Significance
ATP, the fuel of life, is produced in living cells by a complex molecular machine consisting of two motors linked by a rotor. One motor generates rotation by consuming energy derived from oxidative metabolism or photosynthesis; the other uses energy transmitted by the rotor to put ATP molecules together from their building blocks, ADP and phosphate. One such intact machine from the α-proteobacterium Paracoccus denitrificans has been induced to form crystals, providing the means of deducing a blueprint of the machine, giving details of how its components are organized, and providing insights into how it works. The mechanistic principles deduced from the bacterial machine apply to similar molecular machines found in all living organisms.
Keywords: Paracoccus denitrificans, ATP synthase, structure, regulation, proton translocation
Abstract
The structure of the intact ATP synthase from the α-proteobacterium Paracoccus denitrificans, inhibited by its natural regulatory ζ-protein, has been solved by X-ray crystallography at 4.0 Å resolution. The ζ-protein is bound via its N-terminal α-helix in a catalytic interface in the F1 domain. The bacterial F1 domain is attached to the membrane domain by peripheral and central stalks. The δ-subunit component of the peripheral stalk binds to the N-terminal regions of two α-subunits. The stalk extends via two parallel long α-helices, one in each of the related b and b′ subunits, down a noncatalytic interface of the F1 domain and interacts in an unspecified way with the a-subunit in the membrane domain. The a-subunit lies close to a ring of 12 c-subunits attached to the central stalk in the F1 domain, and, together, the central stalk and c-ring form the enzyme’s rotor. Rotation is driven by the transmembrane proton-motive force, by a mechanism where protons pass through the interface between the a-subunit and c-ring via two half-channels in the a-subunit. These half-channels are probably located in a bundle of four α-helices in the a-subunit that are tilted at ∼30° to the plane of the membrane. Conserved polar residues in the two α-helices closest to the c-ring probably line the proton inlet path to an essential carboxyl group in the c-subunit in the proton uptake site and a proton exit path from the proton release site. The structure has provided deep insights into the workings of this extraordinary molecular machine.
The ATP synthases (F-ATPases) found in eubacteria, chloroplasts, and mitochondria are multiprotein molecular machines with a rotary action that provide most cellular ATP. Our understanding of how they work has come mainly from single-molecule studies of rotation made almost entirely on bacterial F-ATPases (1) and from structures of their constituent domains determined predominantly with enzymes from mitochondria (2–5). The most complete high-resolution structure contains about 85% of the bovine F-ATPase, built up from substructures determined by X-ray crystallography (2), within the constraints of an overall structure determined by cryo-EM (6). This model provides many details about the catalytic mechanism of the F1 domain (2, 5); its mode of inhibition by the natural inhibitor protein of F1-ATPase, IF1 (2, 4); and the design of the rotor, an ensemble of a membrane-bound ring of eight c-subunits, and the elongated central stalk, which penetrates into the catalytic F1 domain. However, it lacks a crucial region that would help to explain how the enzyme uses the transmembrane proton-motive force produced by respiration or photosynthesis to generate the turning of the rotor in its membrane domain and other features that keep the proton-motive force coupled to the synthesis of ATP.
Few structural studies have been carried out on the F-ATPases from eubacteria. Their subunit compositions are simpler than the subunit compositions of mitochondrial enzymes (7–9). They contain the same or analogous eight or nine core subunits that constitute the catalytic domain, rotor, and stator, but they lack the six or more supernumerary membrane subunits of the mitochondrial enzyme that have no known role in catalysis (2). Structures have been described of the F1 domains of the enzymes from Escherichia coli (10, 11), Caldalkalibacillus thermarum (12), and Geobacillus stearothermophilus (formerly Bacillus PS3) (13); of the α3β3-subcomplex of the F1 domain from G. stearothermophilus (14); and of isolated c-rings from the rotors of several species (15–19). There is also structural information on the peripheral stalk region of the F-ATPase from E. coli and on the N-terminal domain of the δ-subunit and its interaction with the N-terminal region of the α-subunit (20) and segments of the b-subunit (21–23).
Many attempts have been made to crystallize intact F-ATPases, as a prelude to structural analysis, without success until the recent crystallization of the F-ATPase from Paracoccus denitrificans (24). This enzyme can only synthesize ATP, and inhibition of hydrolysis involves the ζ-inhibitor protein, found only in α-proteobacteria. As described here, the structure of the inhibited complex has been determined at 4.0 Å resolution. It reveals new features about the mechanism of inhibition by the ζ-protein and about the coupling of the proton-motive force to the synthesis of ATP.
Results and Discussion
Structure Determination.
The structure of the P. denitrificans F-ATPase–ζ-inhibitor complex was determined by molecular replacement at 4.0 Å resolution. The asymmetrical unit of the crystals contains one inhibited complex. The data processing and refinement statistics are summarized in Table S1. The final model (Fig. 1) contains the following residues (where E, TP, and DP denote the subunits comprising the empty, diphosphate-containing, and triphosphate-containing catalytic interfaces, respectively): αE, 2–190 and 196–511; αTP, 7–193, 198–405, and 411–511; αDP, 28–511; βE, 3–468; βTP, 4–469; βDP, 2–471; γ, 3–62, 64–73, 78–110, 115–143, 147–166, 170–199, and 212–289; δ, 5–114; ε, 9–83; subunit a, 35 residues in aH3 and aH4 modeled as poly-Ala (residues 1,001–1,035), aH5 (residues 166–198), and aH6 (residues 217–246); and each c-subunit in the c12-rotor ring (3–76). Also, it contains five segments of secondary structure that are not assigned to any specific subunit, defined as follows: chain V, residues 1,001–1,078 (probably either subunit b or b′); W, residues 1,001–1,124 (subunit b or b′); Y, residues 1,001–1,054 (two antiparallel transmembrane α-helices); 1, residues 1,001–1,020 (subunit δ or αDP); 2, residues 1,001–1,015 (subunit δ or αDP or b or b′); and 3, residues 1,001–1,019 (an α-helix parallel to the plane of the membrane). The structure also contains two additional α-helical segments containing residues 1–32 and 82–103 of the ζ-inhibitor. The nucleotide binding sites in the catalytic βDP- and βTP-subunits and the noncatalytic αTP- and αDP-subunits each contain ATP-Mg, and the nucleotide binding site in the αE-subunit contains ADP-Mg. Neither substrates nor products are associated with the βE-subunit.
