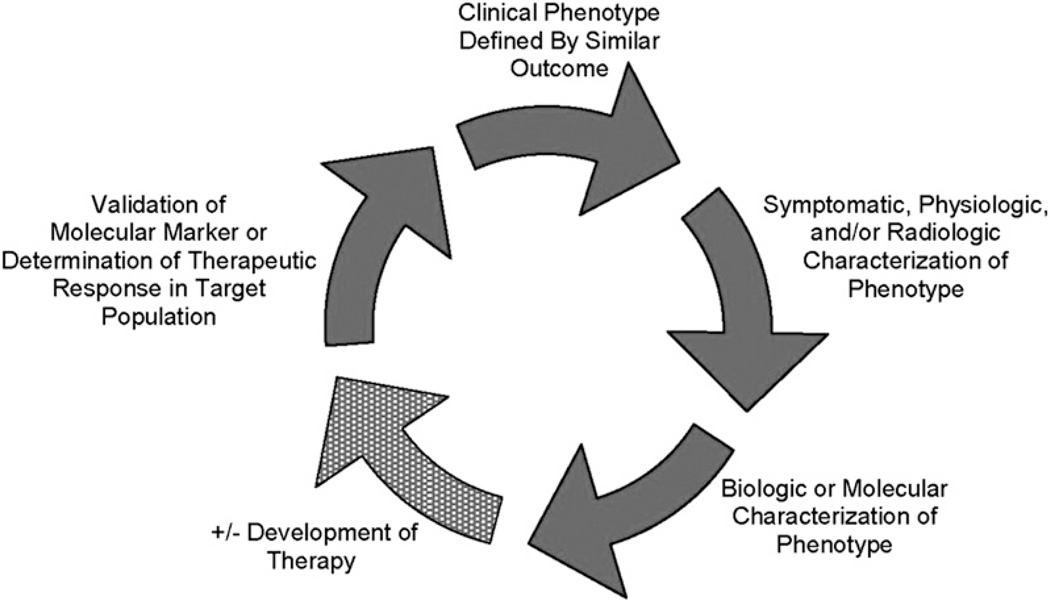
Chronic obstructive pulmonary disease (COPD) is a syndrome characterized by significant disease heterogeneity.1 Whereas the presence of airflow obstruction that is not completely reversible is a hallmark of the disease, in the individual patient this obstruction may be caused by airway inflammation and remodeling, emphysema, or both. Clinicians have identified 2 basic subtypes of COPD: “pink puffers” characterized by emphysema, significant dyspnea, hyperinflation, and weight loss but with adequate oxygenation; and “blue bloaters” characterized by chronic bronchitis, hypoventilation, and obesity. It is likely that many other phenotypes exist in COPD but their relevance has not been adequately validated.2 To provide some clarity, a recent consensus group defined COPD phenotypes as, “a single or combination of disease attributes that describe differences between individuals with COPD as they relate to clinically meaningful outcomes (symptoms, exacerbations, response to therapy, rate of disease progression, or death).”2 The validation of phenotypes will ultimately require an iterative process (Fig. 1) so that groups of patients may initially be identified either by similar clinical outcome, radiologic or physiologic characteristics, biological or molecular signature, or response to therapy. Ultimately, the hope is that these patient subgroups share similar pathophysiologic processes, so that specific therapies can be developed. The goal of this article is to highlight the accumulated data regarding environmental and host factors, in particular comorbid diseases, that may help to identify and refine patient phenotypes in COPD.
Fig. 1.
Ideal phenotyping construct wherein candidate phenotypes are validated once their relevance to clinical outcomes is established. There are multiple potential points of entry into this iterative process of phenotype identification. For instance, similar clinical outcomes may define a subpopulation that leads to the identification of a biologic target and focused therapy. Alternatively, the process might begin with the differentiation of subgroups based on a biologic marker that is then validated by similar clinical response within subgroups. (From Han MK, Agusti A, Calverley PM, et al. Chronic obstructive pulmonary disease phenotypes: the future of COPD. Am J Respir Crit Care Med 2010;182(5):598–4; with permission.)
ENVIRONMENT
Tobacco is the principal risk factor and environmental toxin responsible for the development of the disease in most patients with COPD. Most of what we know about the natural history of COPD is in tobacco smokers. The prevalence of COPD in smokers is approximately 20% as compared with 4% in nonsmokers.3 This association is supported by the acceptance of the preventive effects of smoking cessation. The Lung Health Study, an interventional smoking cessation study in smokers with COPD with mild airflow obstruction, demonstrated that the decline in the rate of forced expiratory volume in 1 second (FEV1) was greatest in patients who smoked the most and least in those who achieved sustained smoking cessation.4 However, the fact that only one in five smokers develops COPD, points towards additional factors that contribute to its development. The importance of second-hand smoke exposure, also known as environmental tobacco smoke (ETS), should not be overlooked. Higher cumulative lifetime ETS at home and work has been associated with increased risk of COPD, even after adjustment for personal smoking history and occupational exposure.5
The prevalence of COPD in nonsmokers in the United States, according to data from the Third National Health and Nutrition Epidemiologic Survey is approximately 6.6%.6 Worldwide figures for nontobacco related COPD vary around 20% to 25% of all COPD cases, with estimates as high as 40% to 50% in countries such as Colombia and South Africa.7 The risk factors associated with COPD in nonsmokers are numerous and incompletely understood, but a history of asthma or tuberculosis, exposure to traffic and outdoor pollution, and exposure to biomass smoke show the strongest associations.8,9 How disease presentation and course differs by risk factor is not well described. One study has suggested that life expectancy for nonsmokers with COPD is only modestly reduced (0.7 years for the Global Initiative for Chronic Obstructive Lung Disease (GOLD) stage 2 patients, and 1.3 years for GOLD stages 3–4) compared with substantially reduced life expectancy in current smokers with COPD (2.2 years for GOLD stage 2, and 5.8 years for GOLD stages 3–4) and former smokers with COPD (1.4 years for GOLD stage 2 and 5.6 years for GOLD stages 3–4).10
Whereas it has been estimated that COPD attributable to cigarette smoking is between 80% and 90%, COPD attributed to occupational exposures (vapors, gas, dust, or fumes) is close to 15%.11 Whereas specific industries, such as coal mining, have been related to the development of airflow obstruction, increasing data from across various industries and occupations are being collected.11 Outdoor air pollution also probably contributes to the development of COPD. A 1958 study of postmen in the United Kingdom documented the prevalence of COPD to be higher in those working in more polluted areas, independent of personal smoking history.12 Data from other population studies of individuals living close to roads with heavy motor vehicle traffic also support these findings.13
In the developing world, exposure to smoke from biomass fuels is an important cause of COPD, particularly in women who use biomass fuels for cooking.14 It is unclear whether the phenotype of COPD in this group of nonsmokers differs from that of tobacco-exposed individuals, although studies of women exposed to biomass fuel smoke suggest that disease presentation and mortality are similar to those with COPD attributable to tobacco smoking.15
Nutrition may also be an important factor that influences the development of COPD. Vitamin D levels, in particular, demonstrate an inverse relationship with pulmonary function in COPD.16 Genetic polymorphisms in vitamin D metabolic pathways have also been associated with COPD.17 An association has also been described between higher levels of vitamin C in plasma and protection against incident COPD.18 Similar information has been reported between vitamin A intake and prevalence of COPD.19 Lately, a protective function has been attributed to a diet high in cereal fiber.20 Further studies of the relationship between nutritional factors and COPD may offer mechanistic insights into the pathogenesis and treatment of this disease.
