Abstract
Two articles published by Pauling and Coryell in PNAS nearly 80 years ago described in detail the magnetic properties of oxy- and deoxyhemoglobin, as well as those of closely related compounds containing hemes. Their measurements revealed a large difference in magnetism between oxygenated and deoxygenated forms of the protein and, along with consideration of the observed diamagnetism of the carbonmonoxy derivative, led to an electronic structural formulation of oxyhemoglobin. The key role of hemoglobin as the main oxygen carrier in mammalian blood had been established earlier, and its allosteric behavior had been described in the 1920s. The Pauling–Coryell articles on hemoglobin represent truly seminal contributions to the field of bioinorganic chemistry because they are the first to make connections between active site electronic structure and the function of a metalloprotein.
Keywords: heme, bioinorganic, magnetism, oxygen transport, metalloproteins
Hemoglobin occupies a very special place in the annals of chemistry. Work on the protein began in the early 1800s, when it was found to be a major component of mammalian blood cells and, importantly, found to contain iron. Isolation of hemoglobin was achieved by Hünefeld in 1840 (1); shortly thereafter, it was determined to have four one-iron subunits, each with a relative molecular mass of 16,000 (an unprecedentedly large value at the time). Research aimed at discovery of other properties of hemoglobin followed almost immediately, including observations in 1845 by Michael Faraday that it was not magnetic, in contrast to most other iron-containing matter (2). These measurements were conducted by placement of a sample between the poles of a magnet and measuring its change in weight. Magnetic samples were drawn into the field, giving an increase in weight; nonmagnetic (diamagnetic) materials were repelled by the field, accompanied by a negligible or slight decrease in weight. In this context, remember that it was fully 80 years later that the notion of electron spin would be proposed as the principal basis of magnetic behavior.
Our story is based on the work of Linus Pauling and Charles Coryell, who, in 1936, published two articles in PNAS on the magnetic properties of hemoglobin and related substances (3, 4). Pauling and Coryell confirmed earlier findings that oxygenated as well as carbonmonoxy forms of hemoglobin were diamagnetic but discovered that the deoxygenated protein was magnetic, having four unpaired electrons per Fe atom. This result would have major implications in establishing the role of the protein as an oxygen transporter in the red blood of mammalian systems. In this Perspective, we discuss the Pauling–Coryell articles and their role in the subsequent molecular-based understanding of oxygen transport, as well as in the binding of other small molecules to heme iron centers.
The measurements by Pauling and Coryell were conducted using a Gouy balance in which the weight of a sample was determined in the absence and presence of a magnetic field. The field was provided by an electromagnet, and sample weights were measured at two different field strengths. The specific examples examined included ferroheme, ferriheme, oxyferroheme, carbonmonoxyferroheme, and a variety of other heme compounds. The balance that was used for these measurements resides in the Chair's office of the Division of Chemistry and Chemical Engineering at the California Institute of Technology (Caltech) (Fig. 1). In the data analysis, the samples were corrected for their small inherent diamagnetism. Based on these measurements, the previously observed diamagnetism of hemoglobin in the form of oxyferroheme, as well as that of carbonmonoxyferroheme, was confirmed whereas deoxyferroheme was determined to possess magnetism characteristic of unpaired electrons. Therefore, arterial and venous blood were found to have different magnetic properties, the basis of which related directly to the function of the protein as the principal oxygen carrier in mammalian blood.
Fig. 1.
The balance used by Coryell and Pauling in 1936 to do the Gouy magnetic susceptibility experiments on oxy- and deoxyhemoglobins, as well as other hemochromogens and hemoglobin derivatives. Image courtesy of J. Barton.
During the decade after the development of quantum mechanics, great strides were made in understanding bonding in molecules. In 1931, Pauling authored a compelling article entitled “The Nature of the Chemical Bond” that brought together the concepts of quantum mechanics, the electron pair bond, and the structures of molecules (5). The title of this paper would be used by Pauling in subsequent publications, including his classic textbook on the subject. In his 1931 treatise, which appeared in the Journal of the American Chemical Society, Pauling introduced the notion of hybridization of metal atomic orbitals and different hybridization schemes that correlated with observed or implied structures of metal-containing compounds. Most notable were the d2sp3 scheme that generated six equivalent hybrids directed to the vertices of an octahedron and dsp2 hybridization that gave four equivalent orbitals directed to the corners of a square, in striking contrast with the sp3 hybrids of tetrahedral carbon in saturated organic compounds.
