Abstract
Background
Green approach in synthesizing metal nanoparticles has gain new interest from the researchers as metal nanoparticles were widely applied in medical equipment and household products. The use of plants in the synthesis of nanoparticles emerges as a cost effective and eco-friendly approach. A green synthetic route for the production of stable silver nanoparticles (Ag-NPs) by using aqueous silver nitrate as metal precursor and Artocarpus elasticus stem bark extract act both as reductant and stabilizer is being reported for the first time.
Results
The resultant Ag-NPs were characterized by UV–vis spectroscopy, powder X-Ray diffraction, transmission electron microscopy (TEM), scanning electron microscopy (SEM), and Fourier-transform infra-red (FT-IR). The morphological study by TEM and SEM shows resultant Ag-NPs in spherical form with an average size of 5.81 ± 3.80, 6.95 ± 5.50, 12.39 ± 9.51, and 19.74 ± 9.70 nm at 3, 6, 24, and 48 h. Powder X-ray diffraction showed that the particles are crystalline in nature, with a face-centered cubic structure. The FT-IR spectrum shows prominent peaks appeared corresponds to different functional groups involved in synthesizing Ag-NPs.
Conclusions
Ag-NPs were synthesized using a simple and biosynthetic method by using methanolic extract of A. elasticus under room temperature, at different reaction time. The diameters of the biosynthesis Ag-NPs depended on the time of reaction. Thus, with the increase of reaction time in the room temperature the size of Ag-NPs increases. From the results obtained in this effort, one can affirm that A. elasticus can play an important role in the bioreduction and stabilization of silver ions to Ag-NPs.
Graphical abstract:
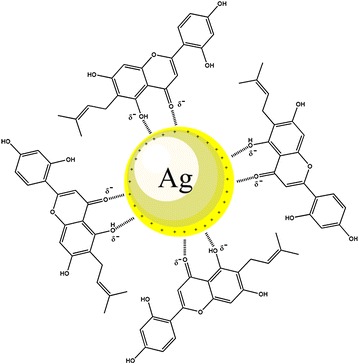
Figure illustrates stabilization of silver nanoparticles after formation by A. elasticus stem bark extract.
Keywords: Biosynthesis, Artocarpus elasticus, Silver nanoparticles, Stem bark, Transmission electron microscopy
Background
Nanotechnology has been emerged as a new technology which design, characterize, produce and applied in the structures, devices and systems by controlling the shape and size at nanometer scale, range from 100 nm down to 1 nm [1].
Metal nanoparticles that have high interest to be synthesized are Ag, Au, Pt and Pb. Silver nanoparticles (Ag-NPs) have the least toxicity to animal cells and highest toxicity to microorganism cells compared to the other metals [2]. Various works have been reported on toxicity of silver nanoparticle against micro-organism such as bacteria [3], fungi [4], viruses [5], and also larvicidal activity [6]. Silver has been widely used in household products such as paint [7], cotton fabrics [8], and in water purification [9]. It was also been applied in surface enhanced raman spectroscopy [10], optical sensor [11], catalyst [12] and in biomedical application [13].
Metal nanoparticles have been synthesized in various techniques in reducing the silver into Ag-NPs including conventional chemical reduction [14], electrochemical [15], irradiation [16, 17], laser ablation [18], polysaccharide [19]. Synthesis of metallic nanoparticles by using living organism is the new approach towards green technology, denominate as biosynthesis.
Biosynthesis of metal nanoparticles includes algae [20], bacteria [21], fungi [22], yeast [23], actinomycetes [24], and plants [25]. From the plant itself, various parts have been explored to give different properties of Ag-NPs. It includes leaf, stem bark, root, flower, vegetable oil, fruit, peel, leaf bud, seed, and callus [26–28]. In addition, biosynthetic process is clearly abiding the three rules of green principles compared to conventional method of chemical reduction.
