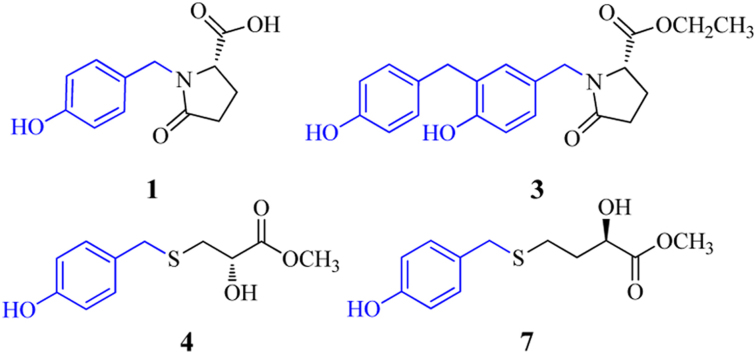
Abstract
Seven new 4-hydroxybenzyl-substituted amino acid derivatives (1−7), together with 11 known compounds, were isolated from an aqueous extract of the rhizomes of Gastrodia elata Blume. Their structures were determined by spectroscopic and chemical methods. Compounds 1−3 are pyroglutamate derivatives containing 4-hydroxybenzyl units at the N atom and 4−7 are the first examples of natural products with the 4-hydroxybenzyl unit linked via a thioether bond to 2-hydroxy-3-mercaptopropanoic acid (4−6) and 2-hydroxy-4-mercaptobutanoic acid (7), which would be biogenetically derived from cysteine and homocysteine, respectively. The structures of 1 and 2 were verified by synthesis, while the absolute configurations of 4, 5 and 7 were assigned using Mosher’s method based on the MPA determination rule of ΔδRS values. The known compound 4-(hydroxymethyl)-5-nitrobenzene-1,2-diol (8) exhibited activity against Fe2+-cysteine induced rat liver microsomal lipid peroxidation with IC50 values of 9.99×10−6 mol/L.
KEY WORDS: Gastrodia elata Blume, Orchidaceae, 4-Hydroxybenzyl- substituted amino acid derivatives, Pyroglutamate deriva tives, Inhibitory activity
Graphical abstract
Seven new 4-hydroxybenzyl-substituted amino acid derivatives, together with 11 known compounds, were isolated from an aqueous extract of the rhizomes of Gastrodia elata.
1. Introduction
Gastrodia elata Blume is a holomycotrophic perennial plant of the Orchidaceae family, and is widely cultivated in several provinces of China to meet the demands of pharmaceutical and food industries1. The steamed and dried rhizome of G. elata, known as “Tianma” in Chinese, is used for the treatment of neuralgic and nervous disorders, such as headaches, migraine, dizziness, tetanus, epilepsy, neuralgia and paralysis. It is also considered to have health benefits enhancing strength and virility and improving memory and blood circulation2. Chemical and pharmacological studies indicated that 4-hydroxybenzyl analogs and 4-hydroxybenzyl-substituted metabolites were main active constituents of ethanol or methanol extracts of this medicine3, 4, 5, 6, 7, 8. As part of a program to assess the chemical and biological diversity of traditional Chinese medicines9, 10, 11, 12, 13, 14, 15, 16, a detailed chemical study was conducted on the aqueous extract of G. elata rhizomes, together with biological assays, since their decoctions are practically used in a variety of formulations. A fraction mainly contained parishin and parishins B and C (total content >50%), and at dosages of 10.00−0.25 mg/kg, the purified parishins improved the impaired memory in mice caused by scopolamine or cycloheximide17. In addition, 23 known compounds were characterized from the extract18, and a minor component N6-(4-hydroxybenzyl)-adenosine (NHBA)19 was isolated as the key sedative and hypnotic constituent of the extract, exhibiting significant activity at a dosage of 0.2 mg/kg (i.p.)20. Therefore, we carried out further investigation on other minor components in the extract. This has resulted in isolation and characterization of seven new 4-hydroxybenzyl-substituted amino acid derivatives 1−7 (Fig. 1), along with 11 known compounds. Reported herein are the isolation, structure determination, and biological activity of these isolates.
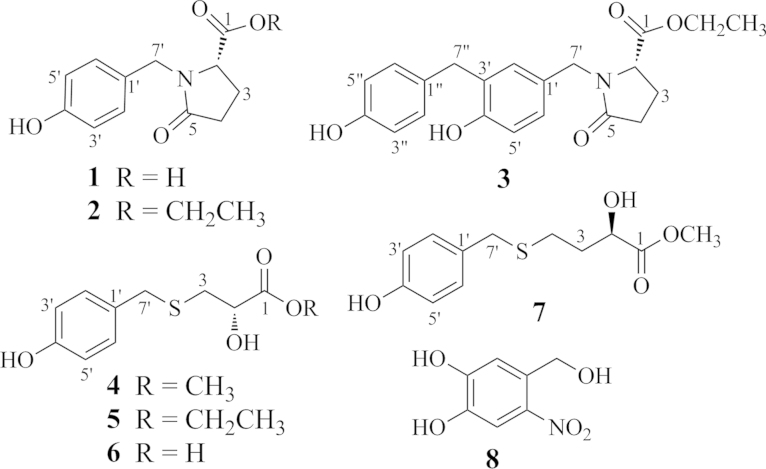
Figure 1.
The structures of compounds 1−8.
2. Results and discussion
Compound 1 showed IR absorptions due to hydroxyl (3216 cm−1), carboxyl (1734 cm−1 and 1658 cm−1), and aromatic ring (1616 cm−1 and 1516 cm−1) functionalities. Its molecular formula C12H13NO4 was indicated by HR-ESI-MS at m/z 236.0925 [M+H]+ (Calcd. for C12H14NO4 236.0917) and the NMR spectral data (Table 1). The NMR spectral data of 1 showed that this compound consisted of 4′-hydroxybenzyl and pyroglutamate moieties21. This was verified by the 1H–1H COSY correlations of H-2/H2-3/H2-4 and HMBC correlations from H-2 to C-1, from H2-3 to C-1 and C-5, and from H2-4 to C-5. In particular, the HMBC correlations from H2-7′ to C-2 and C-5 located the 4′-hydroxybenzyl unit at the N atom of the pyroglutamate moiety. Thus, the planar structure of 1 was determined as N-(4′-hydroxybenzyl)pyroglutamate. The absolute configuration of 1 was assigned by synthesis of enantiomers (+)-(S)- and (−)-(R)-[N-(4′-hydroxybenzyl)]pyroglutamates, starting with l- and d-glutamic acids, respectively. The CD and specific rotation data of 1 were consistent with those of (+)-(S)-[N-(4′-hydroxybenzyl)]pyroglutamate. Therefore, the structure of compound 1 was determined as shown.
Table 1.
