Abstract
Background
Motivational physical activity (PA) interventions are effective in increasing PA behavior among healthy adults; however, the impact of these interventions on cardiorespiratory fitness (CRF) has not yet been examined.
Objective
The purpose of this meta-analysis is to quantitatively synthesize CRF outcomes of motivational PA interventions among healthy adults.
Methods
Comprehensive searching identified studies testing motivational PA interventions with CRF outcomes. Two independent coders extracted data. Data were synthesized using standardized mean difference effect sizes (ESs, d) under a random-effects model. Heterogeneity was assessed and moderator analyses were conducted using subgroup analyses and meta-regression.
Results
Data were analyzed from 11,458 primary study subjects. The overall mean ES for CRF was 0.48 (p < .01), which corresponds to a difference in VO2 max of 2.5 mL/kg/min between treatment and control groups. Studies were significantly heterogeneous (Q = 133.29, p < .01). Significant moderators included age (β = −0.02, p = .01) and recommending endurance plus resistance exercises (d = 1.04) versus recommending only endurance exercise (d = .47).
Discussion
Motivational interventions designed to increase PA can improve CRF among healthy adults. Clinicians should recommend endurance and resistance exercise to improve CRF in this population. Future primary research should test interventions longitudinally and across more diverse populations. Although other moderators examined in this study did not demonstrate a significant effect on ES, the number of comparisons available for moderator analyses was small.
Keywords: Meta-analysis, exercise, physical endurance, cardiorespiratory fitness
Chronic illnesses are the leading cause of death and disability in the United States and are a significant public health issue (Centers for Disease Control and Prevention [CDC], 2012). The most common chronic illnesses among adults are type 2 diabetes mellitus, heart disease, stroke, and chronic respiratory illnesses (CDC, 2012). However, these chronic illnesses are largely preventable through behavioral risk modification.
Physical inactivity has been cited as one of the primary causes of chronic illness; therefore, increasing physical activity (PA) can be an optimal target for chronic illness prevention (CDC, 2012). One mechanism by which PA contributes to chronic illness risk reduction is cardiorespiratory fitness (CRF), defined as the capacity of the cardiovascular and respiratory systems to provide oxygen during sustained PA (US Department of Health and Human Services, 2008). CRF has been linked with improvements in cardiometabolic risk markers (Rhéaume et al., 2011), reduced visceral adiposity (Farrell, Finley, McAuley, & Frierson, 2011; Rhéaume et al., 2011), improvement in other anthropometric measures (Crist et al., 2012), maintenance of normal insulin function and secretion (Larsen, Anderson, Ekblom, & Nyström, 2012), and lower systolic blood pressure (Chen, Das, Barlow, Grundy, & Lakoski, 2010). Furthermore, higher levels of CRF have been associated with reduced risk of sudden cardiac death (Laukkanen et al., 2010), heart disease (Williams, 2010), metabolic syndrome (Crist et al., 2012), cognitive impairment (Sattler, Erickson, Toro, & Schröder, 2011), and cardiovascular and all-cause mortality (Blair & Kampert, 1996; Myers et al., 2002). Therefore, CRF can be an important clinical target and health indicator.
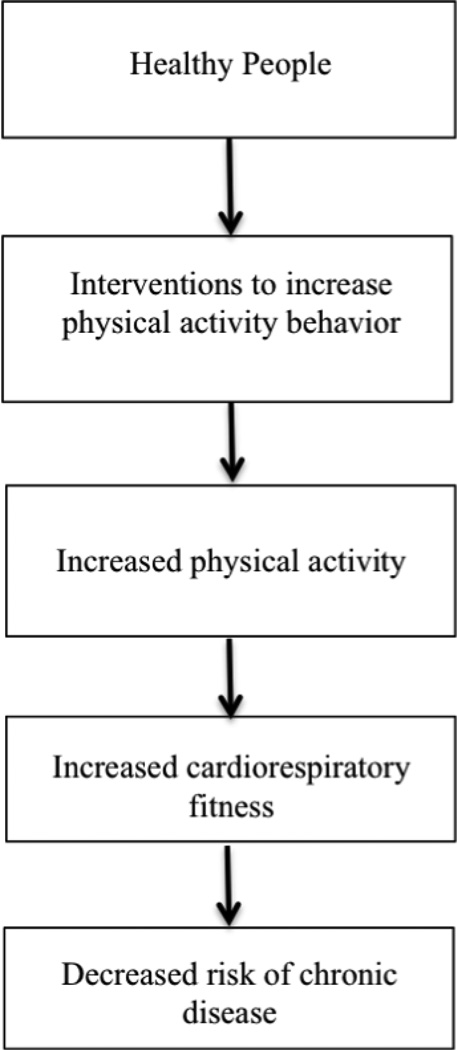
Figure 1 depicts the potential relationships between PA interventions, PA behavior, CRF, and chronic disease risk. Interventions to enhance PA behavior may be divided into two broad, though conceptually distinct categories: supervised or motivational PA interventions. Supervised PA interventions are highly dependent upon strictly formatted and monitored PA sessions to change PA behavior. These interventions may require costly trained personnel to deliver and supervise sessions, as well as specialized equipment and locations. Motivational PA interventions focus on strategies such as educational and motivational content to promote independent PA behavior change. Examples of this type of intervention would include educational sessions, clinician recommendations, exercise prescriptions, or counseling. These interventions do not necessarily require specific equipment or personnel, and may even be delivered in the community or home environment. As a result, these types of interventions may be more feasible to implement in diverse locations. For example, nurses may deliver a motivational PA intervention in a clinic setting during a routine patient visit.
Figure 1.
Framework of Relationships Between Physical Activity Interventions, Cardiorespiratory Fitness and Chronic Disease Risk.
While it is known that interventions intended to educate and motivate healthy adult subjects to independently increase PA behavior are modestly effective in changing PA behavior (Conn, Hafdahl, & Mehr, 2011), it is not yet known to what extent these motivational PA interventions may contribute to improvements in CRF. There have been very few reviews of the literature examining the effects of motivational/educational PA interventions on CRF among healthy adults (Müller-Riemenschneider, Reinhold, Nocon, & Willich, 2008; Oja, 2001; Simons-Morton, Calfas, Oldenburg, & Burton, 1998). All prior reviews combined outcomes from both motivational-type interventions and interventions involving supervised exercise sessions. Most of the older reviews did not include a quantitative synthesis of PA intervention effects on CRF (Oja, 2001; Simons-Morton, et al., 1998). The most recent meta-analysis of PA interventions that investigated effects on CRF only included three studies from 2001 to 2007, thereby excluding older, potentially eligible studies with valuable data (Müller-Riemenschneider et al., 2008). Moreover, moderator analyses were not conducted. Thus knowledge related to the effects of sample characteristics and doses of motivational PA interventions on increasing CRF is yet unknown. Furthermore, a research gap exists regarding the effect of these interventions’ varying PA behavior recommendations on CRF.
The benefits of higher CRF are known, especially in the attenuation and prevention of cardiovascular disease. The purpose of this meta-analysis is to provide new research knowledge related to the effectiveness of motivational PA interventions on improving CRF among healthy adults. Determining the effectiveness of these types of interventions on increasing CRF and identifying specific characteristics that impact effectiveness will help clinicians understand motivational PA strategies that can promote higher CRF among their healthy adult clients. Thus the researchers have conducted a comprehensive search of the PA intervention literature to identify potentially eligible studies for inclusion in the quantitative synthesis. Furthermore, moderator analyses were performed to investigate factors that may impact effectiveness. The research questions for this study were: 1) What is the overall effectiveness of motivational PA intervention on CRF? 2) Do CRF outcomes vary depending on the design, sample, or intervention characteristics of the studies?
