Abstract
EZH2 is over-expressed in human colon cancer and is closely associated with tumor proliferation, metastasis and poor prognosis. Targeting and inhibiting EZH2 may be an effective therapeutic strategy for colon cancer. 3-Deazaneplanocin A (DZNep), as an EZH2 inhibitor, can suppress cancer cell growth. However, the anti-cancer role of DZNep in colon cancer cells has been rarely studied. In this study, we demonstrate that DZNep can inhibit the growth and survival of colon cancer HCT116 cells by inducing cellular senescence and apoptosis. The study provides a novel view of anti-cancer mechanisms of DZNep in human colon cancer cells.
KEY WORDS: EZH2, Human colon cancer HCT116 cells, DZNep, Anti-cancer mechanisms
Graphical abstract
3-Deazaneplanocin A (DZNep), as an EZH2 inhibitor, can suppress cancer cell growth. However, the anti-cancer role of DZNep in colon cancer cells has been rarely studied. In this study, we demonstrate that DZNep can inhibit the growth and survival of colon cancer cells HCT116 by inducing cellular senescence and apoptosis. The study provides a novel view of anti-cancer mechanisms of DZNep in human colon cancer cells.
1. Introduction
Colon cancer has become the third leading cause of tumor-related deaths in the world1, and its incidence is rapidly rising in Chinese cities along with economic development2. Colon cancer normally develops through a multistep process that results from the progressive accumulation of mutations and epigenetic alterations in tumor suppressor genes and oncogenes, which include both DNA hypermethylation and chromatin modifications such as histone methylation and deacetylation3. Recently, the study of epigenetic gene silencing by histone methylation has gained attention in the development of colon cancer.
Enhancer of zeste homolog-2 (EZH2), a histone methyltransferase, is the catalytic subunit of polycom repressive complex 2 (PRC2) that methylates H3K27me3 and results in epigenetic control of cancer genes expression4, 5. Up-regulation of EZH2 has been found in many malignant human tumors, including colorectal carcinomas, and a high level of EZH2 positively correlates with colon cancer metastasis6. Thus, EZH2 has become a promising therapeutic target for colon cancer treatment.
3-Deazaneplanocin A (DZNep), a S-adenosyl-l-homocysteine (AdoHcy) hydrolase, has been shown to be capable of reducing the expression of EZH27. Although DZNep was shown to be able to inhibit the proliferation as well as the metastasis and invasion of a variety of cancer cells, such as lung cancer and breast cancer8, 9, little is known with regard to the effects and mechanisms of DZNep on colon cancer cells.
Cellular senescence has become a vital anti-cancer mechanism due to irreversible cell cycle arrest and permanent inhibition of cancer cell growth10, 11. Inhibition of EZH2 was shown to promote senescence and apoptosis induced by doxorubicin in gastric cancer cells harboring mutant p5312. However, direct evidence linking EZH2 inhibition by pharmacological intervention with cell senescence in colon cancer cells is still lacking. Here, we investigated the role of DZNep in colon cancer HCT116 cells, and found that both senescence and apoptosis contribute to the anti-cancer effects of the compound.
2. Materials and methods
2.1. Reagents and materials
3-Deazaneplanocin A hydrochloride (DZNep) and propidium iodide (PI) were purchased from Sigma-Aldrich. MTT was purchased from Amresco (Solon, OH). AnnexinV-PI was purchased from Beijing 4A Biotech Co., Ltd. Senescence associated β-galactosidase (SA-β-gal) staining kit was purchased from Beyotime Institute of Biotechnology. Antibody for β-actin and EED were purchased from Sigma. EZH2, SUZ12 and other antibodies were purchased from Cell Signaling Technology.
2.2. Cell culture
HCT116 was cultured and maintained in DMEM (Hyclone, Logan, UT) supplemented with 10% (v/v) fetal bovine serum (FBS, Life Technology), 100 U/mL penicillin G (Amresco) and 100 μg/mL streptomycin (Amresco). The cells were cultured in a humidified atmosphere of 5% CO2 at 37 °C.
