Significance
Perceiving things that are not there and holding unfounded, bizarre beliefs (hallucinations and delusions, respectively) are psychotic symptoms that occur in particular syndromes including affective psychoses, paranoid states, and schizophrenia. We studied the emergence of this loss of contact with reality based on current models of normal brain function. Working with clinical individuals experiencing early psychosis and nonclinical individuals with high levels of psychosis proneness, we show that their visual perception is characterized by a shift that favors prior knowledge over incoming sensory evidence. Given that these alterations in information processing are evident early on in psychosis and even in association with subtle perceptual changes indicating psychosis proneness, they may be important factors contributing to the emergence of severe mental illnesses.
Keywords: visual perception, psychosis, top-down processing, predictive coding, schizophrenia
Abstract
Many neuropsychiatric illnesses are associated with psychosis, i.e., hallucinations (perceptions in the absence of causative stimuli) and delusions (irrational, often bizarre beliefs). Current models of brain function view perception as a combination of two distinct sources of information: bottom-up sensory input and top-down influences from prior knowledge. This framework may explain hallucinations and delusions. Here, we characterized the balance between visual bottom-up and top-down processing in people with early psychosis (study 1) and in psychosis-prone, healthy individuals (study 2) to elucidate the mechanisms that might contribute to the emergence of psychotic experiences. Through a specialized mental-health service, we identified unmedicated individuals who experience early psychotic symptoms but fall below the threshold for a categorical diagnosis. We observed that, in early psychosis, there was a shift in information processing favoring prior knowledge over incoming sensory evidence. In the complementary study, we capitalized on subtle variations in perception and belief in the general population that exhibit graded similarity with psychotic experiences (schizotypy). We observed that the degree of psychosis proneness in healthy individuals, and, specifically, the presence of subtle perceptual alterations, is also associated with stronger reliance on prior knowledge. Although, in the current experimental studies, this shift conferred a performance benefit, under most natural viewing situations, it may provoke anomalous perceptual experiences. Overall, we show that early psychosis and psychosis proneness both entail a basic shift in visual information processing, favoring prior knowledge over incoming sensory evidence. The studies provide complementary insights to a mechanism by which psychotic symptoms may emerge.
To interact successfully with our physical and social environment, we need appropriate information about relevant states of the world, such as the size, location, or distance of an object. However, there is no direct access to this information, only to sensory stimulation caused by the environment. This sensory information is inherently ambiguous and, on its own, rarely suffices to uniquely specify our surroundings (1). The human visual system overcomes this challenge by combining ambiguous sensory information with prior knowledge of the environment to generate a robust and unambiguous representation of the world around us (1–7). This insight has been formalized under the tenets of Bayesian decision theory and is typically modeled within a predictive coding framework. Here, the notion is that expectations based on prior knowledge are fed back from higher to lower levels of information processing, thereby shaping the way incoming signals are treated by lower-level mechanisms. This influence is labeled top-down processing. The present study tests the hypothesis that psychotic experiences arise from an increased use of prior knowledge in constructing meaningful percepts from ambiguous sensory inputs.
Psychosis—a loss of contact with external reality—is characterized by delusions (irrational, often bizarre beliefs) and hallucinations (perceptions in the absence of causative stimuli). Conceptual and computational models of psychosis have hypothesized that an imbalance in the combination of bottom-up sensory evidence and top-down prior knowledge is at the core of this altered state of mind (8–12). According to such models, at the perceptual level, an undue reliance on prior knowledge in perception may lead to the emergence of aberrant perceptions such as hallucinations. The current study tests this hypothesis in the visual domain by characterizing the impact of prior knowledge on the perception of ambiguous stimuli in two groups of people: a clinical group with early psychotic experiences (study 1) and healthy volunteers showing differing levels of proneness to such experiences (study 2). Although the conventional view focuses preferentially on auditory hallucinations in psychosis, epidemiological evidence indicates that hallucinations in the visual domain are very common in, for example, schizophrenia (13). In fact, vision seems to play a prominent role in the development of psychosis given that basic visual symptoms identified before illness onset are one of the most powerful predictors of the emergence of later psychotic disorders (14).
To determine mechanisms for the emergence of perceptual psychotic symptoms as purely as possible, we conducted two complementary studies. First, using a case-control study design, we characterized the balance between visual bottom-up and top-down processing in a group of patients with early psychotic experiences and matched healthy controls (SI Materials and Methods and Table S1). Individuals in our clinical group were recruited from a dedicated mental health service identifying help-seeking people who have low-level but measurable psychotic experiences. Although, at the time of testing, these individuals fell below the threshold for a categorical diagnosis, they already showed symptoms and have an increased risk for transitioning to a severe mental illness such as schizophrenia or an affective disorder (15). Importantly, working with such a group of patients and comparing them to controls enabled us to focus on the features of early psychosis before any formal categorical diagnosis. Moreover, and also critically, this comparison is not confounded by the effects of antipsychotic medication or the impact of chronic illness, allowing us, as purely as possible, to explore the mechanisms of early psychosis.
Table S1.
Characteristics of the clinical (n = 18) and the control group (n = 16)
| Attribute | Clincal group | Control group | P value |
| Age (y) | 21 ± 3 | 19 ± 2 | NS* |
| IQ | 112 ± 16 | 121 ± 18 | NS* |
| Sex | 5 female, 13 male | 7 female, 9 male | NS† |
| Urbanicity | 24.4 ± 11.4 | 32.6.± 8.4 | <0.05* |
| CAARMS visual aberrations | 2.0 ± 2.0 | 0.4 ± 0.9 | <0.01‡ |
| CAARMS auditory aberrations | 3.1 ± 2.2 | 0.3 ± 1.1 | <0.01‡ |
| CAARMS unusual thought content | 2.6 ± 2.1 | 0 ± 0 | <0.01‡ |
| BDI | 23.25 ± 11.67 | 4.83 ± 4.18 | <0.01* |
| STAI | 38.1 ± 8.0 | 29.7 ± 6.3 | <0.01* |
BDI, Beck Depression Inventory; the number is the mean for the 2 testing days (58); CAARMS, Comprehensive Assessment of At-Risk Mental States, and all CAARMS values are means of maximal symptom intensity (15); NS, not significant; STAI, State-Trait Anxiety Inventory; the number is the mean for the 2 testing days (59).
All data except those for sex show mean ± SD.
Welch’s two-sample test.
Pearson’s Chi Square test.
Mann-Whitney u test.
In a second study, we explored psychosis proneness in healthy participants characterized according to the presence of perceptual (16) and belief-related schizotypal features (17). Schizotypy refers to a personality measure that has established predictive value for psychotic and other mental illnesses (18). Although it has been traditionally considered a specific risk measure for schizophrenia, more recently it has been proposed to reflect a general psychosis proneness. A number of schizotypy scales have been devised to characterize various dimensions of psychosis. In the current study, we focused on individual variation in measures relating to perception and belief (16, 17) because they most clearly relate to the key features of psychosis. These measures provided us with a fine-grained index for relevant perceptual experience and beliefs, allowing us to characterize the bottom-up/top-down balance in relation to subtle, nonclinical but specific and measurable markers associated with psychosis proneness.