Methods
Data from this meta-analysis were obtained from a larger National Institutes of Health funded parent study examining the overall effects of PA interventions on increasing PA behavior among healthy adults (Conn et al., 2011). An exhaustive search of the literature was conducted to locate potentially eligible studies. Multiple search strategies were employed, such as online database searches, author, and ancestry searching. The 13 databases searched were MEDLINE, PubMed, EMBASE, Healthstar, Cochrane Controlled Trials Register, SPORTDiscus, PsychINFO, Nursing and Allied Health Database, Dissertation Abstracts International, Combined Health Information Database, Database of Abstracts of Reviews of Effectiveness, Educational Resources Information Center, and Google Scholar. Sample MEDLINE intervention search terms were: “adherence, behavior therapy, clinical trial, compliance, counseling, evaluation, evaluation study, evidence-based medicine, health care evaluation, health behavior, health education, health promotion, intervention, outcome & process assessment, patient education, program, program development, program evaluation, self care, treatment outcome, validation study.” Sample MEDLINE search terms for PA were: “exercise, exertion, exercise therapy, physical activity, physical fitness, physical education & training, walking.” No specific search terms were used for fitness outcomes because these have been inconsistently applied; furthermore, the primary focus of the parent study was related to PA outcomes. Multiple research registers were also searched, such as the National Institutes of Health Computer Retrieval of Information on Scientific Projects, Australian/New Zealand Clinical Trials Registry, and the metaRegister of Controlled Trials. Both published and unpublished literature was searched through these databases and registries.
Additionally, 82 journal titles from 1960–2007 were hand searched by research staff. The journals searched were selected based on relevance to PA intervention research and PA behavior among healthy adults. These diverse search strategies were used to minimize bias (Hopewell, Clarke, & Mallett, 2005; Rothstein & Hopewell, 2009). Years searched were limited to 2007 due to the years included in the parent study. The review protocol was not registered but is available from the corresponding author. In summary, published and unpublished studies were included in this meta-analysis if they 1) described motivational interventions designed to increase PA behavior among adults age 18 and older; 2) measured CRF as an outcome; 3) provided enough data to calculate and effect size (ES); and 4) were written in English. Research staff attempted to contact authors of primary studies that met inclusion criteria but lacked usable data to calculate an ES.
“Motivational PA intervention” was conceptually defined as any deliberate intervention intended to motivate subjects to independently increase PA behavior, exclusive of supervised exercise interventions. An intentionally broad definition of motivational PA interventions was used to capture the diversity of PA interventions, allowing for subsequent moderator analyses. Physical activity was defined as “any bodily movement produced by the contraction of skeletal muscle that increases energy expenditure above a basal level” (CDC, 2011). Specific search terms related to motivational PA interventions were not used because specific search terms for this topic are not available. Instead, the comprehensive searching focused on interventions studies with fitness outcomes. This broader searching was necessary given the diverse interventions which could be defined as motivational interventions. Studies were evaluated for type of PA intervention (e.g., motivational or supervised exercise intervention) during the coding and data extraction phase of the parent study.
CRF was defined as the capacity of the cardiovascular and respiratory systems to provide oxygen during sustained PA (US Department of Health and Human Services, 2008). The gold standard of CRF measurement is maximum oxygen uptake, or VO2 max, and is closely related to the functional capacity of an individual’s cardiovascular system (McArdle, Katch, & Katch, 2010; Vanhees et al., 2005). VO2 max captures not only an individual’s ability to achieve a specified intensity, but also to exercise to a specified duration at that intensity. Direct measures of VO2 max occur in a laboratory setting and involve cardiopulmonary monitoring while an individual exercises to exhaustion using incremental increases in intensity. VO2 max is subsequently calculated based on the physiologic data collected, as well as the duration, and maximum intensity of exercise endured (McArdle et al., 2010). Because measuring direct VO2 max is difficult and an expensive test to perform, several other tests have been used to indirectly measure and predict VO2 max with varying degrees of error (Vanhees et al., 2005). Submaximal exercise testing using an exercise treadmill or cycle ergometry protocol are the most commonly used methods in the clinical setting. These tests base predicted VO2 max on heart rate response to graded exercise testing and tend to underpredict VO2 max (Dabney & Butler, 2006; Grant, Corbett, Amjad, Wilson, & Aitchison, 1995; McArdle et al., 2010). Various patient-related (e.g., motivation, age, gender, medications, current health and physical fitness) and operator-related variables (e.g., ability to manage equipment, specific protocol used, ability to motivate patient) impact the validity and reliability of these tests in predicting VO2 max.
Metabolic equivalents (METs) are convenient and clinically useful descriptors for exercise intensity. VO2 max may be converted to METs by dividing the VO2 max value by 3.5 ml/kg/min (McArdle et al., 2010). The result is an individual’s maximum MET capacity. The amount of METs ascribed to various exercise types is available from a wide variety of resources, and clinicians may use this information to recommend specific activities to improve CRF (Ainsworth et al., 1993; McArdle et al., 2010; Thompson, Medicine, Gordon, & Pescatello, 2009).
Study Quality
Primary study quality impacts the validity of meta-analysis research (Valentine, 2009). Determination of primary study quality for meta-analyses is a contentious issue. Various ways of determining study quality in meta-analysis research exist, but there is no standard (Balk et al., 2002; Conn & Rantz, 2003). While the most common method of determining primary study quality for meta-analyses involves using a quality scale, these numerous different measures unreliably capture dimensions of quality; furthermore, study quality determined by these scales may not be associated with primary study outcomes (Conn & Rantz, 2003). Inadequate reporting of quality measures (e.g., randomization, treatment fidelity, sample size) can also impact the management of primary study quality for all meta-analyses (Valentine, 2009). As a result, using a combination of strategies to manage primary study quality is recommended (Conn & Rantz, 2003; Valentine, 2009). For the present study, inclusion criteria, type of study for the main and moderator analyses, and empirical examination of quality features (e.g., randomized allocation) were used.
Data Extraction
Data were extracted using a coding frame developed from prior and related PA intervention research (Abraham & Michie, 2008; Conn et al., 2011; Conn, Valentine, & Cooper, 2002; Michie, Johnston, Francis, Hardeman, & Eccles, 2008). The coding frame was constructed to capture sample, design, and intervention characteristics, as well as outcome data from the studies. The resultant codebook was pilot tested on 30 studies prior to use on the entire sample. Coding items reported in this paper are available from the corresponding author. Subsequently, two extensively trained staff independently coded each study. Coders compared data to achieve consensus. A third, doctorally prepared researcher verified all effect size data.
VO2 max was coded according to direct or indirect measurement. Coding items with precise directions on how to differentiate between the two types of measures were provided for coders. For example, VO2 max was coded as a direct measure if oxygen consumption by the subjects was measured directly by a machine that tests their expired air in one of the following scenarios: 1) subjects exercised to exhaustion or very near exhaustion (symptom-limited exercise); and/or 2) subjects exercised to a predetermined percent of maximum age-predicted heart rate; and/or 3) subjects exercised to a predetermined respiratory exchange ratio; and/or 4) subjects exercise goes to a 19 to 20 on a rate of perceived exertion scale. VO2 max was coded as an indirect measure if it was estimated or predicted by any means, which may or may not involve spirometry.