Table S1.
Data collection and refinement statistics
| Space group | P21 |
| Unit cell dimensions a, b, c; Å | 112.0, 187.9, 164.7 |
| Resolution range, Å | 3.97–36.84 |
| High-resolution bin, Å | 3.97–4.08 |
| No. of unique reflections | 57,347 |
| Multiplicity | 9.5 (8.3) |
| Completeness, % | 98.3 (87.9) |
| Rmerge* | 0.253 (1.604) |
| <I/σ(I)> | 7.7 (1.9) |
| B factor from Wilson plot,† Å | 155.9 |
| R factor,† % | 29.6 |
| Free R factor,‡ % | 32.4 |
| rmsd of bonds, Å | 0.008 |
| rmsd of angles, ° | 1.17 |
Parentheses denote the statistics for the high-resolution bin.
, where I(h) is the mean weighted intensity after rejection of outliers.
, where Fobs and Fcalc are the observed and calculated structure factor amplitudes, respectively.
, where Fobs and Fcalc are the observed and the calculated structure factor amplitudes, respectively, and T is the test set of data omitted from refinement.
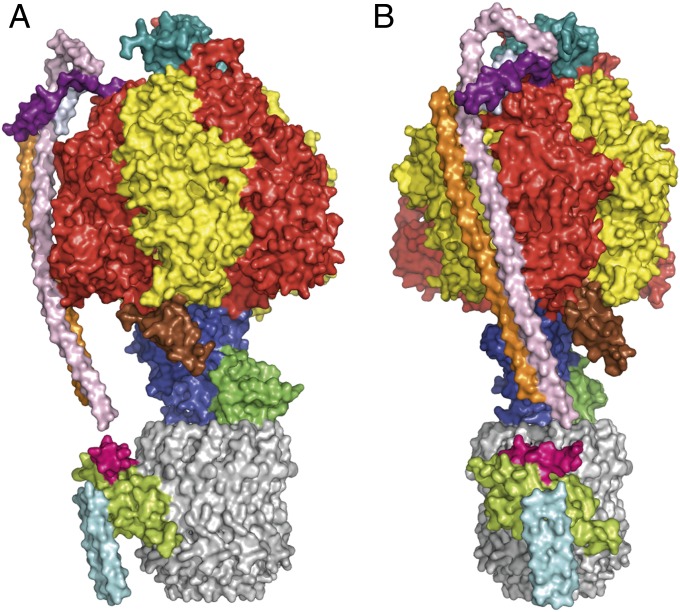
Fig. 1.
Structure of the complex of the F-ATPase from P. denitrificans with the bound ζ-inhibitor protein. (A and B) Side views of the enzyme–inhibitor complex in surface representation. B is rotated right by 90° relative to A. (Upper) Membrane extrinsic F1 catalytic domain (red, yellow, blue, and green corresponding to three α-subunits, three β-subunits, and single γ- and ε-subunits, respectively). In the peripheral stalk, the δ-subunit (top) is sky blue and the long and approximately parallel α-helical segments in orange and pink (chains V and W) extending down the surface of the interface between the α- and β-subunits are parts of the b- and b′-subunits (undistinguished). Unassigned α-helical segments (chains 1 and 2) in the vicinity of the junction between the δ-subunit and b- and b′-subunits are purple and light gray, respectively. Helix-1 of the ζ-inhibitor is brown. (Lower) In the membrane domain, the ring of 12 c-subunits is gray and a bundle of four resolved α-helices assigned to the a-subunit is lemon green. An unassigned α-helical segment in magenta (chain 3) lies approximately parallel to α-helices in subunit a, and two unassigned side-by-side transmembrane α-helices (chain Y) are colored light blue.
Mode of Binding of the ζ-Inhibitor.
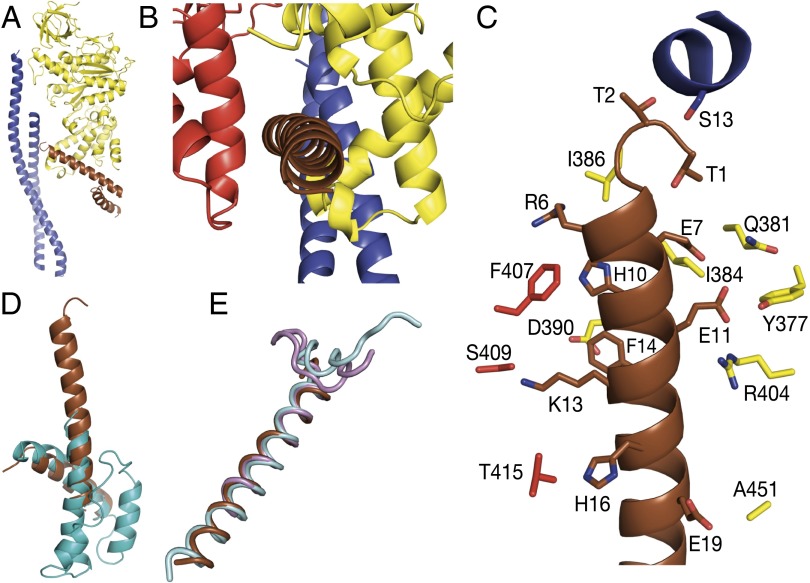
The inhibitor is bound to the F1 domain via residues 1–19 of the N-terminal α-helix, which occupy a cleft in the lower region of the αDPβDP-catalytic interface (Fig. 2 A and B), with the rest of the α-helix (residues 20–32) extending from the surface of the enzyme. Residues 1 and 2 are close to residue Ser13 in the N-terminal α-helix of the γ-subunit, along the central axis of the F1 domain. Residues 3–19 probably form polar and hydrophobic interactions with other residues in α-helices in the C-terminal domains of the αDP- and βDP-subunits (Fig. 2C and Table S2). α-Helix 4 (residues 82–103) is also resolved, stabilized by contacts with α-helix 1 and the αDP-subunit (Table S3). In solution, residues 1–18 of the 107-aa chain of the ζ-inhibitor are unstructured, with the rest of the chain folded into a four-helix bundle (residues 19–42, 46–53, 66–77, and 81–103) (25). A complete fold of the ζ-inhibitor was constructed from the combined solution and crystal structures (Fig. 2D).