The Artocarpus elasticus (A. elasticus) is a distinctive tree in nature, easy to grow, possess anticancer [29, 30], and antimalarial properties [31]. Locals have been using the leaves to nursing mothers, young shoots in curing vomiting blood problems, inner bark used in treating ulcers, and its latex used for dysentery disease [32]. Artocarpus are sources of phenolic-derived secondary metabolites which includes flavonoid compounds, particularly of prenylated flavones that exist as the main group of the phenolic constituents [33]. Some of the compounds that have been isolated were artelastin, artelastochromene, artelasticin and artocarpesin [34].
To the best of our knowledge, there is no work reported on Ag-NPs or any other metal nanoparticles synthesized by using A. elasticus at ambient temperature. Here, we demonstrate the biosynthesis and characterization of Ag/A. elasticus nanoparticles by using silver nitrate and stem bark extract of A. elasticus.
Results and discussion
The reduction of silver ion to Ag-NPs by using A. elasticus stem bark extract as both reducing and stabilizing agent and silver nitrate (0.01 M) as a silver precursor was indicated by colour changes of A. elasticus extract when incubated with silver nitrate at certain time, as shown in Fig. 1. The solution changed colour from yellow to light brown, and going darker with increasing time (1, 3, 6, 12, 24, and 48 h), at room temperature. It was known that silver nanoparticles colloidal solutions shows intense yellow–brown colour, which occur only in nanoparticles, not in the case of bulk materials due to strong interaction between light and conduction electron of silver in the solution.
Fig. 1.

Photograph of synthesized Ag/A. elasticus nanoparticles at different reaction time
The A. elasticuswith different component and functional groups proved to be able to reduce silver ions to Ag-NPs. The possible chemical equations for synthesizing the Ag-NPs are:
| 1 |
| 2 |
After dispersion of silver ions in the A. elasticus aqueous solution matrix (Eq. 1), the extract was reacted with the Ag+ (aq) to form [Ag/A. elasticus)]+ complex, which reacted with functional groups of A. elasticus components to form [Ag/A. elasticus)] (Eq. 2) after left stirred for 48 h [35, 36].
UV–visible spectroscopy analysis
The formation of Ag-NPs was followed by measuring the surface plasmon resonance (SPR) of the A. elasticus and Ag/A. elasticus emulsions over the wavelength range from 300 to 700 nm. The preparation of Ag-NPs was studied by UV–visible spectroscopy, which has proven to be a useful spectroscopic method for the detection of prepared metallic nanoparticles. It was known that spherical Ag-NPs display a SPR band at around 400–450 nm, depending on its size [37]. The SPR band characteristics of Ag-NPs were detected around 406–460 nm (Fig. 2), which strongly suggests that the Ag-NPs were spherical in shape and have been confirmed by the TEM results of this study. As shown in Fig. 2, the intensity of the SPR peak increased as the reaction time increased, which indicated the continued reduction of the silver ions, and the increase of the absorbance indicates that the concentration of Ag-NPs increases.
Fig. 2.
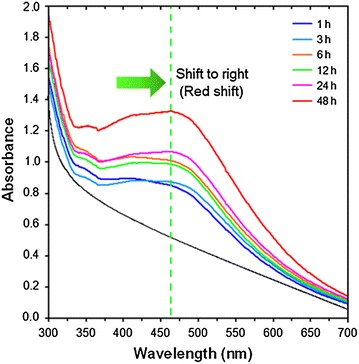
UV-Visible absorption spectra of A. elasticus and Ag/A. elasticus emulsion prepared at 1, 3, 6, 12, 24, 48 and 72 h
At 1 h of reaction time, low intensity of maximum SPR was recorded at 406 nm. However, with increasing time, particles aggregates, causing the conduction electrons near each particle surface become delocalized and shared among neighbouring particles, thus red-shifting the SPR into longer wavelengths from 406 to 424, 420, 433, 455 and 460 nm. At the end of the reaction (48 h), the absorbance was considerably increased and the λmax value was slightly red-shifted to 460 nm, compared with the 24 h reaction time.