HOST FACTORS
Gender is an important factor that also may affect disease phenotype.14 Conflicting data exist on whether women are more susceptible to developing COPD for a similar amount of tobacco smoke exposure.14 However, women with COPD report more dyspnea, similar degrees of cough, but less sputum compared with men with similar degrees of airflow obstruction.21 It has been suggested that women are more likely to display a chronic bronchitic phenotype of COPD whereas men display a more emphysematous phenotype.14 Radiologic data from the National Emphysema Treatment Trial among patients with severe emphysema supports this hypothesis whereby women demonstrated less overall emphysema, particularly in the peripheral portions of the lung.22 Histologic data from the same study revealed that the bronchioles of women have significantly thicker airway walls and smaller lumen than the bronchioles of men.22 Recent data also suggest that COPD exacerbations are more frequent in women but it is unknown whether this represents a difference in disease biology or reporting patterns.23,24
Whereas genetic and epigenetic contributions to COPD are discussed in more detail elsewhere, genetics must also play a role in disease phenotype. The best known genetic risk factor for development of COPD is alpha-1 anti-trypsin (AAT) deficiency, whereby patients are more likely to develop lower lobe predominant emphysema patterns compared with patients without AAT deficiency, who are more likely to present with upper lobe emphysema. More data in this regard are likely to come from the ongoing COPDGene Study, a genome-wide association study of more than 10000 subjects, with the goal of identifying the genetic determinants of specific disease phenotypes (http://www.copdgene.org). Information derived from such studies may help determine the reason for these phenotypic expressions of the disease and their clinical relevance.
Another host factor that probably contributes to the COPD phenotype but is often overlooked is the lung microbiome. Alteration in the lung and the gut microbiomes has been associated with the development of asthma.25–27 This leads to the consideration of the role of the microbiome in COPD, wherein bacterial colonization has been associated with FEV1 decline and increased rates of acute exacerbations of COPD (AECOPD).28 Recent data also support a similar process with the genesis of emphysema and airway structural abnormality.28,29 Although investigations of COPD using advanced, culture-independent techniques are limited, a predominance of Pseudomonas species in patients with more impaired lung function in COPD has been reported.30
COMORBIDITIES
Accumulating data suggest that comorbidities must be included in the assessment of patients with COPD. Some comorbid conditions, such as cardiovascular disease and osteoporosis, are more common in patients with COPD compared with the general population. Although comorbidities such as obstructive sleep apnea may not be more prevalent in COPD, they are important because they may modify the course of the disease.31 Because comorbid conditions can alter the course of the disease or the need for specific therapies in COPD, they remain important considerations in the development and refinement of COPD phenotypes. Systemic inflammation may be pathogenically related to many comorbidities seen in COPD including cardiovascular disease, osteoporosis, metabolic syndrome, and depression.32,33 There is not enough confirmatory data to support the specific treatment of systemic inflammation in patients with COPD but current studies aimed at capturing the nature and extent of the importance of this pathobiological mechanism, may lead to the development of specific therapy for patients presenting with this phenotype.
Cardiovascular Disease
Ischemic cardiovascular disease continues to be a leading cause of death in COPD.34 Whereas shared risk factors, such as tobacco use, contribute to this association, epidemiologic evidence suggests that impaired lung function itself is an independent risk factor for increased cardiovascular mortality. Data from the National Health and Nutrition Examination Survey demonstrated that patients in the lowest FEV1 quintile had the highest risk of cardiovascular mortality (risk ratio [RR] of 3.36), even after adjustment for pertinent risk factors including smoking status, blood pressure, body mass index (BMI), and diabetes.35 In a meta-analysis of studies relating cardiovascular mortality to reduced FEV1 adjusted for smoking status, the RR of death for patients with COPD was 1.77 (95% confidence interval [CI], 1.46–1.97).35 In the Lung Health Study, cardiovascular events accounted for 42% of first hospitalizations and 48% of second hospitalizations in patients with mild to moderate COPD.36 An inverse relationship between FEV1 and the presence of atherosclerosis has also been documented. 37 In addition to the degree of airflow obstruction in COPD, the actual rate of FEV1 decline is also an independent predictor of cardiovascular mortality.38,39
Because atherosclerosis, like COPD, is a disease of systemic inflammation,40 an increase in systemic inflammation may explain the association between the two diseases. Elevated C-reactive protein (CRP) levels correlate not only with the presence of COPD but also with the presence of exacerbations, severity of lung function, and risk for hospitalization and death.41 The interaction between COPD and cardiovascular disease may also have important therapeutic implications. In one study, the withdrawal of inhaled steroids in patients with COPD with moderate-to-severe disease was associated with a 71% increase in serum CRP levels.42 Subsequent treatment with inhaled corticosteroids resulted in a 50% reduction in CRP levels (95% CI, 9%–73%); prednisone-treated patients had a reduction of CRP levels of 63% (95% CI, 29%–81%) whereas the patients receiving placebo experienced no significant change in CRP levels. Medications used to treat cardiovascular disease may affect COPD outcomes. In an analysis of a large Canadian database of patients with COPD, the combination of statin and either angiotensin-converting inhibitor or angiotensin receptor blocker therapy was associated with a reduction in hospitalization (RR 0.66; 95% CI, 0.51–0.85) and total mortality (RR 0.42; 95% CI, 0.33–0.52).43 A separate cohort study also found that statin therapy in COPD was associated with reduced risk of death, (hazard ratio 0.57; 95% CI, 0.38–0.87).44 Although beta-blockers have been used with caution in obstructive lung disease, a retrospective analysis of a COPD cohort recently reported that there was a 22% overall reduction in all-cause mortality in patients with COPD using beta-blockers.45
Musculoskeletal Disease
Although prevalence estimates vary, low BMI is clearly associated with COPD, and in particular with disease severity.46 Low BMI is also an important independent prognostic factor for increased mortality in patients with COPD.47 A relationship between low fat-free mass index (FFMI) and increased mortality in COPD has been demonstrated, even in subjects with a normal BMI, after controlling for age and spirometric severity.48 The prognostic effect of the BMI is further supported by its importance in the calculation of the BMI, obstruction, dyspnea, exercise capacity (BODE) index, one of the best mortality prediction indices in COPD.49
