Abstract
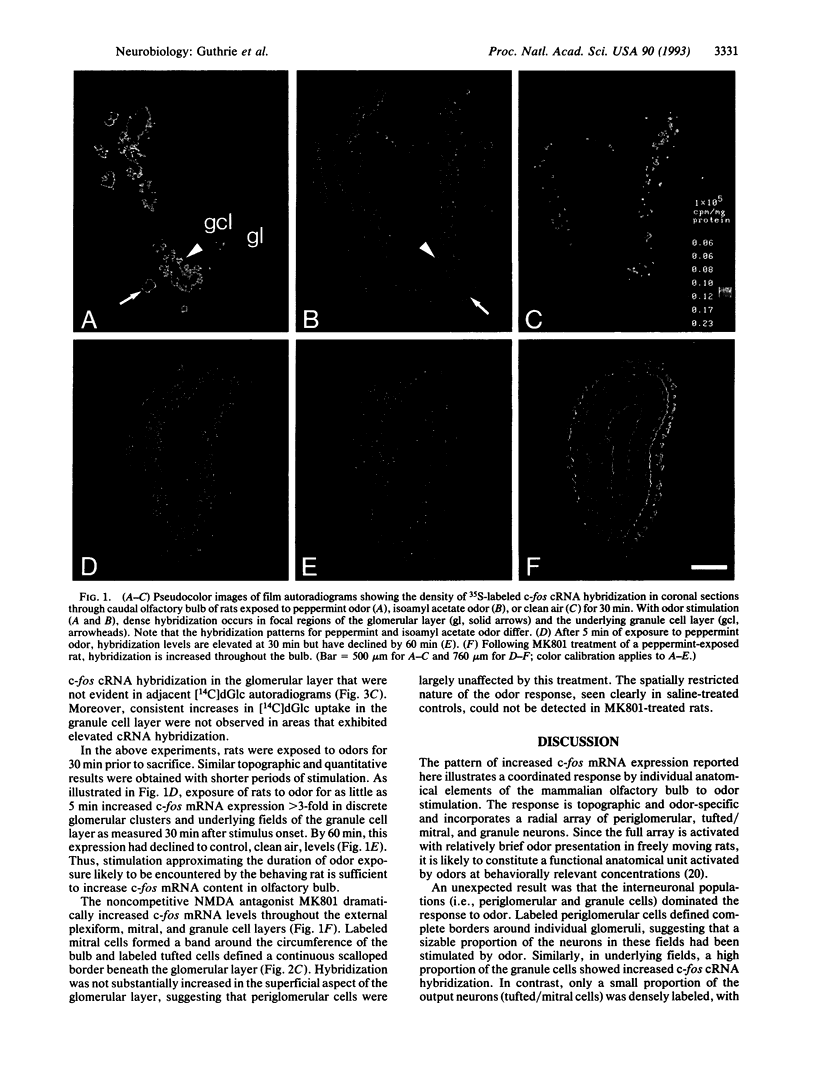
Expression of the immediate-early gene c-fos was used to evaluate the coordinate activation of olfactory bulb neurons by brief exposure to specific odors in the alert rat. In situ hybridization to c-fos mRNA was compared to regional increases in 2-deoxy-D-[14C]glucose incorporation in an adjacent section analysis. Levels of c-fos mRNA in olfactory bulb were high in rats recently removed from their home cage but were low in animals placed in a relatively odor-free chamber for 30 min. Presentation of specific odors to alert rats for as little as 5 min increased c-fos mRNA in radially distributed neuronal ensembles that spanned the lamina of the main olfactory bulb. The complementary RNA (cRNA)-labeled neuronal collectives consisted of cells in the glomerular layer that precisely defined the borders of individual glomeruli and underlying tufted, mitral, and granule cells. The activated fields were much broader in the granule cell layer than in the overlying glomerular layer and thus exhibited a flask-like, as opposed to a columnar, contour. The bulbar distribution of cRNA-labeled cell arrays differed with different odors and, in the glomerular layer, corresponded to focal regions of high 2-deoxy-D-[14C]glucose uptake. Administration of the noncompetitive N-methyl-D-aspartate receptor antagonist MK801 did not attenuate the odor induction of c-fos but, instead, increased c-fos mRNA levels throughout the bulb. We propose that the neuronal ensembles expressing increased c-fos mRNA with odor stimulation represent principal functional units of sensory processing in the main olfactory bulb of the behaving rat.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ambros-Ingerson J., Granger R., Lynch G. Simulation of paleocortex performs hierarchical clustering. Science. 1990 Mar 16;247(4948):1344–1348. doi: 10.1126/science.2315702. [DOI] [PubMed] [Google Scholar]
- Anderson K. J., Monaghan D. T., Cangro C. B., Namboodiri M. A., Neale J. H., Cotman C. W. Localization of N-acetylaspartylglutamate-like immunoreactivity in selected areas of the rat brain. Neurosci Lett. 1986 Dec 3;72(1):14–20. doi: 10.1016/0304-3940(86)90610-5. [DOI] [PubMed] [Google Scholar]
- Buonviso N., Chaput M. A. Response similarity to odors in olfactory bulb output cells presumed to be connected to the same glomerulus: electrophysiological study using simultaneous single-unit recordings. J Neurophysiol. 1990 Mar;63(3):447–454. doi: 10.1152/jn.1990.63.3.447. [DOI] [PubMed] [Google Scholar]
- Coopersmith R., Leon M. Enhanced neural response to familiar olfactory cues. Science. 1984 Aug 24;225(4664):849–851. doi: 10.1126/science.6474157. [DOI] [PubMed] [Google Scholar]
- Curran T., Gordon M. B., Rubino K. L., Sambucetti L. C. Isolation and characterization of the c-fos(rat) cDNA and analysis of post-translational modification in vitro. Oncogene. 1987;2(1):79–84. [PubMed] [Google Scholar]
