Abstract
Neocryptotanshinone (NCTS) is a natural product isolated from traditional Chinese herb Salvia miltiorrhiza Bunge. In this study, we investigated its anti-inflammatory effects in lipopolysaccharide (LPS)-stimulated mouse macrophage (RAW264.7) cells. MTT results showed that NCTS partly reversed LPS-induced cytotoxicity. Real-time PCR results showed that NCTS suppressed LPS-induced mRNA expression of inflammatory cytokines, including tumor necrosis factor α (TNFα), interleukin-6 (IL-6) and interleukin-1β (IL-1β). Moreover, NCTS could decrease LPS-induced nitric oxide (NO) production. Western blotting results showed that NCTS could down-regulate LPS-induced expression of inducible nitric oxide synthase (iNOS), p-IκBα, p-IKKβ and p-NF-κB p65 without affecting cyclooxygenase-2 (COX-2). In addition, NCTS inhibited LPS-induced p-NF-κB p65 nuclear translocation. In conclusion, these data demonstrated that NCTS showed anti-inflammatory effect by suppression of NF-κB and iNOS signaling pathways.
KEY WORDS: Neocryptotanshinone, Inflammation, NF-κB, Inducible nitric oxide synthase
Graphical abstract
Neocryptotanshinone (NCTS) is a natural product isolated from traditional Chinese herb Salvia miltiorrhiza Bunge. Its anti-inflammatory effects in lipopolysaccharide (LPS)-stimulated mouse macrophage (RAW264.7) cells were investigated by suppression of NF-κB and iNOS signaling pathways.
1. Introduction
Inflammation is a complex reaction of the systemic immune vascularized tissues at sites of an infection, toxin exposure or cell injury and is associated with many pathological conditions1, 2. Commonly, inflammation was accompanied by the activation of various immune cells such as macrophages, neutrophils and lymphocytes. Particular, macrophages play important roles in the regulation of inflammation and immune response and are involved in various disease processes2, 3, 4. Activated macrophages secrete lots of inflammatory mediators such as nitric oxide (NO), which are produced by inducible nitric oxide synthase (iNOS), as well as inflammatory cytokines such as tumor necrosis factor α (TNFα), interleukin-6 (IL-6) and interleukin-1β (IL-1β)5, 6, 7, 8. These inflammatory mediators and cytokines are essential for host survival following infection and are also required for the repair of tissue injury. Among these cytokines, TNFα and IL-6 are known as important mediators involved in the progress of many inflammatory diseases5. Therefore, LPS-stimulated macrophage is a widely used model to study the inflammation and the mechanisms of action of potential anti-inflammatory mediators.
These cytokines and their genes can be modulated by activation of transcription factor NF-κB, which plays a critical role in expression of inflammatory genes. Therefore, NF-κB is crucially involved in the pathogenesis of many chronic inflammatory diseases9. In unstimulated state, NF-κB is presented in the cytosol, combined with inhibitory protein IκB. Specific stimuli such as LPS give rise to free NF-κB via the degradation of IκB through phosphorylation by IκB kinase (IKK)10. Activated NF-κB translocates from cytoplasm to nucleus and binds to promoter and modulates the expression of inflammatory genes including iNOS, inflammatory cytokines and chemokines11, 12, 13, 14. Most anti-inflammatory drugs have been shown to repress the expression of inflammatory mediator genes by inhibiting NF-κB activation pathway.
NO, which is produced from l-arginine by three nitric oxide synthase (NOS) enzymes, is increased in inflammation and has pro-inflammatory and regulatory effects15. iNOS, induced by bacterial products and inflammatory cytokines in macrophages, is responsible for prolonged production of larger amounts of NO. The experimental data support the idea that compounds inhibiting expression or activity of iNOS are potential anti-inflammatory agents16. Expression of the iNOS gene is regulated at the transcriptional level, notably through the action of NF-κB.
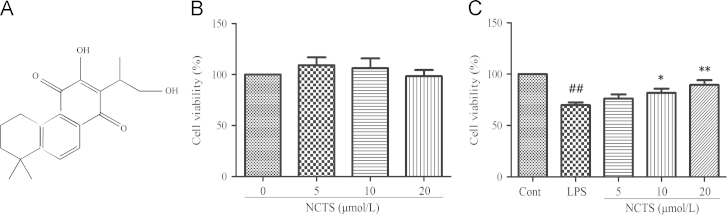
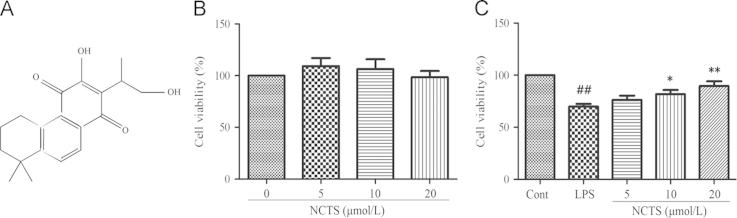
The root of Salvia miltiorrhiza Bunge (Danshen in Chinese), is a traditional Chinese herb used in the treatment of cardiovascular diseases for centuries. It was reported that the lipophilic ingredients isolated from Danshen, such as tanshinone II A17, tanshinone I18 and cryptotanshinone19 showed anti-inflammatory effect in vitro. Neocryptotanshinone (NCTS, Fig. 1A), a diterpenoid with similar structure to these tanshinones, was firstly isolated by Lee et al. from the roots of Salvia miltiorrhiza in 198720. However, its biological activities remain to be clear. In this study, we evaluated the anti-inflammatory effect of NCTS in RAW264.7 induced by LPS.
