Significance
Mushroom bodies are prominent structures of the insect brain that have been associated with the storage and retrieval of elemental forms of memory. We took advantage of the unique cognitive capabilities of honey bees to uncover a previously unidentified function for these structures. Because the honey bee is the only insect in which higher-order, configural learning has been demonstrated, we asked whether the mushroom bodies of this insect are necessary for this task. Disrupting reversibly mushroom body function upon configural conditioning, we show that these structures are required for the acquisition of configural, but not of elemental, learning. This functionality resembles that of the vertebrate brain, where different brain regions are recruited for learning forms of different complexity.
Keywords: learning, configural learning, mushroom bodies, honey bee, Apis mellifera
Abstract
Learning theories distinguish elemental from configural learning based on their different complexity. Although the former relies on simple and unambiguous links between the learned events, the latter deals with ambiguous discriminations in which conjunctive representations of events are learned as being different from their elements. In mammals, configural learning is mediated by brain areas that are either dispensable or partially involved in elemental learning. We studied whether the insect brain follows the same principles and addressed this question in the honey bee, the only insect in which configural learning has been demonstrated. We used a combination of conditioning protocols, disruption of neural activity, and optophysiological recording of olfactory circuits in the bee brain to determine whether mushroom bodies (MBs), brain structures that are essential for memory storage and retrieval, are equally necessary for configural and elemental olfactory learning. We show that bees with anesthetized MBs distinguish odors and learn elemental olfactory discriminations but not configural ones, such as positive and negative patterning. Inhibition of GABAergic signaling in the MB calyces, but not in the lobes, impairs patterning discrimination, thus suggesting a requirement of GABAergic feedback neurons from the lobes to the calyces for nonelemental learning. These results uncover a previously unidentified role for MBs besides memory storage and retrieval: namely, their implication in the acquisition of ambiguous discrimination problems. Thus, in insects as in mammals, specific brain regions are recruited when the ambiguity of learning tasks increases, a fact that reveals similarities in the neural processes underlying the elucidation of ambiguous tasks across species.
Learning can be categorized into two levels of complexity termed elemental and configural (nonelemental) (1–3). Simple and unambiguous links between events characterize elemental learning (4). By contrast, ambiguity and nonlinearity characterize configural learning, where associations involve conjunctions of elemental stimuli, which may have different, contradictory outcomes. As a consequence, solving configural tasks typically requires treating stimulus conjunctions as being different from the simple sum of their elemental components (5–8). For example, in a negative patterning task (9–11), subjects have to discriminate a nonreinforced conjunction of two elements A and B from its reinforced elements (i.e., AB– vs. A+ and B+), which requires treating AB as being different from the simple sum of A and B (12, 13). The ambiguity of the task lies in the fact that each element (A and B) is as often reinforced (when presented alone) as nonreinforced (when presented as a compound). In mammals, different brain structures have been associated with these two learning forms: Whereas the hippocampus seems to be dispensable for learning elemental associations (6, 8), it is required for fast formation of conjunctive representations during learning tasks, such as spatial learning or contextual fear conditioning (6, 8, 10, 14–19). Moreover, the cortical system is necessary to form configural representations over extended training, thus supporting the learning of nonlinear discriminations,
Here, we ask whether the specialization of different brain centers for learning tasks of different complexity is a property that can be extended to an insect brain. Insects offer the possibility of studying sophisticated behaviors and simultaneously accessing the neural bases of these behaviors (20). Several studies have shown that insects, in particular the honey bee Apis mellifera, possess higher-order cognitive abilities (5, 21), which raises the question of which neural mechanisms support these capacities in a brain whose size is only 1 mm3 (22).
The mushroom bodies (MBs) are paired structures in the insect brain that have been historically associated with olfactory learning and memory. Their function has been extensively studied in a variety of elemental learning protocols, mainly in the honey bee and the fruit fly Drosophila melanogaster (23–29). In both species, MBs play a fundamental role for the encoding, storing, and retrieval of appetitive and aversive elemental memories, but no study has clearly established their role for nonelemental learning and memory (30). In fruit flies, this missing information may be due to the incapacity of these insects to solve nonelemental problems, such as negative patterning (31). By contrast, honey bees exhibit elaborated nonelemental learning abilities (32–36), which have been suggested to require intact MB function (5).
Here, we used a combination of nonelemental conditioning protocols, disruption of MB function, and optophysiological recordings of neural activity to determine whether MBs are necessary for nonelemental forms of learning. Our results show that acquisition of olfactory patterning discriminations is impaired in bees in which neural activity in the MBs was blocked by procaine injection (37, 38), but not in control animals injected with saline solution. By contrast, MB blockade by procaine affected neither olfactory processing upstream of the MBs nor elemental olfactory discriminations. To uncover the neural mechanisms underlying the necessity of MBs for patterning discriminations, we focused on GABAergic feedback neurons (39), which provide inhibitory feedback to the MBs of the bee (40–43). We blocked GABAergic signaling by locally injecting picrotoxin (PTX), a GABA antagonist, into the MB calyces or into the MB lobes. We show that GABAergic feedback to the calyces—but not to the lobes—is required for patterning discriminations. These results uncover a previously unidentified role for MBs: namely, the disambiguation between elemental and conjunctive odor representations, thus supporting the learning of nonlinear discriminations.
