Abstract
Ginsenoside Rg1 (GR), a major bioactive compound of traditional Chinese medicine, such as Panax ginseng or Radix Notoginseng, has been shown to exert neuroprotective effects against ischemic stroke. However, pharmacokinetic studies have suggested that GR could not be efficiently transported through the blood–brain barrier. The mechanism by which GR attenuates cerebral ischemic injury in vivo remains largely unknown. Therefore, this study explored potential neuro-protective effects of GR through its systemic metabolic regulating mechanism by using mass spectrometry–based metabolomic profiling. Rats with middle cerebral artery occlusion (MCAO) were treated with GR intravenously. Their metabolic profiles in serum were measured by gas chromatography coupled with mass spectrometry on 1 and 3 days after MCAO. GR exhibited a potent neuro-protective effect by significantly decreasing the neurological scores and infarct volume in the MCAO rats. Moreover, 18 differential metabolites were tentatively identified, all of which appeared to correlate well with these disease indices. Our findings suggested that GR carries a therapeutic potential in stroke possibly through a feed-back mechanism by regulating systematic metabolic mediation.
KEY WORDS: Ginsenoside Rg1, MCAO, Metabolites, Biomarkers
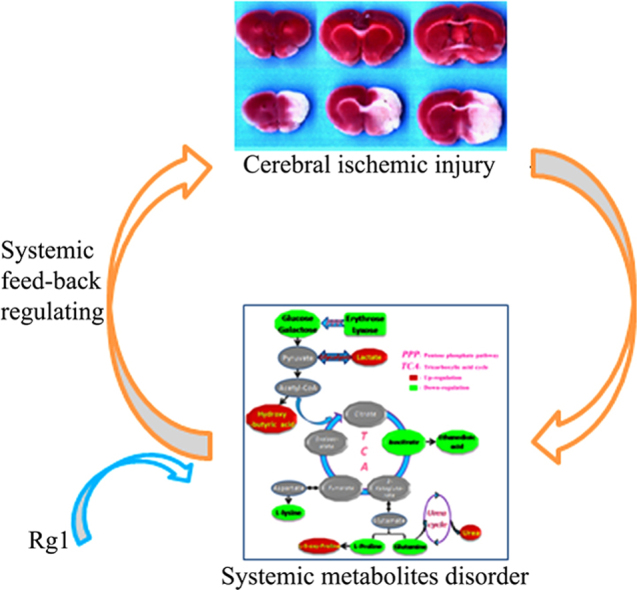
Graphical abstract
The paper demonstrates that the systemic metabolites was significantly differentiated by cerebral ischemic injury, and Rg1 exhibited its neuro-protective effects by a potential systemic feed-back regulating mechanisms.
1. Introduction
Ischemic stroke, a complex devastating disease, is caused by a drastic disruption of cerebral blood flow that triggers a multistep pathophysiological ischemic cascade sufficient to cause metabolic disorder or functional deficit. Benchmark studies have reported quite a few neuro-protective agents with moderate efficacy in stroke management. However, the treatment of stroke remains considerably unsatisfactory as the performance of almost all neuro-protective agents in the clinical treatment of patients with stroke had been disappointing1, 2, 3. Therefore, new pharmacological strategies for stroke management are needed to improve its outcome and elucidate the physiological mechanism of this disease.
Ginsenoside Rg1 (GR; Fig. 1), a panaxatriol saponin, is the major active compound in Panax ginseng and Radix Notoginseng4, which are two distinguished herbs that have been used for thousands of years in clinical treatment in China. Research has shown that GR exerts neurotrophic and neuro-protective effects on ischemic stroke5, and its possible mechanism has been attributed to its anti-oxidative effects on neurons, anti-edema properties, and regulation of various signaling pathways6, 7. However, pharmacokinetic studies have demonstrated that GR could neither be efficiently transported through the blood-brain barrier nor be efficiently distributed to reach its effective concentration in brain tissue8. Therefore, the mechanism by which GR affects cerebral ischemic injury in vivo remains largely unknown and warrants further investigation.
Figure 1.
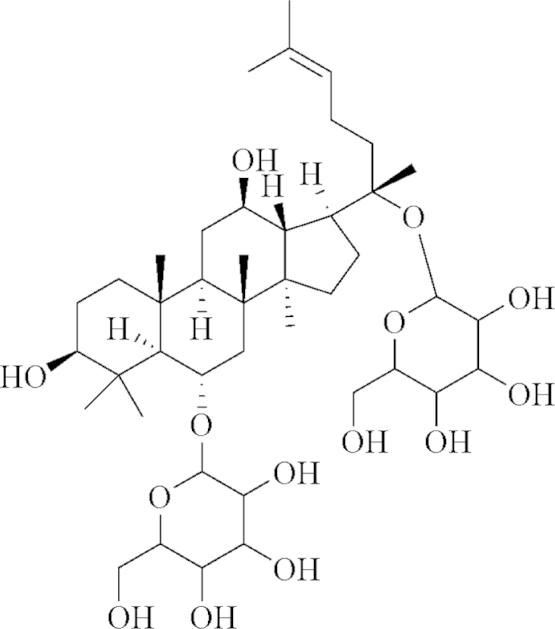
Chemical structure of GR.
