Abstract
Kallistatin, which protects organs and cells against inflammation, fibrosis and oxidative stress, is mainly synthesized and secreted in liver. However, its relationship to human liver disease remains unclear. The purpose of this study was to explore the relationship between serum kallistatin and clinical evidence of both cirrhosis and hepatocellular carcinoma (HCC), and to determine if serum kallistatin levels could be used as a diagnostic indicator of hepatic health status, especially human liver cirrhosis (LC). Our cohort consisted of 115 patients with clinically proven liver fibrosis (LF), LC, or HCC by liver biopsies, and 31 healthy controls (CON). Serum kallistatin levels were quantified by ELISA. Results of the present study demonstrated that irrespective of the underlying etiology, serum kallistatin levels were significantly lower in the LF/LC group when compared with the CON group. A decrease in serum kallistatin levels appeared to reflect the extent of cirrhosis, with the lowest levels associated with higher grades of cirrhosis. Patients with LC had a noticeable correlation between serum kallistatin levels and other serum biochemical indicators. The area under the curve (AUC) for LC, viral liver cirrhosis (VLC) and alcoholic liver cirrhosis (ALC) was 0.845, 0.757 and 0.931, respectively. In conclusion, our findings demonstrated that kallistatin, a plasma protein produced by the liver, can be a useful and reliable diagnostic indicator of hepatic health status, especially for LC.
Abbreviations: ALT, alanine transaminase; ALB, albumin; ALC, alcoholic liver cirrhosis; ALP, alkaline phosphatase; AUC, area under the curve; AST, aspartate aminotransferase; CE, choline esterase; CAP, community-acquired pneumonia; CON, controls; DBIL, direct bilirubin; GGT, gamma-glutamyl transpeptidase; GLB, globulin; HCC, hepatocellular carcinoma; IBIL, indirect bilirubin; KBP, kallikrein-binding protein; LC, liver cirrhosis; LF, liver fibrosis; NASH, non-alcoholic steatohepatitis; PA, prealbumin; STP, serum total protein; TBA, total bile acid; TBIL, total bilirubin; VLC, viral liver cirrhosis
KEY WORDS: Kallistatin, Liver cirrhosis, Biomarker, Liver fibrosis, Hepatocellular carcinoma
Graphical abstract
Kallistatin is mainly synthesized and secreted in liver, its average concentration was reduced significantly in patients with LC of different etiologies and can be a useful and reliable diagnostic indicator of hepatic health status, especially for LC.
1. Introduction
Liver cirrhosis (LC), characterized by the histological development of regenerative nodules surrounded by fibrous bands, is the most frequent long term consequence of all chronic liver diseases. Cirrhosis is often asymptomatic and unsuspected until complications emerge (e.g. liver failure, portal hypertension, ascites, hepatic encephalopathy, variceal hemorrhage, hepatocellular carcinoma (HCC)). The annual incidence rates of liver failure (decompensation), HCC, and death in patients with cirrhosis associated with chronic hepatitis B or C infection are approximately 4%, 3% and 3%, respectively1. The majority of patients with LC die from life-threatening complications, which occurred relatively early in the course of the disease. Thus, the early diagnosis of LC is important for patients with chronic liver disease.
The definitive diagnosis of cirrhosis relies on the histological examination of liver tissue. The use of liver biopsies in clinical practice, however, has several limitations. In particular, sample errors are a significant problem, with an estimated mean of 24% of false negatives being reported in series of blind liver biopsies2. Therefore routine clinical investigation of liver function employs serum analysis of indicators of liver dysfunction, such as increases in alanine transaminase (ALT) and aspartate aminotransferase (AST) amongst others. The complexity of LC and the influence of a multitude of genetic and environmental factors on the course of disease progression and response to therapy suggest the need to extend the battery of serum based functional assays that could add to the sensitivity and accuracy of currently employed diagnostic and prognostic markers.
Kallistatin, a tissue-kallikrein, has vasodilatory, anti-angiogenic, anti-inflammatory, anti-tumor and anti-oxidant effects3, 4, 5, 6. It is present in many human tissues, including the eye, kidney, liver, heart, arteries and veins, atheroma, blood cells and body fluids7, 8. As several studies have shown that the liver represents the major site of synthesis and secretion of kallistatin7, 9, we therefore hypothesized that serum kallistatin levels could be a potential biomarker for liver cirrhosis. We have explored the relationship between serum kallistatin and clinical evidence of both human cirrhosis and HCC in this study.
2. Participants and methods
2.1. Participants
Serum samples were collected at the Fujian Strait Hospital from 12 patients with LF, 93 with LC and 10 with HCC (without LC) proven by liver biopsies (Figs. S1 and S2 in Supporting information). LC patients had diverse etiologies: 41 patients were diagnosed with hepatitis B or C virus infection, 7 with alcoholic liver cirrhosis (ALC), and 45 had liver cirrhosis of uncertain cause. Eighteen of the patients with viral liver cirrhosis (VLC) (the cause of LC came from HBV infection) received different anti-cirrhotic drugs (Lamivudine, Sebivo or Adefovir dipivoxil, respectively) and had their serum samples re-collected 30 days after the treatment. Thirty healthy volunteers served as controls. The study was approved by the local ethics committee (Quanzhou, No. 201001) and written informed consent was obtained from all patients involved.
