–S131. doi: 10.1111/jgh.12021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Tzivion G, Dobson M, Ramakrishnan G. FoxO transcription factors; regulation by AKT and 14-3-3 proteins. Biochim Biophys Acta. 2011;1813:1938–1945. doi: 10.1016/j.bbamcr.2011.06.002. [DOI] [PubMed] [Google Scholar]
- 118.Sengupta A, Molkentin JD, Paik JH, DePinho RA, Yutzey KE. FoxO transcription factors promote cardiomyocyte survival upon induction of oxidative stress. J Biol Chem. 2011;286:7468–7478. doi: 10.1074/jbc.M110.179242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Sengupta A, Molkentin JD, Yutzey KE. FoxO transcription factors promote autophagy in cardiomyocytes. J Biol Chem. 2009;284:28319–28331. doi: 10.1074/jbc.M109.024406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Mammucari C, Milan G, Romanello V, Masiero E, Rudolf R, Del Piccolo P. FoxO3 controls autophagy in skeletal muscle in vivo. Cell Metab. 2007;6:458–471. doi: 10.1016/j.cmet.2007.11.001. [DOI] [PubMed] [Google Scholar]
- 121.Zhao J, Brault JJ, Schild A, Cao P, Sandri M, Schiaffino S. FoxO3 coordinately activates protein degradation by the autophagic/lysosomal and proteasomal pathways in atrophying muscle cells. Cell Metab. 2007;6:472–483. doi: 10.1016/j.cmet.2007.11.004. [DOI] [PubMed] [Google Scholar]
- 122.Tumurbaatar B, Tikhanovich I, Li Z, Ren J, Ralston R, Kuravi S. Hepatitis C and alcohol exacerbate liver injury by suppression of FOXO3. Am J Pathol. 2013;183:1803–1814. doi: 10.1016/j.ajpath.2013.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Tikhanovich I, Kuravi S, Campbell RV, Kharbanda KK, Artigues A, Villar MT. Regulation of FOXO3 by phosphorylation and methylation in hepatitis C virus infection and alcohol exposure. Hepatology. 2014;59:58–70. doi: 10.1002/hep.26618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Zhang G, Park MA, Mitchell C, Walker T, Hamed H, Studer E. Multiple cyclin kinase inhibitors promote bile acid-induced apoptosis and autophagy in primary hepatocytes via p53-CD95-dependent signaling. J Biol Chem. 2008;283:24343–24358. doi: 10.1074/jbc.M803444200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Roesly HB, Khan MR, Chen HDR, Hill KA, Narendran N, Watts GS. The decreased expression of Beclin-1 correlates with progression to esophageal adenocarcinoma: the role of deoxycholic acid. Am J Physiol Gastrointest Liver Physiol. 2012;302:G864–G872. doi: 10.1152/ajpgi.00340.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Payne CM, Crowley-Skillicorn C, Holubec H, Dvorak K, Moyer MP, Garewal H. Deoxycholate, an endogenous cytotoxin/genotoxin, induces the autophagic stress-survival pathway: implications for colon carcinogenesis. J Toxicol. 2009;2009:785907. doi: 10.1155/2009/785907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Manley S, Ni HM, Kong B, Apte U, Guo G, Ding WX. Suppression of autophagic flux by bile acids in hepatocytes. Toxicol Sci. 2014;137:478–490. doi: 10.1093/toxsci/kft246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Ma K, Saha PK, Chan L, Moore DD. Farnesoid X receptor is essential for normal glucose homeostasis. J Clin Invest. 2006;116:1102–1109. doi: 10.1172/JCI25604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Calkin AC, Tontonoz P. Transcriptional integration of metabolism by the nuclear sterol-activated receptors LXR and FXR. Nat Rev Mol Cell Biol. 2012;13:213–224. doi: 10.1038/nrm3312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Thomas C, Pellicciari R, Pruzanski M, Auwerx J, Schoonjans K. Targeting bile-acid signalling for metabolic diseases. Nat Rev Drug Discov. 2008;7:678–693. doi: 10.1038/nrd2619. [DOI] [PubMed] [Google Scholar]