Low BMI has been recognized as a distinguishing feature of pink puffers for many years and several studies correlating low BMI with greater extent of emphysema on high resolution computed tomography (HRCT) help to confirm this association.50 The fat-free mass index (FFMI) has also been demonstrated to have an inverse association with extent of emphysema on HRCT, the six minute walk test distance, and CRP levels.51 In contrast to the observed association between low BMI and emphysema, the presence of chronic bronchitis has been statistically associated not only with higher BMI but also with HRCT indicators of airway disease.52 Different mechanisms have been postulated for the loss of body mass in COPD including systemic inflammation and oxidative stress, tissue hypoxia, disuse atrophy, energy imbalance, hormone insufficiency, and genetic factors but no definitive explanation has been identified.53 There have been several attempts to reverse the cachexia observed in a significant proportion of patients with COPD. The results have been disappointing with nutrition-based interventions, the use of nocturnal ventilation to decrease energy expenditure, and the use of growth-hormone releasing factors.53 However, there are data supporting the capacity of COPD patients with low FFI (<17 kg/m2) to remodel muscle and improve cachexia in response to exercise training.54
Whereas estimates vary, the prevalence of osteoporosis is increased in patients with COPD. Estimates ranging between two- and fivefold increase have been made for patients with COPD compared with age-matched controls without airflow obstruction. 55,56 The frequency of osteoporosis also seems to be correlated to the severity of airflow obstruction. Vertebral fractures are more prevalent as the severity of spirometrically-defined COPD increases.55,57 Whereas there are multiple shared-risk factors such as the use of oral steroids, smoking, and low BMI that may contribute to osteoporosis in COPD, these risk factors do not appear to completely explain the association, as several studies have documented lower bone mineral density (BMD) even in the absence of systemic steroids.57,58
As with low BMI, osteoporosis and low BMD also seem to be more strongly related to the presence and severity of emphysema but not related to the severity of chronic bronchitis or airway wall thickness as assessed by HRCT.59 Whereas an adequate understanding of this association at the cellular and molecular level is incomplete, better insights could lead to novel diagnostic and therapeutic interventions. Although matrix metallopeptidase-9 (MMP-9), interleukin-6 (IL-6) and adipokines have all been implicated, no clear causal link has emerged.60–62 Participation in pulmonary rehabilitation is an option to improve the functional status of patients with advanced COPD. Whereas exercise programs have not been demonstrated to increase BMD in COPD, they may diminish the risk of fracture by decreasing the risk of falls.63
Diabetes
Diabetes is another frequent comorbidity in COPD. Many studies have reported a relationship between increased risk of the development of diabetes and the presence of COPD. Lung function impairment, measured either by a low FEV1 or a low forced vital capacity (FVC), has been associated with the coexistence of the metabolic syndrome, the development of insulin resistance, and development of diabetes.64–66 The association between lung-function impairment and metabolic syndrome remains, even after adjustment for BMI and smoking history. The independent risk for metabolic syndrome conferred by a low FEV1 was estimated to be 1.28 (95% CI, 1.20–1.37) in a study of more than 121,000 cases.64 In the Nurse’s Health Study, the RR of incident diabetes among participants with COPD was 1.8 (95% CI, 1.1–2.8),67 a value very similar to that reported in the Women’s Health Study cohort, wherein the relative risk of incident diabetes after a diagnosis of COPD was 1.38 (95% CI, 1.14–1.67).65
The link between COPD and diabetes is not clear. Certainly the role of therapies, such as inhaled corticosteroids (ICS) frequently used by patients with COPD, must be considered. At least two independent studies point toward the existence of a dose-dependent association between the use of ICS and diabetes control or new-onset diabetes,68 but this is unlikely to be the sole explanation responsible for the association. Certain inflammatory mediators, Interleukin 6 (IL-6) and tumor necrosis factor-alpha (TNF-α) in particular, have been implicated in both bronchial inflammation and insulin resistance. In a study of the HRCT characteristics of patients with COPD who experienced exacerbations, a higher prevalence of diabetes was associated with an airway-disease-predominant COPD phenotype compared with an emphysema-predominant disease phenotype.69 Not surprisingly, patients with predominant airway disease also demonstrated higher BMI and less frequent osteoporosis. These data support a possible relationship between certain metabolic pathways and COPD phenotypes, although the exact pathways and potential therapeutic implications are yet to be defined.
Anemia
Because COPD is a disease of chronic inflammation, it is not surprising to find that anemia is another common comorbidity. The prevalence of anemia in COPD has been reported to be between 7% and 17% in outpatients,70,71 12% in patients receiving long-term oxygen therapy,72 and 23% in hospitalized patients.73 Anemia in COPD patients has significant implications, and is particularly associated with increased health-care related costs.71,74 Anemia also has an independent impact on 3-year survival72 and all-cause mortality.74 The pathophysiology of anemia in COPD patients is probably similar to that of other chronic inflammatory diseases.75 The mediators responsible for the airway and pulmonary parenchymal inflammation, especially IL-6, can interfere with the intestinal absorption of iron.75 Interleukin 1 (IL-1) and TNF-α have been implicated in abnormalities in the production and peripheral effect (or resistance) of erythropoietin.75 There are no specific recommendations for the treatment of the anemia seen in patients with COPD.
Gastroesophageal Reflux Disease
The association between gastroesophageal reflux disease (GERD) and COPD has been long recognized. In cross-sectional studies using validated questionnaires, the prevalence of heartburn, regurgitation, and dysphagia have been found with higher frequency in patients with COPD compared with controls.76,77 In one series using 24-h continuous esophageal pH monitoring, acid reflux was recorded in 62% of patients with COPD compared with 19% of controls.78 This study highlighted the fact that the actual proportion of reflux in the COPD patient population was probably higher than estimates based on symptoms alone. However, even GERD detected by symptoms in the COPD patient population has been associated with poorer outcomes. Both cross-sectional and longitudinal studies have reported associations between the presence of reflux and poor quality of life in COPD.79 GERD has also been identified as a risk factor for COPD exacerbations23,80 and seems to be specifically associated with the chronic bronchitic phenotype in COPD.52
One hypothesis attempting to explain the association between GERD and COPD is that aspiration may lead to persistent airway inflammation resulting in bronchial remodeling. In support of this hypothesis is human data demonstrating a relationship between elevated C-reactive protein levels and swallowing dysfunction in patients with COPD.81 An association between lower exhaled breath condensate pH, and GERD symptoms in COPD has also been documented.77 Whether COPD precedes GERD or vice versa is still open to debate, although epidemiologic evidence supports the idea that a diagnosis of COPD is a risk factor for the development of GERD.82 Whereas robust evidence that GERD treatment alters COPD outcomes is lacking, in a small, randomized, single-blind, study the proton pump inhibitor lansoprazole reduced the risk of COPD exacerbations and adjusted the odds ratio of 0.23, P = .004.83 Thus, common sense suggests that GERD should be suspected in patients with COPD and once diagnosed it should be treated because there are effective treatments for this condition.
