Abstract
Background
The largest-ever outbreak of Ebola virus disease (EVD), ongoing in West Africa since late 2013, has led to export of cases to Europe and North America. Clinicians encountering ill travelers arriving from countries with widespread Ebola virus transmission must be aware of alternate diagnoses associated with fever and other nonspecific symptoms.
Objective
To define the spectrum of illness observed in persons returning from areas of West Africa where EVD transmission has been widespread.
Design
Descriptive, using GeoSentinel records.
Setting
57 travel or tropical medicine clinics in 25 countries.
Patients
805 ill returned travelers and new immigrants from Sierra Leone, Liberia, or Guinea seen between September 2009 and August 2014.
Measurements
Frequencies of demographic and travel-related characteristics and illnesses reported.
Results
The most common specific diagnosis among 770 nonimmigrant travelers was malaria (n = 310 [40.3%]), with Plasmodium falciparum or severe malaria in 267 (86%) and non–P. falciparum malaria in 43 (14%). Acute diarrhea was the second most common diagnosis among nonimmigrant travelers (n = 95 [12.3%]). Such common diagnoses as upper respiratory tract infection, urinary tract infection, and influenza-like illness occurred in only 26, 9, and 7 returning travelers, respectively. Few instances of typhoid fever (n = 8), acute HIV infection (n = 5), and dengue (n = 2) were encountered.
Limitation
Surveillance data collected by specialist clinics may not be representative of all ill returned travelers.
Conclusion
Although EVD may currently drive clinical evaluation of ill travelers arriving from Sierra Leone, Liberia, and Guinea, clinicians must be aware of other more common, potentially fatal diseases. Malaria remains a common diagnosis among travelers seen at GeoSentinel sites. Prompt exclusion of malaria and other life-threatening conditions is critical to limiting morbidity and mortality.
Primary Funding Source
Centers for Disease Control and Prevention.
The emergence of Ebola virus disease (EVD) in eastern Guinea in December 2013 was followed by rapid spread to neighboring Liberia and Sierra Leone (1, 2). This outbreak has since grown into the largest Ebola virus epidemic in history, with more than 25 000 confirmed cases and 10 445 deaths as of 1 April 2015 (1, 3). Global spread of EVD, which has been a concern since the Guinean Ministry of Health declared the outbreak and the World Health Organization began monitoring it in March 2014 (4), has recently escalated, with almost 20 case patients now cared for outside West Africa (5, 6). The resulting international response and fear have sometimes led to delayed evaluation and management of non-Ebola-related febrile illnesses among travelers arriving from West Africa (7–10).
Patients with EVD typically present with fever; fatigue; myalgia; headache; and gastrointestinal symptoms, such as abdominal pain, diarrhea, and vomiting, which worsen over time; overt hemorrhage is less common (11–15). These nonspecific symptoms are shared by many other frequently encountered infectious diseases (16). A recent analysis of illness among travelers from Africa presenting at GeoSentinel sites revealed that those who had been in West Africa were most commonly diagnosed with Plasmodium falciparum malaria, viral syndromes, acute and chronic diarrhea, unspecified febrile illness, and respiratory tract infection (17). Of the 6 deaths in travelers returning from West Africa described in the analysis, 5 were due to severe P. falciparum malaria and 1 was due to disseminated tuberculosis (17).
Current EVD case definitions are relatively nonspecific and generally consist of a history of travel to a country with widespread Ebola virus transmission and presence of fever or other symptoms (18, 19). To characterize the types of illness commonly encountered in travelers arriving from Sierra Leone, Liberia, or Guinea (the countries with widespread Ebola virus transmission), we analyzed GeoSentinel data from September 2009 through August 2014 for travelers and new immigrants from these 3 West African countries. Our aim was to provide an evidence base to inform the differential diagnosis of sick travelers from the Ebola epidemic region and improve evaluation and medical management.
Methods
Data Source
Data were collected using the GeoSentinel Global Surveillance Network platform. This network comprises 57 specialized travel and tropical medicine clinics on 6 continents that provide routine clinical care to ill travelers and contribute anonymous, delinked travel surveillance data on these patients to a centralized database (additional details are available at www.geosentinel.org) (20, 21). GeoSentinel’s data collection protocol is classified as public health surveillance and not as human subjects research requiring submission to institutional review boards.
GeoSentinel sites are staffed by specialists in travel and tropical medicine and are typically secondary or tertiary points of care for patients who self-refer or are referred from emergency departments or primary care providers or who are admitted to site-associated hospitals. Physicians at the sites determine the final diagnoses and diagnostic codes, which are selected from a standardized list of 522 diagnostic entities, some of which are etiologic (such as Streptococcus pneumoniae) and others of which are syndromic (such as cough). Syndromic codes are entered when an etiologic code cannot be assigned because of use of empirical therapy, self-limited disease, or inability to justify complete or sophisticated work-up as part of routine clinical practice. GeoSentinel sites contribute microbiologically confirmed data, where available, based on the best reference diagnostic tests (including molecular diagnostics) available at the time. “Probable” diagnoses are restricted to patients with pathognomonic physical findings (such as tick eschar), clinical response to highly specific therapy, or classic presentation and exposure history with laboratory exclusion of other possible causes.
Definitions and Classifications
Six possible travel purpose designations were used in this analysis: tourism; business; missionary, volunteer, research, or aid work; visiting friends and relatives (VFR); student; and immigration (including refugees). A VFR traveler was defined as a first- or second-generation immigrant who returned to their or their parents’ homeland to “visit friends and relatives.” This term usually applies to travelers going from a high-income country of current residence to a low-income country of origin (22). Country of exposure was determined only when ascertainable by the evaluating clinician and was based on travel itinerary, disease endemicity, and incubation periods.