Figure 1.
The chemical structure of NCTS and its effect on cell viability with or without LPS co-treatment in RAW264.7 macrophages. RAW264.7 cells were treated with or without 500 ng/mL of LPS and NCTS (5, 10 and 20 μmol/L) for 24 h. Data were presented as the means±SD of three independent experiments. ##P<0.01, vs. the control group; **P<0.01, *P<0.05 vs. LPS-stimulated group. NCTS, Neocryptotanshinone; Cont, Control group.
2. Materials and methods
2.1. Reagents
NCTS (>98% in purity) was purchased from ChemFaces Co., Ltd. (China). Dulbecco׳s modified Eagle׳s medium (DMEM), penicillin and streptomycin were purchased from Gibco. Fetal bovine serum (FBS) was purchased from Zhejiang Tianhang Biological Technology Co., Ltd. (China). LPS and 3-(4,5- dimethyl-2,5-diphenyl-2-H-tetrazolium bromide (MTT)) were purchased from Sigma Aldrich (USA), DAF-FM DA (NO fluorescence probe), S-methylisothiourea sulfate (SMT) and Griess reagent kit were purchased from Beyotime Institute of Biotechnology (China). PrimeScript™ RT reagent kit with gDNA eraser (Perfect real time) was purchased from TaKaRa Biotechnology Co., Ltd. (China). TNFα, IL-6, IL-1β and β-actin oligonucleotide primers were purchased from Sangon Biotech (China). Primary antibody for COX-2, iNOS, p-IκBα, p-IKKβ, p-NF-κB p65, and second antibodies for anti-rabbit IgG HRP-linked antibody and anti-mouse IgG HRP-linked antibody were purchased from Cell Signaling Technology (USA), Alexa Fluor 488-conjugated goat anti-rabbit IgG was purchased from Invitrogen (USA).
2.2. Cell culture
Murine RAW264.7 macrophages obtained from the American Type Culture Collection (ATCC) (USA) were cultured in complete DMEM containing 10% heat-inactivated FBS, 100 U/mL penicillin, and 100 μg/mL streptomycin. Cells were incubated at 37 °C in a humidified atmosphere of 5% CO2 in air.
2.3. Cell viability assay
Cell viability was determined using MTT assay. RAW264.7 cells (5×104 cells/well) were cultured in 96-well plates for 24 h after NCTS treatment with or without LPS (500 ng/mL). MTT solution (10 μL; 5 mg/mL) was added, and the cells were incubated at 37 °C for an additional 4 h. After washing out the supernatant, the insoluble formazan product was dissolved in DMSO. Then, the optical density was measured using a microplate reader at 570 nm.
2.4. Real-time PCR assay
RAW264.7 cells were seeded in 6-well plates treated with NCTS and 500 ng/mL LPS for 24 h. The total RNA was isolated and the RNA concentration was detected using a spectrophotometer. Total RNA (1 μg) was converted to cDNA and Real-time PCR (RT-PCR) was performed with PrimeScript™ RT reagent kit. The PCR primers were as follows:
-
TNFα: 5′-TTCTGTCTACTGAACTTCGGGGTGATCGGTCC-3′ (F)
5′-GTATGAGATAGCAAATCGGCTGACGGTGTGGG-3′ (R)
-
IL-6: 5′-TCCAGTTGCCTTCTTGGGAC-3′ (R)
5′-GTGTAATTAAGCCTCCGACTTG-3′ (F)
-
IL-1β: 5′-GAAAGACGGCACACCCACCCT-3′ (F)
5′-GCTCTGCTTGTGAGGTGCTGATGTA-3′ (R)
The amplification sequence protocol was conducted with three steps: 1st step: cycle 1, 95 °C 30 s; 2nd step: cycle 40, 95 °C 5 s, 60 °C 34 s; 3rd step: cycle 1, 95 °C 15 s, 60 °C 1 min, 95 °C 15 s.
2.5. NO assay
RAW264.7 cells (2.5×105 cells/mL) were cultured in 6-well plates with or without LPS pretreatment for 30 min and then incubated with NCTS (5–20 μmol/L) or SMT (15 μmol/L) for another 24 h. 50 μL of supernatants were collected and mixed with equal volumes of Griess reagent for 10 min at room temperature. Optical density was measured at 540 nm. The sodium nitrite (NaNO2) was used to generate a standard curve21. The NO production in the cultured medium was estimated by the NO2− concentration.