2.3. Cellular viability measurement
Cellular viability was performed with the MTT assay as described previously7. The cells were seeded at 5000 cells/well in 96-well plates for 24 h and treated with different concentrations of DZNep for another 48 h, then incubated in MTT solution for 4 h at 37 °C. MTT solution was then carefully removed and replaced by 100 μL DMSO to dissolve formazan crystals. Absorbance was measured using a microplate reader (Bio-Rad) at a wavelength of 570 nm. The control group was set as 100%, and IC50 values were calculated and plotted with Graphpad Prism 5. This experiment was conducted in triplicates three times.
2.4. Colony formation and cell growth assay
Cellular sensitivity to DZNep was determined using a previously described protocol13. In brief, HCT116 cells were seeded at a density of 500 cells/well into 35 mm dishes and treated as indicated. After incubation for 2 weeks, the colonies obtained were fixed in 3% paraformaldehyde for 15 min and then washed with PBS followed by staining with hematoxylin. HCT116 cells were seeded into 96-well plate with 104 cells/well in triplicate for cell counting at indicated time points with a Countess Cell Counter (Life Technology).
2.5. SA-β-gal staining
SA-β-gal activity staining was performed using SA-β-gal staining kit (Beyotime Institute of Biotechnology, Haimen, China) according to the manufacturer׳s protocol. The cells were seeded at 5×104 cells/well in 6-well plates, and treated with DZNep at 5 μmol/L for the indicated time. The cells were washed three times with PBS and fixed with 4% paraformaldehyde for 15 min at room temperature. The cells then were incubated overnight at 37 °C with the working solution containing 0.05 mg/mL X-gal using SA-β-gal staining kit (Beyotime Biotechnology).
2.6. Apoptosis assay
Apoptosis assay was performed using the Annexin V Alexa Fluor 488/PI apoptosis detection Kit (Beijing 4A Biotech Co., Ltd.) according to the manufacturer׳s instructions. Briefly, after treated as indicated, both floating and non-floating cells were collected after trypsinization, and then washed twice in PBS, followed by re-suspension in Annexin-V binding buffer at a concentration of 106 cells/mL. An aliquot of 100 μL of this suspension was stained with 5 μL of Annexin-V Alexa Fluor 488 and 5 μL PI, and incubated at room temperature in the darkness for 15 min. Subsequently, the cells were added to 400 μL PBS and subjected to FACS analysis in a FACS Calibur (Becton Dickinson instruction, BD Bioscience).
2.7. Flow cytometry and cell cycle analysis
The cells were harvested and fixed in 70% ethanol at −20 °C overnight. The fixed cells were stained with PI for 1 h after treatment with RNase at 37 °C for 30 min in the darkness. The stained cells were analyzed for DNA content by FACS. The cell cycle fractions were quantified using the Cell Quest Acquisition and Analysis (BD Bioscience).
2.8. Immunoblotting analysis
Immunoblotting analysis was determined using a previously described protocol14. The cells were washed with icy PBS and lysed with lysis buffer containing protease inhibitors cocktail (CST) plus 1 mmol/L PMSF. Protein concentration was determined by Bradford assay. Equal amounts of protein were separated by 10%–15% sodium dodecyl sulfate polyacrylamide gel electrophoresis and transferred onto PVDF membranes (Whatman). The membranes were blocked with 5% nonfat milk PBS-T buffer at room temperature for 1 h, and then incubated with primary antibodies at 4 °C overnight. The membranes were then incubated for 1 h with an appropriate horseradish peroxidase linked secondary antibody, and electrochemiluminescence was performed with a ChemiImager 5500 imaging system (Alpha Innotech Corporation).
2.9. Statistical analysis
SPSS 19.0 statistical software was used for statistical analysis. Values are shown as the mean±SD. Statistical analysis was carried out using Student׳s t-test. Differences between groups were identified as statistically significant at P<0.05.