- 131.Evans RM, Barish GD, Wang YX. PPARs and the complex journey to obesity. Nat Med. 2004;10:355–361. doi: 10.1038/nm1025. [DOI] [PubMed] [Google Scholar]
- 132.Göttlicher M, Widmark E, Li Q, Gustafsson JA. Fatty acids activate a chimera of the clofibric acid-activated receptor and the glucocorticoid receptor. Proc Natl Acad Sci U S A. 1992;89:4653–4657. doi: 10.1073/pnas.89.10.4653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Keller H, Dreyer C, Medin J, Mahfoudi A, Ozato K, Wahli W. Fatty acids and retinoids control lipid metabolism through activation of peroxisome proliferator-activated receptor-retinoid X receptor heterodimers. Proc Natl Acad Sci U S A. 1993;90:2160–2164. doi: 10.1073/pnas.90.6.2160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Seok S, Fu T, Choi SE, Li Y, Zhu R, Kumar S. Transcriptional regulation of autophagy by an FXR-CREB axis. Nature. 2014;516:108–111. doi: 10.1038/nature13949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Lee JM, Wagner M, Xiao R, Kim KH, Feng D, Lazar MA. Nutrient-sensing nuclear receptors coordinate autophagy. Nature. 2014;516:112–115. doi: 10.1038/nature13961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Kim I, Morimura K, Shah Y, Yang Q, Ward JM, Gonzalez FJ. Spontaneous hepatocarcinogenesis in farnesoid X receptor-null mice. Carcinogenesis. 2007;28:940–946. doi: 10.1093/carcin/bgl249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Yang F, Huang X, Yi T, Yen Y, Moore DD, Huang W. Spontaneous development of liver tumors in the absence of the bile acid receptor farnesoid X receptor. Cancer Res. 2007;67:863–867. doi: 10.1158/0008-5472.CAN-06-1078. [DOI] [PubMed] [Google Scholar]
- 138.Inami Y, Waguri S, Sakamoto A, Kouno T, Nakada K, Hino O. Persistent activation of NRF2 through p62 in hepatocellular carcinoma cells. J Cell Biol. 2011;193:275–284. doi: 10.1083/jcb.201102031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Takamura A, Komatsu M, Hara T, Sakamoto A, Kishi C, Waguri S. Autophagy-deficient mice develop multiple liver tumors. Genes Dev. 2011;25:795–800. doi: 10.1101/gad.2016211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Ni HM, Woolbright BL, Williams J, Copple B, Cui W, Luyendyk JP. NRF2 promotes the development of fibrosis and tumorigenesis in mice with defective hepatic autophagy. J Hepatol. 2014;61:617–625. doi: 10.1016/j.jhep.2014.04.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Nakajima T, Kamijo Y, Tanaka N, Sugiyama E, Tanaka E, Kiyosawa K. Peroxisome proliferator-activated receptor α protects against alcohol-induced liver damage. Hepatology. 2004;40:972–980. doi: 10.1002/hep.20399. [DOI] [PubMed] [Google Scholar]
- 142.Fischer M, You M, Matsumoto M, Crabb DW. Peroxisome proliferator-activated receptor alpha (PPARα) agonist treatment reverses PPARα dysfunction and abnormalities in hepatic lipid metabolism in ethanol-fed mice. J Biol Chem. 2003;278:27997–28004. doi: 10.1074/jbc.M302140200. [DOI] [PubMed] [Google Scholar]
- 143.Kong L, Ren W, Li W, Zhao S, Mi H, Wang R. Activation of peroxisome proliferator activated receptor alpha ameliorates ethanol induced steatohepatitis in mice. Lipids Health Dis. 2011;10:246. doi: 10.1186/1476-511X-10-246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Settembre C, De Cegli R, Mansueto G, Saha PK, Vetrini F, Visvikis O. TFEB controls cellular lipid metabolism through a starvation-induced autoregulatory loop. Nat Cell Biol. 2013;15:647–658. doi: 10.1038/ncb2718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Aroor AR, Jackson DE, Shukla SD. Dysregulated phosphorylation and nuclear translocation of cyclic AMP response element binding protein (CREB) in rat liver after chronic ethanol binge. Eur J Pharmacol. 2012;679:101–108. doi: 10.1016/j.ejphar.2011.12.045. [DOI] [PubMed] [Google Scholar]