Abstract
Licorice is a common herb which has been used in traditional Chinese medicine for centuries. More than 20 triterpenoids and nearly 300 flavonoids have been isolated from licorice. Recent studies have shown that these metabolites possess many pharmacological activities, such as antiviral, antimicrobial, anti-inflammatory, antitumor and other activities. This paper provides a summary of the antiviral and antimicrobial activities of licorice. The active components and the possible mechanisms for these activities are summarized in detail. This review will be helpful for the further studies of licorice for its potential therapeutic effects as an antiviral or an antimicrobial agent.
Abbreviations: CCEC, cerebral capillary vessel endothelial; CCL5, chemokine (C-C motif) ligand 5; CVA16, coxsackievirus A16; CVB3, coxsackievirus B3; CXCL10, chemokine, (C-X-C motif) ligand 10; DGC, dehydroglyasperin C; DHV, duck hepatitis virus; EV71, enterovirus 71; GA, 18β-glycyrrhetinic acid; GATS, glycyrrhizic acid trisodium salt; GL, glycyrrhizin; GLD, glabridin; HBV, hepatitis B virus; HCV, hepatitis C virus; HIV, human immunodeficiency virus; HMGB1, high-mobility-group box1; HRSV, human respiratory syncytial virus; HSV, herpes simplex virus; HSV1, herpes simplex virus type 1; IFN, interferon; IL-6, interleukin-6; LCA, licochalcone A; LCE, licochalcone E; ISL, isoliquiritigenin; LTG, liquiritigenin; MgIG, magnesium isoglycyrrhizinate; MRSA, methicillin-resistant Staphylococcus aureus; MSSA, methicillin-sensitive Staphylococcus aureus; PMN, polymorph nuclear; PrV, pseudorabies virus; TCM, traditional Chinese medicine
KEY WORDS: Licorice, Antiviral, Antimicrobial, Glycyrrhizin, Glycyrrhetinic acid, Chalcone
Graphical abstract
Licorice is a common herb which has been used in traditional Chinese medicine for centuries. This paper provides a summary of the antiviral and antimicrobial activities of licorice. The active components and the possible mechanisms for these activities are summarized in detail.
1. Introduction
Licorice is a very well known herb in traditional Chinese medicine (TCM). In China, it is called “gancao” (meaning “sweet grass”) and has been recorded in the Shennong׳s Classic of Materia Medica around 2100 BC. In this book, licorice was supposed to have life-enhancing properties. During the following thousands of years licorice has been present in most of Chinese traditional prescriptions. It was believed to have the functions of nourishing qi, alleviating pain, tonifying spleen and stomach, eliminating phlegm, and relieving coughing1.
Glycyrrhiza uralensis Fisch., Glycyrrhiza inflate Bat. and Glycyrrhiza glabra L. were prescribed as licorice in Chinese pharmacopoeia2. They are widespread in Inner Mongolia, Gansu, Heilongjiang, Ningxia, Qinghai and many other provinces in China3. The roots and rhizomes are the main medicinal parts of licorice. Numerous studies have revealed many pharmacological activities of licorice, such as antiviral4, 5, anti-inflammatory6, 7, antitumor8, 9, antimicrobial10, 11 and many other activities12, 13. Among the pharmacological activities of licorice mentioned above, the antiviral and antimicrobial activities have been most commonly reported. Viral and other microbial infections play a critical role in many highly prevalent diseases, especially in developing countries. The development of safe and effective antiviral or antimicrobial agents is very important, and licorice deserves more attention for its outstanding activities.
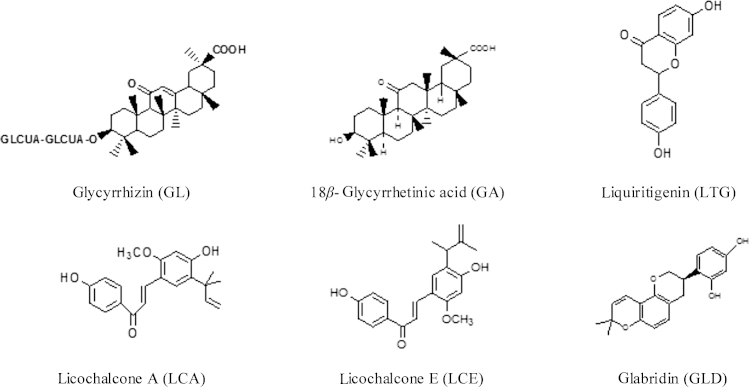
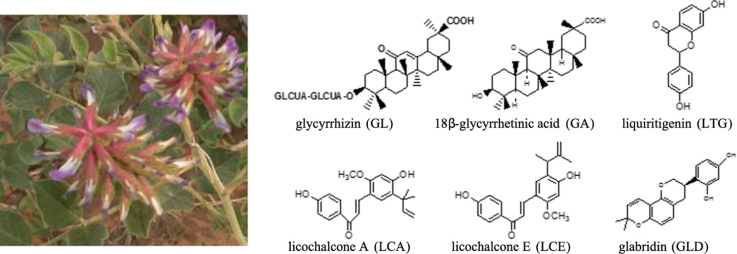
Licorice contains more than 20 triterpenoids and nearly 300 flavonoids. Among them, glycyrrhizin (GL), 18β-glycyrrhetinic acid (GA), liquiritigenin (LTG), licochalcone A (LCA), licochalcone E (LCE) and glabridin (GLD) are the main active components which possess antiviral and antimicrobial activities. Their chemical structures are listed in Fig. 1.