2.2. Serum separation
Serum was collected from clotted blood using serum separator tubes centrifuged at 4000 rpm for 10 min at 4 °C. The serum was snap-frozen and stored at −80 °C until required. All serum samples were thawed only once.
2.3. Determination of serum kallistatin
Kallistatin levels were quantified by ELISA (R&D Systems, Inc. Minneapolis, USA) as previously described10. Briefly, standards and clinical samples were added in duplicate at appropriate dilutions into 96-well microtiter plate coated with capture antibodies.
2.4. Determination of other serum biochemical indicators
The clinical serum samples were also used to measure: serum prealbumin (PA), serum total protein (STP), albumin (ALB), albumin/globulin ratio (ALB/GLB), choline esterase (CE), globulin (GLB), total bilirubin (TBIL), direct bilirubin (DBIL), indirect bilirubin (IBIL), ALT, AST, gamma-glutamyl transpeptidase (GGT), alkaline phosphatase (ALP) and total bile acid (TBA). All of the parameters were measured using a conventional automated analyzer (Beckman Au680).
2.5. Statistical analysis
Data were expressed as mean±SD. All statistical analyses were performed using SPSS 16.0 software. The t-test was used for univariate comparisons between patients and the CON group with P<0.05 considered significant. The Pearson correlation test, Spearman׳s rank correlation test and Logistic regression analysis were used to determine associations between variables. Receiver operating characteristic (ROC) curve analyses were performed to determine the diagnostic sensitivity and specificity of parameters. The area under the ROC curve (AUC) was calculated for prediction of patients with LC and the best cut-off values were established for the serum kallistatin levels.
3. Results
3.1. The main features and laboratory characteristics of all subjects
The serum levels of liver function tests, including PA, STP, ALB, GLB, ALT, AST, TBLI, DBIL, IBIL, GGT, ALP and TBA in the LC groups were significantly different from those of the CON groups (Table 1).
Table 1.
Demographic and clinical characteristics of the study population with LF, LC and HCC.
| CON |
LF |
LC |
HCC |
|
|---|---|---|---|---|
| Parameter | (n=31) | (n=12) | (n=93) | (n=10) |
| Age (years) | 35.10±10.40 | 41.58±12.75 | 52.29±11.98 | 59.40±10.84 |
| Gender (male; female) | 8;23 | 9;3 | 77;16 | 7;3 |
| Kallistatin (μg/mL) | 29.03±8.07 | 22.77±5.02 | 16.78±8.65 | 25.05±7.20 |
| Prealbumin (g/L) | 74.29±3.18 | 24.89±5.89 | 11.67±5.49 | 14.58±5.61 |
| STP (g/L) | 43.67±2.24 | 71.71±6.28 | 67.33±9.92 | 70.00±6.74 |
| Albumin (g/L) | 30.05±3.13 | 44.46±3.37 | 33.52±7.18 | 37.75±5.40 |
| Globulin (g/L) | 21.54±2.82 | 27.25±4.96 | 33.80±8.02 | 32.25±4.81 |
| Albumin/Globulin | 1.45±0.12 | 1.67±0.27 | 1.05±0.33 | 1.20±0.29 |
| ALT (U/L) | 24.81±14.21 | 99.89±146.17 | 48.97±69.74 | 98.76±156.35 |
| AST (U/L) | 18.87±5.49 | 48.76±44.62 | 64.27±120.38 | 76.76±94.59 |
| AST/ALT | 0.93±0.43 | 0.77±0.34 | 1.34±0.52 | 1.08±0.57 |
| Choline esterase (KU/L) | 8.38±2.2 | 8.19±1.94 | 6.38±3.22 | 8.14±4.03 |
| Total bilirubin (μmol/L) | 13.86±5.45 | 15.90±5.65 | 48.64±63.13 | 32.76±48.84 |
| Direct bilirubin (μmol/L) | 5.03±2.17 | 4.09±2.07 | 20.95±34.73 | 16.29±26.34 |
| Indirect bilirubin (μmol/L) | 10.34±6.82 | 11.81±3.99 | 27.36±31.37 | 21.01±21.58 |
| GGT (U/L) | 25.13±13.84 | 160±424.66 | 71.44±83.45 | 169.33±284.42 |
| ALP (U/L) | 90.42±32.71 | 121.50±169.57 | 135.10±70.82 | 307.90±355.98 |
| TBA (μmol/L) | 6.13±3.85 | 7.96±6.6 | 57.85±73.64 | 34.63±49.82 |
3.2. Serum kallistatin levels in patients with LF/LC
Serum kallistatin levels were significantly lower in the 12 LF patients and 93 LC patients as compared to the CON groups (LF 22.77±5.02 & LC 16.78±8.65 vs. CON 29.03±8.07 μg/mL; P<0.05, P<0.01) (Fig. 1). This trend did not appear to be influenced by etiology: whether the cause of LC was viral infection or excessive alcohol consumption, the serum levels of kallistatin were significantly lower than the CON (VLC 19.84±9.71 & ALC 16.38±3.82 vs. CON 29.03±8.07 μg/mL; P<0.01). Kallistatin levels were similar for the VLC and ALC groups. In addition, various age or sex groups had no significant differences in serum kallistatin levels (data not shown).