In this context, the electronic structure of carbonmonoxyhemoglobin was easiest to understand. The porphyrin ring of the protein was known to contain four pyrrole N atoms capable of binding to Fe in a plane, with one axial bond to the globin and the other connecting to carbon monoxide. The six d2sp3 hybrids of Fe were used to form these bonds, leaving the remaining three d orbitals to accommodate the six electrons of Fe(II). A completely spin-paired arrangement was thus achieved, consistent with the observed diamagnetism of carbonmonoxyhemoglobin. The diamagnetic behavior of oxygenated hemoglobin was similarly accommodated, but, according to Pauling and Coryell, “The oxygen molecule undergoes a profound change in electronic structure on combination with hemoglobin” in light of the S = 1 paramagnetism of the O2 ground state (4). The coordination of O2 to Fe was represented in terms of two “resonating” structures shown below, but without any discussion of resultant charges on either the Fe(II) ion or the O2 addend. More about that later.

The observation of magnetism for deoxyhemoglobin was the key experimental feature of the Pauling–Coryell work. From magnetic susceptibility measurements, it was determined that the magnetic moment (μ) for the deoxy protein was 5.46 Bohr magnetons (B.M.), which is only slightly larger than the 4.90 B.M. value expected for four unpaired spins per Fe(II) based on a spin-only formula. Although the result had a major impact on the subject of hemoglobin and oxygen transport, the Pauling—Coryell interpretation proved incorrect. Specifically, they wrote, “this (the magnetic susceptibility) shows that there are present in each heme four unpaired electrons, and that consequently the iron atom is not attached to the four porphyrin nitrogen atoms and the globin molecule by covalent bonds, but is present as a ferrous ion, the bonds to the neighboring atoms being essentially ionic bonds.” The particular nature of the neighboring atoms is not specified, but discussion of the magnetic properties of ferrous compounds is presented, suggesting that significant quenching of orbital angular momentum to the total magnetic moment is seen in such Fe(II) compounds. The authors added, “It is not yet possible to discuss the significance of these structural differences in detail, but they are without doubt closely related to and in a sense responsible for the characteristic properties of hemoglobin (for oxygen transport).” (4)
One of the most important of the characteristic properties was discovered in the 1920s: namely, that the hemoglobin/O2 equilibrium was affected by pH and that the four heme subunits of hemoglobin did not bind O2 with the same formation constant. In work by Adair (6, 7) and Ferry and Green (8), oxygen binding was found to proceed in a cooperative way: the more O2 that was bound, the easier it was to bind the next O2. Pauling published an analysis of the problem in PNAS in 1935, deriving an equation using only two variables rather than the four different equilibrium binding constants used by Adair (9). The basis of the cooperativity, or allosterism as it is also called, was not known at the time but related directly to the change in hemoglobin spin state in going from the paramagnetic deoxy form to the diamagnetic oxy form. It took another 33 years before the basis for the spin state change and the allosteric behavior would be elucidated through the seminal work of Max Perutz.
Pauling's 1935 analysis of hemoglobin cooperativity postulated that the protein bound four equivalent hemes on the protein surface arranged in a square formation (9). Cooperativity induced by ligand binding to the hemes in this arrangement would require communication between and among the sites over relatively large distances. The first X-ray crystal structure of human hemoglobin, one of low resolution (5.5 Å) determined by Perutz in 1959, provided a test of Pauling's predictions (10). Although the hemes were found to be encapsulated by globin and not on the surface, Pauling was correct in the sense that they were not in direct contact and, indeed, were more than 25 Å apart. Furthermore, changes in quaternary structure as a function of heme ligation state were observed. The determination of the hemoglobin structure was the basis for the 1962 Nobel Prize in Chemistry to Perutz, which he shared with John Kendrew, who had solved the structure of myoglobin. Incidentally, these structures played a role in confirming the details of an unrelated 1951 prediction by Pauling in the Proceedings of the National Academy of Sciences by revealing the structure of the alpha helix (11, 12).