1H NMR and 13C NMR spectral data (δ) for compounds 1−7.a.
| No. |
1 |
2 |
3b |
4 |
5 |
6 |
7 |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| δH | δC | δH | δC | δH | δC | δH | δC | δH | δC | δH | δC | δH | δC | |
| 1 | 173.4 | 172.7 | 172.6 | 173.0 | 172.5 | 176.7 | 174.3 | |||||||
| 2 | 4.01 dd (9.0, 3.6) | 58.9 | 3.97 dd (9.0, 3.0) | 59.4 | 3.90 dd (8.4, 3.6) | 59.2 | 4.19 m | 70.4 | 4.16 m | 70.4 | 4.26 brs | 72.1 | 4.11 m | 68.6 |
| 3a | 2.33 m | 23.4 | 2.28 m | 23.4 | 2.22 m | 23.3 | 2.67 dd (13.5, 5.5) | 34.5 | 2.67 dd (14.0, 5.5) | 34.5 | 2.83 brd (13.8) | 36.3 | 1.82 m | 33.7 |
| 3b | 2.08 m | 2.01 m | 1.99 m | 2.58 dd (13.5, 6.5) | 2.57 dd (14.0, 6.0) | 2.69 dd (13.8, 6.6) | 1.76 m | |||||||
| 4a | 2.43 m | 30.2 | 2.38 m | 30.3 | 2.37 m | 30.5 | 2.41 m | 26.3 | ||||||
| 4b | 2.36 m | 2.30 m | 2.26 m | |||||||||||
| 5 | 175.5 | 174.9 | 174.9 | |||||||||||
| 1′ | 127.9 | 128.1 | 127.9 | 128.3 | 128.3 | 130.4 | 128.5 | |||||||
| 2′ | 7.08 d (8.4) | 130.5 | 7.05 d (8.4) | 130.5 | 6.88 d (2.4) | 131.3 | 7.09 d (8.0) | 130.0 | 7.09 d (8.5) | 130.0 | 7.15 d (8.4) | 131.3 | 7.08 d (8.5) | 129.9 |
| 3′ | 6.79 d (8.4) | 116.1 | 6.78 d (8.4) | 116.1 | 129.4 | 6.69 d (8.0) | 115.1 | 6.69 d (8.5) | 115.1 | 6.71 d (8.4) | 116.1 | 6.68 d (8.5) | 115.1 | |
| 4′ | 157.7 | 157.7 | 155.3 | 156.2 | 156.2 | 157.5 | 156.2 | |||||||
| 5′ | 6.79 d (8.4) | 116.1 | 6.78 d (8.4) | 116.1 | 6.79 d (8.4) | 116.0 | 6.69 d (8.0) | 115.1 | 6.69 d (8.5) | 115.1 | 7.15 d (8.4) | 116.1 | 6.68 d (8.5) | 115.1 |
| 6′ | 7.08 d (8.4) | 130.5 | 7.05 d (8.4) | 130.5 | 6.87 dd (8.4, 2.4) | 128.0 | 7.09 d (8.0) | 130.0 | 7.09 d (8.5) | 130.0 | 6.71 d (8.4) | 131.3 | 7.08 d (8.5) | 129.9 |
| 7′a | 4.94 d (15.0) | 45.2 | 4.82 d (15.0) | 45.3 | 4.80 d (14.4) | 45.3 | 3.67 s | 35.3 | 3.67 s | 35.3 | 3.73 s | 37.1 | 3.60 s | 34.5 |
| 7′b | 3.85 d (15.0) | 3.87 d (15.0) | 3.79 d (14.4) | |||||||||||
| OCH3 | 3.63 s | 51.6 | 3.62 s | 51.5 | ||||||||||
| OCH2CH3 | 4.11 q (7.2) | 61.7 | 4.08 q (7.2) | 61.7 | 4.09 q (7.0) | 60.2 | ||||||||
| OCH2CH3 | 1.21 t (7.2) | 14.4 | 1.18 t (7.2) | 14.4 | 1.19 t (7.0) | 14.1 | ||||||||
| 2-OH | 5.75 d (6.0) | 5.71 d (6.0) | 5.49 d (6.0) | |||||||||||
| 4′-OH | 8.31 s | 8.44 s | 9.32 s | 9.33 s | 9.34 s | |||||||||
NMR data (δ) were measured in Me2CO-d6 for 1−3 at 600 MHz for 1H and at 150 MHz for 13C, in DMSO-d6 for 4, 5, and 7 at 500 MHz for 1H and at 125 MHz for 13C and in MeOH-d4 for 6 at 600 MHz for 1H and at 150 MHz for 13C. Proton coupling constants (J) in Hz are given in parentheses. The assignments were based on DEPT, 1H-1H COSY, HSQC, and HMBC experiments.
Data for 4″-hydroxybenzyl in 3: δH 8.16 (s, 1H, OH-4″), 7.05 (d, 2H, J=8.4 Hz, H-2″/6″), 6.71 (d, 2H, J=8.4 Hz, H-3″/5″), 3.82 (s, 2H, H2-7″); δC 132.7 (C-1″), 130.6 (C-2″/6″), 115.8 (C-3″/5″), 156.4 (C-4″), 35.4 (C-7″).
Compound 2 has the molecular formula C14H17NO4 as indicated by the HR-ESI-MS and NMR data (Table 1 and Section 4). Comparison of the NMR data of 2 and 1 indicated the presence of an ethoxy group [δH 4.11 (q, 2H, J=7.2 Hz) and δH 1.21 (t, 3H, J=7.2 Hz); δC 61.7 and δC 14.4] and shielded shifts of C-1 and C-5 by ΔδC −0.7 ppm and −0.6 ppm, respectively in 2. This revealed that 2 is the ethyl ester of 1, which was confirmed by the HMBC correlation from OCH2CH3 to C-1 in the HMBC spectrum of 2. The CD and specific rotation data of 2 were similar with those of 1, indicating that the two compounds have the same configuration, which was further confirmed by synthesis of the enantiomers, ethyl (+)-(S)- and (−)-(R)-[N-(4′-hydroxybenzyl)]pyroglutamates. The CD and specific rotation data of 2 were in agreement with those of the former enantiomer. Thus, the structure of compound 2 was determined as shown.
The molecular formula C21H23NO5 of compound 3 was determined from its HR-ESI-MS and NMR data (Table 1 and Section 4). Comparison of the NMR data of 3 and 2 suggests that 3 is an analog of 2 with an additional 4″-hydroxybenzyl unit substituted at C-3′. This was confirmed by the 1H-1H COSY correlations of H-2/H2-3/H2-4 and HMBC correlations of H-2/C-1 and C-5; H2-3/C-1 and C-5; H2-4/C-5; OCH2CH3/C-1; 4′′-OH/C-3′′, C-4′′, and C-5′′; H-7′′/C-2′, C-3′, C-4′, C-1′′, C-2′′, and C-6′′; 4′-OH/C-3′, C-4′, and C-5′; H-7′/C-1′, C-2, C-2′, C-5, and C-6′. Similarity of the CD and specific rotation data between 3 and 2 suggested the same 2(S) configuration for the two compounds. Thus, compound 3 was determined as ethyl (+)-(S)-{N-[4′-hydroxy-3′-(4″-hydroxybenzyl)benzyl]}pyroglutamate.