Results
Harnessed bees learn the association between an odorant [the conditioned stimulus (CS)] and sugar reward [the unconditioned stimulus (US)] (25, 44, 45). In subsequent retention tests, bees exhibit a typical proboscis extension response (PER) (the conditioned response) to the odorant that predicts the sucrose reward. Different variants of this Pavlovian protocol have been conceived to study nonelemental olfactory learning in honey bees. In particular, bees effectively learn negative patterning discriminations (A+, B+ vs. AB−; see the Introduction) and their reversed counterpart, positive patterning (A−, B− vs. AB+) (equivalent to the XOR and AND problems, respectively) (32–34, 46). To determine whether MBs are required for both patterning discriminations, we combined these two conditioning problems with local procaine anesthesia to block neural activity specifically in these brain centers in a transient and reversible way (37, 38).
Learning of Positive Patterning Is Impaired Under Blockade of Mushroom Bodies.
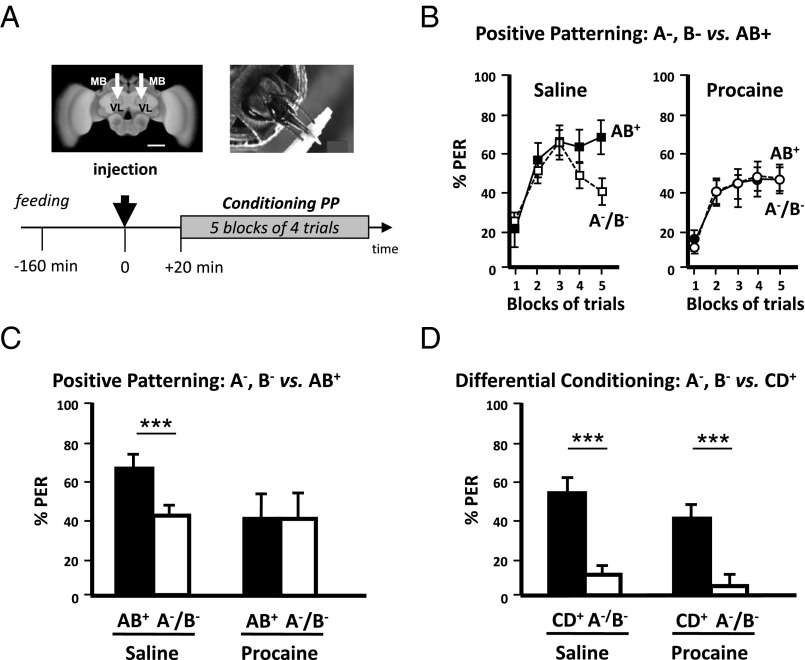
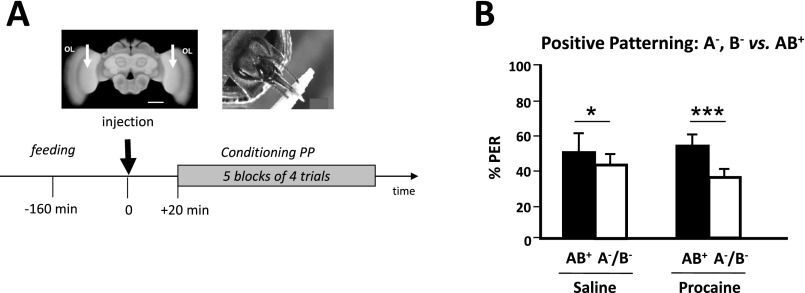
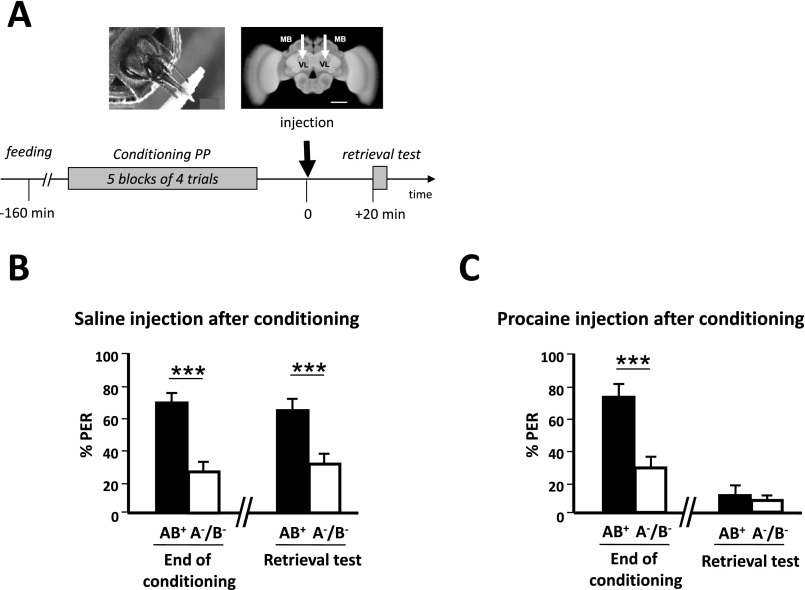
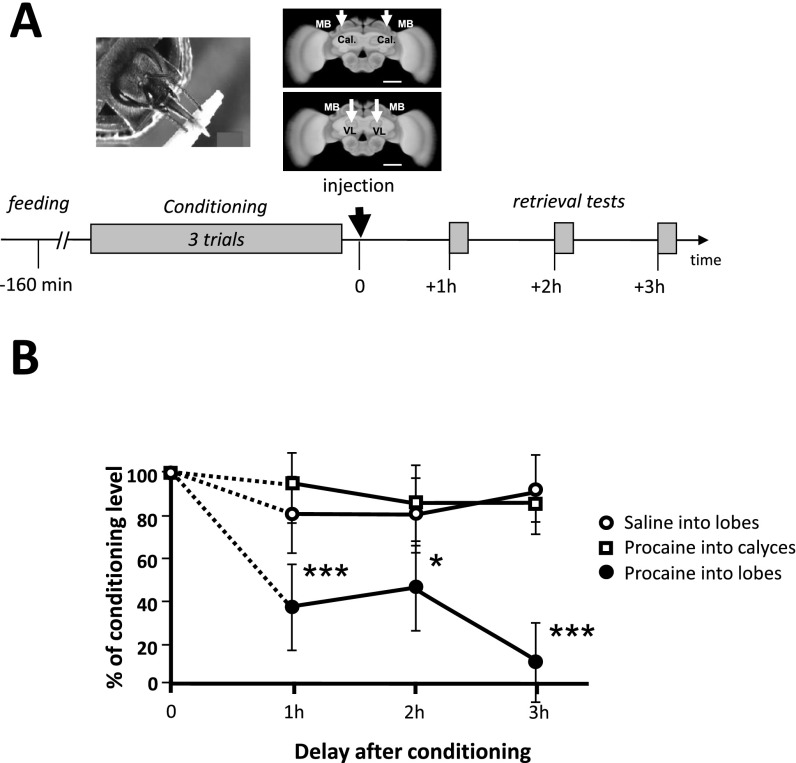
We first studied whether honey bees learn a positive patterning discrimination (A−, B− vs. AB+) under procaine-induced blockade of neural activity in both MB vertical lobes (Fig. 1A). This anesthetic was previously shown to efficiently suppress voltage-gated channels in bee MB neurons (37, 38). Bees injected with saline solution in the vertical lobes were used as controls. The responses of bees during training varied according to the stimuli presented and the injection treatment (ANOVA for repeated measurements; stimulus effect, F1,78 = 6.56, P < 0.05; treatment × stimulus interaction, F1,78 = 3.72, P < 0.01). Saline-injected bees increased their responses to AB+ and reduced their responses to A− and B− (Fig. 1B, Left). By contrast, procaine-injected bees kept responding equally to all three stimuli (Fig. 1B, Right). As a result, by the end of conditioning (fifth block of trials) (Fig. 1C), only bees injected with saline responded significantly more to AB+ than to A− and B− (Z43,1 = 3.62, P < 0.0005) whereas procaine-injected bees did not achieve the discrimination [Z371 = 0.00, nonsignificant (NS)]. Thus, MB anesthesia prevented bees from learning a positive patterning task. To verify that the impairment of learning was due to a specific blockade of MBs by procaine rather than to a nonspecific, diffusive action on adjacent neuropils, we checked the effect of procaine injections into the optic lobes (i.e., outside the MBs) (Fig. S1A). The inactivation of both optic lobes by procaine did not affect the capacity to learn olfactory positive patterning, thus confirming the localized action of the blocker (Fig. S1B). We also confirmed that procaine indeed blocked MB activity by performing injections into the MB lobes immediately after conditioning and before retrieval (Fig. S2A). In accordance with the known role of MBs for retrieval (26, 47), bees that had learned the initial nonelemental discrimination and received subsequent procaine injection failed to differentiate between the compound and the elements in the retrieval tests, unlike saline-injected bees (Fig. S2 B and C). Impairment of retrieval was extensible to an elemental discrimination because bees injected with procaine into the MB lobes after a simple odor–sucrose association (A+) exhibited reduced retrieval up to 3 h after injection (Fig. S3B), a period that corresponds to the duration of the patterning protocols used in our study. This result confirms that a functional output from the MB lobes is required for olfactory memory retrieval (47) and shows that procaine remains confined to the injection site; indeed, injecting the drug into the MB calyces had no impact on retrieval, similarly to saline injection (Fig. S3B). Thus, any diffusion that may occur between these two regions of the MBs is negligible. To sum up, in our experimental conditions, procaine injections into the MB lobes impaired both elemental (Fig. S3) and nonelemental retrieval (Fig. S2), in accordance with the known role of MBs for memory retrieval, but, in addition, they impaired specifically and significantly nonelemental acquisition.
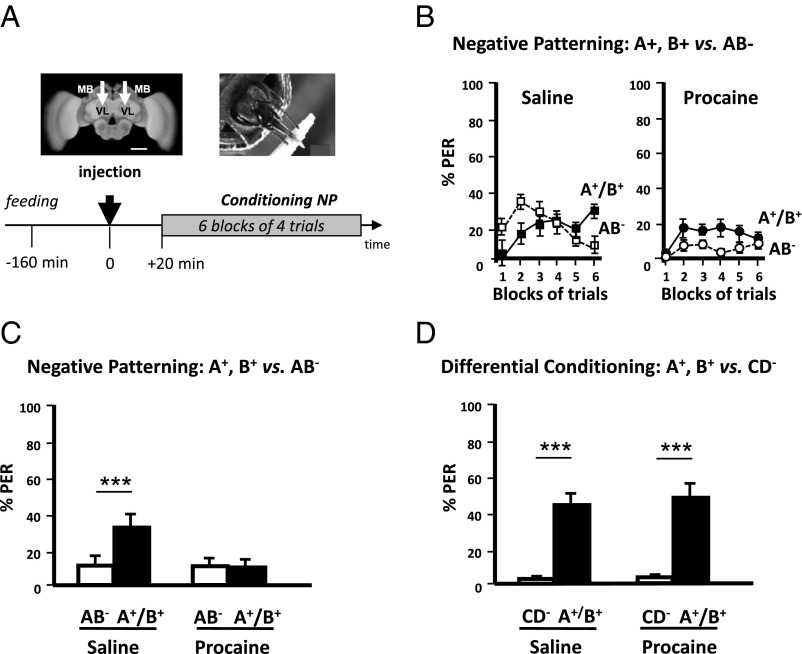
Fig. 1.
Mushroom body blockade impairs positive patterning (PP). (A, Upper Left) Frontal view of a honey bee brain. MB, mushroom body; VL, vertical lobe. The white arrows indicate the sites (VL) of bilateral injections of the anesthetic procaine (or saline solution for control bees). (Scale bar: 250 μm.) (Upper Right) Harnessed honey bee exhibiting the proboscis extension response (PER) and licking a toothpick imbibed in sucrose solution. (Lower) Sequence of the PP experiment. The black arrow at time 0 indicates the moment of procaine injection (saline solution for controls). (B, Left) Percentage of conditioned PER of a group of bees injected with saline solution (controls) in response to unrewarded pure odorants (pooled curve A−/B−, white squares) and to a rewarded compound (AB+, black squares) during five blocks of acquisition trials. Saline-injected bees learned to respond significantly more to the compound AB+ than to its unrewarded elements (A−/B−). (Right) Percentage of conditioned PER of a group of bees injected with procaine in response to unrewarded pure odorants (pooled curve A−/B−, white circles) and to a rewarded compound (AB+, black circles) during five blocks of acquisition trials. Procaine-injected bees did not learn the PP discrimination. (C) Performance (% conditioned PER) in the last block of conditioning trials of a PP discrimination (last block of training in B). Although control bees (Left; n = 43) learned the discrimination between AB+ and A−/B−, procaine-injected bees (Right; n = 37) were unable to learn it. ***P < 0.001. (D) Performance (% conditioned PER) in the last block of conditioning trials of an elemental differential conditioning (CD+ vs. A−/B−). Both control (Left; n = 37) and procaine-injected bees (Right; n = 39) learned the discrimination between CD+ and A−/B−. ***P < 0.001.
Fig. S1.