Inclusion Criteria
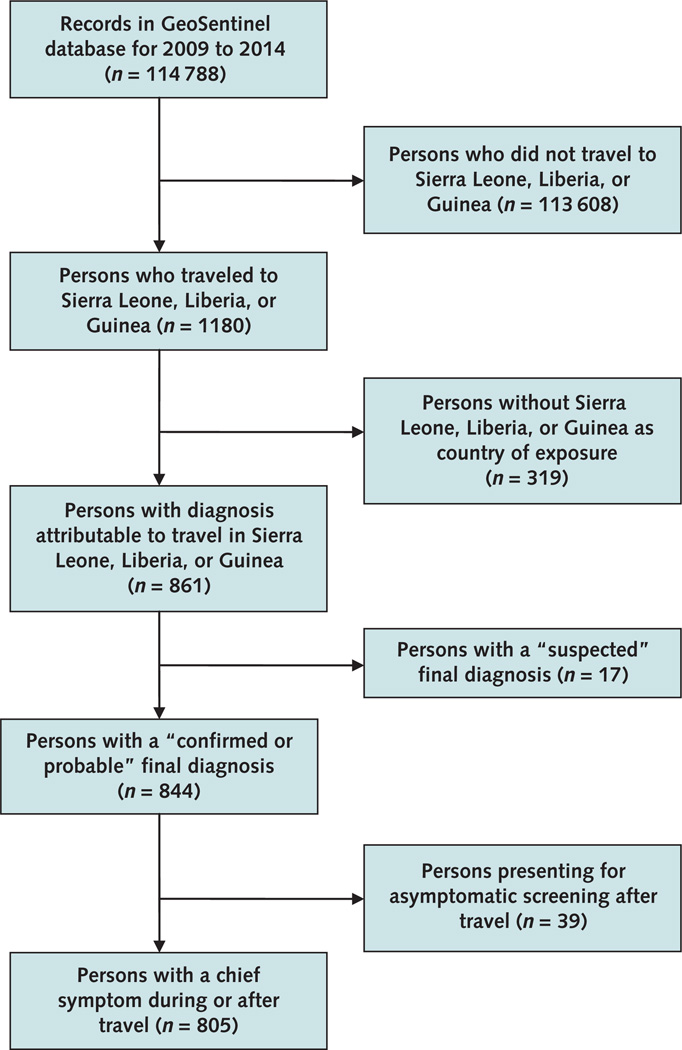
We analyzed demographic, clinical, and travel-related data on travelers arriving or immigrating from Sierra Leone, Liberia, or Guinea and seen at any GeoSentinel Surveillance Network site from 1 September 2009 through 31 August 2014. We included only patients with a probable or confirmed final diagnosis (specific etiologic or syndromic diagnosis as described earlier) and a presenting chief symptom other than asymptomatic screening (Figure).
Figure. 1.
Study flow diagram.
Statistical Analysis
Data were managed in a Structured Query Language database and analyzed for the frequency of travel-related diagnoses by using descriptive statistics. SPSS software (IBM) was used for all statistical computations.
Role of the Funding Source
The GeoSentinel Surveillance Network has an independent Data Use and Publication Committee that oversees analyses of the database from conception to final knowledge product and comprises 5 site directors outside the Centers for Disease Control and Prevention (CDC). Staff from the CDC were involved in the study design; collection, analysis, and interpretation of data; and writing of the manuscript. The final manuscript also received CDC internal clearance.
Results
Patients and Demographic Characteristics
For the surveillance period reported, more than 100 000 records were entered into the GeoSentinel Surveillance Network database (Figure). Of those, 1180 persons had traveled to or immigrated from Sierra Leone, Liberia, or Guinea. We evaluated 805 patients who had confirmed or probable diagnoses and at least 1 presenting chief symptom (>1 diagnosis was possible for each patient). Major demographic characteristics are summarized in Table 1. The most common purpose of travel was VFR (29%), followed by business (27%) and the collective group of missionary, volunteer, research, or aid work (27%) (Table 1). Duration of travel was highly variable, although nonimmigrants traveled for a median of 36 days (range, 0 to 5111 days). Systemic febrile illness was the most common syndrome classification, occurring more than twice as often as the second most frequent syndrome (acute diarrhea) (Table 1).
Table 1.
Demographic Characteristics of 805 Returned Travelers or New Immigrants Presenting at a GeoSentinel Surveillance Network Site for Care of an Illness Related to Travel to Sierra Leone, Liberia, or Guinea, 2009–2014*
| Characteristic | All Travelers (n= 805) |
Purpose of Travel |
|||||
|---|---|---|---|---|---|---|---|
| Visiting Friends and Relatives (n= 235) |
Business (n= 220) |
Missionary Volunteer Research, or Aid Work (n= 216) |
Tourism (n= 83) |
Immigration (n= 35) |
Student (n= 16) |
||
|
Sex† | |||||||
| Male | 503 (62.5) | 153 (65.1) | 181 (82.3) | 91 (42.1) | 49 (59.0) | 12 (34.3) | 6 (37.5) |
| Female | 301 (37.4) | 81 (34.5) | 39 (17.7) | 125 (57.9) | 34 (41.0) | 23 (65.7) | 10 (62.5) |
| Median age (range)y | 34 (1–95) | 33 (1–88) | 41 (4–72) | 33 (7–67) | 36 (1–95) | 28 (4–61) | 20.5 (13–27) |
| Patient type | |||||||
| Inpatient | 219 (27.2) | 102 (43.4) | 62 (28.2) | 27 (12.5) | 24 (28.9) | 2 (5.7) | 2 (12.5) |
| Outpatient | 586 (72.8) | 133 (56.6) | 158 (71.8) | 189 (87.5) | 59 (71.1) | 33 (94.3) | 14 (87.5) |
| Median travel duration (range)d | 36 (0–5111) | 38 (7–4880) | 33 (2–5111) | 57 (2–3115) | 22 (0–3712) | NA | 40 (27–1320) |
|
Pretravel medical encounter | |||||||
| Yes | 420 (52.2) | 77 (32.8) | 123 (55.9) | 144 (66.7) | 43 (51.8) | NA | 12 (75.0) |
| No | 221 (27.5) | 110 (46.8) | 51 (23.2) | 26 (12.0) | 24 (28.9) | NA | 1 (6.3) |
| Unknown | 164 (20.4) | 48 (20.4) | 46 (20.9) | 46 (21.3) | 16 (19.3) | NA | 3 (18.7) |
| Syndromic diagnoses‡ | |||||||
| Systemic febrile illness | 396 (49.2) | 161 (68.5) | 112 (50.9) | 70 (32.4) | 41 (49.4) | 6 (17.1) | 6 (37.5) |
| Acute diarrhea | 158 (19.6) | 20 (8.5) | 50 (22.7) | 56 (25.9) | 17 (20.5) | 8 (22.9) | 7 (43.8) |
| Other gastrointestinal | 94 (11.7) | 29 (12.3) | 13 (5.9) | 15 (6.9) | 3 (3.6) | 31 (88.6) | 3 (18.7) |
| Respiratory | 54 (6.7) | 15 (6.4) | 9 (4.1) | 20 (9.3) | 7 (8.4) | 3 (8.6) | 0 (0) |
| Birth country | |||||||
| Guinea | 117 (14.5) | 102 (43.4) | 5 (2.3) | 1 (0.5) | 3 (3.6) | 6 (17.1) | 0 (0) |
| Liberia | 45 (5.6) | 25 (10.6) | 0 (0) | 2 (0.9) | 2 (2.4) | 16 (45.7) | 0 (0) |
| Sierra Leone | 59 (7.3) | 41 (17.4) | 2 (0.9) | 2 (0.9) | 4 (4.8) | 10 (28.6) | 0 (0) |
| Other§ | 584 (72.5) | 67 (28.5) | 213 (96.8) | 211 (97.7) | 74 (89.2) | 3 (8.6) | 16 (100) |
NA = not available.