Characterizing these two situations enabled us to pursue our central aim of exploring information-processing mechanisms that are altered in association with the occurrence of early symptoms (study 1) and also identifiable even before such symptoms arise (study 2). As well as offering a purer assessment of the emergence of psychotic experiences, this approach is inspired by growing evidence suggesting that psychosis lies on a continuum with normality (19, 20) and is associated with a range of different psychiatric disorders (15, 21). According to this perspective, existing diagnostic categories group biologically heterogeneous syndromes with potentially different pathophysiological mechanisms into one disorder (22); this may obfuscate our attempts to understand the neurobiological underpinnings of mental illness. In keeping with a broader move within the field, the aim of this approach is therefore to characterize deeper dimensions in their own right, such as psychosis as in the current study, irrespective of diagnostic categorization to advance our mechanistic understanding of specific symptom clusters.
In summary, we explored how the use of prior knowledge in visual information processing is related to early psychosis and to psychosis proneness. Importantly, given our hypothesis, we predicted that the putative mechanism associated with the emergence of psychosis would confer a relative advantage in this task, given that successful performance required the use of prior knowledge to discriminate ambiguous stimuli. Together, the two studies provide evidence to suggest that early psychosis and psychosis proneness is associated with a shift in visual processing that favors prior knowledge over incoming sensory evidence. We also demonstrate that this relation is specific to atypical perceptual experiences rather than being linked to psychotic experiences more generally.
SI Materials and Methods
Participants in Experiment 1.
Thirty-four individuals from the database of the Cambridge-based CAMEO early intervention in psychosis service (www.cameo.nhs.uk) consented to take part in the study: 16 healthy volunteers (9 males) and 18 at-risk mental state (ARMS) individuals (13 males). Between 2010 and 2012, this specialized mental health service identified 60 help-seeking individuals who met criteria for high risk according to the Comprehensive Assessment of At-Risk Mental States (CAARMS); all individuals in this cohort fulfilled criteria for the attenuated psychotic symptoms group (15). The exclusion criteria were (i) acute intoxication or withdrawal associated with drug or alcohol abuse or any delirium; (ii) confirmed intellectual disability (Wechsler Adult Intelligence Scale tested IQ < 70); and (iii) prior total treatment with antipsychotics for more than 1 wk.
In the same time period, a random sample of 60 healthy volunteers was recruited by CAMEO, ensuring that clinical and control groups resided in the same geographical location and came from the same age groups. Healthy volunteers did not have previous contact with mental health services. Table S1 provides various background data and clinical characteristics for the specific clinical and control individuals that took part in the current study. The groups were matched on age and IQ, using Cattell’s Culture Fair Intelligence Test (54).
Participants in Experiment 2.
Forty-three healthy volunteers were recruited from the local population through local mailing lists. Three individuals had to be excluded because they failed to fill out the questionnaires or their questionnaire scores were more than 3 SDs above the mean. The remaining forty healthy observers [14 males; age (mean ± SD), 24 ± 4 y] were right-handed, had normal or corrected-to-normal vision, and were native English speakers. They had no history of, or currently suffered from, psychiatric illness or drug/ alcohol abuse.
Stimuli.
Ideal two-tone images have the following two properties: (i) they should be impossible or very difficult to disambiguate before being given appropriate image information; and (ii) once participants have seen the template, they should give the strong experience of a coherent percept. Very few studies using two-tone images use stimuli that have both of these properties.
In the current experiments, we adopted the following strategy to create appropriate pictures. Two-tone images were generated using custom-written Matlab code. Grayscale versions of more than 500 photographs of people (experiment 1) from the Corel Photo Library were used as templates (due to copyright issues, we were not allowed to publish an example of the actual stimuli; the image shown in Fig. 2 is therefore only an illustration of our stimuli, not an actual stimulus). For experiment 2, we used ∼500 additional photographs of animals. These images were multiplied in the frequency domain with a Gaussian kernel of varying width and were thresholded at various black-white cutoffs. This procedure resulted in a large set of two-tone images for every template, which were inspected by two experimenters who manually chose an approximately appropriate level of filtering and threshold for every stimulus. The chosen images were then extensively piloted in naïve observers, who were asked to name the object in these two-tone images before and after having seen the template. Images that were too easy to disambiguate before or too difficult after having seen the templates for most observers were either excluded, or the filter and threshold were adapted accordingly. The new images were then again piloted with a new group of observers. We went through several rounds of piloting these images. Ultimately, this lengthy procedure resulted in ∼200 images of suitable quality. Experiment 1 used 120 images of people only. In experiment 2, we used 160 images, choosing the best images from experiment 1 and then topping up this stimulus pool with the best two-tone images of animals.
Fig. 2.
Illustration of a template image. This template image was used to generate the two-tone image shown in Fig. 1A, Left. To experience the effect of knowledge on perception, the reader should look back at Fig. 1A after having viewed this template for some time. The perceptual experience of the two-tone image in Fig. 1A, Left, should have drastically changed from the initial viewing: rather than looking like meaningless patches, a coherent percept of the child and parts of the puzzle should now be experienced. The control image in Fig. 1A, Right, should still appear as meaningless patches.
The resulting images were used as test stimuli in both experiments. We also generated control images that were similar to the test images with respect to their low-level statistical properties but differed in that they did not contain an embedded object. For this purpose, we inverted the test images and slightly moved some black or white patches that were crucial in conveying configural information. Pilot experiments confirmed that this procedure entirely destroyed the possibility for observers to disambiguate the objects that were embedded in the original images. The control images appeared to consist of meaningless black and white patches, independent of viewing time or whether observers had seen the color images that were used to generate the original test images. Moreover, the procedure also ensured that the low-level statistical properties of test and control images were very similar.
For each of the template images, we thus had a two-tone test version and a two-tone control version. In experiment 1, half of the observers saw a set of 60 two-tone images as test images and the respective second set of 60 images as control. For the remaining observers, it was the other way around. The same procedure was used in experiment 2, except that sets consisting of 80 test and 80 control images were used.
Experimental Procedure.
Both experiments were part of a larger study. In addition to the reported experiments, participants in experiment 1 also took part in learning studies inside and outside an MRI scanner. Participants in experiment 2 took part in a behavioral auditory discrimination task. The order, in which participants took part, was counterbalanced as far as possible. Counterbalancing was identical for the clinical and the control group in experiment 1.
In experiment 1, stimuli were presented on a Dell precision M4600 laptop using Matlab (The MathWorks) with the Psychophysics Toolbox Version 3 (55, 56). Working with a clinical group, we did not want to constrain observers by a chin rest; nevertheless, we ensured that they kept a roughly constant distance from the screen of ∼60 cm. This distance corresponded to a stimulus size of ∼7.2° × 10.8° of visual angle. The characteristics of experiment 2 were identical, except that we used a PC desktop computer with a Samsung SyncMaster 2233 screen. A chin rest ensured a constant distance of 60 cm from the screen.