The intracellular NO level was measured using a NO-sensitive fluorescence probe DAF-FM DA22. Briefly, cells were loaded with DAF-FM DA (5 μmol/L) at 37 °C for 20 min. Then cells were gently washed for three times with PBS. The fluorescence was detected by an IN Cell 2000 Analyser.
2.6. Western blot analysis
RAW264.7 cells after NCTS treatment were collected and the total protein was extracted. The protein concentrations were determined by Bio-Rad protein assay reagent according to the manufacturer׳s instructions. Thirty micrograms of cellular protein from each group was electro-blotted onto PVDF membrane following separation on 8% SDS-PAGE. The immune-blot was incubated with blocking solution (5% skim milk) at room temperature for 1 h, followed by incubation with a primary antibody overnight at 4 °C. After washed with Tween 20/Tris-buffered saline (TBST) and incubated with second antibodies (1:10,000) for 2 h at room temperature. Blots were developed by enhanced chemiluminescence (GE healthcare). Bio-Rad Quantity One Software was used for the densitometric analysis.
2.7. Immunofluorescence staining
Cell viability was determined using MTT assay. RAW264.7 cells (1×104 cells/well) were cultured in 96-well plates for 24 h after NCTS treatment with or without LPS (500 ng/mL). The cells were then washed and fixed with 4% paraformaldehyde for 15 min at 37 °C, permeabilized with 0.5% Triton X-100 for 20 min, and blocked with 3% BSA for 30 min at room temperature. Next, cells were incubated with primary p-NF-κB p65 antibody (1:200) overnight at 4 °C, and Alexa Fluor 488-conjugated goat anti-rabbit IgG as a secondary antibody (1:1000) performed at room temperature for 1 h. After being washed with PBS, cells were incubated in Hoechst 33342 for 5 min in the dark to dye the nucleus. The p-NF-κB protein and nuclei will be displayed as fluorescence of green and blue, respectively. Cell imaging was simultaneously viewed on the In Cell 2000 Analyser to analysis the translocation of p-NF-κB p6523.
2.8. Statistical analysis
The data were expressed as means±SD and analyzed by SPSS (17.0) for statistical significance using one-way analysis of variance (ANOVA). P<0.05 was considered statistically significant.
3. Results
3.1. NCTS inhibited LPS-induced cell viability decrease
To measure the anti-inflammatory effect of NCTS, we treated RAW264.7 cells with three concentrations of NCTS (5, 10 and 20 μmol/L) after pretreatment with or without LPS as shown in Fig. 1B. NCTS showed no obvious cytotoxic effect on RAW264.7 cells. LPS (500 ng/mL) could significantly reduce the cell viability of RAW264.7 after 24 h treatment. NCTS could reverse the reduced cell viability of RAW264.7 induced by LPS. Furthermore, a dose-dependent manner was observed (Fig. 1C).
3.2. NCTS inhibited LPS-induced mRNA expression of TNFα, IL-6 and IL-1β
To evaluate the effect of NCTS on pro-inflammatory cytokines, we examined the mRNA expression of TNFα, IL-6 and IL-1β. LPS treatment dramatically increased the mRNA expression of TNFα, IL-6 and IL-1β. NCTS could significantly suppresses the LPS-induced mRNA expression of TNFα, IL-6 and IL-1β. (Fig. 2).
Figure 2.
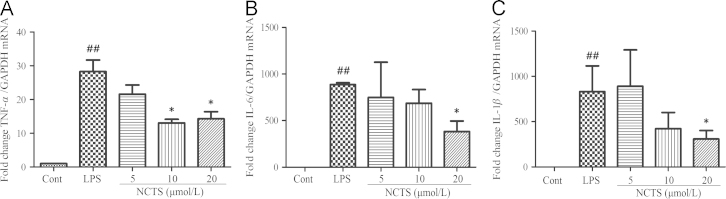
Effects of NCTS on LPS-induced mRNA expression of TNFα, IL-6 and IL-1β in RAW264.7 macrophages. Cells were treated with LPS with or without NCTS co-incubation for 24 h. The total RNA was prepared and the mRNA expression of TNFα, IL-6 and IL-1β were measured with RT-PCR. Data were presented as the means±SD of three independent experiments. ##P<0.01 vs. the control group; *P<0.05 vs. LPS-stimulated group. NCTS, Neocryptotanshinone; Cont, Control group.
