Significance
People are not only concerned about climate change and its effects on plant and animal diversity but also about how humans are fundamentally changing the globe’s largest ecosystem that sustains economic revenue and food for many countries. We show that many species communities and ocean habitats will change from their current states. Ocean acidification and warming increase the potential for an overall simplification of ecosystem structure and function with reduced energy flow among trophic levels and little scope for species to acclimate. The future simplification of our oceans has profound consequences for our current way of life, particularly for coastal populations and those that rely on oceans for food and trade.
Keywords: ocean acidification, climate change, metaanalysis, diversity, acclimation
Abstract
Rising anthropogenic CO2 emissions are anticipated to drive change to ocean ecosystems, but a conceptualization of biological change derived from quantitative analyses is lacking. Derived from multiple ecosystems and latitudes, our metaanalysis of 632 published experiments quantified the direction and magnitude of ecological change resulting from ocean acidification and warming to conceptualize broadly based change. Primary production by temperate noncalcifying plankton increases with elevated temperature and CO2, whereas tropical plankton decreases productivity because of acidification. Temperature increases consumption by and metabolic rates of herbivores, but this response does not translate into greater secondary production, which instead decreases with acidification in calcifying and noncalcifying species. This effect creates a mismatch with carnivores whose metabolic and foraging costs increase with temperature. Species diversity and abundances of tropical as well as temperate species decline with acidification, with shifts favoring novel community compositions dominated by noncalcifiers and microorganisms. Both warming and acidification instigate reduced calcification in tropical and temperate reef-building species. Acidification leads to a decline in dimethylsulfide production by ocean plankton, which as a climate gas, contributes to cloud formation and maintenance of the Earth’s heat budget. Analysis of responses in short- and long-term experiments and of studies at natural CO2 vents reveals little evidence of acclimation to acidification or temperature changes, except for microbes. This conceptualization of change across whole communities and their trophic linkages forecast a reduction in diversity and abundances of various key species that underpin current functioning of marine ecosystems.
We have entered an era of increasing uncertainty about the effect of human activities on the function and services of ecological systems, particularly the effect of human greenhouse gas emissions on marine ecosystems (1–4). Progress on understanding of how climate change affects marine ecosystems has been slower than that of terrestrial ecosystems (4), partly because of the vast coverage of the oceans and renowned complexity of species interactions. Until now, there has been almost total reliance on qualitative reviews and perspectives about potential global ocean community and ecosystem change. Where quantitative assessments exist, they typically focus on single global stressors, single ecosystems, single-species assessments, or small subsets of species interactions that provide information on species performance and key species interactions (5). Recent assessments of multistressor effects at global scales show that more than half of the world’s ocean has experienced an increase in cumulative human impact over a 5-y time span, predominantly driven by increasing climate stressors such as sea surface temperature, ocean acidification, and UV radiation (6). Under a “business-as-usual” emission scenario, these stressors elevate the risk of substantial change to marine organisms and ecosystem services by 2100 (7).
The effects of warming on ecological processes and ecosystem functioning is substantially researched, but there is a tremendous knowledge gap in terms of the effects of ocean acidification. This gap is exacerbated by the inevitable combination of increasing ocean acidification and temperature and their potential interactive effects (8). Previous metaanalyses on the combined effects of climate change and ocean acidification typically targeted single-species responses (9, 10), single ecosystems (11), or life-stages (8). Such analyses detected a lack of difference between the effect sizes of acidification alone and the combined effect of acidification and elevated temperature (9) or identified variation in single-species responses, such as calcification, growth, photosynthesis, reproduction, and survival (10). Because species interactions play a key role in how organisms and their communities respond to global change (12), there remains a critical need for multispecies studies. However, a quantitative metaanalysis of community-level responses and insights in the potential underlying processes is absent. Here, we conceptualize change, based on peer-reviewed outcomes, by quantitatively assessing the direction and magnitude of near-future change from the perspective of system-wide productivity, diversity, and function.
Our metaanalysis builds on previous analyses of single species by studying the combined effects between global stressors, incorporating species interactions, evaluating key ecosystem processes not previously considered, and evaluating scope for acclimation, to demonstrate how these stressors may alter global species abundances and diversity and modify species community structures and food webs. Our metaanalysis used a statistical approach that calculated a weighted mean effect size of the stressor response and its significance (95% confidence interval using bootstrapping) across 632 different experiments published through to early 2014, restricted to studies that used experimental CO2 or temperature elevations as predicted for around year 2100. Response to stressors was quantified on the basis of the natural logarithm of the response ratio (LnR), a metric commonly used in metaanalysis (9).