Neuropsychiatric Disorders
Like patients afflicted with other chronic diseases, patients with COPD tend to have a significant burden of coexistent depression and anxiety. Assessments of the prevalence of neuropsychiatric disorders demonstrate wide variation, given the difficulty of exploring this sensitive issue in large population samples, the diversity of the instruments available for assessment, and variations in the severity of the disease detected in different surveys.84 Conservative estimates suggest that the prevalence of anxiety and depression is at least 10% in the nonhospitalized, general, COPD patient population. Higher estimates have been reported of the prevalence of symptoms of depression (37%–71%) and anxiety (51%–75%) in patients with severe COPD.85
Factors associated with increased risk for depression in COPD include disease severity, limited mobility, low body mass, coexistent comorbid conditions, and the need for supplementary oxygen therapy.86,87 Women are particularly susceptible, with the frequency of depression and anxiety being nearly two times greater in women compared with men.88 More importantly, depression and anxiety have also been associated with poorer disease outcomes in patients with COPD. Anxiety has been associated with lower functional capacity measured by the six minute walk test89 Anxiety and depression are also associated with greater dyspnea at rest, worse scores in quality of life questionnaires, and reduced exercise performance at both the beginning and end of pulmonary rehabilitation.90 Anxiety has been associated with increased risk for COPD exacerbations and greater mortality.91 Depressive symptoms have been associated with two to three times greater risk of death in COPD.92,93 Unfortunately, only a small proportion of COPD patients with anxiety and depression receive effective treatment.86 Specific note should be made that pulmonary rehabilitation can also improve symptoms of anxiety and depression, although its effects beyond the treatment period are not well defined.86
A frequently overlooked comorbidity in COPD is cognitive dysfunction. Data on the prevalence of cognitive dysfunction in COPD is limited by methodological issues, including the need for normative data, the use of different cognitive evaluation instruments, and the interaction of shared risk factors. Even with these limitations, the finding of abnormal cognitive function in COPD has been consistent and includes decreased cognitive performance (story recall, addition tests) compared with healthy controls, lower scores on the Mini-Mental State Examination, slower reaction time, and impaired verbal and logical thinking.94–96 Common factors associated with cognitive dysfunction in cross-sectional studies of patients with COPD include low FEV1, coexistent cardiovascular disease, diabetes, depression, and also obstructive sleep apnea (OSA), hypoxemia, and hypercapnia, all with the potential to affect the cerebral metabolism. 97 COPD exacerbations may also affect cognitive dysfunction, in particular those requiring mechanical ventilation.97 The mechanisms behind cognitive dysfunction probably vary with each of these associations; some studies have demonstrated that participation in rehabilitation programs and the use of supplementary oxygen in patients with hypoxemia may decrease the risk of cognitive abnormalities.94,98
Obstructive Sleep Apnea
The coexistence of COPD and OSA, commonly described as overlap syndrome, has gained significant attention in recent years. Poor sleep quality, decreased sleep efficiency, and difficulties in initiating and maintaining sleep have been reported in more than 40% of patients with COPD.99 The frequency of sleep-disordered breathing in the general population has been estimated to be 9% for women and 24% for men.100 In patients with COPD, the prevalence of OSA been estimated to be about 16%.101 In general, COPD patients with OSA are more severely hypercapnic, demonstrate more profound and frequent nocturnal oxygen desaturation, and have higher risk of pulmonary hypertension.102 Untreated OSA is also a risk factor for poor quality of life, development of AECOPD, and increased all-cause mortality.31,103 COPD and OSA share some pathophysiological characteristics and risk factors, including hyper-inflation with parenchymal destruction and increased traction on the upper airway, the presence of systemic inflammation, particularly TNF-α, IL-6 and Interleukin-8 and increased oxidative stress.102 The importance of OSA assessment in COPD is underscored by the recent finding that continuous positive airway pressure (CPAP) therapy during sleep in patients with overlap syndrome, was associated with both decreased risk of death (RR 1.79; 95% CI, 1.16–2.77) and decreased risk of severe AECOPD leading to a hospitalization (RR 1.70; 95% CI, 1.21–2.38).31
PROGRESS IN DISEASE PHENOTYPES
Only a few of the proposed phenotypes in COPD meet the premise that these phenotypes relate to clinically relevant outcomes or response to therapy. One such example is the subgroup of patients with upper-lobe-predominant emphysema and low exercise tolerance. This unique group benefits from lung-volume-reduction surgery with significant improvements in functional status, quality of life, and survival, although the mechanism of benefit in this specific group is not well understood.104 Another phenotype is composed of patients with frequent exacerbations who produce significant sputum at baseline. These patients specifically demonstrate reduction of exacerbation in response to the phosphodiesterase-4 inhibitor, roflumilast.105 The premise that combinations of comorbid conditions help define disease phenotypes is supported by the data presented here. Two general patterns of clinical features and comorbidities that share some association include (1) emphysema, low BMI and osteoporosis and (2) chronic bronchitis, airway disease, high BMI, OSA, and diabetes. It remains unknown whether these associations are related to specific mechanistic pathways that will lead to the development of targeted therapies.
SUMMARY
COPD is a heterogeneous disease, modified by environmental and intrinsic host factors. The interaction between COPD and its comorbidities is complex and bidirectional.
It has been estimated that the proportion of patients with COPD caused by cigarette smoking is between 80% and 90%. Risk factors associated with COPD in nonsmokers are numerous and incompletely understood, but a history of asthma or tuberculosis, exposure to traffic and outdoor pollution, and exposure to biomass smoke show the strongest associations. Other factors that may contribute to COPD phenotypes include gender, genetics, and the lung microbiome.
Certain comorbid conditions, such as cardiovascular disease and osteoporosis, are more common in the COPD patient population. Other comorbidities, such as overlap syndrome, the coexistence of COPD, and obstructive sleep apnea may not be as prevalent in COPD but are important because they may modify disease course.
Systemic inflammation may be pathogenically related to many comorbidities seen in COPD including cardiovascular disease, osteoporosis, metabolic syndrome, and depression.
Based on the data presented here, two general patterns of clinical features and comorbidities that share some associations are (1) emphysema, low BMI and osteoporosis and (2) chronic bronchitis, airway disease, high BMI, OSA, and diabetes.
The classification of patients with COPD into subgroups with shared characteristics and outcomes offers the potential for specific interventions. New research tools from the fields of epidemiology, immunology, imaging, and data analysis will be helpful in accomplishing this goal.
KEY POINTS.
The heterogeneity implicit to COPD suggests that a wide variety of influences including environmental and biologic factors likely contribute to an individual’s disease presentation and progression.
Certain comorbid conditions such as cardiovascular disease and osteoporosis also contribute to the heterogeneity of the patient population.
Systemic inflammation may be pathogenically related to many of the comorbidities seen in COPD including cardiovascular disease, osteoporosis, metabolic syndrome and depression.
New research in COPD using large patient populations with extensive clinical and biologic characterization along with advanced analytic methods will hopefully further expand our potential to identify disease phenotypes such that targeted therapies can be developed.
Acknowledgments
Disclosures: C.H.M None. M.K.H Dr Han has received lecture fees from Boehringer-Ingelheim, Pfizer and GlaxoSmithKline. She has received consulting fees from Novartis, Genentech, GlaxoSmithKline, Pfizer, Boehringer-Ingelheim and Medimmune.
