Abstract
This review focuses on various components of bile acid signaling in relation to cholangiocytes. Their roles as targets for potential therapies for cholangiopathies are also explored. While many factors are involved in these complex signaling pathways, this review emphasizes the roles of transmembrane G protein coupled receptor (TGR5), farnesoid X receptor (FXR), ursodeoxycholic acid (UDCA) and the bicarbonate umbrella. Following a general background on cholangiocytes and bile acids, we will expand the review and include sections that are most recently known (within 5–7 years) regarding the field of bile acid signaling and cholangiocyte function. These findings all demonstrate that bile acids influence biliary functions which can, in turn, regulate the cholangiocyte response during pathological events.
Abbreviations: ABCB4, ATP-binding cassette, sub-family B; AE2, anion exchanger 2; AKT, protein kinases B; ASBT, apical sodium bile acid transporter; BA, bile acid; BASIC, bile acid sensitive ion channel; Ca2+, intracellular calcium; Cl−/HCO3−, chloride bicarbonate exchanger; COX-2, cyclooxygenase-2; CYP27, sterol-27-hydroxylase; CYP7A1, cholesterol 7α-hydroxylase; EGFR, epidermal growth factor receptor; ERK, extracellular regulated protein kinases; FGF, fibroblast growth factor; FXR, farnesoid X receptor; HGF, hepatocyte growth factor; IL-6, interleukin-6; MAPK, mitogen-activated protein kinase; OST, organic solute transporter; PBC, primary biliary cirrhosis; PC-1, polycystin-1; PM, plasma membrane; PSC, primary sclerosing cholangitis; S1P, sphingosine-1-phosphate; S1PR2, sphingosine 1-phosphate receptor 2; SR, secretin receptor; TCA, taurocholic acid; TGR5, transmembrane G protein coupled receptor; UDCA, ursodeoxycholic acid
KEY WORDS: Bile acids, Cholangiocytes, Receptors, Signaling
Graphical abstract
This review focuses on various components of bile acid signaling in relation to cholangiocytes. Their role as targets for potential therapies for cholangiopathies is also explored. While many factors are involved in these complex signaling pathways, this review emphasizes the roles of transmembrane G protein coupled receptor (TGR5), farnesoid X receptor (FXR), ursodeoxycholic acid (UDCA) and the bicarbonate umbrella.
1. Introduction
Cholangiocytes are a variable group of epithelial cells which line the biliary epithelium, a three-dimensional network of ducts responsible for bile acid transport and ductal bicarbonate secretion1, 2. Cholangiocytes are found in both intrahepatic and extrahepatic bile ducts with size, number and histological type varying based on the size and location of the ducts. In small bile ducts, which are defined as having a lumen diameter less than 15 μm, cholangiocytes are either flattened or cuboidal. In large bile ducts, defined as having a lumen diameter greater than 15 μm, cholangiocytes are columnar and significantly more abundant3, 4. Cholangiocytes contain multiple transporters on both their apical and basolateral plasma membranes, which assist in modifying bile acids via both secretion and absorption5. As bile flows through the duct, the primary cilium on the cholangiocyte apical membranes senses the flow via mechanoreceptors1. This is partly due to the expression of polycystin-1 (PC-1) on the primary cilium. Intra-cholangiocyte Ca2+ levels also increase in response to flow due to PC-21. Modification of the primary bile involves secretion of water, Cl− and HCO3− into the duct, and extraction of glucose, bile acids and amino acids. This is accomplished via several transporters, channels and exchangers, which act based upon the content of the bile and intracellular space1, 6. These modified bile acids are then delivered to the duodenum to be further metabolized by enteric bacteria, or returned to the hepatocytes via the cholehepatic shunt.
Cholangiocytes and bile acid signaling have become a topic of increasing interest due to their roles in the immune defense. The secretion of bicarbonate and other molecules creates the bicarbonate umbrella on the apical membrane of cholangiocytes, which makes cholangiocytes less susceptible to injury and subsequent apoptosis7, 8. A main component of the bicarbonate umbrella is the peptide, secretin. Cholangiocytes are the sole cell type in the liver that express secretin receptor (SR) which, when activated, leads to an opening of the chloride bicarbonate (Cl−/HCO3−) exchanger9. In fact, when animals are infused with secretin they produce increased amounts of bile and bicarbonate demonstrating that secretin is a key player in the regulation of cholangiocyte-induced bile modification9, 10. Further, previous studies have linked bile acids and cholangiocytes by demonstrating that bile acid treatment influences cholangiocyte proliferation and bile flow. Numerous studies from Alpini et al.11, 12, 13 showed that specific bile acids like taurocholic acid (TCA) and taurolithocholic acid stimulate cholangiocyte proliferation, bicarbonate secretion and bile flow.