3. Results
3.1. DZNep inhibits the growth of HCT116 cells
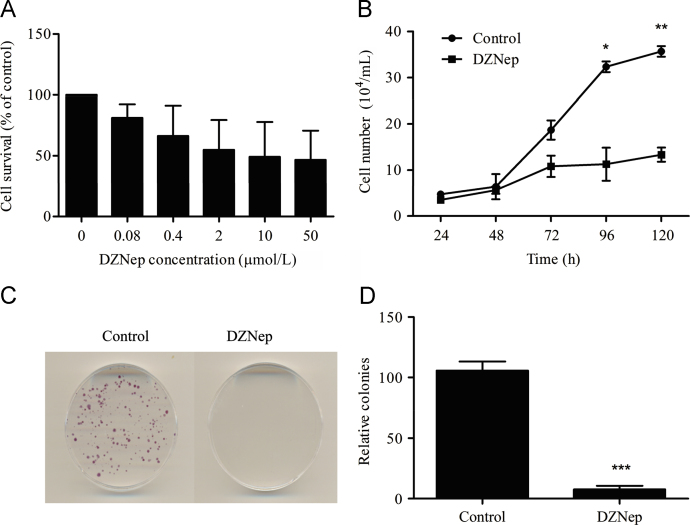
MTT assay was used to screen the effective concentration of DZNep on human colon cancer HCT116 cells. The cells were treated with DZNep for 48 h at the concentration of 0, 0.08, 0.4, 2, 10 and 50 μmol/L. DZNep induced potent growth inhibition in a dose-dependent manner (Fig. 1A). We used 5 μmol/L DZNep at IC50 range for further study, and found that DZNep significantly inhibited the cellular proliferation in a time-dependent way, as indicated by the growth curve (Fig. 1B). Longer treatment of DZNep also displayed significant growth inhibition with colony formation assay (Fig. 1C).
Figure 1.
DZNep inhibited the proliferation of HCT116 cells. (A) Effect of DZNep on the proliferation of HCT116 determined by MTT assay. The cells were treated in 96-well plates for 48 h with DZNep at the indicated concentration. (B) Effect of DZNep on the growth curve. The cells were seeded into 24-well plates with 1×104 cells/well for 24 h, and then treated with or without 5 μmol/L DZNep and counted at indicated points (*P<0.05, **P<0.01 vs. control, n=3). (C) Colony formation upon DZNep treatment. The cells were seeded in 35 mm dishes with 500 cells per dish, and then treated with 5 μmol/L DZNep for 2 weeks. The percentage of the colonies was normalized relative to the control (***P<0.001 vs. control, n=3).
3.2. DZNep induces G1 cell cycle arrest in HCT116 cells
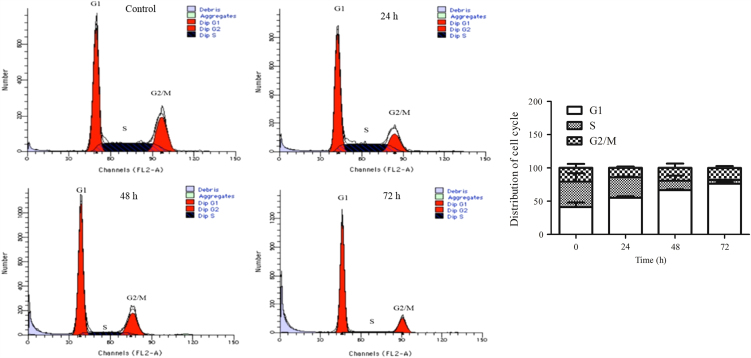
We next used flow cytometry to determine whether the suppression of proliferation was due to cell cycle arrest in HCT116 cells. After treatment with DZNep for 24 h, the cellular ratio in G1 phase increased from 41.09% to 55.14%, and the ratio in S phase decreased from 38.24% to 30.73% (Fig. 2). Higher ratio of G1 phase was seen upon DZNep treatment for 48 h and 72 h (Fig. 2). This suggests that DZNep induces G1 cell cycle arrest in HCT116 cells.
Figure 2.
DZNep induced cell cycle arrest in HCT116 cells. The cells were treated with 5 μmol/L DZNep for the indicated time before being subjected to PI staining and FACS analysis as described in Section 2.