Fig. 2.
Mode of binding of the ζ-inhibitor to the F-ATPase from P. denitrificans. The inhibitor is bound in the αDPβDP-catalytic interface of the enzyme. (A) Cross-sectional side view of the F1 domain showing the interaction of the ζ-inhibitor protein (brown) with the C-terminal domain of the βDP-subunit (yellow) and the coiled-coil of α-helices in the γ-subunit (blue). (B) View from outside the complex toward the αDPβDP-catalytic interface with the N-terminal α-helix of the ζ-inhibitor in a cleft between the αDP- and βDP-subunits. (C) Potential interactions between side chains of the ζ-inhibitor protein with residues in the αDP-, βDP-, and γ-subunits (Table S2). (D) Composite structure of the ζ-inhibitor by combination of residues 1–32 and 82–103 from the current study (brown) with residues 15–104 of the solution structure (cyan). (E) Superposition of the N-terminal region of the ζ-inhibitor (brown) with the corresponding inhibitory regions of IF1 from bovine and yeast mitochondria (cyan and pink, respectively). The α-helical regions are residues 21–49, 16–36, and 3–24, respectively.
Table S2.
Interactions of the ζ-inhibitor protein with the γ-, αDP-, and βDP-subunits of the F-ATPase from P. dentrificans
| ζ | γ | αDP | βDP |
| T1 | S13 | D382 | |
| T2 | R10, S13, V14 | ||
| R6 | F407 | I386 | |
| E7 | Q381, I384, A385 | ||
| A9 | A403, A404 | ||
| H10 | F407 | I384, A385, G388 | |
| E11 | Y377, I384, Q381, R404 | ||
| K13 | A404, S409, L411 | ||
| F14 | M389, D390 | ||
| A15 | R404, E450 | ||
| H16 | D412, T415 | ||
| E19 | D451 | ||
| L20 | D412 | ||
| F22 | H447, P449 |
Contacts ≤4.0 Å were calculated with CONTACT.
Table S3.
Interactions between the ζ-inhibitor with itself and with the αDP-subunit of the F-ATPase from P. denitrificans
| ζ | ζ | αDP |
| H16 | D412, T415 | |
| L20 | I99 | |
| N21 | R96 | |
| K23 | I99 | |
| A24 | I99 | |
| R27 | A95, Q98, I99 | |
| R28 | M88, L91, A95 | |
| L31 | L91, Q98 | |
| L32 | L91 | |
| I100 | D412 | |
| E102 | K417 |
Contacts ≤4.0 Å were calculated with CONTACT.
The N-terminal α-helix of the ζ-inhibitor protein is bound in a very similar way to how the inhibitory regions of bovine and yeast IF1 bind to their cognate F1-ATPases, and the structure of the bacterial F1 domain resembles the bovine and yeast F1-ATPases in their complexes with IF1. The rmsd values for α-carbon superpositions of the P. denitrificans F1 domain with the bovine and yeast F1 domains in the inhibited complexes (3, 26) are 1.3 Å and 1.7 Å, respectively. The sequences of the inhibitory regions of the inhibitor proteins are also related weakly (Fig. S1), and their structures are very similar (Fig. 2E). All three inhibitors occupy equivalent positions in the αDPβDP-catalytic interface, interacting with α-helices in the lower regions of their C-terminal domains.
Fig. S1.

Alignment of the N-terminal sequence of the ζ-inhibitor from P. denitrificans with the N-terminal sequences of F-ATPase inhibitor proteins bIF1 and yIF1 from bovine and yeast mitochondria, respectively. Green, red, blue, and ochre indicate residues that are polar but uncharged, acidic, basic, and hydrophobic, respectively.
The inhibitory regions of free bovine IF1 and the free ζ-inhibitor are intrinsically disordered (4, 25), and the inhibitory region of yeast IF1 is predicted to be so also (Table S4). It seems likely that the pathway of binding and folding of the inhibitory region of the ζ-inhibitor resembles the pathway of binding and folding of the inhibitory region of bovine IF1, where the disordered inhibitory region interacts initially with the αEβE-catalytic interface, the most open of the three catalytic interfaces, and closure of the interface is then driven by the hydrolysis of two ATP molecules first to the αTPβTP-catalytic state and then to the αDPβDP-catalytic state, accompanied by the progressive folding of the disordered region (4).
Table S4.
Regions of the ζ-inhibitor from P. denitrificans and of the ATPase inhibitor proteins bIF1 and yIF1 from bovine and yeast mitochondria predicted to be intrinsically disordered
| Inhibitor | PrDOS | DisEMBL |
| ζ | 1–14 | 1–23 |
| bIF1 | 1–24 | 1–26 |
| yIF1 | 1–21 | 1–23 |
The predictions were made with PrDOS and DisEMBL. bIF1, bovine IF1; yIF1, yeast IF1.
Connections with the Peripheral Stalk.