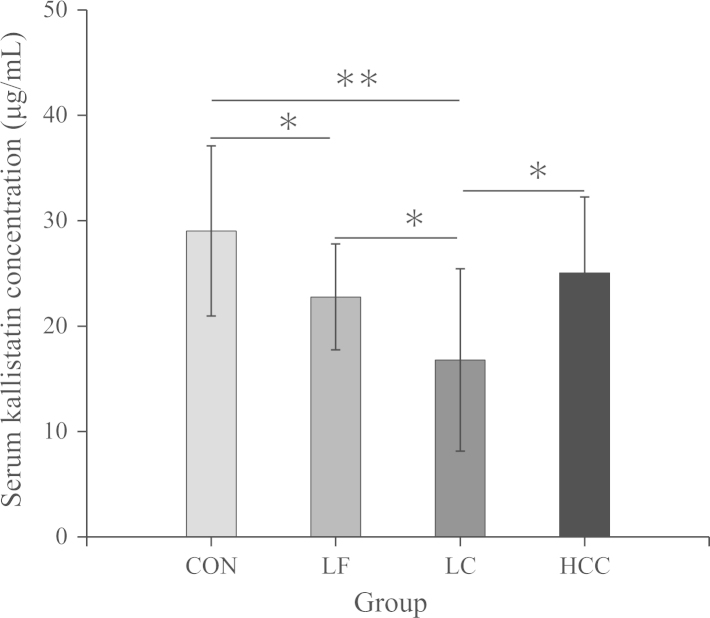
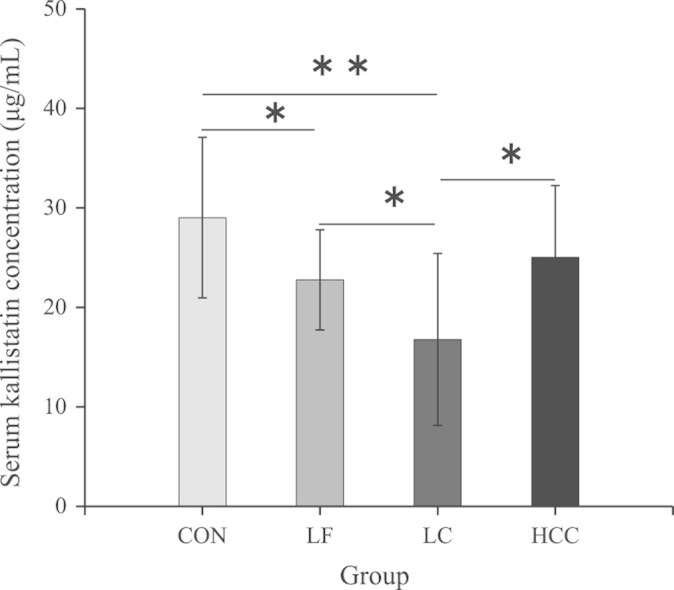
Figure 1.
Mean concentration of serum kallistatin in the patients with LF, LC and HCC. Kallistatin levels were significantly different between LF, LC, HCC and controls (*P<0.05, **P<0.01).
Comparison of the LF and LC groups showed that serum kallistatin levels were further decreased (P<0.05) (Fig. 1). Analysis of kallistatin levels in patients with different severities of LC revealed higher serum kallistatin concentrations in patients with compensated LC compared to patients with decompensated LC (19.98±10.14 vs. 12.25±6.22 μg/mL; P<0.05), suggesting that serum levels of kallistatin may reflect the degree of liver dysfunction.
A follow-up study of 18 patients with VLC who received the anti-cirrhotic treatment (Lamivudine, Sebivo or Adefovir dipivoxil respectively) for 30 days showed elevated kallistatin levels after the treatment (13.67±7.81 prior to vs. 17.09±6.89 µg/mL following the treatment).
3.3. Serum kallistatin levels in patients with HCC
HCC groups had lower serum kallistatin levels than CON groups (25.05±7.20 vs. 29.03±8.06 μg/mL), but higher than the LC groups (P<0.05) (Fig. 1). These results suggest that decreases in serum kallistatin levels are not unique to patients with LC, but may reflect different etiologies of liver disease.