Data Analysis
All analyses were conducted using Comprehensive Meta-Analysis software (Borenstein, Hedges, Higgins, & Rothstein, 2005). Study outcome data were used to calculate standardized mean difference effect sizes (ES, d) among studies. Conceptually, the standardized mean difference is the mean of the treatment group minus the mean of the control group divided by a pooled standard deviation. Some studies reported outcomes as mean change scores. To calculate ESs for these studies, each mean change score were added to the baseline mean score to produce a post-test score, and the baseline standard deviation was used. Two-group comparison, treatment versus control post intervention data were analyzed separately from single-group pre-post intervention data. To control for pre-intervention CRF among two-group, treatment versus control, pre-post test comparisons, additional data are needed regarding the correlation between treatment pre and post data, as well as the control group pre and post data. However, none of the studies included in this analysis reported the necessary correlation data. A moderate correlation was assumed for this additional analysis; however, due to the uncertainty of assumptions regarding the actual correlations within each study, the post-intervention data is reported as the main analysis. Study ESs were weighted by the inverse of the variance so that studies with larger samples weighed more heavily in the analyses. Study ESs were synthesized using a random-effects model. This type of model accounts for variation between studies in addition to within-study sampling error. Given the expected diversity between studies in this area of research in terms of study design, sample, and intervention characteristics, this statistical model is more appropriate compared to a fixed-effect analysis (Borenstein, Hedges, Higgins, & Rothstein, 2009). Effect sizes are presented with 95% confidence intervals (CI) and p value. Statistical significance was set at a p value of ≤ .05. The overall mean ES of two-group, treatment versus control post-intervention data was converted to the original metric of VO2 max. This measure of VO2 max was subsequently translated into METs, for further clinical interpretation. Single-group pre-post intervention overall mean ES was used to supplement two-group findings.
Heterogeneity of ESs was examined using Q and I2 statistics and moderator analyses. The Q statistic is the weighted sum of squares produced by determining and squaring the deviation of each study’s ES from the mean ES, multiplying by each study’s inverse of the variance, and summing the values (Borenstein et al., 2009). As such, the Q statistic is a standardized measure of the total amount of variation observed across studies. This value may be compared to the amount of expected variation due to within-study differences, expressed as degrees of freedom (df). The amount of heterogeneity of ES due to between study differences is determined by subtracting expected variation (df) from the observed variation (Q) (Borenstein et al., 2009). Although the Q statistic is useful in determining heterogeneity of effects, it is highly dependent on the number of studies analyzed; therefore, a measure of the proportion of the observed variation due to true differences among study ESs, expressed as the statistic I2, can be informative. I2 is calculated by dividing the amount of observed excess variation (Q – df) by Q, and multiplying the value by 100 to produce a ratio (Borenstein et al., 2009).
Moderator analyses further examines sources of variation by exploring differences in characteristics across studies. Exploratory moderator analyses were conducted on several variables among the two-group comparison studies to determine the impact, if any, on ES. Potential moderator variables included aspects of motivational PA intervention dose and PA behavior recommendation. Additional variables were selected based on their prior impact in the literature (Conn et al., 2011; Conn, Phillips, Ruppar, & Chase, 2012; Conn et al., 2002). Meta-analytic analogues of ANOVA and regression were used for dichotomous and continuous variables, respectively. Statistical significance for moderators was set at a p value of ≤ .05. Only two-group, treatment versus control studies were used for moderator analyses, because the greater scientific rigor inherent within this research design versus single-group pre-post designs would increase validity of these moderator analyses findings. This study also complies with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses checklist (Moher, Liberati, Teztlaff, & Altman, 2009).
Results
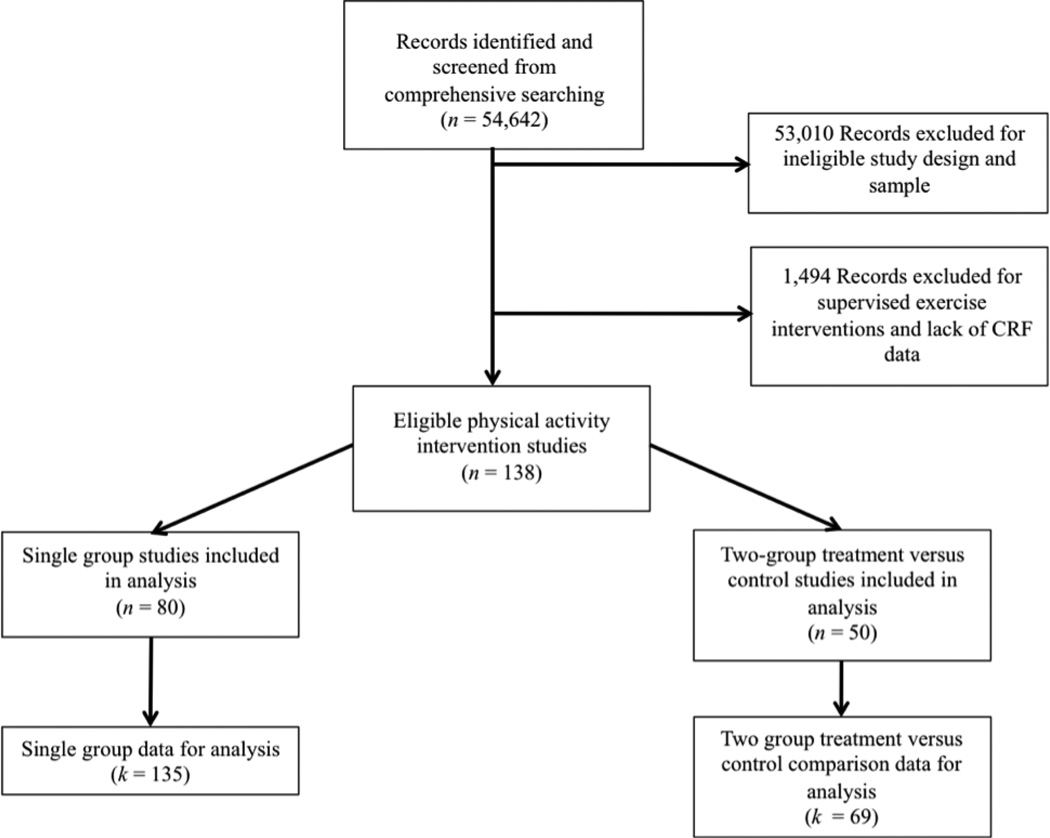
Extensive search strategies identified 54,642 potentially eligible studies. Figure 2 depicts study selection. The total number of independent, two-group comparison studies included in the main analysis was 50, with data available for 69 treatment versus control comparisons. To supplement findings from the main analysis, 78 studies from which 135 single group, pre-post test data were included. Effect sizes were calculated from a total of 11,458 subjects. Given the large number of studies included in the meta-analysis, only a forest plot of the two-group comparison studies will be presented. Additional information regarding characteristics and ESs of all included studies is available as supplemental digital content.
Figure 2.
Diagram of Study Selection.