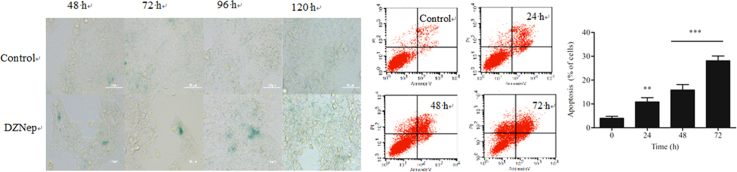
3.3. DZNep induces cellular senescence in HCT116 cells
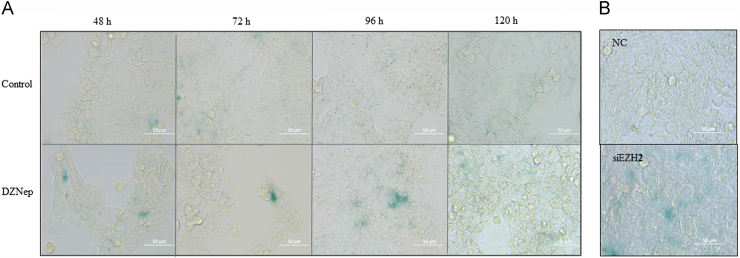
Inhibition of EZH2 is suggested to induce cancer cell senescence15. We thus checked whether DZNep was able to induce cell senescence in HCT116 cells as well. After the cells were treated with DZNep for 48 h, 72 h and 96 h, we used SA-β-gal staining to observe the senescence-like phenotype, and found that many cells became positively stained upon DZNep exposure (Fig. 3A). This suggests that DZNep induces cellular senescence simultaneously under these conditions. Similarly, senescence was observed upon EZH2 siRNA treatment for 72 h (Fig. 3B).
Figure 3.
Inhibition the expression of EZH2 induced cellular senescence in HCT116 cells. The cells were treated with 5 μmol/L DZNep (A) or with siRNA (B) for the indicated time, and senescence was analyzed with SA-β-gal staining and photographed (40×). Scale bar: 50 μm.
3.4. DZNep regulates cell cycle and senescence related proteins expression in HCT116 cells
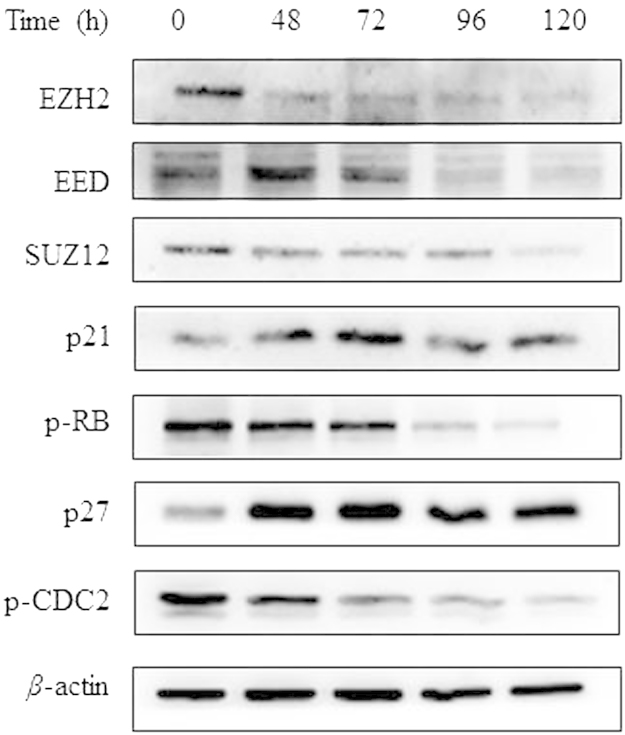
We further investigated the possible molecular mechanisms involved. The expression levels of PRC2 proteins (EZH2, EED and SUZ12) were confirmed to be suppressed after treatment with DZNep for 48 h, 72 h, 96 h and 120 h, as shown by immunoblot analysis in HCT116 cells (Fig. 4). Cell cycle and senescence regulatory proteins were investigated to clarify the underlying mechanism16, and a significant increase of p21 and decrease of p-RB protein expression were found upon exposure to the agent. In addition, we examined the expression of p27 and p-CDC2, which are cell cycle regulators as well17, and found that DZNep increased p27 and decreased p-CDC2 expression in a time-dependent manner (Fig. 4).
Figure 4.
Relative protein expression levels affected by DZNep in HCT116 cells. The cells were treated with 5 μmol/L DZNep for 72 h before being subjected to immunoblot assay as described in Section 2.