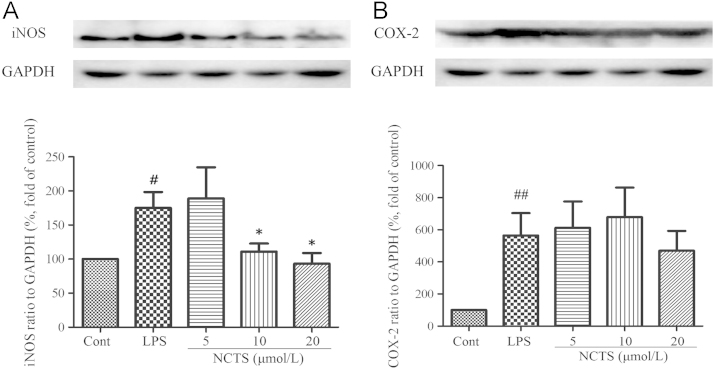
3.3. NCTS inhibited LPS-induced iNOS protein expression but not COX-2
iNOS and cyclooxygenase (COX-2) were responsible for the production of NO and prostaglandin E2 (PGE2), two important inflammatory mediators. LPS treatment significantly up-regulated the protein expression of iNOS and COX-2. NCTS, at the concentration of 10 μmol/L and 20 μmol/L, could decrease the expression of iNOS remarkably (Fig. 3A). However, NCTS showed no effect on LPS-induced up-regulation of COX-2 at all three tested concentrations (Fig. 3B).
Figure 3.
Effect of NCTS on LPS-induced protein expression of iNOS and COX-2 in RAW264.7 macrophages. Cells were treated with LPS with or without NCTS co-incubation for 24 h. The protein expression of iNOS and COX-2 was determined by western blotting. Data were presented as the means±SD of three independent experiments. ##P<0.01, #P<0.05 vs. the control group; *P<0.05 vs. LPS-stimulated group. NCTS, Neocryptotanshinone; Cont, Control group.
3.4. NCTS inhibited LPS-induced NO production
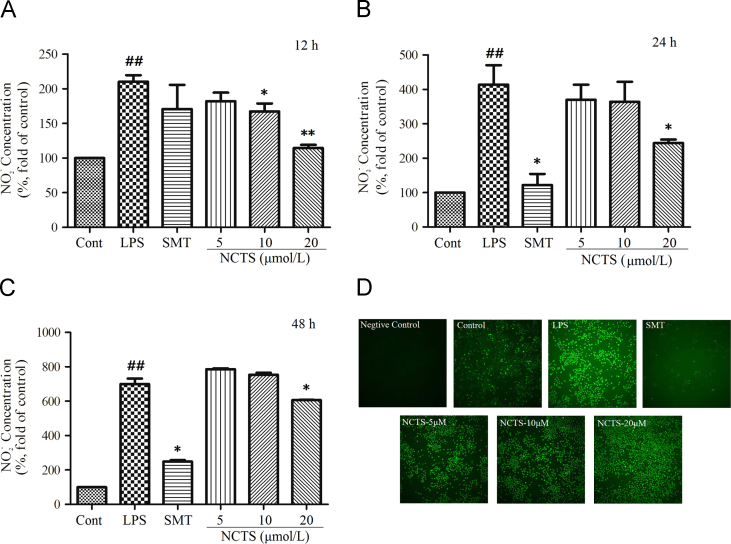
NO is a free radical involved in the regulation of many physiological processes such as vascular relaxation, neurotransmission, platelet aggregation, and the immune response24. In the progress of inflammation, NO is generated by activated inducible iNOS, and participated in the innate response along with other macrophage mediators in many mammal. The cellular NO could be quickly oxidized to NO2− in the culture medium. In this study, we investigated the NO2− production in the cultured medium and the NO formation in the cells.
As shown in Fig. 4, LPS treatment induced large amount of NO2− production in cell supernatant. Furthermore, a time-dependent manner was observed (Fig. 4A-C). SMT, an iNOS inhibitor, dramatically suppressed LPS induced NO2− production after 24 h treatment. Compared with the LPS treated groups, NCTS at the concentration of 20 μmol/L remarkably reduced the NO2− production (P<0.01, 12 h; P<0.05, 24 h and 48 h) NCTS, at the concentration of 10 μmol/L decreased NO2− production at 12 h (P<0.05).
Figure 4.
Effects of NCTS on LPS-induced production of NO2− in the cell supernatants and intracellular NO formation in RAW264.7 macrophages. Cells were treated with LPS with or without NCTS or SMT co-incubation for 12, 24 and 48 h. The NO2− contents in the cell supernatants were determined with Griess reagent (A-C); the intracellular NO formation was monitored with fluorescence probe DAF-DA (D). Data were presented as the means±SD of three independent experiments. ##P<0.01 vs. the control group; **P<0.01, *P<0.05 vs. LPS-stimulated group. NCTS, Neocryptotanshinone; Cont, Control group; SMT, S-methylisothiourea sulfate.
NO generated in RAW264.7 cells was also measured using fluorescence probe DAF-FM DA. In Fig. 4D, negative and unstimulated cell showed weak green fluorescence while LPS-treated group showed intensive green fluorescence. SMT significantly decreased the fluorescence intensity in cells. Furthermore, NCTS also decreased the fluorescence intensity in cells.