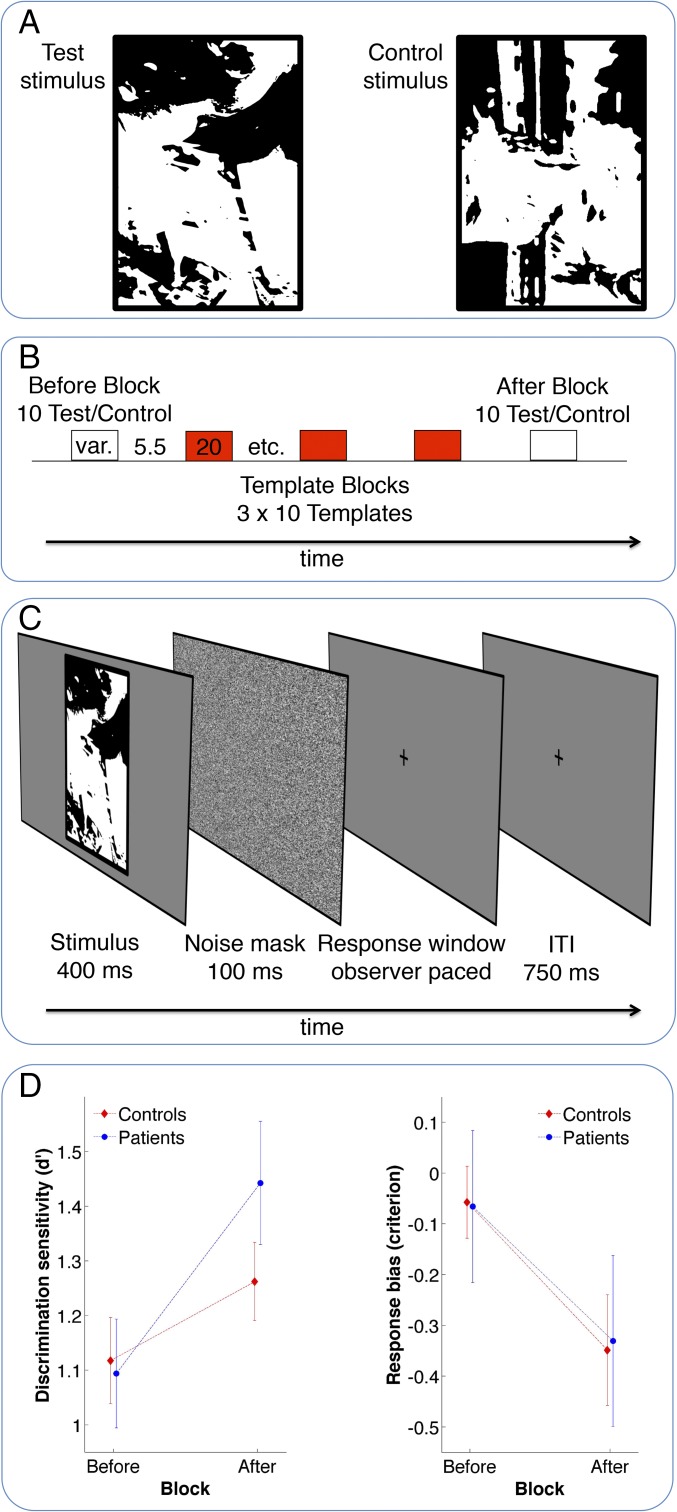
Each experiment started with a practice run to ensure that observers knew the structure of the task. The practice run consisted of one session, which was identical to the experimental sessions (Fig. 1B) except that 6 instead of 10 trials were presented to speed up the practice. Half of the trials were test trials and the other half were control trials.
Fig. 1.
The case-control study (study 1). (A) Illustration of a test (Left) and a control stimulus (Right). Test and control images had similar low-level statistical properties (SI Materials and Methods) but differed in whether or not they contained an embedded person. (B) Illustration of one session. Initially, observers viewed a block of ten two-tone images (Before Block). Across blocks, half of the stimuli were test images that contained an embedded person, the other half were similar-looking control images without a person. The presentation of two-tone images was followed by three blocks of ten color images, presented for 2 s in random order and back-to-back (Template Blocks). For every test image viewed in the Before Block a matching color template was presented, showing the clear version of the same image. Finally, in the After Block, observers saw the same two-tone images as in the Before Block, presented in random order. Numbers along the timeline indicate the length of the different experimental parts in seconds; var. indicates a part of variable length. In total, every observer participated in 12 sessions, separated by self-paced breaks. (C) Illustration of one individual trial. After a brief presentation of either a test or a control image, observers were asked to indicate whether the image contained a person or not. Observers had as much time as needed to respond but in case they had not responded within 1,250 ms, a reminder to respond was shown on the screen. (D) Results of discrimination task. (Left) Discrimination sensitivity in terms of d′ (mean ± SEM) in the Before and the After Block, slightly jittered for both groups to correctly display error bars; the higher the d′ values, the better observers were able to discriminate between test and control images. (Right) Response bias in terms of the observers’ criterion (mean ± SEM); a lower criterion indicates a response bias to report the presence of a person in a given stimulus (independent of whether a test or control image is shown). Healthy controls are plotted in red; the clinical group is shown in blue.
Experiment 1 consisted of 12 experimental sessions (Fig. 1B) and experiment 2 consisted of 16 sessions. Each session started with a Before Block, in which the observer saw 10 two-tone images that were chosen at random from the pool of 60 (experiment 1) or 80 (experiment 2) test images and 60/80 control images assigned to the observer by the counterbalancing schedule described in the previous section. In each trial, a two-tone image was presented followed by a brief mask of Gaussian white noise (Fig. 1C). Observers were then given the opportunity to make a simple decision about the image during an observer-paced response window. In experiment 1, we initially aimed to estimate full ROC curves for each observer. We therefore used a confidence rating paradigm, a standard procedure from signal detection theory, in which the observer had to report how confident they are that a person was present in the image on a scale from 1 to 4. Ideally, this procedure would have allowed us to estimate d′ associated with three criterions depending on the degree of confidence (57). However, many observers did not use response options 2 and 3 at all, resulting in many datasets with empty cells. To deal with this issue, we pooled responses for options 1 and 2, as well as those for options 3 and 4. This procedure essentially transformed the responses into yes/no data, for which we estimated d′ and criterion. Experiment 2 used a yes/no task from the start.
After 10 trials of two-tone images, observers were presented with three blocks of 10 color images to provide them with ample prior knowledge about image content (Fig. 1B). Before each color image block, there was a break of 5.5 s, in which observers were reminded to attentively observe the color images. Each stimulus was shown for 2 s and within one block stimuli were shown back-to-back; the order of presentation was randomized for each block. The color images were those templates used to generate the test and control images shown in the previous part of the study. They could thus be used to disambiguate test images the next time these were viewed; importantly, however, the color images did not provide any information that was useful to disambiguate control images (in fact, there was no person to be disambiguated in these stimuli).