At the initial stage of the reaction, the Ag-NPs formed with a narrow size distribution which led to a SPR peak at about 406 nm. After this stage, the Ag-NPs could associate due to increases of reaction time to form bigger size of Ag-NPs. However, at 48 h of reaction time, the absorbance is the largest but also broad compared to the other reaction time, suggesting bigger silver nanoparticles with stable properties. Shoulder peaks were also observed for all of the samples, at 350 nm [38], indicating the existence of bulk silver. Other works presented a broader peak with maximum at 490 nm that indicating larger size of Ag-NPs [39]. However, at 72 h of reaction time, the particles agglomerate, thus showing no distinguishable maximum SPR band. After reaching certain particle size, the plant extract which act as stabilizer was no longer able to withhold the nanoparticles from agglomeration [40].
Powder X-ray diffraction
The X-ray diffraction pattern of Ag-NPs synthesized by A. elasticus is shown in Fig. 3. The A. elasticus pattern shows no peak assign to crystal structure (Fig. 3a). Broad diffraction peak which was centered at 18.39° could be assigned to organic matters in A. elasticus extract. After silver nitrate was introduced, the peak shifted to 23.70° (Fig. 3b). The Ag/A. elasticus nanoparticles pattern exhibited intense peaks at 38.19°, 44.27°, 64.74°, 77.64° and 81.62° that could be attributed to 111, 200, 220, 311, and 222 crystallographic planes of the face-centered cubic silver crystals, respectively (Powder Diffraction File Card: 00-004-0783) compared to pure silver pattern [41, 42]. There are no other irrelevant peaks observed, indicating only pure crystalline silver exist.
Fig. 3.
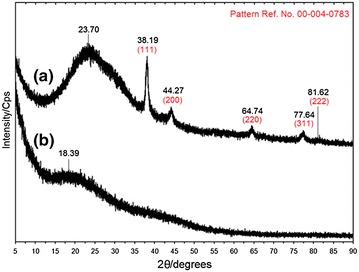
XRD patterns of a crude A. elasticus b synthesized Ag/A. elasticus nanoparticles at 48 h
Morphology study
TEM images and their size distributions (Fig. 4) show the mean diameters and standard deviation of the Ag/A. elasticus nanoparticles as 5.81 ± 3.80, 6.95 ± 5.50, 12.39 ± 9.51, and 19.74 ± 9.70 nm at 3, 6, 24, and 48 h, respectively. It was noted that the size of the nanoparticles increase with increasing time, due to agglomeration of the nanoparticles. At 3 and 6 h of reaction time, the nanoparticles start to develop, indicated by dark clump of nanoparticles together shown on the image taken and proved by SEM image. The reaction completes at 48 h of reaction time.
Fig. 4.
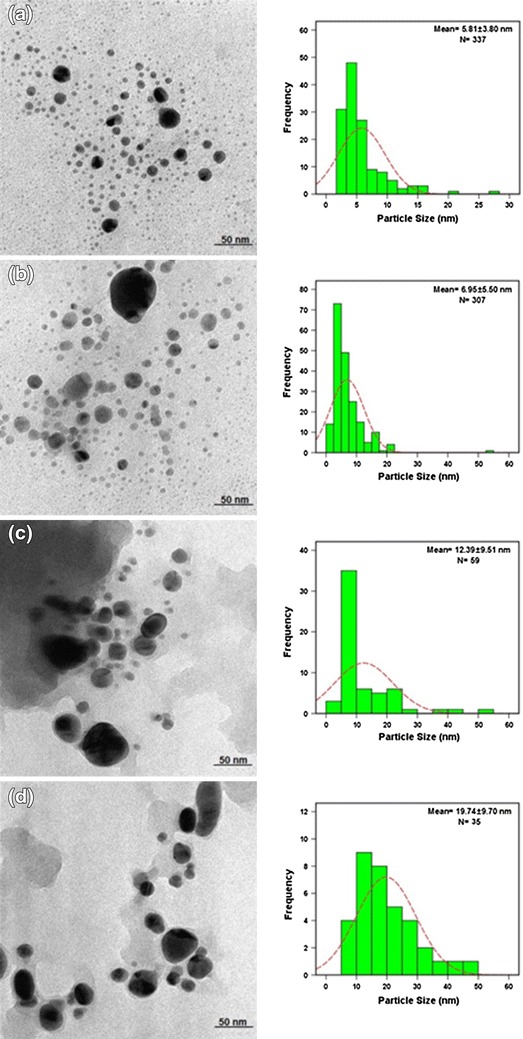
TEM image and histogram of Ag/A. elasticus nanoparticles at 3, 6, 24 and 48 h reaction time (a–d)
Figure 5a show scanning electron microscope (SEM) image of a cloudy-like surface of A. elasticus. After reacted with AgNO3, spherical Ag-NPs had been deposited through reduction by A. elasticus. At 6 h reaction time, the nanoparticles start to form as indicated by formation of bulky and near-spherical nanoparticles. Figure 5d distinctly shows that a large quantity of nanoparticles deposited at 48 h reaction time compared to at 6 and 24 h reaction time, as predicted by UV–vis spectrum.