- Di Prisco G. V., Freeman W. J. Odor-related bulbar EEG spatial pattern analysis during appetitive conditioning in rabbits. Behav Neurosci. 1985 Oct;99(5):964–978. doi: 10.1037//0735-7044.99.5.964. [DOI] [PubMed] [Google Scholar]
- Dragunow M., Faull R. L. MK801 induces c-fos protein in thalamic and neocortical neurons of rat brain. Neurosci Lett. 1990 May 31;113(2):144–150. doi: 10.1016/0304-3940(90)90294-j. [DOI] [PubMed] [Google Scholar]
- Ehrlich M. E., Grillo M., Joh T. H., Margolis F. L., Baker H. Transneuronal regulation of neuronal specific gene expression in the mouse olfactory bulb. Brain Res Mol Brain Res. 1990 Feb;7(2):115–122. doi: 10.1016/0169-328x(90)90088-u. [DOI] [PubMed] [Google Scholar]
- Gall C., Murray K., Isackson P. J. Kainic acid-induced seizures stimulate increased expression of nerve growth factor mRNA in rat hippocampus. Brain Res Mol Brain Res. 1991 Jan;9(1-2):113–123. doi: 10.1016/0169-328x(91)90136-l. [DOI] [PubMed] [Google Scholar]
- Guthrie K. M., Gall C. M. Differential expression of mRNAs for the NGF family of neurotrophic factors in the adult rat central olfactory system. J Comp Neurol. 1991 Nov 1;313(1):95–102. doi: 10.1002/cne.903130107. [DOI] [PubMed] [Google Scholar]
- Hengerer B., Lindholm D., Heumann R., Rüther U., Wagner E. F., Thoenen H. Lesion-induced increase in nerve growth factor mRNA is mediated by c-fos. Proc Natl Acad Sci U S A. 1990 May;87(10):3899–3903. doi: 10.1073/pnas.87.10.3899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jourdan F., Duveau A., Astic L., Holley A. Spatial distribution of [14C]2-deoxyglucose uptake in the olfactory bulbs of rats stimulated with two different odours. Brain Res. 1980 Apr 21;188(1):139–154. doi: 10.1016/0006-8993(80)90563-6. [DOI] [PubMed] [Google Scholar]
- Kauer J. S. Contributions of topography and parallel processing to odor coding in the vertebrate olfactory pathway. Trends Neurosci. 1991 Feb;14(2):79–85. doi: 10.1016/0166-2236(91)90025-p. [DOI] [PubMed] [Google Scholar]
- Lancet D., Greer C. A., Kauer J. S., Shepherd G. M. Mapping of odor-related neuronal activity in the olfactory bulb by high-resolution 2-deoxyglucose autoradiography. Proc Natl Acad Sci U S A. 1982 Jan;79(2):670–674. doi: 10.1073/pnas.79.2.670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macrides F., Schneider S. P. Laminar organization of mitral and tufted cells in the main olfactory bulb of the adult hamster. J Comp Neurol. 1982 Jul 10;208(4):419–430. doi: 10.1002/cne.902080410. [DOI] [PubMed] [Google Scholar]
- Meisami E. A proposed relationship between increases in the number of olfactory receptor neurons, convergence ratio and sensitivity in the developing rat. Brain Res Dev Brain Res. 1989 Mar 1;46(1):9–19. doi: 10.1016/0165-3806(89)90139-9. [DOI] [PubMed] [Google Scholar]