REFERENCES
- 1.Agusti A, Calverley PM, Celli B, et al. Characterisation of COPD heterogeneity in the ECLIPSE cohort. Respir Res. 2010;11:122. doi: 10.1186/1465-9921-11-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Han MK, Agusti A, Calverley PM, et al. Chronic obstructive pulmonary disease phenotypes: the future of COPD. Am J Respir Crit Care Med. 2010;182:598–604. doi: 10.1164/rccm.200912-1843CC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lokke A, Lange P, Scharling H, et al. Developing COPD: a 25 year follow up study of the general population. Thorax. 2006;61:935–939. doi: 10.1136/thx.2006.062802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Anthonisen NR, Connett JE, Kiley JP, et al. Effects of smoking intervention and the use of an inhaled anticholinergic bronchodilator on the rate of decline of FEV1. The Lung Health Study. JAMA. 1994;272:1497–1505. [PubMed] [Google Scholar]
- 5.Eisner MD, Balmes J, Katz PP, et al. Lifetime environmental tobacco smoke exposure and the risk of chronic obstructive pulmonary disease. Environ Health. 2005;4:7. doi: 10.1186/1476-069X-4-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Behrendt CE. Mild and moderate-to-severe COPD in nonsmokers: distinct demographic profiles. Chest. 2005;128:1239–1244. doi: 10.1378/chest.128.3.1239. [DOI] [PubMed] [Google Scholar]
- 7.Salvi SS, Barnes PJ. Chronic obstructive pulmonary disease in non-smokers. Lancet. 2009;374:733–743. doi: 10.1016/S0140-6736(09)61303-9. [DOI] [PubMed] [Google Scholar]
- 8.Lamprecht B, McBurnie MA, Vollmer WM, et al. COPD in never smokers: results from the population-based burden of obstructive lung disease study. Chest. 2011;139:752–763. doi: 10.1378/chest.10-1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Silva GE, Sherrill DL, Guerra S, et al. Asthma as a risk factor for COPD in a longitudinal study. Chest. 2004;126:59–65. doi: 10.1378/chest.126.1.59. [DOI] [PubMed] [Google Scholar]
- 10.Shavelle RM, Paculdo DR, Kush SJ, et al. Life expectancy and years of life lost in chronic obstructive pulmonary disease: findings from the NHANES III Follow-up Study. Int J Chron Obstruct Pulmon Dis. 2009;4:137–148. doi: 10.2147/copd.s5237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Blanc PD, Eisner MD, Earnest G, et al. Further exploration of the links between occupational exposure and chronic obstructive pulmonary disease. J Occup Environ Med. 2009;51:804–810. doi: 10.1097/JOM.0b013e3181a7dd4e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fairbairn AS, Reid DD. Air pollution and other local factors in respiratory disease. Br J Prev Soc Med. 1958;12:94–103. doi: 10.1136/jech.12.2.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Andersen ZJ, Hvidberg M, Jensen SS, et al. Chronic obstructive pulmonary disease and long-term exposure to traffic-related air pollution: a cohort study. Am J Respir Crit Care Med. 2011;183:455–461. doi: 10.1164/rccm.201006-0937OC. [DOI] [PubMed] [Google Scholar]
- 14.Han MK, Postma D, Mannino DM, et al. Gender and chronic obstructive pulmonary disease: why it matters. Am J Respir Crit Care Med. 2007;176:1179–1184. doi: 10.1164/rccm.200704-553CC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ramirez-Venegas A, Sansores RH, Perez-Padilla R, et al. Survival of patients with chronic obstructive pulmonary disease due to biomass smoke and tobacco. Am J Respir Crit Care Med. 2006;173:393–397. doi: 10.1164/rccm.200504-568OC. [DOI] [PubMed] [Google Scholar]
- 16.Janssens W, Lehouck A, Carremans C, et al. Vitamin D beyond bones in chronic obstructive pulmonary disease: time to act. Am J Respir Crit Care Med. 2009;179:630–636. doi: 10.1164/rccm.200810-1576PP. [DOI] [PubMed] [Google Scholar]
- 17.Wood AM, Bassford C, Webster D, et al. Vitamin D-binding protein contributes to COPD by activation of alveolar macrophages. Thorax. 2011;66:205–210. doi: 10.1136/thx.2010.140921. [DOI] [PubMed] [Google Scholar]
- 18.Sargeant LA, Jaeckel A, Wareham NJ. Interaction of vitamin C with the relation between smoking and obstructive airways disease in EPIC Norfolk. European prospective investigation into cancer and nutrition. Eur Respir J. 2000;16:397–403. doi: 10.1034/j.1399-3003.2000.016003397.x. [DOI] [PubMed] [Google Scholar]
- 19.Hirayama F, Lee AH, Binns CW, et al. Do vegetables and fruits reduce the risk of chronic obstructive pulmonary disease? A case-control study in Japan. Prev Med. 2009;49:184–189. doi: 10.1016/j.ypmed.2009.06.010. [DOI] [PubMed] [Google Scholar]
- 20.Varraso R, Willett WC, Camargo CA. Prospective study of dietary fiber and risk of chronic obstructive pulmonary disease among US women and men. Am J Epidemiol. 2010;171:776–784. doi: 10.1093/aje/kwp455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Watson L, Vestbo J, Postma DS, et al. Gender differences in the management and experience of chronic obstructive pulmonary disease. Respir Med. 2004;98:1207–1213. doi: 10.1016/j.rmed.2004.05.004. [DOI] [PubMed] [Google Scholar]
- 22.Martinez FJ, Curtis JL, Sciurba F, et al. Sex differences in severe pulmonary emphysema. Am J Respir Crit Care Med. 2007;176:243–252. doi: 10.1164/rccm.200606-828OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hurst JR, Vestbo J, Anzueto A, et al. Susceptibility to exacerbation in chronic obstructive pulmonary disease. N Engl J Med. 2010;363:1128–1138. doi: 10.1056/NEJMoa0909883. [DOI] [PubMed] [Google Scholar]
- 24.Tashkin D, Celli B, Kesten S, et al. Effect of tiotropium in men and women with COPD: results of the 4-year UPLIFT trial. Respir Med. 2010;104:1495–1504. doi: 10.1016/j.rmed.2010.03.033. [DOI] [PubMed] [Google Scholar]
- 25.Bjorksten B, Sepp E, Julge K, et al. Allergy development and the intestinal microflora during the first year of life. J Allergy Clin Immunol. 2001;108:516–520. doi: 10.1067/mai.2001.118130. [DOI] [PubMed] [Google Scholar]
- 26.Kalliomaki M, Kirjavainen P, Eerola E, et al. Distinct patterns of neonatal gut microflora in infants in whom atopy was and was not developing. J Allergy Clin Immunol. 2001;107:129–134. doi: 10.1067/mai.2001.111237. [DOI] [PubMed] [Google Scholar]
