Significance
Axillary meristems are groups of plant pluripotent stem cells responsible for the formation of secondary axes of growth, such as branches and flowers. A crucial step in the initiation of new axillary meristems is the establishment of boundary domains that allow organ separation and prevent fusion defects during development. This work provides clues on the molecular mechanism by which the plant hormone auxin is involved in the formation of axillary meristems in maize inflorescences. Auxin signaling modules containing the AUXIN/INDOLE-3-ACETIC ACID proteins BARREN INFLORESCENCE1 and BARREN INFLORESCENCE4 and AUXIN RESPONSE FACTOR (ARF) transcriptional regulators are involved in the regulation of the boundary basic helix-loop-helix transcription factor BARREN STALK1, suggesting auxin is directly responsible for establishing boundary regions.
Keywords: auxin signaling, inflorescence development, axillary meristems, maize, boundary domains
Abstract
In plants, small groups of pluripotent stem cells called axillary meristems are required for the formation of the branches and flowers that eventually establish shoot architecture and drive reproductive success. To ensure the proper formation of new axillary meristems, the specification of boundary regions is required for coordinating their development. We have identified two maize genes, BARREN INFLORESCENCE1 and BARREN INFLORESCENCE4 (BIF1 and BIF4), that regulate the early steps required for inflorescence formation. BIF1 and BIF4 encode AUXIN/INDOLE-3-ACETIC ACID (Aux/IAA) proteins, which are key components of the auxin hormone signaling pathway that is essential for organogenesis. Here we show that BIF1 and BIF4 are integral to auxin signaling modules that dynamically regulate the expression of BARREN STALK1 (BA1), a basic helix-loop-helix (bHLH) transcriptional regulator necessary for axillary meristem formation that shows a striking boundary expression pattern. These findings suggest that auxin signaling directly controls boundary domains during axillary meristem formation and define a fundamental mechanism that regulates inflorescence architecture in one of the most widely grown crop species.
Plant shoot architecture is primarily determined by small groups of pluripotent stem cells called meristems. Throughout their life cycle, plants generate different types of meristems whose main function is to drive postembryonic organ initiation. In particular, reproductive axillary meristems (AMs) form branches and flowers that contribute to naturally occurring variations in inflorescence architecture. Genes regulating AM function have been frequent targets during crop domestication (1), and several recent examples have demonstrated how modulation of meristem activity can directly affect yields (2, 3).
Mutations that affect the initial steps in reproductive AM formation often result in the formation of characteristic pin-like inflorescences. Several such mutants, first described in Arabidopsis, are predominantly affected in genes related to the hormone auxin, including PIN-FORMED1 (PIN1) and MONOPTEROS (MP) (4–6). Analysis of these and other mutants has established that auxin is central to the generation of all primordia. Auxin is polarly transported to the site of primordia initiation, where it is perceived by the nuclear auxin receptor TRANSPORT INHIBITOR RESPONSE1/AUXIN SIGNALING F-BOX PROTEIN (TIR1/AFB), part of an E3 ligase that rapidly degrades AUXIN/INDOLE-3-ACETIC ACID (Aux/IAA) coreceptor proteins and disrupts their recruitment of TOPLESS (TPL) corepressors. The auxin-dependent degradation of Aux/IAAs frees interacting activating AUXIN RESPONSE FACTOR (ARF) transcription factors from TPL repression, allowing them to activate downstream genes (7). Although it is known that ARFs bind to auxin-responsive cis-regulatory elements (AuxREs) composed of the core TGTC sequence, few downstream developmental pathways have been characterized (8–13). All components of the auxin signaling machinery are encoded by multimember gene families, and the combinatorial complexity afforded by the various members may contribute to auxin’s capacity to regulate multiple aspects of plant development (14–19). How auxin regulatory components work together to trigger specific developmental responses in reproductive tissues, including grain-bearing inflorescences, remains an unaddressed aspect with important implications for crop productivity and improvement.
Grasses such as maize and rice contain inflorescences with multiple types of specialized reproductive AMs. In maize, these AMs give rise to two types of inflorescences: kernel-laden ears and tassels optimized for pollen dispersal. Maize inflorescence mutants with a pin-like phenotype are classically called barren mutants, with the founding member, barren stalk1 (ba1), originally described more than 85 years ago. BA1 encodes a basic helix-loop-helix (bHLH) transcription factor (20), and loss-of-function ba1 mutants produce earless plants with tassels devoid of reproductive AMs. Additional barren mutants led to the discovery of proteins involved in auxin transport and biosynthesis (21–23), indicating that auxin-related defects often underlie this family of mutants.
Here we provide insight into the molecular mechanisms of auxin signaling during reproductive AM initiation by characterizing two barren mutants of maize. We identify the Aux/IAA proteins BARREN INFLORESCENCE1 and BARREN INFLORESCENCE4 (BIF1 and BIF4) and show that they are essential for organogenesis in maize inflorescences. We demonstrate that BIF1 and BIF4 are integral parts of functionally redundant signaling modules that directly control the transcription of BA1, thereby establishing critical boundary domains that ensure the formation of new AMs.
Results
Bif1 and Bif4 Are Semidominant Mutants Affected in Reproductive Organogenesis.
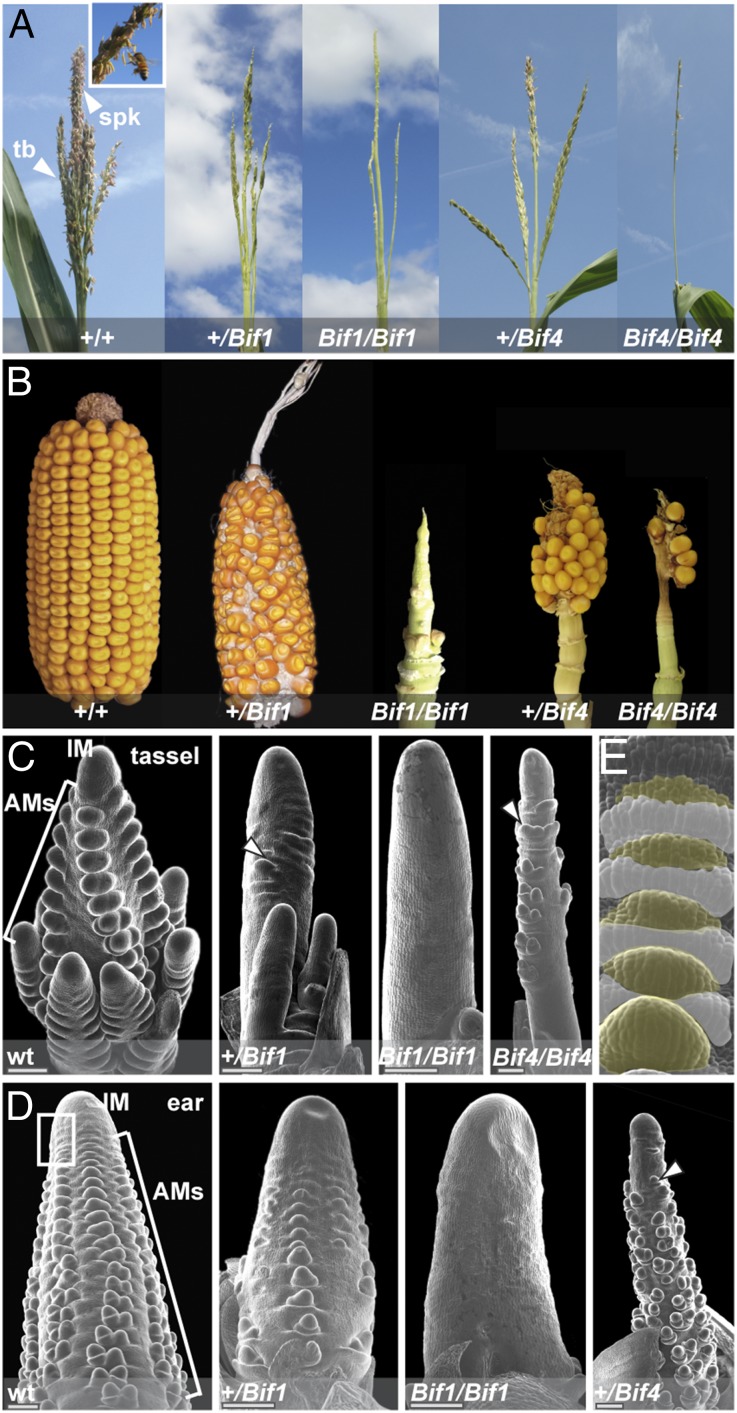
The semidominant barren mutants Bif1 (24, 25) and Bif4 were originally isolated from ethyl methanesulfonate (EMS) mutagenesis screens and displayed similar inflorescence defects. After undergoing a normal vegetative-to-reproductive transition, Bif1 and Bif4 plants developed tassels with reduced numbers of branches and spikelets, the floral unit of grass inflorescences (Fig. 1A and Fig. S1). Ears appeared shortened and displayed disorganized rows of kernels, as well as areas completely devoid of kernels (Fig. 1B). These defects were more pronounced in homozygous Bif1 and Bif4 plants (Fig. 1 A and B and Fig. S1).
Fig. 1.
Bif1 and Bif4 mutant phenotype. (A) Mature tassel phenotype. Normal tassels produce spikelets and flowers that are reduced in both mutants. (Inset) Spikelets with protruding anthers. tb, tassel branch. spk, spikelet. (B) Mature ear phenotype. (C–E) Scanning electron microscope image of early tassel and ear development in normal and mutant plants. Arrowheads point to a few axillary meristems forming in mutant plants. Primordia are absent in homozygous Bif1 mutants. Boxed region in D marks the peripheral zone of the IM. (E) Close-up of the peripheral zone of the IM. White and yellow colors mark suppressed bract primordia and AMs, respectively. Note the acropetal development of primordia (from top, younger, to bottom, older).
Fig. S1.