Metabolomics has been used for identifying potential diagnostic biomarkers, and elucidating mechanism for complex disease and toxicity associated with the treatment9, 10, 11. Metabolomics can comprehensively measure the metabolites present in the circulation, which could reflect the balance between blood and the internal environment of brain tissue. Yang et al.12 reported that their comprehensive analysis of a wide range of metabolites in various physiological states apparently showed promising perspectives in complex disease, whereas Jiang et al.13 suggested that metabolomics might effectively aid in the diagnosis of ischemic stroke and prognostic estimation.
We hypothesized that there is a systemic feed-back regulating mechanism, through which GR indirectly exerts its effects on neurons. The results of this study further explain the neuro-protective mechanism of GR on cerebral ischemic injury. In addition, with the help of potential systemic metabolites in the circulation screened and identified using gas chromatography coupled with mass spectrometry (GC-MS) based on a metabolomic approach, the present study was also to explore potential nontargeted biomarkers for the diagnosis of ischemic stroke.
2. Materials and methods
2.1. Animal care
The experimental protocol was approved by the Ethics Committee for Animal Experimentation of Capital Medical University (Beijing, China) and conformed to internationally accepted standards. Sixty male Sprague-Dawley rats (280–320 g) were supplied by the Vital River Laboratories (Beijing, China) and housed under controlled conditions with a 12-h light/12-h dark photoperiod, at 21±2 °C, and with a relative humidity of 60%±5% for at least 1 week before the experiment. They were allowed free access to a standard rodent diet and tap water.
2.2. Chemicals and reagents
GR was purchased from Ronghe Technology Co., Ltd. (≥98% purity, Shanghai, China). N-methyl-N-(trimethylsilyl)-trifluoroacetamide and trimethylchlorosilane were purchased from Sigma-Aldrich (St. Louis, USA). Ribitol (internal standard) was purchased from Sinopharm Chemical Reagent Corporation (Shanghai, China), and 2,3,5-triphenyltetrazolium chloride was obtained from Sigma-Aldrich.
2.3. Transient focal cerebral ischemia
Middle cerebral artery occlusion (MCAO) was induced using the intraluminal suture method14, 15. Briefly, rats were anesthetized using chloral hydrate (400 mg/kg); a 2.0 cm skin incision was made in the ventral neck muscles. A 3–0 MCAO monofilament (Beijing Sunbio Biotech Co., Ltd.) was advanced to block the origin of the MCA. Regional cerebral blood flow (rCBF) was monitored by a laser Doppler computerized main unit (Perimed AB, Sweden). The MCAO was set up with rCBF sharp decreasing to 20% of baseline level before ischemia; otherwise, animals were excluded. Reperfusion was performed by withdrawing the monofilament after 120 min ischemia, and then wounds were closed. During the surgery and reperfusion, rectal temperature was maintained at 37±0.5 °C by means of a heating blanket. The mortality of the MCAO model is around 15%. A similar procedure without MCAO was conducted in control rats.
2.4. Animal treatments
After MCAO, rats were randomly divided into the following groups: sham operation (control); MCAO treated with water (model); MCAO treated with 30 mg/kg GR per day (GR 30 mg/kg); and MCAO treated with 60 mg/kg GR per day (GR 60 mg/kg). After reperfusion for 2 h, rats were treated with GR intravenously twice daily for 3 days. The control and model groups were treated with an equal volume of saline.
2.5. Neurological function evaluation
The neurological function of the conscious rats was blindly evaluated 1 day and 3 day after MCAO. A five-point scale reported previously16 was carried out to evaluated the neurological deficit scores (score 0, no deficit being observed; score 1, failing to fully extend left forepaw; score 2, circling to the left side; score 3, falling to the left; score 4, with no walking and consciousness being depressed) by an observer who was blind to the group.
2.6. Infarct volume measurement
The animals were euthanized on day 3 after MCAO. Their brains were collected and sliced into seven coronal sections with 2-mm thickness each stained with 2% solution of 2,3,5-triphenyltetrazolium chloride in saline at 37 °C for 30 min14, 15.
2.7. Sample collection and preparation
On day 1 and day 3 after MCAO, the rats were anesthetized by ether inhalation after their treatment. Their blood was collected from orbital venous and centrifuged at 1500×g for 10 min. Serum was stored at −80 °C until use.