In addition to postulating that each of the hemes must somehow communicate its ligation state at a distance, Pauling also correctly predicted that the change in magnetism upon oxygen binding was important in the function of the protein, based on expected differences in bonding linked to spin state (3). Structural studies on the proteins and on synthetic model compounds were both important in testing the hypothesis that iron spin state change triggers geometrical changes essential for allostery. It should be emphasized that model compounds led the way to understanding this notion, with J. Lynn Hoard's structural characterization of high-spin and low-spin iron porphyrin derivatives in the middle to late 1960s. Hoard, a former student of Pauling, found that the high-spin ferric ion lies 0.4–0.5 Å out of the plane of the four porphyrin nitrogens in model compounds, forming relatively long bonds (2.06–2.07 Å) (13, 14). In contrast, low-spin ferric porphyrins have Fe(III) nearly in the heme plane and with shorter (1.989 Å) Fe-N(porphyrin) bonds (15). Although these studies were on ferric porphyrins, Hoard predicted that spin-state changes in ferrous globins would lead to similar changes in stereochemistry, and thus O2 binding would yield substantial movement of Fe relative to the porphyrin ring. There were no structures of ferrous porphyrins available; and, sadly, this situation would remain unchanged for some time.
During the same period (1968), Perutz solved structures of hemoglobin at higher (2.8-Å) resolution, permitting a more detailed analysis of the structural basis for cooperativity (16–18). His analysis relied on comparison of horse deoxy- and methemoglobin [high-spin Fe(III)] and a derivative modified to stabilize the quaternary structure of the oxy ferrous state. A structure of oxyhemoglobin had not been determined at this time (contrary to the earlier report by Perutz), owing to problems with oxidation to the met or Fe(III) form during data collection. Although this structure allowed analysis of differences in tertiary and quaternary structure between the derivatives, referred to as the relaxed (R) state (oxy) and tense (T) state (deoxy), the resolution was still insufficient to define changes clearly in heme stereochemistry. Nevertheless, Perutz made use of a ruler in a reflecting box to estimate that there was a 0.75-Å displacement of Fe from the heme plane in horse deoxyhemoglobin, and a 0.3-Å displacement in horse methemoglobin, which is high-spin and six-coordinate. The suggestion was that these properties fall between those of deoxyhemoglobin (high-spin, five-coordinate) and oxyhemoglobin (low-spin, six-coordinate), which was met with significant skepticism, although the conclusions would later prove to be qualitatively correct. In making his case for the “trigger” hypothesis, Perutz also drew on Hoard's results on model systems. A concluding statement in his analysis made a clear link between the properties of the iron and the function of hemoglobin: “It is remarkable that there should be such an exceedingly complex, subtle and elegant instrument of respiratory transport, exploiting a difference in atomic radius of 13 per cent between the covalent and ionic forms of iron.” (19)
The limitations of the hemoglobin structures, including their modest resolution and the instability of the oxy derivative over the experimental time frame, necessitated the use of alternative approaches to test the trigger hypothesis. In particular, researchers drew on a number of spectroscopic methods. Extended X-ray absorption fine structure (EXAFS) studies by Eisenberger et al. (1978) (20) and Perutz et al. (1982) (21) on deoxyhemoglobin provided measures of Fe-N(porphyrin) bond lengths, showing an increase for deoxy relative to the oxy protein. However, the extent of displacement of Fe from the porphyrin core could not be determined from these data. Perutz took advantage of the link between hemoglobin structure and spin state to monitor the effects of the R-to-T transition on the absorption spectrum of ferric iron. He prepared partially reduced hemoglobin in the presence of CO, resulting in hemoglobin Fe(II)-CO (low-spin)/Fe(III) (high-spin) hybrids. If CO binding to the Fe(II) subunits induces a transition to the R state in the unligated Fe(III) subunits, the ferric ions will shift toward a low-spin configuration, as seen by a decrease in intensity and a red-shift of the 620-nm absorption band. Indeed, this red-shift is the behavior that Perutz observed (22). Support for the model also came from NMR (23) and EPR (24) investigations.