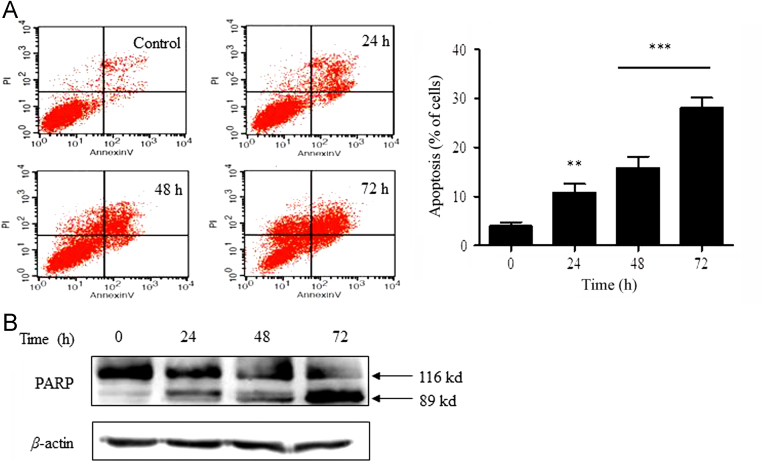
3.5. DZNep induces apoptosis in HCT116 cells
We also asked whether apoptosis is involved. Flow cytometry analysis using Annexin V/PI demonstrated that the apoptotic fraction of HCT116 cells was increased in a time-dependent manner following treatment with DZNep (Fig. 5A). We also analyzed PARP cleavage, a well-known molecular event indicating apoptosis induction18, and found a significant increase of PARP cleavage upon treatment with 5 μmol/L DZNep for 24 h, 48 h and 72 h (Fig. 5B). This indicates that DZNep induces apoptosis as well.
Figure 5.
DZNep induced apoptosis in HCT116 cells. (A) Apoptosis induced by DZNep was detected by AnnexinV/PI staining. The cells were seeded in 6-well plates and treated with 5 μmol/L DZNep for the indicated time, and then subjected to FACS analysis to determine the percentage of early apoptosis cells (lower right quadrant) and late apoptosis cells (upper right quadrant) (**P<0.01, ***P<0.001 vs. control). (B) PARP cleavage analysis was performed in HCT116 cells following treatment with DZNep (5 μmol/L) as indicated. β-actin was used as loading control.
4. Discussion
EZH2-targeted therapy has become an effective strategy for cancer treatment. For example, blocking the expression of EZH2 by siRNA leads to G1/S cell cycle arrest and growth inhibition in colon cancer19. Moreover, decreased EZH2 expression is found to be able to increase the sensitivity to TRAIL-mediated apoptosis in human colon cancer HT29 cells20. In the present work, we provide first evidence to show that inhibition of EZH2 by DZNep can directly induce cell senescence, along with apoptosis, in human colon cancer HCT116 cells.
Cellular senescence of HCT116 cells induced by DZNep is demonstrated by G1 phase arrest and an increased number of cells with positive SA-β-gal staining, which is one of most commonly used senescence markers. We found that the cell cycle arrest caused by DZNep can be addressed by the increase of p21 expression and decrease of p-RB expression. Moreover, p27 and p-CDC2, which blocks cell cycle progression at the G1 transition and promotes G1 and G2/M transition17, 21, respectively, are found to be separately up-regulated and down-regulated as well. In line with our data, silencing of EZH2 is reported to be linked to the increase of p27 expression in pancreatic cancer cells and HCT116 cells11, 22. We also checked the expression of p16, another molecular marker associated with senescence and cell cycle, but did not find any significant change in the protein level (data not shown).
In our work, we also found that DZNep can dose- and time-dependently induce colon cancer cell apoptosis, as confirmed by FACS analysis and PARP cleavage (Fig. 5). Accordingly, DZNep-induced apoptosis has been described in osteosarcoma U2OS and breast cancer MCF7 cells23, 24. More importantly, DZNep at a similar dose range was found to induce apoptosis in human colon cancer stem cells as well25. Therefore, combined mechanisms are involved in the anti-cancer effects of DZNep on colon cancer. A study by Wu et al.23 has shown that DZNep induces apoptosis and G1 cell cycle arrest in response to DNA damage in both p53-proficient and p53-deleted HCT116 cells, resulting in induction of FBXO32, a decrease of p21 and an increase of PARP cleavage. This seems to be inconsistent with our data which suggests an increase of p21 upon DZNep exposure. The discrepancy in p21 regulation may rely on the different stresses imposed on the cells.
