Abstract
Background:
This study was designed to examine the effects of purslane seeds on biomarkers of oxidative stress in type 2 diabetic patients.
Methods:
This cross-over randomized controlled clinical trial was conducted on 40 patients with type 2 diabetes. Participants were randomly assigned to receive either 10 g/day purslane seeds with 240 cc low-fat yogurt (intervention group) or only 240 cc low-fat yogurt (as a control group) for 5 weeks. After a 2-week washout period, subjects were moved to the alternate arm for an additional 5 weeks. At baseline and end of each study phase, fasting blood samples were collected to quantify biomarkers of oxidative stress.
Results:
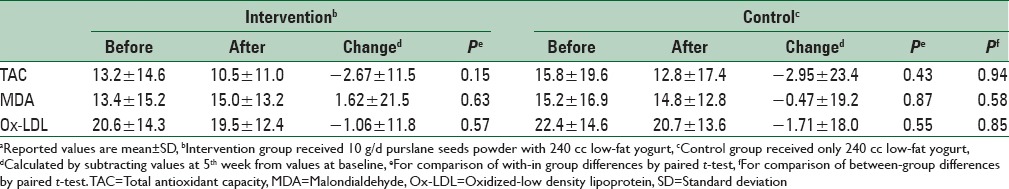
We observed a slight decrease in plasma total antioxidant capacity in both groups, however, between-group changes were not significant (−2.67 vs. −2.95 μg/dL, P = 0.94). Plasma malondialdehyde in purslane group increased slightly, however, we observed no significant effect in the control group (1.62 vs. −0.47 μg/dL, P = 0.58). Although both groups had a slight reduction in plasma oxidized low-density lipoprotein (ox-LDL), we failed to find any significant effect of purslane on plasma ox-LDL (−1.06 vs. −1.71 μg/dL, P = 0.85).
Conclusions:
This cross-over clinical trial revealed that consumption of purslane seeds for 5 weeks in type 2 diabetic patients did not result in improved oxidative stress.
Keywords: Diabetes, medicinal plant, oxidative stress, purslane
INTRODUCTION
Diabetes is one of the most common endocrine disorders with increasing prevalence worldwide.[1] It has been estimated that 7.7% of Iranian adult population is affected by type 2 diabetes.[2] Diabetic patients are at greater risk of morbidity and mortality from microvascular and macrovascular complications.[3] It seems that oxidative stress plays a key role in the pathogenesis of diabetes complications.[4] Patients with diabetes are susceptible to increased oxidative stress due to the excessive production of reactive oxygen species and impaired antioxidant defense mechanism.[5,6] Hyperglycemia in turn results in increased production of free radicals which cause membrane damage due to peroxidation of membrane lipids and protein glycation.[7] In order to prevent complications of diabetes, the control of oxidative stress is necessary in diabetic patients.
Earlier studies have shown that the use of medications, consumption of antioxidants, as well as herbal medicine, might help control the oxidative stress.[8] The use of medicinal plants for the management of diabetes has been common in Iranian population.[9] Among others, the use of purslane, Portulaca oleracea, has been more prevalent by diabetic patients and has recently attracted great attention by scientists. Purslane is a rich source of antioxidants including Vitamins A, C, and E, and beta-carotene, which might help strengthening body's total antioxidant capacity (TAC).[10,11] Previous studies have indicated that phenolic alkaloids of purslane, including oleracin A, B, and E increased scavenging of free radicals in rat brains.[12] Administration of purslane aqueous juice was beneficial for hepatic, renal, and testicular tissue due to its antioxidant properties.[13] Other studies have also demonstrated that polysaccharides from purslane led to lower exercise-induced oxidative stress.[14] In diabetic rats, administration of purslane extract has been resulted in reduced lipid peroxidation.[15]
Despite lots of information about the role of oxidative stress in the pathogenesis of diabetic complications, limited data are available on the application of dietary strategies for alleviating oxidative stress in diabetic patients. The antioxidant properties of purslane in prior studies have been mostly examined in animal models, and we are aware of no study that examined the effects of purslane intake on oxidative stress in humans. Assessing the effect of purslane intake on biomarkers of oxidative stress is particularly relevant for diabetic patients who are at increased risk of oxidative stress. The current study was designed to examine the effects of purslane seeds on biomarkers of oxidative stress in type 2 diabetic patients.