Results
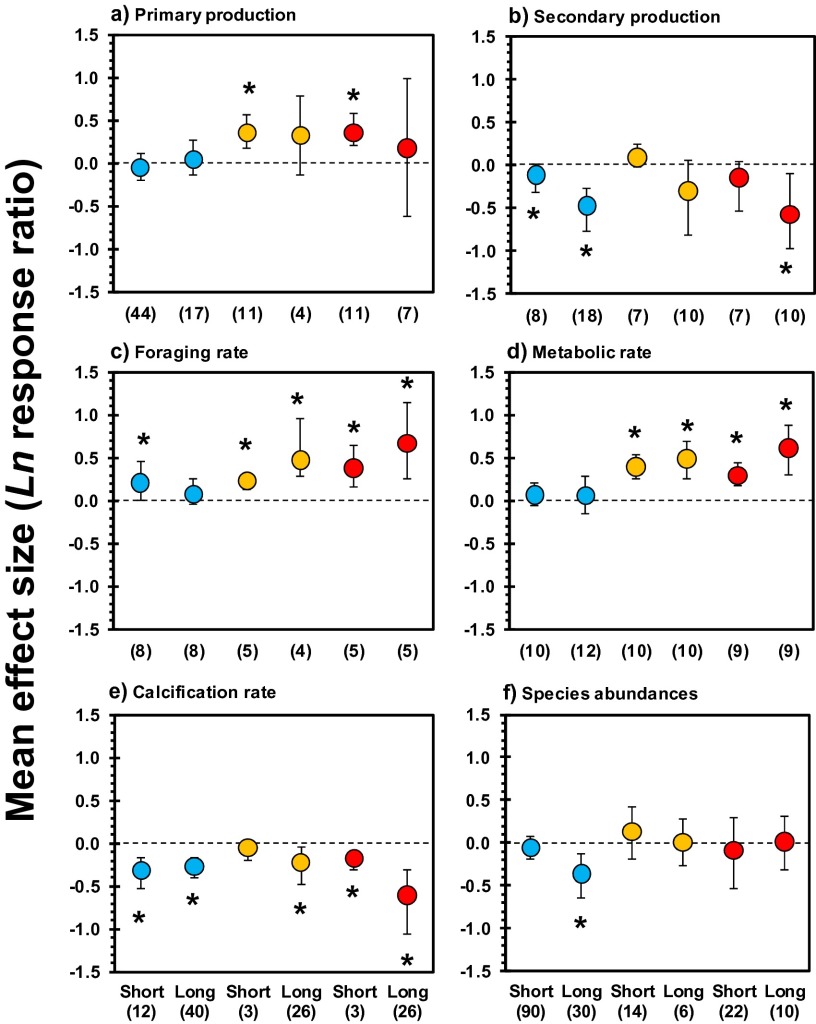
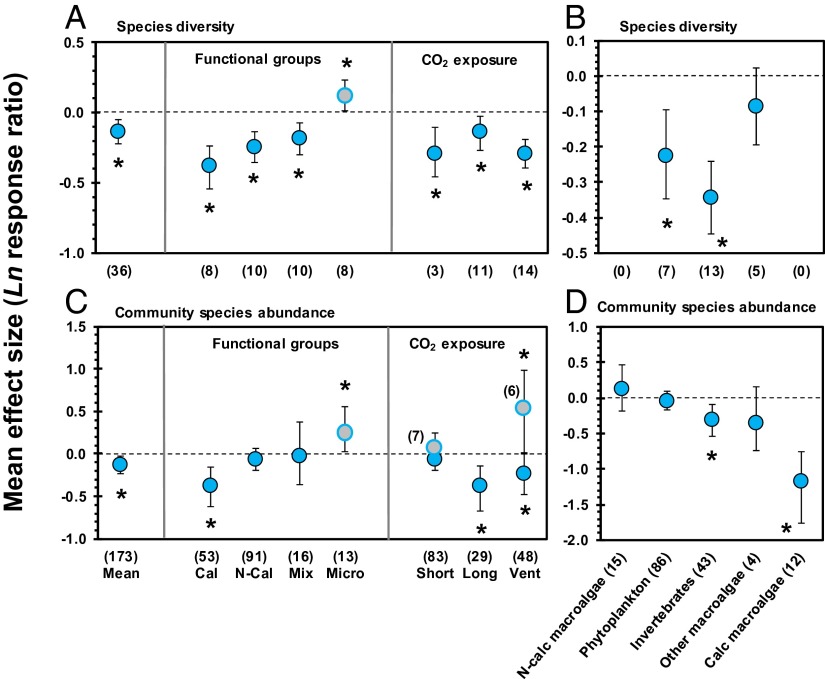
Acclimation to warming, acidification, and their combination was small across the main effects we studied. In general, there was a trend of the effect sizes of longer-term experiments being equal or stronger than those of short-term experiments (Fig. 1 and Fig. S1). Likewise, suppressed species abundances and diversity at natural CO2 vents, where organisms have been exposed to elevated CO2 over extend time periods, are comparable to declines within laboratory experiments (Fig. 2 A and C). Only microorganisms, which are taxa with short generation times and occupy a wide range of (extreme) niches, showed population abundance increases at CO2 vents in (sub)tropical regions (Fig. 2C).
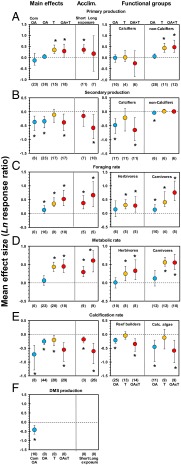
Fig. 1.
Effect of ocean acidification and warming on ecosystem processes and functional groups and scope for acclimation. (A–F) Mean effect size and direction of impacts from ocean acidification (OA) (blue) on species tested in multispecies experiments (Com OA) and of impacts from ocean acidification, warming (T) (orange), and their combined effects (OA × T) (red) on species tested in single-species experiments, for primary production (A), secondary production by invertebrates (B), foraging rate (C), metabolic rate (D), calcification rate (E), and DMS production (F). Scope for acclimation (Acclim.) compares short (<1 mo) vs. long (1–13 mo) experiments on the combined effects of warming and acidification. Error bars represent 95% confidence intervals. Numbers between brackets indicate sample size (no. of experiments). *P ≤ 0.05.
Fig. S1.
Mean effect sizes and direction of impacts of ocean acidification (OA) (blue) and ocean warming (T) (orange) and their combined effects (OA × T) (red) showing the effect of experimental exposure time for primary production (A), secondary production by invertebrates (B), foraging rate (C), metabolic rate (D), calcification rate (E), and species abundances (F). Short: duration of experiment <1 mo; Long: duration of experiment 1–13 mo. Error bars represent 95% confidence intervals. Numbers between brackets indicate sample size (number of experiments). *P ≤ 0.05. For main effects, see Figs. 1 and 2.
Fig. 2.