Figure 1.
The chemical structures of the antiviral activity or antimicrobial components in licorice.
2. The antiviral active components and their possible mechanisms
Among the components isolated from licorice, 73 bioactive components and 91 potential targets have been identified to date14, 15. Many studies have demonstrated that two triterpenoids, GL16, 17 and GA18, are responsible for the antiviral activity. The possible mechanisms for virus prevention of GL and GA, and the viral types are listed in Table 1.
Table 1.
The antiviral active components and their possible mechanisms for virus prevention.
| Component | Antiviral mechanism | Viral type |
|---|---|---|
| GL | Affect release step while infectious HCV particles are infecting cells. | HCV |
| Inhibit HCV full length viral particles and HCV core gene expression. | ||
| Reduce adhesion force and stress between CCEC and PMN. | HSV | |
| Block the degradation of nuclear factor κB inhibitor IκB. | CVB3 | |
| Activate T lymphocyte proliferation. | DHV | |
| Weaken H5N1-induced production of CXCL10, IL-6 and CCL5, and suppress H5N1-induced apoptosis. | H5N1 | |
| Reduce HMGB1 binding to DNA, and inhibit influenza virus polymerase activity. | Influenza virus | |
| Inactivate CVA16 directly, while the effect of anti-EV71 is associated with an event(s) during the virus cell entry. | CVA16 EV71 | |
| Establish a resistance state to HSV1 replication. | HSV1 | |
| GA | Reduce the levels of viral proteins VP2, VP6 and NSP2 at a step or steps subsequent to virus entry. | Rotavirus |
| Prevent viral attachment, internalization and stimulate IFN secretion. | HRSV |
2.1. GL
GL is one of the major compounds isolated from the roots of licorice. In recent years, many studies have confirmed the antiviral activity of GL. Matsumoto et al.16 reported that GL targeted the release step in which infectious anti-hepatitis C virus (HCV) particles were infecting cells. These findings indicated possible novel roles for GL to treat patients suffering from chronic hepatitis C. In another study, researchers also found that GL treatment inhibited HCV titer and caused 50% reduction of HCV at the concentration of 14±2 μg/mL by inhibiting HCV full length viral particles and their core gene expression19.
Previous studies showed that intercellular adhesion molecules played an important role in some viral infections, such as human immunodeficiency virus (HIV)20. Huang et al.5 found that the adhesion force and stress between cerebral capillary vessel endothelial (CCEC) cells and polymorph nuclear (PMN) leukocytes were clearly increased in HSV infection; GL perfusion significantly reduced adhesion force and stress between CCEC and PMN.
Zhang׳s study21 reported that GL showed a significant improvement of coxsackievirus B3 (CVB3)–induced myocarditis by improving weight loss profile, reducing serological levels of cardiac enzymes and increasing survival rate. This effect was evidenced by significantly reduced expression of proinflammatory cytokines, such as nuclear factor–κB, interleukin-1β and interleukin-6. The inhibition of CVB3-induced nuclear factor–κB activity blocks the degradation of nuclear factor–κB inhibitor IκB. All these data suggested that GL had an effect on CVB3-induced myocarditis and may present as a new therapeutic approach for the treatment of viral myocarditis.
Soufy et al.22 found that GL had excellent immunostimulant properties and induced a synergistic effect to duck hepatitis virus (DHV) vaccine by activating T lymphocyte proliferation. Four groups, control, GL treated, vaccinated with live attenuated DHV vaccine and GL treated and vaccinated, were investigated. Among them, treatment with GL alone or with DHV vaccine showed good immune stimulant and antiviral effects against DHV. GL combined with DHV vaccine produced higher antibody titers against DHV than by the use of DHV vaccine alone.