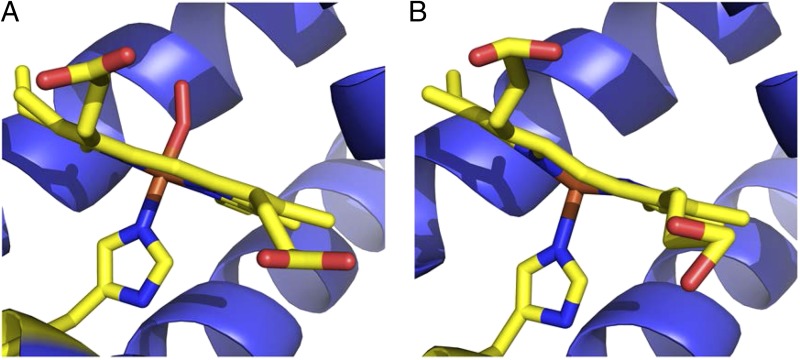
Several years later, higher resolution structures of hemoglobin provided more definitive data on heme stereochemistry. The 2.1-Å structure of oxyhemoglobin was reported in 1983 by Shaanan (also at Cambridge and a collaborator of Perutz) (25), followed by Perutz's report of the 1.74-Å structure of deoxyhemoglobin (26) (Fig. 2). The geometry of the Fe in deoxyhemoglobin was indeed found to be similar to the five-coordinate model compounds of Hoard, with 0.40(5)-Å (alpha subunits) and 0.36(5)-Å (beta subunits) distances of Fe from the porphyrin N mean plane whereas the oxyhemes were close to planar. This work was the first to show definitively the doming of the heme toward the axial His in deoxyhemoglobin, providing strong support for Perutz's trigger hypothesis and Pauling's much earlier proposal that changes in iron spin state lead to the large conformational movements in hemoglobin responsible for allostery. Crystal structures of high-spin Fe(II) porphyrin complexes many years later provided additional evidence in support for the trigger hypothesis. In contrast to a 1973 structure determination of a high-spin, five-coordinate Fe(II) tetraphenylporphyrin, which was complicated by disorder (27), related studies 30 years later yielded detailed structural information on tetraphenyl and octaethyl high-spin Fe(II) porphyrin derivatives (28, 29). The latter of these studies showed different ferrous ion displacements from the heme plane toward the axial imidazole ligand in different derivatives, consistent with the proposal by Perutz that ferrous deoxy heme stereochemistry differs between the alpha and beta subunits of hemoglobin.
Fig. 2.
A view of the oxygen binding sites of oxyhemoglobins (A) and deoxyhemoglobins (B). The view is edge-on with respect to the porphyrin and reveals the subtle structural difference with regard to iron residing in the porphyrin plane in oxyhemoglobin and out of the plane in deoxyhemoglobin, with consequent “doming” of the porphyrin ring. Figure prepared from Protein Data Bank files 1HHO (25) and 4HHB (26) using Pymol (52).
Lively discussions of the nature of iron–oxygen bonding in oxygenated hemoglobin continue to this day. The resonance structures Pauling proposed imply that oxygen is innocent: that is, it has not taken an electron from Fe(II) to form an Fe(III)-superoxo unit. But there is a great body of evidence that oxygen is noninnocent in metal complexes. Arguably the most compelling evidence is the finding that Co(II) packaged by N-donor ligands transfers an electron to oxygen in forming Co(III)-superoxo complexes (30) and that oxidative addition of oxygen to phosphine-Co(I) units produces side-bonded Co(III)-peroxos (31). Of relevance here is that J. J. Weiss formulated the iron–oxygen bond in oxyhemoglobin as an Fe(III)-superoxo unit (32), which led to a spirited exchange between Pauling and Weiss (33, 34). Also of interest is that Irving Klotz insisted on the noninnocence of oxygen in forming bonds to metals in other blood proteins. In formulations of Fe–oxygen bonding in the nonheme reddish purple blood protein hemerythrin and of Cu–oxygen bonding in the blue blood protein oxyhemocyanin, Klotz and Klotz proposed that the oxygen molecule had been reduced to peroxide (35). In 1972, magnetic susceptibility measurements strongly supported this formulation in the case of hemerythrin because the oxygenated protein was found to be weakly paramagnetic, containing a superexchange-coupled Fe(III)-O-Fe(III)-hydroperoxide unit (36). Four years later, resonance Raman experiments carried out by Donald Kurtz et al. confirmed that the oxygen in oxyhemerythrin is an iron-bound peroxide (37).
But if oxyhemoglobin contains an Fe(III)-superoxo unit, how can we account for its diamagnetism? One explanation is that the low-spin Fe(III) is antiferromagnetically coupled to the S = 1/2 superoxide ion, giving an S = 0 ground state with a spin triplet at higher energy. Interestingly, Massimo Cerdonio et al. proposed that a triplet was populated in the temperature range 25–250 K, based on magnetic susceptibility data collected on frozen samples of oxyhemoglobin (38). Pauling insisted that this result could not be correct (39), as did others (40), but the Italian group reported additional experiments at room temperature in support of their model (41). In 1985, however, Cerdonio threw in the towel (42). One of us (H.B.G.) well remembers discussing these papers with Pauling at the Caltech Athenaeum. When told that his early work had not been disproved, his reaction was: “Why did you bother to tell me? I knew I was right.”