Taken together, our results demonstrate that DZNep inhibits the growth and survival of colon cancer HCT116 cells by inducing cellular senescence and apoptosis. The study also provides first evidence showing the direct correlation between inhibition of EZH2 and cellular senescence in colon cancer cells.
Acknowledgments
This project is co-sponsored by Sino-Singapore Collaboration Project from the Ministry of Science and Technology (MOST) of China (No. 2013DFG32990), National Natural Science Foundation of China (NSFC Nos. 81373438 and 31201040), and National Mega-Project for Innovative Drugs by MOST (No. 2012ZX09301002-001-015).
Footnotes
Peer review under responsibility of Institute of Materia Medica, Chinese Academy of Medical Sciences and Chinese Pharmaceutical Association.
Contributor Information
Xiaojian Wu, Email: wxjmqy2003@yahoo.com.
Zhen Wang, Email: wangzhen@imb.pumc.edu.cn.
References
- 1.Siegel R., Desantis C., Jemal A. Colorectal cancer statistics. CA Cancer J Clin. 2014;64:104–117. doi: 10.3322/caac.21220. [DOI] [PubMed] [Google Scholar]
- 2.Li L., Ma B.B. Colorectal cancer in Chinese patients: current and emerging treatment options. Onco Targets Ther. 2014;7:1817–1828. doi: 10.2147/OTT.S48409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bardhan K., Liu K. Epigenetics and colorectal cancer pathogenesis. Cancers. 2013;5:676–713. doi: 10.3390/cancers5020676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tsang D.P., Cheng A.S. Epigenetic regulation of signaling pathways in cancer: role of the histone methyltransferase EZH2. J Gastroenterol Hepatol. 2011;26:19–27. doi: 10.1111/j.1440-1746.2010.06447.x. [DOI] [PubMed] [Google Scholar]
- 5.Tonini T., D׳Andrilli G., Fucito A., Gaspa L., Bagella L. Importance of Ezh2 polycomb protein in tumorigenesis process interfering with the pathway of growth suppressive key elements. J Cell Physiol. 2008;214:295–300. doi: 10.1002/jcp.21241. [DOI] [PubMed] [Google Scholar]
- 6.Shen L., Cui J., Liang S., Pang Y., Liu P. Update of research on the role of EZH2 in cancer progression. Onco Targets Ther. 2013;6:321–324. doi: 10.2147/OTT.S42453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jung H.Y., Jun S., Lee M., Kim H.C., Wang X., Ji H. PAF and EZH2 induce Wnt/β-catenin signaling hyperactivation. Mol Cell. 2013;52:193–205. doi: 10.1016/j.molcel.2013.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kikuchi J., Takashina T., Kinoshita I., Kikuchi E., Shimizu Y., Sakakibara-Konishi J. Epigenetic therapy with 3-deazaneplanocin A, an inhibitor of the histone methyltransferase EZH2, inhibits growth of non-small cell lung cancer cells. Lung Cancer. 2012;78:138–143. doi: 10.1016/j.lungcan.2012.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Crea F., Fornaro L., Bocci G., Sun L., Farrar W.L., Falcone A. EZH2 inhibition: targeting the crossroad of tumor invasion and angiogenesis. Cancer Metastasis Rev. 2012;31:753–761. doi: 10.1007/s10555-012-9387-3. [DOI] [PubMed] [Google Scholar]
- 10.Mosieniak G., Adamowicz M., Alster O., Jaskowiak H., Szczepankiewicz A.A., Wilczynski G.M. Curcumin induces permanent growth arrest of human colon cancer cells: link between senescence and autophagy. Mech Ageing Dev. 2012;133:444–455. doi: 10.1016/j.mad.2012.05.004. [DOI] [PubMed] [Google Scholar]
- 11.Ougolkov A.V., Bilim V.N., Billadeau D.D. Regulation of pancreatic tumor cell proliferation and chemoresistance by the histone methyltransferase enhancer of zeste homologue 2. Clin Cancer Res. 2008;14:6790–6796. doi: 10.1158/1078-0432.CCR-08-1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bai J., Chen J., Ma M., Cai M., Xu F., Wang G. Inhibiting enhancer of zeste homolog 2 promotes cellular senescence in gastric cancer cells SGC-7901 by activation of p21 and p16. DNA Cell Biol. 2014;33:337–344. doi: 10.1089/dna.2014.2340. [DOI] [PubMed] [Google Scholar]