Thawed serum samples (100 μL) were spiked with the internal standard (5 μL of 1 mg/mL ribitol) and mixed with acetone (200 μL) by vortexing for 1 min. After incubation on ice for 10 min, the mixture was centrifuged at 4 °C for 10 min at 10,000×g. The supernatant (200 μL) was transferred to a GC vial and evaporated to dryness under vacuum at 35 °C for approximately 4 h in a concentrator (Thermo Speedvac Concentrator, USA). Derivatization was performed using methoxyamine pyridine (35 μL; 15 mg/mL) at 70 °C for 1 h, followed by N-methyl-N-(trimethylsilyl)trifluoroacetamide (35 μL) with 1% trimethylchlorosilane as the catalyst at room temperature (25 °C) for 1 h. Finally, n-heptane (100 μL) was added to dilute the solution after the derivatization, and the supernatant was used for GC-MS analysis.
2.8. GC-MS analysis
The system consisted of an Agilent 7683 Series mass spectrometer (Electron ionization mass spectra were recorded at 70 eV and 2 scans/s. Full-scan mass spectra were acquired from 60 to 600 m/z at the scan rate of five times per second) coupled to an Agilent 6890 gas chromatograph system (Agilent Technologies, Atlanta, USA). One microliter of sample solution was injected in splitless mode to a ZB-5MS column (30 m×250 μm×0.25 μm) with helium as the carrier gas at the flow rate of 0.8 mL/min. The injector temperature was set at 270 °C. The temperature of the ion source was adjusted to 230 °C, whereas that of the quadrupole was set at 150 °C. The initial temperature of the column was maintained at 70 °C for 5 min. It was then increased at the rate of 10 °C/min to 90 °C where it was held for 1 min, at 5 °C/min to 140 °C for 2 min, at 12 °C/min to 182 °C for 2 min, and then at 2 °C/min to 225 °C for 3 min. Subsequently, the temperature was increased to 300 °C at the rate of 15 °C/min and held for 5 min. The peaks of interest were identified by searching the NIST v1.0.0.12 mass spectra library and compared with the peaks of internal standards.
2.9. Statistical analysis
Statistical analysis was performed in SPSS version 11.5 statistical software (SPSS Inc.). Data were expressed as mean±SEM. For a normal distribution, student׳s t test was used to compare between two groups, whereas one-way ANOVA was conducted for comparisons of multiple groups, followed by Fisher׳s least significant difference test; while non-parameter analysis was used for non-normal distribution. Multiple linear regression analysis was performed to determine the relationship between infarct volume and the metabolites during ischemia, and the predicted and observed infarct volumes in the control group were then compared using analysis of covariance. In addition, principal component analysis (PCA) was performed with SIMCA-P 11.5 (Umetrics AB, Umea, Sweden).
3. Results
3.1. Protective effect of GR on cerebral infarction
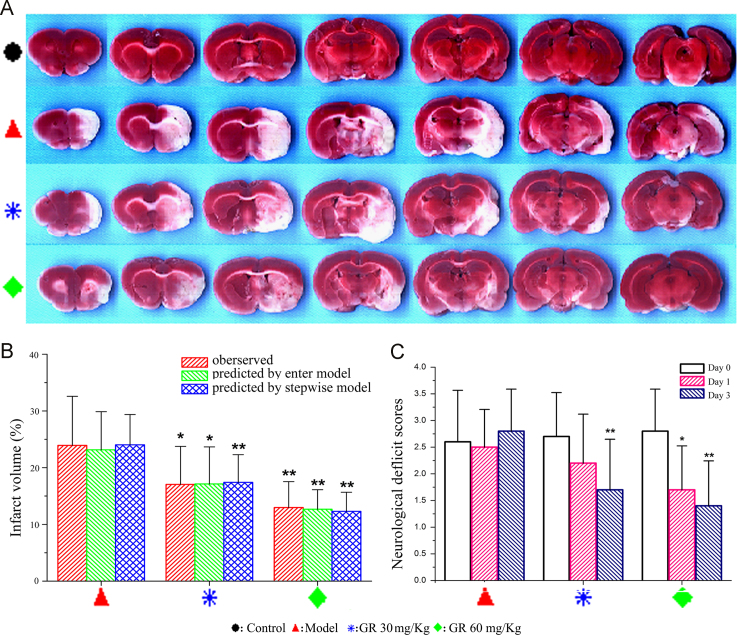
Neurological deficit scores and infarct volume with or without GR treatment were measured to examine the influence of GR on cerebral ischemic injury. The animals were divided into four groups: control, model, GR of 30 mg/kg, and GR of 60 mg/kg. A remarkably decreased pale-colored region was observed in the GR-treated groups compared with the model group (Fig. 2A). The cerebral infarct volumes were significantly reduced after GR treatment compared with model treatment, which was consistent with the results predicted by the enter regression and stepwise models (Fig. 2B). Neurological functional deficits were observed on day 1 and day 3 after MCAO in rats, which could be prevented by GR treatment (Fig. 2C). Compared with the model group, significant reduction in the neurological deficit scores were observed in the GR 60 mg/kg group on day 1, and both GR treatment group on day 3. These results suggested that GR could prevent cerebral ischemic injury with intravenously treatment.