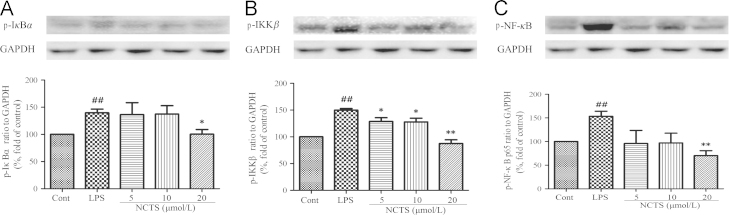
3.5. NCTS inhibited LPS-induced activation of NF-κB pathway
NF-κB is an important transcription factor in the inflammatory response. We examined the protein expression of p-NF-κB p65, p-IκBα and p-IKKβ by Western blot analysis. As shown in Fig. 5, the protein expression of p-IκBα, p-IKKβ and p-NF-κB p65 were remarkably up-regulated by LPS. NCTS at the concentration of 20 μmol/L decreased the expression of p-IκBα, p-IKKβ and p-NF-κB p65 induced by LPS significantly (P<0.01 or P<0.05). Moreover, the expression of p-IKKβ was also decreased by NCTS (5 μmol/L and 10 μmol/L, P<0.05 ).
Figure 5.
Effect of NCTS on LPS-induced protein expression of p-IκBα, p-IKKβ and p-NF-κB p65. Cells were treated with LPS with or without NCTS co-incubation for 24 h. The protein expression of iNOS and COX-2 was determined by western blotting. Data were presented as the means±SD of three independent experiments. ##P<0.01 vs. the control group; **P<0.01, *P<0.05 vs. LPS-stimulated group. NCTS, Neocryptotanshinone; Cont, Control group.
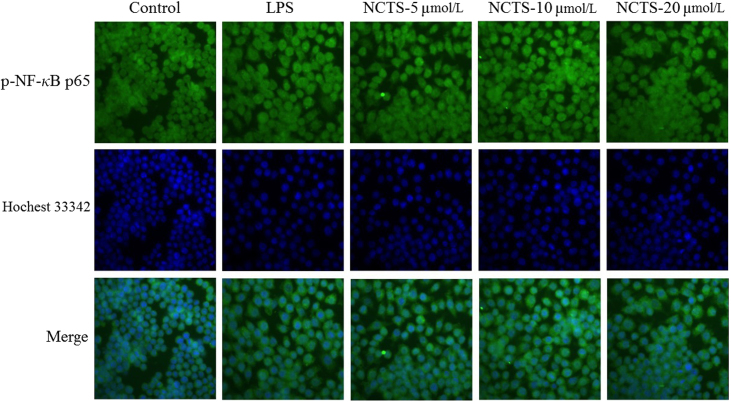
3.6. NCTS inhibited LPS-induced p-NF-κB p65 translocation
To further investigate the impact of NCTS in the NF-κB signaling pathway, the translocation of cytosolic p-NF-κB p65 into the nucleus was evaluated by immunofluorescence assay. As shown in Fig. 6, without LPS stimulation, basal p-NF-κB p65 distributed in the cytoplasm with green fluorescence and nucleus displayed as blue fluorescence by Hoechst 33342. LPS incubation induced nuclear translocation of p-NF-κB p65 as merged images indicated cyan fluorescence in the nucleus. In the presence of three concentrations of NCTS, p-NF-κB p65 translocation induced by LPS was partly inhibited, especially at the concentration of 20 μmol/L.
Figure 6.
Effects of NCTS on the nuclear translocation of p-NF-κB p65. Without LPS stimulation, basal p-NF-κB p65 distributed in cytoplasm with green fluorescence and nucleus in blue fluorescence by Hoechst 33342. 500 ng/mL of LPS incubated with RAW264.7 induced nuclear translocation of p-NF-κB p65, merged images indicated a cyan fluorescence in the nucleus.
4. Discussion
Salvia miltiorrhiza Bunge is a famous traditional Chinese herb used for treating cardiovascular diseases for centuries. A series of lipophilic compounds entitled tanshinones isolated from this herb possess multiple biological activities, such as cardiovascular protection, anti-cancer effect25, 26. Recently, the anti-inflammatory effect of tanshinone IIA27, cryptotanshinone28, tanshinone I29, etc. have been documented. NCTS is a tanshinone identified from Salvia miltiorrhiza Bunge more than 30 years ago. However, its biological activities remain obscure. Here, we investigated the anti-inflammatory effect of NCTS using LPS stimulated RAW264.7 cells and firstly found that NCTS showed anti-inflammatory effect.
In the cell viability assay, NCTS showed no obvious cytotoxic effect in RAW264.7 cells even at 20 μmol/L. This was different from that of other tanshinones with similar structures. It could reverse the reduced cell viability of RAW264.7 induced by LPS in a dose-dependent manner. This result was similar to that of tanshinone II A, which is one of the major extract from Salvia miltiorrhiza Bunge30.