Values are numbers (percentages) unless otherwise indicated.
Missing for 1 traveler visiting friends and relatives.
Travelers could have >1 diagnosis.
Top 5 others were the United States (n = 97), the United Kingdom (n = 87), Germany (n = 62), Canada (n = 61), and France (n = 57).
Diagnoses
We identified 1062 diagnoses (954 confirmed and 108 probable) in the 805 ill travelers and new immigrants coming from countries with widespread Ebola virus transmission. The most frequent diagnoses among nonimmigrant travelers from Sierra Leone, Liberia, or Guinea were malaria (primarily P. falciparum), acute diarrhea, a nonspecific viral syndrome, influenzalike illness or upper respiratory tract infection, and febrile illness not otherwise specified but lasting less than 3 weeks (Table 2). The most frequent diagnoses among immigrants were latent tuberculosis, dental caries, schistosomiasis, strongyloidiasis, and giardiasis (Table 2). There were 57 children (younger than 18 years) among the 805 ill travelers and new immigrants. The most common diagnosis among them was malaria (40.3%), followed by giardiasis (8.8%), anemia (7%), cutaneous fungal infection (7%), and upper respiratory tract infection (5.3%). Table 3 lists the most common travel-related diagnoses for travelers presenting with a chief symptom of fever or gastrointestinal symptoms.
Table 2.
Five Most Common Syndromic and Etiologic Diagnoses, by Purpose of Travel, Among 805 Ill Returned Travelers Seen at a GeoSentinel Surveillance Network Site With Diagnoses Related to Travel to Sierra Leone, Liberia, or Guinea, 2009–2014*
| Rank | Nonimmigrant Travelers | Immigrants With Travel-Related Diagnoses (n= 35) |
|||||
|---|---|---|---|---|---|---|---|
| All (n= 770) | Visiting Friends and Relatives (n= 235) |
Business (n= 220) |
Missionary Volunteer Research, or Aid Work(n= 216) |
Tourism (n= 83) |
Student (n= 16) |
||
| 1 | Malaria: 310 (40.3) (P. falciparum or severe: 267 [34.7]) |
Malaria: 138 (58.7) (P. falciparum or severe: 126 [53.6]) |
Malaria: 87 (39.5) (P. falciparum or severe: 69 [31.4]) |
Malaria: 50 (23.1) (P. falciparum or severe: 39 [18.1]) |
Malaria: 30 (36.1) (P. falciparum or severe: 28 [33.7]) |
Malaria: 5 (31.3) (P. falciparum or severe: 5 [31.3]) |
LTBI: 15 (42.9) |
| 2 | Acute diarrhea: 95 (12.3)† |
Acute diarrhea: 13 (5.5)† |
Acute diarrhea: 34 (15.5)† |
Acute diarrhea: 32 (14.8)† |
Acute diarrhea: 12 (14.5)† |
Acute diarrhea: 4 (25.0)† |
Dental caries: 11 (31.4) |
| 3 | URTI/ILI: 32 (4.2) | Unspecified febrile illness lasting <3 wk: 9 (3.8) |
Viral syndrome: 9 (4.1) |
URTI/ILI: 13 (6.0) | Viral syndrome: 6 (7.2) |
Superficial fungal infection: 3 (18.8) |
Schistosomiasis: 11 (31.4) |
| 4 | Viral syndrome: 29 (3.8) |
URTI/ILI: 8 (3.4) | Unspecified febrile illness lasting <3 wk: 6 (2.7) |
PI-IBS: 11 (5.1) | URTI/ILI: 5 (6.0) | PI-IBS: 2 (12.5) | Strongyloidiasis: 6 (17.1) |
| 5 | Unspecified febrile illness lasting <3 wk: 24 (3.1) |
Viral syndrome: 8 (3.4) |
URTI/ILI: 6 (2.7) | Giardiasis: 8 (3.7) | PI-IBS: 4 (4.8) | Giardiasis: 2 (12.5) | Giardiasis: 5 (14.3) |
ILI = influenza-like illness; LTBI = latent tuberculosis infection; P. falciparum = Plasmodium falciparum; PI-IBS = postinfectious irritable bowel syndrome; URTI = upper respiratory tract infection.
Values are numbers (percentages). Travelers could have >1 diagnosis.
Includes acute bacterial, parasitic, and viral diarrhea and acute diarrhea of unspecified cause.
Table 3.