Several studies have demonstrated that GL showed a significant inhibiting effect to influenza virus. At a concentration of 100 μg/mL (a therapeutically achievable concentration), GL weakened H5N1-induced production of chemokine (C-X-C motif) ligand 10 (CXCL10), interleukin 6 (IL-6) and chemokine (C-C motif) ligand 5 (CCL5), and suppressed H5N1-induced apoptosis23. The high-mobility-group box1 (HMGB1) DNA-binding site was indicated to enhance influenza virus replication. GL could reduce HMGB1 binding to DNA, which inhibited influenza virus polymerase activity24. Smirnov׳s study25 indicated that GL could be considered a promising agent for the treatment of influenza.
Wang׳s study17 revealed that GL was an antiviral component in licorice against enterovirus 71 (EV71) and coxsackievirus A16 (CVA16) infection with defined mechanisms. It activated CVA16 directly, while the effect of anti-EV71 was associated with an event during the cell entry for virus.
GL was also a strong inducer of the autophagy activator Beclin 1. After 24 h of treatment, Beclin 1 production induced by GL was more than two fold higher than that was induced by rapamycin, the reference compound. GL was a strong inducer of Beclin 1, which inhibited the replication of herpes simplex virus type 1 (HSV1)26. Therefore, GL possessed its anti-HSV1 activity by establishing a resistant state to HSV1 replication.
Above all, GL is an effective antiviral compound against HCV, HIV, CVB3, DHV, EV71, CVA16, HSV and H5N1 by weakening virus activity, such as inhibiting virus gene expression and replication, reducing adhesion force and stress, and reducing HMGB1 binding to DNA. The compound also enhances host cell activity, e.g., by blocking the degradation of IκB, activating T lymphocyte proliferation and/or suppressing host cell apoptosis.
2.2. GA
Compared with GL, studies of the antiviral activity of GA are limited. GA treatment inhibited rotavirus replication, which likely occurred at steps subsequent to virus entry. GA reduced rotavirus yields by 99% when it was added to infected cultures post-viral adsorption. The levels of viral proteins VP2, VP6 and NSP2 were substantially reduced27. GA also showed potent anti-human respiratory syncytial virus (HRSV) activity. It inhibited HRSV mainly by internalization, stimulating interferon (IFN) secretion, and preventing viral attachment18.
There is a difference between the antiviral profiles of GA and GL. GA has activity against rotavirus and HRSV. However, the antiviral mechanisms of these compounds are similar. GA exerts its antiviral activity also by inhibiting virus replication, preventing viral attachment or enhancing host cell activity.
3. The antimicrobial active components and their possible mechanisms
Increasing antibiotic resistance has resulted in an urgent need for alternative therapies to treat diseases. In recent years, many studies have shown that licorice aqueous extract28, ethanol extract29 and supercritical fluid extract30 have potent effects in inhibiting the activities of Gram-positive bacteria and Gram-negative bacteria, such as Staphylococcus aureus31, Escherichia coli32, Pseudomonas aeruginosa33, Candida albicans and Bacillus subtilis34. These extracts are also being considered as potential alternatives to synthetic fungicides, or as lead compounds for new classes of synthetic fungicides. Based on the above inhibitory activities against bacteria, licorice may serve as an alternative therapy for treating dental caries, periodontal disease, digestive anabrosis and tuberculosis. The possible mechanisms for antimicrobial effects of the active components and the microorganism types were listed in Table 2.
Table 2.
The antimicrobial active components and their possible mechanisms for microbe prevention.
| Component | Antimicrobial mechanism | Microbial type |
|---|---|---|
| GA | Decrease the expression of SaeR and Hla, which are the key virulence genes of MRSA. | S. aureus |
| Exert the Th1-immunological adjuvant activity. | C. albicans | |
| LCA | Inhibit the biofilm formation and prevent yeast-hyphal transition. | C. albicans |
| LCE | Reduce the production of α-toxin. | S. aureus |
| GLD | Prevent yeast-hyphal transition. | C. albicans |
| LTG | Decrease the production of α-hemolysin. | S. aureus |
3.1. GA
Methicillin-resistant S. aureus (MRSA) has become a main source of infection in both hospitals and the community. Increasing antibiotic resistance in S. aureus strains has created a need for other therapies to treat disease. GA showed bactericidal activity to destroy MRSA by decreasing the expression of SaeR and Hla, the key virulence genes of MRSA31. Studies also indicated that GA produced a better Th1 immune response than Th2 response. This Th1-immunological adjuvant activity would be helpful in the treatment of Th1-related disease caused by C. albican35.