Figure 2.
Protective effect of GR on cerebral ischemic injury. (A) Infarct images observed using 2,3,5-triphenyltetrazolium chloride staining on day 3 after MCAO (n=10). (B) Significant reduction in infarct volume in the GR 30 mg/kg treatment group and 60 mg/kg treatment group compared with the model group consistent with the results predicted by the enter regression and stepwise models. (C) Neurological scores at 24 h after MCAO, the scores of control group was zero, data not shown. ⁎P<0.05; ⁎⁎P<0.01.
3.2. Serum metabolic profiles of control and MCAO rats
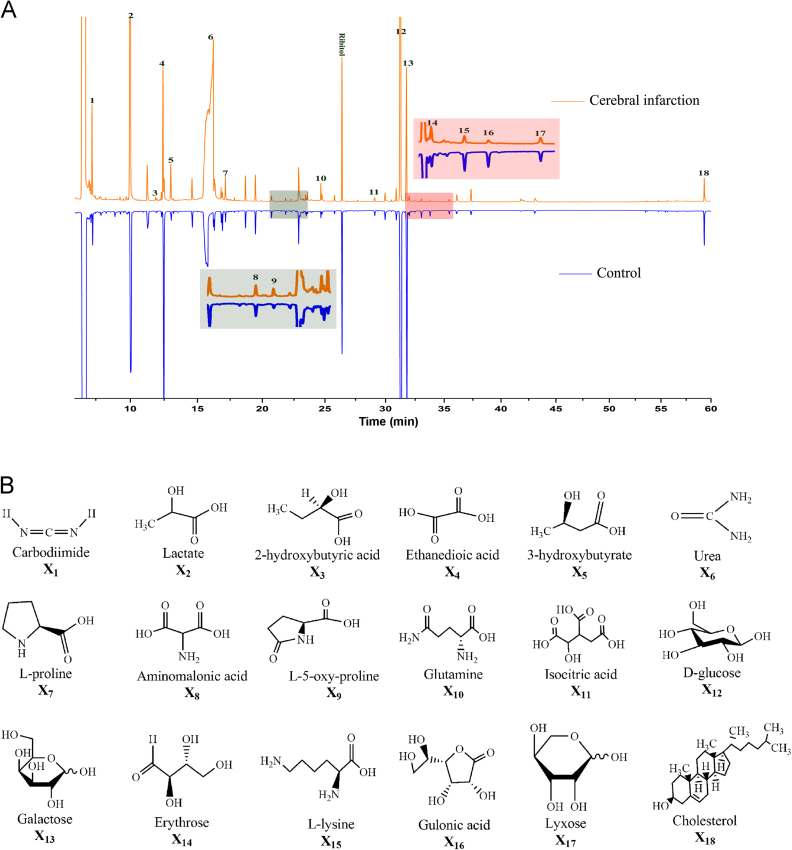
Representative total ion current chromatograms of sera from the control and model (cerebral infarction) groups are shown in Fig. 3A. Forty-five endogenous metabolites in serum were identified by the NIST mass spectra library. According to the peak area ratios of the metabolites to the internal standard (ribitol), 18 metabolites were changed significantly (P<0.05) compared with the control group on day 1 or day 3. The chemical structures of these 18 differential metabolites are shown in Fig. 3B.
Figure 3.
Metabolic profiles in the circulation of MCAO and control rats (n=10). (A) Typical total ion current chromatograms of rat serum from the control and model groups. The numbered peaks represent the metabolites in panel B. (B) Structural information on the differential metabolites identified between the control and model groups.