Table 1 lists characteristics of studies included in this meta-analysis. The median sample size was 21 among all included studies. The median of the mean ages of the studies was about 44 years. Women were overall well represented in this research area with a median of 80.5% of participants included in these studies being women. Minority ethnicity was not consistently reported among studies, however. Many participants in these studies were overweight at baseline with a median baseline body mass index (BMI) of 27.8 and median baseline weight of 78.6 kilograms (172.9 pounds).
Table 1.
Characteristics of Studies Included in Meta-Analysis of Fitness Outcomes for Motivational Physical Activity Interventions, 204 Reports (N=11,458 subjects)
| Characteristic | k | Minimum | Median | Maximum | |
|---|---|---|---|---|---|
| Mean age (years) | 153 | 19.30 | 43.99 | 72.64 | |
| Single group pre-post | 101 | 22.5 | 42.9 | 72.64 | |
| 2-group, treatment versus control, pre-post |
52 | 19.3 | 42.13 | 69.2 | |
| Sample size | 204 | 5 | 21 | 2519 | |
| Single group pre-post | 135 | 5 | 22 | 2519 | |
| 2-group, treatment versus control, pre-post |
69 | 10 | 35 | 298 | |
| Percent female | 173 | 0 | 80.50 | 100 | |
| Single group pre-post | 119 | 0 | 79 | 100 | |
| 2-group, treatment versus control, pre-post |
54 | 0 | 54 | 69.2 | |
| Percent ethnic minority | 40 | 0 | 5 | 100 | |
| Single group pre-post | 35 | 0 | 5 | 100 | |
| 2-group, treatment versus control, pre-post |
5 | 0 | 0 | 17.6 | |
| Mean BMI (kg/m2) | 99 | 20.41 | 27.83 | 37.60 | |
| Single group pre-post | 73 | 20.41 | 28.76 | 37.6 | |
| 2-group, treatment versus control, pre-post |
26 | 22.4 | 27.08 | 32.55 | |
| Mean Weight (kg) | 132 | 41.75 | 78.60 | 102.80 | |
| Single group pre-post | 84 | 41.75 | 78.85 | 102.8 | |
| 2-group, treatment versus control, pre-post |
48 | 56.5 | 74.55 | 93.2 | |
| Total number of sessions | 153 | 1 | 3 | 50 | |
| Single group pre-post | 111 | 1 | 4 | 50 | |
| 2-group, treatment versus control, pre-post |
42 | 1 | 2 | 16 | |
| Session duration (in minutes) | 36 | 7.27 | 60 | 120 | |
| Single group pre-post | 24 | 7.5 | 45 | 75 | |
| 2-group, treatment versus control, pre-post |
12 | 7.27 | 52.5 | 120 | |
| Days over which intervention delivered |
186 | 1 | 84 | 1825 | |
| Single group pre-post | 129 | 1 | 84 | 1460 | |
| 2-group, treatment versus control, pre-post |
57 | 1 | 91.5 | 1825 | |
| Recommended Frequency (days per week) |
122 | 2 | 4.5 | 18 | |
| Single group pre-post | 85 | 2 | 5 | 35 | |
| 2-group, treatment versus control, pre-post |
37 | 2 | 4 | 7 | |
| Recommended duration (minutes per session) |
108 | 5 | 30 | 90 | |
| Single group pre-post | 76 | 5 | 30 | 60 | |
| 2-group, treatment versus control, pre-post |
32 | 7.5 | 30 | 90 | |
Note. k = number of comparisons for which data were available. “Comparisons” refers to the group analyzed within a study. Some studies may have more than one comparison (e.g., multiple treatment groups).
Interventions among studies were varied. The median number of intervention sessions was 3. The median number of minutes per intervention session was 60 minutes, and these interventions were delivered over a median of 84 days. In terms of intervention content, the median number of recommended days of exercise per week was 4.5. Furthermore, the median number of recommended minutes spent exercising per exercise session was 30 minutes.
Effects of Interventions
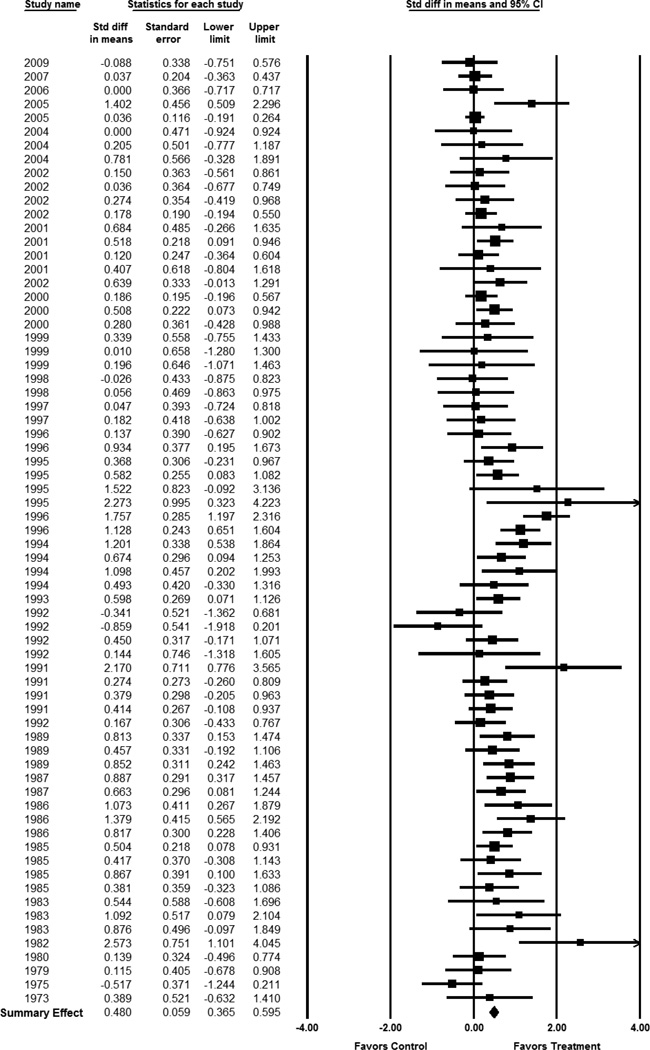
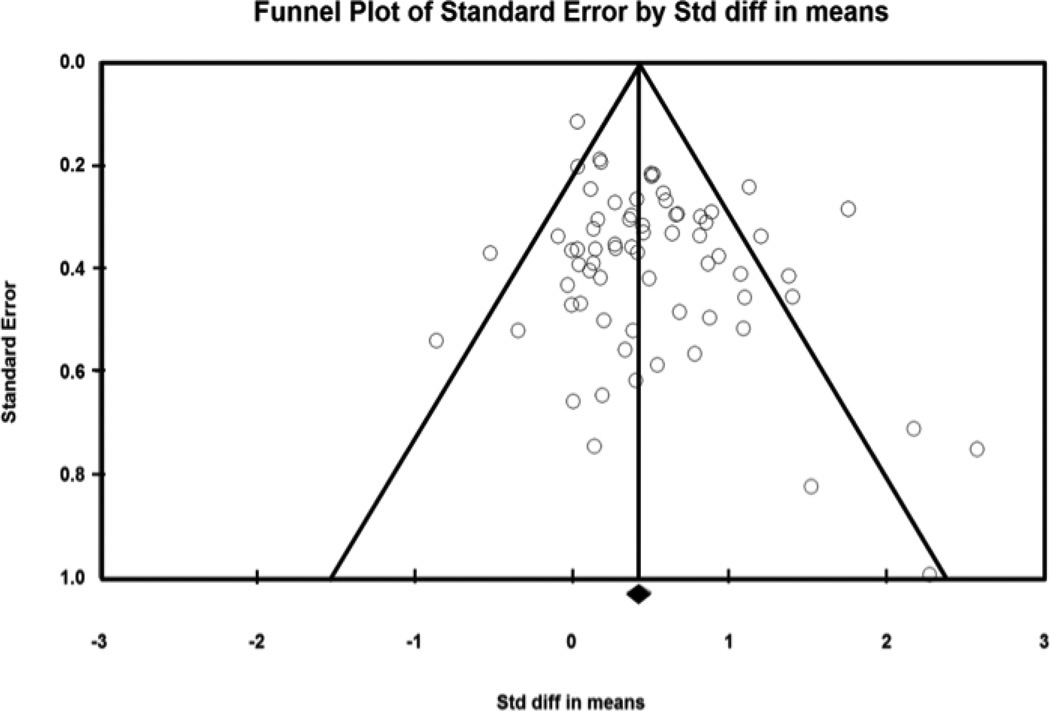
Motivational PA interventions significantly improved CRF among healthy adults (Table 2). The ES for two-group, treatment versus control comparisons at outcome was .48 (95% CI 0.37 – 0.60, p < .01). Figure 3 depicts the forest plot of ESs for each included study. Controlling for pre-test scores, the ES for two-group, treatment versus control, pre-post intervention was .62 (95% CI 0.45–0.78, p < .01). The ES for the treatment group pre-post intervention among the studies designed as two-group comparisons was .42 (95% CI 0.32 – 0.53, p < .01). The ES for single group treatment pre-post intervention in studies without a control group was .32 (95%CI 0.27 – 0.38, p < .01). In contrast, control subjects experienced a decline in CRF with an ES of −.13 (95% CI −0.21 – −0.05, p < .01). The ES of .48 for the treatment versus control comparisons is consistent with a 2.5 mL/kg/min higher VO2 max in the treatment group versus control group. In terms of METs, the difference would be 0.83 METs favoring the treatment group. Significant heterogeneity of effects was present across studies (Q = 133.29, p < .01), with almost half of the observed variation due to true differences in ESs across studies (I2= 48.99). Figure 4 demonstrates the plot for publication bias for this study, in which smaller reports with negative findings may have been missing from this analysis.