Effect of ocean acidification on species diversity and abundances based on multispecies experiments. Mean effect size and direction of impacts of ocean acidification on species diversity (A and B) and abundances of species within communities, for multispecies studies only (C and D). (A and C, Left) Overall mean effects. (A and C, Center) Categorical effects where data are split for various functional groups: calcifying species alone (Cal), noncalcifying species alone (eukaryotes) (N-Cal), mixed communities of calcifiers and noncalcifiers (Mix), and microorganisms (Micro) (blue circles with gray filling). (A and C, Right) Effect sizes for short-term (<1 mo.) vs. longer-term (1–13 mo.) vs. in situ studies on natural CO2 vents, separated for microbes and all other species. (B and D) Effect sizes for different species groups. Error bars represent 95% confidence intervals. Numbers between brackets indicate sample size. *P ≤ 0.05.
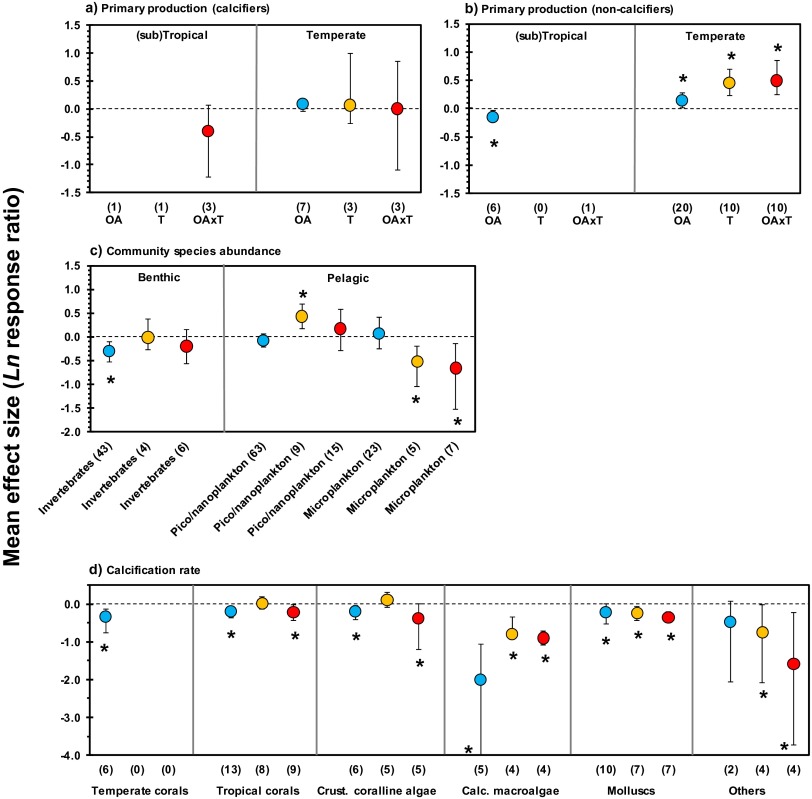
Phytoplankton generate nearly half the planetary net primary production (13), which maintains the diversity and abundance of marine life, ecosystem services, and capacity for fishery yields and influences climate processes per se. Increasing temperature and CO2 could enhance terrestrial primary production (14), although suitable plant-growing days, for example, decrease when changes in other abiotic factors are considered as well (15). In the ocean, elevated temperature has been predicted to increase primary production in polar regions and decrease production in tropical areas (16). Our metaanalysis reveals no effects of ocean acidification on pelagic production by tropical or temperate phytoplankton communities (Fig. 1A and Tables S1 and S2). Single-species experiments, in contrast, show that ocean warming and acidification have a positive effect on primary production by temperate noncalcifying species but that acidification has a negative effect on production by tropical species (Fig. S2 A and B). However, warming also enhances oceanic stratification, exposing phytoplankton to the negative effects of greater levels of harmful UV light (17) and reduced transport of nutrients from ocean depths (18, 19). The disproportionately large global contribution of phytoplankton to primary productivity (13) contributes substantially to the ocean’s net CO2 uptake from the atmosphere (20). Changes in phytoplankton productivity attributable to ocean warming, through the contrasting direct effects of temperature and indirect effects of stratification, could, therefore, be a mechanism through which primary production might be altered in surface oceans. This alteration could consequently modify the demand for atmospheric CO2 as a resource. However, there is no simple relationship between net primary production and net CO2 uptake between the ocean and atmosphere, and a fraction of the production is rapidly respired to CO2 and thus does not contribute to a net CO2 sink.
Fig. S2.
Mean effect sizes and direction of impacts of ocean acidification (OA) (blue) and ocean warming (T) (orange) and their combined effects (OA × T) (red) for various categorical effects. (A and B) Primary production by calcifiers vs. noncalcifiers in (sub)tropical and temperate regions. (C) Community species abundances of various species groups. (D) Calcification rates of several species groups. Error bars represent 95% confidence intervals. Numbers between brackets indicate sample size (number of experiments). *P ≤ 0.05. For main effects, see Figs. 1 and 2.
Predicting the consequences of changing primary productivity is not simple because of the complex interplay among species interactions and their multiple drivers. Nevertheless, whereas warming increases consumption of primary productivity through higher metabolic rates (Fig. 1 C and D), secondary production by invertebrates in tropical as well as temperate regions decreases because of ocean acidification, as established by both single-species and multispecies studies (Fig. 1B and Table S1). Loss of secondary productivity under future scenarios forms a contrast with changing energetic demands of their predators, whose foraging and metabolic rates increase because of acidification as well temperature in tropical, temperate, and polar regions (Fig. 1 C and D and Table S1). Warming can intensify trophic cascades, leading to stronger control by top consumers (21), whereas a reduction in pH imposes energetic costs on acid–base balance (22) that may act as a stressor on many carnivores. Collectively, ocean warming and acidification showed contrasting effects on productivity and consumption at multiple trophic levels, but with higher-order carnivores at clear risk of not meeting increased energetic demands.
