Abstract
The administration of autologous (recipient-derived) tolerogenic dendritic cells (ATDCs) is under clinical evaluation. However, the molecular mechanisms by which these cells prolong graft survival in a donor-specific manner is unknown. Here, we tested mouse ATDCs for their therapeutic potential in a skin transplantation model. ATDC injection in combination with anti-CD3 treatment induced the accumulation of CD8+CD11c+ T cells and significantly prolonged allograft survival. TMEM176B is an intracellular protein expressed in ATDCs and initially identified in allograft tolerance. We show that Tmem176b−/− ATDCs completely failed to trigger both phenomena but recovered their effect when loaded with donor peptides before injection. These results strongly suggested that ATDCs require TMEM176B to cross-present antigens in a tolerogenic fashion. In agreement with this, Tmem176b−/− ATDCs specifically failed to cross-present male antigens or ovalbumin to CD8+ T cells. Finally, we observed that a Tmem176b-dependent cation current controls phagosomal pH, a critical parameter in cross-presentation. Thus, ATDCs require TMEM176B to cross-present donor antigens to induce donor-specific CD8+CD11c+ T cells with regulatory properties and prolong graft survival.
Keywords: Autologous dendritic cells, cellular therapy, cross-presentation, ion channel
Introduction
Innovative therapeutic strategies are needed to diminish the harmful effects of immunosuppressive drugs used to treat patients with autoimmune diseases and transplant recipients. Cell therapy using dendritic cells (DCs) is a promising approach to decrease doses of immunosuppressives in these settings (1,2). Our group has previously characterized, phenotypically and functionally, rat and mouse autologous tolerogenic dendritic cells (ATDCs) (3-6). We showed that ATDC therapy, in association with suboptimal doses of immunosuppression, induces a prolongation of allograft survival in a donor-specific manner (3,4,7,8). It is important to note that, in these studies, ATDCs were not pulsed with donor antigens. This strategy is most clinically relevant because cells can be prepared in advance from patients placed on the transplant waiting list (9). We are currently developing a clinical protocol to inject ATDCs into kidney graft recipients in a phase I/II clinical trial (The ONE Study, 7th Frame Program, European Commission). Other ongoing clinical trials examine the potential of ATDCs in autoimmune patients (10,11).
In the current study, we wished to determine the molecular mechanisms by which mouse ATDCs can prolong graft survival in a donor-specific manner. We observed that ATDC injection generates donor-specific CD8+ regulatory T cells (Tregs). We investigated the role of Tmem176b, a gene encoding for a transmembrane protein of unknown function, in which we previously identified as preferentially expressed in immature DCs (12,13). We found that ATDCs required Tmem176b expression to exert their immunoregulatory function in vivo. We further show that TMEM176B is responsible for a phagosomal cation current that is needed to control phagosomal pH in DCs, a critical parameter in the cross-presentation pathway (14-20).
Materials and Methods
Mice
A Tmem176b−/− (KO) mouse was generated in the 129/SvJ strain and heterozygous mice were backcrossed for 10 generations onto the C57BL/6 background (Janvier, Saint Berthevin, France). WT C57BL/6 littermate controls were obtained in our animal facility. MataHari CD8+ TCR transgenic and β2-microglobulin−/− (B6.129P2-B2mtm1Unc/J) mice were kindly provided by Olivier Lantz. All animal experiments were performed under specific pathogen-free conditions in accordance with the European Union Guidelines. All animal studies were conducted according to the guidelines of the French Agriculture Ministry. The studies were approved by the Veterinary Departmental Services committee, La Chapelle-Sur-Erdre, Paris, France (no. E.44011, 75-1554), and all experiments were carried out in compliance with the ethical rules of the INSERM.
Bone Marrow DCs
Bone marrow DCs (BMDCs) were generated as previously described (6). For the sake of clarity, BMDCs are referred to as ATDCs along the text. Briefly, bone marrow precursors were cultured for 8 days in the presence of low doses of granulocyte macrophage colony-stimulating factor (0.4 ng/mL). By day 8, adherent cells were recovered and used for in vitro and in vivo experiments.