3.2. Chalcones
Zhou et al.36 suggested that licochalcone E (LCE) could be used for chemical synthesis of novel anti–S. aureus compounds which could reduce the production of α-toxin in both methicillin-sensitive S. aureus (MSSA) and MRSA. Licochalcone A (LCA) and glabridin (GLD) showed antifungal activity on C. albicans. They were both potent antifungal agents against C. albicans. LCA (0.2 μg/mL) inhibited biofilm formation by 35%–60% and both LCA and GLD had strong inhibitory effects (>80%) in preventing yeast-hyphal transition in C. albicans37.
3.3. Liquiritigenin
α-Hemolysin is an important exotoxin in the pathogenesis of S. aureus infections. Such infections are associated with a broad spectrum of diseases ranging from endocarditis to minor skin infections, toxinoses, and lethal pneumonia. Liquiritigenin (LTG), one of the most significant active components in licorice, can prevent human lung cells (A549) from α-hemolysin-mediated injury by decreasing α-hemolysin production38. Such data suggest that LTG is potentially useful in developing drugs which target staphylococcal α-hemolysin.
In summary, one triterpene (GA) and four flavones (LCA, LCE, GLD and LTG) seem to account for much of the antimicrobial activity in licorice. These compounds can decrease the expression of microbe genes, inhibit microbe growth and reduce the production of microbe toxin.
4. Discussion
Presently we have summarized the antiviral and antimicrobial activities of licorice. Many studies found that several components were responsible for the antiviral and antimicrobial activities through different mechanisms. Licorice contains more than 20 triterpenoids and nearly 300 flavonoids. Among them, only two triterpenes, GL and GA have been reported to have antiviral effects. They can weaken virus activities by inhibiting virus gene expression and replication, reducing adhesion force and stress, and reducing HMGB1 binding to DNA. They can also enhance host cell activities by blocking the degradation of IκB, activating T lymphocyte proliferation and suppressing host cell apoptosis. In contrast, flavonoids, especially chalcones, play an important role in the treatment of bacterial infection by decreasing expression of bacterial genes, inhibiting bacterial growth and reducing the production of bacterial toxin.
In addition, many studies have reported that the six active compounds listed in this paper, GL, GA, LCA, LCE, GLD and LTG, possess other activities. For example, GL and GA also have antitumor39, 40, anti-inflammatory41, 42, and immunoregulatory activities12, 43, 44. LCA, LCE, LTG and GLD also have inhibitory effects on diabetes45, 46, 47, 48. All of these reports demonstrate potentially broad applications for these agents. In addition, there are many other compounds isolated from licorice with different pharmacological activities. For example, isoliquiritigenin (ISL) shows effective immunoregulatory activity49, glabrol has an inhibitory effect on diabetes50, and dehydroglyasperin C (DGC) has hepatoprotective activity51.
Among the six compounds listed in this paper, only GL has been clinically developed as a drug. As the most important marker component in licorice, the development of GL preparations has a long history in China, from GL tablets to ammonium glycyrrhizinate, diammonium glycyrrhizinate and magnesium isoglycyrrhizinate (MgIG). All of the above GL preparations possess antiviral and antimicrobial activities. Diammonium glycyrrhizinate inhibits cell infection by pseudorabies virus (PrV) and decreases cell apoptosis during PrV infection52. Compared with diammonium glycyrrhizinate, the fourth generation GL preparation, MgIG, has better lipophilic properties, higher targeting activity and fewer adverse reactions. It has been used in treating liver disease53, 54, 55, pulmonary fibrosis56 and testicular injuries57. However, reports about mechanisms of antiviral and antimicrobial activities of MgIG are still very limited. The development of new licorice preparations will improve the safety and efficacy of licorice-related products.
In many African countries with poorly developed health care systems, viruses and bacteria are significant sources of disease. More than 2 billion people have been exposed to HBV over the world, and the situation in some areas of Africa is much more serious58. The development of effective and affordable licorice-related medicines could introduce dramatic improvements in treating the many prevalent diseases of third world populations. It is hoped that the present work will facilitate the development of improved licorice preparations with antiviral and antimicrobial activities.
Acknowledgments
This work was supported by Beijing Project for Young Talents (YETP0819).
Footnotes
Peer review under responsibility of Institute of Materia Medica, Chinese Academy of Medical Sciences and Chinese Pharmaceutical Association.
Contributor Information
Ying Liu, Email: liuyliwd@sina.com.
Chunsheng Liu, Email: maxliucs@263.net.