- 27.Penders J, Thijs C, van den Brandt PA, et al. Gut microbiota composition and development of atopic manifestations in infancy: the KOALA Birth Cohort Study. Gut. 2007;56:661–667. doi: 10.1136/gut.2006.100164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Martinez F, Han M, Flaherty K, et al. Role of infection and antimicrobial therapy in acute exacerbations of chronic obstructive pulmonary disease. Expert Rev Anti Infect Ther. 2006;4:101–124. doi: 10.1586/14787210.4.1.101. [DOI] [PubMed] [Google Scholar]
- 29.Sethi S, Murphy T. Infection in the pathogenesis and course of chronic obstructive pulmonary disease. N Engl J Med. 2008;359:2355–2365. doi: 10.1056/NEJMra0800353. [DOI] [PubMed] [Google Scholar]
- 30.Erb-Downward JR, Thompson DL, Han MK, et al. Analysis of the lung microbiome in the “healthy” smoker and in COPD. PLoS One. 2011;6:e16384. doi: 10.1371/journal.pone.0016384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Marin JM, Soriano JB, Carrizo SJ, et al. Outcomes in patients with chronic obstructive pulmonary disease and obstructive sleep apnea: the overlap syndrome. Am J Respir Crit Care Med. 2010;182:325–331. doi: 10.1164/rccm.200912-1869OC. [DOI] [PubMed] [Google Scholar]
- 32.Agusti AG, Noguera A, Sauleda J, et al. Systemic effects of chronic obstructive pulmonary disease. Eur Respir J. 2003;21:347–360. doi: 10.1183/09031936.03.00405703. [DOI] [PubMed] [Google Scholar]
- 33.Fabbri LM, Rabe KF. From COPD to chronic systemic inflammatory syndrome? . Lancet. 2007;370:797–799. doi: 10.1016/S0140-6736(07)61383-X. [DOI] [PubMed] [Google Scholar]
- 34.Hansell AL, Walk JA, Soriano JB. What do chronic obstructive pulmonary disease patients die from? A multiple cause coding analysis. Eur Respir J. 2003;22:809–814. doi: 10.1183/09031936.03.00031403. [DOI] [PubMed] [Google Scholar]
- 35.Sin DD, Wu L, Man SF. The relationship between reduced lung function and cardiovascular mortality: a population-based study and a systematic review of the literature. Chest. 2005;127:1952–1959. doi: 10.1378/chest.127.6.1952. [DOI] [PubMed] [Google Scholar]
- 36.Anthonisen NR, Connett JE, Enright PL, et al. Hospitalizations and mortality in the Lung Health Study. Am J Respir Crit Care Med. 2002;166:333–339. doi: 10.1164/rccm.2110093. [DOI] [PubMed] [Google Scholar]
- 37.Beaty TH, Newill CA, Cohen BH, et al. Effects of pulmonary function on mortality. J Chronic Dis. 1985;38:703–710. doi: 10.1016/0021-9681(85)90024-4. [DOI] [PubMed] [Google Scholar]
- 38.Speizer FE, Fay ME, Dockery DW, et al. Chronic obstructive pulmonary disease mortality in six U.S. cities. Am Rev Respir Dis. 1989;140:S49–S55. doi: 10.1164/ajrccm/140.3_Pt_2.S49. [DOI] [PubMed] [Google Scholar]
- 39.Hospers JJ, Postma DS, Rijcken B, et al. Histamine airway hyper-responsiveness and mortality from chronic obstructive pulmonary disease: a cohort study. Lancet. 2000;356:1313–1317. doi: 10.1016/S0140-6736(00)02815-4. [DOI] [PubMed] [Google Scholar]
- 40.Ross R. Atherosclerosis–an inflammatory disease. N Engl J Med. 1999;340:115–126. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 41.Han MK, McLaughlin VV, Criner GJ, et al. Pulmonary diseases and the heart. Circulation. 2007;116:2992–3005. doi: 10.1161/CIRCULATIONAHA.106.685206. [DOI] [PubMed] [Google Scholar]
- 42.Sin DD, Lacy P, York E, et al. Effects of fluticasone on systemic markers of inflammation in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2004;170:760–765. doi: 10.1164/rccm.200404-543OC. [DOI] [PubMed] [Google Scholar]
- 43.Mancini GB, Etminan M, Zhang B, et al. Reduction of morbidity and mortality by statins, angiotensin-converting enzyme inhibitors, and angiotensin receptor blockers in patients with chronic obstructive pulmonary disease. J Am Coll Cardiol. 2006;47:2554–2560. doi: 10.1016/j.jacc.2006.04.039. [DOI] [PubMed] [Google Scholar]
- 44.Soyseth V, Brekke PH, Smith P, et al. Statin use is associated with reduced mortality in COPD. Eur Respir J. 2007;29:279–283. doi: 10.1183/09031936.00106406. [DOI] [PubMed] [Google Scholar]
- 45.Short PM, Lipworth SI, Elder DH, et al. Effect of beta blockers in treatment of chronic obstructive pulmonary disease: a retrospective cohort study. BMJ. 2011;342:d2549. doi: 10.1136/bmj.d2549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chailleux E, Laaban JP, Veale D. Prognostic value of nutritional depletion in patients with COPD treated by long-term oxygen therapy: data from the ANTADIR observatory. Chest. 2003;123:1460–1466. doi: 10.1378/chest.123.5.1460. [DOI] [PubMed] [Google Scholar]
- 47.Landbo C, Prescott E, Lange P, et al. Prognostic value of nutritional status in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1999;160:1856–1861. doi: 10.1164/ajrccm.160.6.9902115. [DOI] [PubMed] [Google Scholar]
- 48.Vestbo J, Prescott E, Almdal T, et al. Body mass, fat-free body mass, and prognosis in patients with chronic obstructive pulmonary disease from a random population sample: findings from the Copenhagen City Heart Study. Am J Respir Crit Care Med. 2006;173:79–83. doi: 10.1164/rccm.200506-969OC. [DOI] [PubMed] [Google Scholar]
- 49.Celli BR, Cote CG, Marin JM, et al. The body-mass index, airflow obstruction, dyspnea, and exercise capacity index in chronic obstructive pulmonary disease. N Engl J Med. 2004;350:1005–1012. doi: 10.1056/NEJMoa021322. [DOI] [PubMed] [Google Scholar]
- 50.Marquez-Martin E, Ramos PC, Lopez-Campos JL, et al. Components of physical capacity in patients with chronic obstructive pulmonary disease: relationship with phenotypic expression. Int J Chron Obstruct Pulmon Dis. 2011;6:105–112. doi: 10.2147/COPD.S16646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kurosaki H, Ishii T, Motohashi N, et al. Extent of emphysema on HRCT affects loss of fat-free mass and fat mass in COPD. Intern Med. 2009;48:41–48. doi: 10.2169/internalmedicine.48.1102. [DOI] [PubMed] [Google Scholar]
- 52.Kim V, Han MK, Vance GB, et al. The chronic bronchitic phenotype of chronic obstructive pulmonary disease: an analysis of the COPDGene Study. Chest. 2011;140(3):626–633. doi: 10.1378/chest.10-2948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wagner PD. Possible mechanisms underlying the development of cachexia in COPD. Eur Respir J. 2008;31:492–501. doi: 10.1183/09031936.00074807. [DOI] [PubMed] [Google Scholar]