Compound 4 has the molecular formula C11H14O4S as indicated by the HR-ESI-MS and NMR data (Table 1 and Section 4). Comparing the NMR data between 4 and the synthetic methyl S-(4′-hydroxybenzyl)-l-cysteinate, the chemical shift of C-2 (δC 70.4) and the presence of an exchangeable hydroxy proton [δ 5.75 (d, 1H, J=6.0 Hz)] in the NMR spectra of 4 in DMSO-d6 demonstrated the replacement of the amino group in the synthetic compound by a hydroxyl group in 4. This was proved by the two- and three-bond correlations from H2-3 to C-7′; from H2-7′ to C-1′, C-2′ (C-6′), and C-3; from OH to C-1, C-2, and C-3; and from OCH3 to C-1 in the HMBC spectrum of 4. The absolute configuration at C-2 in 4 was determined by the modified Mosher’s method22. Esterification of 4 with (−)-(R)- and (+)-(S)-α-methoxyphenylacetic acid (MPA) afforded the corresponding derivatives, 4-bis-(R)-MPA and 4-bis-(S)-MPA. Since the MPA moiety at C-4′ of the benzyl unit is away from the chiral center (C-2) in the bis-MPA esters, the chemical shift change of protons around C-2 is mainly induced by the MPA moiety at C-2. From the MPA determination rule based on the ΔδRS values (Fig. 2), the configuration of 4 was assigned as 2S. Therefore, the structure of compound 4 was determined as shown.
Figure 2.
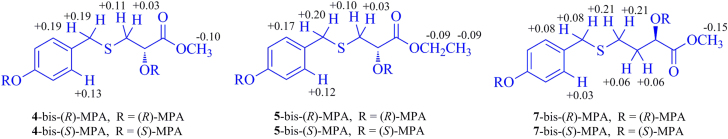
ΔδRS values (δR−δS, black data in ppm) for bis-MPA esters of compounds 4, 5, and 7.
Compound 5 (C12H16O4S) is an analog of 4, as indicated by its spectroscopic data (Table 1 and Section 4). Comparison of the NMR data of 5 and 4 indicated that the methoxyl group in 4 was substituted by an ethoxyl group [δH 4.09 (q, 2H, J=7.0 Hz, OCH2CH3) and δH 1.19 (t, 3H, J=7.0 Hz, OCH2CH3); and δC 60.2 and δC 14.1] in 5. The HMBC correlations from OCH2CH3 to C-1 confirmed the ester linkage of the ethoxyl group. The S-configuration of 5 was verified by Mosher’s method (Fig. 2). Thus, compound 5 was determined as ethyl (+)-(S)-2-hydroxy-3-[(4′-hydroxybenzyl)thio]propanoate.
The spectroscopic data of compound 6 indicated that it is the acid form of 4 and 5. The NMR data of 6 demonstrated a deshielded shift of the C-1 resonance (ΔδC>+3.0 ppm), as compared with that of 4 or 5, in addition to the absence of the methoxyl or ethoxyl group. An ethanol solution of 6 was treated with thionyl chloride (SOCl2) to yield 5. Therefore, compound 6 was determined as (+)-(S)-2-hydroxy-3-[(4′-hydroxybenzyl)thio]-propanoic acid.
The spectroscopic data of compound 7 indicated that it is an isomer of 5. Comparing the NMR data of these two compounds demonstrated that instead of containing an ethyl 2-hydroxypropanoate moiety as in 5, compound 7 contained a methyl 2-hydroxybutyrate moiety. The 1H-1H COSY correlations of H-2/H2-3/H2-4 and HMBC correlations of H-2/C-1, C-3, and C-4; H2-3/C-1, C-2, and C-4; H2-4/C-2 and C-3, and OCH3/C-1, along with their chemical shifts, confirmed the presence of the methyl 2-hydroxybutyrate moiety with the sulfur atom substituted at C-4 in 7. In addition, the HMBC correlations from H2-4 to C-7′ and from H2-7′ to C-4 verified the 4′-hydroxylbenzyl unit located at the sulfur atom. The 2R configuration of 7 was determined by using the same protocol as described for 4 and 5 (Fig. 2). Therefore, compound 7 was determined as methyl (−)-(R)-2-hydroxy-4-[(4′-hydroxybenzyl)thio]butyrate.
The acid/ester pair of 1/2 and the ethyl ester 3 are considered as natural products because HPLC-ESI-MS analysis using the ion extraction method demonstrated their occurrence in the crude extract or an CH3CN-eluted fraction without contacting with EtOH. In addition, methylation or ethylation of the acids and hydrolysis of the esters were unlikely to occur in the isolation procedure because refluxing the EtOH solution of l-[N-(4′-hydroxybenzyl)]glutamic acid only produced 1, whereas 2 was obtained by subsequent addition of thionyl chloride (SOCl2) in the solution. However, the esters 4, 5, and/or 7 may be artifacts because 6 was esterified by keeping the MeOH solution at room temperature for a month, producing the ester with the spectroscopic features identical to that of 4 (Figs. S125–127 in Supporting information).
The known compounds were identified by comparing their spectroscopic data with the reported data as cyclo[glycine-l-S-(4′′-hydroxybenzyl)cysteine]23, 2-[4-(β-d-glucopyranosyl)benzyl]citrate24, 1-ethyl citrate25, 6-ethyl citrate26, parishin E, 4-(hydroxymethyl)-5-nitrobenzene-1,2-diol (8)27, (−)-(6R)-6,7-dihydroxy-3,7-dimethyl-(2E)-octenoic acid28, bis(4-hydroxybenzyl)sulfide29, ethyl (+)-(2S)-2-hydroxy-3-(4-hydroxyphenyl)propanoate30, 1-(4′-hydroxyphenyl)propan-1,2-dione31, and (−)-4-β-d-glucopyranosyl-(1→6)-β-d-glucopyranosyloxybenzyl alcohol32.
In the in vitro bioassays, compound 8 showed activity against Fe2+-cysteine induced rat liver microsomal lipid peroxidation, with IC50 value of 9.99×10−6 mol/L (the positive control, glutathione, gave IC50 20.21×10−6 mol/L). All other compounds isolated in this experiment were inactive at the same concentration. In addition, these compounds were also evaluated for the scavenging activity of 1,1-diphenyl-2-picrylhydrazyl (DPPH) radical and their inhibitory activity against Fe2+/cysteine-induced liver microsomal lipid peroxidation, several human cancer cell lines, HIV-1 replication, and all of them were inactive at a concentration of 10−5 mol/L.
3. Conclusion
Seven new 4-hydroxybenzyl-substituted amino acid derivatives (1−7), together with 11 known compounds, were isolated from an aqueous extract of the rhizomes of G. elata. Blume. Compounds 1−3 are pyroglutamate derivatives containing 4-hydroxybenzyl units at the N atom and 4−7 are the first examples of natural products with the 4-hydroxybenzyl unit linked via a thioether bond to 2-hydroxy-3-mercaptopropanoic acid (4−6) and 2-hydroxy-4-mercaptobutanoic acid (7), which would be biogenetically derived from cysteine and homocysteine, respectively. The enantiomers of compounds 1 and 2 were synthesized, and the absolute configurations of 4, 5 and 7 were assigned using Mosher’s method. These results, combined with our previous studies18, provide an important clue for further studies of chemical transformation, structural modification, and biosynthesis of the diverse 4-hydroxybenzyl-substituted amino acid derivatives from the rhizome of G. elata, as well as for evaluations on other pharmacological models though the new compounds were inactive in the assays carried out in this study.