3.3. Association between differential metabolites expression and cerebral infarct injury
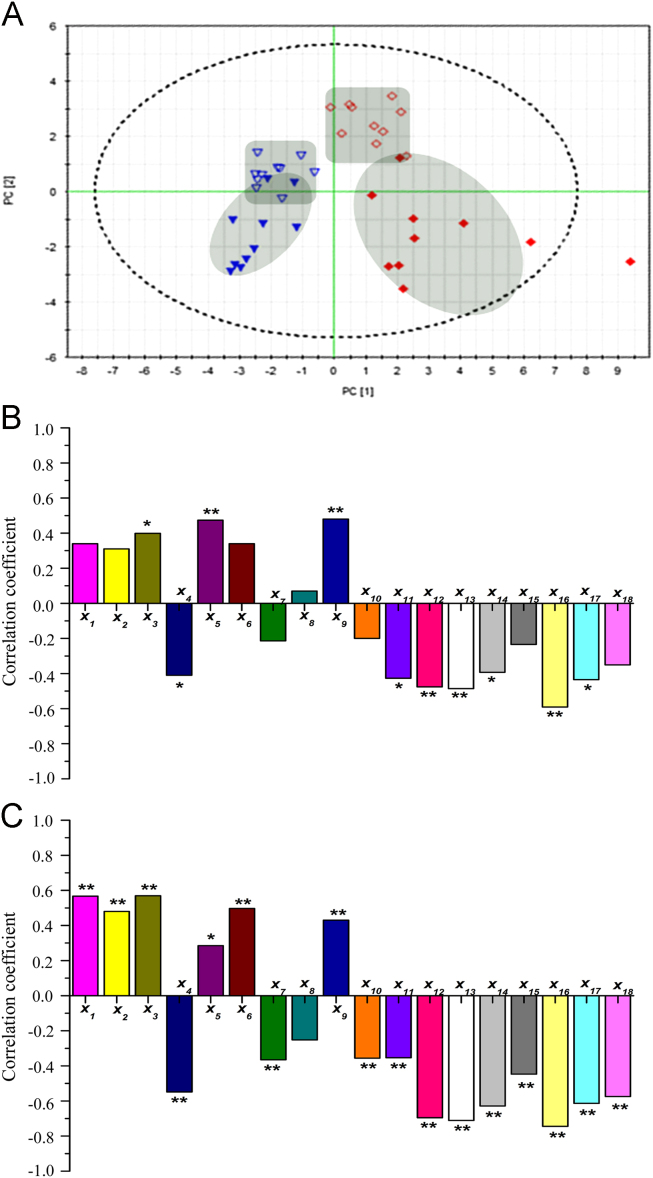
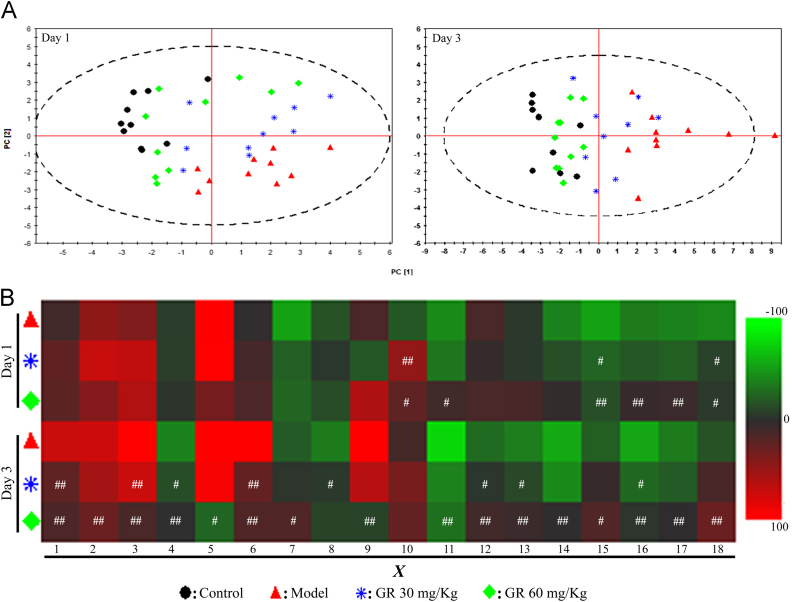
PCA was carried out to evaluate the capability of the GC-MS-based metabolomic approach to differentiate the model group from the control group. The PCA score plots (Fig. 4A) showed marked separation between these groups on days 1 and 3, when time-dependent profile separations could also be observed in the model group.
Figure 4.
Correlation between differential metabolites and cerebral infarct injury (n=10). (A) Score plots of the PCA map for the control group (▼) and model group (♦) on day 1 (open symbols) and day 3 (filled symbols). (B) Pearson׳s correlation coefficients of the 18 differential metabolites and neurological deficit scores on day 1. (C) Pearson׳s correlation coefficients of the 18 differential metabolites and infarct volume on day 3. X1,X2,X3,…,X18 represent the corresponding metabolites identified in Fig. 2. *P<0.05; ⁎⁎P<0.01.
The correlation coefficients between neurological deficit scores (Fig. 4B) and infarct volume (Fig. 4C) with the 18 differential metabolites were listed. Of these metabolites, X3 and X7 were found to positive and negative correlate with neurological scores on day 1, respectively, whereas X6 and X12 were found to positive and negative correlate with infarct volume on day 3, respectively.