METHODS
Participants
Diabetic patients aged 35–65 attending Endocrine Research Center of Isfahan University of Medical Sciences, were included in this randomized cross-over clinical trial. The study was carried out in Isfahan, Iran, during January 2012–July 2012. On the basis of sample size formula suggested for cross-over clinical trials, a sample size of 38 people was needed for the whole trial. Given the high drop-outs in cross-over trials, we enrolled 40 subjects that diagnosed with type 2 diabetes, based on the criteria of American Diabetes Association in this trial.[16] In addition to being a diabetic patient, subjects were needed to be nonsmokers, nonusers of alcohol, nonpregnant, and nonlactating women. They were recruited in the study if they had not changed the dosage and type of medications in the last 2 months prior to the study and had no history of cardiovascular, lung, kidney, and liver diseases. A written informed consent was obtained from all participants. The study protocol was approved by the Ethical Committee of Isfahan University of Medical Sciences, Isfahan, Iran.
Study design
Diabetic patients were randomly assigned to the initial arm of the study to receive either 10 g/day purslane seeds powder, with 240 cc low-fat yogurt (intervention group) or only 240 cc low-fat yogurt (as control group) for 5 weeks. The prescribed dose of 10 g/day purslane seeds powder was selected based on a previous study.[17] After a 2-week washout period, subjects were crossed over to the alternate treatment arm for an additional 5 weeks. Patients were asked not to change their regular diet and routine physical activity levels for the duration of the study. They were also requested not to start insulin injection and not to change the dosage and type of medications throughout the study. We assessed dietary intakes of subjects by the use of dietary records three times during each study phase (2 weeks days and a weekend day). Nutritionist IV software (First Databank) was used to calculate nutrient intakes based on dietary records. Physical activity levels were also assessed through physical activity records during the study once every 2 weeks. Data from physical activity records were expressed as MET-h/day.
Assessment of biochemical measures
Fasting blood samples (10 mL) were taken at baseline and end of each study phase in an early morning after an overnight fast. Blood samples were immediately centrifuged (Hettich D-78532, Tuttlingen, Germany) at 3500 rpm for 10 min to separate serum. Then, the samples were stored at −70°C before analysis at the laboratory. Plasma TAC and plasma oxidized low-density lipoprotein (ox-LDL) was assessed by the use of ELISA kit (Glory Science, USA). Serum malondialdehyde (MDA) levels were quantified using ELISA kit (Glory Science, USA). All inter-and intra-assay coefficients of variation for biochemical indicators were <5%.
Statistical methods
All statistical analyses were done using the Statistical Package for Social Science version 16 (SPSS Inc., Chicago, Illinois, USA). Data on dietary intakes were compared by paired t-test. For each dependent variable, we computed the changes from baseline by subtracting the baseline value from the end-of-trial value. With-in and between-group changes in dependent variables were compared by the use of paired samples t-test. Data are expressed as mean ± standard deviation, and P < 0.05 was considered as statistically significant.
RESULTS
Overall, 40 subjects with type 2 diabetes mellitus who fulfilled the enrollment criteria entered in this study. No adverse events were reported from the subjects throughout the study period. There was no significant difference in dietary intakes of the participants between the two groups [Table 1]. Comparison of physical activity levels in study participants throughout the study revealed no significant differences between participants in the intervention and those in control groups [Figure 1].
Table 1.