Most Common Diagnoses for Specific Causes Within Syndromic Presenting Symptoms Among 805 Ill Returned Travelers Seen at a GeoSentinel Surveillance Network Site With Diagnoses Related to Travel to Sierra Leone, Liberia, or Guinea, 2009–2014
| Diagnosis, by Presenting Symptom |
Patients n (%)* |
Total Patients With Diagnosis in Database n |
|---|---|---|
| Fever(n = 267) | ||
| Malaria | 182 (58.0) | 314 |
| Plasmodium falciparum | 142 (58.4) | 243 |
| Severe/cerebral | 18 (64.3) | 28 |
| Plasmodium vivax | 2 (100) | 2 |
| Plasmodium species unknown | 9 (50.0) | 18 |
| Plasmodium ovale | 8 (50.0) | 16 |
| Plasmodium malariae | 3 (42.9) | 7 |
| Nonspecific viral syndrome | 12 (41.4) | 29 |
| Systemic febrile illness, unspecified | 15 (57.7) | 26 |
| URTI | 6 (23.1) | 26 |
| Acute UTI | 3 (33.3) | 9 |
| Enteric fever | 6 (75.0) | 8 |
| Salmonella enterica serotype Typhi | 1 (50.0) | 2 |
| Typhoid fever, unspecified | 5 (83.3) | 6 |
| Influenza/ILI | 4 (57.1) | 7 |
| Pneumonia | 4 (57.1) | 7 |
| Lobar | 3 (50.0) | 6 |
| Atypical | 1 (100) | 1 |
| Acute HIV infection, febrile | 4 (80.0) | 5 |
| Active tuberculosis | 1 (33.3) | 3 |
| Pulmonary | 0 (0) | 2 |
| Extrapulmonary | 1 (100) | 1 |
| Dengue | 2 (100) | 2 |
| Leptospirosis | 1 (50.0) | 2 |
| Gastrointestinal (n = 186) | ||
| Acute diarrhea† | 54 (56.8) | 95 |
| Giardiasis | 11 (45.8) | 24 |
| PI-IBS | 17 (77.3) | 22 |
| Chronic diarrhea | 8 (53.3) | 15 |
| Campylobacteriosis | 5 (83.3) | 6 |
| Shigellosis | 3 (100) | 3 |
| Dientamoebiasis | 2 (66.7) | 3 |
| Amoebiasis due to Entamoeba histolytica‡ | 2 (66.7) | 3 |
| Salmonellosis, nontyphoidal | 0 (0) | 1 |
ILI = influenza-like illness; PI-IBS = postinfectious irritable bowel syndrome; URTI = upper respiratory tract infection; UTI = urinary tract infection.
Percentages were calculated using the total number of patients in the database as the denominator. Travelers could present with >1 symptom.
Includes acute bacterial, parasitic, and viral diarrhea and acute diarrhea of unspecified cause.
Includes intestinal and extraintestinal amoebiasis.
Malaria was the most common diagnosis for adults and children traveling for all reasons other than immigration (Tables 2 and 3). Only 39% (n = 122) of ill returned travelers with malaria had received pretravel counseling. Most malaria was caused by P. falciparum (Table 3), followed by P. ovale. We recorded only 2 patients with confirmed dengue and 8 with enteric fever due to Salmonella enterica serotype Typhi (Table 3). Notably absent were diagnoses of spotted fever rickettsioses; chikungunya; EVD; and any other severe zoonotic viral hemorrhagic fever (VHF) endemic in Africa, such as Lassa fever, Marburg fever, or yellow fever. Five patients with acute HIV infection presenting with fever were entered into the database (Table 3). Acute diarrheal illness was a common diagnosis; however, confirmation of specific causative bacterial pathogens, including Campylobacter, nontyphoidal Salmonella, and Shigella, was rare (Table 3).
Discussion
Our analysis of GeoSentinel data highlights the spectrum of disease in a large group of travelers and new immigrants arriving from the 3 West African countries with widespread Ebola virus transmission and evaluated at our specialized posttravel clinics. In our analysis, malaria accounted for two fifths of the total diagnoses, in contrast to no diagnoses of EVD or any other severe zoonotic VHF (even those endemic in Africa) during the period analyzed. Travel-acquired zoonotic VHFs are rarely encountered in posttravel medicine clinics because these diseases typically occur in epidemics, are notifiable diseases, and are associated with movement restrictions (23–25). Plasmodium falciparum, a potentially fatal infection, accounted for 86% of the malaria diagnoses reported. Malaria has been estimated to be at least 1000 times more likely than VHF to be diagnosed in febrile travelers arriving from the region (26). Understanding the geographic distribution of febrile illnesses among arriving travelers is essential in the development of a differential diagnosis and care plan (17, 18, 27), and the constellation of signs and symptoms and the incubation period is also helpful.
Current guidance calls for triage and evaluation of ill travelers arriving from countries with widespread Ebola virus transmission or uncertain control measures (28, 29). Patients who present with fever are typically categorized as having high, intermediate, low (but not zero), or no identifiable risk on the basis of their history. Depending on the risk category, it is recommended that clinical evaluation be conducted under strict isolation procedures (19, 28, 29).
In some instances, patients placed in strict isolation while being evaluated for EVD have experienced delays in receipt of timely evaluation and appropriate treatment for alternate diseases (10). These delays were reportedly due to infection control concerns. In addition, testing for alternate infectious diagnoses or other noninfectious conditions has reportedly been delayed pending a negative Ebola virus test result, with associated delays in therapy (10). To further complicate the diagnostic problems, malaria co-infection has been reported in patients with Ebola virus infection (11, 15, 30). In these instances, a positive result on a malaria rapid test or blood smear does not necessarily rule out EVD. Finally, patients presenting within 3 days of symptom onset may be too early in the course of their infection to reliably generate a positive EVD test result. These patients may require additional Ebola virus testing, which may contribute to the additional delays in determining the definitive diagnosis and rapidly administering appropriate therapy. Every effort should be made to avoid delays in diagnosing and treating patients in whom EVD is in the differential diagnosis.
The aforementioned delays may also interfere with the immediate management of patients with undiagnosed illness severe enough to require escalation to critical care support. The risks associated with evaluating and caring for patients with EVD are recognized and debated (31, 32), but delaying such care may limit life-saving treatment for patients who are more likely to have malaria or sepsis syndromes (10, 33).
Where adequate laboratory capacity exists, ruling out malaria in febrile travelers from West Africa is critical to limiting morbidity and mortality. In situations where laboratory infrastructure is inadequate or a diagnostic result will be delayed, administration of empirical antimalarial therapy and broad-spectrum antibiotic coverage for bacteremia is imperative and potentially life-saving (34). In addition, for ill travelers from these regions who are unable to communicate their clinical history (such as those with a diminished level of consciousness), a parenteral antimalarial (if a malaria diagnosis will be delayed or laboratory infrastructure is lacking) and antibiotics are indicated. This indication is supported by our data, which showed that nearly 10% of ill travelers with malaria had severe or cerebral disease, which has a poor prognosis that is most effectively mitigated by prompt initiation of parenteral treatment. Patients with a confirmed diagnosis of malaria who are still considered at risk for EVD may continue to require isolation precautions until EVD is excluded. However, those with malaria who improve clinically and defervesce during antimalarial treatment and who do not have signs of concomitant EVD (such as large-volume diarrhea), even if they are at risk for EVD, may come out of isolation unless their clinical status changes within the 21-day Ebola virus incubation period.