Fig. 5.

SEM image of a crude A. elasticus, b synthesized Ag/A. elasticus nanoparticles at 6, 24 and 48 h reaction time (a–d)
FT-IR chemical analysis
FT-IR measurements were carried out to identify the possible biomolecules responsible for the reduction; capping and stabilization of the Ag-NPs synthesized using A. elasticus extract. For this analysis, solvent was removed to produce Ag/A. elasticus nanoparticles powder in order to remove unbound components.
The control spectrum (A. elasticus) shows numbers of peaks reflecting a complex nature of the compound (Fig. 6a). The peaks corresponding to such bonds such as –C–C–, –C–O–, and –C–O–C– are derived from water soluble phenolic compound of A. elasticus. Some shifts in peak position occur to indicate responsibilities of plant extract in reducing and stabilize silver nitrate to Ag/A. elasticus nanoparticles. The spectrum of the plant extract shows broad and strong absorbance peak at 3222 cm−1 corresponded to O–H stretching. This peak later shift to 3380, 3379 and 3356 cm−1 after reacted with silver nitrate at 6, 24 and 48 h, respectively. Peaks at 2926, 2924, and 2928 cm−1 are assigned as C-H stretch. In the Fig. 6b–d the broad peaks exist in Ag/A. elasticus nanoparticles spectra at 289, 327 and 326 cm−1 represents the Ag…O banding with hydroxyl group in A. elasticus extract, at 6, 24 and 48 h reaction times respectively [43]. The peaks at 1608, 1515, 1368, 1057 cm−1 are shifted to 1603–1606–1606, 1512–1512–1512, 1304–1307–1312, 1046–1041–1042 cm−1 respectively in the Ag/A. elasticus nanoparticles at 6, 24 and 48 h of reaction time. This shifting indicates the interaction of the nanoparticles with the extract. Flavonoids could be adsorbed on the surface of Ag-NPs, possibly by interaction through hydroxyl group.
Fig. 6.
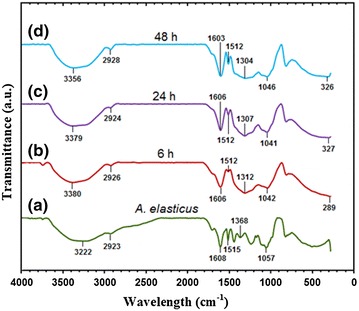
FT-IR spectra of a A. elasticus crude plant extract, Ag/A. elasticus nanoparticles at 6, 24 and 48 h reaction time (b–d)
Methods
Materials
The A. elasticus stem barks were collected from Terengganu, Malaysia. Silver nitrate (99.98 %) was purchased from Merck, Germany and used as silver precursor. All reagents used were of analytical grade. All aqueous solutions were prepared using distilled water. All glassware used were cleaned and washed with distilled water and dried before used.