Dietary intakes of the participants throughout the studya
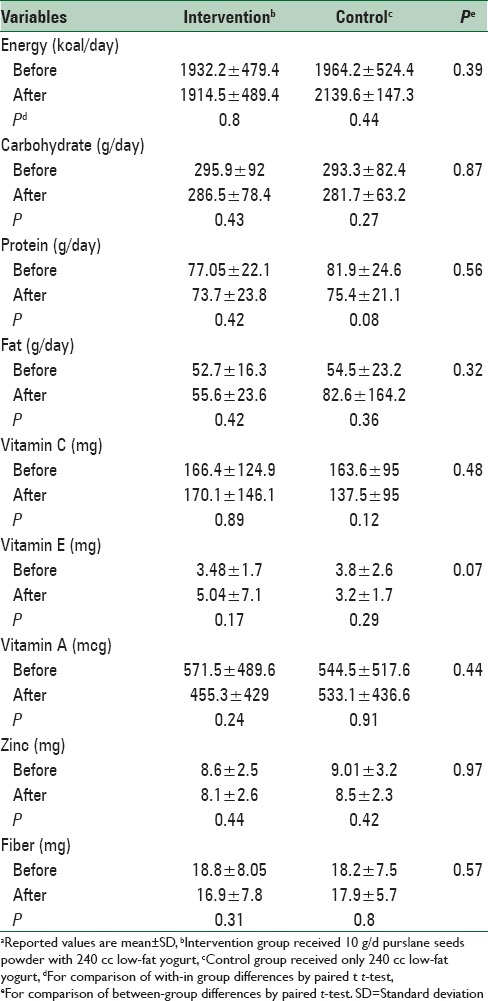
Figure 1.

Comparison of physical activity levels in study participants throughout the study intervention periods
The effects of the 5-week intervention on biomarkers of oxidative stress are presented in Table 2. We observed a slight decrease in plasma TAC in both groups, but between-group changes were not significant (−2.67 vs. −2.95 μg/dL, P = 0.94). Plasma MDA in purslane group increased slightly, but we observed no significant effect in the control group (1.62 vs. −0.47 μg/dL, P = 0.58). Although both groups had a slight reduction in plasma ox-LDL, we failed to find any significant effect of purslane on plasma ox-LDL (−1.06 vs. −1.71 μg/dL, P = 0.85).
Table 2.
Effect of purslane on stress oxidative biomarkers of diabetic patientsa
DISCUSSION
We failed to find any significant effect of purslane on plasma levels of TAC, MDA, and ox-LDL in this randomized cross-over clinical trial. To our knowledge, this study is among the first investigations that examined the effects of purslane seeds on biomarkers of oxidative stress in type 2 diabetic patients.
Type 2 diabetes is the most prevalent metabolic disease all over the world. Oxidative stress plays an important role in the pathogenesis of microvascular and macrovascular complications of diabetes.[15] There are several ways to control the oxidative stress in diabetic patients. Earlier studies have shown that some medicinal plants may protect diabetes against oxidative stress.[8] Due to the high concentrations of antioxidants in purslane, it has potential antioxidant effects.[10,11] However, we did not find any beneficial effect of purslane seeds on oxidative stress in the current study. A limited number of studies has been published about the health effects of purslane. In addition, previous studies have reached opposite findings. Administration of aqueous juice of purslane has been resulted in improvement in antioxidant parameters in rats.[13] Consumption of purslane leaves for 3 weeks led to a significant reduction in TBARS and a tremendous increase in GSH-R in diabetic rats.[15] In addition, Hao et al.[18] reported that purslane administration resulted in the increased levels of superoxide dismutase and decreased levels of MDA in the brains of mice treated with D-galactosamine. Others have also shown that purslane leaves can reduce some cardiovascular risk factors through decreasing ox-LDL and increasing the activity of the paraoxanase-1 enzyme in hyperlipidemic subjects.[19] Previous studies have documented that intake of 5 g/day P. oleracea seeds in type 2 diabetic subjects could decrease serum levels of triglycerides, total cholesterol, LDL-cholesterol, liver alanine aminotransferase, aspartate aminotransferase and gamma-glutamyl transaminase, total and direct bilirubin, fasting and postprandial blood glucose, insulin, body weight, and body mass index while increased high density lipoprotein cholesterol and albumin.[17] The discrepancy between our findings and other studies might be explained by the discrepancies in study design, duration of intervention and participants of the study. Furthermore, it must be kept in mind that almost all previous studies have been done on animals. In addition, we used purslane seeds rather than purslane leaves or other preparations in this study.