4. Experimental
4.1. General experimental procedures
Optical rotations were measured using a Rudolph Research Autopol III polarimeter. UV spectra were measured on a Cary 300 spectrometer. CD spectra were measured on a JASCO J-810 spectropolarimeter. IR spectra were recorded on a Nicolet 5700 FT-IR microscope spectrometer (FT-IR microscope transmission) by microscope transmission method. 1D- and 2D-NMR spectra were obtained on INOVA 400 MHz, 500 MHz, or SYS 600 MHz spectrometers (Varian), with solvent peaks serving as references (unless otherwise noted). ESI-MS data were measured with a Q-Trap LC/MS/MS (Turbo Ionspray source) spectrometer. HR-ESI-MS data were, in turn, measured on an AccuTOF-CS JMS-T100CS spectrometer (JEOL), and HR-EI-MS data were measured using a Micromass Autospec-Ultima ETOF spectrometer. Column chromatography was performed with silica gel (200–300 mesh, Qingdao Marine Chemical Inc., Qingdao, China) and Sephadex LH-20 (Pharmacia Biotech AB, Uppsala, Sweden), Toyopearl HW-40C and HW-40F (Tosoh Bioscience LLC, Tokyo, Japan), and MCI gel (CHP20P) (Mitsubishi Chemical Inc., Tokyo, Japan). HPLC separation was performed on an instrument with a Waters 600 controller, a Waters 600 pump, and a Waters 2487 dual λ absorbance detector (detecting wavelength: 230 nm) on a Grace (250 mm×10 mm, i.d.) semi-preparative column packed with C18 (5 μm), and an YMC-Pack (250 mm×10 mm, i.d.) column packed with Ph (5 μm). Glass precoated silica gel GF254 plates were used for TLC. Spots were visualized under UV light or by spraying with 5% H2SO4 in EtOH, followed by heating.
4.2. Plant material
The rhizomes of G. elata were collected at the plantation field of Xiaocao Ba, Yunnan province, China, in December 2009. Plant identification was verified by Mr. Lin Ma (Institute of Materia Medica, Beijing 100050, China). A voucher specimen (No. ID-S-2384) was deposited at the herbarium of the Department of Medicinal Plants, Institute of Materia Medica, Beijing 100050, China.
4.3. Extraction and isolation
The steamed and air-dried G. elata rhizomes (50 kg) were pulverized and ultrasonicated with H2O (150 L×3×1 h). The aqueous extracts were combined and evaporated under reduced pressure to yield a concentrated solution (50 L), which was loaded on a macroporous adsorbent resin (HPD-100, 30 kg) column (20 cm×200 cm), and eluted successively with H2O (50 L), 30% EtOH (150 L), 50% EtOH (120 L) and 95% EtOH (80 L) to yield four corresponding fractions A−D. After removing the solvent under reduced pressure, fraction C (1.9 kg) was chromatographed over MCI gel (CHP 20P, 10 L), with successive elution using H2O (30 L), 30% EtOH (70 L), 50% EtOH (70 L), 95% EtOH (30 L) and Me2CO (20 L), to afford fractions C1−C5.
Fraction C3 (237 g) was subjected to column chromatography (CC) over silica gel, eluting with a gradient of increasing MeOH concentration (0−100%) in EtOAc followed by 30% EtOH, to yield fractions C3-1–C3-5 based on TLC analysis. Fraction C3-1 (27.3 g) was separated by silica gel CC (petroleum ether-ethyl acetate, 50:1 v/v to 1:1 v/v) to give C3-1-1−C3-1-6. Separation of C3-1-1 (780 mg) by RP flash CC (10%−70% MeOH in H2O) afforded C3-1-1-1–C3-1-1-8. Purification of C3-1-1-1 (120 mg) by HPLC (C18 column, 2.0 mL/min, 45% MeOH in H2O) gave 2 (34 mg, tR=28.7 min), and C3-1-1-4 (42.5 mg) by HPLC (45% MeCN in H2O) gave 5 (22 mg, tR=18.2 min, C18 column, 2.0 mL/min). Separation of C3-1-3 (900 mg) by silica gel CC (CHCl3–MeOH, 15:1 v/v) yielded subfractions C3-1-3-1−C3-1-3-7, of which C3-1-3-7 (234 mg) was further fractioned by CC over Sephadex LH-20 (MeOH-H2O, 1:1 v/v) to obtain C3-1-3-7-1 and C3-1-3-7-2. Fraction C3-1-3-7-1 (27 mg) was purified by HPLC (Ph column, 52% MeOH in H2O, 2.0 mL/min) to obtain 3 (2.7 mg, tR=34.5 min). Fraction C3-1-4 (1.25 g) was further separated by silica gel CC (CHCl3–MeOH, 10:1 v/v to 1:1 v/v) to afford C3-1-4-1–C3-1-4-4, of which C3-1-4-4 (640 mg) was fractionated by CC over Sephadex LH-20 (MeOH–H2O, 1:1 v/v) to yield C3-1-4-4-1–C3-1-4-4-7. Separation of C3-1-4-4-3 (74 mg) by CC over HW-40F (MeOH) gave C3-1-4-4-3-1 and C3-1-4-4-3-2, of which C3-1-4-4-3-2 (37 mg) was purified by RP HPLC (Ph column, 20% MeOH in H2O containing 0.1% TFA, 1.5 mL/min) to yield 1 (19 mg, tR=14.3 min). Fraction C3-1-4-4-6 (46 mg) was chromatographed over HW-40F (MeOH), followed by RP HPLC (C18 column, 25% MeOH in H2O containing 0.1% TFA, 2.0 mL/min), to afford 6 (9 mg, tR=45.3 min). Fraction C3-2 (120 g) was separated by silica gel CC (ethyl acetate-MeOH, 100:1 v/v to 1:1 v/v) to give C3-2-1−C3-2-7. Separation of C3-2-5 (47 g) by CC over Sephadex LH-20 (30% MeOH in H2O) afforded subfractions C3-2-5-1–C3-2-5-21, of which C3-2-5-18 (240 mg) was purified by RP HPLC (C18 column, 55% MeOH in H2O, 2.0 mL/min) to give 4 (19.7 mg, tR=16.5 min), and C3-2-5-6 by reduced pressure HPLC (C18 column, 50% MeOH in H2O, 2.0 mL/min) gave 7 (11.1 mg, tR=21.2 min).
4.3.1. (+)-(S)-[N-(4′-Hydroxybenzyl)]pyroglutamate (1)
Colorless gum; +43.2 (c 0.11, MeOH); UV (MeOH) λmax (logε): 203 (3.16), 226 (3.05), 277 (2.20) nm; CD (MeOH) 224 (Δε +16.3) nm; IR (Nujol): νmax 3216, 3020, 2952, 2719, 1735, 1659, 1616, 1516, 1452, 1420, 1359, 1232, 1174, 1108, 961, 851, 837 cm−1; 1H NMR (CD3OD, 600 MHz) data, see Table 1; 13C NMR (CD3OD, 150 MHz) data, see Table 1; (+)-ESI-MS m/z 236 [M+H]+, 258 [M+Na]+, 274 [M+K]+, 471 [2M+H]+, 493 [2M+Na]+; (+)-HR-ESI-MS m/z 236.0925 [M+H]+ (Calcd. for C12H14NO4, 236.0917).