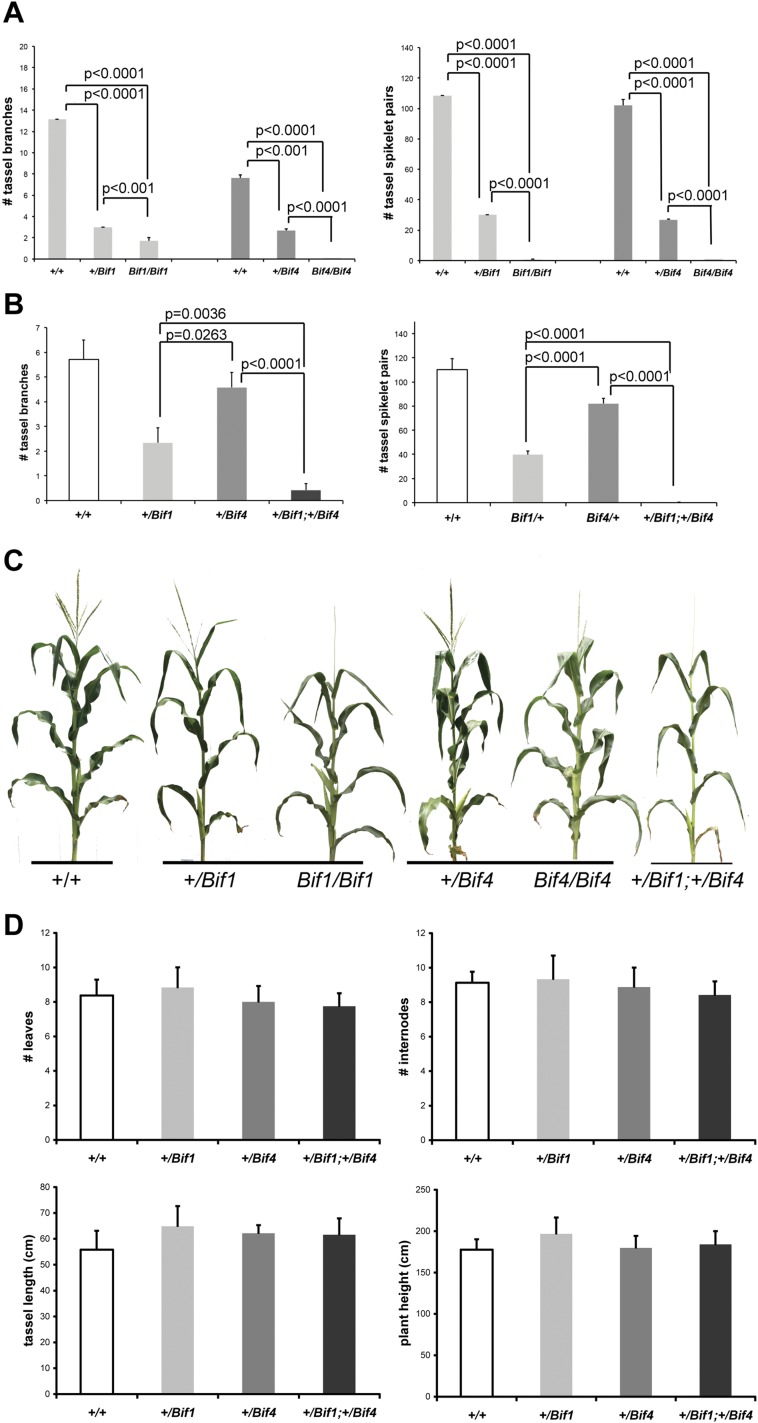
Quantification of the phenotypic defects of Bif1 and Bif4 mutants. (A) Reproductive defects in tassels of single mutants in B73 background (n ≥ 7). (B) Analysis of double mutants in A619 background (n ≥ 7). (C) Whole-plant images. (D) Analysis of vegetative and reproductive phenotypes with no detectable difference (n ≥ 6). Error bars show SD.
SEM analysis of young inflorescences revealed that the Bif1 and Bif4 phenotypes resulted from defects in primordium initiation at the peripheral zone of the apical inflorescence meristem (IM), where organogenesis occurs (Fig. 1 C and D). Normally in maize inflorescences, the first primordia to appear are suppressed bracts (SBs; Fig. 1E). These structures are followed shortly after by the formation of a series of reproductive AMs (branch, spikelet-pair, spikelet and floral meristems) that initiate at the bract axils and eventually give rise to spikelets and flowers (Fig. 1 C and D). In Bif1 tassels and ears, a severe reduction in the initiation of AMs was observed (Fig. 1 C and D). Homozygous mutants produced smooth structures (albeit with a normal IM), indicating that both bract primordia and AM initiation were defective. Similar defects were also observed in Bif4 mutant tassels and ears (Fig. 1 C and D). Double-mutant analysis of Bif1 and Bif4 showed a strong synergistic effect (Fig. 2A). In +/Bif1;+/Bif4 tassels and ears, all primordia were missing, resulting in pin-like inflorescences in which organogenesis was often completely impaired (Fig. 2 A and B). Because of the missing floral organs, we were only able to generate +/Bif1;Bif4/Bif4 plants that resembled +/Bif1;+/Bif4 double heterozygotes. No significant vegetative defects were observed in either single or double mutants (Fig. S1). On the basis of this analysis, we conclude that BIF1 and BIF4 are essential for organogenesis during inflorescence development and function together in the initiation of lateral primordia (SBs and AMs).
Fig. 2.
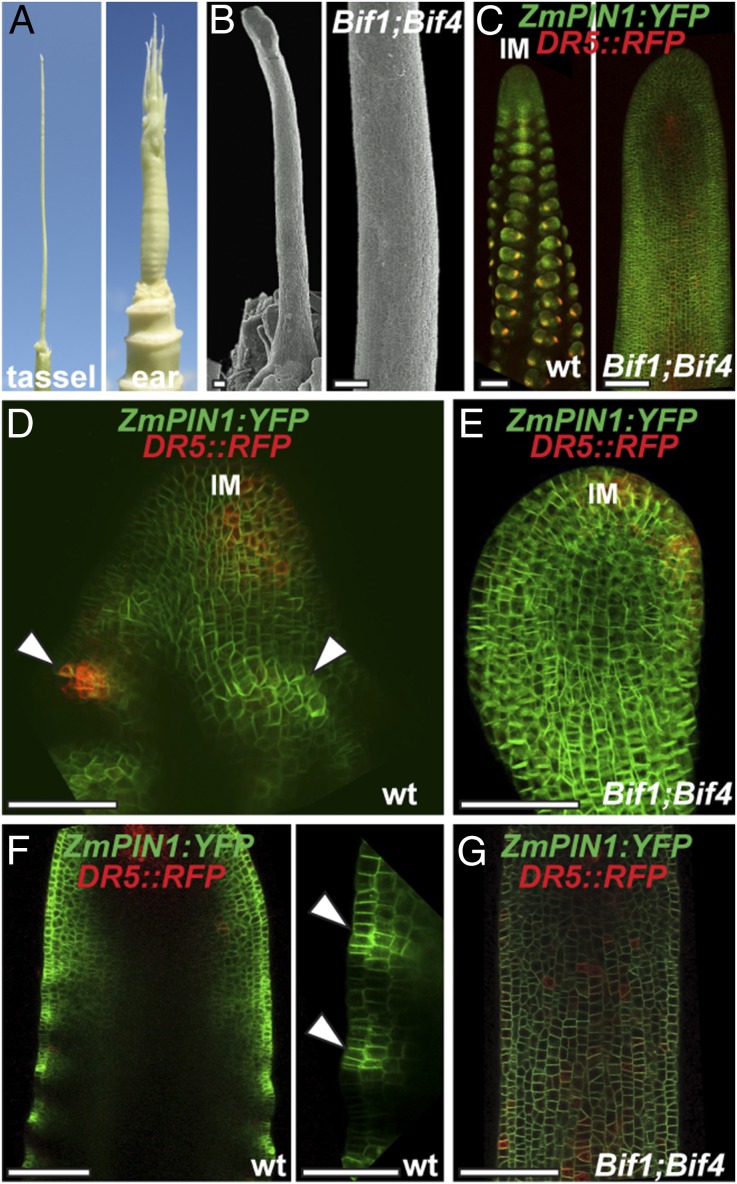
BIF1 and BIF4 are required for patterning of primordium initiation. (A) Mature inflorescence phenotype of the double heterozygous Bif1 and Bif4 mutant. (B) Scanning electron microscope image of a young Bif1;Bif4 tassel showing lack of primordium initiation. (Scale bars, 100 μm.) (C) Confocal images of normal and Bif1;Bif4 tassels expressing ZmPIN1a-YFP fusion proteins and DR5::RFP. (D and E) Maximum projections of confocal images of wild-type and Bif1;Bif4 mutant IMs. (F and G) Confocal images of the peripheral zone of immature tassels showing up-regulation of ZmPIN1a-YFP signals in normal tassels (F, arrowheads in close-up right panel) that is missing in double Bif1;Bif4 mutants (G).
Because of the striking similarity between the inflorescences of the double +/Bif1;+/Bif4 mutant and Arabidopsis auxin transport mutants, we used confocal microscopy to investigate how the expression of the membrane-localized auxin efflux transporter ZmPIN1a:YFP was affected in +/Bif1;+/Bif4 inflorescences. We simultaneously monitored auxin signaling using the DR5rev::RFP reporter (26) (Fig. 2 and Fig. S2). Whereas in wild-type immature tassels the patterning of primordia at the periphery of the IM was marked by increased ZmPIN1a-YFP and RFP signals, in +/Bif1;+/Bif4 plants, this patterning was completely absent (n = 5; Fig. 2 C–G and Fig. S2). This indicates that although ZmPIN1a is still expressed in +/Bif1;+/Bif4 IMs, the normal auxin-driven patterning of primordia is completely disrupted and suggests that BIF1 and BIF4 are required for the up-regulation of PIN-mediated auxin transport. In double-mutant tassels, RFP signal was observed in the IM, confirming that the IM is unaffected (Fig. 2E), and in occasional cells and inner tissue along the main axis (Fig. 2G and Fig. S2), suggesting that auxin signaling is not completely disrupted in these plants.
Fig. S2.
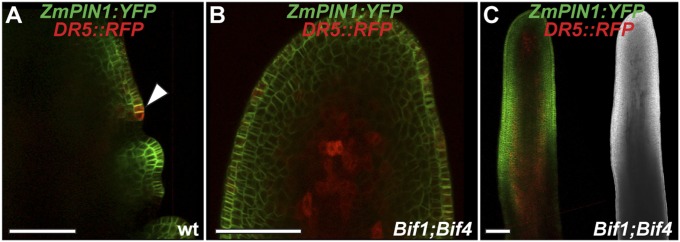
Confocal analysis of ZmPIN1a:YFP and DR5rev::RFP transgenes. (A) Wild-type tassel, showing the peripheral zone of the IM with an emerging primordium (arrowhead). (B and C) +/Bif1;+/Bif4 tassels. In B is a close-up view of the IM. (C, Right) Brightfield image of the confocal sample. (Scale bars, 100 μm.)
Bif1 and Bif4 Harbor Mutations in Aux/IAAs Expressed in the Early Stages of Inflorescence Development.
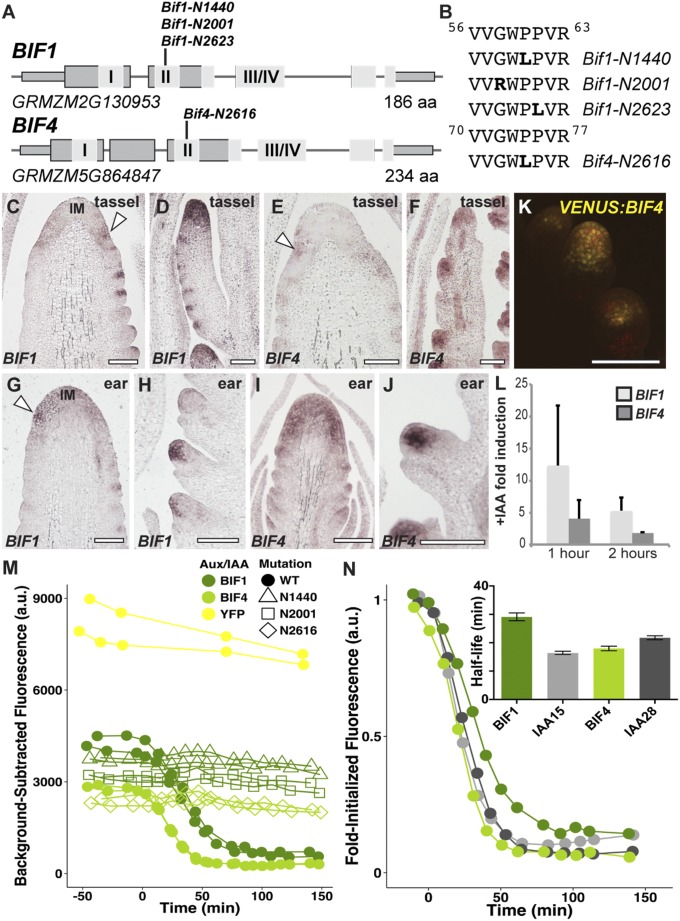
The underlying molecular cause of the Bif1 mutant has remained unknown since its discovery in 1977 (24). On the basis of its auxin-related phenotype and semidominance, we reasoned that BIF1 might encode one of the 38 maize Aux/IAA genes (27, 28), known negative regulators of auxin signaling that give rise to dominant mutants (7). We searched the maize genome for Aux/IAAs that were located in the region of chromosome 8, where the BIF1 locus was previously mapped (25). Single-amino acid substitutions in the degron domain of GRMZM2G130953/IAA27 were observed in all Bif1 alleles (Fig. 3 C and D). The degron domain is a highly conserved amino acid sequence found in Aux/IAA proteins that confers auxin-induced degradation and is consistently mutated in all known dominant aux/iaa mutants. Because Bif4 showed an identical phenotype to Bif1, we used a similar approach, which revealed an amino acid substitution in the degron domain of GRMZM5G864847/IAA20 (Fig. 3B). These results show that BIF1 encodes an Aux/IAA protein, and equally suggest it for BIF4.