Extract preparation
The air-dried stem bark was ground into fine powder. The fine powder (400 g) was extracted with 2500 ml of methanol/water overnight at ratio of 70:30 at room temperature. The solution was then filtered; the residue was collected and re-extracted. The solvent then was removed by using rotary vacuum evaporator under vacuum. The concentrated extract was then kept in dark at 4 °C until used.
Synthesis of Ag/A. elasticus nanoparticles
0.5 g of A. elasticus was added into 0.01 M aqueous solution of AgNO3 (100 ml) with constant stirring at room temperature. Ag-NPs were obtained during the incubation period of 1, 3, 6, 12, 24 and 48 h. Colour changes from light brown to dark brown due to excitation of surface plasmon resonance were observed. The Ag/A. elasticus nanoparticles emulsion obtained were kept at 4 °C.
Characterization methods and instruments
The prepared Ag/A. elasticus nanoparticles were characterized by UV–visible spectroscopy, X-ray diffraction (XRD), transmission electron microscopy (TEM), scanning electron microscopy (SEM), and Fourier-transform infrared (FT-IR) spectroscopy. The reduction of silver ions was confirmed by measuring the UV–vis spectrum at 300–700 nm range with UV-1601 Shimadzu, in a glass cuvette. The structures of the Ag-NPs synthesized after 48 h of incubation were examined with using XRD in powder diffractometer, drop coated onto glass substrates. TEM observation of the Ag-NPs prepared was carried out with LEO 912AB EFTEM. The Ag/A. elasticus nanoparticle solutions were drop onto copper grid and were analyzed. Morphological characterization of the Ag/A. elasticus nanoparticles was performed by Scanning Electron Microscope with using Jeol JSM-7600F Field Emission SEM. The dried powder of Ag/A. elasticus nanoparticles were coated on a carbon tape and coated again with gold before subjected to analysis. The FT-IR spectra were recorded in the range of 280-4000 cm−1 using FT-IR Perkin-Elmer.
Authors’ contributions
NISA carried out the synthesis, and the characterization of the compounds. NISA and KS carried out the acquisition of data, analysis and interpretation of data collected and involved in drafting of manuscript. MA and KS involved in revision of draft for important intellectual content and give final approval of the version to be published. All authors read and approved final manuscript.
Acknowledgements
The authors were gratitude by University Putra Malaysia (UPM) for its facilities and equipment supports. The authors are also grateful to the staff of the Department of Chemistry UPM for their help in this research, Institute of Bioscience (IBS/UPM) for technical assistance.
Competing interests
The authors declare that they have no competing interests.
Contributor Information
Nur Iffah Shafiqah Binti Abdullah, Email: iffahshafiqah@gmail.com.
Mansor B. Ahmad, Email: mansorahmad@upm.edu.my
Kamyar Shameli, Email: kamyarshameli@gmail.com.
References
- 1.Nazeruddin GM, Prasad NR, Prasad SR, Shaikh YI, Waghmare SR, Adhyapak P. Coriandrumsativum seed extract assisted in situ green synthesis of silver nanoparticle and its anti-microbial activity. Ind Crop Prod. 2014;60:212–216. doi: 10.1016/j.indcrop.2014.05.040. [DOI] [Google Scholar]
- 2.Kumar B, Smita K, Cumbal L, Debut A. Sachainchi (Plukenetia volubilis L.) oil for one pot synthesis of silver nanocatalyst: An ecofriendly approach. Ind Crop. Prod. 2014;58:238–243. [Google Scholar]