Our metaanalysis shows an overall decrease of tropical and temperate (but not polar) species abundances and diversity across multiple functional and species groups attributable to ocean acidification (Fig. 2 and Tables S1 and S2). Ocean acidification increases the potential for simplification of species communities for calcifying and noncalcifying species alike (Fig. 2A). Of all taxa, benthic (sub)tropical microorganisms are the clearest “winners” from the effects of ocean acidification (Fig. 2 A and C). Simplification of trophic structure and reduced species diversity has been shown to lead to diminished functional redundancy, which has been coupled to lower ecosystem resistance and resilience to future stress that are both part of natural cycles and human intervention (23, 24).
Ocean acidification has a greater negative effect on abundances of calcifying taxa (e.g., various species of crustaceans, molluscs, and calcifying macroalgae) than noncalcifiers (e.g., various species of noncalcifying macroalgae, sponges, autotrophic and heterotrophic plankton, and benthic invertebrates) (Fig. 2 C and D). A potential community shift toward noncalcifiers is reinforced by the differential effects that the combination of ocean acidification and increasing temperature have on primary and secondary production of noncalcifiers vs. calcifiers (Fig. 1 A and B). Such potential shifts to communities dominated by noncalcifying organisms have profound implications for pelagic and benthic systems.
For pelagic species, warming causes a shift toward smaller pico- and nanoplankton species (to the detriment of microplankton; Fig. S2C), which are less suitable as a food source for zooplankton (25). Furthermore, our results reveal a significant direct negative effect of CO2 on dimethylsulfide (DMS) production by temperate phytoplankton communities (Fig. 1F and Table S1). DMS is a driver of food web structure (26), acting as an antigrazing defense mechanism in phytoplankton (27), while also providing chemical cues to attract predators (e.g., fishes, large zooplankton, birds) to prey that forage on phytoplankton (26, 28). DMS has the potential to mediate trophic interactions that span distances of millimeters (e.g., mesozooplankton attracted to grazing microzooplankton) to thousands of kilometers (e.g., seabirds attracted to oceanic areas with high plankton productivity). Alterations to oceanic DMS release can, therefore, alter the complex trophic interactions in the ocean (29). Reduced DMS production is also linked with potential increases in global temperature because it contributes to cloud formation as a climate gas (30, 31).
For tropical as well as temperate benthic species (Table S1), our analyses show a significant negative effect of acidification, warming, or their combination on calcification rates of key calcifying taxa that construct reefs, such as molluscs and tropical as well as cold water corals, and of calcareous algae that serve as a settlement substratum for coral larvae (Fig. 1E and Fig. S2D). Although there is broad agreement that calcification and abundance of tropical corals will decrease (9, 32), there is uncertainty of the overall effects on other foundation species. Mussel and oyster beds are the dominant reef-building taxa in estuaries and temperate coastal seas (33), whereas cold water corals construct large biogenic deep water reefs (34). A decline in such habitat-forming species at lowered pH and/or elevated temperature is likely to result in loss of secondary productivity, local extinctions, and reduced taxonomic distinctness (35, 36). The extent to which these indirect effects drive future change relative to direct effects is largely unknown (3, 21), although negative effects on habitat formers is likely to affect a greater number of species. Although our metaanalysis highlights negative effects for both habitat formers and users, it remains unclear how these effects will coincide.
Discussion
Ocean warming and acidification have received increasing focus as global change stressors, but marine species will also be impacted in their performance by other emerging stressors such as changes in sea surface height, UV, underwater irradiance, water salinity, and seawater oxygen content (7). Hypoxic zones are becoming widespread in oceanic as well as shelf environments because of climate change and local stressors such as eutrophication (37). Many species will be challenged by the interactive effects of ocean warming, acidification, and deoxygenation, but at present, hardly any (multistressors) studies exist to evaluate the effects of hypoxia on marine species and ecosystems (8, 38). For some species, there are opportunities to move to deeper waters or extend their ranges to higher latitudes, but not all species will be able to keep up with the pace of climate change, leading to alterations in current species distributions (39, 40). Moreover, species that have fewer generations (e.g., k strategists with greater longevity and later maturation) have fewer opportunities to adapt to rapidly changing conditions forecast for the next ∼85 y. Unless longer-lived species relocate to climate refugia, their persistence will rely more on mechanisms of acclimation than adaption. Importantly, if acclimation and adaptation to climate have low potential, the probability for community change is heightened. Hence, variance for adaption among species (41), combined with low scope for acclimation (this study), jointly emphasize the potential for community change.
By integrating multispecies with multifactor experiments of differing acclimation periods, we produce a conceptual insight into how human greenhouse gas emissions may drive change to pelagic and benthic ecosystems from different latitudes. Many of the studies included in our metaanalysis manipulated temperature or CO2 to levels predicted for the end of this century (Table S1 and Dataset S1). It is notable that despite variation in choice of experimental temperatures and CO2 levels among studies, these differences did not translate into detectable differences in the effect size of most processes under study (Table S2). This finding suggests that experimental outcomes are not only robust to such experimental choices but also that the magnitude of our forecast responses are likely to be similar across the range of temperatures and CO2 levels anticipated at the end of the century. Although the magnitude of future change in ocean temperature and pH will be variable at local scales—potentially leading to different outcomes at specific locations and for some species—there are emerging patterns of change in ecosystem processes and species occurrences. We find that ocean warming and acidification increase the potential for an overall simplification of ecosystem structure and function, with reduced energy flow among trophic levels with little scope for acclimation. Ocean acidification per se appears to have the potential to bring penetrating modifications to ecological systems through changes in ecosystem processes and shifts in species community structures. Although some ecosystem processes are affected by ocean acidification only, others are affected by warming alone or by the combination of the two stressors. These results, therefore, provide a conceptual framework toward more inclusive forecasts of future ecological change (Fig. 3).