Current views of iron–oxygen bonding in hemoglobin are mixed. It would seem that reality is more complex than implied by the limiting models proposed by Pauling and Weiss; indeed, our understanding of the Fe(II)–O2 interaction in oxygenated hemoglobin continues to evolve. In 2013, Edward I. Solomon and coworkers reported analyses of iron L-edge X-ray absorption spectra of an oxy-picket fence porphyrin, a model for oxyferrohemoglobin (43). They considered three limiting descriptions of the Fe–O2 unit, for which they also prepared synthetic models: (i) the Pauling model, with an Fe(II)–O2 unit in which both the iron and the oxygen are spin 0; (ii) the Weiss model, with a low-spin ferric ion coupled to a superoxide ion; and (iii) a model put forth by McClure, Harcourt, and Goddard in which intermediate-spin Fe(II) (S = 1) interacts with triplet oxygen. In models ii and iii, the iron and its ligand would couple antiferromagnetically to yield the observed diamagnetic (S = 0) ground state. Their data reveal that the Fe(II) in the iron–oxy complex has a Zeff between that of low-spin Fe(II) and Fe(III), but closer to that of Fe(II). Furthermore, they found that there is up to 15% metal character in the low-energy d(π) hole, indicating a contribution from a state resembling low-spin Fe(III). Of the three models, the intermediate-spin case has the least resemblance to the Fe(II)–O2 compound.
The Stanford group led by Solomon also recognized that hydrogen bonding to the oxygen ligand in the globins will have an effect on spin polarization, which will affect coupling to the iron, so that further studies on the globins and/or models incorporating hydrogen bonding were needed. And, indeed, the Stanford group, this time led by Sarangi, followed up with X-ray absorption spectroscopy and EXAFS studies of oxyhemoglobin (44). The results revealed dominant Fe(III)-O2− (Weiss model) character for solution-state oxy-hemoglobin and dominant Fe(II)-O2 character (Pauling model) in crystalline hemoglobin. The authors emphasized that iron–oxygen bonding in hemoglobin requires a multiconfigurational description that can be tuned by subtle differences in hydrogen bonding and solvation. In discussing their results, the Stanford group underscored the continued complexity of bonding in hemoglobin: In their words (43), “…the electronic structure of the iron in the Fe-O2 center is not simply described by any of these models, emphasizing the limitation of the three oxidation state descriptions … in describing this highly covalent compound.”
But should this conclusion be taken as the last word on the subject? We don’t think so. Work at Caltech by Grinstaff et al. showed that a high potential Fe(II) porphyrin has absolutely no interest in bonding to oxygen (45), supporting the notion that, unless Fe(II) has the ability to transfer an electron to oxygen to make a charge transfer complex, no bond is formed. Thus, the argument about the nature of the bond starts only after the oxygen is reduced. Does the resultant Fe(III) have properties similar to low-spin Fe(II) because the superoxide acts as a good σ donor? Apparently it does because all of the vibrational spectroscopic evidence points to the Fe(III)-superoxo formulation of the iron–oxygen unit in oxygenated hemes (46–48).
Even though current understanding of the electronic structure of the iron–oxygen unit in oxyhemoglobin is not perfect, it should be remembered that Pauling correctly described the bond as highly covalent, referring to extensive electron sharing between O2 and iron. On the other hand, he was wrong about Fe(II)–porphyrin bonding in deoxyhemoglobin, which he described as “ionic.” Pauling did not have much use for ligand field theory (LFT), but, if he had paid more attention to it, he would have realized that the binding of O2 increases the energy separation between the 3d-L π and σ* orbitals, leading to a low-spin iron center, whereas, in deoxyhemoglobin, the smaller separation between the 3d-L π and σ* orbitals produces a high-spin Fe(II) ground state. LFT thus becomes essential to appreciating fully the changes in the iron coordination that are intimately linked to allostery in oxygen binding and release.
Why do we say that Pauling’s work on hemoglobin was a beginning of bioinorganic chemistry? After all, metalloproteins were investigated long before the 1930s, and some would say the field began with the landmark investigations of cytochrome oxidase by Otto Warburg (49) and David Keilin (50). But Pauling occupies a special place in the history of the field because he was the first to attempt to come to grips with the role of structure around the metal in dictating function; and the development of structure/function relationships has been a central theme of modern bioinorganic chemistry, with much attention paid to metal ion electronic structure (51). And the many advances we have witnessed in biochemistry, structural biology, metallosite synthetic chemistry, spectroscopy, and theory all have contributed to the current understanding of the functions of metals in biology.