References
- 1.Zeng L., Li S.H., Lou Z.C. Morphological and histological studies of Chinese licorice. Acta Pharm Sin. 1988;23:200–208. [PubMed] [Google Scholar]
- 2.The state Pharmacopoeia Committee of China . China Medical Science Press; Beijing: 2010. The pharmacopoeia of the people׳s republic of China. Part1; pp. 80–81. [Google Scholar]
- 3.Delectis Florae Reipublicae Popularis Sinicae Agendae Academiae Sinicae Edita . Vol. 42. Science Press; Beijing: 1998. p. 169. (Flora reipublicae popularis sinicae). [Google Scholar]
- 4.Adianti M., Aoki C., Komoto M., Deng L., Shoji I., Wahyuni T.S. Anti-hepatitis C virus compounds obtained from Glycyrrhiza uralensis and other Glycyrrhiza species. Microbiol Immunol. 2014;58:180–187. doi: 10.1111/1348-0421.12127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huang W., Chen X., Li Q., Li P., Zhao G.N., Xu M.M. Inhibition of intercellular adhesion in herpex simplex virus infection by glycyrrhizin. Cell Biochem Biophys. 2012;62:137–140. doi: 10.1007/s12013-011-9271-8. [DOI] [PubMed] [Google Scholar]
- 6.Chandrasekaran C.V., Deepak H.B., Thiyagarajan P., Kathiresan S., Sangli G.K., Deepak M. Dual inhibitory effect of Glycyrrhiza glabra (GutGard™) on COX and LOX products. Phytomedicine. 2011;18:278–284. doi: 10.1016/j.phymed.2010.08.001. [DOI] [PubMed] [Google Scholar]
- 7.Wu T.Y., Khor T.O., Saw C.L.L., Loh S.C., Chen A.I., Lim S.S. Anti-inflammatory/anti-oxidative stress activities and differential regulation of Nrf2-mediated genes by non-polar fractions of tea Chrysanthemum zawadskii and licorice Glycyrrhiza uralensis. AAPS J. 2011;13:1–13. doi: 10.1208/s12248-010-9239-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Choi A.Y., Choi J.H., Hwang K.Y., Jeong Y.J., Choe W., Yoon K.S. Licochalcone A induces apoptosis through endoplasmic reticulum stress via a phospholipase Cγ1-, Ca2+-, and reactive oxygen species–dependent pathway in HepG2 human hepatocellular carcinoma cells. Apoptosis. 2014;19:682–697. doi: 10.1007/s10495-013-0955-y. [DOI] [PubMed] [Google Scholar]
- 9.Khan R., Khan A.Q., Lateef A., Rehman M.U., Tahir M., Ali F. Glycyrrhizic acid suppresses the development of precancerous lesions via regulating the hyperproliferation, inflammation, angiogenesis and apoptosis in the colon of Wistar rats. PLoS One. 2013;8:e56020. doi: 10.1371/journal.pone.0056020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ahn S.J., Cho E.J., Kim H.J., Park S.N., Lim Y.K., Kook J.K. The antimicrobial effects of deglycyrrhizinated licorice root extract on Streptococcus mutans UA159 in both planktonic and biofilm cultures. Anaerobe. 2012;18:590–596. doi: 10.1016/j.anaerobe.2012.10.005. [DOI] [PubMed] [Google Scholar]
- 11.Treutwein J., Cergel S., Runte J., Nowak A., Konstantinidou-Doltsinis S., Kleeberg H. Efficacy of Glycyrrhiza glabra extract fractions against phytopathogenic fungi. Julius-Kühn-Archiv. 2010;428:82. [Google Scholar]
- 12.Bordbar N., Karimi M.H., Amirghofran Z. Phenotypic and functional maturation of murine dendritic cells induced by 18 alpha- and beta-glycyrrhetinic acid. Immunopharm Immunot. 2013;36:52–60. doi: 10.3109/08923973.2013.864670. [DOI] [PubMed] [Google Scholar]
- 13.Hong Y.K., Wu H.T., Ma T., Liu W.J., He X.J. Effects of Glycyrrhiza glabra polysaccharides on immune and antioxidant activities in high-fat mice. Int J Biol Macromol. 2009;45:61–64. doi: 10.1016/j.ijbiomac.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 14.Li Y.J., Chen J., Li Y., Li Q., Zheng Y.F., Fu Y. Screening and characterization of natural antioxidants in four Glycyrrhiza species by liquid chromatography coupled with electrospray ionization quadrupole time-of-flight tandem mass spectrometry. J Chromatogr A. 2011;1218:8181–8191. doi: 10.1016/j.chroma.2011.09.030. [DOI] [PubMed] [Google Scholar]