Finally, the last part of each session, the After Block, was identical to the Before Block except that a new randomization was used for the order of presentation. In other words, observers were presented with the same 10 two-tone images as in the Before Block and were again asked to report whether they saw a person in these images or not.
Note that, in contrast to some previous two-tone image studies, observers did not see one individual two-tone image and the corresponding template back-to-back, a design that is susceptible to alternative interpretations in terms of purely bottom-up priming rather than top-down processing. To minimize the influence of purely bottom-up effects, we presented two-tone and template images in blocks of 10 (repeating the block of template images three times in random order).
Analysis.
As mentioned above, responses were transformed into yes/no data (experiment 1) or were yes/no data to start with (experiment 2). We derived hit rates and false alarm rates from these data, separately for the Before and After Block. Using the standard Eqs. 1 and 2, we derived d′ and criterion (57)
| [S1] |
| [S2] |
where h is the hit rate, f the false alarm rate, and c is criterion.
Results
To quantify top-down processing, we used two-tone images as stimuli (3, 23–26). Without appropriate knowledge, these images appear to depict meaningless 2D black-and-white patches (Fig. 1A). Once an observer is exposed to relevant image information, however, grouping mechanisms in the visual system are able to integrate patches in such a way that a robust and coherent percept of a 3D object forms. In the present study, we provided the prior knowledge necessary to bind a two-tone image into a coherent percept by exposing observers to the color templates that were used to generate the two-tone images (Fig. 2). Importantly, rather than seeing an individual two-tone and the corresponding template image back-to-back, observers freely viewed the stimuli in blocks of 10 (SI Materials and Methods). This procedure ensured that purely bottom-up priming was minimized in the disambiguation of two-tone stimuli after template exposure. In combination with the fact that sensory stimulation is identical before and after template exposure, the perceptual change of the two-tone image in this experimental design thus provides an ideal index of the impact of knowledge on perception. This notion is supported by previous psychophysical and neuroimaging literature, which indicates that disambiguation of two-tone images is due to top-down influences from high-level processes onto low-level visual function (3, 23–26). In particular, two-tone image perception recruits memory processes and object knowledge associated with cortical areas such as precuneus and dorsolateral prefrontal cortex; these processes activate stored, high-level visual representations of the template images and feed back information to lower-level areas to shape perceptual processing, even as early as the primary visual cortex V1.
In study 1, observers performed a yes/no discrimination task, in which they viewed briefly presented two-tone images of people and similar-looking control images without an embedded object (Fig. 1A). Within each session, these images were presented before and after the observer received prior knowledge by viewing template images (Figs. 1B and 2). On every trial observers were required to indicate whether a given image contained a person or not (Fig. 1C and SI Materials and Methods). Based on signal detection theory, we derived two measures from the observers’ performance: (i) d′, a pure index of an observer’s perceptual mechanisms independent of response bias; and (ii) criterion, which captures the general bias of observers to report the presence of an object. As an objective performance measure of the influence of knowledge on perception, we were primarily interested in the change in d′ after having been exposed to relevant image information.
The results support our hypothesis (Fig. 1D). As expected, a mixed 2 × 2 ANOVA with the between-subjects factor group and the within-subjects factor block found an overall increased ability to distinguish between test and control images measured in terms of d′ after having viewed template images in comparison with before having seen them [F(1,32) = 29.27, P < 0.001]. Critically, this main effect was qualified by an interaction between group and block, indicating that the improvement in performance differed between the groups [F(1,32) = 5.02, P < 0.05]. As can be seen in the left plot in Fig. 1D and supported by post hoc tests, both groups showed an improvement in performance (paired t test, controls: df = 15, t = 2.17, P < 0.05; clinical group: df = 17, t = 5.60, P < 0.001), but this was on average more than twice as large in the clinical group (mean ± SE: 0.35 ± 0.06) compared with controls (0.14 ± 0.07). It is noteworthy that the increased benefit in the clinical group was observed in the absence of an overall difference between groups [F(1,32) < 1, not significant] and was not due to a difference in baseline performance in the Before block (Welch’s two-sample t test, before clinical vs. before controls: df = 31.16, t = 0.19, not significant).
Additional analyses showed that, on average, observers in both groups had a stronger response bias (as measured by a criterion shift) to report seeing an object after being given relevant image information compared with before [F(1,32) = 37.77, P < 0.001; Fig. 1D, Right]. This finding did not differ between groups [F(1,32) < 1, not significant) and is an expected change given that observers received additional information to solve the task and were therefore more confident in the After than in the Before Block.
In sum, study 1 shows that, in comparison with healthy controls, observers with early psychosis can call more strongly on prior knowledge at the expense of sensory evidence when faced with a visual signal. This group comparison result thus establishes the clinical importance of this basic information-processing shift but, as is typical for such studies, our clinical group differs from the control group on a range of psychiatric measures (Table S1). It is therefore difficult to determine the specificity of the effect with respect to the two primary psychotic symptom clusters: hallucinations and delusions. In study 2, we sought to gain a more specific and nuanced understanding of the relationship between psychotic symptoms and the use of knowledge in perception. We capitalized on subtle alterations in perception and belief across the healthy population that show a graded similarity with psychotic experiences. This variability can be characterized independently for perception and belief by two schizotypy scales, the Cardiff Anomalous Perception Scale (CAPS) (16) and the Peters Delusion Inventory (PDI) (17). Note that, although other schizotypy scales such as Oxford-Liverpool Inventory of Feelings and Experiences (O-LIFE) (27) and Schizotypal Personality Questionnaire (SPQ) (28) characterize a number of schizotypal features, CAPS and PDI are specific to clinical features relating to hallucinations and delusions respectively. In study 2, we thus recruited a larger sample of individuals from the general population and related their tendency to rely on prior knowledge in visual perception to their schizotypy scores. The task was identical to that used in the previous experiment except for two aspects: Given that study 2 involved healthy observers only, we extended testing to 16 rather than 12 sessions to obtain a more precise estimate of every observer’s d′; second, an improved stimulus set was used that contained two image categories (people and animals) rather than one (people) as in study 1.