Acknowledgments
We thank Jay R. Winkler for a critical reading of the manuscript and for several helpful suggestions. Research in biological inorganic chemistry at the California Institute of Technology is supported by the National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health under Award R01DK019038 (to H.B.G. and J. R. Winkler) and at the University of Rochester is supported by National Science Foundation Award CHE-1409929 (to K.L.B.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article is part of the special series of PNAS 100th Anniversary articles to commemorate exceptional research published in PNAS over the last century. See the companion articles, “The magnetic properties and structure of the hemochromogens and related substances” on page 159 in issue 3 of volume 22, and “The magnetic properties and structure of hemoglobin, oxyhemoglobin and carbonmonoxyhemoglobin” on page 210 in issue 4 of volume 22.
References
- 1.Hünefeld FL. Der Chemismus in der Thierischen Organisation. Brockhaus; Leipzig: 1840. [Google Scholar]
- 2.Faraday M. II. Experimental researches in electricity–twentieth series: On new magnetic actions and on the magnetic condition of all matter. Philos Trans R Soc Lond. 1846;136:29. [Google Scholar]
- 3.Pauling L, Coryell CD. The magnetic properties and structure of the hemochromogens and related substances. Proc Natl Acad Sci USA. 1936;22(3):159–163. doi: 10.1073/pnas.22.3.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pauling L, Coryell CD. The magnetic properties and structure of hemoglobin, oxyhemoglobin and carbonmonoxyhemoglobin. Proc Natl Acad Sci USA. 1936;22(4):210–216. doi: 10.1073/pnas.22.4.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pauling L. The nature of the chemical bond: Application of results obtained from the quantum mechanics and from a theory of paramagnetic susceptibility to the structure of molecules. J Am Chem Soc. 1931;53:1367–1400. [Google Scholar]
- 6.Adair GS. The osmotic pressure of haemoglobin in the absence of salts. Proc R Soc Lond, A Contain Pap Math Phys Character. 1925;109(750):292–300. [Google Scholar]
- 7.Adair GS. The hemoglobin system. VI. The oxygen dissociation curve of hemoglobin. J Biol Chem. 1925;63(2):529–545. [Google Scholar]
- 8.Ferry RM, Green AA. Studies in the chemistry of hemoglobin. III. The equilibrium between oxygen and hemoglobin and its relation to changing hydrogen ion activity. J Biol Chem. 1929;81(1):175–203. [Google Scholar]
- 9.Pauling L. The oxygen equilibrium of hemoglobin and its structural interpretation. Proc Natl Acad Sci USA. 1935;21(4):186–191. doi: 10.1073/pnas.21.4.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Perutz MF, et al. Structure of haemoglobin: A three-dimensional Fourier synthesis at 5.5-Å resolution, obtained by X-ray analysis. Nature. 1960;185(4711):416–422. doi: 10.1038/185416a0. [DOI] [PubMed] [Google Scholar]
- 11.Pauling L, Corey RB, Branson HR. The structure of proteins: Two hydrogen-bonded helical configurations of the polypeptide chain. Proc Natl Acad Sci USA. 1951;37(4):205–211. doi: 10.1073/pnas.37.4.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Perutz MF. New x-ray evidence on the configuration of polypeptide chains. Nature. 1951;167(4261):1053–1054. doi: 10.1038/1671053a0. [DOI] [PubMed] [Google Scholar]
- 13.Hoard JL, Hamor MJ, Hamor TA, Caughey WS. Crystal structure and molecular sterochemistry of methoxyiron(III) mesoporphyrin-9 dimethyl ester. J Am Chem Soc. 1965;87(11):2312–2319. [Google Scholar]
- 14.Hoard JL, Cohen GH, Glick MD. Stereochemistry of coordination group in an iron(III) derivative of tetraphenylporphine. J Am Chem Soc. 1967;89(9):1992–1996. [Google Scholar]