- 15.Liu H., Wang J.N., Zhou W., Wang Y.H., Yang L. Systems approaches and polypharmacology for drug discovery from herbal medicines: an example using licorice. J Ethnopharmacol. 2013;146:773–793. doi: 10.1016/j.jep.2013.02.004. [DOI] [PubMed] [Google Scholar]
- 16.Matsumoto Y., Matsuura T., Aoyagi H., Matsuda M., Hmwe S.S., Date T. Antiviral activity of glycyrrhizin against hepatitis C virus in vitro. PLoS One. 2013;8:e68992. doi: 10.1371/journal.pone.0068992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang J.J., Chen X.Q., Wang W., Zhang Y.T., Yang Z.Y., Jin Y. Glycyrrhizic acid as the antiviral component of Glycyrrhiza uralensis Fisch. against coxsackievirus A16 and enterovirus 71 of hand foot and mouth disease. J Ethnopharmacol. 2013;147:114–121. doi: 10.1016/j.jep.2013.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yeh C.F., Wang K.C., Chiang L.C., Shieh D.E., Yen M.H., Chang J.S. Water extract of licorice had anti-viral activity against human respiratory syncytial virus in human respiratory tract cell lines. J Ethnopharmacol. 2013;148:466–473. doi: 10.1016/j.jep.2013.04.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ashfaq U.A., Masoud M.S., Nawaz Z., Riazuddin S. Glycyrrhizin as antiviral agent against Hepatitis C Virus. J Transl Med. 2011;9:112. doi: 10.1186/1479-5876-9-112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang J.H., Kwas C., Wu L. Intercellular adhesion molecule 1 (ICAM-1), but not ICAM-2 and -3, is important for dendritic cell-mediated human immunodeficiency virus type 1 transmission. J Virol. 2009;83:4195–4204. doi: 10.1128/JVI.00006-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang H.C., Song Y.X., Zhang Z.C. Glycyrrhizin administration ameliorates coxsackievirus B3–induced myocarditis in mice. Am J Med Sci. 2012;344:206–210. doi: 10.1097/MAJ.0b013e31823e2867. [DOI] [PubMed] [Google Scholar]
- 22.Soufy H., Yassein S., Ahmed A.R., Khodier M.H., Kutkat M.A., Nasr S.M. Antiviral and immune stimulant activities of glycyrrhizin against duck hepatitis virus. Afr J Tradit Complement Altern Med. 2012;9:389–395. doi: 10.4314/ajtcam.v9i3.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Michaelis M., Geiler J., Naczk P., Sithisarn P., Ogbomo H., Altenbrandt B. Glycyrrhizin inhibits highly pathogenic H5N1 influenza A virus–induced pro-inflammatory cytokine and chemokine expression in human macrophages. Med Microbiol Immunol. 2010;199:291–297. doi: 10.1007/s00430-010-0155-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moisy D., Avilov S.V., Jacob Y., Laoide B.M., Ge X.Y., Baudin F. HMGB1 protein binds to influenza virus nucleoprotein and promotes viral replication. J Virol. 2012;86:9122–9133. doi: 10.1128/JVI.00789-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Smirnov V.S., Zarubaev V.V., Anfimov P.M., Shtro A.A. Effect of a combination of glutamyl-tryptophan and glycyrrhizic acid on the course of acute infection caused by influenza (H3H2) virus in mice. Vopr Virusol. 2012;57:23–27. [PubMed] [Google Scholar]
- 26.Laconi S., Madeddu M.A., Pompei R. Autophagy activation and antiviral activity by a licorice triterpene. Phytother Res. 2014;28:1890–1892. doi: 10.1002/ptr.5189. [DOI] [PubMed] [Google Scholar]
- 27.Hardy M.E., Hendricks J.M., Paulson J.M., Faunce N.R. 18β-glycyrrhetinic acid inhibits rotavirus replication in culture. Virol J. 2012;9:96. doi: 10.1186/1743-422X-9-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Al-Turki A.I., El-Ziney M.G., Abdel-Salam A.M. Chemical and anti-bacterial characterization of aqueous extracts of oregano, marjoram, sage and licorice and their application in milk and labneh. J food Agric Environ. 2008;6:39–44. [Google Scholar]