Fig. 3.
BIF1 and BIF4 encode Aux/IAA proteins. (A) Schematic representation of BIF1 and BIF4 genes. Exons are depicted as gray rectangles. I and II represent the EAR repressor motif and the degron domain; III/IV corresponds to the dimerization domain. (B) The amino acid sequence of the degron domains of BIF1 and BIF4 and the mutations identified. (C–J) mRNA in situ hybridizations of immature inflorescences with BIF1 and BIF4 antisense probes. Arrowheads, localized signals at the peripheral zone of the IM. (D and F) Branch meristems are shown; (H and J) spikelet meristems. (Scale bars, 100 μm.) (K) Confocal image of VENUS-BIF4 in spikelet meristems. (L) Auxin inducibility of BIF1 and BIF4. Error bars show SD. (M and N) Auxin-induced degradation profiles of normal and mutant BIF1 and BIF4 proteins.
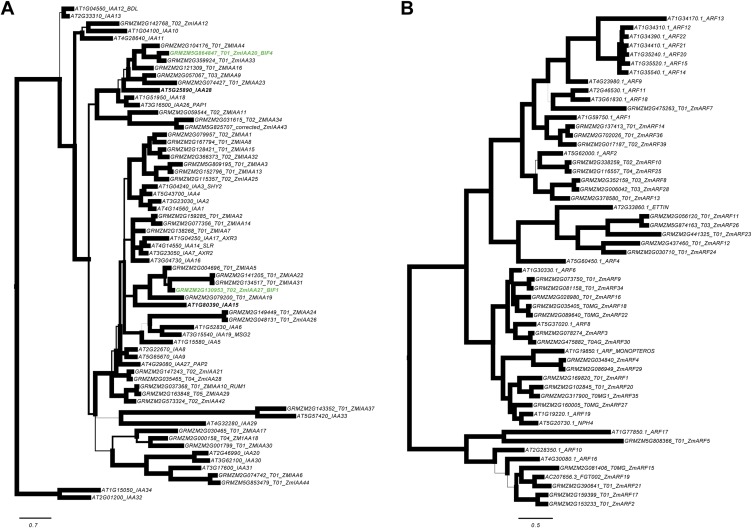
Phylogenetic analysis of BIF1/IAA27 and BIF4/IAA20 revealed that the two genes belong to separate clades and share only 39% amino acid identity (Fig. S3A). To examine their expression pattern, we carried out RNA in situ hybridizations in developing inflorescences (Fig. 3 C–J). Both genes were broadly expressed in the IM, and in its peripheral zone in both tassels and ears (Fig. 3 C, E, G, and I). As the newly formed AMs developed, BIF1 and BIF4 showed strong expression in the central zone of all AMs (Fig. 3 D, F, H, and J). Similarly, maize transgenic lines expressing a VENUS-BIF4 fusion protein driven by the endogenous BIF4 promoter showed VENUS-BIF4 protein in AMs (Fig. 3K). These expression patterns are consistent with the mutant phenotypes and support a role for BIF1 and BIF4 in initiating reproductive primordia.
Fig. S3.
Phylogenetic analysis. (A) Bayesian consensus phylogram of 67 Arabidopsis (At) and maize (GRMZM) Aux/IAA protein sequences. The maize BIF1 and BIF4 sequences and their closest Arabidopsis relatives, ATIAA15, and ATIAA28, respectively, are bolded. Phylogram is rooted using Arabidopsis IAA32 and IAA34 based on Remington (61). Branch widths are proportional to support with bold branches equivalent to greater than or equal to 0.95 clade credibility. (B) Bayesian consensus phylogram of 55 Arabidopsis (At) and maize (GRMZM) ARF protein sequences. Phylogram is rooted using the Arabidopsis ARF10, ARF16, and ARF17 clade, based on Remington (61). Branch widths are proportional to support with bold branches equivalent to greater than or equal to 0.95 clade credibility. (Scale bars, substitution per site.)
BIF1 and BIF4 Show Distinct Auxin-Response Dynamics.
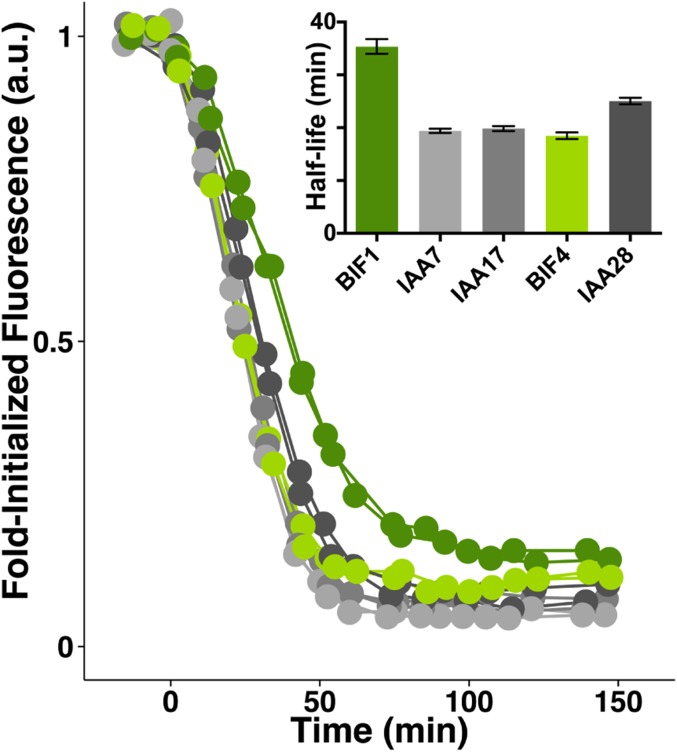
Despite having similar phenotypes and localization patterns, BIF1 and BIF4 displayed different degrees of auxin inducibility when subjected to exogenous auxin treatments (Fig. 3L), as previously reported for other Aux/IAA genes. Because auxin signaling relies on the rapid degradation of Aux/IAA proteins, we monitored the stability of the two proteins in the presence of auxin, using a yeast synthetic assay (29, 30). We engineered yeast expressing BIF1 or BIF4 and monitored their degradation dynamics in combination with the Arabidopsis auxin receptor TIR1 (Fig. 3M). This analysis revealed that BIF1 displayed a slower rate of degradation than BIF4. We also tested the degradation rates of mutant alleles of BIF1 and BIF4, and all showed strong auxin insensitivity (Fig. 3M). These data, together with the observation that the same mutation in IAA20 and BIF1-N1440 stabilizes both proteins, provided additional confirmation that the Bif4 phenotype is caused by a mutation in IAA20. The degradation rate of BIF1 was slower compared with the closest putative co-ortholog AtIAA15 and with other closely related Aux/IAAs (Fig. 3N and Fig. S4). In contrast, we observed similar degradation rates of BIF4 compared with its respective co-orthologs in Arabidopsis, suggesting there may be an evolutionarily conserved sequence-based bias for the stability of certain Aux/IAAs. Overall, this analysis indicates that BIF1 and BIF4 have unique auxin-response dynamics, suggesting the two genes may have subtle functional differences.
Fig. S4.
Auxin-induced degradation of wild-type BIF1 and BIF4 proteins in a yeast synthetic system compared with similar Arabidopsis proteins.
Maize-Activating ARFs Are Expressed in Defined Domains of the Inflorescence Meristem.
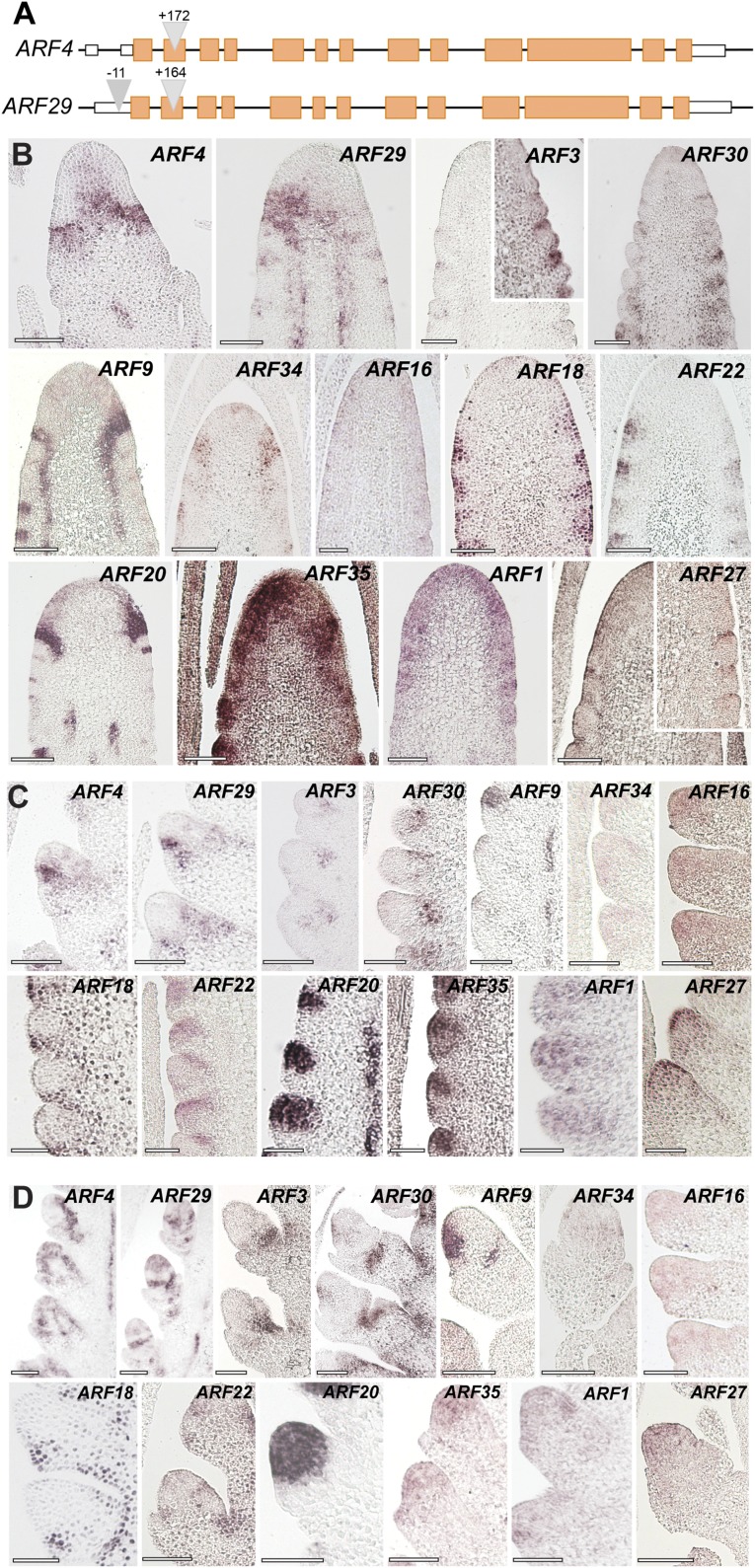
Aux/IAA proteins interact with and regulate the activity of ARF transcription factors. To determine which ARFs function with BIF1 and BIF4, we first took a reverse genetic approach. When grown to the adult stage, the Arabidopsis mp mutant shows pin-like inflorescences (5). Therefore, we hypothesized that the closest maize homologs of MP, ZmARF4, and ZmARF29 (duplicated genes with 96% aa similarity; Fig. S3B) would be likely candidates to work with BIF1 and BIF4 in reproductive organogenesis. We identified exonic transposon insertions in both genes (Fig. S5A); however, double arf4;arf29 mutants showed no phenotype in either shoot or reproductive development.