- 15.Countryman R, Collins DM, Hoard JL. Stereochemistry of the low-spin iron porphyrin, bis(imidazole)-alpha,beta,gamma,tetraphenylporphinatoiron(III) chloride. J Am Chem Soc. 1969;91(18):5166–5167. doi: 10.1021/ja01046a042. [DOI] [PubMed] [Google Scholar]
- 16.Bolton W, Cox JM, Perutz MF. Structure and function of haemoglobin. IV. A three-dimensional Fourier synthesis of horse deoxyhaemoglobin at 5.5 Å resolution. J Mol Biol. 1968;33(1):283–297. doi: 10.1016/0022-2836(68)90294-5. [DOI] [PubMed] [Google Scholar]
- 17.Perutz MF, Muirhead H, Cox JM, Goaman LCG. Three-dimensional Fourier synthesis of horse oxyhaemoglobin at 2.8 Å resolution: The atomic model. Nature. 1968;219(5150):131–139. doi: 10.1038/219131a0. [DOI] [PubMed] [Google Scholar]
- 18.Perutz MF, et al. Three-dimensional Fourier synthesis of horse oxyhaemoglobin at 2.8 A resolution: (1) X-ray analysis. Nature. 1968;219(5149):29–32. doi: 10.1038/219029a0. [DOI] [PubMed] [Google Scholar]
- 19.Perutz MF. Stereochemistry of cooperative effects in haemoglobin. Nature. 1970;228(5273):726–739. doi: 10.1038/228726a0. [DOI] [PubMed] [Google Scholar]
- 20.Eisenberger P, Shulman RG, Kincaid BM, Brown GS, Ogawa S. Extended X-ray absorption fine structure determination of iron nitrogen distances in haemoglobin. Nature. 1978;274(5666):30–34. doi: 10.1038/274030a0. [DOI] [PubMed] [Google Scholar]
- 21.Perutz MF, Hasnain SS, Duke PJ, Sessler JL, Hahn JE. Stereochemistry of iron in deoxyhaemoglobin. Nature. 1982;295(5849):535–538. doi: 10.1038/295535a0. [DOI] [PubMed] [Google Scholar]
- 22.Perutz MF. Nature of haem-haem interaction. Nature. 1972;237(5357):495–499. doi: 10.1038/237495a0. [DOI] [PubMed] [Google Scholar]
- 23.Lindström TR, Ho C, Pisciotta AV. Nuclear magnetic resonance studies of haemoglobin M Milwaukee. Nat New Biol. 1972;237(78):263–264. doi: 10.1038/newbio237263a0. [DOI] [PubMed] [Google Scholar]
- 24.Smith DW, Williams RJP. The spectra of ferric haems and haemoproteins. Struct Bond. 1970;7:1–49. [Google Scholar]
- 25.Shaanan B. Structure of human oxyhaemoglobin at 2.1 Å resolution. J Mol Biol. 1983;171(1):31–59. doi: 10.1016/s0022-2836(83)80313-1. [DOI] [PubMed] [Google Scholar]
- 26.Fermi G, Perutz MF, Shaanan B, Fourme R. The crystal structure of human deoxyhaemoglobin at 1.74 Å resolution. J Mol Biol. 1984;175(2):159–174. doi: 10.1016/0022-2836(84)90472-8. [DOI] [PubMed] [Google Scholar]
- 27.Hoard JL, Scheidt WR. Stereochemical trigger for initiating cooperative interaction of the subunits during the oxygenation of cobaltohemoglobin. Proc Natl Acad Sci USA. 1973;70(12):3919–3922. doi: 10.1073/pnas.70.12.3919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ellison MK, Schulz CE, Scheidt WR. Structure of the deoxymyoglobin model [Fe(TPP)(2-MeHIm)] reveals unusual porphyrin core distortions. Inorg Chem. 2002;41(8):2173–2181. doi: 10.1021/ic020012g. [DOI] [PubMed] [Google Scholar]
- 29.Hu C, An J, Noll BC, Schulz CE, Scheidt WR. Electronic configuration of high-spin imidazole-ligated iron(II) octaethylporphyrinates. Inorg Chem. 2006;45(10):4177–4185. doi: 10.1021/ic052194v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Miskowski VM, Robbins JL, Treitel IM, Gray HB. Electronic structure and spectra of μ-superoxo-dicobalt(III) complexes. Inorg Chem. 1975;14(10):2318–2321. [Google Scholar]
- 31.Miskowski VM, Robbins JL, Hammond GS, Gray HB. Preparation and spectroscopic properties of cobalt(III) complexes containing phosphine ligands: Electronic structural description of side-bonded dioxygen. J Am Chem Soc. 1976;98(9):2477–2483. [Google Scholar]
- 32.Weiss JJ. Nature of the iron-oxygen bond in oxyhaemoglobin. Nature. 1964;202(4927):83–84. doi: 10.1038/202083b0. [DOI] [PubMed] [Google Scholar]
- 33.Pauling L. Nature of the iron-oxygen bond in oxyhaemoglobin. Nature. 1964;203:182–183 (lett). [PubMed] [Google Scholar]
- 34.Weiss JJ. 1964. Nature of the iron-oxygen bond in oxyhaemoglobin. Nature 203:183 (lett)