4.3.2. Ethyl (+)-(S)-[N-(4′-hydroxybenzyl)]pyroglutamate (2)
Colorless gum; +40.2 (c 0.09, MeOH); UV (MeOH) λmax (logε): 203 (3.36), 226 (3.22), 277 (2.84) nm; CD (MeOH) 224 (Δε +7.8) nm; IR (Nujol): νmax 3241, 2982, 2935, 1740, 1669, 1615, 1597, 1517, 1451, 1419, 1367, 1269, 1230, 1203, 1107, 1031, 959, 849, 838 cm−1; 1H NMR (DMSO-d6, 600 MHz) data, see Table 1; 13C NMR (DMSO-d6, 150 MHz) data, see Table 1; (+)-ESI-MS m/z 264 [M+H]+, 286 [M+Na]+, 527 [2M+H]+, 549 [2M+Na]+; (+)-HR-ESI-MS m/z 264.1235 [M+H]+ (Calcd. for C14H18NO4, 264.1230), 286.1052 [M+Na]+ (Calcd. for C14H17NO4Na, 286.1050).
4.3.3. Ethyl (+)-(S)-{N-[4′-hydroxy-3′-(4′′-hydroxybenzyl)benzyl]}pyroglutamate (3)
Colorless gum; +3.1 (c 0.19, MeOH); UV (MeOH) λmax (logε): 204 (3.39), 227 (2.82), 279 (2.16) nm; CD (MeOH) 222 (Δε +1.7) nm; IR (Nujol): νmax 3334, 3017, 2981, 2930, 1739, 1668, 1612, 1513, 1444, 1368, 1265, 1209, 1111, 1017, 963, 913, 827 cm−1; 1H NMR (Me2CO-d6, 600 MHz) data, see Table 1; 13C NMR (Me2CO-d6, 150 MHz) data, see Table 1; (+)-ESI-MS m/z 370 [M+H]+, 392 [M+Na]+, 408 [M+K]+; (+)-HR-ESI-MS m/z 370.1661 [M+H]+ (Calcd. for C21H24NO5, 370.1649), 392.1487 [M+Na]+ (Calcd. for C21H23NO5Na, 392.1468).
4.3.4. Methyl (+)-(S)-2-hydroxy-3-[(4′-hydroxybenzyl)thio]propanoate (4)
White amorphous powder; +68.7 (c 0.02, MeOH); UV (MeOH) λmax (logε): 226 (4.32), 279 (2.73) nm; IR (Nujol): νmax 3366, 3021, 2955, 2924, 1893, 1738, 1677, 1612, 1514, 1443, 1225, 1143, 1098, 1012, 970, 837 cm−1; 1H NMR (DMSO-d6, 500 MHz) data, see Table 1; 13C NMR (DMSO-d6, 125 MHz) data, see Table 1; (+)-HR-ESI-MS m/z 265.0508 [M+Na]+ (Calcd. for C11H14O4SNa, 265.0505).
4.3.5. Ethyl (+)-(S)-2-hydroxy-3-[(4′-hydroxybenzyl)thio]propanoate (5)
White powder; +34.2 (c 0.04, MeOH); UV (MeOH) λmax (logε): 227 (4.28), 279 (2.83) nm; IR (Nujol): νmax 3372, 2983, 2927, 1890, 1733, 1679, 1612, 1596, 1514, 1446, 1370, 1224, 1097, 1024, 837 cm−1; 1H NMR (DMSO-d6, 500 MHz) data, see Table 1; 13C NMR (DMSO-d6, 125 MHz) data, see Table 1; (+)-HR-ESI-MS m/z 257.0837 [M+H]+ (Calcd. for C12H17O4S, 257.0842), 279.0659 [M+Na]+ (Calcd for C12H16O4SNa, 279.0662).
4.3.6. (+)-(S)-2-Hydroxy-3-[(4′-hydroxybenzyl)thio]propanoate (6)
Colorless gum; +8.51 (c 0.80, MeOH); UV (MeOH) λmax (logε): 204 (3.43), 227 (3.31), 280 (2.49) nm; IR (Nujol): νmax 3290, 3020, 2921, 1894, 1729, 1612, 1597, 1514, 1445, 1368, 1235, 1097, 1044, 1022, 980, 835 cm−1; 1H NMR (MeOH-d4, 600 MHz) data, see Table 1; 13C NMR (MeOH-d4, 150 MHz) data, see Table 1; (−)-ESI-MS m/z 227 [M–H]−, 455 [2M–H]−; (+)-HR-ESI-MS m/z 251.0349 [M+Na]+ (Calcd. for C10H12O4SNa, 251.0349), 267.0080 [M+K]+ (Calcd. for C10H12O4SK, 267.0088).
4.3.7. (−)-(R)-2-Hydroxy-4-[(4′-hydroxybenzyl)thio]butyrate (7)
White powder; −61.7 (c 0.01, MeOH); UV (MeOH) λmax (logε): 222 (4.14), 278 (2.77) nm; IR (Nujol): νmax 3352, 2956, 2919, 1891, 1733, 1680, 1613, 1597, 1514, 1443, 1364, 1305, 1234, 1206, 1142, 1097, 1024, 925, 838, 802 cm−1; 1H NMR (DMSO-d6, 500 MHz) data, see Table 1; 13C NMR (DMSO-d6, 125 MHz) data, see Table 1; (+)-HR-ESI-MS m/z 279.0662 [M+Na]+ (Calcd. for C12H16O4SNa, 279.0662).
4.4. Synthesis of 1 and 2
To a solution of l- or d-glutamic acid (2 g) in MeOH (30 mL), 4-hydroxybenzaldehyde (3 g) and anhydrous Na2CO3 (3 g) were added. The mixture was stirred at r.t. for 4 h, cooled to 0 °C, and NaBH4 (1 g) was slowly added by keeping the temperature at 0−5 °C. The mixture was stirred at r.t. for 40 min, and acidified with 2 mol/L HCl to pH 3 at 0−5 °C to produce precipitate, which was collected by filtration, washed with cold water, and dried to afford l- or d-[N-(4-hydroxybenzyl)]glutamic acid (~1.4 g)33.