Table 2.
Overall Effect Size of Motivational Physical Activity Interventions on Fitness among Healthy Adults
| k | d | 95% CI | SE | Q | ||
|---|---|---|---|---|---|---|
| LL | UL | |||||
| Two-group, treatment versus control comparison, post-intervention |
69 | 0.48** | .37 | .60 | 0.59 | 133.29** |
| Treatment group, within-group, pre post intervention |
69 | 0.42** | .32 | .53 | 0.05 | 213.90** |
| Single group, pre-post intervention | 135 | 0.32** | .27 | .37 | 0.03 | 415.16** |
| Control group, within-group pre-post intervention |
69 | −0.13** | −.21 | −.05 | 0.04 | 87.52* |
Note. k=number of comparisons; d=standardized mean difference calculated under random-effects model; CI=confidence interval; SE=standard error of d; Q= heterogeneity statistic; LL=lower limit; UL=upper limit
p<.05;
p<.001
Figure 3.
Meta-Analysis of 2-group Post-Intervention Fitness Outcomes
Studies listed by year of publication. Effect sizes calculated under a random-effects model. The area of each square is proportional to study weight.
Figure 4.
Funnel Plot for Publication Bias for Two-Group, Post-Intervention Analysis
Lack of publication bias would be evident if the symmetrical distribution of studies (data points) around the mean effect size (center line) was present. This plot suggests that some publication bias may be present, evidenced by missing data from the lower left corner of the funnel. Larger studies would appear at the top of the funnel, and positive studies would appear to the right of the mean effect size. Thus smaller studies with negative findings are likely missing.
Moderator Analyses
Table 3 lists findings for exploratory moderator analyses among dichotomous variables. Presence or absence of study funding or randomized allocation of subjects into groups did not impact overall effectiveness. Studies that recommended endurance plus resistance exercises (d = 1.04) had significantly larger ESs than studies that only recommended endurance exercises (d = .47). Recommended intensity level (e.g., moderate or high level) did not appear to impact ESs. Studies that targeted PA behavior alone did not demonstrate statistically different ESs from studies that targeted PA behavior in combination with another behavioral target (e.g., diet). Furthermore, ESs were equally effective regardless of the type of interventionist (e.g., exercise specialist or not). ESs did not significantly differ between studies that used a direct versus indirect method of measuring fitness.
Table 3.
Moderator Analyses of Dichotomous Variables on Cardiorespiratory Fitness
| Variable | 0 | 1 | k0 | k1 | d0 | d1 | QB |
|---|---|---|---|---|---|---|---|
| Funding | No | Yes | 28 | 43 | .62** | .47** | 1.07 |
| Behavioral target | PA only | PA + other behavior |
52 | 19 | .47** | .62** | .93 |
| Participants were prior exercisers | No | Yes | 58 | 13 | .48** | .69** | 1.48 |
| Interventionist type | Not exercise specialist |
Exercise specialist |
64 | 7 | .55** | .32 | 1.20 |
| Randomized allocation into groups | No | Yes | 15 | 54 | .74** | .46** | 2.68 |
| Direct measure of VO2 max | Indirect or other | Direct | 32 | 39 | .42** | .61** | 2.01 |
| Level of intensity recommended | Moderate level | High level | 35 | 6 | .58** | .76* | .43 |
| Recommended endurance alone or endurance plus resistance exercise |
Endurance alone | Endurance + Resistance |
66 | 5 | .47** | 1.04** | 5.69* |
Note. k0=number of studies with characteristic under column 0; k1=number of studies with characteristic under column 1; d0=standardized mean difference calculated under random-effects model of studies with characteristic under column 0; d1=standardized mean difference calculated under random-effects model of studies with characteristic under column 1; QB = Q between
p<.05;
p<.001
Table 4 lists findings for moderator analyses among continuous variables. Among continuous moderators, mean age was a significant moderator with studies of younger subjects demonstrating larger improvements (β = −0.02, 95% CI −0.03 – −0.00, p = .01). Mean BMI, mean weight, and percent of the sample that were women were not significant moderators. Duration of recommended exercise (β = 0.01, 95% CI 0.00 – 0.02, p = .03) and days over which the intervention was delivered (β = 0.00, 95% CI 0.000 – 0.001, p = .04) were significant moderators. However, while statistically significant, these findings did not represent clinically significant changes. Similarly, while year of publication was a statistically significant moderator, the impact on ES appeared small (β = −0.01, 95% CI −0.02 – −0.00, p = .05). Other intervention characteristics, such as number of intervention sessions, and recommended frequency of exercise in days per week were not significant moderators in this analysis.
Table 4.