- 29.Park I.K., Kim J., Lee Y.S., Shin S.C. In vivo fungicidal activity of medicinal plant extracts against six phytopathogenic fungi. Int J Pest Manag. 2008;54:63–68. [Google Scholar]
- 30.Bodet C., La V.D., Gafner S., Bergeron C., Grenier D. A licorice extract reduces lipopolysaccharide-induced proinflammatory cytokine secretion by macrophages and whole blood. J Periodontol. 2008;79:1752–1761. doi: 10.1902/jop.2008.080052. [DOI] [PubMed] [Google Scholar]
- 31.Long D.R., Mead J., Hendricks J.M., Hardy M.E., Voyich J.M. 18β-Glycyrrhetinic acid inhibits methicillin-resistant Staphylococcus aureus survival and attenuates virulence gene expression. Antimicrob Agents Chemother. 2013;57:241–247. doi: 10.1128/AAC.01023-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Awandkar S.P., Mendhe M.S., Badukale D.M., Kulkarni M.B. Antimicrobial action of cold aqueous and cold ethanolic extracts of Glycyrrhiza glabra against bovine mammary pathogens. Anim Sci Report. 2012;6:88–91. [Google Scholar]
- 33.Yoshida T., Yoshida S., Kobayashi M., Herndon D.N., Suzuki F. Pivotal advance: glycyrrhizin restores the impaired production of β-defensins in tissues surrounding the burn area and improves the resistance of burn mice to Pseudomonas aeruginosa wound infection. J Leukoc Biol. 2010;87:35–41. doi: 10.1189/jlb.1208760. [DOI] [PubMed] [Google Scholar]
- 34.Irani M., Sarmadi M., Bernard F., Ebrahimi P.G.H., Shaker B.H. Leaves antimicrobial activity of Glycyrrhiza glabra L. Iran J Pharm Res. 2010;9:425–428. [PMC free article] [PubMed] [Google Scholar]
- 35.Kim J., Joo I., Kim H., Han Y. 18β-Glycyrrhetinic acid induces immunological adjuvant activity of Th1 against Candida albicans surface mannan extract. Phytomedicine. 2013;20:951–955. doi: 10.1016/j.phymed.2013.04.008. [DOI] [PubMed] [Google Scholar]
- 36.Zhou T.Z., Deng X.M., Qiu J.Z. Antimicrobial activity of licochalcone E against Staphylococcus aureus and its impact on the production of staphylococcal alpha-toxin. J Microbiol Biotechnol. 2012;22:800–805. doi: 10.4014/jmb.1112.12020. [DOI] [PubMed] [Google Scholar]
- 37.Messier C., Grenier D. Effect of licorice compounds licochalcone A, glabridin and glycyrrhizic acid on growth and virulence properties of Candida albicans. Mycoses. 2011;54:e801–e806. doi: 10.1111/j.1439-0507.2011.02028.x. [DOI] [PubMed] [Google Scholar]
- 38.Dai X.H., Li H.E., Lu C.J., Wang J.F., Dong J., Wei J.Y. Liquiritigenin prevents Staphylococcus aureus–mediated lung cell injury via inhibiting the production of α-hemolysin. J Asian Nat Prod Res. 2013;15:390–399. doi: 10.1080/10286020.2013.771344. [DOI] [PubMed] [Google Scholar]
- 39.Kim H.J., Seo J.Y., Suh H.J., Lim S.S., Kim J.S. Antioxidant activities of licorice-derived prenylflavonoids. Nutr Res Pract. 2012;6:491–498. doi: 10.4162/nrp.2012.6.6.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yasukawa K. Inhibitory effect of a combined treatment of glycyrrhizin and caffeine on tumor promotion by 12-O-tetradecanoylphorbol-13-acetate in two-stage carcinogenesis in mouse skin. J Pharm Nutr Sci. 2013;3:202–205. [Google Scholar]
- 41.Wang C.Y., Kao T.C., Lo W.H., Yen G.C. Glycyrrhizic acid and 18β-glycyrrhetinic acid modulate lipopolysaccharide-induced inflammatory response by suppression of NF-κB through PI3K p110δ and p110γ inhibitions. J Agric Food Chem. 2011;59:7726–7733. doi: 10.1021/jf2013265. [DOI] [PubMed] [Google Scholar]
- 42.Imai K., Takagi Y., Iwazaki A., Nakanishi K. Radical scavenging ability of glycyrrhizin. Free Radic Antioxid. 2013;3:40–42. [Google Scholar]
- 43.Li W., Li J.H., Sama A.E., Wang H.C. Carbenoxolone blocks endotoxin-induced protein kinase R (PKR) activation and high mobility group box 1 (HMGB1) release. Mol Med. 2013;19:203–211. doi: 10.2119/molmed.2013.00064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kim M.E., Kim H.K., Kim D.H., Yoon J.H., Lee J.S. 18β-Glycyrrhetinic acid from licorice root impairs dendritic cells maturation and Th1 immune responses. Immunopharmacol Immunotoxicol. 2013;35:329–335. doi: 10.3109/08923973.2013.768636. [DOI] [PubMed] [Google Scholar]