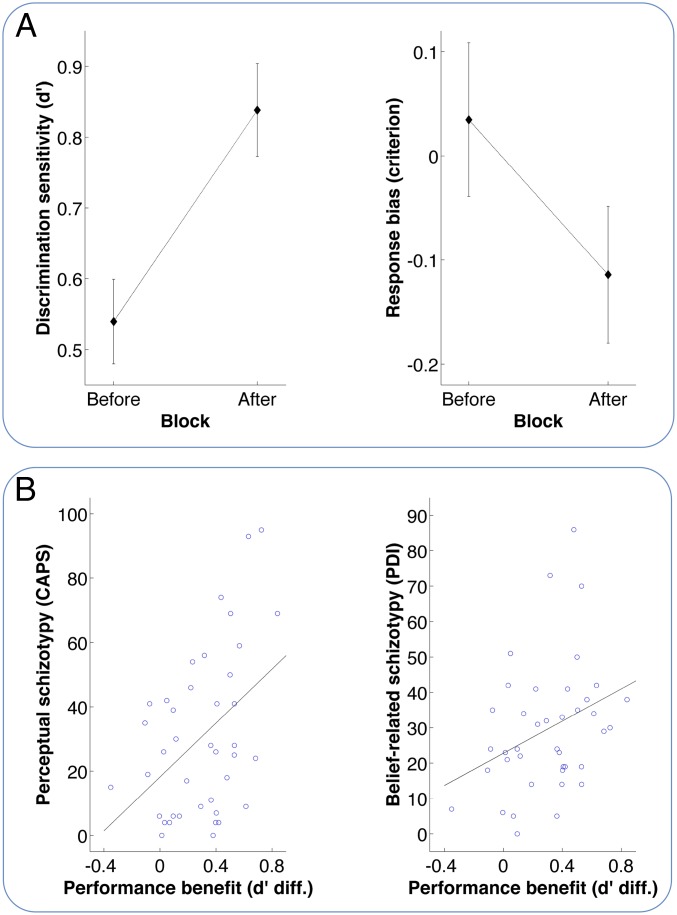
The results of study 2 are plotted in Fig. 3A. As expected, prior knowledge resulted in a significant improvement in the ability to distinguish between test and control images (Fig. 3A left plot; paired t test, df = 39, t = 7.08, P < 0.001). Similar to the previous experiment, we also observed a criterion shift (Fig. 3A, Right; paired t test, df = 39, t = 4.11, P < 0.001). Due to the differences in experimental design, we cannot directly compare performance across experiments. Nevertheless, as expected, it is evident that inclusion of a second image category in study 2 made the task more difficult, explaining the performance differences between the two experiments in healthy observers. Importantly and in line with the results of study 1, Fig. 3B indicates that the performance benefit due to prior knowledge was related to both the extent to which an observer experienced anomalous perceptual phenomena similar to those of psychotic patients as measured by CAPS (Fig. 3B, Left; product-moment, r = 0.42, df = 38, t = 2.98, P < 0.01) and the extent to which they showed a delusion-like style of thinking as measured by PDI (Fig. 3B, Right; r = 0.33, df = 38, t = 2.14, P < 0.05). As was expected, however, the schizotypy scales for perception and belief were positively correlated (r = 0.40, df = 38, t = 2.67, P < 0.05), and we therefore conducted a first-order partial correlation analyses to determine the amount of variability in performance that can be explained by schizotypal features related to perception or belief alone. This analysis indicated a very specific effect. The relation between a delusion-like style of thinking and performance benefit due to prior knowledge disappeared once an observer’s anomalous perceptual experiences were taken into account (first-order partial correlation, r = 0.19, df = 37, t = 1.17, not significant). By contrast, a significant relation between CAPS and performance benefit remained even when PDI was controlled for (r = 0.35, df = 37, t = 2.28, P < 0.05).
Fig. 3.
The individual-differences study (study 2). (A) Results of discrimination task. (Left) Discrimination sensitivity in terms of d′ (mean ± SEM) in the Before and After Block; again, the higher the d′ values, the better observers were able to discriminate between test and control images. (Right) Response bias in terms of the observers’ criterion (mean ± SEM); as in Fig. 1, a lower criterion indicates a response bias to report the presence of a person in a given stimulus (independent of whether a test or control image is shown). (B) Relation of task performance with schizotypy. (Left) Performance benefit due to prior knowledge against the global CAPS score, which captures perceptual alterations that show graded similarity with perceptual atypicalities experienced during psychosis. Performance benefit was calculated as the difference in d′ between After and Before Blocks. (Right) Relation between performance benefit and global PDI scores of the participants. PDI captures belief-related alterations similar to those seen during psychosis.
Discussion
Our studies were designed to characterize, in complementary ways, the balance between visual bottom-up and top-down processing in clinical individuals with early psychosis and healthy people prone to developing psychotic symptoms. A relative advantage in using prior knowledge to discriminate between ambiguous images was observed in both situations. This finding is especially striking in the clinical group in study 1 given that performance in this group (as in psychiatrically ill individuals more generally) is typically impaired. Such a result is rare and revealing in that it highlights a specific information-processing atypicality rather than a general performance deficit. Study 2 allowed us to characterize these alterations in visual function more completely by adopting an individual differences approach with healthy participants and by capitalizing on subtle variations in perception and belief that exhibit graded similarity with psychotic experiences. In line with our clinical findings, we uncovered a relation between an individual’s visual performance benefit due to prior knowledge and their scores on two scales of psychosis proneness. Importantly, also, our data suggest that this relation is primarily driven by perceptual alterations rather than unusual beliefs. Taken together, these results indicate that visual function in early psychosis and in healthy people who are prone to such experiences is characterized by a basic information-processing shift that favors existing knowledge over incoming sensory evidence. Although, in the current experimental task, this shift conferred a performance benefit, under most natural viewing situations, it may provoke anomalous perceptual experiences. Specifically, it might impose prior expectations on inputs to the extent that, ultimately, formed percepts are generated that have no direct sensory cause: hallucinations.
These findings fit neatly with and support current conceptual and computational models of psychotic symptoms (8–12). For instance, it has been hypothesized that a single core disturbance relating to the balance between bottom-up and top-down processing can explain both the hallucinatory experiences and the bizarre delusional beliefs of psychotic patients (8, 11). Importantly, we show that, on the perceptual level, a shift in this balance toward prior knowledge is present both in a clinical group of individuals with early psychosis and even associated with psychosis proneness in the general population. Although schizotypy is a marker for psychosis proneness as ascertained by previous longitudinal studies (18), it is important to acknowledge that individuals in study 2 were not suffering from psychosis or even a diagnosed mental illness. Rather, those individuals scoring high on the scales identified a number of unusual perceptual experiences. It is therefore striking that the same information-processing shift was observed as was found in early psychosis. Indeed, even in the early psychosis group, no formal, categorical diagnosis was applicable (although it is known that such groups have a high risk of transition to full psychiatric illness) (15). The findings may therefore suggest that the altered balance is a fundamental trait that contributes to the emergence of psychosis rather than a reflection or consequence of the psychotic state.
The specificity of the relation between performance on our task and perceptual aspects of schizotypy is of particular interest. It has long been known that altered perceptual experiences form a key part of the emergence of psychosis (29). Given that the CAPS is selective for measuring schizotypal perceptual phenomena rather than targeting schizotypy in general (16), our findings indicate that a shift in visual information processing that favors prior knowledge over sensory evidence might be a marker for the mechanisms underlying this observation. The finding that healthy individuals that score high on this scale share this marker with our clinical group is in line with the growing belief that psychotic mental illnesses are part of a continuum with normality (19, 20). It supports the idea that the putative atypicality underlying the emergence of perceptual psychotic experiences relates directly to normal function of the system. In other words, the potential for psychotic experiences such as hallucinations might be a logical consequence of the way in which our brain deals with the inherent ambiguity of sensory information by incorporating prior knowledge into our perceptual processing. The current study uncovered an imbalance of this processing type that shows its effects at the perceptual level. However, within a hierarchical and recurrent information-processing system such as the human brain, an imbalance at any level will, in time, propagate up and down the hierarchy and affects the whole system (8, 30), a notion that might ultimately account for atypicalities in both lower-level perceptual processing and higher-level belief formation in severe mental illnesses and psychosis proneness (30).