Skin transplantation and treatments
C57BL/6 male tail skin was grafted on female recipients as previously described (21). One million WT or Tmem176b−/− (KO) female ATDCs were injected intravenously (i.v.) the day before transplantation. One microgram anti-CD3 antibody (145-2C11, kindly provided by J. Bluestone) per mouse was injected intraperitoneally at days −1, +1, +3, +5 and +7 following skin transplantation. Graft survival was followed every other day.
Reagents and antibodies
Endotoxin-free OVA protein was from Profos (Regensberg, Germany). OVA (SIINFEKL), Smcy (KCSRNRQYL) and Uty (WMHHNMDLI) peptides were from Polypeptide (Strasbourg, France). Fluorescein isothiocyanate (FITC), PKH-26 and latex beads amine-modified polystyrene fluorescent red were from Sigma (St. Quentin Fallavier, France). Fluoprobes 647 was from Fluoprobes (Montluçon, France). DDAO-SE and OVA-Alexa 647 were from Molecular Probes (Montluçon, France). Anti-CD4 Pacific blue, anti-CD8 PECy7, anti-CD69 Biotin, anti-Vα2 FITC, CD19 APC and Annexin V APC were from BD (Le Pont-De-Claix, France). Anti-cathepsin S antibody was from Santa Cruz Biotechnology (Santa Cruz, CA). H-2Db WMHHNMDLI (Uty), H-2Db KCSRNRQYL (Smcy), H-2Kb SIINFEKL (OVA), H-2Kb VNHRFTLV (Trypanosoma Cruzi, control) pentamers were from Proimmune (Oxford, UK). Pentamers stainings were performed according to the manufacturer’s instructions (Pro5®Recombinant murine MHC Pentamer; ProImmune, Oxford, UK). The pentamers used in this study were H-2Db WMHHNMDLI (Uty) and H-2Db KCSRNRQYL (Smcy).
Adoptive cell transfer
CD8+ T cells from graft-draining lymph nodes (axillary lymph node) were purified using a FACSAria (BD). Purified CD8+ T cells (>95% purity) were injected i.v. (2 × 105 cells) the day before grafting male skin on female recipients that did not receive any additional treatment.
Antigen presentation assays
Five thousand WT or KO ATDCs were plated in triplicate in 96-well plates and incubated with indicated concentrations of soluble OVA, OVA-coated beads or SIINFEKL peptide for 45 min at 37°C. After extensive washing, cells were treated overnight with 0.25 μg/mL lipopolysaccharide, then washed a second time before adding 5 × 104 DDAO-labeled OT-1 CD8+ or OT-2 CD4+ T cells purified with the AutoMACS device (Miltenyi Biotec, Paris, France). Upon a 4-day culture, proliferation of T cells was analyzed by assessing DDAO dilution in CD8+Vα2+ (OT-1) or CD4+Vα2+ cells (OT-2). In assays based on direct presentation of OVA as an endogenous antigen, ATDCs were electroporated at 300 V, 150 μF (4-mm gap electroporation cuvette, Gene Pulser II apparatus; BioRad, Hercules, CA) with different doses of an in vitro synthesized mRNA (mMESSAGE mMACHINE Ultra Kit; Ambion, Austin, TX) coding for a protein fusioning the OVA peptides (for OT-1 and OT-2) and green fluorescent protein (GFP). In HY antigen experiments, 5 × 103 female WT or KO ATDCs were incubated with male β2-microglobulin−/− splenocytes at different ratios for 2 h in 96-well plates. After extensive washing, the splenocytes were eliminated while adherent ATDCs remained attached. Purified Uty-specific TCR transgenic CD8+ MataHari T cells were then added (5 × 104 cells). After a 20 h culture, CD69 expression was assessed by flow cytometry on CD8+ T cells.
Endocytosis and phagocytosis measurement by flow cytometry analysis
WT and KO ATDCs were pulsed with different doses of OVA-Alexa 647 for 15 min and then chased for 30 min at 37 or 4°C. To study phagocytosis, ATDCs were pulsed with fluorescent beads (Sigma) at different dilutions.
Measurement of phagosomal pH
Phagosomal pH was measured by flow cytometry analysis as previously described (14). Briefly, 3 μm polybeads amino were covalently coupled with FITC (pH sensitive) and FluoProbes 647 (pH insensitive) and used to pulse/chase cells at 37°C.