Fig. 3.
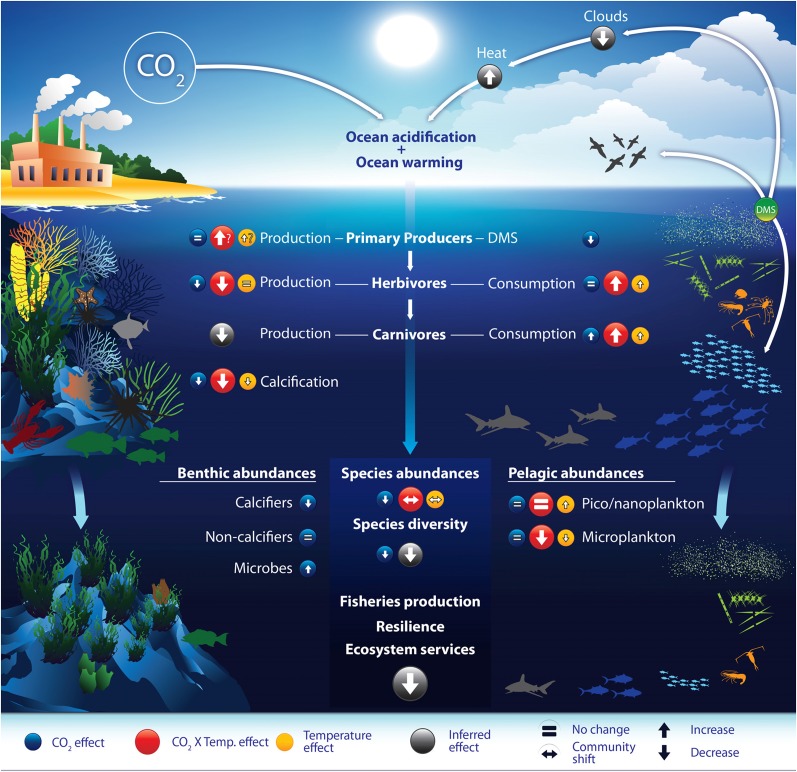
Conceptual diagram illustrating the main effects of ocean acidification, warming, and their combination on ecosystem processes and species groups, based on the metaanalysis results as shown in the various figures of our study. Circled arrows indicate the direction of change, and question marks (?) indicate less certain responses. The most likely feedback responses that exacerbate the direct effects of these two global stressors are indicated with white arrows. Two model ecosystems are shown here (reefs and surface-ocean) to visually capture potential change [present day (Upper Left and Upper Right) vs. future (Lower Left and Lower Right)] in species abundance, species diversity, and community shifts, as revealed by our metaanalysis for ecosystems in general. The changes shown here for reefs and surface-ocean are not exact outcomes of future states but merely emphasize overall responses for (relative) abundance of species.
Materials and Methods
Data Selection.
We searched the literature for studies published through to early 2014 on effects of ocean acidification on marine biota using Thomson Reuters’ Web of Science. By using the search string “ocean acidification” we explicitly incorporated studies that placed their experimental designs and results within this broader context of climate change as distinct from those testing the effects of changes in pH per se. We screened the titles and abstracts of ∼2,300 published articles, of which 151 studies (covering 632 experiments) met the requirements for inclusion (Dataset S1). We selected studies that investigated the effect of ocean acidification on species diversity, species community abundances, and DMS production and studies that investigated the effects of ocean acidification and warming on species performance (primary production, secondary production, foraging, metabolism, calcification). In addition to our own literature survey, we also cross-referenced our database with some more taxon-targeted metaanalyses on ocean acidification (5, 9, 10, 42), but this procedure added only a limited number of studies, suggesting that our search string in Web of Knowledge was very effective.
We focused our analyses on studies that used increases in CO2 and temperature, as predicted for year ∼2100, typically based on the representative concentration pathway (RCP) 8.5 emission scenario (business-as-usual). Under this scenario, global ocean surface temperatures are predicted to rise by an average (±1 SD) of ∼3.7 ± 0.7 °C (43) compared with the 1990s, whereas CO2 into the atmosphere will more than triple relative to preindustrial conditions, increasing from the current levels of ∼400 to ∼936 ppm by the end of the century. This scenario will lead to a decrease in ocean surface pH of approximately –0.33 ± 0.003 units by 2100 compared with the 1990s (43). Regarding the less likely high mitigation scenario RCP2.6, corresponding changes would be +0.7 (± 0.5) °C and –0.07 (± 0.001) pH units, respectively. A few studies that we included used somewhat higher values than predicted for the RCP8.5 emission scenario, because their present-day conditions already showed above-average values for these stressors (e.g., enhanced acidification attributable to seasonal upwelling or shallow coastal areas that warm up faster during summertime), reflecting the variability as typically observed across ecosystems, latitudes, and water depths. Studies that used extreme temperature elevations or pH reductions that are well beyond the predictions for year 2100 were excluded from the analyses, following previous approaches (9). Average (SD) reduction in pH and enhancement of CO2 and temperature levels across all studies included in our metaanalysis were –0.3 (0.1) units, +508 (230) ppm CO2, and +3.8 °C (1.1 °C), respectively, which closely match the average and range in projections for RCP8.5. Nevertheless, there was variability across studies in the treatment levels used. Elevation levels (Δ treatment vs. control of the experiment) and their SD for CO2 and temperature per main factor tested are shown in Table S1, and values per experiment are shown in Dataset S1. We did not normalize the data for experimental elevation of CO2 and temperature levels, because in almost all cases regression analyses (see procedure description under Metaanalysis) showed lack of a significant correlation between the response variables and stressor levels (see regression results in Table S2).