Moderator Analyses of Continuous Variables on Cardiorespiratory Fitness
| Variable | β | SE | p |
|---|---|---|---|
| Year of publication | −0.01 | 0.01 | 0.05 |
| Mean age | −0.02 | 0.01 | 0.01 |
| Mean BMI (kg/m2) | 0.02 | 0.03 | 0.71 |
| Mean weight | 0.00 | 0.01 | 0.79 |
| Percent sample women | −.00 | 0.00 | 0.60 |
| Days over which intervention delivered | 0.00 | 0.00 | 0.04 |
| Number of minutes per intervention session | −0.00 | 0.00 | 0.53 |
| Number of sessions | −0.00 | 0.01 | 0.65 |
| Duration of recommended exercise (in minutes) | 0.01 | 0.00 | 0.03 |
| Recommended frequency of exercise (days per week) | −0.04 | 0.06 | 0.51 |
Note. β=meta-regression coefficient for slope; SE=standard error of β; p refers to statistical signficance for β
Discussion
This is the first study to employ extensive literature search strategies to identify motivational PA intervention studies among healthy adults from which CRF outcome data were quantitatively synthesized. This study provides evidence supporting the effectiveness of motivational PA interventions in improving CRF among healthy adults. Study findings are similar to past literature reviews (Simons-Morton et al., 1998), including a limited meta-analysis by Müller-Riemenschneider and colleagues (2008). This study goes beyond the previous reviews because the researchers not only expanded the years of inclusion for studies, but also conducted moderator analyses. ESs have also been converted to meaningful CRF metrics to facilitate clinical interpretation.
Clinicians should recognize CRF as an important health indicator and a useful clinical target because higher CRF has been linked with numerous positive health outcomes and reduced risk of chronic diseases (Chen et al., 2010; Farrell et al., 2011; Larsen et al., 2012; Rhéaume et al., 2011). For example, among healthy middle-aged women, the risk of cancer mortality is increased among obese women in the lower percentile of CRF (Farrell et al., 2011). Improvement in CRF over time is associated with decrease visceral adiposity and risk of metabolic syndrome among healthy men and women (Rhéaume et al., 2011). Risk of elevated systolic BP also decreases with a moderate level of CRF and normal weight (Chen et al., 2010).
Improvement in CRF is also associated with a decrease in all-cause mortality and cardiovascular events in healthy adults (Blair et al., 1995; Kodama et al., 2009). In a recent meta-analysis, Kodama and colleagues (2009) suggest that that an increase of 1 MET of maximal aerobic capacity conferred a reduction in risk of 13% for all-cause mortality and 15% for cardiovascular events (Kodama et al., 2009). This present meta-analysis demonstrated an almost 1 MET difference between treatment and control groups. Thus findings from this meta-analysis are clinically significant and useful for practitioners. For example, simply recommending or educating sedentary adult patients on engaging in activities, such as fast-paced walking, that meet the duration of the most recent PA guidelines (Physical Activity Guidelines Advisory Committee, 2008), may improve CRF to achieve important health benefits (Anton, Duncan, Limacher, Martin, & Perri, 2011).
Our moderator analyses presented additional interesting findings. Published guidelines for the quantity and types of PA from the American College of Sports Medicine, American Heart Association, and the Department of Health and Human Services include both endurance and resistance training (Chodzko-Zajko et al., 2009; Physical Activity Guidelines Advisory Committee, 2008). Examples of endurance exercise would be aerobic activities such as walking, bicycling, swimming, and running. Examples of resistance training would include weight training or body weight exercises. In this analysis, motivational PA interventions that recommended endurance plus resistance exercises demonstrated higher ESs than studies that only recommended endurance exercises. These findings are similar to a recent meta-analysis examining the effectiveness of PA interventions using aerobic alone versus combined resistance and aerobic training among people with coronary artery disease (Brennan, 2012). Thus findings from this meta-analysis support the importance of recommending and incorporating both types of PA to improve CRF.
A particularly surprising finding was the lack of impact of recommended intensity in affecting CRF. Progressive and higher intensity PA has been linked with increased CRF (Anton et al., 2011; Metcalfe, Babraj, Fawkner, & Vollaard, 2012). This moderator analysis finding should be interpreted with caution, and may be due to the imprecision of describing and recommending exercise intensity to participants across studies. Furthermore, since participant PA behavior was not actually observed as part of the interventions, no accurate, objective measure of intensity could be obtained.
Method of CRF measurement, either direct or indirect, did not appear to be a significant moderator. This is an important finding because direct testing of VO2 max is often not feasible in the clinical setting. Furthermore, direct testing of VO2 max may only capture data for specific populations. For example, directly measuring of VO2 max among older adults, who may have some mobility or cognitive issues, may be more difficult than among younger adults. Although more commonly used in the clinical setting, submaximal predictive tests, such as exercise treadmill and cycle ergometry protocols, have demonstrated varying levels of criterion validity (Dabney & Butler, 2006; Grant et al., 1995, McArdle et al., 2010). Findings from this meta-analysis suggest that motivational PA interventions improve CRF regardless of CRF testing method. Consequently, clinicians and researchers may consider tracking their patients’ or participants’ progress in CRF using typical clinical measures, as opposed to considering a more expensive and burdensome study.
Additional non-significant moderators were found. Moderator analyses findings suggest that targeting multiple health behaviors such as diet and PA may be equally effective in improving CRF as interventions solely targeting PA behavior. Furthermore, motivational PA interventions may be effective in improving CRF among patients with varied exercise histories. Effectiveness in improving CRF does not appear to be dependent upon intervention delivery by an exercise specialist. Thus, nurses, who operate on the front lines of preventive care, may be adequately poised to successfully implement these types of interventions. These moderator findings should be interpreted with care, however, as absence of statistically significant differences among the variables may be related to the limited number of comparisons available for analysis. Moreover, moderator analyses are hypotheses generating and do not demonstrate cause and effect relationships. Rather these findings warrant further primary research directly comparing motivational PA interventions with the presence or absence of these specific variables.
In terms of population characteristics, moderator analyses of these motivational PA interventions demonstrated that younger populations might experience better CRF outcomes than older populations. Aging results in an incremental decrease in CRF starting at the age of 25 (McArdle et al., 2010; Spirduso, Francis, & MacRae, 2005). To improve and maintain CRF, older adults may need PA interventions that focus on different motivational and educational targets than younger populations (Spirduso et al, 2005). Future research could examine the efficacy of improving CRF through motivational PA interventions that might be more appropriate for older populations.
This study does have some limitations. Meta-analyses are observational studies, and the moderator analyses findings from this study are intended to encourage further research rather than imply causation. Additionally, findings of this meta-analysis may be limited in terms of generalization to diverse populations. The samples from the included studies were all healthy, community-dwelling, predominantly middle-aged adults, with women being fairly well represented. In contrast, ethnically diverse populations as well as older adult populations were less represented. As a result, the findings of this study should be interpreted with caution among these populations. Future primary research is needed to examine the effectiveness of motivational and educational interventions populations who are at higher risk for chronic illnesses and potential disability.
Another limitation of this meta-analysis is related to the primary studies included. While several moderators were examined in this study, data to potentially link actually achieved doses of PA behavior from motivational PA interventions to changes in CRF were not consistently reported across studies. Very few studies reported PA behavior outcome data; thus meta-regression is not recommended (Borenstein, et al., 2009). As a result, the potential dose-response connection between actually achieved PA behavior and CRF changes is yet unclear. Future studies testing motivational PA interventions effects on CRF should strive to incorporate valid and reliable measures of PA behavior dose, such as frequency, duration, and intensity.