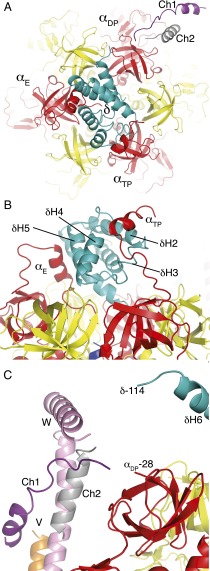
The main interactions between the peripheral stalk and the F1 domain of the enzyme involve the δ-subunit, which sits on top of the crown of the F1 domain (Fig. 3A). The N-terminal domain of the P. denitrificans δ-subunit is folded into a bundle of six α-helices, as in the E. coli δ-subunit (20) and the orthologous bovine oligomycin sensitivity conferral protein subunit, OSCP (27, 28). The N-terminal regions of α-subunits project from the top of the crown, and α-helices in the N-terminal regions of the αE- and αTP-subunits (residues 2–22 and 7–22, respectively) interact with α-helices δH1 (residues 8–27) and δH5 (residues 79–91), and δH2 (residues 30–45), δH3 (residues 47–53), and δH4 (residues 61–68), respectively; only the first interaction was observed in the structure of the bovine F1–peripheral stalk complex (28). The structure of the N-terminal region of the αDP-subunit is unclear. It appears to be in a region of contiguity of structural elements from the N terminus of the αDP-subunit and the C-terminal regions of the δ-, b-, and b′-subunits (Fig. 2C). Although they are not fully resolved in the current electron density map, this region involves the two unassigned element chains 1 and 2 that could be part of any one of the αDP-, δ-, b-, or b′-subunits (Fig. 2C). It appears that this partially resolved region is mobile, and it may act as a hinge or elbow connecting to the well-defined, and probably rigid, α-helical region of the peripheral stalk. This region is composed of the approximately parallel α-helices in the b- and b′-subunits, but in the current map, these subunits cannot be distinguished with certainty. As in the structure of the bovine F1–peripheral stalk complex, this extensive α-helical region is associated along the external surface of the noncatalytic αTPβDP-interface, and so this conformation of the F-ATPase appears to represent an abundant, probably low-energy, state that allows the enzyme to crystallize from the many structural conformers of the F-ATPase complex. The α-helical region of the peripheral stalk extends almost to the membrane domain, where the electron density again becomes difficult to interpret, suggesting that this region may also be flexible.
Fig. 3.
Interactions of the δ-subunit with N-terminal regions of α-subunits in the F-ATPase from P. denitrificans. (A) View from above the F-ATPase toward the “crown” of the F1 domain depicting the N-terminal regions of the αE-, αTP-, and αDP-subunits (red) with the δ-subunit (blue), β-subunits (yellow) and chains 1 and 2 (Ch1 and Ch2; purple and gray, respectively). (B) Side view of the interactions of the αE-subunit (residues 7–22) and the αTP-subunit (residues 2–22) with helices δH1 and δH5 and helices δH2, δH3, and δH4, respectively. (C) Side view of the region around the N-terminal part of the αDP-subunit with structural elements from peripheral stalk subunits.
Structure of the Membrane Domain.
The resolved structure of the membrane domain of the F-ATPase from P. denitrificans (Figs. 1 and 4A) consists of a c12-ring (gray); an associated bundle of four α-helices (green) with its axis tilted at about 30° to the plane of the membrane, with a fifth α-helix (magenta) sitting on top of the bundle close to the inner surface of the bacterial membrane; and two side-by-side α-helices (cyan) normal to the plane of the membrane. The c-ring is made of an inner ring of N-terminal α-helices and an outer ring of C-terminal α-helices, and the loops joining the helices make an extensive interface with the foot of the central stalk with a buried surface area of 522 Å2. Together, the c-ring and the central stalk constitute the rotor of the enzyme.
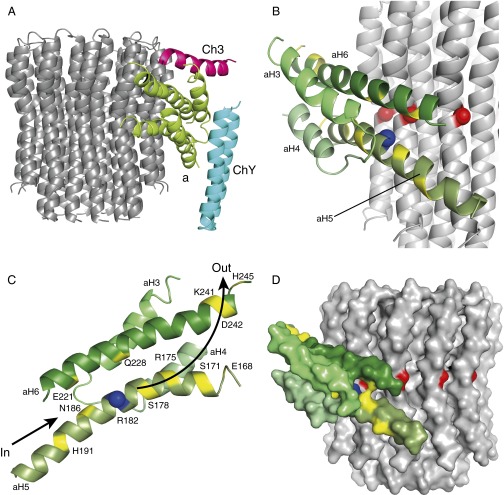
Fig. 4.
Topography of the membrane domain of the F-ATPase from P. dentrificans and a potential pathway of transmembrane proton translocation. (A) View of the c12-rotor ring, and an associated bundle of α-helices (green), assigned to the a-subunit and named aH3–aH4 (residues 1,001–1,035), and aH5 and aH6 containing residues 166–198 and 217–246, respectively (Figs. S2 and S3). Unassigned α-helix Ch3 and α-helical hairpin ChY are shown in magenta and blue, respectively. (B) View of the association of the tilted bundle of four α-helices in subunit a with the c-ring showing residue Glu60 (red) in the c-subunit in the proton transfer site and residue Arg182 (blue) in aH5. (C) View from the c-ring of the tilted bundle of four α-helices in subunit a showing conserved polar residues (yellow) that could provide the access path (In) for protons from the bacterial periplasm to reach the proton transfer site and the exit path (Out) for protons to be released into the bacterial cytoplasm. Residue Arg182 is colored blue. (D) View in solid representation of the tilted bundle of four α-helices in the a-subunit in juxtaposition with the c-ring showing the potential inlet pathway for protons (yellow) leading through the bundle to the proton transfer site containing a negatively charged Glu60 (red).
Based on the conservation of six hydrophobic segments in their sequences (Figs. S2 and S3), it is likely that the a-subunit has six transmembrane α-helices (aH1–aH6), whereas it has previously been considered to have five (corresponding to aH1–aH3, aH5, and aH6) (28). The four-helix bundle in the current structure has been attributed to α-helices aH3–aH6, where aH5 and aH6 consist of 29 and 32 residues, respectively, corresponding to the unusually long hydrophobic segments 5 and 6 in the sequences of a-subunits (Figs. S2 and S3). Segment 5 contains the absolutely conserved Arg residue Arg182, known from studies in E. coli to be essential for proton translocation through the membrane domain of the enzyme, and in the structure, as required, this residue is close to another essential residue Glu60 in the c-subunit (Fig. 4). Tilted α-helices aH3 and aH4 are shorter, as expected from the shorter hydrophobic sequence segments 3 and 4, and are packed close to aH5 and aH6. A tilted four-helix bundle has been observed also by cryo-EM, nominally at 7 Å resolution, in the membrane domain of the F-ATPase from Polytomella, but aH5 and aH6 in P. denitrificans were assigned as aH6 and aH5, respectively, in Polytomella (29).
Fig. S2.