- 54.Vogiatzis I, Simoes DC, Stratakos G, et al. Effect of pulmonary rehabilitation on muscle remodelling in cachectic patients with COPD. Eur Respir J. 2010;36:301–310. doi: 10.1183/09031936.00112909. [DOI] [PubMed] [Google Scholar]
- 55.Nuti R, Siviero P, Maggi S, et al. Vertebral fractures in patients with chronic obstructive pulmonary disease: the EOLO Study. Osteoporos Int. 2009;20:989–998. doi: 10.1007/s00198-008-0770-4. [DOI] [PubMed] [Google Scholar]
- 56.Bolton CE, Ionescu AA, Shiels KM, et al. Associated loss of fat-free mass and bone mineral density in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2004;170:1286–1293. doi: 10.1164/rccm.200406-754OC. [DOI] [PubMed] [Google Scholar]
- 57.Sin DD, Man JP, Man SF. The risk of osteoporosis in Caucasian men and women with obstructive airways disease. Am J Med. 2003;114:10–14. doi: 10.1016/s0002-9343(02)01297-4. [DOI] [PubMed] [Google Scholar]
- 58.Dam TT, Harrison S, Fink HA, et al. Bone mineral density and fractures in older men with chronic obstructive pulmonary disease or asthma. Osteoporos Int. 2010;21:1341–1349. doi: 10.1007/s00198-009-1076-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bon J, Fuhrman CR, Weissfeld JL, et al. Radiographic emphysema predicts low bone mineral density in a tobacco-exposed cohort. Am J Respir Crit Care Med. 2011;183:885–890. doi: 10.1164/rccm.201004-0666OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bolton CE, Stone MD, Edwards PH, et al. Circulating matrix metalloproteinase-9 and osteoporosis in patients with chronic obstructive pulmonary disease. Chron Respir Dis. 2009;6:81–87. doi: 10.1177/1479972309103131. [DOI] [PubMed] [Google Scholar]
- 61.Bon JM, Leader JK, Weissfeld JL, et al. The influence of radiographic phenotype and smoking status on peripheral blood biomarker patterns in chronic obstructive pulmonary disease. PLoS One. 2009;4:e6865. doi: 10.1371/journal.pone.0006865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Vondracek SF, Voelkel NF, McDermott MT, et al. The relationship between adipokines, body composition, and bone density in men with chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis. 2009;4:267–277. doi: 10.2147/copd.s2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Beauchamp MK, O’Hoski S, Goldstein RS, et al. Effect of pulmonary rehabilitation on balance in persons with chronic obstructive pulmonary disease. Arch Phys Med Rehabil. 2010;91:1460–1465. doi: 10.1016/j.apmr.2010.06.021. [DOI] [PubMed] [Google Scholar]
- 64.Leone N, Courbon D, Thomas F, et al. Lung function impairment and metabolic syndrome: the critical role of abdominal obesity. Am J Respir Crit Care Med. 2009;179:509–516. doi: 10.1164/rccm.200807-1195OC. [DOI] [PubMed] [Google Scholar]
- 65.Song Y, Klevak A, Manson JE, et al. Asthma, chronic obstructive pulmonary disease, and type 2 diabetes in the Women’s Health Study. Diabetes Res Clin Pract. 2010;90:365–371. doi: 10.1016/j.diabres.2010.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lazarus R, Sparrow D, Weiss ST. Baseline ventilatory function predicts the development of higher levels of fasting insulin and fasting insulin resistance index: the Normative Aging Study. Eur Respir J. 1998;12:641–645. doi: 10.1183/09031936.98.12030641. [DOI] [PubMed] [Google Scholar]
- 67.Rana JS, Mittleman MA, Sheikh J, et al. Chronic obstructive pulmonary disease, asthma, and risk of type 2 diabetes in women. Diabetes Care. 2004;27:2478–2484. doi: 10.2337/diacare.27.10.2478. [DOI] [PubMed] [Google Scholar]
- 68.Suissa S, Kezouh A, Ernst P. Inhaled corticosteroids and the risks of diabetes onset and progression. Am J Med. 2010;123:1001–1006. doi: 10.1016/j.amjmed.2010.06.019. [DOI] [PubMed] [Google Scholar]
- 69.Han MK, Kazerooni EA, Lynch DA, et al. Chronic obstructive pulmonary disease exacerbations in the COPDGene Study: associated radiologic phenotypes. Radiology. 2011;261(1):274–282. doi: 10.1148/radiol.11110173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Cote C, Zilberberg MD, Mody SH, et al. Haemoglobin level and its clinical impact in a cohort of patients with COPD. Eur Respir J. 2007;29:923–929. doi: 10.1183/09031936.00137106. [DOI] [PubMed] [Google Scholar]
- 71.Ershler WB, Chen K, Reyes EB, et al. Economic burden of patients with anemia in selected diseases. Value Health. 2005;8:629–638. doi: 10.1111/j.1524-4733.2005.00058.x. [DOI] [PubMed] [Google Scholar]
- 72.Chambellan A, Chailleux E, Similowski T. Prognostic value of the hematocrit in patients with severe COPD receiving long-term oxygen therapy. Chest. 2005;128:1201–1208. doi: 10.1378/chest.128.3.1201. [DOI] [PubMed] [Google Scholar]
- 73.John M, Lange A, Hoernig S, et al. Prevalence of anemia in chronic obstructive pulmonary disease: comparison to other chronic diseases. Int J Cardiol. 2006;111:365–370. doi: 10.1016/j.ijcard.2005.07.043. [DOI] [PubMed] [Google Scholar]
- 74.Halpern MT, Zilberberg MD, Schmier JK, et al. Anemia, costs and mortality in chronic obstructive pulmonary disease. Cost Eff Resour Alloc. 2006;4:17. doi: 10.1186/1478-7547-4-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Weiss G, Goodnough LT. Anemia of chronic disease. N Engl J Med. 2005;352:1011–1023. doi: 10.1056/NEJMra041809. [DOI] [PubMed] [Google Scholar]
- 76.Mokhlesi B, Morris AL, Huang CF, et al. Increased prevalence of gastroesophageal reflux symptoms in patients with COPD. Chest. 2001;119:1043–1048. doi: 10.1378/chest.119.4.1043. [DOI] [PubMed] [Google Scholar]
- 77.Terada K, Muro S, Sato S, et al. Impact of gastrooesophageal reflux disease symptoms on COPD exacerbation. Thorax. 2008;63:951–955. doi: 10.1136/thx.2007.092858. [DOI] [PubMed] [Google Scholar]
- 78.Casanova C, Baudet JS, del Valle Velasco M, et al. Increased gastrooesophageal reflux disease in patients with severe COPD. Eur Respir J. 2004;23:841–845. doi: 10.1183/09031936.04.00107004. [DOI] [PubMed] [Google Scholar]
- 79.Rascon-Aguilar IE, Pamer M, Wludyka P, et al. Poorly treated or unrecognized GERD reduces quality of life in patients with COPD. Dig Dis Sci. 2011;56:1976–1980. doi: 10.1007/s10620-010-1542-5. [DOI] [PubMed] [Google Scholar]