While once thought to be a type of dormant, passive cells, cholangiocytes are now recognized to be active, hormone-responsive cells that are the target of biliary pathologies known collectively as cholangiopathies14, 15, 16. Numerous studies have indicated that targeting of cholangiocytes may lead to new therapies for cholangiopathies, especially primary sclerosing cholangitis (PSC) and primary biliary cirrhosis (PBC)1, 6, 7, 17, 18. PSC and PBC are both end-stage cholangiopathies that, if left untreated, are likely to develop into the primary liver cancer, cholangiocarcinoma19. It is known that cholangiocytes express adhesion molecules, which are hormone-responsive, secrete inflammatory mediators and immunoglobulins, and can act as antigen presenting cells. These functions have the potential to create beneficial effects for both adaptive and innate immunity, and also to provide potential therapeutic advances for patients suffering from cholangiopathies.
2. Bile acids
Bile acids are cholanic acid derivatives, which share the chemical structure of steroids20. They act as detergents and are responsible for facilitating the absorption of dietary lipids and fat-soluble vitamins, and for maintaining cholesterol homeostasis in the body. Secretion of bile is mediated by secretin, which is released by S cells in the duodenum and jejunum in response to a meal1, 18. The formation of bile acids is initiated in hepatocytes. Classic and alternative pathways can be initiated by cholesterol 7α-hydroxylase (CYP7A1)20. Initially, bile composed primarily of water and various ions, and solutes is released into bile canaliculi via the apical side of hepatocytes. The bile acids flow through the canals of Hering before continuing through the biliary epithelium, where they are subsequently modified primarily by cholangiocytes1, 20. Large cholangiocytes are thought to play the primary role in this process, but small cholangiocytes may also contribute to some extent1. After modification by cholangiocytes, bile acids are stored in the gallbladder and most are then secreted into the duodenum where they are metabolized by enteric bacteria. Approximately 95% of these bile acids are absorbed in the terminal ileum, and are then transported back to the liver via the portal vein for recycling20. These conjugated bile acids will be secreted back into the bile pool. This process is known as the enterohepatic shunt. However, unconjugated bile acids are absorbed by cholangiocytes and returned to the hepatocytes via the peribiliary vascular plexus in a process known as the cholehepatic shunt1. The cations found in bile are primarily calcium, sodium and potassium, while the anions are chloride and bicarbonate. Bile contains several things, which are found naturally throughout the body, including lipids, carbohydrates, vitamins, minerals and proteins20. After synthesis, bile acids are conjugated with either glycine or taurine, which decreases the toxicity of bile and makes it more soluble. This is important for the relationship among cholangiocytes, bile acids and bile acid signaling.
3. Transmembrane G protein coupled receptor (TGR5)
TGR5 is a G-protein coupled receptor, which is activated in response to bile acids. It transmits bile acid signals via the cAMP-signaling pathway in various cells throughout the body. While our focus is on its activity in relation to cholangiocytes, it can also be found in macrophages of the liver (Kupffer cells), intestine, lung and peritoneum, epithelial cells of the intestine and CD14 positive monocytes in peripheral blood6, 21. Overall, the highest levels of expression are found in the gallbladder and intestine. There are no studies that indicate the functional significance of the diverse localization of TGR5 throughout the body and cholangiocytes. TGR5 is found in many areas of the cholangiocyte, including the apical plasma membrane, primary cilia and multiple locations in the intracellular space. While TGR5 is most highly expressed on the outer and inner nuclear membranes of the cholangiocyte intracellularly, one study noted that it could also be found in multivesicular bodies, intracellular vesicles and endoplasmic reticulum21. However, in addition to the nuclear membrane, TGR5 is highly expressed on the apical plasma membrane and cilia21. This finding is significant because activation of TGR5 has also been shown to contribute to the formation of the bicarbonate umbrella located on the apical surface of cholangiocytes6.