3.4. Prediction of infarct volume using metabolic profiling
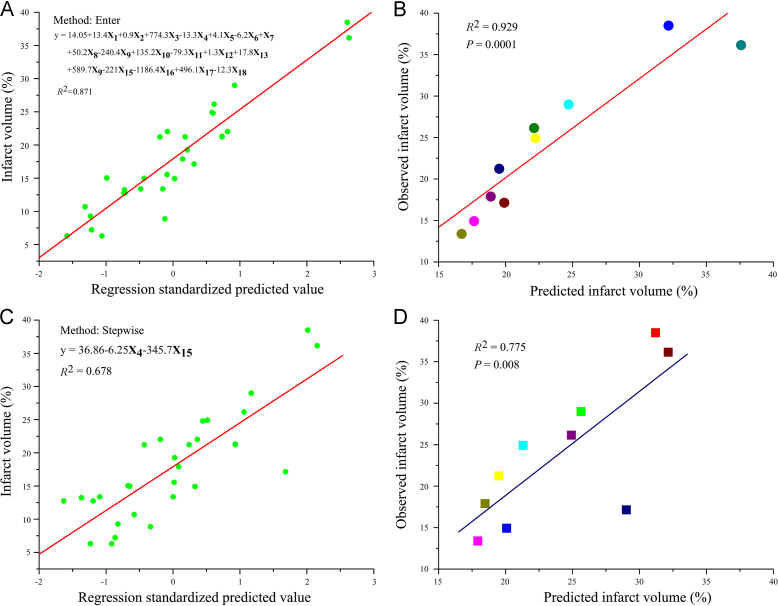
Multiple linear regression analysis was performed to build a prediction model of infarct volume based on the differential metabolites. As shown in Fig. 5A, a linear regression model exhibited a significantly high R2 value (0.871; P<0.05) using the enter selection method with all factors entered. The enter model was further validated by covariance analysis of predicted and observed infarct volumes in MCAO rats (P<0.05) (Fig. 5B), indicating that the infarct volume might be predicted using this model with these metabolites.
Figure 5.
Prediction model for cerebral infarction built based on the differential metabolites using multiple regression analysis. (A) Model built using the enter selection method (n=30). (B) Results of the covariance analysis of predicted and observed infarct volumes in MCAO rats, with the predicted infarct volume calculated using the equation in panel A (n=10). (C) Model built using the stepwise selection method (n=30). (D) Results of the covariance analysis of predicted and observed infarct volumes in MCAO rats, with the predicted infarct volume calculated using the equation in panel C (n=10).
A stepwise regression selection procedure was performed to build a compact model (Fig. 5C) with two factors finally entered (R2=0.678; P<0.05) to avoid high R2 values using the enter procedure due to overfitting with a high number of factors. As shown in Fig. 5D, the model was validated by covariance analysis using the infarct volume in MCAO rats as the test set (P<0.05). These results suggested that metabolite X4 (Ethanedioic acid) and X18 (Cholesterol) are potential major biomarkers for the prediction of infarct volume and that all the metabolites might be systemic nontargeted biomarkers for the prediction of cerebral ischemic injury.
3.5. Intervention of GR in the differential metabolites in the circulation
Differential metabolites, which have been previously confirmed to be correlated with the evolution of ischemic injury, were used to investigate the potential neuro-protective mechanism of GR. Good classifications were observed in the PCA score plots for days 1 and 3 (n=10) (Fig. 6A). The model group was distributed on the bottom right side, well separated from the control group on the top left side. Sample plots from the model+GR-treated groups were moved from the bottom right side to the top left side in a time- and dose-dependent fashion, indicating that the differential metabolites in the circulation after MCAO could be regulated back to normal levels.
Figure 6.
Intervention of GR in the differential metabolites in the circulation after MCAO (n=10). (A) PCA score plots of differential metabolites in serum on days 1 and 3. (B) Heat maps indicating the recovery condition of the differential metabolites after GR treatment on days 1 and 3. The color intensity represents changes in the percentages of relative peak area ratios compared with the control. Green indicates decreased expression, whereas red signifies increased expression. #P<0.05; ##P<0.01, compared with the model group.
As shown in Fig. 6B, changes in the percentages of relative peak area ratios in the control group were calculated and compared with those in the model group to evaluate the recovery condition of each metabolite after GR treatment. With GR 30 mg/kg treatment, X3 and X8 metabolites were significantly regulated back to their normal status on days 1 and 3, respectively. Meanwhile, with GR 60 mg/kg treatment, X6 and X16 metabolites were regulated significantly back on days 1 and 3, respectively.
4. Discussion
Stroke is the leading cause of disability and second major cause of death in China17, 18. However, until now, its treatment remains considerably unsatisfactory. Traditional Chinese medicines have been practiced in stroke prevention and management for thousands of years19. GR, an active constituent of protopanaxatriol found in traditional Chinese medicine agents, such as Ginseng and Radix Notoginseng, is known to have potent neuro-protective effects against brain injury20. Most experimental studies have focused on neurons as neuro-protective targets of GR5, 21, 22. However, research has also demonstrated that GR is poorly distributed to the brain because it could not be efficiently transported through the blood-brain barrier and reach the effective concentration in brain tissue8. Accordingly, whether GR could be effectively distributed into brain tissue and directly carry out potent neuro-protective effects on neurons remains unclear. We thus hypothesized that the neuro-protective effects of GR could be attributed to a potential systemic regulating mechanism. Following cerebral infarction, the microenvironment of blood circulation is disturbed by central nervous system disorders, which might react by affecting the injury course of brain tissue. Then small molecule metabolites transported through the blood-brain barrier, the degradation of the microenvironment of the brain enhances cerebral tissue damage, which showed that the metabolites were significantly correlated with cerebral damages23.