Acute diarrhea was the second most common diagnosis after malaria, including diarrhea caused by Campylobacter; nontyphoidal Salmonella; Shigella; and protozoa, such as Giardia intestinalis. Travelers’ diarrhea typically has a short incubation period (2 to 7 days), with fever usually persisting for only a few days (26). In contrast, EVD-associated diarrhea is frequently large-volume and severe, is usually not bloody, develops after the emergence of high fever, and persists for several days (13, 14). By the time severe EVD-associated diarrhea develops, other more suggestive signs of EVD may be present, such as truncal maculopapular rash; conjunctival injection; and, in fewer than 30% of patients, bleeding (13, 14).
The third to fifth most common illnesses among travelers in our analysis were nonspecific febrile, viral, or mononucleosis-like syndromes; influenza-like illnesses; and upper respiratory tract infection. Collection of a complete vaccine history among travelers presenting with fever must also be a component of the medical evaluation. Respiratory illnesses are common in travelers from all regions (20, 21), including West Africa, and influenza viruses circulate year-round in the tropics (35). Hence, it is important to verify up-to-date influenza vaccination in travelers, although vaccine efficacy may vary individually and seasonally (35). Meningococcal vaccine provides coverage only for the A, C, Y, and W135 serogroups. The B serogroup rarely circulates in West Africa, but the X serogroup is increasingly reported (36). Although both the live, attenuated oral typhoid vaccine and the killed parenteral typhoid vaccine provide only 55% to 70% protection against illness caused by S. enterica serotype Typhi, they have higher efficacy against fatal forms of disease (37).
Although typical travel-acquired infections, such as dengue, chikungunya, measles, spotted fever rickettsioses, and brucellosis, often begin with a nonspecific febrile illness similar to EVD, they were infrequent or absent among travelers from Sierra Leone, Liberia, and Guinea in this analysis. Similarly, we found no diagnoses of meningococcal meningitis.
Risk for travel-related illness is particularly high in VFR travelers (20–22, 38). Although such travelers made up only 29% of our sample, they accounted for 44% of malaria diagnoses and 2 of 5 patients with acute HIV infection. They also were more likely to return with a systemic febrile illness (68.5% vs. 41% of non-VFR travelers); had higher rates of hospitalization for their illness (43% vs. 21% of non-VFR travelers); and had the lowest rates of pretravel encounter (33%) of any type of traveler, a finding that past studies have noted (22, 39). Because malaria is preventable with chemoprophylaxis and insect precautions, poor uptake of pretravel advice and intervention may translate into a proportionately higher incidence of malaria among VFR travelers. Travelers to a country with widespread transmission should have a pretravel consultation to ensure that malaria chemoprophylaxis is prescribed and vaccinations are administered. These key interventions will not only prevent disease but also inform the differential diagnosis. Thus, it is important to extend educational efforts in West African immigrant communities to improve coverage of pretravel care.
This analysis has limitations. Our results pertain to ill arriving travelers and new immigrants presenting for care at GeoSentinel Surveillance Network sites and may not apply to all ill international travelers. GeoSentinel is an epidemiologic surveillance system that is not designed to capture comprehensive and objective clinical and laboratory details. Latent tuberculosis was the most common diagnosis among ill immigrants but would not have accounted for their presenting symptoms; thus, in some cases, the final diagnosis may not reflect the chief symptom. Travelers who have illnesses with incubation periods that are short (such as influenza) or very long (such as hepatitis B virus) may be underrepresented because they sought care in alternate locations. Similarly, we are unable to provide data on incubation periods for the represented diagnoses because the date of symptom onset was only recently added to the GeoSentinel data collection instrument. In addition, calculation of incidence rates is impossible because the database lacks a denominator of all travelers from all countries to a specific region (40). Finally, because of the nature of many GeoSentinel sites, children are probably underrepresented in the database.
In summary, ill travelers from Sierra Leone, Liberia, or Guinea presenting at GeoSentinel surveillance sites are most frequently diagnosed with malaria, a clinical entity that requires prompt diagnosis and rapid initiation of treatment to limit morbidity and mortality and one whose diagnosis is almost necessarily delayed by the procedures and protocols in place for evaluating suspected EVD. Therefore, the differential diagnosis of illness in travelers arriving from countries with widespread Ebola virus transmission must include not only EVD but also malaria and other more common infections, such as influenza, other respiratory tract infections, and travelers’ diarrhea. The optimal strategy is preventing infections through comprehensive pretravel interventions and, for ill travelers, promptly diagnosing and treating illnesses, such as malaria, and initiating empirical treatment if bacteremia, influenza, or meningitis is suspected.
EDITORS’ NOTES.
Context
When evaluating ill travelers arriving from West Africa, physicians must be aware of infections other than Ebola virus disease (EVD) that travelers are likely to have acquired there.
Contribution
In 57 travel clinics in 25 countries, malaria was diagnosed in 40% of nonimmigrant travelers arriving from West Africa and was usually serious. Diagnosis and therapy of malaria should not be delayed during evaluation for EVD.
Caution
Travelers seeking care in specialty travel clinics may not be representative of all ill returned travelers.
Implication
Early diagnosis and therapy for malaria is essential in ill travelers returning from West Africa. Co-infection with malaria and EVD has been reported.
Acknowledgments
Financial Support: GeoSentinel, the Global Surveillance Network of the International Society of Travel Medicine, is supported by cooperative agreement U50/CCU412347 from the CDC. Dr. Gkrania-Klotsas was supported by the National Institute for Health Research Cambridge Biomedical Research Centre. Dr. Beeching was funded in part by the National Institute for Health Research Health Protection Research Unit in Emerging and Zoonotic Infections, a partnership among the University of Liverpool, the Liverpool School of Tropical Medicine, and Public Health England.