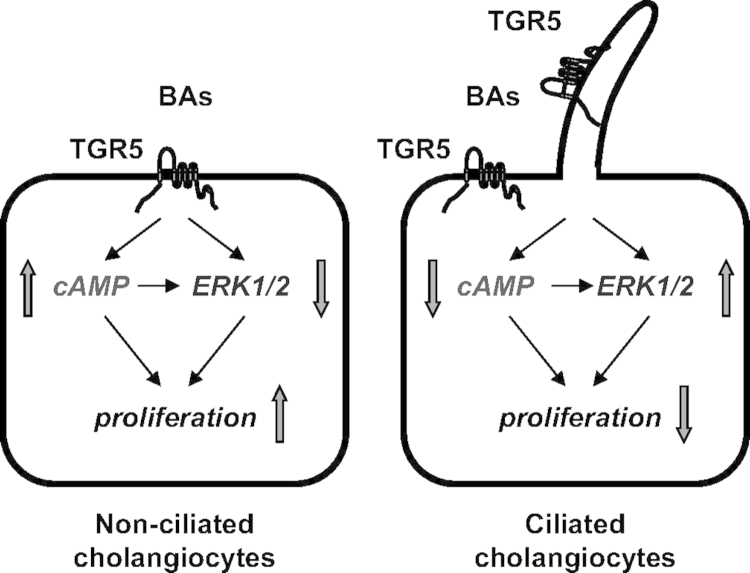
Cholangiocyte cilia have been determined to have mechanoreceptive, chemoreceptive and osmosensory properties. It is important to note that not all cholangiocytes have a primary cilium, and this presumably influences the different effects that TGR5 activation has on cholangiocytes. This further supports the idea that TGR5 has a strong effect at the apical membrane. It has been shown that the presence of cilia is a result of a longer duration of cell growth21. Studies using TGR5 agonists such as taurolithocholic acid, lithocholic acid, oleanolic acid and cholic acid were used to identify these differences21. The presence or absence of cilia has also been determined to have an effect on cellular proliferation. This was validated by demonstrating that suppression of apical sodium bile acid transporter (ASBT), a transporter with significant involvement in cholangiocyte proliferation, has no effect on the inhibitory effects induced by TGR5 agonists on ciliated cholangiocyte proliferation21.
In ciliated cholangiocytes, TGR5 binds to a Gαi protein, which is found at the base of the cilia. This results in a decrease in cAMP signaling, which suggests that an inhibitory signaling pathway exists in ciliated cholangiocytes in response to stimuli, such as bile flow21. It has also been shown that this mechanism is associated with decreased proliferation in ciliated cells in response to TGR5. It is also important to note that ciliated cholangiocytes contain more TGR5, Gαi and Gαs than non-ciliated cells. Experiments involving mitogen-activated protein kinase (MAPK), also known as extracellular regulated protein kinases (ERK), have demonstrated a positive relationship between TGR5 signaling and MAPK activation21. This is of particular interest because of the association of defects in the MAPK pathway with a variety of cancers.
In non-ciliated cholangiocytes, TGR5 binds to a Gαs protein. This relationship has been studied more than that of TGR5 and Gαi proteins. Binding to this G protein leads to an increase in cAMP signaling, suggesting that there is a stimulatory relationship between TGR5 and non-ciliated cholangiocyte cells21. As opposed to the effect of TGR5 on ciliated cholangiocytes, the binding to non-ciliated cholangiocytes leads to increased cellular proliferation. In contrast to ciliated cholangiocytes, TGR5 in non-ciliated cholangiocytes inhibits MAPK activation, which also makes it especially interesting for certain fields of cancer research. To date, no studies have demonstrated what mechanisms affect the binding of TGR5 to different Gα proteins21.
Because TGR5 inhibits the secretion and expression of cytokines in immune cells, it has been studied as a potential target for immune-mediated cholangiopathies. Other protective qualities of TGR5 include the prevention of apoptosis and secretion of components of the bicarbonate umbrella. Such mechanisms have led to the idea that TGR5 agonists could be helpful in diseases such as PSC, which involve inflammatory changes within the biliary tree. One study demonstrated that, while the activation of TGR5 led to decreased systemic inflammation and enhanced cholangiocyte protection, it did not improve PSC in mice6. However, it was noted to increase cholestatic-induced pruritus. It was also noted that the lack of TGR5 activation increased inflammatory hepatic infiltrates, but decreased the incidence of cholesterol gallstones6. Mutations in the coding region of TGR5, such as 2q35, have been found in patients with PSC and ulcerative colitis, but have not been found to enhance one׳s susceptibility to these diseases. While the primary source of injury in PSC is yet to be determined, one concept involves gut-derived inflammatory leakage. This is secondary to the absence of the ABCB4 (ATP-binding cassette, sub-family B) phospholipid transporter, which results in a lack of phospholipids in bile. This allows bile acids to leak across tight junctions and initiate inflammatory damage. Gut-derived lymphocytes have been found in the portal infiltrate of patients with PSC6. Support for this concept stems from the fact that PSC is frequently associated with IBD, and that proctocolectomy has been shown to reduce the recurrence of PSC after liver transplantation for this illness6.
While the activation of TGR5 has many beneficial effects, it can also lead to detrimental outcomes. Because of its pro-proliferation and anti-apoptotic properties, it has been implicated as a contributing factor in the pathogenesis of several gastrointestinal tumors, including cholangiocarcinoma and colorectal cancer6. One study noted that TGR5 was highly expressed in human cholangiocarcinoma samples taken from patients who did not have PSC6. A summary figure of TGR5 signaling is found in Fig. 1. While TGR5 has the potential to offer new and beneficial alternatives for patients with cholangiopathies, further studies are needed, not only to explore potential negative effects but also to demonstrate specific mechanisms by which it can be utilized.