As previously discussed, our aim in conducting these studies was specifically to try to understand the processes that contribute to the emergence of psychotic symptoms rather than to examine a particular categorical diagnosis within which psychosis may occur. This approach is part of a more general move in translational research aimed at developing our understanding of the mechanisms by which symptoms arise (22); this is seen as a necessary prelude to a successful classificatory system in psychiatry. We do not advocate that we can do without diagnostic systems in psychiatry but rather selected our approach to fit the specific question we aimed to address. Ultimately, it will be extremely interesting to determine whether the shift in the bottom-up/top-down balance identified here is a transdiagnostic effect, occurring generally in early psychosis and psychosis proneness, or whether it is specific to a particular diagnostic category such as schizophrenia. This future work will be important and we suggest that having identified such a cognitive biomarker will prove very useful in examining, and validating or challenging existing taxonomies of mental illness.
Computational explanations of the balance between sensory evidence and prior knowledge as highlighted by our study are typically cast in terms of Bayesian models of perception and formalized within a predictive coding framework (5, 31, 32). In this setting, the integration of bottom-up and top-down signals is mediated by the relative amount of information each of these components provides: the stronger the sensory evidence is relative to prior knowledge, the more it will impact on the final processing output; conversely, if prior knowledge provides a relatively greater amount of information it will be weighted more strongly. Our findings fit comfortably with such a computational account: a stronger reliance on top-down processing in psychosis-prone individuals as identified in both of our experiments suggests that prior knowledge provides a large amount of information relative to the amount of sensory evidence. Importantly, this could either be due to atypically strong knowledge representations or, alternatively, to an unusually noisy sensory system. Given well-established psychotic deficits in early visual processing as discussed below, the latter explanation might be more likely. However, the findings cannot distinguish between these hypotheses. In this context, it is interesting to note that the same theme of an imbalance between bottom-up and top-down processing figures prominently in current debates about autism (33, 34). This similarity highlights the notion that predictive coding might provide a common framework within which to understand mental illness more generally. An important task for the future will be to explain the specificities of the different pathological trajectories associated with different diagnostic categories within such a unifying approach.
Some authors argue that, on a neuronal level, the bottom-up/top-down balance is implemented by neuromodulatory gain control of feedforward and feedback neuronal circuitries; adopting this approach, psychosis may thus be understood in terms of atypicalities in these control mechanisms (35). Although some models have focused on the emergence of delusions as a consequence of enhanced gain of feed-forward connections (and thus of the bottom-up signal) (8, 35, 36), the current findings suggest that a shift in the opposite direction—i.e., either enhanced gain of feedback connections or reduced gain of feedforward connections—may account for perceptual changes associated with psychosis. Given that our study did not directly measure neuromodulatory gain control, this assertion is speculative but it might provide a fruitful avenue for future research.
In schizophrenia, one important diagnostic category associated with psychosis, both increased and decreased susceptibility to various visual illusions is observed (37–41). Although most studies implicate atypicalities in early visual function as the source of these differences, some authors argue that certain illusions rely on some form of top-down processing (40, 41), a claim that has been contested (42, 43). Critically, however, most researchers agree that these visual illusions can be explained by processes within the visual system, rather than by influences on visual function from higher-level knowledge areas outside the visual system. Our findings are therefore not at odds with decreased susceptibility to some visual illusions in schizophrenic patients (37, 38, 40, 41). In fact, we argue that our findings dovetail with and complement these previous results in a surprising way: independently of the underlying mechanisms, atypicalities within the visual system result in the outputs of the perceptual analysis being less well structured and less adaptive. This could lead to an increased reliance on sources of information from outside the visual system to structure visual percepts and might make psychotic patients “hungry” for prior object knowledge. It is this latter effect that was demonstrated in the current study.
We should add that an explanation of these findings in terms of better memory for the information provided by the templates is implausible in both studies. Existing evidence suggests that both early psychosis and high schizotypal characteristics may be associated with poorer working memory (44, 45).
Although performance improvements due to prior knowledge were increased in the clinical group in study 1, these individuals were no worse (than controls) in their ability to discriminate ambiguous two-tone images without prior knowledge. This finding is not directly relevant to the main question addressed in this study but it is interesting because it contrasts with previous studies in patients with established schizophrenia who show reduced ability to spontaneously disambiguate two-tone images of faces without prior knowledge (46, 47). This previous effect is most likely related to well-established schizophrenic deficits in early and midlevel vision that affect perceptual organization, context processing and integration (37, 38, 48–53) rather than to top-down influences from high-level visual cognition as in the current study. We did not directly probe early and midlevel visual function in our participants, but it seems most likely that the absence of impairments in spontaneous disambiguation of two-tone images in the clinical group might be due to the specific nature of our stimulus material, which was extensively piloted to be difficult to disambiguate without prior knowledge (for details, see SI Materials and Methods).
To conclude, if we are to make progress in understanding the nature of psychotic experiences and how they relate to cognitive and neural markers, we have to identify candidate mechanisms for how they may arise based on a growing understanding of the relevant perceptual and cognitive systems. Here, we identified a shift in visual information-processing in early psychosis and in psychosis-prone healthy individuals. In both cases, top-down prior knowledge was favored over bottom-up sensory evidence. These alterations directly relate to visual function in healthy individuals and our findings support the notion of a continuum between normality and psychotic experiences. The changes in information processing we report here might be among the influences that, in concert with other factors, contribute to the emergence of major mental illnesses such as schizophrenia and affective disorders.
Materials and Methods
Observers.
In study 1, 34 individuals from the database of the Cambridge and Peterborough Assessing, Managing, and Enhancing Outcomes (CAMEO) early intervention in psychosis service (www.cameo.nhs.uk) based in Cambridge consented to take part in the study: 16 healthy volunteers (9 males) and 18 at-risk mental state (ARMS) individuals (13 males). The groups were matched on age and IQ, using Cattell’s Culture Fair Intelligence Test. For full description of the recruitment procedure and the clinical and demographic profile of participants, see SI Materials and Methods and Table S1.
In study 2, the 40 healthy observers who participated in the study were recruited from the local population through local mailing lists. For further details, see SI Materials and Methods.
Stimuli.
Ideal two-tone images have the following two properties: (i) they should be impossible or very difficult to disambiguate before being given prior knowledge about image content; and (ii) once participants have seen the template, the two-tone images should give the strong experience of a coherent object. Very few studies using two-tone images use stimuli that have both of these properties. In the current study, we ran an extensive piloting phase to generate appropriate images. For details, see SI Materials and Methods.
Experimental Procedure and Analysis.
As described here and in Fig. 1, we used a yes/no task with an analysis derived from signal detection theory to characterize the balance between bottom-up and top-down processing in our observers. A complete description of experimental procedures and analyses is presented in SI Materials and Methods.