A suspension of l- or d-[N-(4-hydroxybenzyl)]glutamic acid (200 mg) in ethanol (15 mL) was refluxed for 5 h. The resulting solution was filtrated, followed by evaporation of the filtrate, to afford (+)-(S)-[N-(4-hydroxybenzyl)]pyroglutamate (126 mg) from l-[N-(4-hydroxybenzyl)]glutamic acid or (−)-(R)-[N-(4-hydroxybenzyl)]pyroglutamate (138 mg) from d-[N-(4-hydroxybenzyl)]glutamic acid. (+)-(S)-[N-(4-Hydroxybenzyl)]pyroglutamate: colorless gum; +49.8 (c 1.6, MeOH); CD (MeOH) 223 (Δε +11.9) nm; 1H NMR (400 MHz, CD3COCD3): δ 7.06 (d, 2H, J=7.6 Hz, H-2′/6′), 6.78 (d, 2H, J=7.6 Hz, H-3′/5′), 4.93 (d, 1H, J=14.8 Hz, H-7′a), 3.97 (d, 1H, J=8.4 Hz, H-2), 3.82 (d, 1H, J=14.8Hz, H-7′b), 2.32–2.41 (m, 3H, H-3a/4a/4b), 2.08 (m, 1H, H-3b); 13C NMR (100 MHz, CD3COCD3): δ 175.6 (C-5), 173.4 (C-1), 157.7 (C-4′), 130.5 (C-2′/6′), 128.0 (C-1′), 116.2 (C-3′/5′), 59.0 (C-2), 45.3 (C-7′), 30.2 (C-4), 23.5 (C-5); (+)-ESI-MS m/z 236 [M+H]+, 258 [M+Na]+. (−)-(R)-[N-(4-Hydroxybenzyl)]pyroglutamate: colorless gum; −46.5 (c 1.1, MeOH); CD (MeOH) 223 (Δε −31.3) nm; 1H NMR (400 MHz, CD3COCD3): δ 7.07 (d, 2H, J=8.0 Hz, H-2′/6′), 6.78 (d, 2H, J=8.0 Hz, H-3′/5′), 4.94 (d, 1H, J=14.8 Hz, H-7′a), 4.00 (d, 1H, J=8.0 Hz, H-2), 3.84 (d, 1H, J=14.8 Hz, H-7′b), 2.30–2.45 (m, 3H, H-3a/4a/4b), 2.07 (m, 1H, H-3b); 13C NMR (100 MHz, CD3COCD3): δ 175.9 (C-5), 173.4 (C-1), 157.8 (C-4′), 130.6 (C-2′/6′), 127.8 (C-1′), 116.2 (C-3′/5′), 59.0 (C-2), 45.3 (C-7′), 30.4 (C-4), 23.5 (C-5); (+)-ESI-MS m/z 236 [M+H]+, 258 [M+Na]+. The NMR data of the synthetic compounds (Figs. S16−18 and S21−23 in Supporting information) were consistent with those of 1.
To the suspension of l-[N-(4-hydroxybenzyl)]glutamic acid (200 mg) in ethanol (15 mL), SOCl2 (5 mL) was slowly added at 0 °C, and stirred for 1 h. Then, the mixture was refluxed for 5 h. The resulting solution was filtrated, followed by evaporation of the filtrate, to afford ethyl (+)-(S)-[N-(4-hydroxybenzyl)]pyroglutamate (167 mg). By changing the starting material to d-[N-(4-hydroxybenzyl)]glutamic acid, ethyl (−)-(R)-[N-(4-hydroxy benzyl)]pyroglutamate (124 mg) was obtained. Ethyl (+)-(S)-[N-(4-hydroxybenzyl)]pyroglutamate: colorless gum; +47.5 (c 1.7, MeOH); CD (MeOH) 222 (Δε +10.0) nm; 1H NMR (400 MHz, CD3COCD3): δ 8.31 (s, 1H, OH-4′), 7.05 (d, 2H, J=7.6 Hz, H-2′/6′), 6.78 (d, 2H, J=7.6 Hz, H-3′/5′), 4.82 (d, 1H, J=14.8 Hz, H-7′a), 4.11 (q, 2H, J=7.2 Hz, OCH2CH3), 3.97 (d, 1H, J=6.0 Hz, H-2), 3.87 (d, 1H, J=14.8 Hz, H-7′b), 2.39 (m, 1H, H-4a), 2.27 (m, 2H, H-3a/4b), 2.00 (m, 1H, H-3b), 1.21 (t, 3H, J=7.2 Hz, OCH2CH3); 13C NMR (150 MHz, CD3COCD3): δ 174.9 (C-5), 172.7 (C-1), 157.7 (C-4′), 130.5 (C-2′/6′), 128.1 (C-1′), 116.1 (C-3′/5′), 61.7 (OCH2CH3), 59.3 (C-2), 45.2 (C-7′), 30.2 (C-4), 23.4 (C-5), 14.4 (OCH2CH3); (+)-ESI-MS m/z 263 [M+H]+, 286 [M+Na]+, 527 [2M+H]+, 549 [2M+Na]+. Ethyl (−)-(R)-[N-(4-hydroxybenzyl)]pyroglutamate: colorless gum; −45.7 (1.5, MeOH); CD (MeOH) 222 (Δε −17.0) nm; 1H NMR (600 MHz, CD3COCD3): δ 8.45 (s, 1H, OH-4′), 7.07 (d, 2H, J=8.0 Hz, H-2′/6′), 6.80 (d, 2H, J=8.0 Hz, H-3′/5′), 4.85 (d, 1H, J=14.4 Hz, H-7′a), 4.14 (q, 2H, J=7.2 Hz, OCH2CH3), 4.00 (dd, 1H, J=8.4, 2.4 Hz, H-2), 3.91 (d, 1H, J=14.4 Hz, H-7′b), 2.43 (m, 1H, H-4a), 2.32 (m, 1H, H-4b), 3.20 (m, 1H, H-3a), 2.05 (m, 1H, H-3b), 1.23 (t, 3H, J=7.2 Hz, OCH2CH3); 13C NMR (150 MHz, CD3COCD3): δ 174.8 (C-5), 172.6 (C-1), 157.6 (C-4′), 130.4 (C-2′/6′), 128.0 (C-1′), 116.0 (C-3′/5′), 61.6 (OCH2CH3), 59.3 (C-2), 45.1 (C-7′), 30.3 (C-4), 23.3 (C-5), 14.3 (OCH2CH3); (+)-ESI-MS m/z 263 [M+H]+, 286 [M+Na]+, 527 [2M+H]+, 549 [2M+Na]+. The NMR data of the synthetic compounds (Figs. S39−41 and S44−46 in Supporting information) were identical with those of 2.