- 3.Elechiguerra JL, Burt JL, Morones JR, Camacho-Bragado A, Gao X, Lara HH, Yacaman M. Interaction of silver nanoparticles with HIV-1. J Nanobiotechnol. 2005;3(6):1–10. doi: 10.1186/1477-3155-3-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kim KJ, Sung WS, Moon SK, Choi JS, Kim JG, Lee DG. Antifungal effect of silver nanoparticles on dermatophytes. J Microbiol Biotechnol. 2008;18(8):1482–1484. [PubMed] [Google Scholar]
- 5.Rogers JV, Parkinson CV, Choi YW, Speshock JL, Hussain SM. A preliminary assessment of silver nanoparticle inhibition of Monkeypox Virus Plaque Formation. Nanoscale ResLett. 2008;3(4):129–133. doi: 10.1007/s11671-008-9128-2. [DOI] [Google Scholar]
- 6.Roopan SM, Madhumitha G, Rahuman AA, Kamaraj C, Bharathi A, Surendra TV. Low-cost and eco-friendly phyto-synthesis of silver nanoparticles using Cocos nucifera coir extract and its larvicidal activity. Ind Crop Prod. 2013;43:631–635. doi: 10.1016/j.indcrop.2012.08.013. [DOI] [Google Scholar]
- 7.Kumar A, Vemula PK, Ajayan PM, John G. Silver-nanoparticle-embedded antimicrobial paints based on vegetable oil. Nat Mater. 2008;7(3):236–241. doi: 10.1038/nmat2099. [DOI] [PubMed] [Google Scholar]
- 8.El-Rafie MH, Shaheen TI, Mohamed AA, Hebeish A. Bio-synthesis and applications of silver nanoparticles onto cotton fabrics. Carbohyd Polym. 2012;90(2):915–920. doi: 10.1016/j.carbpol.2012.06.020. [DOI] [PubMed] [Google Scholar]
- 9.Davies RL, Etris SF. The development and functions of silver in water purification and disease control. Catal Today. 1997;36(1):107–114. doi: 10.1016/S0920-5861(96)00203-9. [DOI] [Google Scholar]
- 10.Meyer MW, Smith EA. Optimization of silver nanoparticles for surface enhanced Raman spectroscopy of structurally diverse analytes using visible and near-infrared excitation. Analyst. 2011;136(17):3542–3549. doi: 10.1039/c0an00851f. [DOI] [PubMed] [Google Scholar]
- 11.Haes AJ, Van Duyne RP. A nanoscale optical biosensor: sensitivity and selectivity of an approach based on the localized surface plasmon resonance spectroscopy of triangular silver nanoparticles. J Am Chem Soc. 2002;124(35):10596–10604. doi: 10.1021/ja020393x. [DOI] [PubMed] [Google Scholar]
- 12.Ernest V, Shiny PJ, Mukherjee A, Chandrasekaran N. Silver nanoparticles: a potential nanocatalyst for the rapid degradation of starch hydrolysis by α-amylase. Carbohyd Res. 2012;352:60–64. doi: 10.1016/j.carres.2012.02.009. [DOI] [PubMed] [Google Scholar]
- 13.Chaloupka K, Malam Y, Seifalian A. Nanosilver as a new generation of nanoproduct in biomedical applications. Trends Biotechnol. 2010;28(11):580–588. doi: 10.1016/j.tibtech.2010.07.006. [DOI] [PubMed] [Google Scholar]
- 14.Szczepanowicz K. Preparation of silver nanoparticles via chemical reduction and their antimicrobial activity. Physicochem Physicochem ProblMi. 2010;45:85–98. [Google Scholar]
- 15.Khaydarov RA, Khaydarov RR, Gapurova O, EstrinY Scheper T. Electrochemical method for the synthesis of silver nanoparticles. J Nanopart Res. 2009;11(5):1193–1200. doi: 10.1007/s11051-008-9513-x. [DOI] [Google Scholar]