Whereas initial studies primarily focused on single-species experiments, there has been a rapid increase in multispecies experiments in in situ as well as laboratory-based mesocosms in the last few years. These studies have not yet been specifically tested using metaanalysis, even though they are much more realistic than single-species experiments because they incorporate complex species interactions. Where present, we therefore included multispecies (i.e., “community”-level) experiments that manipulated CO2 even if the experiments were not tested in a factorial design with temperature. For species-level studies, we focused predominantly on factorial experiments on ocean acidification and warming.
We focused on several key processes that underpin the persistence, functioning, health, and productivity of ocean ecosystems. Primary production at community level involved experiments using multispecies phytoplankton assemblages and typically measuring 14C fixation rates across the entire assemblage. Primary production at a species level involved experiments on monocultures of phytoplankton and were primarily based on cellular growth rates followed by changes in cell biomass (Dataset S1). Secondary production at community level was measured as changes in the total density or biomass of all animals combined within the type of assemblage under consideration. At a species level, production was predominantly based on changes in biomass of single species. Foraging rates were based on per capita consumption rates. Metabolic rates were largely measured as resting/routine metabolic rates. Calcification rates at community level were measured as the net accumulation of CaCO3 or 14C uptake for the entire assemblage, whereas at the species level, this was usually measured as accumulation of CaCO3 for individual species. Where studies selected solely calcifying or noncalcifying species to test community-level effects, we refer to these groups as calcifying species and noncalcifying species, respectively. In cases where both groups were present in the experiment and their responses were not separated, we refer to them as mixed communities of calcifiers and noncalcifiers. For DMS, studies on dimethylsulfoniopropionate (DMSP) or dimethylsulfoxide (DMSO) were not included because photolysis and DMS gas exchange are linear functions of the DMS concentration (30). Species diversity was largely based on changes between treatments in total number of species, number of operational taxonomic units, or the Shannon–Wiener diversity index for complete species assemblages. Community species abundances reflect the changes in abundances (typically measured as densities, cover, or abundances) of individual species in multispecies experiments, providing a more realistic measure of species changes because these experiments incorporate species interactions. In cases where multiple response variables were reported within a study for the processes we considered, only one response variable was included to avoid pseudoreplication. For example, if a study reported growth rate for an organism as a change in length and biomass, biomass was selected as the more meaningful response variable (9).
Metaanalysis.
For each experiment, we calculated both the individual and combined effect sizes of acidification and warming using the natural logarithm of the response ratio, a response metric commonly used in metaanalyses (9, 10). The effect size based on LnR represents the ratio of the response variable measured in an experimental group to that of the control group. The effect size of individual experiments were weighted by the reciprocal of their sampling variance, followed by a random-effects model to calculate the mean (“overall”) effect size for comparisons across treatments for the various response variables (Table S2). The mean effect size is thus a weighted average of individual effect sizes to reduce bias attributable to studies with few vs. large sample sizes (44). Confidence intervals around mean effect sizes were generated using bootstrapping methods (4,999 iterations). We used biased-corrected bootstrap confidence intervals to reduce bias attributable to small sample sizes. If the confidence intervals do not overlap zero, then the effect size is considered significant.
The total heterogeneity of a weighted mean effect size is represented by the QT statistic, which is a weighted sum of squares, comparable to the total sum of squares in an ANOVA. For each mean effect size, QT was calculated and tested against a χ2 distribution (Table S2). A significant QT indicates that the variance among individual effect sizes is larger than expected by sampling error and that there may be an underlying structure to the data, and therefore other explanatory variables should be tested. Consequently, we investigated several categorical factors for the overall mean effect sizes that showed significant heterogeneity using a categorical random-effects model, which is analogous to a mixed-effects model in ANOVA. For this model, total heterogeneity QT can be partitioned in the variance explained by the model (QM) and the residual error variance not explained by the model (QE). QM was tested against a χ2 distribution using a randomization procedure (4,999 iterations), with a significant QM (Table S2) indicating statistical differences in the mean effects sizes among categories (within a factor).
We first tested for the effect of latitude as a categorical factor for all main response variables that showed a significant QT (Table S2). Except for primary production, the response for the various main factors considered was similar for the different latitudes and the respective QM was nonsignificant and/or very low, indicating other factors were more important. Hence, for secondary production, calcifiers vs. noncalcifiers was tested as a categorical factor instead (Table S2). For calcification rate, taxon was tested as a categorical factor, and the results are shown in Fig. S2D; in Fig. 1E, aggregated results are shown for comparative purposes only. For species diversity, functional group was tested as a categorical factor, and taxon-level responses were shown for comparative purposes only. For ocean acidification effects on species abundances, functional group was tested as a categorical factor, and taxon-level responses were shown for comparative purposes only. For effects of temperature on species abundances, taxon was tested as a categorical factor. For primary productivity of single-species studies, either latitude or calcifiers vs. noncalcifiers or both categories were significant in independent categorical analyses, and therefore calcifiers vs. noncalcifiers was tested for the different latitudes separately (i.e., category calcifier within category latitude; the results are shown in Fig. S2 A and B, whereas the aggregated results for calcifiers vs. noncalcifiers (across latitudes) are shown in Fig. 1A for comparative purposes only). In all other cases where QT was nonsignificant, this finding implies that the various categories (functional groups/latitudes) showed a similar direction of response as the main effects analysis (either all positive or all negative); nevertheless, these responses are also plotted for some categories (Fig. 1) and reported (Table S2) to facilitate the understanding of the complex dataset.
To assess whether normalization of effect sizes to particular levels of altered pH or temperature would improve interpretive value, we performed a continuous random-effects metaanalysis on effect sizes across their different combinations using the relative differences between treatment and control as the explanatory variable. The relationship between effects sizes and predictor variables is calculated on the basis of a least-squares regression. In almost all cases, no significant correlations were detected (see regression results in Table S2) and the original data were analyzed without normalizing to pH or temperature.