Electrophysiology and intra-oocyte pH measurements
Oocytes were surgically removed from MS222 (0.4%)-anesthetized Xenopus laevis female and dissociated under gentle agitation by a 2–3 h incubation in an OR2 solution (in mM NaCl 82; KCl, 2; MgCl2, 1; HEPES, 5; pH 7.2) supplemented with collagenase 1A (1 mg/mg). Oocytes were then injected with 40 nL of in vitro synthesized Tmem176b mRNA at 1 μg/μL (mMESSAGE mMACHINE Ultra Kit). Tmem176b was fused to a signal peptide sequence (N-terminal) from pSecTag2B (Invitrogen, Carlsbad, CA) and to V5 + 6-His tags (C-terminal). The day after injection, the oocytes were placed in a pH 8 solution (in mM, NaCl, 100; KCl, 3; MgCl2, 2; HEPES, 15; pH 8) that was changed daily. Two to three days later, currents were recorded in two-electrode voltage-clamp using a genclamp500 amplifier (AxonInst.,Foster City,CA) interfaced to a personal computer using the Digidata 1200 interface and the pClamp software (ver 7.0; Axon Inst.). Prior to recording, oocytes were incubated in phorbol myristate acetate (PMA) at 100 nM in the pH 8 solution for 20–30 min. Currents were filtered at 100 Hz and digitized at 0.5 kHz before storage and further analysis. During recording, oocytes were continuously superfused with the pH 8 solution. On TMEM176B-expressing oocytes, induction of an inward current was obtained by switching to a pH 5 solution (in mM NaCl, 100; KCl, 3; MgCl2, 2; MES, 15; pH 5).
Statistical analysis
Results were expressed as mean ± SD. Statistical significance was evaluated using a one-way analysis of variance test. p < 0.05 was considered significant. Kaplan–Meier tests were performed for survival analysis.
Results
Tolerogenic DCs prolong allograft survival in combination with anti-CD3 treatment
The main objective of this work was to determine the molecular mechanisms that allow ATDCs to prolong graft survival in a donor-specific manner. We used a mouse minor histocompatibility transplantation model in which male skin is grafted onto female recipients. We examined the effect of ATDC injection in recipient mice the day before transplantation. Injection of WT (female) ATDCs had no effect on graft survival as compared to untreated mice (Figure 1A). However, a synergistic effect was observed when female recipients were co-treated with WT ATDCs and peritransplant anti-CD3 antibody therapy. In fact, anti-CD3 antibody treatment alone prolonged skin graft survival, but its effect was significantly improved when associating ATDC injection the day before transplantation (Figure 1A). To understand the mechanism by which ATDC + anti-CD3 therapy prolongs allograft survival we carried out experiments aiming at tracking injected ATDCs in vivo. We analyzed the graft, the draining-lymph node and the spleen 7 and 15 days after transplantation. ATDCs were found in skin graft both at days 7 and 15 posttransplantation whereas they could be detected in the draining lymph node only at day 15 (Figure S1). ATDCs were not found in the spleen (data not shown). These observations strongly suggest that injected ATDCs uptake donor antigens in the graft and subsequently migrate to the draining lymph node.