4.5. Synthesis of bis-(R)-MPA and bis-(S)-MPA esters of 4, 5, and 7
R- or S-MPA (~10 mg) was added to solutions of 4, 5, or 7 (~0.5 mg), EDCI (~10 mg), and DMAP (~5 mg) in freshly distilled methylene chloride (3 mL), and kept at r.t. overnight. The reaction mixtures were separated by preparative TLC (mobile phase: petroleum ether/Me2CO= 2:1 v/v) to yield 4-bis-(R)-MPA or 4-bis-(S)-MPA from 4, 5-bis-(R)-MPA or 5-bis-(S)-MPA from 5, and 7-bis-(R)-MPA or 7-bis-(S)-MPA from 7. 4-Bis-(R)-MPA: 1H NMR (400 MHz, CDCl3): δ 7.23 (d, 2H, J=8.4 Hz, H-2′/6′), 6.92 (d, 2H, J=8.4 Hz, H-3′/5′), 5.28 (dd, 1H, J=7.6, 4.0 Hz, H-2), 3.69 (s, 2H, H2-7′), 3.61 (s, 3H, OCH3), 2.84 (dd, 1H, J=14.4, 4.0 Hz, H-3a), 2.76 (dd, 1H, J=14.4, 7.6 Hz, H-3b). 4-Bis-(S)-MPA: 1H NMR (400 MHz, CDCl3): δ 7.10 (d, 2H, J=8.8 Hz, H-2′/6′), 6.88 (d, 2H, J=8.8 Hz, H-3′/5′), 5.28 (dd, 1H, J=6.8, 4.8 Hz, H-2), 3.71 (s, 3H, –OCH3), 3.50 (s, 2H, H2-7′), 2.73 (m, 2H, H2-3). 5-Bis-(R)-MPA: 1H NMR (400 MHz, CDCl3): δ 7.23 (d, 2H, J=8.8 Hz, H-2′/6′), 6.92 (d, 2H, J=8.8 Hz, H-3′/5′), 5.26 (dd, 1H, J=7.2, 3.6 Hz, H-2), 4.08 (q, 2H, J=7.2 Hz, OCH2CH3), 3.70 (s, 2H, H2-7′), 2.84 (dd, 1H, J=14.8, 3.6 Hz, H-3a), 2.77 (dd, 1H, J=14.8, 7.2 Hz, H-3b), 1.12 (t, 3H, J=7.2 Hz, OCH2CH3). 5-Bis-(S)-MPA: 1H NMR (400 MHz, CDCl3): δ 7.11 (d, 2H, J=8.4 Hz, H-2′/6′), 6.88 (d, 2H, J=8.4 Hz, H-3′/5′), 5.25 (dd, 1H, J=6.8, 5.2 Hz, H-2), 4.17 (q, 2H, J=7.2 Hz, OCH2CH3), 3.53 (d, 1H, J=13.2, H-7a′), 3.50 (d, 1H, J=13.2, H-7b′), 2.74 (m, 2H, H2-3), 1.21 (t, 3H, J=7.2Hz, OCH2CH3). 6-Bis-(R)-MPA: 1H NMR (400 MHz, CDCl3): δ 7.23 (d, 2H, J=8.4 Hz, H-2′/6′), 6.93 (d, 2H, J=8.4 Hz, H-3′/5′), 5.18 (dd, 1H, J=6.4, 6.0 Hz, H-2), 3.60 (s, 2H, H2-7’), 3.56 (s, 3H, OCH3), 2.36 (m, 2H, H2-4), 2.06 (m, 2H, H2-3). 6-Bis-(S)-MPA: 1H NMR (400 MHz, CDCl3): δ 7.20 (d, 2H, J=8.4 Hz, H-2′/6′), 6.94 (d, 2H, J=8.4 Hz, H-3′/5′), 5.16 (dd, 1H, J=7.6, 4.8 Hz, H-2), 3.71 (s, 3H, OCH3), 3.52 (s, 2H, H2-7’), 2.15 (m, 2H, H2-4), 2.00 (m, 2H, H2-3).
4.6. Synthesis of methyl S-(4-hydroxybenzyl)-l-cysteinate
Methyl l-cysteinate (10 mg) and 4-hydroxybenzylalcohol (13 mg) were added to 5 mL of 2 mol/L HCl. The mixture was stirred at r.t. for 20 min, and extracted with EtOAc (5×3 mL). The organic layer was evaporated under reduced pressure. The residue was chromatographed over Toyopearl HW-40F, using H2O as the mobile phase to afford methyl S-(4-hydroxybenzyl)]-l-cysteinate (14 mg): white amorphous powder, +37.6 (c 2.43, MeOH); 1H NMR (DMSO-d6, 400 MHz): δ 2.94 (m, 2H, H2-3), 3.70 (s, 2H, H2-7′), 3.73 (s, 3H, OCH3), 4.23 (brt, 1H, J=6.0 Hz, H-2), 6.73 (d, 2H, J=8.4 Hz, H-3′/5′), 7.12 (d, 2H, J=8.4 Hz, H-2′/6′), 8.85 (s, 3H, OH and NH2). 13C NMR (DMSO-d6, 100 MHz): δ 30.7 (C-3), 35.1 (C-7′), 51.8 (OCH3), 52.9 (C-2), 115.3 (C-3′/5′), 127.5 (C-1′), 130.1 (C-2′/6′), 156.6 (C-4′), 168.7 (C-1). (+)-ESI-MS m/z 242 [M+H]+, 242 [M+Na]+.
4.7. Antioxidant activity assay against Fe2+/cysteine-induced liver microsomal lipid peroxidation
See Ref. 34.
Acknowledgments
Financial support from the National Natural Science Foundation of China (NNSFC; Nos. 30825044 and 20932007), the Program for Changjiang Scholars and Innovative Research Team in University (PCSIRT, No. IRT1007), and the National Science and Technology Project of China (Nos. 2012ZX09301002-002 and 2011ZX09307-002-01) is acknowledged.
Footnotes
Peer review under responsibility of Institute of Materia Medica, Chinese Academy of Medical Sciences and Chinese Pharmaceutical Association.
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.apsb.2015.02.002.
Appendix A. Supplementary materials
Supplementary data
References
- 1.Xu J.T., Guo S.X. Retrospect on the research of the cultivation of Gastrodia elata Bl, a rare traditional Chinese medicine. Chin Med J. 2000;113:686–692. [PubMed] [Google Scholar]
- 2.Jiangsu New Medical College. Dictionary of traditional Chinese medicine. Shanghai: Shanghai Science and Technology PublishingHouse; 1997, Vol.1:315–7.
- 3.Zhou J., Pu X.Y., Yang Y.B. Phenolic constituents of fresh Gastrodia elata Blume. Chin Sin Bull. 1981;18:1118–1120. [Google Scholar]
- 4.Shin E.J., Whang W.K., Kim S., Bach J.H., Kim J.M., Nguyen X.K. Psychosis in mice: involvements of 5-HT1A receptor. J Pharmacol Sci. 2010;113:404–408. doi: 10.1254/jphs.10040sc. [DOI] [PubMed] [Google Scholar]
- 5.Huang N.K., Lin J.H., Lin J.T., Lin C.I., Liu E.M., Lin C.J. A new drug design targeting the adenosinergic system for Huntington’s disease. PLoS One. 2011;6:e20934. doi: 10.1371/journal.pone.0020934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kam K.Y., Yu S.J., Jeong N., Hong J.H., Anthony Jalin A.M.A., Lee S. p-Hydroxybenzyl alcohol prevents brain injury and behavioral impairment by activating Nrf2, PDI, and neurotrophic factor genes in a rat model of brain ischemia. Mol Cells. 2011;31:209–215. doi: 10.1007/s10059-011-0028-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhao X., Zou Y., Xu H., Fan L., Guo H., Li X. Gastrodin protect primary cultured rat hippocampal neurons against amyloid-β peptide-induced neurotoxicity via ERK1/2-Nrf2 pathway. Brain Res. 2012;1482:13–21. doi: 10.1016/j.brainres.2012.09.010. [DOI] [PubMed] [Google Scholar]
- 8.Kim B.W., Koppula S., Kim J.W., Lim H.W., Hwang J.W., Kim I.S. Modulation of LPS-stimulated neuroinflammation in BV-2 microglia by Gastrodia elata: 4-hydroxybenzyl alcohol is the bioactive candidate. J Ethnopharmacol. 2012;139:549–557. doi: 10.1016/j.jep.2011.11.048. [DOI] [PubMed] [Google Scholar]
- 9.Chen M., Lin L., Li L., Zhu C., Wang X., Wang Y. Enantiomers of an indole alkaloid containing unusual dihydrothiopyran and 1,2,4-thiadiazole rings from the root of Isatis indigotica. Org Lett. 2012;14:5668–5671. doi: 10.1021/ol302660t. [DOI] [PubMed] [Google Scholar]
- 10.Zhao F., Wang S., Lin S., Zhu C., Yue Z., Yu Y. Natural and unnatural anthraquinones isolated from the ethanol extract of the roots of Knoxia valerianoides. Acta Pharm Sin B. 2012;2:260–266. [Google Scholar]
- 11.Yu Y., Zhu C., Wang S., Song W., Yang Y., Shi J. Homosecoiridoid alkaloids with amino acid units from the flower buds of Lonicera japonica. J Nat Prod. 2013;76:2226–2233. doi: 10.1021/np4005773. [DOI] [PubMed] [Google Scholar]
- 12.Wang F., Jiang Y.P., Wang X.L., Wang S.J., Bu P.B., Lin S. Aromatic glycosides from the flower buds of Lonicera japonica. J Asian Nat Prod Res. 2013;15:492–501. doi: 10.1080/10286020.2013.785531. [DOI] [PubMed] [Google Scholar]
- 13.Tian Y., Guo Q., Xu W., Zhu C., Yang Y., Shi J. A minor diterpenoid with a new 6/5/7/3 fused-ring skeleton from Euphorbia micractina. Org Lett. 2014;16:3950–3953. doi: 10.1021/ol501760h. [DOI] [PubMed] [Google Scholar]
- 14.Xu W.D., Tian Y., Guo Q.L., Yang Y.C., Shi J.G. Secoeuphoractin, a minor diterpenoid with a new skeleton from Euphorbia micractina. Chin Chem Lett. 2014;25:1531–1534. doi: 10.1021/ol501760h. [DOI] [PubMed] [Google Scholar]
- 15.Song WX, Yang YC, Shi JG. Two new β-hydroxy amino acid-coupled secoiridoids from the flower buds of Lonicera japonica: isolation, structure elucidation, semisynthesis, and biological activities. Chin Chem Lett 2014;25:1215–9.