To test for the potential of species to acclimate to changing stressors, we compared short-term experiments (<1 mo) to longer-term experiments (>1 mo; range, 4–56 wk; mean ± SD: 11.6 ± 11.6 wk) as well as data collected from natural CO2 vents where many sessile or low-motility species have typically been exposed to decreased pH conditions over significant parts of their life cycle. For this specific analysis alone, we combined the data from single-species and multiple-species studies, and our interpretation is based on comparing the mean effect size and their 95% confidence intervals between short- and long-term experiments (rather than testing if their means differ from 0).
Sensitivity Analyses.
Because data selection and weighing might affect the outcome of the overall effect size (44), we also calculated the unweighted effect sizes using a fixed-effects model. Because both approaches revealed similar trends and significances (test outcomes reported in Table S2), we report the weighted mean effect sizes. We tested for publication bias for main effects that were significant using Rosenthal’s method of fail-safe numbers. The fail-safe number represents the number of studies with a nonsignificant outcome that needs to be added to change the effect sizes from significant to nonsignificant. Fail-safe numbers ranged between 7 and 1,151, with almost all cases >19 (Table S2), which is relatively large compared with the sample sizes of main effects tested in our study.
Supplementary Material
Acknowledgments
We thank T. Rossi for help with designing Fig. 3. This study was supported by Australian Research Council Future Fellowship Grants FT120100183 (to I.N.) and FT0991953 (to S.D.C.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1510856112/-/DCSupplemental.
References
- 1.Walther GR, et al. Ecological responses to recent climate change. Nature. 2002;416(6879):389–395. doi: 10.1038/416389a. [DOI] [PubMed] [Google Scholar]
- 2.Schröter D, et al. Ecosystem service supply and vulnerability to global change in Europe. Science. 2005;310(5752):1333–1337. doi: 10.1126/science.1115233. [DOI] [PubMed] [Google Scholar]
- 3.Nagelkerken I, Russel BD, Gillanders BM, Connell SD. Ocean acidification alters fish populations indirectly through habitat modification. Nat Clim Chang. August 10, 2015 doi: 10.1038/nclimate2757. [DOI] [Google Scholar]
- 4.Hoegh-Guldberg O, Bruno JF. The impact of climate change on the world’s marine ecosystems. Science. 2010;328(5985):1523–1528. doi: 10.1126/science.1189930. [DOI] [PubMed] [Google Scholar]
- 5.Wernberg T, Smale DA, Thomsen MS. A decade of climate change experiments on marine organisms: Procedures, patterns and problems. Glob Change Biol. 2012;18(5):1491–1498. [Google Scholar]
- 6.Halpern BS, et al. Spatial and temporal changes in cumulative human impacts on the world’s ocean. Nat Commun. 2015;6:7615. doi: 10.1038/ncomms8615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gattuso J-P, et al. OCEANOGRAPHY. Contrasting futures for ocean and society from different anthropogenic CO₂ emissions scenarios. Science. 2015;349(6243):aac4722. doi: 10.1126/science.aac4722. [DOI] [PubMed] [Google Scholar]
- 8.Przeslawski R, Byrne M, Mellin C. A review and meta-analysis of the effects of multiple abiotic stressors on marine embryos and larvae. Glob Change Biol. 2015;21(6):2122–2140. doi: 10.1111/gcb.12833. [DOI] [PubMed] [Google Scholar]
- 9.Kroeker KJ, et al. Impacts of ocean acidification on marine organisms: Quantifying sensitivities and interaction with warming. Glob Change Biol. 2013;19(6):1884–1896. doi: 10.1111/gcb.12179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Harvey BP, Gwynn-Jones D, Moore PJ. Meta-analysis reveals complex marine biological responses to the interactive effects of ocean acidification and warming. Ecol Evol. 2013;3(4):1016–1030. doi: 10.1002/ece3.516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Strain EMA, Thomson RJ, Micheli F, Mancuso FP, Airoldi L. Identifying the interacting roles of stressors in driving the global loss of canopy-forming to mat-forming algae in marine ecosystems. Glob Change Biol. 2014;20(11):3300–3312. doi: 10.1111/gcb.12619. [DOI] [PubMed] [Google Scholar]
- 12.Gilman SE, Urban MC, Tewksbury J, Gilchrist GW, Holt RD. A framework for community interactions under climate change. Trends Ecol Evol. 2010;25(6):325–331. doi: 10.1016/j.tree.2010.03.002. [DOI] [PubMed] [Google Scholar]
- 13.Field CB, Behrenfeld MJ, Randerson JT, Falkowski P. Primary production of the biosphere: Integrating terrestrial and oceanic components. Science. 1998;281(5374):237–240. doi: 10.1126/science.281.5374.237. [DOI] [PubMed] [Google Scholar]
- 14.Hooper DU, et al. A global synthesis reveals biodiversity loss as a major driver of ecosystem change. Nature. 2012;486(7401):105–108. doi: 10.1038/nature11118. [DOI] [PubMed] [Google Scholar]
- 15.Mora C, et al. Suitable days for plant growth disappear under projected climate change: Potential human and biotic vulnerability. PLoS Biol. 2015;13(6):e1002167. doi: 10.1371/journal.pbio.1002167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chust G, et al. Biomass changes and trophic amplification of plankton in a warmer ocean. Glob Change Biol. 2014;20(7):2124–2139. doi: 10.1111/gcb.12562. [DOI] [PubMed] [Google Scholar]