Acknowledgments
We thank all the members of the Cambridge and Peterborough Assessing, Managing, and Enhancing Outcomes (CAMEO) services for their help and support in conducting this study. This work was carried out within the Behavioural and Clinical Neuroscience Institute (BCNI), funded by the Wellcome Trust and the Medical Research Council, and the Cambridgeshire and Peterborough National Health Service Foundation Trust (CPFT). This study was supported by grants from the Wellcome Trust and the Bernard Wolfe Health Neuroscience fund (to P.C.F.). I.M.G. is supported by the Wellcome Trust.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1503916112/-/DCSupplemental.
References
- 1.Kersten D, Mamassian P, Yuille A. Object perception as Bayesian inference. Annu Rev Psychol. 2004;55(1):271–304. doi: 10.1146/annurev.psych.55.090902.142005. [DOI] [PubMed] [Google Scholar]
- 2.Neri P. Semantic control of feature extraction from natural scenes. J Neurosci. 2014;34(6):2374–2388. doi: 10.1523/JNEUROSCI.1755-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cavanagh P. Visual cognition. Vision Res. 2011;51(13):1538–1551. doi: 10.1016/j.visres.2011.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dakin SC. Vision: Thinking globally, acting locally. Curr Biol. 2009;19(18):R851–R854. doi: 10.1016/j.cub.2009.08.021. [DOI] [PubMed] [Google Scholar]
- 5.Friston K. The free-energy principle: A unified brain theory? Nat Rev Neurosci. 2010;11(2):127–138. doi: 10.1038/nrn2787. [DOI] [PubMed] [Google Scholar]
- 6.Schmack K, et al. Delusions and the role of beliefs in perceptual inference. J Neurosci. 2013;33(34):13701–13712. doi: 10.1523/JNEUROSCI.1778-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Teufel C, Subramaniam N, Fletcher PC. The role of priors in Bayesian models of perception. Front Comput Neurosci. 2013;7(25):25. doi: 10.3389/fncom.2013.00025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fletcher PC, Frith CD. Perceiving is believing: A Bayesian approach to explaining the positive symptoms of schizophrenia. Nat Rev Neurosci. 2009;10(1):48–58. doi: 10.1038/nrn2536. [DOI] [PubMed] [Google Scholar]
- 9.Grossberg S. Adaptive Resonance Theory: How a brain learns to consciously attend, learn, and recognize a changing world. Neural Netw. 2013;37:1–47. doi: 10.1016/j.neunet.2012.09.017. [DOI] [PubMed] [Google Scholar]
- 10.Jardri R, Denève S. Computational models of hallucinations. In: Jardri R, Cachia A, Thomas P, Pins D, editors. The Neuroscience of Hallucinations. Springer, Berlin: 2013. pp. 289–313. [Google Scholar]
- 11.Corlett PR, Frith CD, Fletcher PC. From drugs to deprivation: A Bayesian framework for understanding models of psychosis. Psychopharmacology (Berl) 2009;206(4):515–530. doi: 10.1007/s00213-009-1561-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Clark A. Whatever next? Predictive brains, situated agents, and the future of cognitive science. Behav Brain Sci. 2013;36(3):181–204. doi: 10.1017/S0140525X12000477. [DOI] [PubMed] [Google Scholar]
- 13.Waters F, et al. Visual hallucinations in the psychosis spectrum and comparative information from neurodegenerative disorders and eye disease. Schizophr Bull. 2014;40(Suppl 4):S233–S245. doi: 10.1093/schbul/sbu036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Klosterkötter J, Hellmich M, Steinmeyer EM, Schultze-Lutter F. Diagnosing schizophrenia in the initial prodromal phase. Arch Gen Psychiatry. 2001;58(2):158–164. doi: 10.1001/archpsyc.58.2.158. [DOI] [PubMed] [Google Scholar]
- 15.Hui C, et al. Psychiatric morbidity, functioning and quality of life in young people at clinical high risk for psychosis. Schizophr Res. 2013;148(1-3):175–180. doi: 10.1016/j.schres.2013.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bell V, Halligan PW, Ellis HD. The Cardiff Anomalous Perceptions Scale (CAPS): A new validated measure of anomalous perceptual experience. Schizophr Bull. 2006;32(2):366–377. doi: 10.1093/schbul/sbj014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Peters E, Joseph S, Day S, Garety P. Measuring delusional ideation: The 21-item Peters et al. Delusions Inventory (PDI) Schizophr Bull. 2004;30(4):1005–1022. doi: 10.1093/oxfordjournals.schbul.a007116. [DOI] [PubMed] [Google Scholar]
- 18.Kwapil TR, Gross GM, Silvia PJ, Barrantes-Vidal N. Prediction of psychopathology and functional impairment by positive and negative schizotypy in the Chapmans’ ten-year longitudinal study. J Abnorm Psychol. 2013;122(3):807–815. doi: 10.1037/a0033759. [DOI] [PubMed] [Google Scholar]
- 19.Johns LC, van Os J. The continuity of psychotic experiences in the general population. Clin Psychol Rev. 2001;21(8):1125–1141. doi: 10.1016/s0272-7358(01)00103-9. [DOI] [PubMed] [Google Scholar]
- 20.van Os J, Linscott RJ, Myin-Germeys I, Delespaul P, Krabbendam L. A systematic review and meta-analysis of the psychosis continuum: Evidence for a psychosis proneness-persistence-impairment model of psychotic disorder. Psychol Med. 2009;39(2):179–195. doi: 10.1017/S0033291708003814. [DOI] [PubMed] [Google Scholar]
- 21.Stochl J, et al. Mood, anxiety and psychotic phenomena measure a common psychopathological factor. Psychol Med. 2015;45(7):1483–1493. doi: 10.1017/S003329171400261X. [DOI] [PubMed] [Google Scholar]
- 22.Cuthbert BN, Insel TR. Toward the future of psychiatric diagnosis: The seven pillars of RDoC. BMC Med. 2013;11(1):126. doi: 10.1186/1741-7015-11-126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moore C, Cavanagh P. Recovery of 3D volume from 2-tone images of novel objects. Cognition. 1998;67(1-2):45–71. doi: 10.1016/s0010-0277(98)00014-6. [DOI] [PubMed] [Google Scholar]
- 24.Hegdé J, Kersten D. A link between visual disambiguation and visual memory. J Neurosci. 2010;30(45):15124–15133. doi: 10.1523/JNEUROSCI.4415-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dolan RJ, et al. How the brain learns to see objects and faces in an impoverished context. Nature. 1997;389(6651):596–599. doi: 10.1038/39309. [DOI] [PubMed] [Google Scholar]
- 26.Hsieh PJ, Vul E, Kanwisher N. Recognition alters the spatial pattern of FMRI activation in early retinotopic cortex. J Neurophysiol. 2010;103(3):1501–1507. doi: 10.1152/jn.00812.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mason O, Claridge G, Jackson M. New scales for the assessment of schizotypy. Pers Individ Dif. 1995;18(1):7–13. [Google Scholar]
- 28.Raine A. The SPQ: A scale for the assessment of schizotypal personality based on DSM-III-R criteria. Schizophr Bull. 1991;17(4):555–564. doi: 10.1093/schbul/17.4.555. [DOI] [PubMed] [Google Scholar]
- 29.Chapman J. The early symptoms of schizophrenia. Br J Psychiatry. 1966;112(484):225–251. doi: 10.1192/bjp.112.484.225. [DOI] [PubMed] [Google Scholar]
- 30.Leitman DI, et al. Sensory deficits and distributed hierarchical dysfunction in schizophrenia. Am J Psychiatry. 2010;167(7):818–827. doi: 10.1176/appi.ajp.2010.09030338. [DOI] [PubMed] [Google Scholar]
- 31.Huang Y, Rao RPN. Predictive coding. Wiley Interdiscip Rev Cogn Sci. 2011;2(5):580–593. doi: 10.1002/wcs.142. [DOI] [PubMed] [Google Scholar]
- 32.Spratling MW. Predictive coding as a model of response properties in cortical area V1. J Neurosci. 2010;30(9):3531–3543. doi: 10.1523/JNEUROSCI.4911-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Van de Cruys S, et al. Precise minds in uncertain worlds: Predictive coding in autism. Psychol Rev. 2014;121(4):649–675. doi: 10.1037/a0037665. [DOI] [PubMed] [Google Scholar]
- 34.Lawson RP, Rees G, Friston KJ. An aberrant precision account of autism. Front Hum Neurosci. 2014;8:302. doi: 10.3389/fnhum.2014.00302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Adams RA, Stephan KE, Brown HR, Frith CD, Friston KJ. 2013. The computational anatomy of psychosis. Front Integr Neurosci 4(47):1–26.