Procaine injections in the optic lobes do not impair olfactory patterning discrimination. (A, Upper Left) Frontal view of a honey bee brain. OL, optic lobes. The white arrows indicate the sites of bilateral injections of the anesthetic procaine (or saline solution for control bees). (Scale bar: 250 μm.) (Lower) Sequence of the positive patterning (PP) experiment. The black arrow at time 0 indicates the moment of procaine (saline solution for controls) injection. (B) Performance (% conditioned PER) in the last block of conditioning trials of a PP discrimination (AB+ vs. A−/B−). Both saline-injected control bees (Left; Z56,1 = 2.49, P < 0.05; n = 56) and procaine-injected bees (Right; Z57,1 = 3.60, P < 0.0005; n = 57) learned the discrimination (Wilcoxon test; *P < 0.05; ***P < 0.001) and did so equally well (Mann–Whitney test, U > 359.0, NS for both stimuli).
Fig. S2.
Procaine injections into the mushroom body lobes impair configural retrieval. To verify that procaine blocks mushroom body output when injected into the vertical lobes, we checked that bees injected either with saline or procaine immediately after conditioning differ in their ability to recall configural information later. (A, Upper Right) Frontal view of a honey bee brain. MB, mushroom body; VL, vertical lobe. The white arrows indicate the sites (VL) of bilateral injections of the anesthetic procaine (or saline solution for control bees). (Scale bar: 250 μm.) (Lower) Sequence of the positive patterning (PP) experiment. The black arrow at time 0 indicates the moment of procaine (saline solution for controls) injection, which in this case occurred after conditioning and before retrieval. (B) Performance (% conditioned PER) in the last block of conditioning trials of a PP discrimination and in a retrieval test performed postinjection of saline solution. Bees learned the discrimination between AB+ and A−/B− (Z30,1 = 4.17, P < 0.005; n = 30) and retrieved efficiently the learned information 20 min after saline injection (Z30,1 = 3.62, P = 0.0005; n = 30). (C) Performance (% conditioned PER) in the last block of conditioning trials of a PP discrimination and in a retrieval test performed postinjection of procaine solution. Although bees learned the discrimination between AB+ and A−/B− (Z32,1 = 3.68, P < 0.005; n = 32), they were unable to retrieve the learned information 20 min after procaine injection (Z32,1 = 1.10, NS; n = 32). Wilcoxon test, ***P < 0.005.
Fig. S3.
Procaine injections into the mushroom body lobes, but not into the calyces, impair elemental retrieval in a durable way. To verify the localization and the duration of action of procaine within the mushroom bodies, we injected procaine either into the vertical lobes or into the calyces of bees previously trained in an elementary conditioning (A+). Control bees trained similarly were injected with saline solution into the lobes. Retrieval was tested 1 h, 2 h, and 3 h after injection. (A, Upper Right) Frontal view of a honey bee brain. MB, mushroom body; VL, vertical lobe; Cal., calyces. The white arrows indicate the sites of bilateral injections of the anesthetic procaine (VL or Cal., depending of the experimental group) or saline (for controls). (Scale bar: 250 μm.) (Lower) Sequence of experiment. The black arrow at time 0 indicates the moment of procaine injection, which in this case, occurred after absolute conditioning and before three consecutive retrieval tests, 1 h apart from each other. (B) Performance of bees (expressed as % of the conditioned response level at the end of conditioning, for each condition) in retrieval tests after conditioning. Although procaine injection into the calyces (n = 25) had no impact on recall compared with saline-injected controls (n = 28), it led to a significant impairment of recall when injected into the lobes (n = 23), which lasted at least 3 h compared with the two other groups (vs. saline, 1 h, χ2 = 10.00, P < 0.005; 2 h, χ2 = 6.65, P < 0.01; 3 h, χ2 = 27.22, P < 0.005; vs. procaine into the calyces, 1 h, χ2 = 32.92, P < 0.005; 2 h, χ2 = 8.60, P < 0.05; 3 h, χ2 = 17.15, P < 0.0001). *P < 0.05; ***P < 0.005.
The inability to solve positive patterning in the absence of functional MBs may be due to the nonelemental nature of this discrimination. However, bees could have used an elemental strategy to solve this task. If they learned the elements A and B per se, the associative strength of each nonreinforced element may not be sufficient to elicit a response whereas their sum could reach the response threshold. In this case, the reinforced compound AB would elicit behavioral responses through summation (32, 48), and the animals would solve positive patterning trough elemental learning. To test this hypothesis and the derived conclusion that MBs would be required for any elemental learning task, we trained procaine- and saline-injected bees in an elemental discrimination task in which they had to differentiate between the same single, nonreinforced odorants (A−, B−) and a rewarded compound made of two new odorants different from A and B (CD+). This task preserved the features of positive patterning (nonrewarded odorants vs. rewarded compound) but was purely elemental and unambiguous because each odorant was predictable in terms of its positive or negative outcome. Procaine- and saline-injected bees performed equally well (Fig. 1D): No group difference was detected (U > 164.0, NS for both stimuli), and both responded significantly more to CD+ than to A− and B− in the last block of trials (saline, Z37,1 = 4.00, P < 0.0001; procaine, Z39,1 = 3.42, P < 0.001). Thus, solving this elemental discrimination was possible under MB blockade. We thus conclude that bees used different strategies to solve the elemental differential conditioning and the positive patterning tasks. Intact MB function was required only for acquiring the latter whereas it was dispensable for acquiring an elemental discrimination.
Learning of Negative Patterning Is Impaired Under Blockade of Mushroom Bodies.
Although positive patterning admits pure elemental interpretations, it is not the case for negative patterning (A+, B+ vs. AB−) (32, 48): Because each single element is reinforced, elemental summation would result in the compound AB eliciting twice as much responding as each component. As a consequence, an animal would never show lower responses to the nonreinforced compound than to the components. Negative patterning is therefore an appropriate protocol to determine whether performance impairment in procaine-injected bees does indeed reflect a failure in nonelemental computations.
We thus next studied the requirement of MBs for learning a negative patterning discrimination (Fig. 2A). Saline-injected bees solved the task (Fig. 2B, Left) and responded more to A+ and B+ than to AB− at the end of acquisition (sixth block) (Z43,1 = 3.18, P < 0.005) (Fig. 2C). Procaine-injected bees, on the contrary, did not learn the discrimination at the end of training and maintained equally low response levels to all three stimuli (Z45,1 = 0.27, NS) (Fig. 2B, Right and 2C). As a result, the two groups differed in their response levels to the rewarded elements (U = 688.5, P < 0.05) but responded at similarly low levels to the unrewarded compound (U = 944.0, NS).
Fig. 2.
Mushroom body blockade impairs negative patterning (NP). (A, Upper Left) Frontal view of a honey bee brain. MB, mushroom body; VL, vertical lobe. The white arrows indicate the sites (VL) of bilateral injections of the anesthetic procaine (or saline solution for control bees). (Scale bar: 250 μm.) (Lower) Sequence of the NP experiment. The black arrow at time 0 indicates the moment of procaine (saline solution for controls) injection. (B, Left) Percentage of conditioned PER of a group of bees injected with saline solution (controls) in response to rewarded pure odorants (pooled curve A+/B+, black squares) and to an unrewarded compound (AB−, white squares) during six blocks of acquisition trials. Saline-injected bees learned to respond significantly more to the odorants A+ and B+ than to its unrewarded compound (AB−). (Right) Percentage of conditioned PER of a group of bees injected with procaine in response to rewarded pure odorants (pooled curve A+/B+, black circles) and to an unrewarded compound (AB−, white circles) during six blocks of acquisition trials. Procaine-injected bees did not learn the NP discrimination. (C) Performance (% conditioned PER) in the last (sixth) block of conditioning trials of an NP discrimination (last block of training in B). Although control bees (Left; n = 43) learned the discrimination between AB− and A+/B+, procaine-injected bees (Right; n = 45) were unable to learn it. ***P < 0.005. (D) Performance (% conditioned PER) in the last block of conditioning trials of an elemental differential conditioning (CD− vs. A+/B+). Both control (Left; n = 37) and procaine-injected bees (Right; n = 39) learned the discrimination between CD+ and A−/B−. ***P < 0.001.
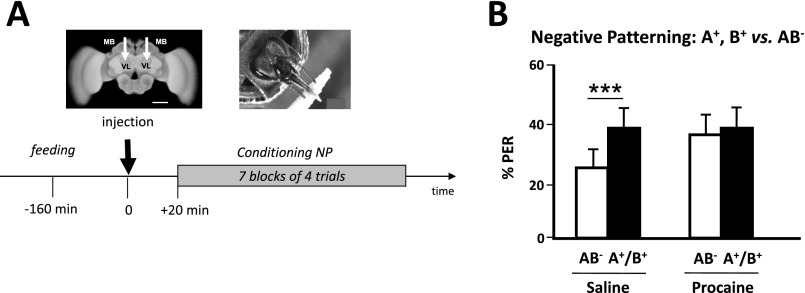
Considering the difficulty of the negative patterning task, we tested the possibility that the absence of MB function caused by procaine could simply delay learning instead of impairing it. To do so, we trained procaine-injected bees in a longer version of the negative patterning task, which included an additional block of trials to increase the possibility for bees to learn the task (Fig. S4A). Still, these bees did not learn the discrimination whereas saline-injected bees did (Fig. S4B). Thus, MB blockade via procaine injection resulted in an effective impairment of negative patterning.