Figure 1.
Working model of TGR-mediated bile acid signaling in nonciliated and ciliated cholangiocytes. The localization of TGR5 on the plasma membrane (PM) or ciliary membrane determines the cholangiocyte functional response to bile acid (BA) signaling. In nonciliated cholangiocytes, TGR5 agonists increase cAMP levels and cell proliferation but inhibit ERK signaling. In ciliated cholangiocytes, the effects of TGR5 agonists are opposite, i.e., cAMP levels and cell proliferation decreased, but ERK signaling is activated. Reprinted with permission from Masyuk et al.21.
4. Farnesoid X receptor (FXR)
Due to their location, cholangiocytes are able to modify bile quickly and efficiently as it travels through the biliary tree. FXR is a nuclear receptor, encoded by NR1H4, which is found in the liver and intestines22. Bile acids, specifically hydrophobic bile acids, act as ligands for this receptor and utilize it in their negative feedback loop17. Fibroblast growth factor 19, which is highly expressed in human cholangiocytes, is activated by FXR. This activation, via fibroblast growth factor receptor 4, leads to the repression of cholangiocyte sterol-27-hydroxylase (CYP27), which is the rate-limiting enzyme in the acidic pathway of bile acid synthesis17. While it was determined that p38 kinase inhibitor is not directly involved in the repression of CYP27, it has been shown to be an important mediator of the fibroblast growth factor 19 (FGF19) signal via the ability to down-regulate FXR17, 23. Inhibition of CYP27 prevents the basolateral cholesterol efflux mediated by liver X receptor and 27-hydrocholesterol, the product of Cyp27, in the cholangiocyte17. FXR has also been noted to have paracrine effects. While it is known that bile acids can inhibit hepatocyte CYP27, a recent study also demonstrated that bile acids have the ability to repress hepatocyte CYP7A1 via the cholangiocyte FXR/FGF pathway17, 24. This leads to a reduction in bile acid by the liver24. Studies have shown that defects in FXR, and its associated factors, are linked to gallstone disease17. This further emphasizes the importance of the cholangiocyte in bile acid modification.
Several studies have demonstrated the protective effects of FXR in relation to liver transplantation23, 24. This is a topic of interest due to the complications, such as nonanastomotic strictures, which can result from bile acid toxicity post-transplant. Many such injuries are the result of hypoxia and ischemia, which are unavoidable components of the procedure. It has been shown that FXR is repressed in the setting of hypoxia and ischemia post-transplant23. However, the release of inflammatory cytokines post-transplant has also been shown to decrease the expression of FXR23. While it is not the sole factor, the down-regulation of FXR is a contributing cause in biliary epithelial damage due to the associated disturbance in bile acid transport. Hypoxia induces the repression of FXR via p38, leading to changes in bile acid transporters17, 23. ASBT, a transporter located on the apical surface of cholangiocytes, is slightly down-regulated by FXR. When levels of FXR are reduced, the expression of this transporter is increased, leading to enhanced absorption of bile acids into the cholangiocyte. In addition, the activity of organic solute transporter α/β (OSTα/β), one transporter responsible for basolateral efflux of bile acids, is reduced in response to lower levels of FXR. These factors lead to an unbalanced accumulation of intracellular bile acids and have been shown to enhance apoptosis and the expression of profibrotic factors. Increased biliary transit time is associated with enhanced bile duct injury, and has been linked to reduced levels of FXR23. The demonstrated capacity of bile acid signaling to induce toxic effects and cellular disruption suggests a possible role in the development of cholangiopathies.
The hepato-protective effects of FXR can also be noted in the setting of partial hepatectomy. In this setting, it is particularly important to control the level of bile acid synthesis in order to reduce bile acid induced toxicity. FGF19 down-regulates CYP7A1 expression, thereby preventing cellular injury via the suppression of bile acid synthesis. One study demonstrated that the reduction of FXR, and thereby FGF15 expression in mice, led to a significantly high mortality after partial hepatectomy24. It was also noted that mice deficient in FGF15 activity had a higher mortality overall24. In addition to regulation of bile acid synthesis, FGF stimulation enhances the expression of hepatocyte growth factor (HGF), a regenerative growth factor, and decreases levels of some inflammatory chemokines. While HGF and EGF are known to be mitogens in terms of liver growth, studies have demonstrated that FGF19 can act as a growth factor for hepatocytes and cholangiocytes, and promote wound healing in the biliary system24. FGF19 acts on the MAPK/ERK1/2 pathway to stimulate proliferation of hepatocytes and cholangiocytes. Measured growth via this pathway was found to be comparable to that induced by more established growth factors24. In addition to these findings, it was also noted that mice lacking Fgf15 did not demonstrate the expected amount of liver growth in response to cholic acid feeding. This data was verified using a human hepatocyte cell line. These findings suggest a possible role for FGF19 in the setting of liver transplant, in which the administration could prevent cholestasis and the detrimental effects associated with it.