- 45.Yao K., Chen H.Y., Lee M.H., Li H.T., Ma W.Y., Peng C. Licochalcone A, a natural inhibitor of c-Jun N-terminal kinase 1. Cancer Prev Res. 2014;7:139–149. doi: 10.1158/1940-6207.CAPR-13-0117. [DOI] [PubMed] [Google Scholar]
- 46.Park H.G., Bak E.J., Woo G.H., Kim J.M., Quan Z.J., Kim J.M. Licochalcone E has an antidiabetic effect. J Nutr Biochem. 2012;23:759–767. doi: 10.1016/j.jnutbio.2011.03.021. [DOI] [PubMed] [Google Scholar]
- 47.Gaur R., Yadav K.S., Verma R.K., Yadav N.P., Bhakuni R.S. In vivo anti-diabetic activity of derivatives of isoliquiritigenin and liquiritigenin. Phytomedicine. 2014;21:415–422. doi: 10.1016/j.phymed.2013.10.015. [DOI] [PubMed] [Google Scholar]
- 48.Wu F.H., Jin Z.G., Jin J. Hypoglycemic effects of glabridin, a polyphenolic flavonoid from licorice, in an animal model of diabetes mellitus. Mol Med Rep. 2013;7:1278–1282. doi: 10.3892/mmr.2013.1330. [DOI] [PubMed] [Google Scholar]
- 49.Park S.J., Song H.Y., Youn H.S. Suppression of the TRIF-dependent signaling pathway of toll-like receptors by isoliquiritigenin in RAW264.7 macrophages. Mol Cells. 2009;28:365–368. doi: 10.1007/s10059-009-0130-z. [DOI] [PubMed] [Google Scholar]
- 50.Choi J.H., Choi J.N., Lee S.Y., Lee S.J., Kim K., Kim Y.K. Inhibitory activity of diacylglycerol acyltransferase by glabrol isolated from the roots of licorice. Arch Pharm Res. 2010;33:237–242. doi: 10.1007/s12272-010-0208-3. [DOI] [PubMed] [Google Scholar]
- 51.Seo J.Y., Han J.H., Kim Y.J., Lim S.S., Kim J.S. Protective effects of dehydroglyasperin c against carbon tetrachloride–induced liver damage in mice. Food Sci Biotechnol. 2014;23:547–553. [Google Scholar]
- 52.Sui X.W., Yin J.C., Ren X.F. Antiviral effect of diammonium glycyrrhizinate and lithium chloride on cell infection by pseudorabies herpesvirus. Antiviral Res. 2010;85:346–353. doi: 10.1016/j.antiviral.2009.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Huang X.L., Qin J.J., Lu S. Magnesium isoglycyrrhizinate protects hepatic L02 cells from ischemia/reperfusion induced injury. Int J Clin Exp Pathol. 2014;7:4755–4764. [PMC free article] [PubMed] [Google Scholar]
- 54.Chen K.J., Chen W.Y., Chen X., Jia Y.M., Peng G.Q., Chen L. Increased elimination of paclitaxel by magnesium isoglycyrrhizinate in epithelial ovarian cancer patients treated with paclitaxel plus cisplatin: a pilot clinical study. Eur J Drug Metab Pharmacokinet. 2014;39:25–31. doi: 10.1007/s13318-013-0136-y. [DOI] [PubMed] [Google Scholar]
- 55.Cheng Y., Zhang J., Shang J., Zhang L.Y. Prevention of free fatty acid-induced hepatic lipotoxicity in HepG2 cells by magnesium isoglycyrrhizinate in vitro. Pharmacology. 2009;84:183–190. doi: 10.1159/000235873. [DOI] [PubMed] [Google Scholar]
- 56.Xiao Z.W., Zhang W., Ma L., Qiu Z.W. Therapeutic effect of magnesium isoglycyrrhizinate in rats on lung injury induced by paraquat poisoning. Eur Rev Med Pharmacol Sci. 2014;18:311–320. [PubMed] [Google Scholar]
- 57.He Y.Q., Zeng F.Q., Liu Q., Ju W., Fu H.J., Hao H. Protective effect of magnesium isoglycyrrhizinate on ethanol-induced testicular injuries in mice. J Biomed Res. 2010;24:153–160. doi: 10.1016/S1674-8301(10)60024-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rey-Cuille M.A., Njouom R., Bekondi C., Seck A., Gody C., Bata P. Hepatitis B virus exposure during childhood in Cameroon, Central African Republic and Senegal after the integration of HBV vaccine in the expanded program on immunization. Pediatr Infect Dis J. 2013;32:1110–1115. doi: 10.1097/INF.0b013e31829be401. [DOI] [PubMed] [Google Scholar]