This study is also limited by the primary studies excluded from the analysis. Some potential studies were excluded because they inadequately reported data. However, these studies may have included some important characteristics of interventions and study designs that could have contributed to identifying potential moderators. Publication bias, which results in unintentional exclusion of the unpublished literature, may also limit this meta-analysis (Conn, Valentine, Cooper, & Rantz, 2003; Cooper, Hedges, & Valentine, 2009). While researchers for this study employed extensive and diverse search strategies to uncover all available literature in this research area, the grey literature, such as dissertations and conference abstracts, is still difficult to find and obtain (Conn et al., 2003; Rothstein & Hopewell, 2009). This meta-analysis used data from a larger parent study; thus limitations related to year of included published studies exist. Moderator analysis of year of publication demonstrated only minimal impact on ES, however.
Despite these limitations, this study has several strengths and adds to the body of research knowledge related to PA interventions and CRF. Specific strengths include the comprehensive strategies used to identify eligible studies and the rigorous methods utilized for data extraction and data analysis. This is the first study to quantitatively synthesize the effectiveness of motivational PA interventions to increase CRF. Furthermore, the moderator analyses conducted for this project have uncovered research gaps that may inspire further studies. This study also has implications for clinical practice and future research. Clinicians should consider implementing motivational PA interventions among their healthy adult clients, not only to increase their PA behavior, but also to improve CRF, which could improve health outcomes. Future research should focus on testing these motivational and educational PA interventions among diverse populations to determine their efficacy on CRF and to reduce health disparities. Finally, to contribute to long-term reduction in chronic illness and disability, researchers should examine ways to extend and maintain PA behavior among healthy adults as they age.
Acknowledgements
Conflicts of Interest and Source of Funding:
This work was supported by the National Institutes of Health [grant number R01NR009656 to Vicki Conn], the National Institute Of Nursing Research [grant number F31NR013586 to Jo-Ana Chase], and the John A. Hartford Foundation's National Hartford Centers of Gerontological Nursing Excellence Award Program [Patricia G. Archbold Scholarship to Jo-Ana Chase]. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute Of Nursing Research or the National Institutes of Health.
The authors would like to thank Lorraine Phillips, PhD, RN, Assistant Professor, University of Missouri Sinclair School of Nursing, for her valuable input during preparations for this manuscript.
Contributor Information
Jo-Ana D. Chase, Doctoral Student, University of Missouri Sinclair School of Nursing.
Vicki S. Conn, Associate Dean for Research, Potter-Brinton Professor, University of Missouri Sinclair School of Nursing.
References
- Abraham C, Michie S. A taxonomy of behavior change techniques used in interventions. Health Psychology: Official Journal of the Division of Health Psychology, American Psychological Association. 2008;27(3):379–387. doi: 10.1037/0278-6133.27.3.379. [DOI] [PubMed] [Google Scholar]
- Ainsworth BE, Haskell WL, Leon AS, Jacobs DR, Jr, Montoye HJ, Sallis JF, Paffenbarger RS., Jr Compendium of physical activities: classification of energy costs of human physical activities. Medicine and Science in Sports and Exercise. 1993;25(1):71–80. doi: 10.1249/00005768-199301000-00011. [DOI] [PubMed] [Google Scholar]
- Anton SD, Duncan GE, Limacher MC, Martin AD, Perri MG. How much walking is needed to improve cardiorespiratory fitness? An examination of the 2008 Physical Activity Guidelines for Americans. Research Quarterly for Exercise and Sport. 2011;82(2):365–370. doi: 10.1080/02701367.2011.10599766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balk EM, Bonis PAL, Moskowitz H, Schmid CH, Ioannidis JPA, Wang C, Lau J. Correlation of quality measures with estimates of treatment effect in meta-analyses of randomized controlled trials. The Journal of the American Medical Association. 2002;287(22):2973–2982. doi: 10.1001/jama.287.22.2973. [DOI] [PubMed] [Google Scholar]
- Blair SN, Kampert JB. Influences of cardiorespiratory fitness and other precursors on cardiovascular disease and all-cause mortality in men and women. The Journal of the American Medical Association. 1996;276(3):205–210. [PubMed] [Google Scholar]
- Borenstein M, Hedges LV, Higgins JPT, Rothstein HR. Comprehensive meta-analysis. Englewood, NJ: Biostat; 2005. [Google Scholar]
- Borenstein M, Hedges LV, Higgins JPT, Rothstein HR. Introduction to meta-analysis. 1st ed. Chinchester, UK: Wiley; 2009. [Google Scholar]
- Brennan B. Combined resistance and aerobic training is more effective than aerobic training alone in people with coronary artery disease. Journal of Physiotherapy. 2012;58(2):129. doi: 10.1016/S1836-9553(12)70095-4. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control Prevention. Physical activity for everyone: Glossary of terms. 2011 Retrieved September 29, 2012, from http://www.cdc.gov/physicalactivity/everyone/glossary/
- Centers for Disease Control Prevention. Chronic disease: Overview. 2012 Retrieved September 29, 2012, from http://www.cdc.gov/chronicdisease/overview/index.htm.
- Chen J, Das S, Barlow CE, Grundy S, Lakoski SG. Fitness, fatness, and systolic blood pressure: data from the Cooper Center Longitudinal Study. American Heart Journal. 2010;160(1):166–170. doi: 10.1016/j.ahj.2010.04.014. [DOI] [PubMed] [Google Scholar]
- Chodzko-Zajko WJ, Proctor DN, Fiatarone Singh MA, Minson CT, Nigg CR, Salem GJ, Skinner JS. American College of Sports Medicine position stand: Exercise and physical activity for older adults. Medicine and Science in Sports and Exercise. 2009;41(7):1510–1530. doi: 10.1249/MSS.0b013e3181a0c95c. [DOI] [PubMed] [Google Scholar]
- Conn VS, Hafdahl AR, Mehr DR. Interventions to increase physical activity among healthy adults: Meta-analysis of outcomes. American Journal of Public Health. 2011;101(4):751–758. doi: 10.2105/AJPH.2010.194381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conn VS, Phillips LJ, Ruppar TM, Chase JD. Physical activity interventions with healthy minority adults: meta-analysis of behavior and health outcomes. Journal of Health Care for the Poor and Underserved. 2012;23(1):59–80. doi: 10.1353/hpu.2012.0032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conn VS, Rantz MJ. Research methods: managing primary study quality in meta-analyses. Research in Nursing & Health. 2003;26(4):322–333. doi: 10.1002/nur.10092. [DOI] [PubMed] [Google Scholar]
- Conn VS, Valentine JC, Cooper HM. Interventions to increase physical activity among aging adults: A meta-analysis. Annals of Behavioral Medicine: A Publication of the Society of Behavioral Medicine. 2002;24(3):190–200. doi: 10.1207/S15324796ABM2403_04. [DOI] [PubMed] [Google Scholar]