Appendix. The GeoSentinel Surveillance Network
Additional members of the GeoSentinel Surveillance Network who did not author the article but contributed data (in descending order) are Vanessa Field, InterHealth Worldwide, London, United Kingdom; Michael D. Libman, McGill University, Montréal, Québec, Canada; Camilla Rothe, Bernhard-Nocht-Institute for Tropical Medicine, Hamburg, Germany; Elizabeth D. Barnett, Boston University, Boston, Massachusetts; Eli Schwartz, Chaim Sheba Medical Center, Tel Hashomer, Israel; Philippe Gautret, Hôpital Nord, Marseille, France; Frank von Sonnenburg, University of Munich, Munich, Germany; Perry van Genderen, Havenziekenhuis, Rotterdam, the Netherlands; Mogens Jensenius, Ullevål University Hospital, Oslo, Norway; William M. Stauffer, University of Minnesota, Minneapolis, Minnesota; Frank Mockenhaupt, University of Berlin, Berlin, Germany; Kevin C. Kain, University of Toronto, Toronto, Ontario, Canada; Shuzo Kanagawa, International Medical Center of Japan, Tokyo, Japan; Christina M. Coyle, Albert Einstein School of Medicine, Bronx, New York; Bradley A. Connor, Cornell University, New York, New York; Johan Ursing, Karolinska University Hospital, Stockholm, Sweden; Karin Leder, Royal Melbourne Hospital, Melbourne, Australia; Jean Haulman, University of Washington, Seattle, Washington; Patricia Schlagenhauf, University of Zurich, Zurich, Switzerland; Jean Vincelette, Central Hospital of the University of Montréal, Montréal, Québec, Canada; Anne McCarthy, University of Ottawa, Ottawa, Ontario, Canada; Prativa Pandey, CIWEC Clinic Travel Medicine Center, Kathmandu, Nepal; Lin H. Chen, Mount Auburn Hospital, Harvard University, Cambridge, Massachusetts; John D. Cahill, St. Luke’s–Roosevelt Hospital Center, New York, New York; Christophe Rapp, Begin Military Hospital of Vincennes, Paris, France; Brian Kendall, University of Utah, Salt Lake City, Utah; David Lalloo, Liverpool School of Tropical Medicine, Liverpool, United Kingdom; Yukihiro Yoshimura, Yokohama Municipal Citizen’s Hospital, Yokohama, Japan; Emanuel Bottieau, Institute of Tropical Medicine, Antwerp, Belgium; Wayne Ghesquiere, Vancouver General Hospital, Vancouver, British Columbia, Canada; Kitty Smith, Health Protection Scotland, Glasgow, United Kingdom; Watcharapong Piyaphanee, Mahidol University, Bangkok, Thailand; Carmelo Licitra, Orlando Regional Health Center, Orlando, Florida; Johnnie Yates, Kaiser Permanente Honolulu Clinic, Honolulu, Hawaii; Luis Manuel Valdez, Clinica Anglo Americana, Lima, Peru; Marc Shaw, Worldwise Travellers Health and Vaccination Centre, Auckland, New Zealand; Andy Wang, United Family Hospital and Clinics, Beijing, People’s Republic of China; and Sarah Borwein, Central Health Medical Practice, Hong Kong SAR, China.
Footnotes
Disclaimer: The findings and conclusions in this article are those of the authors and do not necessarily represent the official position of the CDC, the National Health Service, the National Institute for Health Research, the Department of Health, or Public Health England.
Disclosures: Disclosures can be viewed at www.acponline.org/authors/icmje/ConflictOfInterestForms.do?msNum=M15-0074.
Reproducible Research Statement:Study protocol and statistical code: Available from Dr. Boggild (andrea.boggild@utoronto.ca). Data set: Not available.
Author Contributions: Conception and design: A.K. Boggild, D.H. Esposito, P.E. Kozarsky, V. Ansdell, E. Gkrania-Klotsas, M.P. Grobusch, S.H.F. Hagmann, N.A. Hynes, P.L. Lim, M.J. Sotir, D.H. Hamer.
Analysis and interpretation of the data: A.K. Boggild, D.H. Esposito, P.E. Kozarsky, V. Ansdell, D. Campion, F. Castelli, J.P. Cramer, E. Gkrania-Klotsas, M.P. Grobusch, S.H.F. Hagmann, N.A. Hynes, P.L. Lim, D.J.M. Malvy, M. Mendelson, P. Parola, M.J. Sotir, D.H. Hamer.
Drafting of the article: A.K. Boggild, D.H. Esposito, P.E. Kozarsky, V. Ansdell, N.J. Beeching, M.P. Grobusch, N.A. Hynes, M.J. Sotir, D.H. Hamer.
Critical revision of the article for important intellectual content: A.K. Boggild, D.H. Esposito, P.E. Kozarsky, V. Ansdell, N.J. Beeching, D. Campion, F. Castelli, E. Caumes, F. Chappuis, J.P. Cramer, E. Gkrania-Klotsas, S.H.F. Hagmann, N.A. Hynes, P.L. Lim, D.J.M. Malvy, M. Mendelson, M.J. Sotir, H.M. Wu, D.H. Hamer.
Final approval of the article: A.K. Boggild, D.H. Esposito, P.E. Kozarsky, V. Ansdell, N.J. Beeching, D. Campion, F. Castelli, E. Caumes, J.P. Cramer, E. Gkrania-Klotsas, M.P. Grobusch, S.H.F. Hagmann, N.A. Hynes, P.L. Lim, R. López-Vélez, D.J.M. Malvy, M. Mendelson, P. Parola, M.J. Sotir, H.M. Wu, D.H. Hamer.
Provision of study materials or patients: A.K. Boggild, V. Ansdell, N.J. Beeching, F. Castelli, E. Caumes, F. Chappuis, J.P. Cramer, E. Gkrania-Klotsas, M.P. Grobusch, S.H.F. Hagmann, P.L. Lim, R. López-Vélez, D.J.M. Malvy.
Statistical expertise: A.K. Boggild, M.J. Sotir.
Obtaining of funding: D.H. Hamer.
Administrative, technical, or logistic support: D.H. Esposito, M.J. Sotir, D.H. Hamer.
Collection and assembly of data: A.K. Boggild, V. Ansdell, N.J. Beeching, F. Castelli, E. Gkrania-Klotsas, M.P. Grobusch, P. Parola, H.M. Wu.