- Monaghan D. T., Cotman C. W. Distribution of N-methyl-D-aspartate-sensitive L-[3H]glutamate-binding sites in rat brain. J Neurosci. 1985 Nov;5(11):2909–2919. doi: 10.1523/JNEUROSCI.05-11-02909.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan J. I., Curran T. Calcium as a modulator of the immediate-early gene cascade in neurons. Cell Calcium. 1988 Dec;9(5-6):303–311. doi: 10.1016/0143-4160(88)90011-5. [DOI] [PubMed] [Google Scholar]
- Morgan J. I., Curran T. Stimulus-transcription coupling in neurons: role of cellular immediate-early genes. Trends Neurosci. 1989 Nov;12(11):459–462. doi: 10.1016/0166-2236(89)90096-9. [DOI] [PubMed] [Google Scholar]
- Moriyoshi K., Masu M., Ishii T., Shigemoto R., Mizuno N., Nakanishi S. Molecular cloning and characterization of the rat NMDA receptor. Nature. 1991 Nov 7;354(6348):31–37. doi: 10.1038/354031a0. [DOI] [PubMed] [Google Scholar]
- Murphy T. H., Worley P. F., Baraban J. M. L-type voltage-sensitive calcium channels mediate synaptic activation of immediate early genes. Neuron. 1991 Oct;7(4):625–635. doi: 10.1016/0896-6273(91)90375-a. [DOI] [PubMed] [Google Scholar]
- Onoda N., Mori K. Depth distribution of temporal firing patterns in olfactory bulb related to air-intake cycles. J Neurophysiol. 1980 Jul;44(1):29–39. doi: 10.1152/jn.1980.44.1.29. [DOI] [PubMed] [Google Scholar]
- Onoda N. Odor-induced fos-like immunoreactivity in the rat olfactory bulb. Neurosci Lett. 1992 Mar 30;137(2):157–160. doi: 10.1016/0304-3940(92)90393-l. [DOI] [PubMed] [Google Scholar]
- Orona E., Scott J. W., Rainer E. C. Different granule cell populations innervate superficial and deep regions of the external plexiform layer in rat olfactory bulb. J Comp Neurol. 1983 Jun 20;217(2):227–237. doi: 10.1002/cne.902170209. [DOI] [PubMed] [Google Scholar]
- Pilgrim C., Stumpf W. E. Applications of autoradiography in neurobiological research. J Histochem Cytochem. 1987 Aug;35(8):917–928. doi: 10.1177/35.8.2439585. [DOI] [PubMed] [Google Scholar]
- Rusak B., Robertson H. A., Wisden W., Hunt S. P. Light pulses that shift rhythms induce gene expression in the suprachiasmatic nucleus. Science. 1990 Jun 8;248(4960):1237–1240. doi: 10.1126/science.2112267. [DOI] [PubMed] [Google Scholar]
- Sagar S. M., Sharp F. R., Curran T. Expression of c-fos protein in brain: metabolic mapping at the cellular level. Science. 1988 Jun 3;240(4857):1328–1331. doi: 10.1126/science.3131879. [DOI] [PubMed] [Google Scholar]
- Sariban E., Luebbers R., Kufe D. Transcriptional and posttranscriptional control of c-fos gene expression in human monocytes. Mol Cell Biol. 1988 Jan;8(1):340–346. doi: 10.1128/mcb.8.1.340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider S. P., Scott J. W. Orthodromic response properties of rat olfactory bulb mitral and tufted cells correlate with their projection patterns. J Neurophysiol. 1983 Aug;50(2):358–378. doi: 10.1152/jn.1983.50.2.358. [DOI] [PubMed] [Google Scholar]