Several mechanisms might explain the antioxidant effects of purslane. It has been shown that purslane intake results in better glycemic control in diabetic patients.[17] Purslane mechanism can be in connection with increasing insulin secretion by closing of the channel gate ATP-K+, membrane depolarization, and Ca2+ entry stimulation as the first key step in insulin secretion.[20] Improved homeostasis of glucose in diabetic patients might explain the beneficial effects of purslane on biomarkers of oxidative stress. However, as we published previously,[21] we did not find any significant effect of purslane seeds on plasma glucose levels in these patients. Purslane has been shown to elevate antioxidant enzymes through which it might lead to reduced oxidative stress.[22]
The strength of the present study was the use of a crossover design which decreased the effect of confounders. Also, the study was among the first investigations that examined the effects of purslane seeds on biomarkers of oxidative stress in patients with type 2 diabetic. Some limitations need to be taken into account in the interpretation of our findings. One of our study limitations is the method of assessment of compliance to purslane seeds intake. We assessed compliance through phone interviews as well as asking participants to deliver the empty sachets to study personnel. Future studies are better to assess the compliance through the use of an appropriate biomarker. Although finding a suitable biomarker for purslane intake is difficult, measurement of the alpha-linolenic acid content of red blood cells’ membranes might provide some information in this regard.[23,24] The short duration of intervention might prohibit us to reach significant effects of purslane on biomarkers of oxidative stress. In addition, a single measurement of the metabolic profile, which has day-to-day variations, in the current study, might lead to misleading findings.
CONCLUSIONS
This cross-over clinical trial revealed that consumption of purslane seeds for 5 weeks in type 2 diabetic patients did not result in improved oxidative stress. Additional studies with a much longer experimental period and a larger sample size are needed to further explore the effects of purslane on oxidative stress.
ACKNOWLEDGEMENTS
This study was extracted from an MSc thesis which was approved by Isfahan University of Medical Sciences (No. 190151).
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Wild S, Roglic G, Green A, Sicree R, King H. Global prevalence of diabetes: Estimates for the year 2000 and projections for 2030. Diabetes Care. 2004;27:1047–53. doi: 10.2337/diacare.27.5.1047. [DOI] [PubMed] [Google Scholar]
- 2.Esteghamati A, Gouya MM, Abbasi M, Delavari A, Alikhani S, Alaedini F, et al. Prevalence of diabetes and impaired fasting glucose in the adult population of Iran: National Survey of Risk Factors for Non-Communicable Diseases of Iran. Diabetes Care. 2008;31:96–8. doi: 10.2337/dc07-0959. [DOI] [PubMed] [Google Scholar]
- 3.Jarald E, Joshi SB, Jain DC. Diabetes vs. herbal medicines. Iran J Pharmacol Ther. 2008;7:97–106. [Google Scholar]
- 4.Hunt JV, Smith CC, Wolff SP. Autoxidative glycosylation and possible involvement of peroxides and free radicals in LDL modification by glucose. Diabetes. 1990;39:1420–4. doi: 10.2337/diab.39.11.1420. [DOI] [PubMed] [Google Scholar]
- 5.West IC. Radicals and oxidative stress in diabetes. Diabet Med. 2000;17:171–80. doi: 10.1046/j.1464-5491.2000.00259.x. [DOI] [PubMed] [Google Scholar]
- 6.Laudat A, Lecourbe K, Guéchot J, Palluel AM. Values of sperm thiobarbituric acid-reactive substance in fertile men. Clin Chim Acta. 2002;325:113–5. doi: 10.1016/s0009-8981(02)00289-9. [DOI] [PubMed] [Google Scholar]
- 7.Baynes JW. Role of oxidative stress in development of complications in diabetes. Diabetes. 1991;40:405–12. doi: 10.2337/diab.40.4.405. [DOI] [PubMed] [Google Scholar]