- 35.Klotz IM, Klotz TA. Oxygen-carrying proteins: A comparison of the oxygenation reaction in hemocyanin and hemerythrin with that in hemoglobin. Science. 1955;121(3145):477–480. doi: 10.1126/science.121.3145.477. [DOI] [PubMed] [Google Scholar]
- 36.Dawson JW, et al. A magnetic susceptibility study of hemerythrin using an ultrasensitive magnetometer. Biochemistry. 1972;11(3):461–465. doi: 10.1021/bi00753a026. [DOI] [PubMed] [Google Scholar]
- 37.Kurtz DM, Jr, Shriver DF, Klotz IM. Letter: Resonance Raman spectroscopy with unsymmetrically isotopic ligands: Differentiation of possible structures of hemerythrin complexes. J Am Chem Soc. 1976;98(16):5033–5035 (lett). doi: 10.1021/ja00432a064. [DOI] [PubMed] [Google Scholar]
- 38.Cerdonio M, et al. Magnetic properties of oxyhemoglobin. Proc Natl Acad Sci USA. 1977;74(2):398–400. doi: 10.1073/pnas.74.2.398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pauling L. Magnetic properties and structure of oxyhemoglobin. Proc Natl Acad Sci USA. 1977;74(7):2612–2613. doi: 10.1073/pnas.74.7.2612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Boso B, Debrunner PG, Wagner GC, Inubushi T. High-field, variable-temperature Mössbauer effect measurements on oxyhemeproteins. Biochim Biophys Acta. 1984;791(2):244–251. doi: 10.1016/0167-4838(84)90015-3. [DOI] [PubMed] [Google Scholar]
- 41.Cerdonio M, et al. Room-temperature magnetic properties of oxy- and carbonmonoxyhemoglobin. Proc Natl Acad Sci USA. 1978;75(10):4916–4919. doi: 10.1073/pnas.75.10.4916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cerdonio M, et al. Reexamination of the evidence for paramagnetism in oxy- and carbonmonoxyhemoglobins. Proc Natl Acad Sci USA. 1985;82(1):102–103. doi: 10.1073/pnas.82.1.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wilson SA, et al. Iron L-edge X-ray absorption spectroscopy of oxy-picket fence porphyrin: Experimental insight into Fe-O2 bonding. J Am Chem Soc. 2013;135(3):1124–1136. doi: 10.1021/ja3103583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wilson SA, et al. X-ray absorption spectroscopic investigation of the electronic structure differences in solution and crystalline oxyhemoglobin. Proc Natl Acad Sci USA. 2013;110(41):16333–16338. doi: 10.1073/pnas.1315734110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Grinstaff MW, Hill MG, Labinger JA, Gray HB. Mechanism of catalytic oxygenation of alkanes by halogenated iron porphyrins. Science. 1994;264(5163):1311–1313. doi: 10.1126/science.8191283. [DOI] [PubMed] [Google Scholar]
- 46.Potter WT, Tucker MP, Houtchens RA, Caughey WS. Oxygen infrared spectra of oxyhemoglobins and oxymyoglobins: Evidence of two major liganded O2 structures. Biochemistry. 1987;26(15):4699–4707. doi: 10.1021/bi00389a016. [DOI] [PubMed] [Google Scholar]
- 47.Das TK, Couture M, Ouellet Y, Guertin M, Rousseau DL. Simultaneous observation of the O—O and Fe—O2 stretching modes in oxyhemoglobins. Proc Natl Acad Sci USA. 2001;98(2):479–484. doi: 10.1073/pnas.98.2.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Spiro TG, Strekas TC. Resonance Raman spectra of heme proteins: Effects of oxidation and spin state. J Am Chem Soc. 1974;96(2):338–345. doi: 10.1021/ja00809a004. [DOI] [PubMed] [Google Scholar]
- 49.Warburg O. On iron, the oxygen-transferring element of breathing-enzymes. Biochem Z. 1924;152:479–494. [Google Scholar]
- 50.Keilin D. On cytochrome, a respiratory pigment, common to animals, yeast, and higher plants. Proc R Soc Lond, B. 1925;98(690):312–339. [Google Scholar]
- 51.Gray HB. Biological inorganic chemistry at the beginning of the 21st century. Proc Natl Acad Sci USA. 2003;100(7):3563–3568. doi: 10.1073/pnas.0730378100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Schrödinger, LLC (2012) The PyMOL Molecular Graphics System (Schrödinger, LLC, New York), Version 1.5.