3.4. Relationship between kallistatin serum levels and liver function
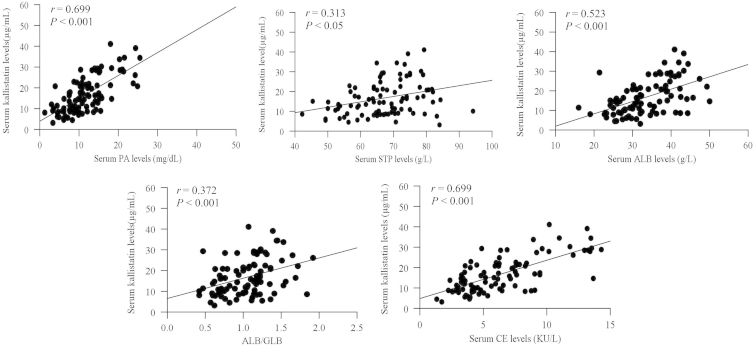
Patients with LC had a noticeable positive correlation between serum kallistatin levels and serum PA, STP, ALB, ALB/GLB and CE. Also, they had a significantly negative correlation between serum kallistatin levels and TBIL, ALT and AST levels, as determined by Pearson and Spearman rank correlation tests (Table 2) and linear regression analysis (Fig. 2).
Table 2.
Pearson correlation (r-value) and spearman rank correlation (ρ) between biochemical indices of liver sufficiency and kallistatin concentration in all cirrhotic patients without HCC.
| Pearson correlation |
Spearman rank correlation |
|||
| Parameter | r | P | ρ | P |
| Prealbumin (g/L) | 0.699 | <0.001 | 0.671 | <0.001 |
| STP (g/L) | 0.523 | <0.001 | 0.536 | 0.005 |
| Albumin (g/L) | 0.313 | 0.002 | 0.326 | <0.001 |
| ALB/GLB | 0.372 | <0.001 | 0.392 | <0.001 |
| Choline esterase (U/L) | 0.699 | <0.001 | 0.645 | <0.001 |
| ALT (U/L) | −0.224 | 0.031 | ||
| AST (U/L) | −0.348 | 0.001 | ||
| Total bilirubin (μmol/L) | −0.313 | 0.002 | ||
| Direct bilirubin (μmol/L) | −0.329 | 0.001 | ||
| Indirect bilirubin (μmol/L) | −0.288 | 0.005 | ||
| TBA (μmol/L) | −0.257 | 0.013 | ||
Figure 2.
Correlation between serum kallistatin levels and other biomarkers in 93 patients with LC. The vertical axis represents serum kallistatin levels in μg/mL and the horizontal axis represents the expression levels of serum PA, STP, ALB, ALB/GLB and CE.
Table 3 presents the association of each characteristic (serum kallistatin levels and other biochemical indicators) with LC (unadjusted odds ratios). It seemed that all the variables except PA and STP were significantly associated with liver cirrhosis. But only ALB and GLB were included in the final model, which was determined by performing multivariate logistic regression with stepwise selection of LC, and significant variables at level of 0.05. It was indicated from the final model that for 1 μg/mL increase in serum kallistatin concentration, the odds of liver cirrhosis decreases by (1−0.682)×100%=31.8% after controlling the effect of all the other covariates.
Table 3.
Logistic regression analysis of liver cirrhosis.
| Variable | Unadjusted | Unadjusted OR |
Final model |
|||
|---|---|---|---|---|---|---|
| 95% CI | P-value | Unadjusted OR | 95% CI | P-value | ||
| PA | 0.72 | 0.325–1.594 | 0.4172 | |||
| STP | 1.00 | 0.993–1.007 | 0.9254 | |||
| ALB | 1.09 | 1.019–1.174 | 0.0133 | 1.36 | 1.072–1.716 | 0.01 |
| GLB | 1.73 | 1.387–2.159 | <0.0001 | 1.95 | 1.272–2.990 | 0.00 |
| ALB/GLB | 0.00 | <0.001–0.033 | <0.0001 | |||
| CE | 1.00 | 1.0001–1.0008 | 0.0027 | |||
| ALT | 1.04 | 1.008–1.071 | 0.0122 | |||
| AST | 1.22 | 1.121–1.328 | <0.0001 | |||
| AST/ALT | 9.82 | 2.777–34.723 | 0.0004 | |||
| TBIL | 1.22 | 1.114–1.334 | <0.0001 | |||
| DBIL | 1.46 | 1.178–1.815 | 0.0006 | |||
| IBIL | 1.19 | 1.092–1.298 | <0.0001 | |||
| GGT | 1.04 | 1.012–1.063 | 0.0037 | |||
| ALP | 1.02 | 1.008–1.035 | 0.0015 | |||
| TBA | 1.19 | 1.080–1.319 | 0.0005 | |||
3.5. Reliability of serum kallistatin level as predictor of LC
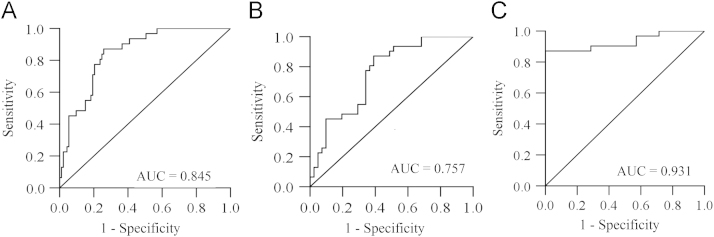
The serum kallistatin level predicted the presence of LC estimated with AUC of 0.845 in 93 patients (Fig. 3A). Based on the ROC curve, an optimal cut-off value for LC diagnosis was set <21.64 μg/mL of kallistatin. Sensitivity (Se), specificity (Sp), positive predictive value (PPV) and negative predictive value (NPV) values were 87.1%, 74.2%, 91% and 62.3%, respectively (Table S1 in supporting information).