- 13.Luo Z., Yu G., Lee H.W., Li L., Wang L., Yang D. The Nedd8-activating enzyme inhibitor MLN4924 induces autophagy and apoptosis to suppress liver cancer cell growth. Cancer Res. 2012;72:3360–3371. doi: 10.1158/0008-5472.CAN-12-0388. [DOI] [PubMed] [Google Scholar]
- 14.Wang Y., Zhan Y., Xu R., Shao R., Jiang J., Wang Z. Src mediates extracellular signal-regulated kinase 1/2 activation and autophagic cell death induced by cardiac glycosides in human non-small cell lung cancer cell lines. Mol Carcinog. 2014 doi: 10.1002/mc.22147. [DOI] [PubMed] [Google Scholar]
- 15.Bai J., Ma M., Cai M., Xu F., Chen J., Wang G. Inhibition enhancer of zeste homologue 2 promotes senescence and apoptosis induced by doxorubicin in p53 mutant gastric cancer cells. Cell Prolif. 2014;47:211–218. doi: 10.1111/cpr.12103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Choi H.J., Lim do Y., Park J.H. Induction of G1 and G2/M cell cycle arrests by the dietary compound 3,3′-diindolylmethane in HT-29 human colon cancer cells. BMC Gastroenterol. 2009;9:39. doi: 10.1186/1471-230X-9-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chu I.M., Hengst L., Slingerland J.M. The Cdk inhibitor p27 in human cancer: prognostic potential and relevance to anticancer therapy. Nat Rev Cancer. 2008;8:253–267. doi: 10.1038/nrc2347. [DOI] [PubMed] [Google Scholar]
- 18.Kumari A., Iwasaki T., Pyndiah S., Cassimere E.K., Palani C.D., Sakamuro D. Regulation of E2F1-induced apoptosis by poly(ADP-ribosyl)ation. Cell Death Differ. 2015;22:311–322. doi: 10.1038/cdd.2014.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fussbroich B., Wagener N., Macher-Goeppinger S., Benner A., Falth M., Sultmann H. EZH2 depletion blocks the proliferation of colon cancer cells. PLoS One. 2011;6:e21651. doi: 10.1371/journal.pone.0021651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Benoit Y.D., Laursen K.B., Witherspoon M.S., Lipkin S.M., Gudas L.J. Inhibition of PRC2 histone methyltransferase activity increases TRAIL-mediated apoptosis sensitivity in human colon cancer cells. J Cell Physiol. 2013;228:764–772. doi: 10.1002/jcp.24224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ferraro A., Mourtzoukou D., Kosmidou V., Avlonitis S., Kontogeorgos G., Zografos G. EZH2 is regulated by ERK/AKT and targets integrin α2 gene to control epithelial–mesenchymal transition and anoikis in colon cancer cells. Int J Biochem Cell Biol. 2013;45:243–254. doi: 10.1016/j.biocel.2012.10.009. [DOI] [PubMed] [Google Scholar]
- 22.Ishikawa S., Hayashi H., Kinoshita K., Abe M., Kuroki H., Tokunaga R. Statins inhibit tumor progression via an enhancer of zeste homolog 2-mediated epigenetic alteration in colorectal cancer. Int J Cancer. 2014;135:2528–2536. doi: 10.1002/ijc.28672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wu Z., Lee S.T., Qiao Y., Li Z., Lee P.L., Lee Y.J. Polycomb protein EZH2 regulates cancer cell fate decision in response to DNA damage. Cell Death Differ. 2011;18:1771–1779. doi: 10.1038/cdd.2011.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tan J., Yang X., Zhuang L., Jiang X., Chen W., Lee P.L. Pharmacologic disruption of polycomb-repressive complex 2-mediated gene repression selectively induces apoptosis in cancer cells. Genes Dev. 2007;21:1050–1063. doi: 10.1101/gad.1524107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Benoit Y.D., Witherspoon M.S., Laursen K.B., Guezguez A., Beausejour M., Beaulieu J.F. Pharmacological inhibition of polycomb repressive complex-2 activity induces apoptosis in human colon cancer stem cells. Exp Cell Res. 2013;319:1463–1470. doi: 10.1016/j.yexcr.2013.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]