- Senba E., Simmons D. M., Swanson L. W. Localization of neuropeptide precursor-synthesizing neurons in the rat olfactory bulb: a hybridization histochemical study. Neuroscience. 1990;38(3):629–641. doi: 10.1016/0306-4522(90)90057-b. [DOI] [PubMed] [Google Scholar]
- Sheng M., McFadden G., Greenberg M. E. Membrane depolarization and calcium induce c-fos transcription via phosphorylation of transcription factor CREB. Neuron. 1990 Apr;4(4):571–582. doi: 10.1016/0896-6273(90)90115-v. [DOI] [PubMed] [Google Scholar]
- Shepherd G. M. Synaptic organization of the mammalian olfactory bulb. Physiol Rev. 1972 Oct;52(4):864–917. doi: 10.1152/physrev.1972.52.4.864. [DOI] [PubMed] [Google Scholar]
- Shin C., McNamara J. O., Morgan J. I., Curran T., Cohen D. R. Induction of c-fos mRNA expression by afterdischarge in the hippocampus of naive and kindled rats. J Neurochem. 1990 Sep;55(3):1050–1055. doi: 10.1111/j.1471-4159.1990.tb04595.x. [DOI] [PubMed] [Google Scholar]
- Sonnenberg J. L., Macgregor-Leon P. F., Curran T., Morgan J. I. Dynamic alterations occur in the levels and composition of transcription factor AP-1 complexes after seizure. Neuron. 1989 Sep;3(3):359–365. doi: 10.1016/0896-6273(89)90260-2. [DOI] [PubMed] [Google Scholar]
- Sonnenberg J. L., Mitchelmore C., Macgregor-Leon P. F., Hempstead J., Morgan J. I., Curran T. Glutamate receptor agonists increase the expression of Fos, Fra, and AP-1 DNA binding activity in the mammalian brain. J Neurosci Res. 1989 Sep;24(1):72–80. doi: 10.1002/jnr.490240111. [DOI] [PubMed] [Google Scholar]
- Sonnenberg J. L., Rauscher F. J., 3rd, Morgan J. I., Curran T. Regulation of proenkephalin by Fos and Jun. Science. 1989 Dec 22;246(4937):1622–1625. doi: 10.1126/science.2512642. [DOI] [PubMed] [Google Scholar]
- Stewart W. B., Kauer J. S., Shepherd G. M. Functional organization of rat olfactory bulb analysed by the 2-deoxyglucose method. J Comp Neurol. 1979 Jun 15;185(4):715–734. doi: 10.1002/cne.901850407. [DOI] [PubMed] [Google Scholar]
- Wellis D. P., Scott J. W. Intracellular responses of identified rat olfactory bulb interneurons to electrical and odor stimulation. J Neurophysiol. 1990 Sep;64(3):932–947. doi: 10.1152/jn.1990.64.3.932. [DOI] [PubMed] [Google Scholar]
- Wilson D. A., Leon M. Evidence of lateral synaptic interactions in olfactory bulb output cell responses to odors. Brain Res. 1987 Aug 4;417(1):175–180. doi: 10.1016/0006-8993(87)90196-x. [DOI] [PubMed] [Google Scholar]
- Worley P. F., Cole A. J., Saffen D. W., Baraban J. M. Regulation of immediate early genes in brain: role of NMDA receptor activation. Prog Brain Res. 1990;86:277–285. doi: 10.1016/s0079-6123(08)63184-2. [DOI] [PubMed] [Google Scholar]