Figure 3.
Serum kallstatin levels can accurately diagnose liver disease. A: LC; B: VLC; C: ALC. The AUC is shown for the performance of the serum kallistatin levels for discriminating LC, VLC and ALC from the healthy control groups. The vertical axis represents the sensitivity and the horizontal axis represents the 1-specificity.
3.6. Receiver operating characteristic curves for predicting VLC and ALC
In the cases of discriminating VLC and ALC from the CON patients based on the kallistatin level, the AUCs were 0.757 (P=0.001) and 0.931 (P<0.01) respectively. The results indicate that the predictive value is significantly better for patients with ALC than for the ones with VLC (Fig. 3B, C and Table S1 in Supporting information).
3.7. Disease specificity for LC
Using similar ROC analyses, we found that kallistatin level was not a reliable predictor of HCC, even though the levels in those patients were lower than in the CON groups. Serum kallistatin is therefore a good biomarker for different etiologies of LC with exception of HCC.
4. Discussion
To our knowledge, the present study is the first to demonstrate kallistatin to be a useful potential biomarker for the detection of human LC. The data presented in this study showed that serum kallistatin levels in patients with LC were significantly lower than those in healthy controls, demonstrating a close correlation between the reduction in serum kallistatin levels and severity of early hepatic disease. Serum kallistatin levels could therefore provide an additional biomarker for the detection of LC and progressive loss of liver function along in response to the therapy.
The biology of LC is characterized by a constant stimulus for hepatocellular regeneration by a microenvironment associated with chronic inflammation and tissue fibrosis. Cirrhosis represents the final common pathological outcome for the majority of chronic liver diseases. Previous studies have shown that 30%-40% of non-alcoholic steatohepatitis (NASH) patients have advancing bridging fibrosis or cirrhosis11, 12, 13. Most patients with cirrhosis die from one or more clinical complications including ascites, hepatic encephalopathy and variceal hemorrhage14. Among the 1.4 million liver-disease-related deaths that occur each year worldwide, over 55%, or 796,000, are directly attributable to cirrhosis15.
Liver transplantation has been the most effective therapy for patients with many types of advanced liver disease. Unfortunately, many patients cannot obtain transplantation due to the limited availability of donor livers, with over 10% of patients dying whilst on the waiting list. Of those patients who receive liver transplants, 94% survive after three months, 88% after one year and 79% after three years16. Furthermore, evidence of either fibrotic or cirrhotic regression has now been reported in chronic liver diseases of different etiologies, including viral hepatitis17, 18, 19, 20, 21, 22, 23, 24, autoimmune hepatitis25, alcoholic and NASH26, 27. While traditionally considered irreversible, recent evidence from animal studies and human clinical observations indicated that even advanced fibrosis can be reversed17, 28. This makes it important to identify reliable biomarkers for the early detection of liver disease and subsequent evaluation of response to therapeutic intervention. The ability of serum biomarkers of liver function to further discriminate between LC and HCC would add a major advantage.
Since first identified as a tissue kallikrein-binding protein (KBP), kallistatin mRNA was found in varying amounts in human liver, stomach, pancreas, kidney, aorta, testes, prostate, artery, atrium, ventricle and lung29. By far, the most important site of synthesis of kallistatin is the liver30, explaining the significantly reduced levels of serum kallistatin in patients with LC.
Kallistatin can inhibit tumor growth5, reduce blood pressure31, suppress arthritis6, 32 and protect organs and cells against inflammation, fibrosis, and oxidative stress33, 34, 35. The anti-oxidative effect of kallistatin can also inhibit salt-induced renal injury, inflammation, and fibrosis34.