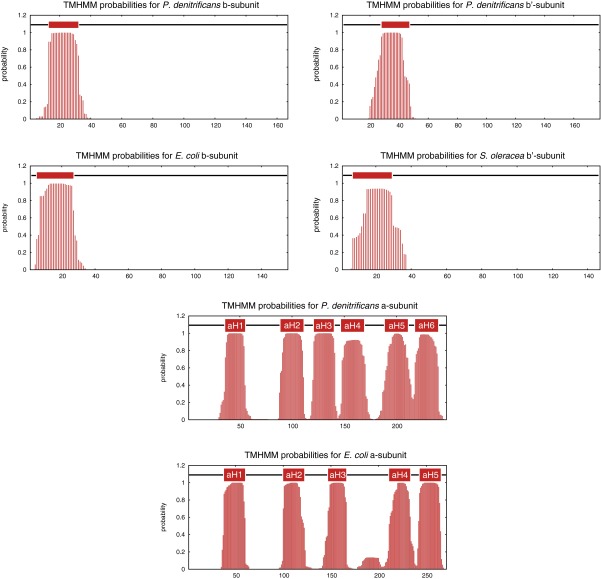
Predicted transmembrane α-helices in the b-, b′-, and a-subunits of the F-ATPase from P. denitrificans. For comparison, the predictions for b- and a-subunits from E. coli and for the b′-subunit from Spinacea oleracea are shown also. The predictions were calculated by TMHMM.
Fig. S3.
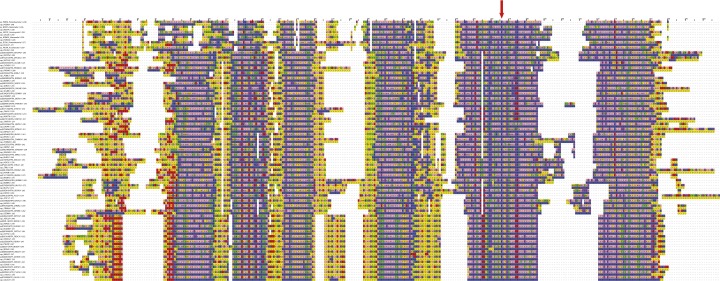
Alignment of sequences of a-subunits. The sequences cover a wide range of species. The arrow denotes the position of the Arg residue (Arg210 in E. coli, Arg182 in P. denitrificans) that is an essential part of the transmembrane proton translocation pathway.
The high conservation of the sequences of a-subunits in the regions of aH5 and aH6 suggests that their structures will be conserved also (Fig. S3), and because there was no significant side-chain density, the sequence register assigned to these α-helices was based on biochemical data. The tilted transmembrane α-helix closer to the periplasm was identified as aH5 (containing the essential Arg182), because this interpretation agrees better with cross-linking experiments between the E. coli a- and c-subunits (30). However, the reported cross-link between E. coli residues 55 in subunit c and 207 in subunit a (residues 54 in subunit c and 179 in subunit a in P. denitrificans) does not fit with this model, but the yield of this cross-link was significantly lower (11–20%) than the remainder (20–40%). Additional cross-links involving residues in E. coli aH5 (residues 239–260) also suggest that residues in the upper part of cH2 are closer to E. coli aH5 (P. denitrificans aH6) (31). Moreover, the placement of aH5 is consistent with the demonstration that residues Ser206 and Asn214 in E. coli (Ser178 and Asn186 in P. denitrificans) are accessible from the cytoplasm and periplasm, respectively (32). Finally, the lower α-helix (Fig. 4) is significantly more curved (by 20°) than the upper α-helix, and it is in close contact with the c-ring over three adjacent c-subunits. This enhanced curvature is consistent with the assignment of aH5 as the lower helix because of the presence of a Pro residue at position 176 (conserved as Pro or Ala in many sequences), whereas there is no Pro in aH6 in P. denitrificans or in an appropriate region of aH5 in E. coli (the only Pro is at position 240). Crucially, this model of aH5 in the α-helical density places the essential Arg182 residue close to the essential carboxylate of Glu60 in the c-subunit. Moreover, it defines the position of the many polar residues N-terminal to Arg182, with their side chains occupying a narrow cleft between the a-subunit and the c-ring that leads to the cytoplasm (Fig. 4). In the E. coli enzyme, the activity of the second site suppressor mutant, Arg210Gln-Gln252Arg, in the a-subunit suggests that Gln252 is near to Arg210 (30). Therefore, P. denitrificans aH6 was modeled with the equivalent Gln228 close to Arg182 in aH5. Although there is strong electron density for both aH5 and aH6, it is relatively featureless (i.e., sausage-like), and because of the absence of either clear side-chain density or clear α-helical features for the polypeptide backbone, it is possible that this part of the model could be in error by as much as 3–4 Å. Therefore, side chains in this region have been truncated to the β-carbon atoms. It is not possible to assign residue numbers reliably to the α-helical hairpin region of the a-subunit based on the available data, and this poly-Ala chain has been given residue numbers 1,001–1,035.
Currently, neither α-helices aH1 and aH2 nor single anticipated membrane α-helices in the N-terminal regions of subunits b and b′ have been assigned (Fig. S2). Thus, the three remaining resolved hydrophobic segments in Ch3 and ChY (Fig. 4) could represent any of these unassigned α-helices. At the current level of detail, it is not possible to know whether the loop between aH3 and aH4 lies close to ChY or whether the loop (residues 199–216) between aH5 and aH6 extends to the periplasmic side of the membrane, as has been suggested in the E. coli protein (30).
Pathway of Transmembrane Proton Translocation.
In current models to explain the generation of rotation of the c-ring, during ATP synthesis (33), protons from the bacterial periplasm access a negatively charged carboxylate of a Glu60 residue in the interface region between the c-ring and the a-subunit. Once neutralized, driven by the proton-motive force, the neutralized carboxyl makes a rotary substep by moving anticlockwise, as viewed from the cytoplasmic side of the ring, into the hydrophobic environment of the membrane. Neutralization of further negatively charged carboxylates brought successively into the proton transfer site in the interface region generates further rotary substeps. Following an almost complete rotation of the ring, each neutralized carboxyl reenters the interface between subunit a and the c-ring, and becomes reionized in a process mediated by the essential Arg182, releasing the protons through a second half-channel leading to the bacterial cytoplasm. A series of conserved polar residues in helices aH5 and aH6 could be part of a sloping entry half-channel leading through the a-subunit to the proton transfer site, and other conserved polar residues in the same region could be part of the exit half-channel (Fig. 4 C and D). Many of the human pathogenic mutations in the human a-subunit are located in residues in the proposed proton inlet pathway (34).
Perspectives.
The current structure of the F-ATPase from P. dentrificans has provided new information about its mode of regulation by the ζ-inhibitor protein and about the association of the static a-subunit and the rotating c-ring, and the possible pathways by which the protons cross the membrane domain of the enzyme during the generation of rotation of the c-ring. To function, and for ATP synthesis to be coupled to the proton-motive force, the a-subunit has to be held in position against the rotating ring by being a component of the enzyme’s stator (subunits a, b, b′, and δ, and the α3β3-domain). Currently, crucial connections in the stator are unresolved, and the proton pathways lack the detail required for a full understanding of the coupling mechanism and the generation of rotation. Better diffracting crystals may provide a solution.
Materials and Methods
Protein Methods.
The complex of the F-ATPase with the ζ-inhibitor was purified from cells of P. denitrificans and crystallized as described previously (24). These initial crystals diffracted X-rays to 6.8 Å resolution. Their diffraction properties were improved by seeding and by treatment of the resulting crystals with a solution of dicyclohexylcarbodiimide in DMSO, as follows. Crystals in a single well were broken into small fragments with a glass rod. The tip of a human hair was dipped into this suspension of microcrystals and drawn across the surface of a new well containing the F-ATPase and corresponding to the initial crystallization conditions. Crystals were grown at 25 °C for 10 d. Then, they were soaked for 8 h in a solution of dicyclohexylcarbodiimide in DMSO [final concentrations 5 mM and 1% (wt/vol)], respectively.
Data Collection and Structure Determination.
Information on data collection and structure determination is provided in SI Materials and Methods.
SI Materials and Methods
X-ray diffraction data were collected from two flash-frozen crystals at Beamline I04-1 (wavelength = 0.9174 Å, beam size = 70 μm2) at the Diamond Light Source. The data were processed with programs from the CCP4 suite (35). Diffraction images were integrated with iMOSFLM (36), and the data were reduced with AIMLESS (37) and CTRUNCATE (35). Molecular replacement was carried out with PHASER (38). An initial model for the α3β3-assembly was constructed from the structure of the αEβE-heterodimer of the F-ATPase from P. denitrificans (39) using the SSM and LSQ features of Coot (40) and the structure of bovine F1-ATPase inhibited with dicyclohexylcarbodiimide [Protein Data Bank (PDB) ID code 1E79] (41) as a guide. The γ-subunit for this initial model was taken from the structure of yeast F1-ATPase inhibited by its natural inhibitor protein IF1 (PDB ID code 3ZIA) (3). Rigid body refinement of the molecular replacement solution, with each domain of the α3β3-assembly treated as a rigid group, improved the model, and the resulting electron density maps allowed a complete model to be constructed as follows. The δ-subunit was modeled based on the NMR structure of the corresponding subunit from E. coli (PDB ID code 2A7U) (20). Models for the ε- and γ-subunits were based on the crystal structure of the ε–γ complex from E. coli (PDB ID code 1FS0) (42) and the γ-subunit from the revised structure of F1-ATPase from C. thermarum. The ζ-inhibitor protein was derived from the solution structure (PDB ID code 2LL0) (25). Electron density for the a-, b-, and b′-subunits was modeled as poly-Ala helices. The c-ring was built from a single c-subunit from the c-ring of the F-ATPase of Acetobacterium woodii (PDB ID code 4BEM) (18). Because the stoichiometry of the c-ring was initially uncertain, both 11-fold and 12-fold rings were modeled. Because the temperature factors of the c-subunits are very high (250–330 Å2), there was no significant difference in refinement statistics between the two models, but the 12-fold ring had far fewer features in the difference electron density map and lower average temperature factors. The sequence register for the α-, β-, γ-, δ-, ε-, and c-subunits was unambiguous based on sequence alignment with homologous structures, but side chains in the δ-, ε-, and c-subunits were truncated back to the β-carbons because the electron density was very weak. The complete model was refined in REFMAC5 (43) using additional restraints provided by ProSMART (44) derived from the initial models for the α-, β-, γ-, δ-, ε-, and c-subunits; α-helical restraints for the a-, b-, and b′-subunits; and strict noncrystallographic symmetry restraints for the 12 c-subunits. Alternate rounds of refinement with REFMAC5 (43) and manual rebuilding with Coot (40) were carried out. Following each round of refinement, the stereochemistry of the model was assessed with Coot and MolProbity (45). Residues forming potential interactions between the ζ-inhibitor and subunits in the F1 domain of the F-ATPase from P. denitrificans were identified with CONTACT (35). Figures were made with PyMOL Molecular Graphics System (version 1.7; Schrodinger, LLC). The sequences of the ζ-inhibitor and yeast IF1 were analyzed for the presence of intrinsically unfolded regions with PrDOS (46) and DisEMBL (47). Buried surface area calculations were performed using ePISA (48). Transmembrane α-helices were predicted with TMHMM (49).
Acknowledgments
We thank Dr. T. B. Walpole for making the multiple sequence alignment of the a-subunit and the staff at Beamline I04-1 at the Diamond Light Source for their help. This work was funded by Medical Research Council (MRC) Intramural Program U105663150 and Program Grant MR/M009858/1 (to J.E.W.) and by MRC Intramural Program U105184325 (for A.G.W.L.). E.M.-R. was supported by an MRC Career Development Fellowship.
Footnotes
The authors declare no conflict of interest.
Data deposition: The atomic coordinates have been deposited in the Protein Data Bank, www.pdb.org (PDB ID code 5DN6).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1517542112/-/DCSupplemental.
References
- 1.Watanabe R, Noji H. Chemomechanical coupling mechanism of F1-ATPase: catalysis and torque generation. FEBS Lett. 2013;587(8):1030–1035. doi: 10.1016/j.febslet.2013.01.063. [DOI] [PubMed] [Google Scholar]
- 2.Walker JE. The ATP synthase: The understood, the uncertain and the unknown. Biochem Soc Trans. 2013;41(1):1–16. doi: 10.1042/BST20110773. [DOI] [PubMed] [Google Scholar]
- 3.Robinson GC, et al. The structure of F1-ATPase from Saccharomyces cerevisiae inhibited by its regulatory protein IF1. Open Biol. 2013;3(2):120164. doi: 10.1098/rsob.120164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bason JV, Montgomery MG, Leslie AGW, Walker JE. Pathway of binding of the intrinsically disordered mitochondrial inhibitor protein to F1-ATPase. Proc Natl Acad Sci USA. 2014;111(31):11305–11310. doi: 10.1073/pnas.1411560111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bason JV, Montgomery MG, Leslie AGW, Walker JE. How release of phosphate from mammalian F1-ATPase generates a rotary substep. Proc Natl Acad Sci USA. 2015;112(19):6009–6014. doi: 10.1073/pnas.1506465112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baker LA, Watt IN, Runswick MJ, Walker JE, Rubinstein JL. Arrangement of subunits in intact mammalian mitochondrial ATP synthase determined by cryo-EM. Proc Natl Acad Sci USA. 2012;109(29):11675–11680. doi: 10.1073/pnas.1204935109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Foster DL, Fillingame RH. Energy-transducing H+-ATPase of Escherichia coli. Purification, reconstitution, and subunit composition. J Biol Chem. 1979;254(17):8230–8236. [PubMed] [Google Scholar]
- 8.Foster DL, Fillingame RH. Stoichiometry of subunits in the H+-ATPase complex of Escherichia coli. J Biol Chem. 1982;257(4):2009–2015. [PubMed] [Google Scholar]
- 9.Walker JE, Saraste M, Gay NJ. The unc operon. Nucleotide sequence, regulation and structure of ATP-synthase. Biochim Biophys Acta. 1984;768(2):164–200. doi: 10.1016/0304-4173(84)90003-x. [DOI] [PubMed] [Google Scholar]
- 10.Cingolani G, Duncan TM. Structure of the ATP synthase catalytic complex (F1) from Escherichia coli in an autoinhibited conformation. Nat Struct Mol Biol. 2011;18(6):701–707. doi: 10.1038/nsmb.2058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Roy A, Hutcheon ML, Duncan TM, Cingolani G. Improved crystallization of Escherichia coli ATP synthase catalytic complex (F1) by introducing a phosphomimetic mutation in subunit ε. Acta Crystallogr Sect F Struct Biol Cryst Commun. 2012;68(Pt 10):1229–1233. doi: 10.1107/S1744309112036718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stocker A, Keis S, Vonck J, Cook GM, Dimroth P. The structural basis for unidirectional rotation of thermoalkaliphilic F1-ATPase. Structure. 2007;15(8):904–914. doi: 10.1016/j.str.2007.06.009. [DOI] [PubMed] [Google Scholar]
- 13.Shirakihara Y, et al. Structure of a thermophilic F1-ATPase inhibited by an ε-subunit: deeper insight into the ε-inhibition mechanism. FEBS J. 2015;282(15):2895–2913. doi: 10.1111/febs.13329. [DOI] [PubMed] [Google Scholar]
- 14.Shirakihara Y, et al. The crystal structure of the nucleotide-free α3β3 subcomplex of F1-ATPase from the thermophilic Bacillus PS3 is a symmetric trimer. Structure. 1997;5(6):825–836. doi: 10.1016/s0969-2126(97)00236-0. [DOI] [PubMed] [Google Scholar]
- 15.Meier T, Polzer P, Diederichs K, Welte W, Dimroth P. Structure of the rotor ring of F-Type Na+-ATPase from Ilyobacter tartaricus. Science. 2005;308(5722):659–662. doi: 10.1126/science.1111199. [DOI] [PubMed] [Google Scholar]
- 16.Pogoryelov D, Yildiz O, Faraldo-Gómez JD, Meier T. High-resolution structure of the rotor ring of a proton-dependent ATP synthase. Nat Struct Mol Biol. 2009;16(10):1068–1073. doi: 10.1038/nsmb.1678. [DOI] [PubMed] [Google Scholar]
- 17.Preiss L, et al. The c-ring stoichiometry of ATP synthase is adapted to cell physiological requirements of alkaliphilic Bacillus pseudofirmus OF4. Proc Natl Acad Sci USA. 2013;110(19):7874–7879. doi: 10.1073/pnas.1303333110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Matthies D, et al. High-resolution structure and mechanism of an F/V-hybrid rotor ring in a Na⁺-coupled ATP synthase. Nat Commun. 2014;5:5286. doi: 10.1038/ncomms6286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Preiss L, et al. The c-ring ion binding site of the ATP synthase from Bacillus pseudofirmus OF4 is adapted to alkaliphilic lifestyle. Mol Microbiol. 2014;92(5):973–984. doi: 10.1111/mmi.12605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wilkens S, Borchardt D, Weber J, Senior AE. Structural characterization of the interaction of the delta and alpha subunits of the Escherichia coli F1F0-ATP synthase by NMR spectroscopy. Biochemistry. 2005;44(35):11786–11794. doi: 10.1021/bi0510678. [DOI] [PubMed] [Google Scholar]
- 21.Dmitriev O, Jones PC, Jiang W, Fillingame RH. Structure of the membrane domain of subunit b of the Escherichia coli FoF1 ATP synthase. J Biol Chem. 1999;274(22):15598–15604. doi: 10.1074/jbc.274.22.15598. [DOI] [PubMed] [Google Scholar]
- 22.Del Rizzo PA, Bi Y, Dunn SD, Shilton BH. The “second stalk” of Escherichia coli ATP synthase: Structure of the isolated dimerization domain. Biochemistry. 2002;41(21):6875–6884. doi: 10.1021/bi025736i. [DOI] [PubMed] [Google Scholar]