Fig. S5.
The maize ARF gene family. (A) Gene structure of ZmARF4 and ZmARF29, the maize co-orthologs of Arabidopsis MONOPTEROS. Triangles represent transposon insertions. Orange boxes, exons; white boxes, UTR regions. (B–D) Inflorescence-specific expression of maize-activating ARF genes by mRNA in situ hybridizations in immature tassels. Expression pattern in inflorescence meristems (B), spikelet-pair (C), and spikelet meristems (D). (Scale bars, 100 μm.)
Suspecting ARFs may work redundantly, we mined public transcriptome databases and found 13 maize-activating ARFs expressed in inflorescences. To obtain an expression map of these ARFs and assess whether they were coexpressed with BIF1 and BIF4, we performed in situ hybridizations on immature inflorescences (Fig. S5 B–D). All ZmARFs except ARF16 were expressed in specific domains of the IM: ARF1 and 35 showed broad expression; ARF4, ARF18, ARF20, ARF22, ARF29, and ARF34 showed strong expression at the peripheral zone of the IM; and ARF3, ARF27, and ARF30 showed narrow expression in developing primordia. Expression patterns of the different ARFs also varied in developing AMs; the majority were predominantly restricted to the meristematic core of the different types of AMs (ARF1, ARF4, ARF9, ARF16, ARF20, ARF22, ARF29, ARF34, ARF35), and others such as ARF3 and ARF30 appeared localized in more restricted domains at the base of AMs and at their boundary, whereas ARF18 and ARF22 localized to the suppressed bracts and glume primordia (Fig. S5). Strong vasculature expression was also observed for ARF4, ARF9, ARF20, and ARF29. Overall, these domains largely overlapped with those of BIF1 and BIF4.
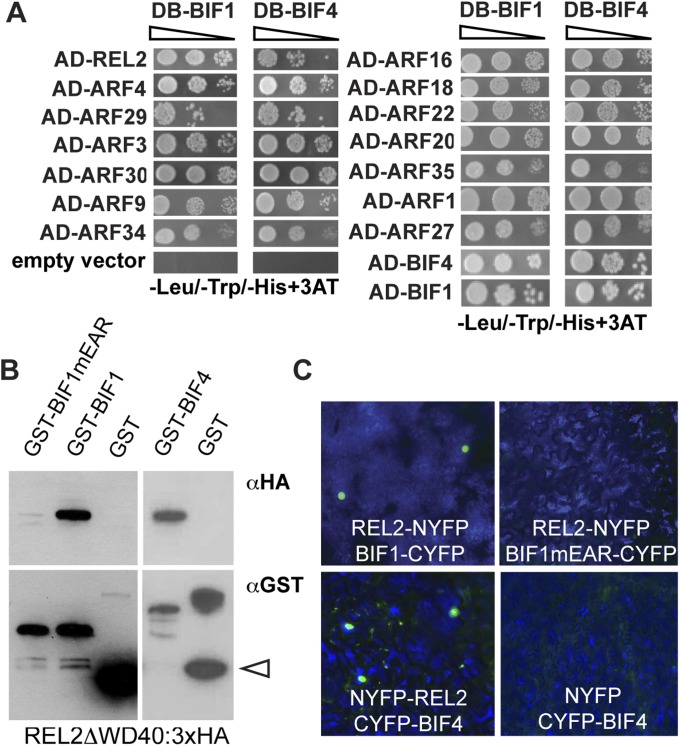
To determine whether all activating ARFs were capable of physically interacting with BIF1 and BIF4 proteins, we performed yeast 2-hybrid (Y2H) assays and detected interaction of BIF1 and BIF4 with all activating ARFs (Fig. S6). We verified by Y2H, BiFC, and in vitro pull-down that BIF1 and BIF4 interacted with REL2, a functional homolog of the Arabidopsis TPL corepressor (31). Furthermore, we showed that BIF1 and BIF4 homo and heterodimerize (Fig. S6 A–C). Overall, our expression and protein interaction data suggest functional redundancy among BIF1/BIF4-ARFs transcriptional repression modules and that multiple ARFs work together with BIF1 and BIF4 during the initial stages of reproductive organogenesis.
Fig. S6.
Protein–protein interaction assays. (A) Y2H analysis of BIF1 and BIF4 with the 13 activating ARFs and REL2. (B) The interaction of BIF1 and BIF4 with REL2 in in vitro pull-down assays. Arrowhead points to GST. (C) BiFC assay by transient expression in tobacco leaves.
BARREN STALK1 Is an Early Target of the Auxin Signaling Pathway.
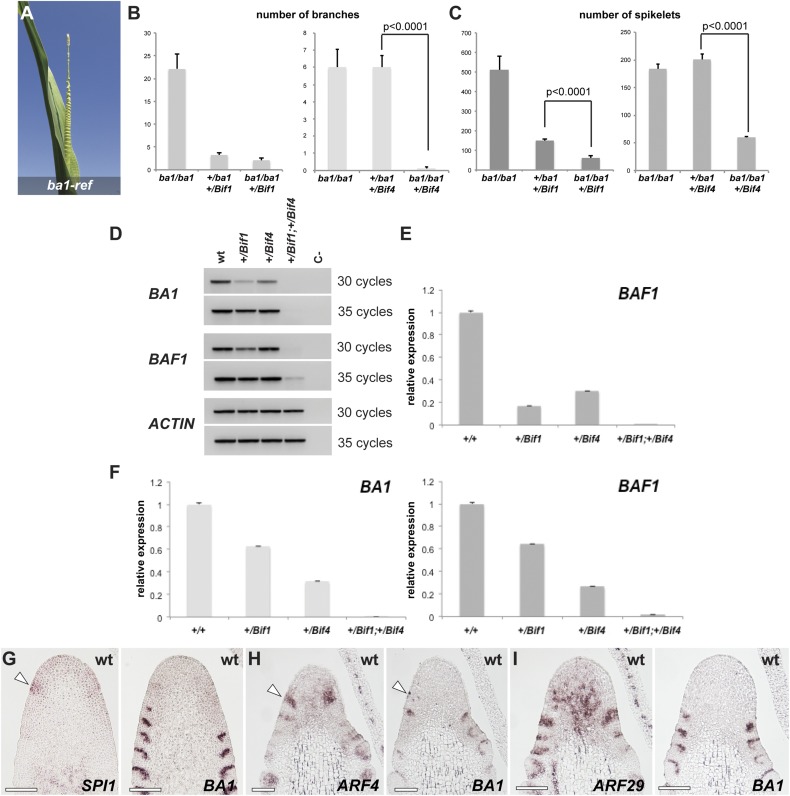
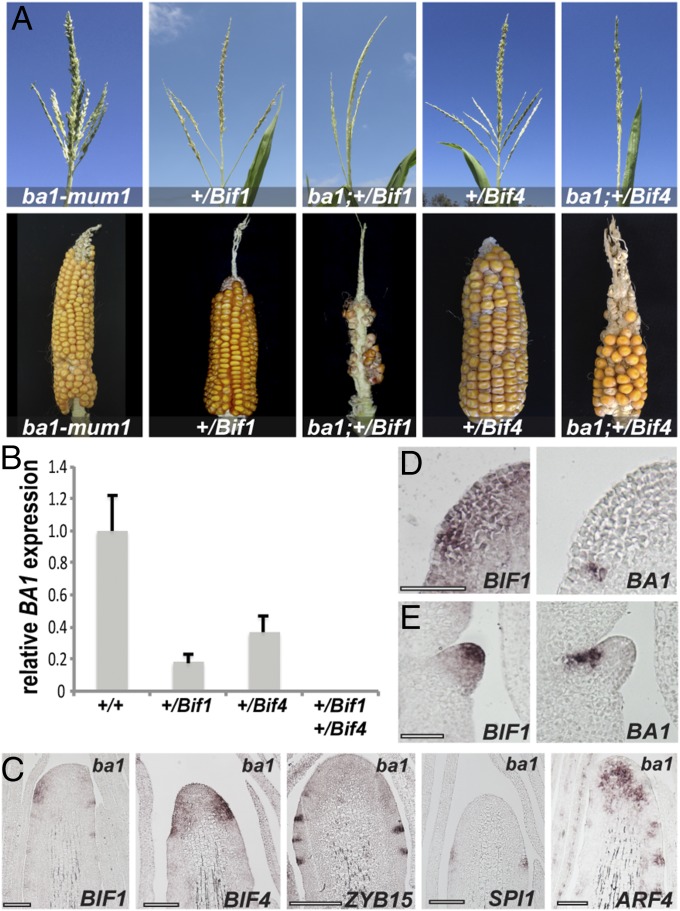
One of the earliest genes expressed at the peripheral zone of the IM is BA1, whose mRNA expression marks a boundary domain in newly forming AMs that is necessary for meristem formation (20). Severe ba1 mutants lack all AMs but form enlarged suppressed bracts (Fig. S7A). Because of the phenotypic resemblance between ba1 and Bif1;Bif4 mutants, we hypothesized that BA1 may be directly regulated by transcriptional repressor complexes containing BIF1 and BIF4. To investigate this possibility, we first checked the genetic interaction between Bif1, Bif4, and ba1, using a weak, fertile allele of ba1 (ba1-mum1) (20). Analysis of double +/Bif1;ba1-mum1/ba1-mum1 and +/Bif4;ba1-mum1/ba1-mum1 mutants showed that ba1 strongly enhanced the phenotype of heterozygous Bif1 and Bif4 mutants in both tassels and ears, impairing both branch and spikelet formation (Fig. 4A and Fig. S7 B and C). These data suggest that BIF1, BIF4, and BA1 function either in the same or in parallel pathways contributing to AM formation.
Fig. S7.
Genetic and expression analysis. (A) ba1-ref tassel. Note the enlarged suppressed bracts. (B and C) Quantification of tassel phenotypes (branch and spikelet-pair number) in Bif1;ba1-mum1 and Bif4;ba1-mum1 double mutants (n ≥ 6). Error bars, SEM. (D) Semiquantitative RT-PCRs of BA1, and BAF1 and ACTIN controls in single- and double-mutant tassels. BAF1 is a boundary-expressed gene that functions upstream of BA1 (50). (E) Quantitative RT-PCR of BAF1 (same samples as in Fig. 4; background A619). (F) Quantitative RT-PCR of BA1 and BAF1 in a B73 background. (G–I) Consecutive sections of young IMs hybridized with different in situ probes. (G) Note that SPI1 expression (arrowhead) appears before BA1. (H and I) ARF expression precedes BA1 expression (arrowheads), but later overlaps with it in slightly broader domains.