References
- 1.WHO Ebola Response Team. Ebola virus disease in West Africa—the first 9 months of the epidemic and forward projections. N Engl J Med. 2014;371:1481–1495. doi: 10.1056/NEJMoa1411100. [PMID: 25244186] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Briand S, Bertherat E, Cox P, Formenty P, Kieny MP, Myhre JK, et al. The international Ebola emergency. N Engl J Med. 2014;371:1180–1183. doi: 10.1056/NEJMp1409858. [PMID: 25140855] [DOI] [PubMed] [Google Scholar]
- 3.Baize S, Pannetier D, Oestereich L, Rieger T, Koivogui L, Magassouba N, et al. Emergence of Zaire Ebola virus disease in Guinea. N Engl J Med. 2014;371:1418–1425. doi: 10.1056/NEJMoa1404505. [PMID: 24738640] [DOI] [PubMed] [Google Scholar]
- 4.World Health Organization. Ebola virus disease in Guinea. Geneva: World Health Organization; 2014. Accessed at www.who.int/csr/don/2014_03_23_ebola/en on 15 December 2014. [Google Scholar]
- 5.Medical evacuations. Solna, Sweden: European Centre for Disease Prevention and Control; 2015. European Centre for Disease Prevention and Control. Accessed at www.ecdc.europa.eu/en/healthtopics/ebola_marburg_fevers/Pages/medical-evacuations.aspx on 7 January 2015. [Google Scholar]
- 6.Centers for Disease Control and Prevention. Key messages—Ebola virus disease, West Africa. Updated 7 January 2015. Atlanta, GA: Centers for Disease Control and Prevention; 2015. [Google Scholar]
- 7.McCarthy M. US nurse says she will fight Ebola quarantine. BMJ. 2014;349:g6555. doi: 10.1136/bmj.g6555. [PMID: 25360033] [DOI] [PubMed] [Google Scholar]
- 8.Gonsalves G, Staley P. Panic, paranoia, and public health—the AIDS epidemic’s lessons for Ebola. N Engl J Med. 2014;371:2348–2349. doi: 10.1056/NEJMp1413425. [PMID: 25372947] [DOI] [PubMed] [Google Scholar]
- 9.Inglis T. Developed nations must not fear sending Ebola help. Nature. 2014;514:537. doi: 10.1038/514537a. [PMID: 25355324] [DOI] [PubMed] [Google Scholar]
- 10.Karwowski MP, Meites E, Fullerton KE, Ströher U, Lowe L, Rayfield M, et al. Centers for Disease Control and Prevention (CDC) Clinical inquiries regarding Ebola virus disease received by CDC– United States, July 9-November 15, 2014. MMWR Morb Mortal Wkly Rep. 2014;63:1175–1179. [PMID: 25503923] [PMC free article] [PubMed] [Google Scholar]
- 11.Bah EI, Lamah MC, Fletcher T, Jacob ST, Brett-Major DM, Sall AA, et al. Clinical presentation of patients with Ebola virus disease in Conakry, Guinea. N Engl J Med. 2015;372:40–47. doi: 10.1056/NEJMoa1411249. [PMID: 25372658] [DOI] [PubMed] [Google Scholar]
- 12.Bwaka MA, Bonnet MJ, Calain P, Colebunders R, De Roo A, Guimard Y, et al. Ebola hemorrhagic fever in Kikwit, Democratic Republic of the Congo: clinical observations in 103 patients. J Infect Dis. 1999;179(Suppl 1):S1–S7. doi: 10.1086/514308. [PMID: 9988155] [DOI] [PubMed] [Google Scholar]
- 13.Kortepeter MG, Bausch DG, Bray M. Basic clinical and laboratory features of filoviral hemorrhagic fever. J Infect Dis. 2011;204(Suppl 3):S810–S816. doi: 10.1093/infdis/jir299. [PMID: 21987756] [DOI] [PubMed] [Google Scholar]
- 14.Chertow DS, Kleine C, Edwards JK, Scaini R, Giuliani R, Sprecher A. Ebola virus disease in West Africa—clinical manifestations and management. N Engl J Med. 2014;371:2054–2057. doi: 10.1056/NEJMp1413084. [PMID: 25372854] [DOI] [PubMed] [Google Scholar]
- 15.Lyon GM, Mehta AK, Varkey JB, Brantly K, Plyler L, McElroy AK, et al. Emory Serious Communicable Diseases Unit. Clinical care of two patients with Ebola virus disease in the United States. N Engl J Med. 2014;371:2402–2409. doi: 10.1056/NEJMoa1409838. [PMID: 25390460] [DOI] [PubMed] [Google Scholar]
- 16.Fichet-Calvet E, Rogers DJ. Risk maps of Lassa fever in West Africa. PLoS Negl Trop Dis. 2009;3:e388. doi: 10.1371/journal.pntd.0000388. [PMID: 19255625] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mendelson M, Han PV, Vincent P, von Sonnenburg F, Cramer JP, Loutan L, et al. GeoSentinel Surveillance Network. Regional variation in travel-related illness acquired in Africa, March 1997-May 2011. Emerg Infect Dis. 2014;20:532–541. doi: 10.3201/eid2004.131128. [PMID: 24655358] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Centers for Disease Control and Prevention. Key messages— Ebola virus disease, West Africa. Updated 5 November 2014. Atlanta, GA: Centers for Disease Control and Prevention; 2014. [Google Scholar]
- 19.World Health Organization. Case definition recommendations for Ebola or Marburg virus diseases. Geneva: World Health Organization; 2014. Accessed at www.who.int/csr/resources/publications/ebola/ebola-case-definition-contact-en.pdf?ua=1 on 27 December 2014. [Google Scholar]
- 20.Freedman DO, Weld LH, Kozarsky PE, Fisk T, Robins R, von Son-nenburg F, et al. GeoSentinel Surveillance Network. Spectrum of disease and relation to place of exposure among ill returned travelers. N Engl J Med. 2006;354:119–130. doi: 10.1056/NEJMoa051331. [PMID: 16407507] [DOI] [PubMed] [Google Scholar]
- 21.Leder K, Torresi J, Libman MD, Cramer JP, Castelli F, Schlagenhauf P, et al. GeoSentinel Surveillance Network. GeoSentinel surveillance of illness in returned travelers, 2007–2011. Ann Intern Med. 2013;158:456–468. doi: 10.7326/0003-4819-158-6-201303190-00005. [PMID: 23552375] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Leder K, Tong S, Weld L, Kain KC, Wilder-Smith A, von Sonnenburg F, et al. GeoSentinel Surveillance Network. Illness in travelers visiting friends and relatives: a review of the GeoSentinel Surveillance Network. Clin Infect Dis. 2006;43:1185–1193. doi: 10.1086/507893. [PMID: 17029140] [DOI] [PubMed] [Google Scholar]