It has been well documented that LPS-induced inflammatory mediator production by macrophages can be attenuated by tanshinones like tanshinone I, dihydrotanshinone, cryptotanshinone and tanshinone II A31, 32, 33, 34. Our results showed that NCTS could significantly suppress the LPS-induced mRNA expression of TNFα, IL-6 and IL-1β. These results were consist with the anti-inflammatory effects of other tanshinones28, 32, 35.
Among inflammatory mediators, NO and PGE2, which are produced by iNOS and COX-2, play important roles in the innate response in activated macrophages. Our results revealed that NCTS showed excellent inhibition in the protein expression of iNOS, but not COX-2. This was different with the anti-inflammation effect of tanshinone II A and cryptotanshinone, two of major active components of Salvia miltiorrhiza Bunge. Both compounds have been shown as anti-inflammation agents by inhibiting the protein expression of both iNOS and COX-232. In our study, NCTS did not affect the protein expression of COX-2. However, its effect on the mRNA expression of COX-2 and COX-2 activity needs remain further study to elucidate.
Macrophages produce NO and pro-inflammatory cytokines in response to LPS. This NO production can be controlled by selective pharmacological inhibition of distinct nitric oxide synthase isoforms. NO is recognized as a potential mediator and regulator of inflammatory response36. NO generated by iNOS is involved in immune-modulatory mechanisms. As mentioned above, NCTS successfully inhibited the increased iNOS expression induced by LPS. It also reversed the production of NO in a time-dependent manner, which is similar to that of tanshinone II A and cryptotanshinone19, 32. This suggested that inhibition of NO production could be a potential mechanism in NCTS׳s anti-inflammation effect.
The NF-κB signaling pathway plays a key role in inflammatory process in macrophages. NF-κB is activated by the phosphorylation, ubiquitination, and subsequent proteolytic degradation of the NF-κB-bound IκB via activated IκB kinase (IKK). Here, we found that the phosphorylation of IκBα, IKKβ and NF-κB p65 were inhibited by NCTS treatment. Furthermore, the translocation of p-NF-κB p65 was also inhibited by NCTS. This effect of NCTS on NF-κB pathway was in line with that of tanshinone IIA and cryptotanshinone28, 30. Thus, inhibition of NF-κB pathway might be a potential mechanism of NCTS.
In conclusion, this study provided evidence that NCTS showed anti-inflammatory effect of NCTS in vitro. This function of NCTS might be mediated through suppression of NF-κB and iNOS signaling pathways.
Acknowledgments
This study was supported by the Science and Technology Development Fund of Macau Special Administrative Region (No. 021/2012/A1) and the Research Fund of University of Macau (No. MRG007/CXP/2013/ICMS).
Footnotes
Peer review under responsibility of Institute of Materia Medica, Chinese Academy of Medical Sciences and Chinese Pharmaceutical Association.
References
- 1.Jiang WY, Jeon BH, Kim YC, Lee SH, Sohn DH, Seo GS. PF2401-SF, standardized fraction of Salvia miltiorrhiza shows anti-inflammatory activity in macrophages and acute arthritis in vivo. Int Immunopharmacol. 2013;16:160–164. doi: 10.1016/j.intimp.2013.03.028. [DOI] [PubMed] [Google Scholar]
- 2.Pierce GF. Macrophages: important physiologic and pathologic sources of polypeptide growth factors. Am J Respir Cell Mol Biol. 1990;2:233–234. doi: 10.1165/ajrcmb/2.3.233. [DOI] [PubMed] [Google Scholar]
- 3.Erwig LP, Rees AJ. Macrophage activation and programming and its role for macrophage function in glomerular inflammation. Kidney Blood Press Res. 1999;22:21–25. doi: 10.1159/000025905. [DOI] [PubMed] [Google Scholar]
- 4.Wadleigh DJ, Reddy ST, Kopp E, Ghosh S, Herschman HR. Transcriptional activation of the cyclooxygenase-2 gene in endotoxin-treated RAW 264.7 macrophages. J Biol Chem. 2000;275:6259–6266. doi: 10.1074/jbc.275.9.6259. [DOI] [PubMed] [Google Scholar]