- Conn VS, Valentine JC, Cooper HM, Rantz MJ. Grey literature in meta-analyses. Nursing Research. 2003;52(4):256–261. doi: 10.1097/00006199-200307000-00008. [DOI] [PubMed] [Google Scholar]
- Cooper HM, Hedges LV, Valentine JC. The handbook of research synthesis and meta-analysis. 2nd ed. New York: Russell Sage Foundation; 2009. [Google Scholar]
- Crist LA, Champagne CM, Corsino L, Lien LF, Zhang G, Young DR. Influence of change in aerobic fitness and weight on prevalence of metabolic syndrome. Preventing Chronic Disease. 2012:9. doi: 10.5888/pcd9.110171. Retrieved from http://www.cdc.gov/pcd/issues/2012/11_0171.htm. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dabney U, Butler M. The predictive ability of the YMCA Test and Bruce Test for triathletes with different training backgrounds. Emporia State Research Studies. 2006;43(1):38–44. [Google Scholar]
- Farrell SW, Finley CE, McAuley PA, Frierson GM. Cardiorespiratory fitness, different measures of adiposity, and total cancer mortality in women. Obesity. 2011;19(11):2261–2267. doi: 10.1038/oby.2010.345. [DOI] [PubMed] [Google Scholar]
- Grant S, Corbett K, Amjad AM, Wilson J, Aitchison T. A comparison of methods of predicting maximum oxygen uptake. British Journal of Sports Medicine. 1995;29(3):147–152. doi: 10.1136/bjsm.29.3.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopewell S, Clarke M, Mallett S. Grey literature and systematic reviews. In: Rothstein HR, Sutton AJ, Borenstein M, editors. Publication Bias in Meta-Analysis: Prevention, Assessment and Adjustments. Chinchester, UK: John Wiley & Sons, Ltd.; 2005. pp. 49–70. [Google Scholar]
- Kodama S. Cardiorespiratory fitness as a quantitative predictor of all-cause mortality and cardiovascular events in healthy men and women: A meta-analysis. The Journal of the American Medical Association. 2009;301(19):2024–2035. doi: 10.1001/jama.2009.681. [DOI] [PubMed] [Google Scholar]
- Larsen FJ, Anderson M, Ekblom B, Nyström T. Cardiorespiratory fitness predicts insulin action and secretion in healthy individuals. Metabolism: Clinical and Experimental. 2012;61(1):12–16. doi: 10.1016/j.metabol.2011.05.010. [DOI] [PubMed] [Google Scholar]
- Laukkanen JA, Mäkikallio TH, Rauramaa R, Kiviniemi V, Ronkainen K, Kurl S. Cardiorespiratory fitness is related to the risk of sudden cardiac death: a population-based follow-up study. Journal of the American College of Cardiology. 2010;56(18):1476–1483. doi: 10.1016/j.jacc.2010.05.043. [DOI] [PubMed] [Google Scholar]
- McArdle WD, Katch FI, Katch VL. Exercise physiology: Nutrition, energy, and human performance. Lippincott Williams & Wilkins; 2010. [Google Scholar]
- McArdle WD, Katch FI, Pechar GS. Comparison of continuous and discontinuous treadmill and bicycle tests for VO2 max. Medicine and Science in Sports. 1973;5(3):156–160. [PubMed] [Google Scholar]
- Metcalfe RS, Babraj JA, Fawkner SG, Vollaard NBJ. Towards the minimal amount of exercise for improving metabolic health: beneficial effects of reduced-exertion high-intensity interval training. European Journal of Applied Physiology. 2012;112(7):2767–2775. doi: 10.1007/s00421-011-2254-z. [DOI] [PubMed] [Google Scholar]
- Michie S, Johnston M, Francis J, Hardeman W, Eccles M. From theory to intervention: Mapping theoretically derived behavioural determinants to behaviour change techniques. Applied Psychology. 2008;57(4):660–680. [Google Scholar]
- Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. British Medical Journal. 2009;339:2535. [PMC free article] [PubMed] [Google Scholar]
- Müller-Riemenschneider F, Reinhold T, Nocon M, Willich SN. Long-term effectiveness of interventions promoting physical activity: A systematic review. Preventive Medicine. 2008;47(4):354–368. doi: 10.1016/j.ypmed.2008.07.006. [DOI] [PubMed] [Google Scholar]
- Myers J, Prakash M, Froelicher V, Do D, Partington S, Atwood JE. Exercise capacity and mortality among men referred for exercise testing. New England Journal of Medicine. 2002;346(11):793–801. doi: 10.1056/NEJMoa011858. [DOI] [PubMed] [Google Scholar]
- Oja P. Dose response between total volume of physical activity and health and fitness. Medicine and Science in Sports and Exercise. 2001;33(6 Suppl):S428–S437. doi: 10.1097/00005768-200106001-00011. [DOI] [PubMed] [Google Scholar]
- Physical Activity Guidelines Advisory Committee. Physical activity guidelines advisory committee report. Washington, DC: Department of Health and Human Services; 2008. [DOI] [PubMed] [Google Scholar]
- Rhéaume C, Arsenault BJ, Dumas M-P, Pérusse L, Tremblay A, Bouchard C, … Després J-P. Contributions of cardiorespiratory fitness and visceral adiposity to six-year changes in cardiometabolic risk markers in apparently healthy men and women. The Journal of Clinical Endocrinology and Metabolism. 2011;96(5):1462–1468. doi: 10.1210/jc.2010-2432. [DOI] [PubMed] [Google Scholar]
- Rothstein HR, Hopewell S. Grey literature. In: Cooper HM, Hedges LV, Valentine JC, editors. The handbook of research synthesis and meta-analysis. 2nd ed. New York, NY: Russell Sage Foundation; 2009. pp. 103–129. [Google Scholar]
- Sattler C, Erickson KI, Toro P, Schröder J. Physical fitness as a protective factor for cognitive impairment in a prospective population-based study in Germany. Journal of Alzheimer’s Disease. 2011;26(4):709–718. doi: 10.3233/JAD-2011-110548. [DOI] [PubMed] [Google Scholar]
- Simons-Morton DG, Calfas KJ, Oldenburg B, Burton NW. Effects of interventions in health care settings on physical activity or cardiorespiratory fitness. American Journal of Preventive Medicine. 1998;15(4):413–430. doi: 10.1016/s0749-3797(98)00078-6. [DOI] [PubMed] [Google Scholar]
- Spirduso WW, Francis KL, MacRae PG. Physical dimensions of aging. 2nd ed. Champaign, IL: Human Kinetics; 2005. [Google Scholar]
- Thompson WR, Gordon NF, Pescatello LS, editors. ACSM’s guidelines for exercise testing and prescription. 8th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2009. [Google Scholar]
- US Department of Health and Human Services. 2008 Physical activity guidelines for Americans. 2008 Retrieved from http://www.health.gov/paguidelines/pdf/paguide.pdf.
- Valentine JC. Judging the quality of primary research. In: Cooper HM, Hedges LV, Valentine JC, editors. The handbook of research synthesis and meta-analysis. 2nd ed. New York, NY: Russell Sage Foundation; 2009. pp. 122–146. [Google Scholar]
- Vanhees L, Lefevre J, Philippaerts R, Martens M, Huygens W, Troosters T, Beunen G. How to assess physical activity? How to assess physical fitness? European Journal of Cardiovascular Prevention and Rehabilitation: Official Journal of the European Society of Cardiology, Working Groups on Epidemiology & Prevention and Cardiac Rehabilitation and Exercise Physiology. 2005;12(2):102–114. doi: 10.1097/01.hjr.0000161551.73095.9c. [DOI] [PubMed] [Google Scholar]
- Williams PT. Usefulness of cardiorespiratory fitness to predict coronary heart disease risk independent of physical activity. The American Journal of Cardiology. 2010;106(2):210–215. doi: 10.1016/j.amjcard.2010.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]