- 16.Yu Y., Jiang Z., Song W., Yang Y., Li Y., Jiang J. Glucosylated caffeoylquinic acid derivatives from the flower buds of Lonicera japonica. Acta Pharm Sin B. 2015 doi: 10.1016/j.apsb.2015.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang K, Shi J, Liu Y, Zhao L, Zhang M, Chen M. Gastrodia elata Blume extract for preventing vascular dementia and the preparation method. China patent CN 200810132404.0. 2008 July 15.
- 18.Wang Y., Lin S., Chen M., Jiang B., Guo Q., Zhu C. Chemical constituents from aqueous extract of Gastrodia elata. China J Chin Mater Med. 2012;37:1775–1781. [PubMed] [Google Scholar]
- 19.Huang N.K., Chern Y., Fang J.M., Lin C.I., Chen W.P., Lin Y.L. Neuroprotective principles from Gastrodia elata. J Nat Prod. 2007;70:571–574. doi: 10.1021/np0605182. [DOI] [PubMed] [Google Scholar]
- 20.Zhang Y., Li M., Kang R.X., Shi J.G., Liu G.T., Zhang J.J. NHBA isolated from Gastrodia elata exerts sedative and hypnotic effects in sodium pentobarbital-treated mice. Pharmacol Biochem Behav. 2012;102:450–457. doi: 10.1016/j.pbb.2012.06.002. [DOI] [PubMed] [Google Scholar]
- 21.Zhao J., Ma M., Wang S., Li S., Cao P., Yang Y. Bromophenols coupled with derivatives of amino acids and nucleosides from the red alga Rhodomela confervoides. J Nat Prod. 2005;68:691–694. doi: 10.1021/np040234m. [DOI] [PubMed] [Google Scholar]
- 22.Trost B.M., Belletire J.L., Godleski S., McDougal P.G., Balkovec J.M. On the use of the O-methylmandelate ester for establishment of absolute configuration of secondary alcohols. J Org Chem. 1986;51:2370–2374. [Google Scholar]
- 23.Zi J.C., Lin S., Zhu C.G., Yang Y.C., Shi J.G. Minor constituents from the tubers of Gymnadenia conopsea. J Asian Nat Prod Res. 2010;12:477–484. doi: 10.1080/10286020.2010.491476. [DOI] [PubMed] [Google Scholar]
- 24.Wang L., Xiao H.B., Yang L., Wang Z.T. Two new phenolic glycosides from the rhizome of Gastrodia elata. J Asian Nat Prod Res. 2012;14:457–462. doi: 10.1080/10286020.2012.669755. [DOI] [PubMed] [Google Scholar]
- 25.Dias C., Dias M., Borges C., Almoster Ferreira M.A., Paulo A., Nascimento J. Structural elucidation of natural 2-hydroxy di- and tricarboxylic acids and esters, phenylpropanoid esters and a flavonoid from Autonoe madeirensis using gas chromatographic/electron ionization, electrospray ionization and tandem mass spectrometric techniques. J Mass Spectrom. 2003;38:1240–1244. doi: 10.1002/jms.554. [DOI] [PubMed] [Google Scholar]
- 26.Tang L., Li G., Yang B., Kuang H. Studies on the chemical constituents of Choerospondias axillaris. Chin Tradit Herbal Drugs. 2009;40:541–543. [Google Scholar]
- 27.Lu G., Burgess K. A diversity oriented synthesis of 3′-O-modified nucleoside triphosphates for DNA sequencing by synthesis. Bioorg Med Chem Lett. 2006;16:3902–3905. doi: 10.1016/j.bmcl.2006.05.035. [DOI] [PubMed] [Google Scholar]
- 28.Chen J., Zhu C., Xu H., Ni X., Yang P. Study on chemical constituents of the root of Litsea cubeba. II. Chloroform portion and ethyl acetate portion from methanol extract. Chin J Pharm. 2010;41:504–508. [Google Scholar]
- 29.Xiao Y., Li L., You X. Studies on chemical constituents of effective part of Gastrodia elata. China J Chin Mater Med. 2002;27:35–36. [PubMed] [Google Scholar]
- 30.Takaya Y., Furukawa T., Miura S., Akutagawa T., Hotta Y., Ishikawa N. Antioxidant constituents in distillation residue of awamori spirits. J Agric Food Chem. 2007;55:75–79. doi: 10.1021/jf062029d. [DOI] [PubMed] [Google Scholar]
- 31.Metzler M., Haaf H. Dearylation and other cleavage reactions of diethylstilbestrol: novel oxidative pathways mediated by peroxidases. Xenobiotica. 1985;15:41–49. doi: 10.3109/00498258509045333. [DOI] [PubMed] [Google Scholar]
- 32.Zi J., Li S., Liu M., Gan M., Lin S., Song W. Glycosidic constituents of the tubers of Gymnadenia conopsea. J Nat Prod. 2008;71:799–805. doi: 10.1021/np070670j. [DOI] [PubMed] [Google Scholar]
- 33.Maechalin S., Kadlecinkova K., Bar N., Decroix B. The improved synthesis of enantiopure (S)-N-arylmethyl-5-oxoprolines. Synth Commun. 1998;28:3619–3624. [Google Scholar]
- 34.Xie P., Jiao X.Z., Liang X.T., Feng W.H., Wei H.L., Liu G.T. Synthesis and antioxiactivity of squamosamide cyclic analogs. Acta Acad Med Sin. 2004;26:372–378. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary data