The emergence of the Isthmus of Panama left a major imprint on the biodiversity of the Americas. The connection between South and North America facilitated dispersal of terrestrial and freshwater organisms, while separating marine species between the eastern Pacific and Caribbean seas. Recent geological data have questioned the long-standing view of a Pliocene emergence of the Isthmus (1) and show that the Central American Seaway, defined as the deep oceanic seaway along the tectonic boundary of the South American plate and Panama arc, was already closed by 15–13 Ma (2). Caribbean–Pacific shallow water exchange probably continued, albeit intermittently, until a full closure at 3.5 Ma (1–3). Recently Bacon et al. (3) used molecular and fossil data to evaluate the timing, tempo, and directionality of biotic exchange and vicariance across the Isthmus, and tested whether biological data are congruent with recent geological evidence. Significant increases in terrestrial dispersals were found at ca. 20 and 6 Ma, and increases in marine vicariance at ca. 23 and 7 Ma. Similar patterns prevailed despite intrinsic differences among the taxonomic groups surveyed. This led Bacon et al. (3) to reject the assumption of a single closure of the Isthmus at ca. 3.5 Ma in favor of an older, more complex model of land emergence and biotic interchange.
Letters to the Editor by Lessios (4) and Marko et al. (5) point to the possibility that the results and conclusions presented by Bacon et al. (3) are compromised by the underlying data used in the analyses, particularly with respect to marine taxa. We address their concerns and reanalyze the original and new data, showing that our results are robust to the issues raised and our conclusions remain unchanged.
Lessios (4) describes cases of excluded data from his (6) and other previous studies. An exhaustive compilation of trans-Isthmian biogeographic events was beyond the scope of our paper, in which we aimed to produce a reliable and adequately sized dataset for the statistical analyses performed (3). In the data presented by Lessios (6), we strictly used the Cytochrome c oxidase subunit 1 because it was the most completely sampled gene for which we could apply a standard molecular clock. This made results directly comparable across taxa but required the exclusion of redundant data (other genes from the single mitochondrial coalescent unit), shorter fragments, and nuclear genes. During data compilation, some Cytochrome c oxidase subunit 1 sequences (table 4 in ref. 6) were inadvertently excluded, but the other claimed errors concern marine sister (not strictly geminate) species that are not found on either side of the Isthmus, according to the original distribution maps (7, 8), or derive from conflicting dates between Lessios* (4) and the original literature.
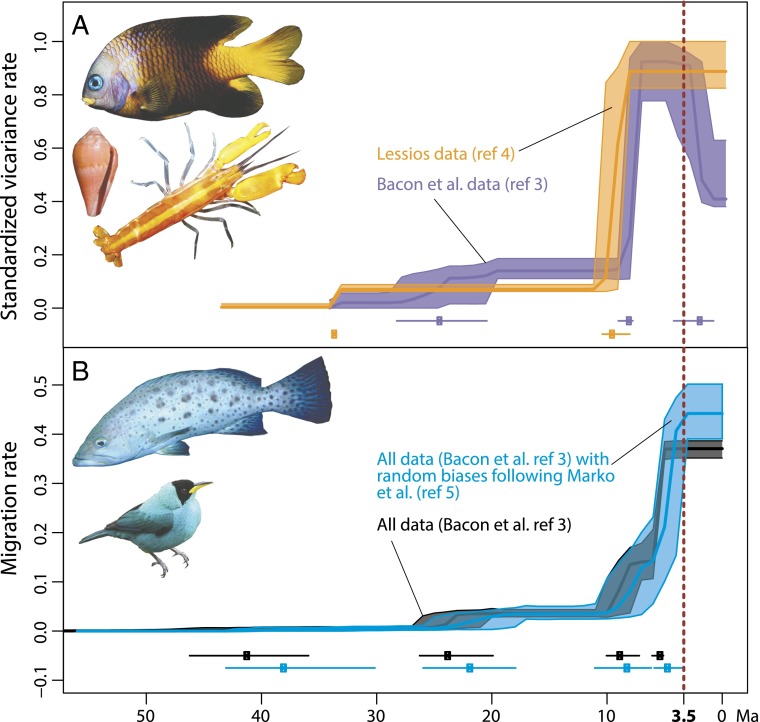
We performed and compared migration (vicariance) through time plots using both the original (3) dataset and that compiled by Lessios (4). Results obtained from Lessios’ data (4) recovered the same drastic increase in marine vicariance at ca. 9 Ma that we previously reported (Fig. 1A) (3). Lessios’ data (4) also recovered the early, albeit smaller increase in marine vicariance in the Oligocene (3), but erased the significant decrease in vicariance at ca. 2 Ma originally reported (3).
Fig. 1.
Biotic movements through time across the Isthmus of Panama. Plots show a comparison of results presented by Bacon et al. (3) with the results from new analyses that account for issues raised by Lessios (4) and Marko et al. (5). (A) Rate of events separating marine organisms between the Caribbean and the eastern Pacific ocean, inferred from the data compiled by Bacon et al. (3) and the dataset provided by Lessios* (4), where rates were standardized in a range (0,1) to facilitate comparison. (B) Biogeographic events inferred from the analysis of all data (including both marine and terrestrial organisms) reproduced from Bacon et al. (3), compared with migration rates based on 1,000 randomized datasets accounting for the potential systematic bias postulated by Marko et al. (5). In both A and B, statistically significant rate shifts are indicated below the rate curves together with their 95% confidence interval. These analyses show that substantial biotic interchange across the Isthmus of Panama started millions of years earlier than commonly assumed (3.5 Ma; the dashed line in both graphs) and in distinct phases. Our original conclusions (3) therefore remain robust, despite concerns about missing data and biological biases. All images are of organisms that migrated across the Isthmus of Panama region. Images courtesy of STRI database.†
Marko et al. (5) express concerns about the potential effects of extinction, incomplete taxon sampling, and use of single genes in molecular clock analyses, suggesting these issues cause an overestimation in the timing of biogeographical events. In our paper we demonstrate the robustness of our results against dating errors through simulations with normally distributed random errors (3), but as correctly pointed out by Marko et al. (5), these simulations do not capture the potential bias deriving from systematically overestimated ages of biogeographic events. Although we cannot know the magnitude of this bias, we repeated these simulations but modeled the error based on a skew-normal distribution, to bias the biogeographic events toward younger ages, thus reflecting the scenario postulated by Marko et al. (5). Every event age m was randomized from a skew-normal distribution with location = m, scale = 0.25 × m, and shape = −6.5. Although location and scale are equivalent to our previous simulations (see equation S4 in ref. 3), the strongly negative shape parameter ensures that the randomized migration events become younger than the original value with ca. 95% probability (>40% of the randomized ages were between 20% and 75% younger than the original). Results from 1,000 simulations (Fig. 1B) remained highly consistent with those presented originally (3), indicating that the estimated trends in migration rates are robust even in the face of potential systematic overestimation. An arguably larger source of dating error is fossil calibration. Calibrations are typically defined as minimum ages for a node, thus fossil-calibrated phylogenies (i.e., the majority of all phylogenies included in our study) should be, if anything, biased toward younger splitting events rather than older.
Although a migration analysis for marine organisms based solely on fossil data would assess the inferences from molecular data as we did for mammals (3), there is still a marked overrepresentation of Caribbean fossils compared with records from the eastern Pacific for the Cenozoic.‡ Similarly, the increasing production of new multilocus datasets will further improve inferences on divergence times and biogeographic history across the Isthmus, but such data remain scarce. All datasets must therefore be considered incomplete because of taxonomic and spatial biases in sampling, undescribed biodiversity, and local as well as global extinction. Biogeographic studies need to be designed to address these biases.
In summary, the analyses presented here indicate that the findings reported by Bacon et al. (3) are robust to the issues raised (4, 5). Recent efforts have shed light on the geological formation of the Isthmus of Panama and its impact on the region’s biodiversity, climate, and oceanic circulation (9, 10). However, many intriguing aspects remain to be fully understood, such as why organisms varied in migration time, which ecosystems dominated through time, and how sea level and climatic changes affected the Great American Biotic Interchange (11).
Acknowledgments
Funding for this work was provided by the Swedish Research Council (B0569601); the European Research Council under the European Union’s Seventh Framework Programme (FP/2007-2013, ERC Grant 331024); and a Wallenberg Academy fellowship (to A.A., C.D.B., and D.S.).
Footnotes
The authors declare no conflict of interest.
‡E.g., https://paleobiodb.org.
References
- 1.Coates AG, Stallard RF. How old is the Isthmus of Panama. Bull Mar Sci. 2013;89(4):801–814. [Google Scholar]
- 2.Montes C, et al. Middle Miocene closure of the Central American Seaway. Science. 2015;348(6231):226–229. doi: 10.1126/science.aaa2815. [DOI] [PubMed] [Google Scholar]
- 3.Bacon CD, et al. Biological evidence supports an early and complex emergence of the Isthmus of Panama. Proc Natl Acad Sci USA. 2015;112(19):6110–6115. doi: 10.1073/pnas.1423853112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lessios HA. Appearance of an early closure of the Isthmus of Panama is the product of biased inclusion of data in the metaanalysis. Proc Natl Acad Sci USA. 2015;112:E5765. doi: 10.1073/pnas.1514719112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Marko PB, Eytan RI, Knowlton N. Do large molecular sequence divergences imply an early closure of the Isthmus of Panama? Proc Natl Acad Sci USA. 2015;112:E5766. doi: 10.1073/pnas.1515048112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lessios HA. The Great American Schism: Divergence of marine organisms after the rise of the Central American Isthmus. Annu Rev Ecol Evol Syst. 2008;39(1):63–91. [Google Scholar]
- 7.Claremont M, Williams ST, Barraclough TG, Reid DG. The geographic scale of speciation in a marine snail with high dispersal potential. J Biogeogr. 2011;38(6):1016–1032. [Google Scholar]
- 8.Williams ST, Reid DG. Speciation and diversity on tropical rocky shores: A global phylogeny of snails of the genus Echinolittorina. Evolution. 2004;58(10):2227–2251. doi: 10.1111/j.0014-3820.2004.tb01600.x. [DOI] [PubMed] [Google Scholar]
- 9.Bacon CD, Mora A, Wagner WL, Jaramillo CA. Testing geological models of evolution of the Isthmus of Panama in a phylogenetic framework. Bot J Linn Soc. 2013;171(1):287–300. [Google Scholar]
- 10.Sepulchre P, et al. Consequences of shoaling of the Central American Seaway determined from modeling Nd isotypes. Paleoceanography. 2014;29(3):176–189. [Google Scholar]
- 11.Molnar P. Closing of the Central American Seaway and the Ice Age: A critical review. Paleoceanography. 2008;23(2):PA2201. [Google Scholar]