It has been shown that many small molecule metabolites in the circulation system may contribute to cerebral ischemic disorders13. In this study, GC-MS-based metabolomic profiling was performed to screen differential metabolites in the circulation after MCAO. Eighteen metabolites in serum were tentatively identified. With PCA, these metabolites can clearly distinguish MCAO rats from the controls. Meanwhile, the multivariate linear regression model showed that these metabolites significantly correlated with the neurological deficit scores on day 1 and with the infarct volume on day 3, implying that these metabolites correlated with the cerebral injury process. Therefore, these differential metabolites might be a potential systemic biomarker for cerebral ischemic injury prediction and potential systemic targets for cerebral injury regulation.
This study has provided preliminary evidence that GR could protect against cerebral ischemic injury, including the reductions in neurological deficit scores and infarct volume. We found that the differential metabolites in the circulation after MCAO might be regulated back to their normal levels after GR treatment. Furthermore, the infarct volumes predicted by the enter and stepwise prediction models were consistent with the observed values. These findings suggested that the neuro-protective effects of GR are correlated with regulating the imbalance of metabolites to a normal status in the circulation. However, further studies are needed in order to determine whether the changes in serum metabolite levels are mechanistically linked to the neurological changes.
In conclusion, by using an integrated analytical approach based on GC-MS, this study found preliminary data in support of the idea that the neuro-protective effects of GR on cerebral ischemic injury might be attributed to a potential indirect systemic regulating mechanism, associated with anaerobic glycolysis, the tricarboxylic acid cycle, the urea cycle and the amino acid metabolism. The differential metabolites detected in this study might be a potential systemic biomarker for the prediction of cerebral infarct injury. These findings may eventually enhance the current understanding the pharmacological targets and mechanism of GR on cerebral infarction. Further research will be carried out to support the above-described hypothesis.
Acknowledgments
This work was supported by research grants from the National Twelfth-Five Year Research Program of China (No. 2012ZX09301002001002) and the National Natural Science Foundation of China (No. 81473398).
Footnotes
Peer review under responsibility of Institute of Materia Medica, Chinese Academy of Medical Sciences and Chinese Pharmaceutical Association.
References
- 1.Cheng Y.D., Al-Khoury L., Zivin J.A. Neuroprotection for ischemic stroke: two decades of success and failure. NeuroRx. 2004;1:36–45. doi: 10.1602/neurorx.1.1.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Curry S.H. Why have so many drugs with stellar results in laboratory stroke models failed in clinical trials? A theory based on allometric relationships. Ann NY Acad Sci. 2003;993:69–74. doi: 10.1111/j.1749-6632.2003.tb07512.x. [DOI] [PubMed] [Google Scholar]
- 3.Gladstone D.J., Black S.E., Hakim A.M. Toward wisdom from failure: lessons from neuroprotective stroke trials and new therapeutic directions. Stroke: J Cereb Circ. 2002;33:2123–2136. doi: 10.1161/01.str.0000025518.34157.51. [DOI] [PubMed] [Google Scholar]
- 4.Kiefer D., Pantuso T. Panax ginseng. Am Fam Physician. 2003;68:1539–1542. [PubMed] [Google Scholar]
- 5.Kehong Y., Shuxing G., Bingying X., Junling Y., Lanou W. Variation of BDNF mRNA on focol cerebral ischemia reperfusion injury in rats with notogisenoside Rg1. J Chin Med Mater. 2007;30:313–316. [PubMed] [Google Scholar]
- 6.Chen X.C., Zhou Y.C., Chen Y., Zhu Y.G., Fang F., Chen L.M. Ginsenoside Rg1 reduces MPTP-induced substantia nigra neuron loss by suppressing oxidative stress. Acta Pharmacol Sin. 2005;26:56–62. doi: 10.1111/j.1745-7254.2005.00019.x. [DOI] [PubMed] [Google Scholar]