- 23.Priya R, Biukovic G, Gayen S, Vivekanandan S, Grüber G. Solution structure, determined by nuclear magnetic resonance, of the b30-82 domain of subunit b of Escherichia coli F1Fo ATP synthase. J Bacteriol. 2009;191(24):7538–7544. doi: 10.1128/JB.00540-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Morales-Ríos E, et al. Purification, characterization and crystallization of the F-ATPase from Paracoccus denitrificans. Open Biol. 2015 doi: 10.1098/rsob.150119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Serrano P, Geralt M, Mohanty B, Wüthrich K. NMR structures of α-proteobacterial ATPase-regulating ζ-subunits. J Mol Biol. 2014;426(14):2547–2553. doi: 10.1016/j.jmb.2014.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gledhill JR, Montgomery MG, Leslie AGW, Walker JE. How the regulatory protein, IF1, inhibits F1-ATPase from bovine mitochondria. Proc Natl Acad Sci USA. 2007;104(40):15671–15676. doi: 10.1073/pnas.0707326104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Carbajo RJ, et al. How the N-terminal domain of the OSCP subunit of bovine F1Fo-ATP synthase interacts with the N-terminal region of an alpha subunit. J Mol Biol. 2007;368(2):310–318. doi: 10.1016/j.jmb.2007.02.059. [DOI] [PubMed] [Google Scholar]
- 28.Rees DM, Leslie AGW, Walker JE. The structure of the membrane extrinsic region of bovine ATP synthase. Proc Natl Acad Sci USA. 2009;106(51):21597–21601. doi: 10.1073/pnas.0910365106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Allegretti M, et al. Horizontal membrane-intrinsic α-helices in the stator a-subunit of an F-type ATP synthase. Nature. 2015;521(7551):237–240. doi: 10.1038/nature14185. [DOI] [PubMed] [Google Scholar]
- 30.Fillingame RH, Steed PR. Half channels mediating H+ transport and the mechanism of gating in the Fo sector of Escherichia coli F1Fo ATP synthase. Biochim Biophys Acta. 2014;1837(7):1063–1068. doi: 10.1016/j.bbabio.2014.03.005. [DOI] [PubMed] [Google Scholar]
- 31.Moore KJ, Fillingame RH. Structural interactions between transmembrane helices 4 and 5 of subunit a and the subunit c ring of Escherichia coli ATP synthase. J Biol Chem. 2008;283(46):31726–31735. doi: 10.1074/jbc.M803848200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dong H, Fillingame RH. Chemical reactivities of cysteine substitutions in subunit a of ATP synthase define residues gating H+ transport from each side of the membrane. J Biol Chem. 2010;285(51):39811–39818. doi: 10.1074/jbc.M110.175844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Junge W, Nelson N. ATP synthase. Annu Rev Biochem. 2015;84:631–657. doi: 10.1146/annurev-biochem-060614-034124. [DOI] [PubMed] [Google Scholar]
- 34.Xu T, Pagadala V, Mueller DM. Understanding structure, function, and mutations in the mitochondrial ATP synthase. Microb Cell. 2015;2(4):105–125. doi: 10.15698/mic2015.04.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Winn MD, et al. Overview of the CCP4 suite and current developments. Acta Crystallogr D Biol Crystallogr. 2011;67(Pt 4):235–242. doi: 10.1107/S0907444910045749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Battye TG, Kontogiannis L, Johnson O, Powell HR, Leslie AGW. iMOSFLM: A new graphical interface for diffraction-image processing with MOSFLM. Acta Crystallogr D Biol Crystallogr. 2011;67(Pt 4):271–281. doi: 10.1107/S0907444910048675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Evans PR, Murshudov GN. How good are my data and what is the resolution? Acta Crystallogr D Biol Crystallogr. 2013;69(Pt 7):1204–1214. doi: 10.1107/S0907444913000061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McCoy AJ, et al. Phaser crystallographic software. J Appl Crystallogr. 2007;40(Pt 4):658–674. doi: 10.1107/S0021889807021206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Morales-Ríos E, Montgomery MG, Leslie AGW, Walker JE. Structure of a catalytic dimer of the α- and β-subunits of the F-ATPase from Paracoccus denitrificans at 2.3 Å resolution. Acta Cryst. 2015 doi: 10.1107/S2053230X15016076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Emsley P, Lohkamp B, Scott WG, Cowtan K. Features and development of Coot. Acta Crystallogr D Biol Crystallogr. 2010;66(Pt 4):486–501. doi: 10.1107/S0907444910007493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gibbons C, Montgomery MG, Leslie AG, Walker JE. The structure of the central stalk in bovine F(1)-ATPase at 2.4 A resolution. Nat Struct Biol. 2000;7(11):1055–1061. doi: 10.1038/80981. [DOI] [PubMed] [Google Scholar]
- 42.Rodgers AJ, Wilce MC. Structure of the gamma-epsilon complex of ATP synthase. Nat Struct Biol. 2000;7(11):1051–1054. doi: 10.1038/80975. [DOI] [PubMed] [Google Scholar]
- 43.Murshudov GN, et al. REFMAC5 for the refinement of macromolecular crystal structures. Acta Crystallogr D Biol Crystallogr. 2011;67(Pt 4):355–367. doi: 10.1107/S0907444911001314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nicholls RA, Fischer M, McNicholas S, Murshudov GN. Conformation-independent structural comparison of macromolecules with ProSMART. Acta Crystallogr D Biol Crystallogr. 2014;70(Pt 9):2487–2499. doi: 10.1107/S1399004714016241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chen VB, et al. MolProbity: All-atom structure validation for macromolecular crystallography. Acta Crystallogr D Biol Crystallogr. 2010;66(Pt 1):12–21. doi: 10.1107/S0907444909042073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li X, Romero P, Rani M, Dunker AK, Obradovic Z. Predicting Protein Disorder for N-, C-, and Internal Regions. Genome Inform Ser Workshop Genome Inform. 1999;10:30–40. [PubMed] [Google Scholar]
- 47.Linding R, et al. Protein disorder prediction: Implications for structural proteomics. Structure. 2003;11(11):1453–1459. doi: 10.1016/j.str.2003.10.002. [DOI] [PubMed] [Google Scholar]
- 48.Krissinel E, Henrick K. Inference of macromolecular assemblies from crystalline state. J Mol Biol. 2007;372(3):774–797. doi: 10.1016/j.jmb.2007.05.022. [DOI] [PubMed] [Google Scholar]
- 49.Krogh A, Larsson B, von Heijne G, Sonnhammer EL. Predicting transmembrane protein topology with a hidden Markov model: Application to complete genomes. J Mol Biol. 2001;305(3):567–580. doi: 10.1006/jmbi.2000.4315. [DOI] [PubMed] [Google Scholar]