- 16.Shameli K, Ahmad MA, Wan Yunus WMZ, Rustaiyan A, Zargar M, Abdullahi Y. Green synthesis of silver/montmorillonite/chitosan bionanocomposites using the UV-irradiation method and evaluation of antibacterial activity. Int J Nanomed. 2010;5:875–887. doi: 10.2147/IJN.S13632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shameli K, Ahmad MA, Wan Yunus WMZ, Gharayebi Y, Sedaghat S. Synthesis of silver/montmorillonite nanocomposites using γ-irradiation. Int J Nanomed. 2010;5:1067–1077. doi: 10.2147/IJN.S15033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mafune F, Kohno JY, Takeda Y, Kondow T. Formation and size control of silver nanoparticles by laser ablation in aqueous solution. J Phys Chem B. 2000;104(39):9111–9117. doi: 10.1021/jp001336y. [DOI] [Google Scholar]
- 19.Huang H, Yang X. Synthesis of polysaccharide-stabilized gold and silver nanoparticles: a green method. Carbohyd Re. 2004;339(15):2627–2631. doi: 10.1016/j.carres.2004.08.005. [DOI] [PubMed] [Google Scholar]
- 20.Thangaraju N, Venkatalakshmi RP, Chinnasamy A, Kannaiyan P. Synthesis of silver nanoparticles and the antibacterial and anticancer activities of the crude extract of Sargassum polycystum C. Agardh. Nano Biomed Eng. 2012;4(2):89–94. [Google Scholar]
- 21.Sadhasivam S, Shanmugam P, Yun K. Biosynthesis of silver nanoparticles by Streptomyces hygroscopicus and antimicrobial activity against medically important pathogenic microorganisms. Colloid Surface B. 2010;81(1):358–362. doi: 10.1016/j.colsurfb.2010.07.036. [DOI] [PubMed] [Google Scholar]
- 22.Li G, He D, Qian Y, Guan B, Gao S, Yokoyama K, Wang L. Fungus-mediated green synthesis of silver nanoparticles using Aspergillus terreus. Inter J Mol Sci. 2012;13(1):466–476. doi: 10.3390/ijms13010466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mourato A, Gadanho M, Lino AR, Tenreiro R (2011) Biosynthesis of crystalline silver and gold nanoparticles by extremophilic yeasts. Bioinorg Chem Appl 2011:546074. http://dx.doi.org/10.1155/2011/546074 [DOI] [PMC free article] [PubMed]
- 24.Sastry M, Ahmad A, Khan M, Kumar R. Biosynthesis of metal nanoparticles using fungi and actinomycete. Curr Sci India. 2003;85(2):162–170. [Google Scholar]
- 25.Shameli K, Ahmad MB, Zamanian A, Sangpour P, Shabanzadeh P, Abdollahi Y, Zargar M. Green biosynthesis of silver nanoparticles using Curcuma longa tuber powder. Inter J Nanomed. 2012;7:5603–5610. doi: 10.2147/IJN.S36786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vidhu VK, Philip D. Spectroscopic, microscopic and catalytic properties of silver nanoparticles synthesized using Saraca indica flower. Spectrochim Acta Part A Mol Biomol Spectrosc. 2014;117:102–108. doi: 10.1016/j.saa.2013.08.015. [DOI] [PubMed] [Google Scholar]
- 27.Ghaffari-Moghaddam M, Hadi-Dabanlou R. Plant mediated green synthesis and antibacterial activity of silver nanoparticles using Crataegus douglasii fruit extract. J Ind Eng Chem. 2014;20:739–744. doi: 10.1016/j.jiec.2013.09.005. [DOI] [Google Scholar]
- 28.Murugan K, Senthilkumar B, Senbagam D, Al-sohaibani S. Biosynthesis of silver nanoparticles using Acacia leucophloea extract and their antibacterial activity. Int J Nanomed. 2014;9:2431–2438. doi: 10.2147/IJN.S61779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ko HH, Lu YH, Yang SZ, Won SJ, Lin CN. Cytotoxic prenylflavonoids from Artocarpus elasticus. J Nat Prod. 2005;68(11):1692–1695. doi: 10.1021/np050287j. [DOI] [PubMed] [Google Scholar]