5. Ursodeoxycholic acid (UDCA) and the bicarbonate umbrella
UDCA is a hydrophilic bile acid found in small amounts in humans. It is the target of many studies due to its therapeutic potential, and is considered a first line treatment for primary biliary cirrhosis18, 25. Accumulation of hydrophobic bile acids in the setting of cholestasis can lead to increased injury of cholangiocytes due to prolonged membrane exposure. It has been demonstrated that, at decreased pH levels, the penetration and accumulation of bile salts into cholangiocytes increase7, 8. The penetrating ability of bile acids is largely determined by their protonation, and can be determined by their pKa7. This increase in biliary acidity also contributes to enhanced rates of apoptosis. It has also been determined that bile acid uptake is influenced by the expression of anion exchanger 2 (AE2)7, 8. Higher rates of bile acid uptake and toxicity are noted in the setting of decreased AE2 expression7. These findings reflect the importance of the bicarbonate umbrella, which produces a more alkaline environment at the cholangiocyte apical membrane. It has been described as a glycocalyx composed of a variety of substances, such as glycosphingolipids, proteoglycans, glycosylated proteins and glycosaminoglycans7. The secretion of bicarbonate rich fluid is mediated via conjugated UDCA by the Cl−/HCO3− exchanger and AE2, in response to secretin acting on the cholangiocyte18. However, this response is not found in those with untreated PBC. Conjugated ursodeoxycholic acid has been shown to promote secretin-stimulated hydrocholresis via multiple mediators, including AE2, PKCα, PI3K, MEK, PKA, intracellular Ca2+ and microtubules18. It has been shown, via studies utilizing colchicine and biliary inhibitors, that this route is highly dependent on microtubule polymerization18. Secretin alone cannot stimulate hydrochloresis. For it to be effective, the bile-acid pool must be maintained in a relatively homeostatic state. In patients with PBC, secretin induced bicarbonate secretion is diminished. When such patients are treated with UDCA, the response to secretin is restored, allowing hydrochloresis to occur18. Treatment with UDCA also improves the expression of AE2, which is diminished in the setting of PBC. One report demonstrated that hydrochloresis via the secretin induced UDCA pathway requires conjugation of UDCA. When given orally, UDCA is conjugated in hepatocytes as it reaches the liver via portal circulation18. It can then reach the basolateral surface of cholangiocytes via enterohepatic circulation. It is important to note that a combination of factors is required to increase bile flow. One study demonstrated that secretin, UDCA and taurine conjugated UDCA were not individually able to enhance bile flow, but were able to do so when in combination with one another18. The cytoprotective mechanisms of UDCA reflect the important role that it may play in the prevention of cholestasis and treatment of cholangiopathies. The bicarbonate umbrella has also been suggested as a target for the treatment of cholangiopathies. Of particular interest is the fact that fucosyltransferase 2, which is an enzyme known to be responsible for maintenance of the biliary glycocalyx, has been determined to be a susceptibility gene for primary sclerosing cholangitis8. A study of bile acid sensitive ion channel (BASIC), which is highly expressed in cholangiocytes, demonstrated that UDCA is a potent activator of this channel. While further studies are needed to better determine the role and cellular activities of BASIC, the study suggested that it may act as a factor in the therapeutic effects provided by UDCA25.