- 7.Ge K.L., Chen W.F., Xie J.X., Wong M.S. Ginsenoside Rg1 protects against 6-OHDA-induced toxicity in MES23.5 cells via AKT and ERK signaling pathways. J Ethnopharmacol. 2010;127:118–123. doi: 10.1016/j.jep.2009.09.038. [DOI] [PubMed] [Google Scholar]
- 8.Wang R., Li Y.N., Wang G.J., Hao H.P., Wu X.L., Zhou F. Neuroprotective effects and brain transport of ginsenoside Rg1. Chin J Nat Med. 2009;7:315–320. [Google Scholar]
- 9.Yao H., Shi P., Zhang L., Fan X., Shao Q., Cheng Y. Untargeted metabolic profiling reveals potential biomarkers in myocardial infarction and its application. Mol Biosyst. 2010;6:1061–1070. doi: 10.1039/b925612a. [DOI] [PubMed] [Google Scholar]
- 10.Chen M., Ni Y., Duan H., Qiu Y., Guo C., Jiao Y. Mass spectrometry-based metabolic profiling of rat urine associated with general toxicity induced by the multiglycoside of tripterygium wilfordii hook. F. Chem Res Toxicol. 2008;21:288–294. doi: 10.1021/tx7002905. [DOI] [PubMed] [Google Scholar]
- 11.Chen M., Zhao L., Jia W. Metabonomic study on the biochemical profiles of a hydrocortisone-induced animal model. J Proteome Res. 2005;4:2391–2396. doi: 10.1021/pr050158o. [DOI] [PubMed] [Google Scholar]
- 12.Yang J., Xu G., Hong Q., Liebich H.M., Lutz K., Schmulling R.M. Discrimination of type 2 diabetic patients from healthy controls by using metabonomics method based on their serum fatty acid profiles. J Chromatogr B Anal Technol Biomed Life Sci. 2004;813:53–58. doi: 10.1016/j.jchromb.2004.09.023. [DOI] [PubMed] [Google Scholar]
- 13.Jiang Z., Sun J., Liang Q., Cai Y., Li S., Huang Y. A metabonomic approach applied to predict patients with cerebral infarction. Talanta. 2011;84:298–304. doi: 10.1016/j.talanta.2011.01.015. [DOI] [PubMed] [Google Scholar]
- 14.Wang Q., Peng Y., Chen S., Gou X., Hu B., Du J. Pretreatment with electroacupuncture induces rapid tolerance to focal cerebral ischemia through regulation of endocannabinoid system. Stroke: J Cereb Circ. 2009;40:2157–2164. doi: 10.1161/STROKEAHA.108.541490. [DOI] [PubMed] [Google Scholar]
- 15.Sasaki M., Honmou O., Kocsis J.D. A rat middle cerebral artery occlusion model and intravenous cellular delivery. Methods Mol Biol. 2009;549:187–195. doi: 10.1007/978-1-60327-931-4_13. [DOI] [PubMed] [Google Scholar]
- 16.Longa E.Z., Weinstein P.R., Carlson S., Cummins R. Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke: J Cereb Circ. 1989;20:84–91. doi: 10.1161/01.str.20.1.84. [DOI] [PubMed] [Google Scholar]
- 17.Dalal P., Bhattacharjee M., Vairale J., Bhat P. International stroke society–who global stroke initiative: a report on population-based mumbai stroke registry (2005–2006), India. Int J Stroke. 2009;4:239–240. doi: 10.1111/j.1747-4949.2009.00313.x. [DOI] [PubMed] [Google Scholar]
- 18.Bonita R., Mendis S., Truelsen T., Bogousslavsky J., Toole J., Yatsu F. The global stroke initiative. Lancet Neurol. 2004;3:391–393. doi: 10.1016/S1474-4422(04)00800-2. [DOI] [PubMed] [Google Scholar]
- 19.Zheng G.Q., Cheng W., Wang Y., Wang X.M., Zhao S.Z., Zhou Y. Ginseng total saponins enhance neurogenesis after focal cerebral ischemia. J Ethnopharmacol. 2010;133:724–728. doi: 10.1016/j.jep.2010.01.064. [DOI] [PubMed] [Google Scholar]
- 20.He L., Chen X., Zhou M., Zhang D., Yang J., Yang M. Radix/rhizoma notoginseng extract (sanchitongtshu) for ischemic stroke: a randomized controlled study. Phytomedicine. 2010;18:437–442. doi: 10.1016/j.phymed.2010.10.004. [DOI] [PubMed] [Google Scholar]
- 21.Liu Q., Kou J.P., Yu B.Y. Ginsenoside Rg1 protects against hydrogen peroxide-induced cell death in PC12 cells via inhibiting NF-κB activation. Neurochem Int. 2011;58:119–125. doi: 10.1016/j.neuint.2010.11.004. [DOI] [PubMed] [Google Scholar]
- 22.Zhang Y.F., Fan X.J., Li X., Peng L.L., Wang G.H., Ke K.F. Ginsenoside Rg1 protects neurons from hypoxic-ischemic injury possibly by inhibiting Ca2+ influx through NMDA receptors and l-type voltage-dependent Ca2+ channels. Eur J Pharmacol. 2008;586:90–99. doi: 10.1016/j.ejphar.2007.12.037. [DOI] [PubMed] [Google Scholar]
- 23.Berthet C., Lei H., Gruetter R., Hirt L. Early predictive biomarkers for lesion after transient cerebral ischemia. Stroke: J Cereb Circ. 2011;42:799–805. doi: 10.1161/STROKEAHA.110.603647. [DOI] [PubMed] [Google Scholar]