- 30.Musthapa I, Juliawaty LD, Syah YM, Hakim EH, Latip J, Ghisalberti EL. An oxepinoflavone from Artocarpus elasticus with cytotoxic activity against P-388 cells. Arch Pharm Res. 2009;32(2):191–194. doi: 10.1007/s12272-009-1134-0. [DOI] [PubMed] [Google Scholar]
- 31.Widyawaruyanti A, Kalauni SK, Awale S, Nindatu M, Zaini NC, Syafruddin D, Asih PBS. New prenylated flavones from some Artocarpus champeden, and their antimalarial activity in vitro. J Nat Med. 2007;61:410–413. doi: 10.1007/s11418-007-0153-8. [DOI] [Google Scholar]
- 32.Lim TK. Edible medicinal and non medicinal plants. Dordrecht: Springer; 2012. pp. 312–315. [Google Scholar]
- 33.Musthapa I, Hakim EH, Juliawaty LD, Syah YM, Achmad SA. Prenylated flavones from some Indonesian Artocarpus and their antimalarial properties. Med Plant. 2010;2(2):157–160. [Google Scholar]
- 34.Kijjoa A, Cidade H, Pinto M, Gonzalez MJTG, Anantachoke C, Gedris TE, Herz W. Prenylflavonoids from Artocarpus elasticus. Phytochemistry. 1996;43(3):691–694. doi: 10.1016/0031-9422(96)00293-2. [DOI] [Google Scholar]
- 35.Shameli K, Ahmad MB, Shabanzadeh P, Al-Mulla EAJ, Zamanian A, Abdullahi Y, Jazayeri SD, Eili M, Jalilian FA. Effect of Curcuma longa tuber powder extract on size of silver nanoparticles prepared by green method. Res Chem Intermediat. 2014;40(3):1313–1325. doi: 10.1007/s11164-013-1040-4. [DOI] [Google Scholar]
- 36.Zargar M, Shameli K, Najafi GR, Farahani F. Plant mediated green biosynthesis of silver nanoparticles using Vitex negundo L. extract. J Ind Eng Chem. 2014;20(25):4169–4175. doi: 10.1016/j.jiec.2014.01.016. [DOI] [Google Scholar]
- 37.Shameli K, Ahmad MB, Al-Mulla EAJ, Shabanzadeh P, Rustaiyan A, Abdullahi Y, Abdolmohammadi S. Green biosynthesis of silver nanoparticles using Callicarpa maingayi stem bark extraction. Molecules. 2002;17(7):8506–8517. doi: 10.3390/molecules17078506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sun Y, Gates B, Mayers B, Xia Y. Crystalline silver nanowires by soft solution processing. Nano Lett. 2002;2(2):165–168. doi: 10.1021/nl010093y. [DOI] [Google Scholar]
- 39.Martinez-Castanon G, Nino-Martinez N, Martinez-Gutierrez F, Martinez-Mendoza JR, Ruiz F. Synthesis and antibacterial activity of silver nanoparticles with different sizes. J Nanopart Res. 2008;10(8):1343–1348. doi: 10.1007/s11051-008-9428-6. [DOI] [Google Scholar]
- 40.Balavandy SK, Shameli K, Biak DRBA, Abidin ZZ. Stirring time effect of silver nanoparticles prepared in glutathione mediated by green method. Chem Cent J. 2014;8(1):11. doi: 10.1186/1752-153X-8-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zargar M, Hamid AA, Bakar FA, Shamsudin MN, Shameli K, Jahanshiri F, Farahani F. Green synthesis and antibacterial effect of silver nanoparticles using Vitex negundo L. Molecules. 2011;16(12):6667–6676. doi: 10.3390/molecules16086667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gong P, Li H, He X, Wang K, Hu J, Tan W, Zhang S, Yang X. Preparation and antibacterial activity of Fe3O4@Ag nanoparticles. Nanotechnology. 2007;18(28):285604. doi: 10.1088/0957-4484/18/28/285604. [DOI] [Google Scholar]
- 43.Shameli K, Ahmad MB, Jazayeri SD, Sedaghat S, Shabanzadeh P, Jahangirian H, Mahdavi M, Abdollahi Y. Synthesis and characterization of polyethylene glycol mediated silver nanoparticles by the green method. Inter J Mol Sci. 2012;13(6):6639–6650. doi: 10.3390/ijms13066639. [DOI] [PMC free article] [PubMed] [Google Scholar]