Fig. S4.
Negative patterning cannot be solved upon mushroom body blockade despite increased training. To verify that mushroom blockade by procaine impairs and not only delays acquisition of a negative (NP) patterning discrimination, we added another block of trials during training, thus totaling to seven blocks instead of 6 (see Fig. 2). (A, Upper Left) Frontal view of a honey bee brain. MB, mushroom body; VL, vertical lobe. The white arrows indicate the sites (VL) of bilateral injections of the anesthetic procaine (or saline solution for control bees). (Scale bar: 250 μm.) (Lower) Sequence of the NP experiment in which seven blocks of trials were used during training. The black arrow at time 0 indicates the moment of procaine (saline solution for controls) injection. (B) Performance (% conditioned PER) in the last (seventh) block of conditioning trials of an NP discrimination. Although control bees (Left) learned the discrimination between AB− and A+/B+ (Z44,1 = 2.45, P < 0.00; n = 44), procaine-injected bees (Right) were unable to learn it despite the additional block of trials (Z44,1 = 0.45, NS; n = 44). ***P < 0.005.
As for positive patterning, we trained another group of bees in an olfactory discrimination that preserved the features of negative patterning (two rewarded odorants vs. one nonrewarded compound) without raising ambiguity at the level of the single odorants (A+, B+ vs. CD−). In this case, both saline-injected and procaine-injected bees performed equally well (U > 164.5, NS) and ended up discriminating the elements from the compound (saline, Z37,1 = 3.72, P < 0.0005; procaine, Z39,1 = 4.06, P < 0.00005) (Fig. 2D). Thus, purely elemental differential conditioning was not impaired by MB blockade. Our results therefore show that MBs are dispensable for elemental olfactory discriminations but are required for the acquisition of nonelemental olfactory discriminations.
Blocking GABAergic Signaling in the Mushroom Body Calyces Impairs Patterning Discrimination.
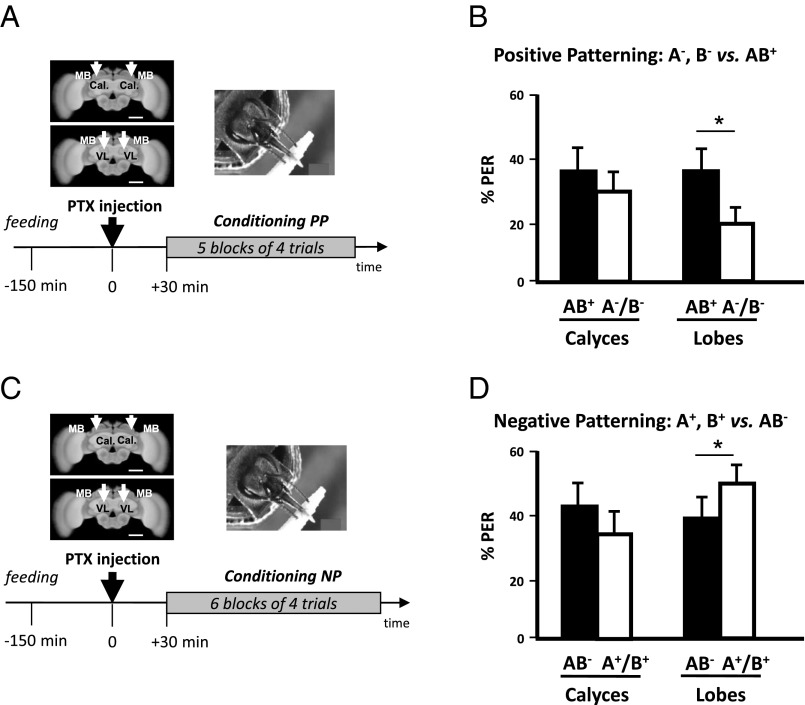
To gain insights into the neural mechanisms involved in nonelemental learning, we focused on GABAergic feedback neurons (39), which provide inhibitory signaling to the MBs of the honey bee (40–43). Two main tracks innervate the MB lobes and feedback either onto the calyces (A3v neurons) or onto the lobes themselves (A3d neurons). We reasoned that these circuits might be crucial to inhibit linear responses to odorants during patterning learning. We thus blocked GABAergic signaling in the MBs by locally injecting picrotoxin (PTX), an efficient GABA antagonist (49), into the calyces or into the lobes. Convergence between the olfactory and the reward pathways exists at the level of the calyces but not of the lobes (50); we thus hypothesized that feedback inhibition from the lobes to the calyces (39, 40–43, 51) might be crucial for nonelemental learning (48).
Fig. 3 shows that learning of both positive (Fig. 3 A and B) and negative patterning (Fig. 3 C and D) tasks was impaired when PTX was injected into the calyces (positive patterning, Z40,1 = 1.47, NS; negative patterning, Z43,1 = 1.49, NS) but was preserved when injections were done into the lobes (positive patterning, Z39,1 = 2.48, P < 0.05; negative patterning, Z54,1 = 2.16, P < 0.05). Thus, GABAergic input to the MBs calyces, but not the lobes, is crucial for the acquisition of patterning tasks.
Fig. 3.
Blocking GABAergic signaling in the mushroom body calyces impairs patterning discrimination. (A, Upper Left) Frontal view of a honey bee brain. MB, mushroom body; VL, vertical lobe; Cal., calyces. The white arrows indicate the sites (VL or Cal.) of bilateral injections of the GABAergic antagonist picrotoxin. (Scale bar: 250 μm.) (Lower) Sequence of the PP experiment. The black arrow at time 0 indicates the moment of picrotoxin injection. (B) Performance (% conditioned PER) in the last (fifth) block of conditioning trials of the PP discrimination. Bees injected in the MB calyces (Left, n = 40) were unable to learn the discrimination between AB+ and A−/B−, contrary to those injected in the vertical lobes (Right; n = 39). (C) Same experiment as in A, with an NP task. (D) Performance (% conditioned PER) in the last (sixth) block of conditioning trials of the NP discrimination. Bees injected in the MB calyces (Left, n = 43) were unable to learn the discrimination between AB− and A+/B+, contrary to those injected in the vertical lobes (Right; n = 54). *P < 0.05.
Neural Processing of Odorants Is Not Impaired Under Procaine Blockade of Mushroom Bodies.
We have shown that bees with both MBs blocked by procaine injection are still capable of learning elemental olfactory discriminations (e.g., A−, B− vs. CD+ or A+,B+ vs. CD−), suggesting that prior stages of the olfactory circuit provided the neural computations necessary for elemental but not for nonelemental discriminations. However, procaine injection might have induced subtle defects in the neural representation of odorants, thereby impairing the correct processing (and learning) of the stimuli by the MBs and possibly accounting for the observed deficits.
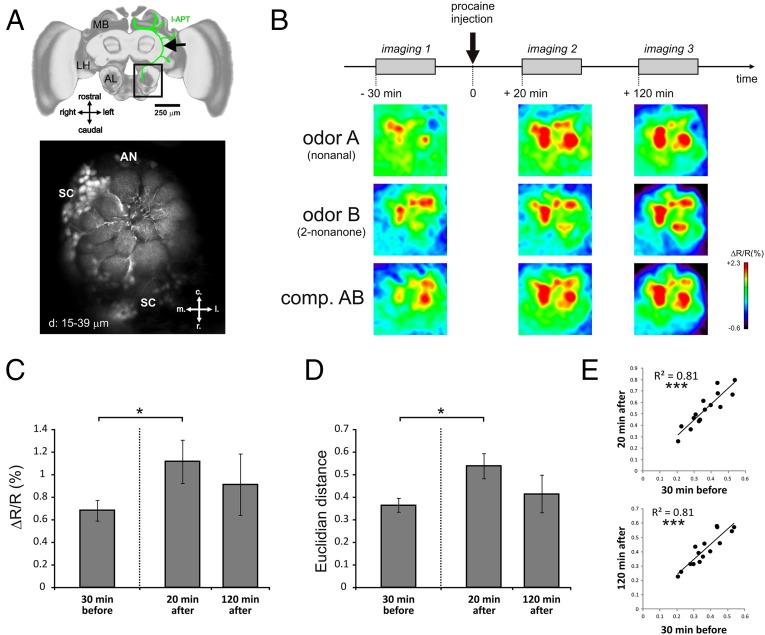
To control for this possibility, we performed optophysiological recordings of neural odor representations at the level of projection neurons (PNs): i.e., just before they reach the MBs. PNs are second-order olfactory neurons (equivalent to mitral/tufted cells in mammals) and convey olfactory information processed within the primary olfactory centers, the antennal lobes (ALs), to the MBs and the lateral horn (LH) (Fig. 4A). Thus, if procaine had undesirable effects on primary olfactory processing (for instance a defect in the separation—i.e., in the discriminability—between odorant representations), they should be measurable at the PN level. Odorant representations can be characterized as spatial activity maps across AL glomeruli, the anatomical and functional units of these olfactory centers, as revealed by functional imaging using fluorescent calcium probes (47). We quantified calcium activity in PNs upon stimulation with the single odorants A, B, C, and D and with the binary compounds AB and CD, before and after procaine injection into the MBs. Recordings were performed 30 min before (−30) and 20 min (+20) and 120 min (+120) after procaine injection (Fig. 4B). Recording times after injections were chosen to match the beginning of conditioning (+20) and the earliest observation of learning impairments (+120, i.e., during the fourth block—compare performances of saline and procaine-injected bees in Fig. 1B).
Fig. 4.
Mushroom body blockade does not impair olfactory coding in the antennal lobe. (A) Selective staining of projection neurons for calcium imaging. (Upper) Honey bee brain showing (in green) the innervation of projection neurons of the lateral tract (l-ALT) from the antennal lobe (AL) to the lateral horn (LH) and the mushroom body (MB) calyces. The location of the calcium-dye (Fura-2 dextran) injection is shown by a black arrow. (Scale bar: 250 μm.) (Lower) Typical retrograde staining of l-ALT projection neurons showing their dendrites in AL glomeruli. AN, antennal nerve location; c, caudal; l, lateral; m, medial; r, rostral; SC, PN somata cluster. d, 15–39 µm = Z-projections of optical slices at 15–39 µm depth. (B) Time course of imaging experiment: Bees were subjected to an imaging session 30 min before, 20 min after, and 120 min after procaine injection in the vertical lobes. Below the time course, typical activity maps are shown for the odorants A (nonanal), B (2-nonanone), and their binary mixture (AB) at the different time points of the experiment. Maps are displayed according to a false color code, from dark blue (no activity) to red (maximum activity)(see lateral bar % ΔR/R). (C) Average intensity (ΔR/R in %) of glomerular calcium responses to the odor panel in the three imaging sessions. A significant increase in response intensity was observed 20 min after injection of procaine (Wilcoxon matched-pairs test, P = 0.038, n = 9), which then returned to baseline 120 min after injection (P = 0.91, n = 6). (D) Average Euclidian distance (dissimilarity measure) between odor-response maps for all odor pairs of our panel. An increase in Euclidian distance (i.e., an increase in dissimilarity) was observed 20 min after injection of procaine (P = 0.011, n = 9), which then returned to baseline 120 min after injection (P = 0.46, n = 6). (E) Similarity relationships between odor response patterns are conserved before and after procaine injection. (Upper) Euclidian distances for all odor pairs are highly correlated 30 min before and 20 min after procaine injection (Pearson correlation, P < 0.001). (Lower) Euclidian distances are likewise highly correlated 30 min before and 120 min after procaine injection (P < 0.001). Error bars correspond to SEM.
At all three recording times, we found consistent and specific glomerular activity maps for each odorant and mixture tested (52) (Fig. 4B). This result constitutes an additional control for the local effect of procaine because diffusion of the drug from the vertical lobes toward the ALs should have determined a reduction of odor-evoked activity in these structures. Pre- and postinjection recordings differed only in the global intensity of activity, which increased after procaine injection (+20; Z5,1 = 1.99, P < 0.05) but then returned to initial levels (+120; Z5,1 = 0.11, NS) irrespective of the odorant considered (Fig. 4C). To further test for any disturbance of odor coding that might explain impaired configural learning, we calculated pixelwise Euclidian distances between the maps obtained for each pair of odorants. Euclidean distances are typical measures of the dissimilarity between odorant-induced activity maps in imaging studies (53–55). They are inversely related to the overlap between activity maps and thus provide an appropriate measure of the bees' capacity to distinguish between odorants (53). Had the procaine injection affected this capacity at the PN level (thus rendering learning difficult), this impairment should be revealed by reduced distances (i.e., increased similarity) between odorant response maps. This situation was never observed (Fig. 4D) because interodorant distances rather increased significantly after procaine injection (+20; Z5,1 = 1.99, P < 0.05) and then returned to basal levels at the time when procaine impaired configural learning (+120; Z5,1 = 0.73, NS). Distances between pairs of odorants before and after procaine injection were strongly correlated (in both cases, R = 0.81, P < 0.01) (Fig. 4E). Thus, odorant separability in the AL output was not impaired by procaine injection, and similarity relationships among odorants were conserved. Therefore, the bees’ inability to solve patterning tasks cannot be attributed to an alteration of olfactory input to the MBs; rather, it was due to MB blockade by procaine.
Discussion
Our results show for the first time, to our knowledge, that blocking the output from the MBs impairs the learning of two configural olfactory discriminations, positive and negative patterning, but not elemental olfactory discriminations. Thus, configural tasks require MB functional integrity. This conclusion adds a different perspective to our understanding of MB function because this structure has been mainly studied and characterized as a site for the encoding, storage, and retrieval of elemental forms of memory (e.g., simple odor–sucrose and odor–shock associations) (23–29). Prior work on cockroaches related MBs with visual place learning in a heat avoidance task (56). However, neurogenetic experiments using Drosophila in a similar task showed that the ellipsoid body, and not the MBs, is the neural structure mediating place learning (57). In the honey bee, MB extrinsic neurons with broad input arborizations at the level of the vertical lobe have the capacity to encode differently odor cues and the context defining their valence (58). This result and other results coupling electrophysiological recordings of MB extrinsic neurons with olfactory learning (see ref. 30 for review) provide valuable information about the mechanisms that may underlie nonelemental learning in the bee. However, they do not address the necessity of MBs for these tasks because they did not block neural activity upon learning. Thus, our results constitute the first demonstration, to our knowledge, that MBs are necessary for the acquisition of configural learning.
Patterning tasks are considered higher-order forms of associative learning due to their nonlinearity and intrinsic stimulus ambiguity (6, 8). They require the ability to establish configural associations: i.e., unique conjunctive representations of compound stimuli that can then be treated as different from the simple sum of their elements. Honey bees, like mammals, learn patterning discriminations using nonelemental strategies (32–34, 46), a capacity that seems absent in other insect models of learning and memory (31, 59). Prior work on patterning discriminations in bees focused on negative rather than on positive patterning (32–34) because elemental accounts exist for the latter but not for the former (32). Our results indicate, however, that both problems are solved by bees using nonelemental processing of odorants because MB blockade impaired the acquisition of both positive and negative patterning but preserved that of elemental discriminations between single odors A and B and a compound CD.
Our results do not question the established role of MBs in memory encoding, storing, and retrieval. When procaine was injected before retrieval and after acquisition, both elemental (Fig. S3) and nonelemental memories (Fig. S2) were impaired. Moreover, they also confirm the known functional distinction between the MB calyces and the MB lobes: In the honey bee (47), disruption of neurotransmission in the lobes impairs memory retrieval, as shown by Figs. S2 and S3, but not the acquisition of elemental discriminations, as shown in our case by the learning of the A,B vs. CD discriminations (Figs. 1 and 2). Contrary to Drosophila (for recent reviews see, for instance, refs. 60 and 61), in the honey bee, the convergence between the olfactory–CS and the appetitive–US pathways occurs at the level of the calyces but not of the lobes (50, 62). It is, therefore, understandable that targeting the lobes with procaine leaves acquisition unaffected in the case of an elemental discrimination. The fact that, on the contrary, it affected configural acquisition indicates that specific MB networks that arise from the MB lobes play an essential role for nonelemental learning.
In the honey bee, the configural representation of odor compounds has been analyzed in calcium imaging studies of neural activity at different stages of the olfactory circuit (63, 64). At the input to the AL (olfactory receptor neurons), olfactory compound representation is linear so that a compound is encoded as the sum of the representations of its odor components (63). Such linear coding does not facilitate the solving of patterning tasks so that compound uniqueness has to arise downstream. Recordings of PNs, which convey an olfactory message reshaped by AL processing to higher-order centers such as the MBs and the lateral horn, show a slight, yet not total, departure from linearity (64). Increased inhibition within the AL leads to more synthetic, less elemental mixture representation at the output level than at the input level. However, because olfactory coding remains mostly linear, the solving of patterning tasks is not facilitated, and compound uniqueness has to arise downstream of the AL. Because procaine injection did not impair PN signals (Fig. 4) but abolished the capacity to solve pattern discriminations, the synthetic, unique signature of olfactory compounds has to arise at the level of the MBs (48).
GABAergic feedback neurons of the MBs have been suggested as key elements for configural learning (48). Two subpopulations of these neurons are known, the A3v and A3d neurons, which provide inhibitory signaling from the lobes to the calyces and to the lobes themselves, respectively (39). Blocking of GABAergic signaling in the calyces, but not in the lobes, impaired patterning tasks (Fig. 3). These results argue in favor of a crucial role for GABAergic feedback to the MB calyces, and thus for the circuitry provided by A3v neurons, which precisely provide such feedback (39–43, 51). In Drosophila, GABAergic input to the MBs is provided by anterior paired lateral neurons, which seem to be presynaptic both to the calyces and lobes (65) so that they do not provide an inhibitory feedback signal to the MBs, contrary to A3v neurons of the bee. If such feedback is crucial for mastering patterning tasks, this difference in connectivity could account for the incapacity of fruit flies to solve a negative patterning discrimination (31).
In the absence of functional MBs, bees were able to learn elemental associations, thus indicating that alternative convergence sites between odor and sucrose pathways may suffice to mediate these simple forms of learning. In the bee, a convergence between odor and sucrose pathways also exists at the level of the AL and the LH because VUMmx1, the instructive neuron that signals the presence of sucrose reward in the bee brain, contacts the olfactory circuit at these stages besides that of the MBs (50). Functional and structural changes have been found in the ALs of adult bees after elemental olfactory learning in the laboratory (66–68) or shortly after emergence in the hive (69, 70). We thus suggest that, in the absence of functional MBs, elemental learning is still possible via the ALs. Moreover, because information processed in the AL is conveyed by PNs not only to the MBs but also to the LH, which is considered to be a premotor center, the information necessary to produce adaptive responses in elemental olfactory learning could be available in the absence of functional MBs.
In mammals, specific brain areas such as the hippocampus or the perirhinal cortex are required for configural learning whereas they seem to be dispensable for elemental learning (71–74). Our results show that, despite its different functional organization and evolutionary history, the insect brain follows a similar principle because distinct areas mediate the learning of problems of different complexity. Such a remarkable similarity may reflect convergence of the mechanisms used by neural systems for problem solving and provides further evidence of comparable functional principles underlying the organization of brains of very different sizes.
Materials and Methods
Animal Preparation.
Honey bee workers (A. mellifera) were caught upon leaving the entrance of the hive in the day of each experiment. They were immobilized on ice for 5 min and harnessed in individual metal tubes that allowed free movements of their antennae and mouthparts. A window was cut in the head cuticle (delimited by the compound eyes, the basal segment of the antennae, and the ocelli) to access the brain and perform local injections. Glands and tracheae were partially removed, leaving intact the neurilemma. After placing the piece of cuticle back in its original position to avoid brain desiccation, bees were fed with 20 μL of sucrose solution (50% wt/wt in water) and allowed to recover until injection in a dark and humid place, at room temperature.
Injections.
Local injections were performed at the level of the vertical lobes, the output region of the MBs, or, in control groups, between the median and lateral calyces, the main input regions of each MB. Procaine injections were performed 150 min after feeding: i.e., 30 min before conditioning, a delay that is sufficient to induce a blockade of neurotransmission that would last during conditioning (37) (in two control experiments, injections were performed immediately after the end of conditioning). In all cases, each animal received a bilateral injection, using a pulled glass capillary (GC 100–10; Harvard Apparatus) connected to a pressure microinjector (IM 300; Narishige). Procaine was dissolved in a saline solution that consisted of (in mmol/L) sucrose, 160; glucose, 25; Hepes, 10; MgCl2, 4; NaCl, 130; KCl, 6; CaCl2, 5 (pH 6.7, 500 mOsmol). A volume of 0.5 nL of either a 20% procaine solution or saline solution alone (control treatment) was injected within each vertical lobe (in a control experiment, injections were done bilaterally within each optic lobe). Methylene blue (2 mM) was added to both solutions to check that the capillary was not obstructed and that the solution actually penetrated into the tissue. In all cases, the staining was confined to the injected structure. After injection, the cuticle piece was put back on the head, and the animal was allowed to recover for approximately 20 min before starting the experiments. In a separate experiment, picrotoxin was injected following the same procedure, either in the vertical lobes or in the calyces of the MBs. The concentration used (5 µM) was chosen based on the dose–response values established for the MBs of the honey bee (49).
Conditioning Protocols.
In all experiments, saline- and procaine-injected bees were conditioned in parallel. Each conditioning trial lasted 40 s and started when the bee was placed in the conditioning setup, facing an odorless airflow. A 4-s odor stimulus was applied 15 s later by passing the airflow through two syringes, an empty syringe and a syringe containing a filter paper soaked with 4 µL of pure odorant [conditioned stimulus, CS). Whenever a binary mixture was used as CS, the airflow was also sent through two syringes, each containing one odorant. In rewarded CS presentations (CS+), both antennae were touched with a toothpick soaked with sucrose solution [unconditioned stimulus (US)], 3 s after odorant onset, to induce PER, followed by ingestion of sucrose solution. The duration of the US was 3 s with a 1-s overlap with the CS.
Bees (n = 37–57; see figure legends) were subjected to one of the following conditioning protocols: (i) In the positive patterning experiment, presentation of either of two pure odorants was not rewarded whereas their mixture (compound stimulus) was rewarded (A−, B−, AB+); (ii) in the negative patterning experiment, individual odorants were rewarded whereas their mixture was not (A+, B+, AB−). Each of these two groups had a control group (n = 32–39; see figure legends) using two additional odorants C and D (i.e., A−, B−, CD+ for positive patterning, and A+, B+, CD− for negative patterning).
The odorants used in positive and negative patterning were nonanal (A) and 2-nonanone (B), which are well discriminated by bees (53). The odorants used in the control groups were 1-hexanol (C) and 1-heptanal (D), which are both well distinguished from nonanal and 2-nonanone (53). All odorants were from Sigma.
Conditioning consisted of five blocks of four trials for positive patterning. Because negative patterning is a more difficult task for bees (32, 48), we included a sixth block of trials for negative patterning. In a control experiment for negative patterning, seven blocks were used. Each block included one presentation of each single odorant (A or B) and two presentations of their mixture AB. Thus, bees experienced 5 A−, 5 B−, and 10 AB+ in the positive patterning discrimination, and 6 A+, 6 B+, and 12 AB− in the negative patterning discrimination, so that positive and negative reinforcements were balanced in both cases. Throughout the procedure, the intertrial interval (interval between trials of a bloc) was 8 min. The sequence of presentations (e.g., A AB AB B) was constant over all blocks for each animal but balanced across animals. The control experiments were performed likewise, using CD instead of AB. After conditioning, bees were checked for their PER by applying sugar to their antennae; bees showing no PER were discarded (< 5%).
Calcium Imaging.
In vivo calcium recordings of the bee brain were performed under standard conditions, as detailed elsewhere (55). Briefly, bees were placed in recording chambers, and the head capsule was opened revealing the brain. Projection neurons of the lateral antennal lobe tract (l-ALT) were specifically stained with the calcium indicator Fura-2 dextran (potassium salt, 10,000 kDa, in 2% BSA; Life Technologies) using a glass electrode coated with dye crystals. The dye was inserted in this axonal path, between the vertical lobe and the border of the optic lobe, rostrally from the lateral horn. After staining, the brain was immersed in standard bee saline solution, and the bee was left in a moist and dark place for 3 h before imaging was performed.
Measurements were obtained using a T.I.L.L. Photonics imaging set up, under an epifluorescent microscope (Olympus BX-51WI) with a 10× water-immersion objective (UMPlanFL, N.A. 0.3; Olympus). Fura-2 was alternatively excited with 340 nm and 380 nm monochromatic light (T.I.L.L. Polychrom IV). Each measurement consisted of 100 double frames, at a rate of 5 Hz (interval between double frames, 200 ms), with 4 × 4 binning on chip (pixel image size corresponded to 4.8 μm × 4.8 μm). Integration time was 10–20 ms at 380 nm excitation and 40–80 ms at 340 nm excitation. Olfactory stimulation started at the 15th frame until the 20th frame, for 1 s.
Each bee was subjected to three imaging sessions, in which the four individual odorants (A–D), both binary mixtures (AB and CD), and an air control were presented in a random order. Thirty minutes after the start of the first session, bees received a procaine injection as above. After 20 min and 120 min, they were subjected to the second and third imaging sessions. Imaging data were analyzed using custom-made software written in IDL 6.4 (Research Systems Inc.) and following standard methods (55). The calcium response to each stimulation was calculated as the average of three frames during odor presentation (frames 17–19) minus the average of three frames just before stimulus delivery (frames 12–14). These responses are shown in a color code from dark blue to red in the activity maps. For analysis, a mask was precisely drawn around the AL of each bee, and analysis was limited to the unmasked region. To analyze the intensity of odor-evoked responses, the average of the intensity of all pixels in the unmasked area was calculated for all presented stimuli. Evaluation of the similarity relationships between neural representations was assessed pixelwise, using a Euclidian metric (measure of dissimilarity) (50).
Data Transformation and Statistical Analysis.
Because the response levels to A and B were overall equivalent, both in positive and negative patterning, the results were pooled and the responses presented as a CS+ vs. CS− discrimination. For each four-trial block (and retrieval test, whenever performed), we quantified the percentage of bees showing a conditioned response (% PER) separately in successive rewarded trials (omitting the randomly interspersed unrewarded trials) and in successive unrewarded trials (omitting the randomly interspersed rewarded trials). Data shown in all graphs are means ± SEM. To check whether bees significantly differentiated between the elements and the compound within a group, responses were compared using a Wilcoxon matched-pair test. Performances between groups, both for the elements and the compound, were performed using a Mann–Whitney test. In the imaging experiments, within-group differences in the intensity of odor responses or in Euclidian distance between odor response maps were evaluated using Wilcoxon matched-pairs tests. Statistical tests were performed with Statistica 7.0 (Statsoft).
Acknowledgments
We thank two anonymous reviewers for constructive criticisms, and Georges di Scala and Alain Marchand for suggestions to improve earlier versions of the manuscript. Special thanks are due to Harald Lachnit for much inspiration and years of fruitful collaboration in the study of nonelemental learning, and to Jonathan Ceccom and Elodie Urlacher for help with preliminary negative-patterning experiments. We also thank Andrew Barron for critical reading of the manuscript. This work was funded by a Deutscher Akademischer Austausch Dienst/French Government PROCOPE grant (to B.G. and M.G.). M.G. thanks the Institut Universitaire de France, the Human Frontier Science Program (Grant RGP022/2014), and the French Research Agency (ANR) (Project MINICOG, Grant 13-BSV4-0004-01). J.-C.S. thanks the ANR (Project EVOLBEE, Grant 2010-BLAN-1712-01). J.-M.D. and M.G. also thank the CNRS and the Université Paul Sabatier.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1508422112/-/DCSupplemental.
References
- 1.Rudy JW, Sutherland RJ. Configural and elemental associations and the memory coherence problem. J Cogn Neurosci. 1992;4(3):208–216. doi: 10.1162/jocn.1992.4.3.208. [DOI] [PubMed] [Google Scholar]
- 2.Pearce JM, Bouton ME. Theories of associative learning in animals. Annu Rev Psychol. 2001;52:111–139. doi: 10.1146/annurev.psych.52.1.111. [DOI] [PubMed] [Google Scholar]
- 3.Delamater AR, Sosa W, Katz M. Elemental and configural processes in patterning discrimination learning. Q J Exp Psychol. 1999;52B:97–124. [Google Scholar]
- 4.Rescorla RA, Wagner AR. A theory of classical conditioning: variations in the effectiveness of reinforcement and non-reinforcement. In: Black AH, Prokasy WF, editors. Classical Conditioning II: Current Research and Theory. Appleton-Century-Crofts; New York: 1972. pp. 64–99. [Google Scholar]
- 5.Giurfa M. Cognitive neuroethology: Dissecting non-elemental learning in a honeybee brain. Curr Opin Neurobiol. 2003;13(6):726–735. doi: 10.1016/j.conb.2003.10.015. [DOI] [PubMed] [Google Scholar]
- 6.Sutherland RJ, Rudy JW. Configural association theory: The role of the hippocampal formation in learning, memory, and amnesia. Psychobiology (Austin Tex) 1989;17(2):129–144. [Google Scholar]
- 7.Pearce JM. Similarity and discrimination: A selective review and a connectionist model. Psychol Rev. 1994;101(4):587–607. doi: 10.1037/0033-295x.101.4.587. [DOI] [PubMed] [Google Scholar]
- 8.Rudy JW, Sutherland RJ. Configural association theory and the hippocampal formation: an appraisal and reconfiguration. Hippocampus. 1995;5(5):375–389. doi: 10.1002/hipo.450050502. [DOI] [PubMed] [Google Scholar]
- 9.O’Reilly RC, Rudy JW. Computational principles of learning in the neocortex and hippocampus. Hippocampus. 2000;10(4):389–397. doi: 10.1002/1098-1063(2000)10:4<389::AID-HIPO5>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 10.Rudy JW, O’Reilly RC. Conjunctive representations, the hippocampus, and contextual fear conditioning. Cogn Affect Behav Neurosci. 2001;1(1):66–82. doi: 10.3758/cabn.1.1.66. [DOI] [PubMed] [Google Scholar]
- 11.O’Reilly RC, Rudy JW. Conjunctive representations in learning and memory: Principles of cortical and hippocampal function. Psychol Rev. 2001;108(2):311–345. doi: 10.1037/0033-295x.108.2.311. [DOI] [PubMed] [Google Scholar]
- 12.Whitlow JW, Wagner AR. Negative patterning in classical conditioning: Summation of response tendencies to isolable and configural components. Psychon Sci. 1972;27:299–301. [Google Scholar]
- 13.Lachnit H, Kimmel HD. Positive and negative patterning in human classical skin conductance response conditioning. Anim Learn Behav. 1993;21(4):314–326. [Google Scholar]
- 14.Stupien G, Florian C, Roullet P. Involvement of the hippocampal CA3-region in acquisition and in memory consolidation of spatial but not in object information in mice. Neurobiol Learn Mem. 2003;80(1):32–41. doi: 10.1016/s1074-7427(03)00022-4. [DOI] [PubMed] [Google Scholar]
- 15.Dusek JA, Eichenbaum H. The hippocampus and memory for orderly stimulus relations. Proc Natl Acad Sci USA. 1997;94(13):7109–7114. doi: 10.1073/pnas.94.13.7109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rudy JW, O’Reilly RC. Contextual fear conditioning, conjunctive representations, pattern completion, and the hippocampus. Behav Neurosci. 1999;113(5):867–880. doi: 10.1037//0735-7044.113.5.867. [DOI] [PubMed] [Google Scholar]
- 17.Bannerman DM, et al. Contextual fear conditioning is disrupted by lesions of the subcortical, but not entorhinal, connections to the hippocampus. Exp Brain Res. 2001;141(3):304–311. doi: 10.1007/s002210100869. [DOI] [PubMed] [Google Scholar]
- 18.Rudy JW, Barrientos RM, O’Reilly RC. Hippocampal formation supports conditioning to memory of a context. Behav Neurosci. 2002;116(4):530–538. doi: 10.1037//0735-7044.116.4.530. [DOI] [PubMed] [Google Scholar]
- 19.Kumaran D, et al. Impaired spatial and non-spatial configural learning in patients with hippocampal pathology. Neuropsychologia. 2007;45(12):2699–2711. doi: 10.1016/j.neuropsychologia.2007.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Giurfa M. Cognition with few neurons: Higher-order learning in insects. Trends Neurosci. 2013;36(5):285–294. doi: 10.1016/j.tins.2012.12.011. [DOI] [PubMed] [Google Scholar]
- 21.Giurfa M. Behavioral and neural analysis of associative learning in the honeybee: A taste from the magic well. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2007;193(8):801–824. doi: 10.1007/s00359-007-0235-9. [DOI] [PubMed] [Google Scholar]
- 22.Avarguès-Weber A, Giurfa M. Conceptual learning by miniature brains. Proc Biol Sci. 2013;280(1772):20131907. doi: 10.1098/rspb.2013.1907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Busto GU, Cervantes-Sandoval I, Davis RL. Olfactory learning in Drosophila. Physiology (Bethesda) 2010;25(6):338–346. doi: 10.1152/physiol.00026.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Davis RL. Traces of Drosophila memory. Neuron. 2011;70(1):8–19. doi: 10.1016/j.neuron.2011.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Giurfa M, Sandoz JC. Invertebrate learning and memory: Fifty years of olfactory conditioning of the proboscis extension response in honeybees. Learn Mem. 2012;19(2):54–66. doi: 10.1101/lm.024711.111. [DOI] [PubMed] [Google Scholar]
- 26.Menzel R. Memory dynamics in the honeybee. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 1999;185:323–340. [Google Scholar]
- 27.Heisenberg M. Mushroom body memoir: From maps to models. Nat Rev Neurosci. 2003;4(4):266–275. doi: 10.1038/nrn1074. [DOI] [PubMed] [Google Scholar]
- 28.Margulies C, Tully T, Dubnau J. Deconstructing memory in Drosophila. Curr Biol. 2005;15(17):R700–R713. doi: 10.1016/j.cub.2005.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Keene AC, Waddell S. Drosophila olfactory memory: Single genes to complex neural circuits. Nat Rev Neurosci. 2007;8(5):341–354. doi: 10.1038/nrn2098. [DOI] [PubMed] [Google Scholar]
- 30.Menzel R. The insect mushroom body, an experience-dependent recoding device. J Physiol Paris. 2014;108(2-3):84–95. doi: 10.1016/j.jphysparis.2014.07.004. [DOI] [PubMed] [Google Scholar]
- 31.Young JM, Wessnitzer J, Armstrong JD, Webb B. Elemental and non-elemental olfactory learning in Drosophila. Neurobiol Learn Mem. 2011;96(2):339–352. doi: 10.1016/j.nlm.2011.06.009. [DOI] [PubMed] [Google Scholar]
- 32.Deisig N, Lachnit H, Giurfa M, Hellstern F. Configural olfactory learning in honeybees: Negative and positive patterning discrimination. Learn Mem. 2001;8(2):70–78. doi: 10.1101/lm.8.2.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Deisig N, Lachnit H, Giurfa M. The effect of similarity between elemental stimuli and compounds in olfactory patterning discriminations. Learn Mem. 2002;9(3):112–121. doi: 10.1101/lm.41002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Deisig N, Lachnit H, Sandoz JC, Lober K, Giurfa M. A modified version of the unique cue theory accounts for olfactory compound processing in honeybees. Learn Mem. 2003;10(3):199–208. doi: 10.1101/lm.55803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schubert M, Lachnit H, Francucci S, Giurfa M. Nonelemental visual learning in honeybees. Anim Behav. 2002;64:175–184. [Google Scholar]
- 36.Giurfa M, Schubert M, Reisenman C, Gerber B, Lachnit H. The effect of cumulative experience on the use of elemental and configural visual discrimination strategies in honeybees. Behav Brain Res. 2003;145(1-2):161–169. doi: 10.1016/s0166-4328(03)00104-9. [DOI] [PubMed] [Google Scholar]
- 37.Devaud JM, Blunk A, Podufall J, Giurfa M, Grünewald B. Using local anaesthetics to block neuronal activity and map specific learning tasks to the mushroom bodies of an insect brain. Eur J Neurosci. 2007;26(11):3193–3206. doi: 10.1111/j.1460-9568.2007.05904.x. [DOI] [PubMed] [Google Scholar]
- 38.Müller D, Staffelt D, Fiala A, Menzel R. Procaine impairs learning and memory consolidation in the honeybee. Brain Res. 2003;977(1):124–127. doi: 10.1016/s0006-8993(03)02760-4. [DOI] [PubMed] [Google Scholar]
- 39.Rybak J, Menzel R. Anatomy of the mushroom bodies in the honey bee brain: The neuronal connections of the alpha-lobe. J Comp Neurol. 1993;334(3):444–465. doi: 10.1002/cne.903340309. [DOI] [PubMed] [Google Scholar]
- 40.Bicker G, Schäfer S, Kingan TG. Mushroom body feedback interneurones in the honeybee show GABA-like immunoreactivity. Brain Res. 1985;360(1-2):394–397. doi: 10.1016/0006-8993(85)91262-4. [DOI] [PubMed] [Google Scholar]
- 41.Gronenberg W. Anatomical and physiological properties of feedback neurons of the mushroom bodies in the bee brain. Exp Biol. 1987;46(3):115–125. [PubMed] [Google Scholar]
- 42.Grünewald B. Physiological properties and response modulations of mushroom body feedback neurons during olfactory learning in the honeybee Apis mellifera. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 1999;185:565–576. [Google Scholar]
- 43.Grünewald B. Morphology of feedback neurons in the mushroom body of the honeybee, Apis mellifera. J Comp Neurol. 1999;404(1):114–126. doi: 10.1002/(sici)1096-9861(19990201)404:1<114::aid-cne9>3.3.co;2-r. [DOI] [PubMed] [Google Scholar]
- 44.Bitterman ME, Menzel R, Fietz A, Schäfer S. Classical conditioning of proboscis extension in honeybees (Apis mellifera) J Comp Psychol. 1983;97(2):107–119. [PubMed] [Google Scholar]
- 45.Matsumoto Y, Menzel R, Sandoz JC, Giurfa M. Revisiting olfactory classical conditioning of the proboscis extension response in honey bees: A step toward standardized procedures. J Neurosci Methods. 2012;211(1):159–167. doi: 10.1016/j.jneumeth.2012.08.018. [DOI] [PubMed] [Google Scholar]
- 46.Komischke B, Sandoz JC, Lachnit H, Giurfa M. Non-elemental processing in olfactory discrimination tasks needs bilateral input in honeybees. Behav Brain Res. 2003;145(1-2):135–143. doi: 10.1016/s0166-4328(03)00105-0. [DOI] [PubMed] [Google Scholar]
- 47.Lozano VC, Armengaud C, Gauthier M. Memory impairment induced by cholinergic antagonists injected into the mushroom bodies of the honeybee. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2001;187(4):249–254. doi: 10.1007/s003590100196. [DOI] [PubMed] [Google Scholar]
- 48.Lachnit H, Giurfa M, Menzel R. Odor processing in honeybees: Is the whole equal to, more than, or different from the sum of its parts? Adv Stud Behav. 2004;34(34):241–264. [Google Scholar]
- 49.Boitard C, Devaud J-M, Isabel G, Giurfa M. GABAergic feedback signaling into the calyces of the mushroom bodies enables olfactory reversal learning in honey bees. Front Behav Neurosci. 2015;9:198. doi: 10.3389/fnbeh.2015.00198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hammer M. An identified neuron mediates the unconditioned stimulus in associative olfactory learning in honeybees. Nature. 1993;366:59–63. doi: 10.1038/366059a0. [DOI] [PubMed] [Google Scholar]
- 51.Haehnel M, Menzel R. Sensory representation and learning-related plasticity in mushroom body extrinsic feedback neurons of the protocerebral tract. Front Syst Neurosci. 2010;4:161. doi: 10.3389/fnsys.2010.00161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Joerges J, Küttner A, Galizia CG, Menzel R. Representation of odours and odour mixtures visualized in the honeybee brain. Nature. 1997;387:285–288. [Google Scholar]
- 53.Guerrieri F, Schubert M, Sandoz JC, Giurfa M. Perceptual and neural olfactory similarity in honeybees. PLoS Biol. 2005;3(4):e60. doi: 10.1371/journal.pbio.0030060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Carcaud J, Hill T, Giurfa M, Sandoz JC. Differential coding by two olfactory subsystems in the honeybee brain. J Neurophysiol. 2012;108(4):1106–1121. doi: 10.1152/jn.01034.2011. [DOI] [PubMed] [Google Scholar]
- 55.Roussel E, Carcaud J, Combe M, Giurfa M, Sandoz J-C. Olfactory coding in the honeybee lateral horn. Curr Biol. 2014;24(5):561–567. doi: 10.1016/j.cub.2014.01.063. [DOI] [PubMed] [Google Scholar]
- 56.Mizunami M, Weibrecht JM, Strausfeld NJ. Mushroom bodies of the cockroach: Their participation in place memory. J Comp Neurol. 1998;402(4):520–537. [PubMed] [Google Scholar]
- 57.Ofstad TA, Zuker CS, Reiser MB. Visual place learning in Drosophila melanogaster. Nature. 2011;474(7350):204–207. doi: 10.1038/nature10131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hussaini SA, Menzel R. Mushroom body extrinsic neurons in the honeybee brain encode cues and contexts differently. J Neurosci. 2013;33(17):7154–7164. doi: 10.1523/JNEUROSCI.1331-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sommerlandt FMJ, Roessler W, Spaethe J. Elemental and non-elemental olfactory learning using PER conditioning in the bumblebee, Bombus terrestris. Apidologie (Celle) 2014;45(1):106–115. [Google Scholar]
- 60.Waddell S. Reinforcement signalling in Drosophila: Dopamine does it all after all. Curr Opin Neurobiol. 2013;23(3):324–329. doi: 10.1016/j.conb.2013.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Guven-Ozkan T, Davis RL. Functional neuroanatomy of Drosophila olfactory memory formation. Learn Mem. 2014;21(10):519–526. doi: 10.1101/lm.034363.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hammer M, Menzel R. Multiple sites of associative odor learning as revealed by local brain microinjections of octopamine in honeybees. Learn Mem. 1998;5(1-2):146–156. [PMC free article] [PubMed] [Google Scholar]
- 63.Deisig N, Giurfa M, Lachnit H, Sandoz JC. Neural representation of olfactory mixtures in the honeybee antennal lobe. Eur J Neurosci. 2006;24(4):1161–1174. doi: 10.1111/j.1460-9568.2006.04959.x. [DOI] [PubMed] [Google Scholar]
- 64.Deisig N, Giurfa M, Sandoz JC. Antennal lobe processing increases separability of odor mixture representations in the honeybee. J Neurophysiol. 2010;103(4):2185–2194. doi: 10.1152/jn.00342.2009. [DOI] [PubMed] [Google Scholar]
- 65.Liu X, Davis RL. The GABAergic anterior paired lateral neuron suppresses and is suppressed by olfactory learning. Nat Neurosci. 2009;12(1):53–59. doi: 10.1038/nn.2235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rath L, Giovanni Galizia C, Szyszka P. Multiple memory traces after associative learning in the honey bee antennal lobe. Eur J Neurosci. 2011;34(2):352–360. doi: 10.1111/j.1460-9568.2011.07753.x. [DOI] [PubMed] [Google Scholar]
- 67.Faber T, Joerges J, Menzel R. Associative learning modifies neural representations of odors in the insect brain. Nat Neurosci. 1999;2(1):74–78. doi: 10.1038/4576. [DOI] [PubMed] [Google Scholar]
- 68.Hourcade B, Perisse E, Devaud JM, Sandoz JC. Long-term memory shapes the primary olfactory center of an insect brain. Learn Mem. 2009;16(10):607–615. doi: 10.1101/lm.1445609. [DOI] [PubMed] [Google Scholar]
- 69.Arenas A, Giurfa M, Farina WM, Sandoz JC. Early olfactory experience modifies neural activity in the antennal lobe of a social insect at the adult stage. Eur J Neurosci. 2009;30(8):1498–1508. doi: 10.1111/j.1460-9568.2009.06940.x. [DOI] [PubMed] [Google Scholar]
- 70.Arenas A, et al. Early olfactory experience induces structural changes in the primary olfactory center of an insect brain. Eur J Neurosci. 2012;35(5):682–690. doi: 10.1111/j.1460-9568.2012.07999.x. [DOI] [PubMed] [Google Scholar]
- 71.Moses SN, Cole C, Driscoll I, Ryan JD. Differential contributions of hippocampus, amygdala and perirhinal cortex to recognition of novel objects, contextual stimuli and stimulus relationships. Brain Res Bull. 2005;67(1-2):62–76. doi: 10.1016/j.brainresbull.2005.05.026. [DOI] [PubMed] [Google Scholar]
- 72.Bussey TJ, Saksida LM, Murray EA. Perirhinal cortex and feature-ambiguous discriminations. Learn Mem. 2006;13(2):103–105, author reply 106–107. doi: 10.1101/lm.163606. [DOI] [PubMed] [Google Scholar]
- 73.Bussey TJ, Saksida LM, Murray EA. Perirhinal cortex resolves feature ambiguity in complex visual discriminations. Eur J Neurosci. 2002;15(2):365–374. doi: 10.1046/j.0953-816x.2001.01851.x. [DOI] [PubMed] [Google Scholar]
- 74.Eichenbaum H. Is the rodent hippocampus just for ‘place’? Curr Opin Neurobiol. 1996;6(2):187–195. doi: 10.1016/s0959-4388(96)80072-9. [DOI] [PubMed] [Google Scholar]