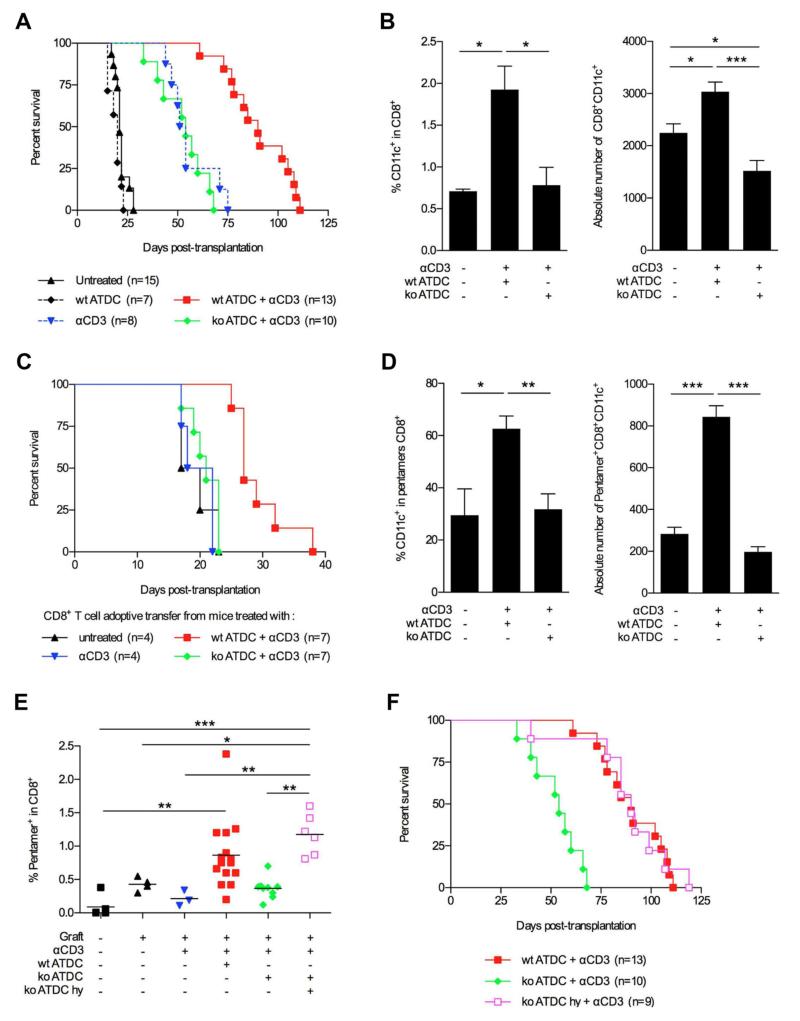
Figure 1. Tmem176b−/− (KO) ATDCs fail to prolong skin allograft survival in anti-CD3-treated mice.
(A) Female C57BL/6 recipients were grafted with skin from male C57BL/6 donors. Groups of mice underwent the indicated treatments detailed in the Materials and Methods section. Graft survival was monitored every other day. (B) At day 15 posttransplantation, the presence of CD8+CD11c+ regulatory T cells was determined by flow cytometry in the graft-draining lymph node of mice left untreated, treated with WT ATDCs anti-CD3 or Tmem176b−/− (KO) ATDCs + anti-CD3. n = 5 for each group. CD8+CD11c+ were defined as CD11cint to exclude CD11chi conventional DCs. Relative (percentage) and absolute numbers are shown.*p < 0.05. (C) Maleskin-graftedfemale recipients were left untreated or injected with WT ATDCs + anti-CD3, Tmem176b−/− (KO) ATDCs + anti-CD3 or anti-CD3 alone. At day 30 posttransplantation, CD8+ cells were purified from the graft-draining lymph node from the different groups and 2 × 105 cells were adoptively transferred into female recipients grafted with male skin, without any other treatment. Graft survival was monitored every other day. ***p < 0.001. (D) At day 15 posttransplantation, the relative (percentage) and absolute numbers of CD11cint cells among donor-specific CD8+ T cells from the graft-draining lymph node were determined by flow cytometry using Uty pentamers in the indicated groups. n = 5 for each group. *p < 0.05; **p < 0.01; ***p < 0.001. (E) At day 15 posttransplantation, graft-draining lymph nodes from mice undergoing the indicated treatments were recovered, and cell suspensions were stained to detect donor-specific CD8+ T cells using Uty pentamers.*p < 0.05; **p < 0.01; ***p < 0.001. (F) KO ATDCs were pulsed with a mix of Uty and Smcy peptides (15 μM) before injection and their effect on graft survival was compared to unpulsed WT or KO ATDCs in combination with anti-CD3 therapy. ATDCs, autologous tolerogenic dendritic cells; DCs, dendritic cells.