- 5.Becker S, Mundandhara S, Devlin RB, Madden M. Regulation of cytokine production in human alveolar macrophages and airway epithelial cells in response to ambient air pollution particles: further mechanistic studies. Toxicol Appl Pharmacol. 2005;207:269–275. doi: 10.1016/j.taap.2005.01.023. [DOI] [PubMed] [Google Scholar]
- 6.Jung HW, Seo UK, Kim JH, Leem KH, Park YK. Flower extract of Panax notoginseng attenuates lipopolysaccharide-induced inflammatory response via blocking of NF-κB signaling pathway in murine macrophages. J Ethnopharmacol. 2009;122:313–319. doi: 10.1016/j.jep.2008.12.024. [DOI] [PubMed] [Google Scholar]
- 7.Kim JB, Han AR, Park EY, Kim JY, Cho W, Lee J. Inhibition of LPS-induced iNOS, COX-2 and cytokines expression by poncirin through the NF-κB inactivation in RAW 264.7 macrophage cells. Biol Pharm Bull. 2007;30:2345–2351. doi: 10.1248/bpb.30.2345. [DOI] [PubMed] [Google Scholar]
- 8.Ronis MJ, Butura A, Korourian S, Shankar K, Simpson P, Badeaux J. Cytokine and chemokine expression associated with steatohepatitis and hepatocyte proliferation in rats fed ethanol via total enteral nutrition. Exp Biol Med. 2008;233:344–355. doi: 10.3181/0707-RM-203. [DOI] [PubMed] [Google Scholar]
- 9.Makarov SS. NF-κB as a therapeutic target in chronic inflammation: recent advances. Mol Med Today. 2000;6:441–448. doi: 10.1016/s1357-4310(00)01814-1. [DOI] [PubMed] [Google Scholar]
- 10.de Martin R, Vanhove B, Cheng Q, Hofer E, Csizmadia V, Winkler H. Cytokine-inducible expression in endothelial cells of an I κB α-like gene is regulated by NF-κB. EMBO J. 1993;12:2773–2779. doi: 10.1002/j.1460-2075.1993.tb05938.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Baeuerle PA, Baltimore D. NF-κB: ten years after. Cell. 1996;87:13–20. doi: 10.1016/s0092-8674(00)81318-5. [DOI] [PubMed] [Google Scholar]
- 12.Chao WW, Hong YH, Chen ML, Lin BF. Inhibitory effects of Angelica sinensis ethyl acetate extract and major compounds on NF-κB trans-activation activity and LPS-induced inflammation. J Ethnopharmacol. 2010;129:244–249. doi: 10.1016/j.jep.2010.03.022. [DOI] [PubMed] [Google Scholar]
- 13.Gilroy DW, Lawrence T, Perretti M, Rossi AG. Inflammatory resolution: new opportunities for drug discovery. Nat Rev Drug Discov. 2004;3:401–416. doi: 10.1038/nrd1383. [DOI] [PubMed] [Google Scholar]
- 14.Pahl HL. Activators and target genes of Rel/NF-κB transcription factors. Oncogene. 1999;18:6853–6866. doi: 10.1038/sj.onc.1203239. [DOI] [PubMed] [Google Scholar]
- 15.Bogdan C. Nitric oxide and the immune response. Nat Immunol. 2001;2:907–916. doi: 10.1038/ni1001-907. [DOI] [PubMed] [Google Scholar]
- 16.Vallance P, Leiper J. Blocking NO synthesis: how, where and why? Nat Rev Drug Discov. 2002;1:939–950. doi: 10.1038/nrd960. [DOI] [PubMed] [Google Scholar]
- 17.Zhang K, Wang J, Jiang H, Xu X, Wang S, Zhang C. Tanshinone IIA inhibits lipopolysaccharide-induced MUC1 overexpression in alveolar epithelial cells. Am J Physiol Cell Physiol. 2014;306:C59–C65. doi: 10.1152/ajpcell.00070.2013. [DOI] [PubMed] [Google Scholar]
- 18.Tao SS, Zheng Y, Lau A, Jaramillo MC, Chau BT, Lantz RC. Tanshinone I activates the Nrf2-dependent antioxidant response and protects against As(III)-induced lung inflammation in vitro and in vivo. Antioxid Redox Signal. 2013;19:1647–1661. doi: 10.1089/ars.2012.5117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li X, Lian LH, Bai T, Wu YL, Wan Y, Xie WX. Cryptotanshinone inhibits LPS-induced proinflammatory mediators via TLR4 and TAK1 signaling pathway. Int Immunopharmacol. 2011;11:1871–1876. doi: 10.1016/j.intimp.2011.07.018. [DOI] [PubMed] [Google Scholar]
- 20.Lee AR, Wu WL, Chang WL, Lin HC, King ML. Isolation and bioactivity of new tanshinones. J Nat Prod. 1987;50:157–160. doi: 10.1021/np50050a004. [DOI] [PubMed] [Google Scholar]
- 21.Lin HC, Ding HY, Chang WL. Two new fatty diterpenoids from Salvia miltiorrhiza. J Nat Prod. 2001;64:648–650. doi: 10.1021/np000345v. [DOI] [PubMed] [Google Scholar]
- 22.Sheng B, Gong K, Niu Y, Liu L, Yan Y, Lu G. Inhibition of γ-secretase activity reduces Abeta production, reduces oxidative stress, increases mitochondrial activity and leads to reduced vulnerability to apoptosis: Implications for the treatment of Alzheimer׳s disease. Free Radical Biol Med. 2009;46:1362–1375. doi: 10.1016/j.freeradbiomed.2009.02.018. [DOI] [PubMed] [Google Scholar]