- 80.Rascon-Aguilar IE, Pamer M, Wludyka P, et al. Role of gastroesophageal reflux symptoms in exacerbations of COPD. Chest. 2006;130:1096–1101. doi: 10.1378/chest.130.4.1096. [DOI] [PubMed] [Google Scholar]
- 81.Terada K, Muro S, Ohara T, et al. Abnormal swallowing reflex and COPD exacerbations. Chest. 2010;137:326–332. doi: 10.1378/chest.09-0482. [DOI] [PubMed] [Google Scholar]
- 82.Garcia Rodriguez LA, Ruigomez A, Martin-Merino E, et al. Relationship between gastroesophageal reflux disease and COPD in UK primary care. Chest. 2008;134:1223–1230. doi: 10.1378/chest.08-0902. [DOI] [PubMed] [Google Scholar]
- 83.Sasaki T, Nakayama K, Yasuda H, et al. A randomized, single-blind study of lansoprazole for the prevention of exacerbations of chronic obstructive pulmonary disease in older patients. J Am Geriatr Soc. 2009;57:1453–1457. doi: 10.1111/j.1532-5415.2009.02349.x. [DOI] [PubMed] [Google Scholar]
- 84.Yohannes AM, Willgoss TG, Baldwin RC, et al. Depression and anxiety in chronic heart failure and chronic obstructive pulmonary disease: prevalence, relevance, clinical implications and management principles. Int J Geriatr Psychiatry. 2010;25:1209–1221. doi: 10.1002/gps.2463. [DOI] [PubMed] [Google Scholar]
- 85.Solano JP, Gomes B, Higginson IJ. A comparison of symptom prevalence in far advanced cancer, AIDS, heart disease, chronic obstructive pulmonary disease and renal disease. J Pain Symptom Manage. 2006;31:58–69. doi: 10.1016/j.jpainsymman.2005.06.007. [DOI] [PubMed] [Google Scholar]
- 86.Maurer J, Rebbapragada V, Borson S, et al. Anxiety and depression in COPD: current understanding, unanswered questions, and research needs. Chest. 2008;134:43S–56S. doi: 10.1378/chest.08-0342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hanania NA, Mullerova H, Locantore NW, et al. Determinants of depression in the ECLIPSE chronic obstructive pulmonary disease cohort. Am J Respir Crit Care Med. 2011;183:604–611. doi: 10.1164/rccm.201003-0472OC. [DOI] [PubMed] [Google Scholar]
- 88.Laurin C, Lavoie KL, Bacon SL, et al. Sex differences in the prevalence of psychiatric disorders and psychological distress in patients with COPD. Chest. 2007;132:148–155. doi: 10.1378/chest.07-0134. [DOI] [PubMed] [Google Scholar]
- 89.Giardino ND, Curtis JL, Andrei AC, et al. Anxiety is associated with diminished exercise performance and quality of life in severe emphysema: a cross-sectional study. Respir Res. 2010;11:29. doi: 10.1186/1465-9921-11-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.von Leupoldt A, Taube K, Lehmann K, et al. The impact of anxiety and depression on outcomes of pulmonary rehabilitation in patients with COPD. Chest. 2011;140(3):730–736. doi: 10.1378/chest.10-2917. [DOI] [PubMed] [Google Scholar]
- 91.Eisner MD, Blanc PD, Yelin EH, et al. Influence of anxiety on health outcomes in COPD. Thorax. 2010;65:229–234. doi: 10.1136/thx.2009.126201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.de Voogd JN, Wempe JB, Koeter GH, et al. Depressive symptoms as predictors of mortality in patients with COPD. Chest. 2009;135:619–625. doi: 10.1378/chest.08-0078. [DOI] [PubMed] [Google Scholar]
- 93.Fan VS, Ramsey SD, Giardino ND, et al. Sex, depression, and risk of hospitalization and mortality in chronic obstructive pulmonary disease. Arch Intern Med. 2007;167:2345–2353. doi: 10.1001/archinte.167.21.2345. [DOI] [PubMed] [Google Scholar]
- 94.Thakur N, Blanc PD, Julian LJ, et al. COPD and cognitive impairment: the role of hypoxemia and oxygen therapy. Int J Chron Obstruct Pulmon Dis. 2010;5:263–269. doi: 10.2147/copd.s10684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Liesker JJ, Postma DS, Beukema RJ, et al. Cognitive performance in patients with COPD. Respir Med. 2004;98:351–356. doi: 10.1016/j.rmed.2003.11.004. [DOI] [PubMed] [Google Scholar]
- 96.Klein M, Gauggel S, Sachs G, et al. Impact of chronic obstructive pulmonary disease (COPD) on attention functions. Respir Med. 2010;104:52–60. doi: 10.1016/j.rmed.2009.08.008. [DOI] [PubMed] [Google Scholar]
- 97.Dodd JW, Getov SV, Jones PW. Cognitive function in COPD. Eur Respir J. 2010;35:913–922. doi: 10.1183/09031936.00125109. [DOI] [PubMed] [Google Scholar]
- 98.Pereira ED, Viana CS, Taunay TC, et al. Improvement of cognitive function after a three-month pulmonary rehabilitation program for COPD patients. Lung. 2011;189:279–285. doi: 10.1007/s00408-011-9303-6. [DOI] [PubMed] [Google Scholar]
- 99.Kinsman RA, Yaroush RA, Fernandez E, et al. Symptoms and experiences in chronic bronchitis and emphysema. Chest. 1983;83:755–761. doi: 10.1378/chest.83.5.755. [DOI] [PubMed] [Google Scholar]
- 100.Young T, Palta M, Dempsey J, et al. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med. 1993;328:1230–1235. doi: 10.1056/NEJM199304293281704. [DOI] [PubMed] [Google Scholar]
- 101.Simon-Tuval T, Scharf SM, Maimon N, et al. Determinants of elevated healthcare utilization in patients with COPD. Respir Res. 2011;12:7. doi: 10.1186/1465-9921-12-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Weitzenblum E, Chaouat A, Kessler R, et al. Overlap syndrome: obstructive sleep apnea in patients with chronic obstructive pulmonary disease. Proc Am Thorac Soc. 2008;5:237–241. doi: 10.1513/pats.200706-077MG. [DOI] [PubMed] [Google Scholar]
- 103.Mermigkis C, Kopanakis A, Foldvary-Schaefer N, et al. Health-related quality of life in patients with obstructive sleep apnoea and chronic obstructive pulmonary disease (overlap syndrome) Int J Clin Pract. 2007;61:207–211. doi: 10.1111/j.1742-1241.2006.01213.x. [DOI] [PubMed] [Google Scholar]
- 104.Criner GJ, Sternberg AL. National Emphysema Treatment Trial: the major outcomes of lung volume reduction surgery in severe emphysema. Proc Am Thorac Soc. 2008;5:393–405. doi: 10.1513/pats.200801-013ET. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Calverley PM, Rabe KF, Goehring UM, et al. Roflumilast in symptomatic chronic obstructive pulmonary disease: two randomised clinical trials. Lancet. 2009;374:685–694. doi: 10.1016/S0140-6736(09)61255-1. [DOI] [PubMed] [Google Scholar]