- 23.Centers for Disease Control and Prevention (CDC) Imported case of Marburg hemorrhagic fever—Colorado, 2008. MMWR Morb Mortal Wkly Rep. 2009;58:1377–1381. [PMID: 20019654] [PubMed] [Google Scholar]
- 24.Amorosa V, MacNeil A, McConnell R, Patel A, Dillon KE, Hamilton K, et al. Imported Lassa fever, Pennsylvania, USA, 2010. Emerg Infect Dis. 2010;16:1598–1600. doi: 10.3201/eid1610.100774. [PMID: 20875288] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Timen A, Koopmans MP, Vossen AC, van Doornum GJ, Günther S, van den Berkmortel F, et al. Response to imported case of Marburg hemorrhagic fever, the Netherland. Emerg Infect Dis. 2009;15:1171–1175. doi: 10.3201/eid1508.090051. [PMID: 19751577] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Beeching NJ, Fletcher TE, Hill DR, Thomson GL. Travellers and viral haemorrhagic fevers: what are the risks? Int J Antimicrob Agents. 2010;36(Suppl 1):S26–S35. doi: 10.1016/j.ijantimicag.2010.06.017. [PMID: 20705436] [DOI] [PubMed] [Google Scholar]
- 27.Johnston V, Stockley JM, Dockrell D, Warrell D, Bailey R, Pasvol G, et al. British Infection Society and the Hospital for Tropical Diseases. Fever in returned travellers presenting in the United Kingdom: recommendations for investigation and initial management. J Infect. 2009;59:1–18. doi: 10.1016/j.jinf.2009.05.005. [PMID: 19595360] [DOI] [PubMed] [Google Scholar]
- 28.Centers for Disease Control and Prevention. Identify, isolate, inform: ambulatory care evaluation of patients with possible Ebola virus disease (Ebola) Atlanta, GA: Centers for Disease Control and Prevention; 2014. Accessed at www.cdc.gov/vhf/ebola/pdf/ambulatory-care-evaluation-of-patients-with-possible-ebola.pdf on 12 November 2014. [Google Scholar]
- 29.Centers for Disease Control and Prevention. Think Ebola. Early recognition is critical for infection control. Atlanta, GA: Centers for Disease Control and Prevention; 2014. Accessed at www.cdc.gov/vhf/ebola/pdf/could-it-be-ebola.pdf 26 December 2014. [Google Scholar]
- 30.Schieffelin JS, Shaffer JG, Goba A, Gbakie M, Gire SK, Colubri A, et al. KGH Lassa Fever Program. Clinical illness and outcomes in patients with Ebola in Sierra Leone. N Engl J Med. 2014;371:2092–2100. doi: 10.1056/NEJMoa1411680. [PMID: 25353969] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fowler RA, Fletcher T, Fischer WA2nd, Lamontagne F, Jacob S, Brett-Major D, et al. Caring for critically ill patients with Ebola virus disease. Perspectives from West Africa. Am J Respir Crit Care Med. 2014;190:733–737. doi: 10.1164/rccm.201408-1514CP. [PMID: 25166884] [DOI] [PubMed] [Google Scholar]
- 32.Canadian Critical Care Society, Canadian Association of Emergency Physicians, Association of Medical Microbiology & Infectious Diseases Canada. Ebola clinical care guidelines: a guide for clinicians in Canada. 2014 Accessed at www.canadiancriticalcare.org/_assets/Ebola%20Clinical%20Care%20Guidelines_ENG.pdf on 14 November 2014.
- 33.Ferrer R, Martin-Loeches I, Phillips G, Osborn TM, Townsend S, Dellinger RP, et al. Empiric antibiotic treatment reduces mortality in severe sepsis and septic shock from the first hour: results from a guideline-based performance improvement program. Crit Care Med. 2014;42:1749–1755. doi: 10.1097/CCM.0000000000000330. [PMID: 24717459] [DOI] [PubMed] [Google Scholar]
- 34.Roddy P, Colebunders R, Jeffs B, Palma PP, Van Herp M, Borchert M. Filovirus hemorrhagic fever outbreak case management: a review of current and future treatment options. J Infect Dis. 2011;204(Suppl 3):S791–S795. doi: 10.1093/infdis/jir297. [PMID: 21987752] [DOI] [PubMed] [Google Scholar]
- 35.Marti F, Steffen R, Mutsch M. Influenza vaccine: a travelers’ vaccine? Expert Rev Vaccines. 2008;7:679–687. doi: 10.1586/14760584.7.5.679. [PMID: 18564022] [DOI] [PubMed] [Google Scholar]
- 36.Xie O, Pollard AJ, Mueller JE, Norheim G. Emergence of sero-group X meningococcal disease in Africa: need for a vaccine. Vaccine. 2013;31:2852–2861. doi: 10.1016/j.vaccine.2013.04.036. [PMID: 23623866] [DOI] [PubMed] [Google Scholar]
- 37.Fraser A, Paul M, Goldberg E, Acosta CJ, Leibovici L. Typhoid fever vaccines: systematic review and meta-analysis of randomised controlled trials. Vaccine. 2007;25:7848–7857. doi: 10.1016/j.vaccine.2007.08.027. [PMID: 17928109] [DOI] [PubMed] [Google Scholar]
- 38.Wilson ME, Weld LH, Boggild A, Keystone JS, Kain KC, von Son-nenburg F, et al. GeoSentinel Surveillance Network. Fever in returned travelers: results from the GeoSentinel Surveillance Network. Clin Infect Dis. 2007;44:1560–1568. doi: 10.1086/518173. [PMID: 17516399] [DOI] [PubMed] [Google Scholar]
- 39.Boggild AK, Castelli F, Gautret P, Torresi J, von Sonnenburg F, Barnett ED, et al. GeoSentinel Surveillance Network. Vaccine preventable diseases in returned international travelers: results from the GeoSentinel Surveillance Network. Vaccine. 2010;28:7389–7395. doi: 10.1016/j.vaccine.2010.09.009. [PMID: 20851081] [DOI] [PubMed] [Google Scholar]
- 40.Leder K, Steffen R, Cramer JP, Greenaway C. Risk assessment in travel medicine: how to obtain, interpret, and use risk data for informing pre-travel advice. J Travel Med. 2015;22:13–20. doi: 10.1111/jtm.12170. [PMID: 25378126] [DOI] [PubMed] [Google Scholar]