6. Sphingosine 1-phosphate receptor 2 (S1PR2)
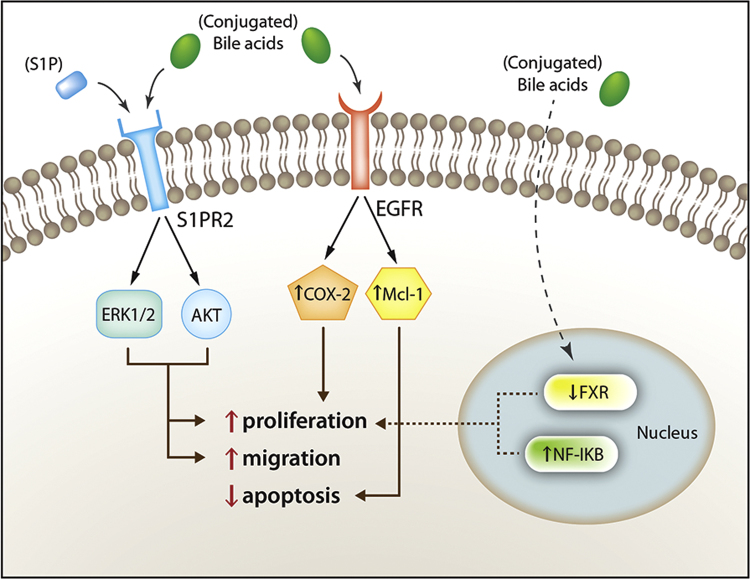
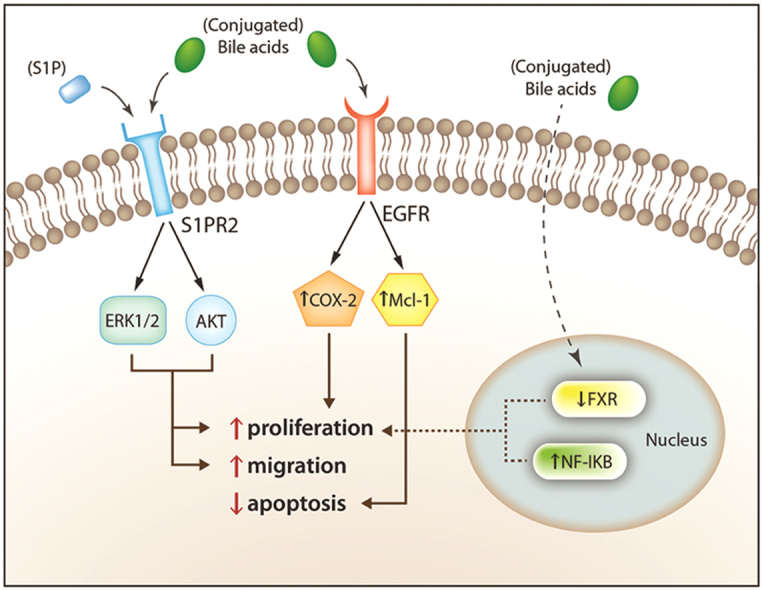
Although it is rare, cholangiocarcinoma, a primary neoplasm arising from cholangiocytes, is the second most common primary hepatic neoplasm2. Due to increasing incidence, targets for potential therapies have become a particular area of interest. Most cases of cholangiocarcinoma are sporadic, having no known risk factors associated. However, it is known that conditions leading to cholangitis and chronic cholestasis, such as choledocholithiasis, PSC, hepatolithiasis and fluke infestation of the biliary tract, are risk factors for the development of cholangiocarcinoma. Conjugated bile acids increase the expression of cyclooxygenase-2 (COX-2), an enzyme implicated in a variety of cancers, via activation of epidermal growth factor receptor (EGFR). Unlike free bile acids, conjugated bile acids increase the activation of nuclear factor-kappa B (NF-κB), which is associated with higher levels of interleukin-6 (IL-6) and COX-2 expression, both of which are implicated in enhanced growth and apoptosis resistance in cholangiocarcinoma cells26. Studies have also shown that, via activation of S1PR2, the growth and invasiveness of cholangiocarcinoma can be sustained by conjugated bile acids26, 27.
S1PR2 is a G-protein coupled receptor act via the sphingosine-1-phosphate (S1P) ligand to exert both proliferative and anti-proliferative effects in various tissues. This effect is cell-type specific. Levels of S1PR2 are higher in human cholangiocarcinoma cells than in non-tumor samples from the same source27. In cholangiocytes, S1PR2 enhances proliferation via activation of ERK1/2 and protein kinases B (AKT). It has also been shown that TCA has the ability to activate S1PR2, thereby enhancing the invasiveness of cholangiocarcinoma. TCA is a hydrophilic bile acid, acting as the most potent stimulator of cholangiocarcinoma cell growth. To further demonstrate the specific effect of S1PR2 on cholangiocytes, JTE-013, a selective S1PR2 agonist, was utilized. It was shown, via utilization of TCA mediated cholangiocarcinoma proliferation, that this antagonist blunted both ERK1/2 and AKT levels, and reduced proliferation of cholangiocarcinoma cell lines26. It has been hypothesized that conjugated bile acids exert the aforementioned effects without ever actually entering the cholangiocyte, and this, if true, could explain dysregulation of FXR expression in the setting of cholangiocarcinoma26. Fig. 2 demonstrates the diverse effects of bile acids on SIPR2 and EGFR. Further studies are needed to determine the therapeutic potential of S1PR2 directed treatments.
Figure 2.
Schematic representation of bile acid-activated intracellular pathways involved in the sustenance of cholangiocarcinoma growth and invasiveness. Reprinted with permission from Maroni et al.27.