- 36.Kapur S. Psychosis as a state of aberrant salience: A framework linking biology, phenomenology, and pharmacology in schizophrenia. Am J Psychiatry. 2003;160(1):13–23. doi: 10.1176/appi.ajp.160.1.13. [DOI] [PubMed] [Google Scholar]
- 37.Dakin S, Carlin P, Hemsley D. Weak suppression of visual context in chronic schizophrenia. Curr Biol. 2005;15(20):R822–R824. doi: 10.1016/j.cub.2005.10.015. [DOI] [PubMed] [Google Scholar]
- 38.Tibber MS, et al. 2013. Visual surround suppression in schizophrenia. Front Integr Neurosci 4(88):1–13.
- 39.Kantrowitz JT, Butler PD, Schecter I, Silipo G, Javitt DC. Seeing the world dimly: The impact of early visual deficits on visual experience in schizophrenia. Schizophr Bull. 2009;35(6):1085–1094. doi: 10.1093/schbul/sbp100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schneider U, et al. Reduced binocular depth inversion in schizophrenic patients. Schizophr Res. 2002;53(1-2):101–108. doi: 10.1016/s0920-9964(00)00172-9. [DOI] [PubMed] [Google Scholar]
- 41.Keane BP, Silverstein SM, Wang Y, Papathomas TV. Reduced depth inversion illusions in schizophrenia are state-specific and occur for multiple object types and viewing conditions. J Abnorm Psychol. 2013;122(2):506–512. doi: 10.1037/a0032110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Barlow HB. The knowledge used in vision and where it comes from. Philos Trans R Soc Lond B Biol Sci. 1997;352(1358):1141–1147. doi: 10.1098/rstb.1997.0097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hill H, Johnston A. The hollow-face illusion: Object-specific knowledge, general assumptions or properties of the stimulus? Perception. 2007;36(2):199–223. doi: 10.1068/p5523. [DOI] [PubMed] [Google Scholar]
- 44.Pawełczyk A, et al. Figural fluency and immediate visual memory in patients with at-risk mental state for psychosis: Empirical study. Early Interv Psychiatry. 2015;9(4):324–330. doi: 10.1111/eip.12116. [DOI] [PubMed] [Google Scholar]
- 45.Schmidt-Hansen M, Honey RC. Working memory and multidimensional schizotypy: Dissociable influences of the different dimensions. Cogn Neuropsychol. 2009;26(7):655–670. doi: 10.1080/02643291003644501. [DOI] [PubMed] [Google Scholar]
- 46.Grützner C, et al. Deficits in high- (>60 Hz) gamma-band oscillations during visual processing in schizophrenia. Front Hum Neurosci. 2013;7(88):88. doi: 10.3389/fnhum.2013.00088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sun L, et al. Evidence for dysregulated high-frequency oscillations during sensory processing in medication-naïve, first episode schizophrenia. Schizophr Res. 2013;150(2-3):519–525. doi: 10.1016/j.schres.2013.08.023. [DOI] [PubMed] [Google Scholar]
- 48.Javitt DC. When doors of perception close: Bottom-up models of disrupted cognition in schizophrenia. Annu Rev Clin Psychol. 2009;5(1):249–275. doi: 10.1146/annurev.clinpsy.032408.153502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Butler PD, Silverstein SM, Dakin SC. Visual perception and its impairment in schizophrenia. Biol Psychiatry. 2008;64(1):40–47. doi: 10.1016/j.biopsych.2008.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Doniger GM, Silipo G, Rabinowicz EF, Snodgrass JG, Javitt DC. Impaired sensory processing as a basis for object-recognition deficits in schizophrenia. Am J Psychiatry. 2001;158(11):1818–1826. doi: 10.1176/appi.ajp.158.11.1818. [DOI] [PubMed] [Google Scholar]
- 51.Robol V, et al. Reduced crowding and poor contour detection in schizophrenia are consistent with weak surround inhibition. PLoS ONE. 2013;8(4):e60951. doi: 10.1371/journal.pone.0060951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Silverstein SM, Keane BP. Perceptual organization impairment in schizophrenia and associated brain mechanisms: Review of research from 2005 to 2010. Schizophr Bull. 2011;37(4):690–699. doi: 10.1093/schbul/sbr052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Uhlhaas PJ, Silverstein SM. Perceptual organization in schizophrenia spectrum disorders: Empirical research and theoretical implications. Psychol Bull. 2005;131(4):618–632. doi: 10.1037/0033-2909.131.4.618. [DOI] [PubMed] [Google Scholar]
- 54.Cattell RB, Cattell AK. Measuring Intelligence with the Culture Fair Tests. Institute for Personality and Ability Testing; Champaign, IL: 1973. [Google Scholar]
- 55.Kleiner M, Brainard D, Pelli D. 2007. What's new in psychtoolbox-3? Perception 36(14):1.
- 56.Brainard DH. The psychophysics toolbox. Spat Vis. 1997;10(4):433–436. [PubMed] [Google Scholar]
- 57.Macmillan NA, Creelman CD. Detection Theory: A User's Guide. 2nd Ed Lawrence Erbaum Associates; Mahwah, NJ: 2005. [Google Scholar]
- 58.Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Arch Gen Psychiatry. 1961;4(6):561–571. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- 59.Spielberger CD, Gorsuch RL, Lushene R, Vagg PR, Jacobs GA. Manual for the State-Trait Anxiety Inventory. Consulting Psychologists Press; Palo Alto, CA: 1983. [Google Scholar]