Fig. 4.
Genetic and expression analysis of ba1 mutants. (A) Double-mutant analysis of Bif1 and Bif4 with ba1-mum1 in A619 background. (B) qRT-PCR of BA1 in double Bif1;Bif4 mutants. Error bars, SD. (C) In situ hybridization of immature ba1-ref tassels with specific markers. (Scale bars, 100 μm.) (D and E) mRNA in situ hybridizations on consecutive sections of immature inflorescences with BIF1 and BA1 antisense probes. (Scale bars, 50 μm.)
If BIF1 and BIF4 formed repressor complexes targeting BA1 transcription, BA1 expression should be down-regulated in auxin-insensitive tassels. Quantitative RT-PCR on immature +/Bif1;+/Bif4 tassels supported this prediction, as no significant expression of BA1 was detected (Fig. 4B and Fig. S7 D and F). Conversely, in situ hybridizations showed that both BIF1 and BIF4 expression were unchanged in strong ba1 mutant tassels, as were SPI1, an auxin biosynthetic gene, ARF4, and ZYB15, a marker for SBs (Fig. 4C) (22, 32), suggesting that auxin biosynthesis, signaling, and SB patterning are unaffected in ba1 mutants. Furthermore, expression of SPI1 was observed in the peripheral zone of the IM before the appearance of BA1, whereas ARFs showed expression patterns that preceded but subsequently partially overlapped with BA1, indicating that BA1 functions downstream of auxin biosynthesis and signaling (Fig. S7 G–I). Finally, in situ hybridizations of BA1 and BIF1 on consecutive sections showed that BIF1 was broadly expressed in the peripheral zone of the IM, whereas BA1 was present only in a small number of cells (Fig. 4D). However, as the AM developed, the two genes showed a striking complementary expression, with BIF1 being expressed in the center of the meristem and BA1 in its characteristic boundary domain (Fig. 4E). This analysis shows that BA1 and BIF1 expression patterns, although initially overlapping, are subsequently partitioned in two distinct domains of the AM: the boundary domain and the meristem center. Overall, these results are consistent with the hypothesis that BIF1 and BIF4 directly repress BA1 transcription.
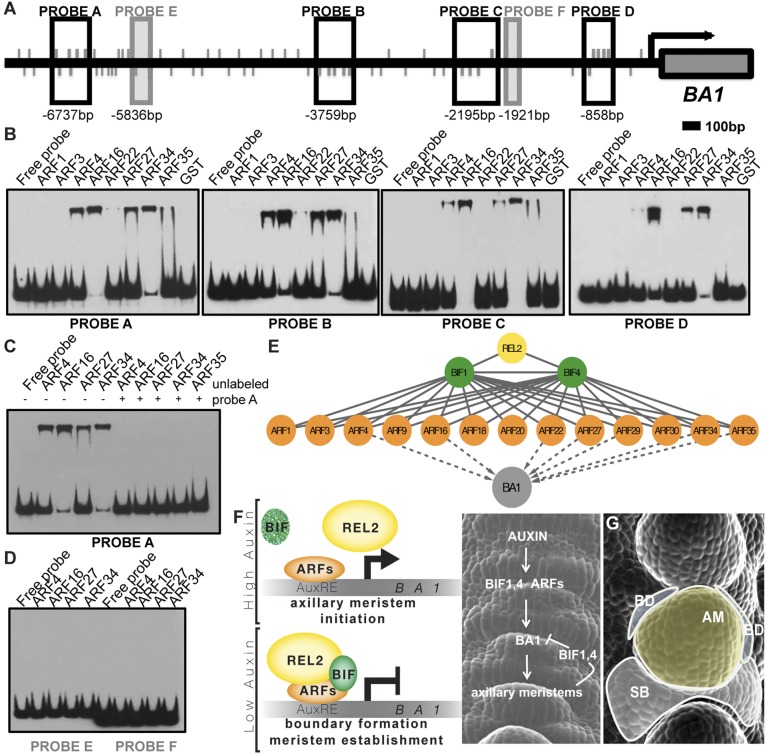
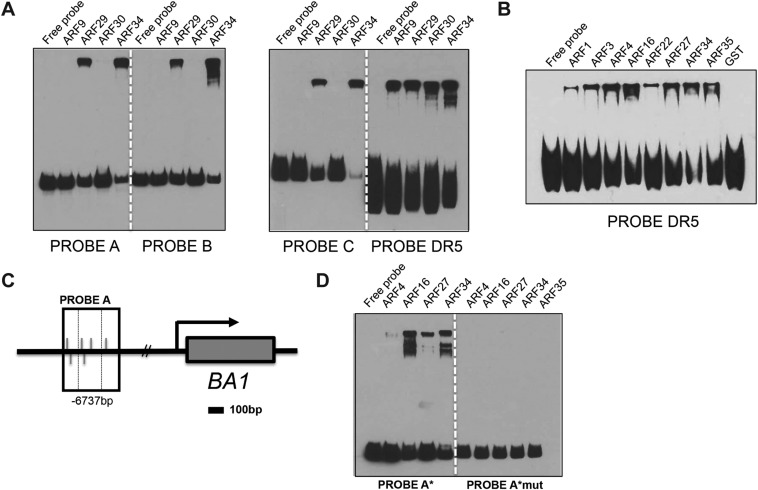
To assess whether coexpressed BIF-ARF repression modules directly bind the BA1 promoter, we expressed a subset of nonparalogous maize-activating ARFs and carried out electrophoretic mobility shift assays (EMSAs) with four regions of the ∼7-kb BA1 promoter enriched for the core TGTC AuxRE element (Fig. 5A). ARF4, ARF16, ARF27, ARF29, and ARF34 strongly bound to all four probes, whereas ARF22 and ARF35 bound only a subset of these regions (Fig. 5B and Fig. S8). Competition with unlabeled probe or mutation of the core TGTC elements inhibited binding (Fig. 5C and Fig. S8). No detectable binding was observed for ARF1, ARF3, ARF9, or ARF30; however, all ARFs bound to the DR5 promoter, albeit with varying intensities (Fig. S8B). No ARFs bound to regions containing only a single AuxRE (Fig. 5D, probes E and F). These results demonstrate that various activating ARFs directly bind to the BA1 promoter and suggest that multiple BIF1,4-ARF modules regulate the expression of BA1 (Fig. 5E).
Fig. 5.
BA1 is a target of BIF/ARF transcriptional regulatory modules. (A) Schematic showing BA1 genomic locus including 7 kb of putative promoter. Promoter fragments used as probes in EMSAs are shown as boxed regions. Values below boxes indicate position relative to BA1 start codon (+1). Gray lines indicate TGTC core AuxRE elements. (B) EMSAs show that various activating ARFs bind to BA1 promoter fragments; GST alone does not. (C) EMSA showing specificity of ARF binding to probe A. Addition of unlabeled probe A outcompetes binding to labeled probe A. (D) EMSA showing ARFs do not bind non-TGTC-enriched promoter fragments E and F. (E) Summary of protein–protein (solid lines) and protein–DNA (dashed lines) interactions identified in this study. (F) Molecular model of organogenesis in the peripheral zone of maize IMs. (G) Diagram of the resulting functional domains (false-colored). SB, suppressed bract; BD, boundary domain; AM, axillary meristem.
Fig. S8.
Multiple-activating ARFs directly bind to the BA1 promoter. (A) EMSAs showing that ARF29 and ARF34 bind to probes A, B, C, and DR5. (B) EMSA showing that all ARFs tested bind to a 9xAuxRE DR5 probe. (C) Schematic showing simplified BA1 genomic locus. Promoter fragment used for probe A in EMSAs is shown as boxed region; dashed box in probe A corresponds to 100-bp probe A*. Values below boxes indicate position relative to BA1 start codon (+1). Gray lines indicate TGTC core AuxRE elements. (D) EMSA showing that mutation of core TGTCs in probe A* eliminates binding of ARF4, ARF16, ARF27, and ARF34.
Discussion
A major outstanding question in auxin signaling is the degree of specificity existing among the various components, and whether combinatorial complexity plays a role in the multitude of processes controlled by auxin. The Bif1 and Bif4 mutants represent a striking case of stabilized Aux/IAAs that specifically confer phenotypes resembling the pin-like inflorescences of Arabidopsis pin1 and mp mutants, indicating a specific and predominant role for both genes in reproductive organogenesis. However, previous analysis suggested a synergistic interaction of Bif1 with bif2, an auxin transport mutant, during vegetative development (25), raising the possibility that other Aux/IAAs may function redundantly with BIF1 and BIF4 during shoot development.
Stabilizing degron mutations in Arabidopsis Aux/IAA genes that are closely related to BIF1 (AXR2/IAA7, AXR3/IAA17, SLR/IAA14, and IAA16) and BIF4 (IAA28) (Fig. S3) were reported to show decreased shoot branching, dwarfism, and partial infertility (33–38). Some of these phenotypes may point to functional homology, as mutations in both species affect reproductive branching. Alternatively, the lack of severe pin-like inflorescence phenotypes in Arabidopsis may indicate that BIF1 and BIF4 were specifically co-opted for patterning maize reproductive AMs. Although Arabidopsis mp mutants display strong pleiotropic defects, no phenotype was observed in the orthologous maize arf4;arf29 double mutants. Overall, our findings from maize suggest specificity among Aux/IAA function, as well as redundancy among activating ARFs. However, ARF expression patterns suggest that although several ARFs are expressed in the peripheral zone of the IM early in inflorescence development, they subsequently acquire more specific domains of expression (AMs vs. suppressed bracts and glumes).
The reproductive defects observed in both mutants suggest that the function of BIF1 and BIF4 is to negatively regulate organogenesis in the peripheral zone of the IM, and that their auxin-induced degradation is necessary for new primordia to initiate. Analysis of the ZmPIN1a-YFP reporter line in +/Bif1;+/Bif4 tassels also indicates that BIF1 and BIF4 are part of a core signaling mechanism that regulates the patterning of maize inflorescences and is required for the local up-regulation of the polar auxin transport components necessary for organogenesis. Previous reports suggested that auxin negatively regulates boundary domain genes during embryo and leaf development (39, 40). Together with a general role in organogenesis, our data support a model in which multiple auxin signaling modules involving BIF1 and BIF4 directly regulate the formation of boundary regions during AM initiation (Fig. 5F). In this model, auxin, first synthesized and transported in the peripheral zone of the IM (22, 26), triggers the transcription of the early-response genes BIF1 and BIF4 (Fig. 3L). Both BIF1 and BIF4 proteins are, in turn, rapidly degraded in the presence of auxin (Fig. 3M), and activating ARFs expressed in this region can promote transcription of their targets to initiate organogenesis. Among these targets, BA1 is specifically required for initiating AMs (20). As meristems develop, auxin is transported to the inner tissue for vasculature formation and to nearby areas to promote new primordia initiation (41, 42). Therefore, in the central zone of developing AMs, BIF1 and BIF4 are no longer efficiently degraded and can form stable repressor complexes on the BA1 promoter. This repression restricts BA1 expression and establishes boundary domains essential for AM formation (Fig. 5 F and G).