The patients with HCC had lower serum kallistatin levels than normal controls, but significantly higher than in patients with LC. Thus, an inverse relationship between oxidative stress and kallistatin level might exist. This would be consistent with data demonstrating that male Sprague-Dawley (SD) rats exposed to either episodic or sustained hypoxia have reduced renal expression of kallistatin36. Moreover, chronic oxidative organ damage can markedly reduce circulating kallistatin levels in rats, while hydrogen peroxide (H2O2) lowers kallistatin mRNA and protein levels in dose-dependent fashion in vitro33. Kallistatin is known to play an important role in prevention of cancer, probably through its anti-angiogenic effects5. The human body can therefore up-regulate kallistatin expression when cancers occur in a self-protective mechanism. In agreement with data in our present study, previous examination of plasma kallistatin concentrations detected 22.1±3.5 µg/mL in 30 normal subjects and 21.1±3.8 µg/mL in five patients with hereditary angioedema7. A significant decrease in kallistatin levels (7.2±2.5 µg/mL, P<0.001) was also found in plasma samples from nine patients with liver disease7. Such a discrepancy may be related to differences in the populations studied or may reflect the relatively small sample size. We have previously demonstrated that transgenic expression of kallistatin can significantly attenuate carbon tetrachloride-induced liver damage37. This anti-inflammatory, anti-fibrotic potential of kallistatin warrants clinical evaluation, either with gene-therapy-based or recombinant-protein-administration-based evaluation of its therapeutic potential in human LC.
Previous studies showed that other clinical situations can also affect serum kallistatin levels, such as adiposity, community-acquired pneumonia (CAP) and type 1 diabetes38, 39. Serum kallistatin levels were significantly increased in diabetic vs. non-diabetic control subjects (12.6±4.2 vs. 10.3±2.8 μg/mL), and showing that levels were higher in diabetic patients with complications but did not differ for complication-free patients38. A study of CAP assessing the correlations of kallistatin with other biomarkers found that kallistatin was significantly consumed in CAP patients (median: 8.3, range 1.3–17.3 μg/mL) compared with healthy subjects (median: 17.2, range 5.3-82.7 μg/mL)39. These studies emphasize the diagnostic value of serum kallistatin levels in human LC, even though CAP patients have lower serum kallistatin levels, and the downgrading is fairly modest. However, the levels were decreased in liver disease but increased in diabetic patients. Therefore, comprehensive evaluation of kallistatin level and patients with obesity and diabetes history may determine their liver health more accurately.
In conclusion, the present study demonstrated the levels of serum kallistatin reduced significantly in patients with LC of different etiologies, but not with HCC. The magnitude of this decrease appeared to be correlated with the degree of LC and disruption of normal liver function. Kallistatin therefore has a potential as a new biomarker for the diagnosis of, and evaluation of the extent of human LC, along with its response to therapeutic intervention. Furthermore, kallistatin may provide a new therapeutic strategy for the management and treatment of cirrhotic liver damage.
Acknowledgments
This work was supported by the National Natural Science Foundation of China, China (Nos. 81072578 and 81271692) and The Hundred Talents Program of Fujian Provincial Government to Ruian Xu, with an additional support from Huaqiao University Grant Committee. Dr. Junping Zhang completed all pathological and histological work of this study.
Footnotes
Peer review under responsibility of Institute of Materia Medica, Chinese Academy of Medical Sciences and Chinese Pharmaceutical Association.
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.apsb.2015.02.003.
Appendix A. Supplementary materials
Supplementary Material
References
- 1.Benvegnu L, Gios M, Boccato S, Alberti A. Natural history of compensated viral cirrhosis: a prospective study on the incidence and hierarchy of major complications. Gut. 2004;53:744–749. doi: 10.1136/gut.2003.020263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nord HJ. Biopsy diagnosis of cirrhosis: blind percutaneous versus guided direct vision techniques—a review. Gastrointest Endosc. 1982;28:102–104. doi: 10.1016/s0016-5107(82)73015-9. [DOI] [PubMed] [Google Scholar]