- 17.Gao K, Walter Helbling E, Haeder D-P, Hutchins DA. Responses of marine primary producers to interactions between ocean acidification, solar radiation, and warming. Mar Ecol Prog Ser. 2012;470:167–189. [Google Scholar]
- 18.Behrenfeld MJ, et al. Climate-driven trends in contemporary ocean productivity. Nature. 2006;444(7120):752–755. doi: 10.1038/nature05317. [DOI] [PubMed] [Google Scholar]
- 19.Boyce DG, Lewis MR, Worm B. Global phytoplankton decline over the past century. Nature. 2010;466(7306):591–596. doi: 10.1038/nature09268. [DOI] [PubMed] [Google Scholar]
- 20.Holligan PM, Robertson JE. Significance of ocean carbonate budgets for the global carbon cycle. Glob Change Biol. 1996;2(2):85–95. [Google Scholar]
- 21.Harley CDG. Climate change, keystone predation, and biodiversity loss. Science. 2011;334(6059):1124–1127. doi: 10.1126/science.1210199. [DOI] [PubMed] [Google Scholar]
- 22.Portner HO. Ecosystem effects of ocean acidification in times of ocean warming: A physiologist’s view. Mar Ecol Prog Ser. 2008;373:203–217. [Google Scholar]
- 23.Worm B, et al. Impacts of biodiversity loss on ocean ecosystem services. Science. 2006;314(5800):787–790. doi: 10.1126/science.1132294. [DOI] [PubMed] [Google Scholar]
- 24.Bascompte J, Melián CJ, Sala E. Interaction strength combinations and the overfishing of a marine food web. Proc Natl Acad Sci USA. 2005;102(15):5443–5447. doi: 10.1073/pnas.0501562102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lewandowska AM, et al. Effects of sea surface warming on marine plankton. Ecol Lett. 2014;17(5):614–623. doi: 10.1111/ele.12265. [DOI] [PubMed] [Google Scholar]
- 26.Hay ME, Kubanek J. Community and ecosystem level consequences of chemical cues in the plankton. J Chem Ecol. 2002;28(10):2001–2016. doi: 10.1023/a:1020797827806. [DOI] [PubMed] [Google Scholar]
- 27.Wolfe GV, Steinke M, Kirst GO. Grazing-activated chemical defence in a unicellular marine alga. Nature. 1997;387(6636):894–897. [Google Scholar]
- 28.Nevitt GA, Veit RR, Kareiva P. Dimethyl sulfide as a foraging cue for antarctic procellariform seabirds. Nature. 1995;376(6542):680–682. [Google Scholar]
- 29.Pohnert G, Steinke M, Tollrian R. Chemical cues, defence metabolites and the shaping of pelagic interspecific interactions. Trends Ecol Evol. 2007;22(4):198–204. doi: 10.1016/j.tree.2007.01.005. [DOI] [PubMed] [Google Scholar]
- 30.Six KD, et al. Global warming amplified by reduced sulphur fluxes as a result of ocean acidification. Nat Clim Chang. 2013;3(11):975–978. [Google Scholar]
- 31.Vallina SM, Simó R. Strong relationship between DMS and the solar radiation dose over the global surface ocean. Science. 2007;315(5811):506–508. doi: 10.1126/science.1133680. [DOI] [PubMed] [Google Scholar]
- 32.Hoegh-Guldberg O, et al. Coral reefs under rapid climate change and ocean acidification. Science. 2007;318(5857):1737–1742. doi: 10.1126/science.1152509. [DOI] [PubMed] [Google Scholar]
- 33.Kirby MX. Fishing down the coast: Historical expansion and collapse of oyster fisheries along continental margins. Proc Natl Acad Sci USA. 2004;101(35):13096–13099. doi: 10.1073/pnas.0405150101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Turley CM, Roberts JM, Guinotte J. Corals in deep-water: Will the unseen hand of ocean acidification destroy cold-water ecosystems? Coral Reefs. 2007;26(3):445–448. [Google Scholar]
- 35.Fabricius KE, De’ath G, Noonan S, Uthicke S. Ecological effects of ocean acidification and habitat complexity on reef-associated macroinvertebrate communities. Proc Biol Sci. 2014;281(1775):20132479. doi: 10.1098/rspb.2013.2479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rogers A, Blanchard JL, Mumby PJ. Vulnerability of coral reef fisheries to a loss of structural complexity. Curr Biol. 2014;24(9):1000–1005. doi: 10.1016/j.cub.2014.03.026. [DOI] [PubMed] [Google Scholar]
- 37.Doney SC. The growing human footprint on coastal and open-ocean biogeochemistry. Science. 2010;328(5985):1512–1516. doi: 10.1126/science.1185198. [DOI] [PubMed] [Google Scholar]
- 38.Crain CM, Kroeker K, Halpern BS. Interactive and cumulative effects of multiple human stressors in marine systems. Ecol Lett. 2008;11(12):1304–1315. doi: 10.1111/j.1461-0248.2008.01253.x. [DOI] [PubMed] [Google Scholar]
- 39.Hiddink JG, Burrows MT, García Molinos J. Temperature tracking by North Sea benthic invertebrates in response to climate change. Glob Change Biol. 2015;21(1):117–129. doi: 10.1111/gcb.12726. [DOI] [PubMed] [Google Scholar]
- 40.Lenoir J, Svenning JC. Climate-related range shifts – A global multidimensional synthesis and new research directions. Ecography. 2015;38(1):15–28. [Google Scholar]
- 41.Kelly MW, Hofmann GE. Adaptation and the physiology of ocean acidification. Funct Ecol. 2013;27(4):980–990. [Google Scholar]
- 42.Hendriks IE, Duarte CM, Alvarez M. Vulnerability of marine biodiversity to ocean acidification: A meta-analysis. Estuar Coast Shelf Sci. 2010;86(2):157–164. [Google Scholar]
- 43.Bopp L, et al. Multiple stressors of ocean ecosystems in the 21st century: Projections with CMIP5 models. Biogeosciences. 2013;10(10):6225–6245. [Google Scholar]
- 44.Gurevitch J, Hedges LV. Statistical issues in ecological meta-analyses. Ecology. 1999;80(4):1142–1149. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.