- 8.Sabu MC, Kuttan R. Anti-diabetic activity of medicinal plants and its relationship with their antioxidant property. J Ethnopharmacol. 2002;81:155–60. doi: 10.1016/s0378-8741(02)00034-x. [DOI] [PubMed] [Google Scholar]
- 9.Hosseini HF, Fakhrzadeh H, Mohammad BA, Samani AS. Review of anti-diabetic medicinal plant used in traditional medicine. J Med Plants. 2006;5:85–60. [Google Scholar]
- 10.Simopoulos AP, Norman HA, Gillaspy JE, Duke JA. Common purslane: A source of omega-3 fatty acids and antioxidants. J Am Coll Nutr. 1992;11:374–82. doi: 10.1080/07315724.1992.10718240. [DOI] [PubMed] [Google Scholar]
- 11.Liu L, Howe P, Zhou YF, Xu ZQ, Hocart C, Zhan R. Fatty acids and beta-carotene in australian purslane (Portulaca oleracea) varieties. J Chromatogr A. 2000;893:207–13. doi: 10.1016/s0021-9673(00)00747-0. [DOI] [PubMed] [Google Scholar]
- 12.Yang Z, Liu C, Xiang L, Zheng Y. Phenolic alkaloids as a new class of antioxidants in Portulaca oleracea. Phytother Res. 2009;23:1032–5. doi: 10.1002/ptr.2742. [DOI] [PubMed] [Google Scholar]
- 13.Dkhil MA, Abdel Moniem A, Al-Quraishy S, Saleh R. Antioxidant effect of purslane (Portulaca oleracea) and its mechanism of action. J Med Plants Res. 2011;5:1589–63. [Google Scholar]
- 14.Xiaojuan L, Yong H, Xueqi G, Fanhui Z, Zhihong J, Yong-Ping Y. Polysaccharides from Portulaca oleracea (purslane) supplementation lowers acute exercise induced oxidative stress in young rats. Afr J Pharm Pharmacol. 2011;5:381–5. [Google Scholar]
- 15.Sharma A, Vijayakumar M, Rao CV, Unnikrishnan M, Reddy G. Action of Portulaca oleracea against streptozotocin-induced oxidative stress in experimental diabetic rats. J Complement Integr Med. 2009;6:1. [Google Scholar]
- 16.Mellitus D. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2006;29:S43. [PubMed] [Google Scholar]
- 17.El-Sayed MI. Effects of Portulaca oleracea L. seeds in treatment of type-2 diabetes mellitus patients as adjunctive and alternative therapy. J Ethnopharmacol. 2011;137:643–51. doi: 10.1016/j.jep.2011.06.020. [DOI] [PubMed] [Google Scholar]
- 18.Hao H, Nancai Y, Lei F, Wen S, Guofu H, Yanxia W, et al. Retracted: Antiaging effect of purslane herb aqueous extracts and its mechanism of action. Phytother Res. 2009;23:i–vii. doi: 10.1002/ptr.2835. [DOI] [PubMed] [Google Scholar]
- 19.Gatreh-Samani K, Farrokhi E, Khalili B, Rafieian M, Moradi M. Purslane (Portulaca oleracea) effects on serum paraoxanase-1 activity. J Shahrekord Univ Med Sci. 2011;13:9–14. [Google Scholar]
- 20.Gong F, Li F, Zhang L, Li J, Zhang Z, Wang G. Hypoglycemic effects of crude polysaccharide from Purslane. Int J Mol Sci. 2009;10:880–8. doi: 10.3390/ijms10030880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Esmaillzadeh A, Zakizadeh E, Faghihimani E, Gohari M, Jazayeri S. The effect of purslane seeds on glycemic status and lipid profiles of persons with type 2 diabetes: A randomized controlled cross-over clinical trial. J Res Med Sci. 2015;20:47–53. [PMC free article] [PubMed] [Google Scholar]
- 22.El-Sherbiny GA, Morsy MA, Mohamed A, Fouad MM. Antidiabetic effect of Portulaca oleracea extract, alone and plus gliclazide, on streptozotocin-nicotinamide-induded type 2 diabetes in rats. El Minia Med Bull. 2005;16:77–82. [Google Scholar]
- 23.Uddin MK, Juraimi AS, Hossain MS, Nahar MA, Ali ME, Rahman MM. Purslane weed (Portulaca oleracea): A prospective plant source of nutrition, omega-3 fatty acid, and antioxidant attributes. ScientificWorldJournal 2014. 2014:951019. doi: 10.1155/2014/951019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Barceló-Coblijn G, Murphy EJ, Othman R, Moghadasian MH, Kashour T, Friel JK. Flaxseed oil and fish-oil capsule consumption alters human red blood cell n-3 fatty acid composition: A multiple-dosing trial comparing 2 sources of n-3 fatty acid. Am J Clin Nutr. 2008;88:801–9. doi: 10.1093/ajcn/88.3.801. [DOI] [PubMed] [Google Scholar]