- 3.Chao JL, Yin H, Yao YY, Shen B, Smith RS, Jr., Chao L. Novel role of kallistatin in protection against myocardial ischemia-reperfusion injury by preventing apoptosis and inflammation. Hum Gene Ther. 2006;17:1201–1213. doi: 10.1089/hum.2006.17.1201. [DOI] [PubMed] [Google Scholar]
- 4.Gao L, Yin H, S Smith R, Jr., Chao L, Chao J. Role of kallistatin in prevention of cardiac remodeling after chronic myocardial infarction. Lab Invest. 2008;88:1157–1166. doi: 10.1038/labinvest.2008.85. [DOI] [PubMed] [Google Scholar]
- 5.Miao RQ, Agata J, Chao L, Chao J. Kallistatin is a new inhibitor of angiogenesis and tumor growth. Blood. 2002;100:3245–3252. doi: 10.1182/blood-2002-01-0185. [DOI] [PubMed] [Google Scholar]
- 6.Wang CR, Chen SY, Wu CL, Liu MF, Jin YT, Chao L. Prophylactic adenovirus-mediated human kallistatin gene therapy suppresses rat arthritis by inhibiting angiogenesis and inflammation. Arthritis Rheum. 2005;52:1319–1324. doi: 10.1002/art.20991. [DOI] [PubMed] [Google Scholar]
- 7.Chao JL, Schmaier A, Chen LM, Yang ZR, Chao L. Kallistatin a novel human tissue kallikrein inhibitor: levels in body fluids, blood cells, and tissues in health and disease. J Lab Clin Med. 1996;127:612–620. doi: 10.1016/s0022-2143(96)90152-3. [DOI] [PubMed] [Google Scholar]
- 8.Wolf WC, Harley RA, Sluce D, Chao L, Chao J. Localization and expression of tissue kallikrein and kallistatin in human blood vessels. J Histochem Cytochem. 1999;47:221–228. doi: 10.1177/002215549904700210. [DOI] [PubMed] [Google Scholar]
- 9.Chen VC, Chao L, Pimenta DC, Bledsoe G, Juliano L, Chao J. Identification of a major heparin-binding site in kallistatin. J Biol Chem. 2001;276:1276–1284. doi: 10.1074/jbc.M005791200. [DOI] [PubMed] [Google Scholar]
- 10.Wolf WC, Harley RA, Sluce D, Chao L, Chao J. Cellular localization of kallistatin and tissue kallikrein in human pancreas and salivary glands. Histochem Cell Biol. 1998;110:477–484. doi: 10.1007/s004180050309. [DOI] [PubMed] [Google Scholar]
- 11.Bacon BR, Farahvash MJ, Janney CG, Neuschwander-Tetri BA. Nonalcoholic steatohepatitis: an expanded clinical entity. Gastroenterology. 1994;107:1103–1109. doi: 10.1016/0016-5085(94)90235-6. [DOI] [PubMed] [Google Scholar]
- 12.Dixon JB, Bhathal PS, O׳Brien PE. Nonalcoholic fatty liver disease: predictors of nonalcoholic steatohepatitis and liver fibrosis in the severely obese. Gastroenterology. 2001;121:91–100. doi: 10.1053/gast.2001.25540. [DOI] [PubMed] [Google Scholar]
- 13.Ratziu V, Giral P, Charlotte F, Bruckert E, Thibault V, Theodorou I. Liver fibrosis in overweight patients. Gastroenterology. 2000;118:1117–1123. doi: 10.1016/s0016-5085(00)70364-7. [DOI] [PubMed] [Google Scholar]
- 14.Bataller R, Brenner DA. Liver fibrosis. J Clin Invest. 2005;115:209–218. doi: 10.1172/JCI24282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Poynard T, Yuen MF, Ratzin V, Lai CL. Viral hepatitis C. Lancet. 2003;362:2095–2100. doi: 10.1016/s0140-6736(03)15109-4. [DOI] [PubMed] [Google Scholar]
- 16.Freeman RB, Jr., Steffick DE, Guidinger MK, Farmer DG, Berg CL, Merion RM. Liver and intestine transplantation in the United States, 1997-2006. Am J Transplant. 2008;8:958–976. doi: 10.1111/j.1600-6143.2008.02174.x. [DOI] [PubMed] [Google Scholar]
- 17.Arthur MJ. Reversibility of liver fibrosis and cirrhosis following treatment for hepatitis C. Gastroenterology. 2002;122:1525–1528. doi: 10.1053/gast.2002.33367. [DOI] [PubMed] [Google Scholar]
- 18.Dienstag JL, Goldin RD, Heathcote EJ, Hann HW, Woessner M, Stephenson SL. Histological outcome during long-term lamivudine therapy. Gastroenterology. 2003;124:105–117. doi: 10.1053/gast.2003.50013. [DOI] [PubMed] [Google Scholar]
- 19.Hui CK, Leung N, Shek TW, Yao H, Lee WK, Lai JY. Sustained disease remission after spontaneous HBeAg seroconversion is associated with reduction in fibrosis progression in chronic hepatitis B Chinese patients. Hepatology. 2007;46:690–698. doi: 10.1002/hep.21758. [DOI] [PubMed] [Google Scholar]
- 20.Kweon YO, Goodman ZD, Dienstag JL, Schiff ER, Brown NA, Burchardt E. Decreasing fibrogenesis: an immunohistochemical study of paired liver biopsies following lamivudine therapy for chronic hepatitis B. J Hepatol. 2001;35:749–755. doi: 10.1016/s0168-8278(01)00218-5. [DOI] [PubMed] [Google Scholar]
- 21.Poynard T, McHutchison J, Manns M, Trepo C, Lindsay K, Goodman Z. Impact of pegylated interferon alfa-2b and ribavirin on liver fibrosis in patients with chronic hepatitis C. Gastroenterology. 2002;122:1303–1313. doi: 10.1053/gast.2002.33023. [DOI] [PubMed] [Google Scholar]
- 22.Serpaggi J, Carnot F, Nalpas B, Canioni D, Guéchot J, Lebray P. Direct and indirect evidence for the reversibility of cirrhosis. Hum Pathol. 2006;37:1519–1526. doi: 10.1016/j.humpath.2006.07.007. [DOI] [PubMed] [Google Scholar]
- 23.Shiratori Y, Imazeki F, Moriyama M, Yano M, Arakawa Y, Yokosuka O. Histological improvement of fibrosis in patients with hepatitis C who have sustained response to interferon therapy. Ann Intern Med. 2000;132:517–524. doi: 10.7326/0003-4819-132-7-200004040-00002. [DOI] [PubMed] [Google Scholar]
- 24.Farci P, Roskams T, Chessa L, Peddis G, Mazzoleni AP, Scioscia R. Long-term benefit of interferon a therapy of chronic hepatitis D: regression of advanced hepatic fibrosis. Gastroenterology. 2004;126:1740–1749. doi: 10.1053/j.gastro.2004.03.017. [DOI] [PubMed] [Google Scholar]
- 25.Dufour JF, DeLellis R, Kaplan MM. Reversibility of hepatic fibrosis in autoimmune hepatitis. Ann Intern Med. 1997;127:981–985. doi: 10.7326/0003-4819-127-11-199712010-00006. [DOI] [PubMed] [Google Scholar]
- 26.Dixon JB, Bhathal PS, Hughes NR, O׳Brien PE. Nonalcoholic fatty liver disease: improvement in liver histological analysis with weight loss. Hepatology. 2004;39:1647–1654. doi: 10.1002/hep.20251. [DOI] [PubMed] [Google Scholar]
- 27.Wakim-Fleming J, Mullen KD. Long-term management of alcoholic liver disease. Clin Liver Dis. 2005;9:135–149. doi: 10.1016/j.cld.2004.10.001. [DOI] [PubMed] [Google Scholar]
- 28.Xu R, Harrison PM, Chen M, Li L, Tsui TY, Fung PC. Cytoglobin overexpression protects against damage-induced fibrosis. Mol Ther. 2006;13:1093–1100. doi: 10.1016/j.ymthe.2005.11.027. [DOI] [PubMed] [Google Scholar]
- 29.Chai KX, Chen LM, Chao J, Chao L. Kallistatin: a novel human serine proteinase inhibitor. Molecular cloning, tissue distribution, and expression in Escherichia coli. J Biol Chem. 1993;268:24498–24505. [PubMed] [Google Scholar]
- 30.Chao J, Chao L. Biochemistry, regulation and potential function of kallistatin. Biol Chem Hoppe-Seyler. 1995;376:705–713. [PubMed] [Google Scholar]
- 31.Chao J, Miao RQ, Chen V, Chen LM, Chao L. Novel roles of kallistatin, a specific tissue kallikrein inhibitor, in vascular remodeling. Biol Chem. 2001;382:15–21. doi: 10.1515/BC.2001.003. [DOI] [PubMed] [Google Scholar]
- 32.Hsieh JL, Shen PC, Shiau AL, Jou IM, Lee CH, Teo ML. Adenovirus-mediated kallistatin gene transfer ameliorates disease progression in a rat model of osteoarthritis induced by anterior cruciate ligament transection. Hum Gene Ther. 2009;20:147–158. doi: 10.1089/hum.2008.096. [DOI] [PubMed] [Google Scholar]
- 33.Shen B, Chao L, Chao J. Pivotal role of JNK-dependent FOXO1 activation in downregulation of kallistatin expression by oxidative stress. Am J Physiol Heart Circ Physiol. 2010;298:H1048–H1054. doi: 10.1152/ajpheart.00826.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shen B, Hagiwara M, Yao YY, Chao L, Chao JL. Salutary effect of kallistatin in salt-induced renal injury, inflammation, and fibrosis via antioxidative stress. Hypertension. 2008;51:1358–1365. doi: 10.1161/HYPERTENSIONAHA.107.108514. [DOI] [PubMed] [Google Scholar]
- 35.Yin H, Gao L, Shen B, Chao L, Chao J. Kallistatin inhibits vascular inflammation by antagonizing tumor necrosis factor-α-induced nuclear factor κB activation. Hypertension. 2010;56:260–267. doi: 10.1161/HYPERTENSIONAHA.110.152330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Thongboonkerd V, Gozal E, Sachleben LR, Jr., Arthur JM, Pierce WM, Cai J. Proteomic analysis reveals alterations in the renal kallikrein pathway during hypoxia-induced hypertension. J Biol Chem. 2002;277:34708–34716. doi: 10.1074/jbc.M203799200. [DOI] [PubMed] [Google Scholar]
- 37.Diao Y, Zhao XF, Lin JS, Wang QZ, Xu RA. Protection of the liver against CCl4-induced injury by intramuscular electrotransfer of a kallistatin-encoding plasmid. World J Gastroenterol. 2011;17:111–117. doi: 10.3748/wjg.v17.i1.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jenkins AJ, McBride JD, Januszewski AS, Karschimkus CS, Zhang B, O׳Neal DN. Increased serum kallistatin levels in type 1 diabetes patients with vascular complications. J Angiogenes Res. 2010;2:19–26. doi: 10.1186/2040-2384-2-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lin WC, Lu SL, Lin CF, Chen CW, Chao L, Chao J. Plasma kallistatin levels in patients with severe community- acquired pneumonia. Crit Care. 2013;17:R27–R36. doi: 10.1186/cc12507. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material