- 23.Xin WY, Huang C, Zhang X, Xin S, Zhou YM, Ma XW. Methyl salicylate lactoside inhibits inflammatory response of fibroblast-like synoviocytes and joint destruction in collagen-induced arthritis in mice. Br J Pharmacol. 2014;171:3526–3538. doi: 10.1111/bph.12715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Park EJ, Kim SA, Choi YM, Kwon HK, Shim W, Lee G. Capric acid inhibits NO production and STAT3 activation during LPS-induced osteoclastogenesis. PLoS One. 2011;6:e27739. doi: 10.1371/journal.pone.0027739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen X, Guo J, Bao J, Lu J, Wang Y. The anticancer properties of Salvia miltiorrhiza Bunge (Danshen): a systematic review. Med Res Rev. 2014;34:768–794. doi: 10.1002/med.21304. [DOI] [PubMed] [Google Scholar]
- 26.Gao S, Liu Z, Li H, Little PJ, Liu P, Xu S. Cardiovascular actions and therapeutic potential of tanshinone IIA. Atherosclerosis. 2012;220:3–10. doi: 10.1016/j.atherosclerosis.2011.06.041. [DOI] [PubMed] [Google Scholar]
- 27.Fan GW, Gao XM, Wang H, Zhu Y, Zhang J, Hu LM. The anti-inflammatory activities of Tanshinone IIA, an active component of TCM, are mediated by estrogen receptor activation and inhibition of iNOS. J Steroid Biochem Mol Biol. 2009;113:275–280. doi: 10.1016/j.jsbmb.2009.01.011. [DOI] [PubMed] [Google Scholar]
- 28.Tang S, Shen XY, Huang HQ, Xu SW, Yu Y, Zhou CH. Cryptotanshinone suppressed inflammatory cytokines secretion in RAW264.7 macrophages through inhibition of the NF-κB and MAPK signaling pathways. Inflammation. 2011;34:111–118. doi: 10.1007/s10753-010-9214-3. [DOI] [PubMed] [Google Scholar]
- 29.Tao JY, Zheng GH, Zhao L, Wu JG, Zhang XY, Zhang SL. Anti-inflammatory effects of ethyl acetate fraction from Melilotus suaveolens Ledeb on LPS-stimulated RAW 264.7 cells. J Ethnopharmacol. 2009;123:97–105. doi: 10.1016/j.jep.2009.02.024. [DOI] [PubMed] [Google Scholar]
- 30.Jang SI, Kim HJ, Kim YJ, Jeong SI, You YO. Tanshinone IIA inhibits LPS-induced NF-κB activation in RAW 264.7 cells: possible involvement of the NIK-IKK, ERK1/2, p38 and JNK pathways. Eur J Pharmacol. 2006;542:1–7. doi: 10.1016/j.ejphar.2006.04.044. [DOI] [PubMed] [Google Scholar]
- 31.Choi HS, Cho DI, Choi HK, Im SY, Ryu SY, Kim KM. Molecular mechanisms of inhibitory activities of tanshinones on lipopolysaccharide-induced nitric oxide generation in RAW 264.7 cells. Arch Pharm Res. 2004;27:1233–1237. doi: 10.1007/BF02975887. [DOI] [PubMed] [Google Scholar]
- 32.Jang SI, Kim KJ, Kim HJ, Yu HH, Park R, Kim HM. Tanshinone IIA from Salvia miltiorrhiza inhibits inducible nitric oxide synthase expression and production of TNF-α, IL-1β and IL-6 in activated RAW 264.7 cells. Planta Med. 2003;69:1057–1059. doi: 10.1055/s-2003-45157. [DOI] [PubMed] [Google Scholar]
- 33.Jeon SJ, Son KH, Kim YS, Choi YH, Kim HP. Inhibition of prostaglandin and nitric oxide production in lipopolysaccharide-treated RAW 264.7 cells by tanshinones from the roots of Salvia miltiorrhiza Bunge. Arch Pharm Res. 2008;31:758–763. doi: 10.1007/s12272-001-1223-4. [DOI] [PubMed] [Google Scholar]
- 34.Kang BY, Chung SW, Kim SH, Ryu SY, Kim TS. Inhibition of interleukin-12 and interferon-γ production in immune cells by tanshinones from Salvia miltiorrhiza. Immunopharmacology. 2000;49:355–361. doi: 10.1016/s0162-3109(00)00256-3. [DOI] [PubMed] [Google Scholar]
- 35.Lee P, Hur J, Lee J, Kim J, Jeong J, Kang I. 15,16-Dihydrotanshinone I suppresses the activation of BV-2 cell, a murine microglia cell line, by lipopolysaccharide. Neurochem Int. 2006;48:60–66. doi: 10.1016/j.neuint.2005.07.004. [DOI] [PubMed] [Google Scholar]
- 36.Nakano H, Shindo M, Sakon S, Nishinaka S, Mihara M, Yagita H. Differential regulation of IκB kinase α and β by two upstream kinases, NF-κB-inducing kinase and mitogen-activated protein kinase/ERK kinase kinase-1. Proc Natl Acad Sci U S A. 1998;95:3537–3542. doi: 10.1073/pnas.95.7.3537. [DOI] [PMC free article] [PubMed] [Google Scholar]