Recent reports in tomato and Arabidopsis have established that low auxin at the adaxial boundary of leaf primordia is necessary for vegetative AM formation (43–45). Whether a similar mechanism is established during reproductive development is not known. In maize inflorescences, SB (modified leaves) and AM primordia, although initially overlapping, subsequently resolve and acquire distinct identities (46), making it inherently difficult to test whether auxin minima exist at the axils of SBs. Nonetheless, the auxin-dependent regulation of BA1 transcription, a key regulator of maize inflorescence architecture, ensures that axillary meristems are established throughout reproductive development. Our results pave the way for future biotechnological strategies aimed at modifying reproductive structures. For example, by modulating the auxin-dependent stability of BIF1 and BIF4 proteins, using engineered variants of their degron motifs (47), it may be possible to alter the position and number of primordia initiated by the IM. Similar strategies could be used in other species as well, allowing optimization of inflorescence architecture in crops.
Methods
All Bif1 and Bif4 alleles were generated by EMS mutagenesis by Gerry Neuffer. The Mutator transposon insertion lines were obtained from the UniformMu collection (mu1021266; ARF4) (48), and the Pioneer TUSC population (BT94 27C-05 and BT94 27E-08; ARF29) (49). Experimentally verified full-length cDNAs of BIF1 and BIF4 genes correspond to GRMZM2G130953_T02 and GRMZM5G864847_T01, respectively (GenBank KT819172 and KT819173).
Full-length ZmARFs ORFs were cloned from B73 mixed-stage inflorescence cDNA. EMSAs were performed using recombinant ARFs and the Lightshift Chemiluminescent kit. Auxin-induced degradation assays were carried out as in Havens et al. (29). In situ hybridizations, qRT-PCRs, analysis of transgenic lines, and detailed description of all methods are provided in SI Experimental Procedures.
SI Experimental Procedures
Plant Material and Phenotyping.
All Bif1 and Bif4 alleles were obtained from the Maize Genetics Cooperation Stock Center. Bif1-N2623 and Bif4-N2616 were previously designated as Bif*-N2623 and Bif*-N2616. The mutants used for all phenotypic analysis were introgressed in B73 or A619 with a minimum of two backcrosses. The ba1-mum1 allele (20) was introgressed in (backcross) A619, where it shows a weak phenotype. Analysis of double Bif1;ba1-mum1 and Bif4;ba1-mum1 were carried out in BC2 and BC4 (backcross) A619 populations, respectively. For quantification of phenotypic defects, we followed previous protocols (50). The quantifications of the single- and double-mutant phenotypes in Fig. S1 were conducted in B73 and A619 backgrounds, respectively. Student t-test was used for statistical analysis.
Cloning of BIF1 and BIF4 and Phylogenetic Analysis of ZmAux/IAAs and ZmARFs.
Bif1 was previously mapped to chromosome 8 (24, 25). Two Aux/IAAs (GRMZM2G126422 and GRMZM2G130953) were found to reside in the mapping window. Sequencing of both genes in homozygous Bif1 mutants revealed single-amino acid substitution in all three alleles of GRMZM2G130953. The Bif4 mutant was rough mapped by bulk segregant analysis in a BC1 mapping population, using 39 simple sequence repeat (SSR) markers across the genome, selected on MaizeGDB. The BIF4 locus mapped between markers umc1014 and umc2170 on chromosome 6, with perfect linkage to bnlg1136 (0 recombinants out of 20 chromosomes).
Thirty-eight ZmAux/IAA gene sequences were compiled from Ludwig et al. and Burdo et al. (27, 28), following the nomenclature adopted in these publications. Gene models were verified or manually corrected using publicly available (51, 52) RNA-seq data visualized in the IGV browser (Broad Institute). ZmIAA35, ZmIAA36, ZmIAA38, ZmIAA39, ZmIAA40, and ZmIAA41 were excluded from our analysis because of the lack of expression evidence. Thirty-two ZmARF gene sequences were compiled from Burdo et al. and Xing et al. (28, 53), using the nomenclature of Burdo et al. Gene models were verified or manually corrected as described earlier for ZmAux/IAAs. ZmARF6, ZmARF31, ZmARF32, ZmARF33, ZmARF37, and ZmARF38 were excluded from our analysis due to a lack of expression evidence in which to confirm the gene model. GRMZM2G017187, which corresponds to ARF6 in Xing et al. (53), was not annotated in Burdo et al. (28). To avoid confusion, we therefore refer to this ARF as ZmARF39.
Sequences were imported into Mesquite 3.02 (mesquiteproject.org) and aligned using MUSCLE (54), before being adjusted manually. Regions that were too divergent to align reliably were removed and not included in subsequent analyses. The resulting ARF matrix comprised 55 taxa and 614 characters, whereas the Aux/IAA matrix comprised 67 taxa and 173 characters. ProtTest 3 (55) identified the Jones, Taylor, and Thornton (JTT) (56) substitution model, estimated portions of invariable states, an estimated gamma distribution parameter, and estimated amino acid frequencies (JTT + I + G + F) as the best-fit model of evolution for both the ARF and Aux/IAA matrices. Bayesian phylogenetic analyses were conducted using MrBayes 3.2.3 (57) on the iPlant parallel processing cluster at the University of Arizona, using a fixed JTT amino acid substitution model, four rate categories approximating a gamma distribution, four separate runs with four chains, and 4,000,000 generations. The first 25% of resulting trees were removed as burn-in. SD of split frequencies at the end of the analyses were 0.002193 (ARF) and 0.009407 (Aux/IAA), respectively.
Expression Analysis, Confocal Microscopy, and Transgenic Line Construction.
For in situ hybridizations, 0.2–0.5 cm inflorescences were fixed in paraformaldehyde acetic acid (PFA). Hybridizations were carried out at 55 °C. The BA1, SPI1, and ZYB15 probes have been described before (20, 22, 50). The antisense in situ probes for both BIF1 and BIF4 were synthesized by in vitro transcription (T7 RNA polymerase, Promega) of both genes cloned in pAD-GAL4 and digested with EcoRI, and encompassed the entire coding sequences. For the maize ARF genes, the vectors and enzymes used for probe design are listed in Table S1.
Table S1.
Vectors and primers used for ARF cloning and expression analysis
| Gene name | Vector | Use | Fwd primer 5′-3′ | Rev primer 5′-3′ | Enzyme |
| GRMZM2G073750/ARF9 | pENTR223-Sfi | Y2H in situ | GAATTCGGCCGTCAAGGCCAATGAACCTCTCACCGCCCAGGATG | AGTCGACGGCCCATGAGGCCGTAGTCCAGAGGCACCACAGGTGCG | SpeI |
| GRMZM2G081158/ARF34 | pENTR223-Sfi | Y2H | GAATTCGGCCGTCAAGGCCAATGAACCTCTCCCCGCCCAAG | AGTCGACGGCCCATGAGGCCGTAGTCCAGAGGCACCACAGG | |
| GRMZM2G081158/ARF34 | pGEM T-Easy | In situ | TCCCGCAGCCCAGCCATTCT | TCAACAGGGCCTGAGACTGAGAG | PstI |
| GRMZM2G028980/ARF16 | pENTR223-Sfi | Y2H in situ | GAATTCGGCCGTCAAGGCCAATGAAGCTCTCGCCGTCGG | AGTCGACGGCCCATGAGGCCGAACTCGACCGAACCCACCG | SmaI |
| GRMZM2G035405/ARF18 | pENTR223-Sfi | Y2H in situ | GAATTCGGCCGTCAAGGCCAATGAGGCTCTCGTCGTCGTCC | AGTCGACGGCCCATGAGGCCTCCCAACTCGACCGAACCGAC | BglII |
| GRMZM2G089640/ARF22 | pENTR223-Sfi | Y2H in situ | GTACAAAAAAGCAGAAGGGCCGTCAAGGCCAATGTCACCAGTTCATTCTCCCATG | ACTTTGTACAAGAAAGCTGGGCCCATGAGGCCAACTCGACCGAACCCACGGACGC | BglII |
| GRMZM2G160005/ARF27 | pENTR223-sfi | Y2H in situ | GAATTCGGCCGTCAAGGCCAATGAAGGATCACGGATCGGGC | AGTCGACGGCCCATGAGGCCGGCCCTCCATGCATTTGCGT | HindIII |
| GRMZM2G169820/ARF1 | pENTR223-sfi | Y2H in situ | GAATTCGGCCGTCAAGGCCAATGGAGGCGCCGGGGACGAGCT | AGTCGACGGCCCATGAGGCCTCGCCGGTGACCCTCCACGGACGA | MluI |
| GRMZM2G102845/ARF20 | pENTR223-sfi | Y2H in situ | GAATTCGGCCGTCAAGGCCAATGAAGCAGTCCCCGGCCA | AGTCGACGGCCCATGAGGCCCTCAAATTGGTCGTAGAACCCGATGG | NotI |
| GRMZM2G317900/ARF35 | pENTR223-sfi | Y2H in situ | GAATTCGGCCGTCAAGGCCAATGATCAAGCAGCAGCAGCAGC | AGTCGACGGCCCATGAGGCCCTGGTCATATGAACCAGTAGATGGG | HindIII |
| GRMZM2G034840/ARF4 | pAD-GAL4 | Y2H in situ | AATTCTCTAATGCTTCTCGAGATGATGACGTCCTCGTACGA | GACTCACTATAGGGCTCTAGATCAAGCCATTTGCATGCAGT | XhoI |
| GRMZM2G086949/ARF29 | pAD-GAL4 | Y2H in situ | AATTCTCTAATGCTTCTCGAGATGATGGCCTCCTCGCAGGAG | GACTCACTATAGGGCTCTAGACTAAGCTATTTGGATGCAGT | XhoI |
| GRMZM2G078274/ARF3 | pENTR223-Sfi | Y2H in situ | GAATTCGGCCGTCAAGGCCAATGAGCTCCTCGTCCGCG | AGTCGACGGCCCATGAGGCCAGATAGGTAGCGTGGATC | NdeI |
| GRMZM2G475882/ARF30 | pENTR223-Sfi | Y2H in situ | GAATTCGGCCGTCAAGGCCAATGAGCTCCTCGTCCGCG | AGTCGACGGCCCATGAGGCCAGATAGGTAGCGTGGATC | NdeI |
For qRT-PCR, dissected immature inflorescences were pooled (at least three per pool) from different genotypes. Two biological replicates were performed for each assay. The qScript cDNA synthesis kit was used for cDNA synthesis, and the PerfeCTa SYBR Green FastMix for amplification (Quanta Biosciences). All reactions were carried out on an Illumina Eco Real-Time PCR System and quantified using the Eco Real-Time PCR System Software v4 (Illumina). The analysis was carried out in two different genetic backgrounds, B73 and A619, with analogous results (Fig. S7). For auxin induction experiments, dissected B73 ears (0.2–0.4 cm) were incubated with 100 μM IAA in 1% DMSO, with gentle shaking at room temperature. The control samples were incubated in 1% DMSO only. After incubation, all samples were treated as described earlier. Expression levels were calculated relative to ubiquitin for IAA inductions and actin in Fig. 4. Primers used in qRT-PCR experiments are listed in Table S2.
Table S2.