7. Conclusions
The role of cholangiocytes encompasses much more than initially considered. Multiple studies have demonstrated ways in which they may be targeted as a therapeutic option in the future. The proper functioning of these cells is necessary to maintain biliary homeostasis. This is underscored by the many detrimental effects that cholestasis has been proven to cause and is thought to cause. Cholangiocytes are the site of many concerted signaling pathways, which demonstrate effects throughout the body. TGR5 acts on both inhibitory and stimulatory pathways in cholangiocytes in an effort to regulate cellular proliferation and apoptosis. It also mediates the immune response by inhibiting the secretion of inflammatory cytokines. While it may provide benefit in the field of cholangiopathies, its possible relationship to malignancies must be studied further as its anti-apoptotic properties could contribute to detrimental side effects. FXR plays a hepato-protective role by helping to regulate bile acid synthesis. It has also been shown to have the ability to enhance proliferation of both hepatocytes and cholangiocytes and promote hepatic healing. The bicarbonate umbrella provides a protective shield for cholangiocytes against bile acids. Because secretion and absorption of bile acids are highly connected to pH and protonation, this alkaline layer protects cholangiocytes from the intracellular accumulation of toxic bile acids. Ursodeoxycholic acid promotes the hydrochloresis necessary to maintain the protective layer. It has also been shown to have the ability to enhance bile flow, which decreases toxicity via decreasing biliary transit time. It is already considered the first line treatment in some cholangiopathies. While cholangiocytes have been determined to have a very important role in many areas, further research is required to reveal their signaling potential.
Acknowledgments
Portions of this work was supported by the Dr. Nicholas C. Hightower Centennial Chair of Gastroenterology from Scott & White, a VA Research Career Scientist Award, a VA Merit award and a VA CDA-2 Award to Heather Francis (No. IK2 BX001760).
Footnotes
Peer review under responsibility of Institute of Materia Medica, Chinese Academy of Medical Sciences and Chinese Pharmaceutical Association.
References
- 1.Tabibian JH, Masyuk AI, Masyuk TV, O’Hara SP, LaRusso NF. Physiology of cholangiocytes. Compr Physiol. 2013;3:541–565. doi: 10.1002/cphy.c120019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alpini G, McGill JM, Larusso NF. The pathobiology of biliary epithelia. Hepatology. 2002;35:1256–1268. doi: 10.1053/jhep.2002.33541. [DOI] [PubMed] [Google Scholar]
- 3.Alpini G, Roberts S, Kuntz SM, Ueno Y, Gubba S, Podila PV. Morphological, molecular, and functional heterogeneity of cholangiocytes from normal rat liver. Gastroenterology. 1996;110:1636–1643. doi: 10.1053/gast.1996.v110.pm8613073. [DOI] [PubMed] [Google Scholar]
- 4.Alpini G, Glaser S, Robertson W, Rodgers RE, Phinizy JL, Lasater J. Large but not small intrahepatic bile ducts are involved in secretin-regulated ductal bile secretion. Am J Physiol. 1997;272:G1064–G1074. doi: 10.1152/ajpgi.1997.272.5.G1064. [DOI] [PubMed] [Google Scholar]
- 5.Xia X, Francis H, Glaser S, Alpini G, LeSage G. Bile acid interactions with cholangiocytes. World J Gastroenterol. 2006;12:3553–3563. doi: 10.3748/wjg.v12.i22.3553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Keitel V, Reich M, Häussinger D. TGR5: pathogenetic role and/or therapeutic target in fibrosing cholangitis? Clin Rev Allergy Immunol. 2015 doi: 10.1007/s12016-014-8443-x. [DOI] [PubMed] [Google Scholar]
- 7.Hohenester S, Maillette de Buy Wenniger L, Jefferson DM, Oude Elferink RP, Beuers U. Biliary bicarbonate secretion constitutes a protective mechanism against bile acid-induced injury in man. Dig Dis. 2011;29:62–65. doi: 10.1159/000324687. [DOI] [PubMed] [Google Scholar]
- 8.Hohenester S, Wenniger LM, Paulusma CC, van Vliet SJ, Jefferson DM, Elferink RP. A biliary HCO3− umbrella constitutes a protective mechanism against bile acid-induced injury in human cholangiocytes. Hepatology. 2012;55:173–183. doi: 10.1002/hep.24691. [DOI] [PubMed] [Google Scholar]
- 9.Alpini G, Glaser S, Baiocchi L, Francis H, Xia XF, Lesage G. Secretin activation of the apical Na+-dependent bile acid transporter is associated with cholehepatic shunting in rats. Hepatology. 2005;41:1037–1045. doi: 10.1002/hep.20653. [DOI] [PubMed] [Google Scholar]