List of primers used in this study
| Primer name | Sequence 5′-3′ | Use |
| ProbeA-FWD | GGCAAACATATGACATTAGTGG | EMSA |
| ProbeA-REV | CTTAGATCAAGAATTCTCACCCC | EMSA |
| ProbeB-FWD | CCAATTCAATGACATGTCTCT | EMSA |
| ProbeB-REV | GGTCGTTGAGAACCGTCAGTAAA | EMSA |
| ProbeC-FWD | CGTGCACACTAGTTATAAGATATGG | EMSA |
| ProbeC-REV | CGACTTCCCTTCGAGGCCAA | EMSA |
| ProbeD-FWD | GCTTAGAGGTGGAAAGTACGAC | EMSA |
| ProbeD-REV | GACAAATACAAACATGTCTGTTAC | EMSA |
| ProbeE-FWD | CACTACACTAAACCAGGGCG | EMSA |
| ProbeE-REV | GGACATAAATTACTCCGACAACC | EMSA |
| ProbeF-FWD | CCGTCGTCGACGTAGCCTCA | EMSA |
| ProbeF-REV | CTCGACTGGCGCCGGAGCTC | EMSA |
| ProbeG-FWD | GATGCAATGTGTTATCGAGTG | EMSA |
| ProbeG-REV | CCATACATGCACCTTTGATCAG | EMSA |
| BA1-F19 | GTGGTTGGTGACAACGAGGT | qRT-PCR |
| BA1-R5 | CGAGGAAGATGCAAGAAGCAG | qRT-PCR |
| BAF1-F3 | CAGCTGCTGCTAAGACTCAATCC | qRT-PCR |
| BAF1-R3 | AGACTGACATGCAGTTCCAAGC | qRT-PCR |
| BIF1-RT-F1 | CAGTACGCACTGGCTTTTAGTGG | qRT-PCR |
| BIF1-RT-R1 | ACAAACTTTCCTCAGGCAAAAGC | qRT-PCR |
| BIF4-RT-F3 | CGTACCCTGGAGTCTCAACTCTG | qRT-PCR |
| BIF4-RT-R3 | ATCTCCTCTAGATTCCTGCTTCC | qRT-PCR |
| ACTIN-F3 | CTCATGCTATTCTCCGTTTGG | qRT-PCR |
| ACTIN-R3 | TCAGGCATCTCGTAGCTCTTC | qRT-PCR |
| UBIQUITIN-F1 | GAGTGCCCCAACGCCGAGTG | qRT-PCR |
| UBIQUITIN-R1 | CTACGCCTGCTGGTTGTAGACGTA | qRT-PCR |
The ZmPIN1a-YFP and DR5::RFP transgenic maize lines (26) were crossed to Bif1 and Bif4 mutants in A619, and subsequently the two heterozygous mutant lines were crossed together and imaged. The VENUS-BIF4 line was imaged in the T0 generation. Reporter genes were visualized in developing tassels (0.3–0.6 cm), using a Leica SP5 confocal microscope. YFP and VENUS signals were imaged using 514 excitation and 520–575 emission. RFP signals were imaged using 594 excitation and 625–655 emission. Images were analyzed using FIJI.
A Leica DM5500B microscope equipped with a DFC450 C digital camera was used to image all in situ hybridizations. Scanning electron microscope analysis was performed as previously described (31).
The pBIF4::VENUS:BIF4 construct was assembled using the Gibson assembly cloning method (58) and cloned into the maize transformation vector pTF101.1 digested with HindIII/EcoRI. The construct includes an 8,018-bp region upstream of the start codon and a 2,467-bp fragment downstream of the stop codon. Transformation of maize embryos by Agrobacterium infection was performed according to published protocols (59). Seven independent lines were obtained, and expression was detected in three lines.
Cloning of ZmARFs, Protein–Protein Interaction Assays, Protein Expression, and Electrophoretic Mobility Shift Assays.
Full-length activating ZmARFs ORFs were cloned into the SfiI sites of pENTR223-Sfi (60), using standard restriction enzyme-based cloning. pENTR-ARF clones were recombined into pDEST-AD using LR clonase II (Life Technologies). BIF1 and BIF4 ORFs were cloned into pENTR223-Sfi and recombined into pDEST-DB, using LR clonase II. pDEST-AD and pDEST-DB clones were transformed into mating compatible yeast strains Y8800 and Y8930, respectively, using the LiAc transformation method. Mating was carried out according to standard procedures. Reporter gene activation was determined by assessing growth on −Leu/−Trp/−His +1 mM 3AT (3-amino-1,2,4-triazole) media after 3 d at 30 °C.
For pull-down experiments, BIF1 was cloned in pGEX-4T-3, using EcoRI/XhoI sites; pENTR223-Sfi-BIF4 was recombined in the pIX-GST vector (60). The EAR motif of BIF1 (LALTLRLP) was changed in FAFTFRFP in the BIF1mEAR clones. The REL2ΔWD40 clone was previously described (31). For BiFC, BIF1 and REL2 were cloned in SPYCE and SPYNE vectors, whereas BIF4 was recombined in a Gateway-compatible pCYFP vector. BiFC and in vitro pull-down experiments were performed as previously described (31).
pENTR-ARF clones were recombined into pDEST15 (Life Technologies), using LR clonase II. GST-ARF clones were subsequently transformed in BL21DE3 codon plus cells (Stratagene). Protein expression was induced with 0.4 mM IPTG (isopropyl β-D-1-thiogalactopyranoside) and carried out at 23°C for 4 h. Cells from 0.5 L media were harvested and resuspended in 10 mL HDB buffer at pH 7.3 [25 mM Hepes, 0.7 mM Na2HPO4, 137 mM NaCl, 5 mM KCl, 1 mM AEBSF, protease inhibitor mixture (Roche), and 2 mM lysozyme] and sonicated. Cleared lysate was applied to GST Gravitrap Glutathione Sepharose 4B (GE Healthcare) columns and washed thoroughly. GST-ARF fusions were eluted with 10 mM reduced glutathione and concentrated using Amicon Ultra 30K concentrators (Millipore).
For EMSAs, selected regions of ∼200–250 bp within the BA1 promoter that were enriched for core TGTC elements were PCR amplified, gel purified, and biotinylated using the Biotin 3′ End DNA Labeling Kit (ThermoScientific) according to the manufacturer’s recommendations. Primers used to amplify the seven probes are listed in Table S1. DNA binding assays were performed using the Lightshift Chemiluminescent EMSA kit (ThermoScientific) as follows: binding reactions containing 1× Binding Buffer, 50 ng/μL polydI/dC, 2.5% glycerol, 2 μL biotinylated probe, and 1 μL purified GST-ARF protein or GST alone were incubated at room temperature for 20 min and loaded on a 6% DNA retardation gel (Life Technologies) before transfer to a nylon membrane. Subsequent detection was carried out according to the manufacturer’s recommendations. Competition with unlabeled probe was carried out using 50-fold excess of unlabeled probe. Mutated probe A* was generated by annealing two complementary oligos in which the core TGTC elements were changed to AAAA. ARF18 and ARF20 were not analyzed by EMSA because of high homology with ARF22 and ARF35.
Aux/IAA Degradation Assays in Yeast.
Diploid yeast strains coexpressing stably integrated Arabidopsis thaliana TIR1 and YFP-tagged Aux/IAA proteins were prepared by transferring a freshly grown colony from YPD plates into Synthetic Complete media. Flow cytometry was used to estimate the cell density and dilute cells to 0.5 events⋅μL−1, such that cultures were in log phase 16 h later and for the duration of the experiment. All cultures were grown at 30 °C with shaking. Preauxin measurements were taken to ascertain baseline expression, followed by addition of auxin (10 μM indole-3-acetic acid) or mock treatment (95% [vol/vol] ethanol). Measurements were acquired over the course of 150 min after auxin treatment, with intervals ranging from 10 min early in the YFP-Aux/IAA degradation phase to 30 min later in the degradation phase. Controls were measured every hour for the duration of the experiment. Data normalization was carried out by subtracting background autofluorescence and normalizing to preauxin fluorescence levels for each yeast strain. Nonlinear regression analysis (plateau followed by one-phase decay) was performed in Prism6 (GraphPad), using raw data to calculate degradation half-lives with 95% confidence intervals.
Acknowledgments
We thank Gerry Neuffer and the Maize Inflorescence Project for generating mutants; the Maize Genetics Cooperation Stock Center for providing seeds; UniformMu for insertion lines; Marc Probasco for field and greenhouse care; David Jackson for SEM use; Sharon Stanfield for preliminary experiments; and Robert Schmidt, Brian Crawford, Rhiannon Macrae, and Mithu Chatterjee for critical reading of the manuscript. We acknowledge funding from National Science Foundation (IOS-08207291/1114484 to A.G. and S.M.; MCB-1411949 to J.L.N.), NIH (R01-GM107084 to J.L.N.; F32CA180514 to B.L.M.), the China Scholarship Council (Q.L.), and the Waksman Institute (A.G.). Any opinion, findings, and conclusions or recommendations expressed in this material are those of the authors and do not necessarily reflect the views of the National Science Foundation.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1516473112/-/DCSupplemental.
References
- 1.Doebley JF, Gaut BS, Smith BD. The molecular genetics of crop domestication. Cell. 2006;127(7):1309–1321. doi: 10.1016/j.cell.2006.12.006. [DOI] [PubMed] [Google Scholar]
- 2.Bommert P, Nagasawa NS, Jackson D. Quantitative variation in maize kernel row number is controlled by the FASCIATED EAR2 locus. Nat Genet. 2013;45(3):334–337. doi: 10.1038/ng.2534. [DOI] [PubMed] [Google Scholar]
- 3.Park SJ, et al. Optimization of crop productivity in tomato using induced mutations in the florigen pathway. Nat Genet. 2014;46(12):1337–1342. doi: 10.1038/ng.3131. [DOI] [PubMed] [Google Scholar]
- 4.Gälweiler L, et al. Regulation of polar auxin transport by AtPIN1 in Arabidopsis vascular tissue. Science. 1998;282(5397):2226–2230. doi: 10.1126/science.282.5397.2226. [DOI] [PubMed] [Google Scholar]
- 5.Przemeck GK, Mattsson J, Hardtke CS, Sung ZR, Berleth T. Studies on the role of the Arabidopsis gene MONOPTEROS in vascular development and plant cell axialization. Planta. 1996;200(2):229–237. doi: 10.1007/BF00208313. [DOI] [PubMed] [Google Scholar]
- 6.Hardtke CS, Berleth T. The Arabidopsis gene MONOPTEROS encodes a transcription factor mediating embryo axis formation and vascular development. EMBO J. 1998;17(5):1405–1411. doi: 10.1093/emboj/17.5.1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Salehin M, Bagchi R, Estelle M. SCFTIR1/AFB-based auxin perception: Mechanism and role in plant growth and development. Plant Cell. 2015;27(1):9–19. doi: 10.1105/tpc.114.133744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Okushima Y, Fukaki H, Onoda M, Theologis A, Tasaka M. ARF7 and ARF19 regulate lateral root formation via direct activation of LBD/ASL genes in Arabidopsis. Plant Cell. 2007;19(1):118–130. doi: 10.1105/tpc.106.047761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schlereth A, et al. MONOPTEROS controls embryonic root initiation by regulating a mobile transcription factor. Nature. 2010;464(7290):913–916. doi: 10.1038/nature08836. [DOI] [PubMed] [Google Scholar]
- 10.Yamaguchi N, et al. A molecular framework for auxin-mediated initiation of flower primordia. Dev Cell. 2013;24(3):271–282. doi: 10.1016/j.devcel.2012.12.017. [DOI] [PubMed] [Google Scholar]
- 11.Oh E, et al. Cell elongation is regulated through a central circuit of interacting transcription factors in the Arabidopsis hypocotyl. eLife. 2014;3:3. doi: 10.7554/eLife.03031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Crawford BC, et al. Plant development. Genetic control of distal stem cell fate within root and embryonic meristems. Science. 2015;347(6222):655–659. doi: 10.1126/science.aaa0196. [DOI] [PubMed] [Google Scholar]
- 13.Ripoll JJ, et al. microRNA regulation of fruit growth. Nature Plants. March 30, 2015 doi: 10.1038/nplants.2015.36. [DOI] [PubMed] [Google Scholar]
- 14.Weijers D, et al. Developmental specificity of auxin response by pairs of ARF and Aux/IAA transcriptional regulators. EMBO J. 2005;24(10):1874–1885. doi: 10.1038/sj.emboj.7600659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vernoux T, et al. The auxin signalling network translates dynamic input into robust patterning at the shoot apex. Mol Syst Biol. 2011;7:508. doi: 10.1038/msb.2011.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guilfoyle TJ, Hagen G. Getting a grasp on domain III/IV responsible for Auxin Response Factor-IAA protein interactions. Plant Sci. 2012;190:82–88. doi: 10.1016/j.plantsci.2012.04.003. [DOI] [PubMed] [Google Scholar]
- 17.Boer DR, et al. Structural basis for DNA binding specificity by the auxin-dependent ARF transcription factors. Cell. 2014;156(3):577–589. doi: 10.1016/j.cell.2013.12.027. [DOI] [PubMed] [Google Scholar]
- 18.Nanao MH, et al. Structural basis for oligomerization of auxin transcriptional regulators. Nat Commun. 2014;5:3617. doi: 10.1038/ncomms4617. [DOI] [PubMed] [Google Scholar]
- 19.Korasick DA, et al. Molecular basis for AUXIN RESPONSE FACTOR protein interaction and the control of auxin response repression. Proc Natl Acad Sci USA. 2014;111(14):5427–5432. doi: 10.1073/pnas.1400074111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gallavotti A, et al. The role of barren stalk1 in the architecture of maize. Nature. 2004;432(7017):630–635. doi: 10.1038/nature03148. [DOI] [PubMed] [Google Scholar]
- 21.McSteen P, et al. barren inflorescence2 Encodes a co-ortholog of the PINOID serine/threonine kinase and is required for organogenesis during inflorescence and vegetative development in maize. Plant Physiol. 2007;144(2):1000–1011. doi: 10.1104/pp.107.098558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gallavotti A, et al. sparse inflorescence1 encodes a monocot-specific YUCCA-like gene required for vegetative and reproductive development in maize. Proc Natl Acad Sci USA. 2008;105(39):15196–15201. doi: 10.1073/pnas.0805596105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Phillips KA, et al. vanishing tassel2 encodes a grass-specific tryptophan aminotransferase required for vegetative and reproductive development in maize. Plant Cell. 2011;23(2):550–566. doi: 10.1105/tpc.110.075267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Neuffer M, Coe E, Wessler S. Mutants of Maize. 1997. Cold Spring Harbor Laboratory Press; Cold Spring Harbor, New York: 1997. [Google Scholar]
- 25.Barazesh S, McSteen P. Barren inflorescence1 functions in organogenesis during vegetative and inflorescence development in maize. Genetics. 2008;179(1):389–401. doi: 10.1534/genetics.107.084079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gallavotti A, Yang Y, Schmidt RJ, Jackson D. The Relationship between auxin transport and maize branching. Plant Physiol. 2008;147(4):1913–1923. doi: 10.1104/pp.108.121541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ludwig Y, Zhang Y, Hochholdinger F. The maize (Zea mays L.) AUXIN/INDOLE-3-ACETIC ACID gene family: Phylogeny, synteny, and unique root-type and tissue-specific expression patterns during development. PLoS One. 2013;8(11):e78859. doi: 10.1371/journal.pone.0078859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Burdo B, et al. The Maize TFome–development of a transcription factor open reading frame collection for functional genomics. Plant J. 2014;80(2):356–366. doi: 10.1111/tpj.12623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Havens KA, et al. A synthetic approach reveals extensive tunability of auxin signaling. Plant Physiol. 2012;160(1):135–142. doi: 10.1104/pp.112.202184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pierre-Jerome E, Jang SS, Havens KA, Nemhauser JL, Klavins E. Recapitulation of the forward nuclear auxin response pathway in yeast. Proc Natl Acad Sci USA. 2014;111(26):9407–9412. doi: 10.1073/pnas.1324147111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gallavotti A, et al. The control of axillary meristem fate in the maize ramosa pathway. Development. 2010;137(17):2849–2856. doi: 10.1242/dev.051748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Whipple CJ, et al. A conserved mechanism of bract suppression in the grass family. Plant Cell. 2010;22(3):565–578. doi: 10.1105/tpc.109.073536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rogg LE, Lasswell J, Bartel B. A gain-of-function mutation in IAA28 suppresses lateral root development. Plant Cell. 2001;13(3):465–480. doi: 10.1105/tpc.13.3.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nagpal P, et al. AXR2 encodes a member of the Aux/IAA protein family. Plant Physiol. 2000;123(2):563–574. doi: 10.1104/pp.123.2.563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Leyser HM, Pickett FB, Dharmasiri S, Estelle M. Mutations in the AXR3 gene of Arabidopsis result in altered auxin response including ectopic expression from the SAUR-AC1 promoter. Plant J. 1996;10(3):403–413. doi: 10.1046/j.1365-313x.1996.10030403.x. [DOI] [PubMed] [Google Scholar]
- 36.Fukaki H, Tameda S, Masuda H, Tasaka M. Lateral root formation is blocked by a gain-of-function mutation in the SOLITARY-ROOT/IAA14 gene of Arabidopsis. Plant J. 2002;29(2):153–168. doi: 10.1046/j.0960-7412.2001.01201.x. [DOI] [PubMed] [Google Scholar]
- 37.Muto H, Watahiki MK, Nakamoto D, Kinjo M, Yamamoto KT. Specificity and similarity of functions of the Aux/IAA genes in auxin signaling of Arabidopsis revealed by promoter-exchange experiments among MSG2/IAA19, AXR2/IAA7, and SLR/IAA14. Plant Physiol. 2007;144(1):187–196. doi: 10.1104/pp.107.096628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rinaldi MA, Liu J, Enders TA, Bartel B, Strader LC. A gain-of-function mutation in IAA16 confers reduced responses to auxin and abscisic acid and impedes plant growth and fertility. Plant Mol Biol. 2012;79(4-5):359–373. doi: 10.1007/s11103-012-9917-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Furutani M, et al. PIN-FORMED1 and PINOID regulate boundary formation and cotyledon development in Arabidopsis embryogenesis. Development. 2004;131(20):5021–5030. doi: 10.1242/dev.01388. [DOI] [PubMed] [Google Scholar]
- 40.Bilsborough GD, et al. Model for the regulation of Arabidopsis thaliana leaf margin development. Proc Natl Acad Sci USA. 2011;108(8):3424–3429. doi: 10.1073/pnas.1015162108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Benková E, et al. Local, efflux-dependent auxin gradients as a common module for plant organ formation. Cell. 2003;115(5):591–602. doi: 10.1016/s0092-8674(03)00924-3. [DOI] [PubMed] [Google Scholar]
- 42.Heisler MG, et al. Patterns of auxin transport and gene expression during primordium development revealed by live imaging of the Arabidopsis inflorescence meristem. Curr Biol. 2005;15(21):1899–1911. doi: 10.1016/j.cub.2005.09.052. [DOI] [PubMed] [Google Scholar]
- 43.Wang Q, Kohlen W, Rossmann S, Vernoux T, Theres K. Auxin Depletion from the Leaf Axil Conditions Competence for Axillary Meristem Formation in Arabidopsis and Tomato. Plant Cell. 2014;26(5):2068–2079. doi: 10.1105/tpc.114.123059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Qi J, et al. Auxin depletion from leaf primordia contributes to organ patterning. Proc Natl Acad Sci USA. 2014;111(52):18769–18774. doi: 10.1073/pnas.1421878112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang Y, et al. The Stem Cell Niche in Leaf Axils Is Established by Auxin and Cytokinin in Arabidopsis. Plant Cell. 2014;26(5):2055–2067. doi: 10.1105/tpc.114.123083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chuck G, Whipple C, Jackson D, Hake S. The maize SBP-box transcription factor encoded by tasselsheath4 regulates bract development and the establishment of meristem boundaries. Development. 2010;137(8):1243–1250. doi: 10.1242/dev.048348. [DOI] [PubMed] [Google Scholar]
- 47.Guseman JM, et al. Auxin-induced degradation dynamics set the pace for lateral root development. Development. 2015;142(5):905–909. doi: 10.1242/dev.117234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Settles AM, et al. Sequence-indexed mutations in maize using the UniformMu transposon-tagging population. BMC Genomics. 2007;8:116. doi: 10.1186/1471-2164-8-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bensen RJ, et al. Cloning and characterization of the maize An1 gene. Plant Cell. 1995;7(1):75–84. doi: 10.1105/tpc.7.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gallavotti A, et al. BARREN STALK FASTIGIATE1 is an AT-hook protein required for the formation of maize ears. Plant Cell. 2011;23(5):1756–1771. doi: 10.1105/tpc.111.084590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bolduc N, et al. Unraveling the KNOTTED1 regulatory network in maize meristems. Genes Dev. 2012;26(15):1685–1690. doi: 10.1101/gad.193433.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Eveland AL, et al. Regulatory modules controlling maize inflorescence architecture. Genome Res. 2014;24(3):431–443. doi: 10.1101/gr.166397.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Xing H, et al. Genome-wide identification and expression profiling of auxin response factor (ARF) gene family in maize. BMC Genomics. 2011;12:178. doi: 10.1186/1471-2164-12-178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Edgar RC. MUSCLE: A multiple sequence alignment method with reduced time and space complexity. BMC Bioinformatics. 2004;5:113. doi: 10.1186/1471-2105-5-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Darriba D, Taboada GL, Doallo R, Posada D. ProtTest 3: Fast selection of best-fit models of protein evolution. Bioinformatics. 2011;27(8):1164–1165. doi: 10.1093/bioinformatics/btr088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jones DT, Taylor WR, Thornton JM. The rapid generation of mutation data matrices from protein sequences. Comput Appl Biosci. 1992;8(3):275–282. doi: 10.1093/bioinformatics/8.3.275. [DOI] [PubMed] [Google Scholar]
- 57.Ronquist F, et al. MrBayes 3.2: Efficient Bayesian phylogenetic inference and model choice across a large model space. Syst Biol. 2012;61(3):539–542. doi: 10.1093/sysbio/sys029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gibson DG, et al. Enzymatic assembly of DNA molecules up to several hundred kilobases. Nat Methods. 2009;6(5):343–345. doi: 10.1038/nmeth.1318. [DOI] [PubMed] [Google Scholar]
- 59.Frame BR, et al. Agrobacterium tumefaciens-mediated transformation of maize embryos using a standard binary vector system. Plant Physiol. 2002;129(1):13–22. doi: 10.1104/pp.000653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Arabidopsis Interactome Mapping C. Arabidopsis Interactome Mapping Consortium Evidence for network evolution in an Arabidopsis interactome map. Science. 2011;333(6042):601–607. doi: 10.1126/science.1203877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Remington DL, Vision TJ, Guilfoyle TJ, Reed JW. Contrasting modes of diversification in the Aux/IAA and ARF gene families. Plant Physiol. 2004;135(3):1738–1752. doi: 10.1104/pp.104.039669. [DOI] [PMC free article] [PubMed] [Google Scholar]