- 10.Glaser S, Meng F, Han Y, Onori P, Chow BK, Francis H. Secretin stimulates biliary cell proliferation by regulating expression of microRNA 125b and microRNA let7a in mice. Gastroenterology. 2014;146:1795–1808. doi: 10.1053/j.gastro.2014.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alpini G, Glaser SS, Ueno Y, Rodgers R, Phinizy JL, Francis H. Bile acid feeding induces cholangiocyte proliferation and secretion: evidence for bile acid-regulated ductal secretion. Gastroenterology. 1999;116:179–186. doi: 10.1016/s0016-5085(99)70242-8. [DOI] [PubMed] [Google Scholar]
- 12.Alpini G, Ueno Y, Glaser SS, Marzioni M, Phinizy JL, Francis H. Bile acid feeding increased proliferative activity and apical bile acid transporter expression in both small and large rat cholangiocytes. Hepatology. 2001;34:868–876. doi: 10.1053/jhep.2001.28884. [DOI] [PubMed] [Google Scholar]
- 13.Mancinelli R, Onori P, Gaudio E, Franchitto A, Carpino G, Ueno Y. Taurocholate feeding to bile duct ligated rats prevents caffeic acid-induced bile duct damage by changes in cholangiocyte VEGF expression. Exp Biol Med. 2009;234:462–474. doi: 10.3181/0808-RM-255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Alvaro D, Mancino MG, Glaser S, Gaudio E, Marzioni M, Francis H. Proliferating cholangiocytes: a neuroendocrine compartment in the diseased liver. Gastroenterology. 2007;132:415–431. doi: 10.1053/j.gastro.2006.07.023. [DOI] [PubMed] [Google Scholar]
- 15.Glaser SS, Gaudio E, Miller T, Alvaro D, Alpini G. Cholangiocyte proliferation and liver fibrosis. Expert Rev Mol Med. 2009;11:e7. doi: 10.1017/S1462399409000994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.LeSage G, Glaser S, Alpini G. Regulation of cholangiocyte proliferation. Liver. 2001;21:73–80. doi: 10.1034/j.1600-0676.2001.021002073.x. [DOI] [PubMed] [Google Scholar]
- 17.Jung DJ, York JP, Wang L, Yang CF, Zhang AJ, Francis HL. FXR-induced secretion of FGF15/19 inhibits CYP27 expression in cholangiocytes through p38 kinase pathway. Pflug Arch. 2014;466:1011–1019. doi: 10.1007/s00424-013-1364-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Úriz M, Sáez E, Prieto J, Medina JF, Banales JM. Ursodeoxycholic acid is conjugated with taurine to promote secretin-stimulated biliary hydrocholeresis in the normal rat. PLoS One. 2011;6:e28717. doi: 10.1371/journal.pone.0028717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Francis H, Alpini G, DeMorrow S. Recent advances in the regulation of cholangiocarcinoma growth. Am J Physiol Gastrointest Liver Physiol. 2010;299:G1–G9. doi: 10.1152/ajpgi.00114.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Reshetnyak VI. Physiological and molecular biochemical mechanisms of bile formation. World J Gastroenterol. 2013;19:7341–7360. doi: 10.3748/wjg.v19.i42.7341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Masyuk AI, Huang BQ, Radtke BN, Gajdos GB, Splinter PL, Masyuk TV. Ciliary subcellular localization of TGR5 determines the cholangiocyte functional response to bile acid signaling. Am J Physiol Gastrointest Liver Physiol. 2013;304:G1013–G1024. doi: 10.1152/ajpgi.00383.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Deuschle U, Birkel M, Hambruch E, Hornberger M, Kinzel O, Perović-Ottstadt S. The nuclear bile acid receptor FXR controls the liver derived tumor suppressor histidine-rich glycoprotein. Int J Cancer. 2015;136:2693–2704. doi: 10.1002/ijc.29312. [DOI] [PubMed] [Google Scholar]
- 23.Cheng L, Tian F, Tian F, Tang L, Chen G, Luo Z. Repression of farnesoid X receptor contributes to biliary injuries of liver grafts through disturbing cholangiocyte bile acid transport. Am J Transplant. 2013;13:3094–3102. doi: 10.1111/ajt.12479. [DOI] [PubMed] [Google Scholar]
- 24.Uriarte I, Fernandez-Barrena MG, Monte MJ, Latasa MU, Chang HC, Carotti S. Identification of fibroblast growth factor 15 as a novel mediator of liver regeneration and its application in the prevention of post-resection liver failure in mice. Gut. 2013;62:899–910. doi: 10.1136/gutjnl-2012-302945. [DOI] [PubMed] [Google Scholar]
- 25.Wiemuth D, Sahin H, Lefèvre CMT, Wasmuth HE, Gründer S. Strong activation of bile acid-sensitive ion channel (BASIC) by ursodeoxycholic acid. Channels. 2013;7:38–42. doi: 10.4161/chan.22406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu RP, Zhao RP, Zhou XQ, Liang XY, Campbell DJ, Zhang XX. Conjugated bile acids promote cholangiocarcinoma cell invasive growth through activation of sphingosine 1-phosphate receptor 2. Hepatology. 2014;60:908–918. doi: 10.1002/hep.27085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Maroni L, Alpini G, Marzioni M. Cholangiocarcinoma development: the resurgence of bile acids. Hepatology. 2014;60:795–797. doi: 10.1002/hep.27223. [DOI] [PubMed] [Google Scholar]