Tolerogenic DCs induce regulatory CD8+ T cells in a Tmem176b-dependent manner
We previously identified the gene Tmem176b (Torid) as overexpressed in tolerated allografts in the rat and preferentially associated with the immature state of DCs (12,13). We generated a Tmem176b-deficient mouse (Figure S2) that showed no obvious developmental abnormalities. Strikingly, when ATDCs were generated from Tmem176−/− (KO) mice, prolongation of skin allograft survival was completely lost (Figure 1A). We then reasoned that injected ATDCs could induce or activate donor-specific Tregs through donor antigen presentation in the graft-draining lymph node. We first quantified the presence of CD4+Foxp3+ T cells. No significant differences were observed between the different treatment groups in the graft-draining lymph nodes (Figure S3). It was reported that male skin graft survival can be prolonged by tolerizing donor-specific CD8+ T cells (22). We then studied different phenotypes associated to CD8+ Tregs. Neither CD8+Foxp3+ (23) nor CD8+CD28− (24) T cells were induced by ATDC + anti-CD3 treatment (Figure S4). However, CD8+CD11c+ T cells, a phenotype previously described in Tregs (25), were found to be significantly increased in the graft-draining lymph node of female recipients treated with WT ATDCs but not in KO ATDC-treated nor in untreated mice. This was true for the percentage and absolute numbers of CD8+CD11c+ T cells (Figure 1B). To functionally demonstrate that WT ATDCs + anti-CD3 treatment does indeed induce CD8+ Tregs, we realized adoptive cell transfer experiments into otherwise untreated females receiving male skin grafts (Figure 1C). Transfer of CD8+ T cells purified from the graft-draining lymph node of WT ATDC-treated mice significantly prolonged the graft survival compared to the ones purified from untreated rejecting mice or mice treated with anti-CD3 alone (p < 0.05). In contrast, CD8+ T cells from animals treated with KO ATDCs as well as the ones from mice treated only with anti-CD3 failed to prolong allograft survival. TMEM176B expression by ATDCs is therefore required for the induction of regulatory CD8+ T cells.
Male antigen cross-presentation by tolerogenic DCs is required for regulatory CD8+ T cell induction and allograft survival prolongation
To further elucidate the mechanisms by which ATDCs prolong allograft survival and induce CD8+CD11c+ Tregs in a Tmem176b-dependent manner, we examined whether Tregs were donor-specific. We first analyzed the presence of donor-specific CD8+ T cells in the graft-draining lymph node by using Uty-MHC class I and Smcy-MHC class I pentamers. Our results showed a significant increase of donor-specific CD8+CD11c+ T cells in WT ATDC-treated mice compared to untreated ones (p < 0.05) but not in Tmem176b−/− ATDC-treated recipients (Figure 1D). The fact that CD8+CD11c+ cells were stained with MHC pentamers strongly suggests that those cells are T cells and not conventional DCs. We then quantified the presence of donor-specific CD8+ T cells among total CD8+ T cells in the draining lymph node of animals undergoing different treatments (Figure 1E). WTATDCs + anti-CD3 treatment was associated with increased donor-specific CD8+ T cells as compared to naïive, untreated and anti-CD3 treated. In contrast, recipients treated with KO ATDCs + anti-CD3 were unable to expand donor-specific CD8+ T cells in the graft-draining lymph node.
We observed that WT and KO ATDCs both migrated to the graft-draining lymph node (Figure S1). Moreover, no phenotypic difference was observed between WT and KO ATDCs (Figure S5). We then hypothesized that Tmem176b−/− ATDCs fail to process donor antigens through the cross-presentation pathway. We speculated that loading Tmem176b−/− ATDCs with the already processed donor minimal peptides issued from Uty and Smcy proteins should rescue the generation of donor-specific CD8+Tcells. Remarkably, when KO ATDCs were loaded with Uty or Smcy peptides before injection, the numbers of donor-specific CD8+ T cells reached the levels observed in recipients treated with WT ATDCs (Figure 1E). Importantly, the injection of KO ATDCs loaded with Uty and Smcy peptides prolonged allograft survival in a similar manner to WT ATDCs (Figure 1F). The fact that the effect of Tmem176b-deficient ATDCs can be rescued by loading them with the minimal Uty and Smcy peptides strongly suggests that they fail to process and present donor antigens through the cross-presentation pathway.
Taken together, our results show that Tmem176b expression is required by ATDCs to prolong allograft survival by inducing CD8+ Tregs through a mechanism probably involving donor antigen cross-presentation.
Tmem176b is required for efficient antigen cross-presentation by DCs
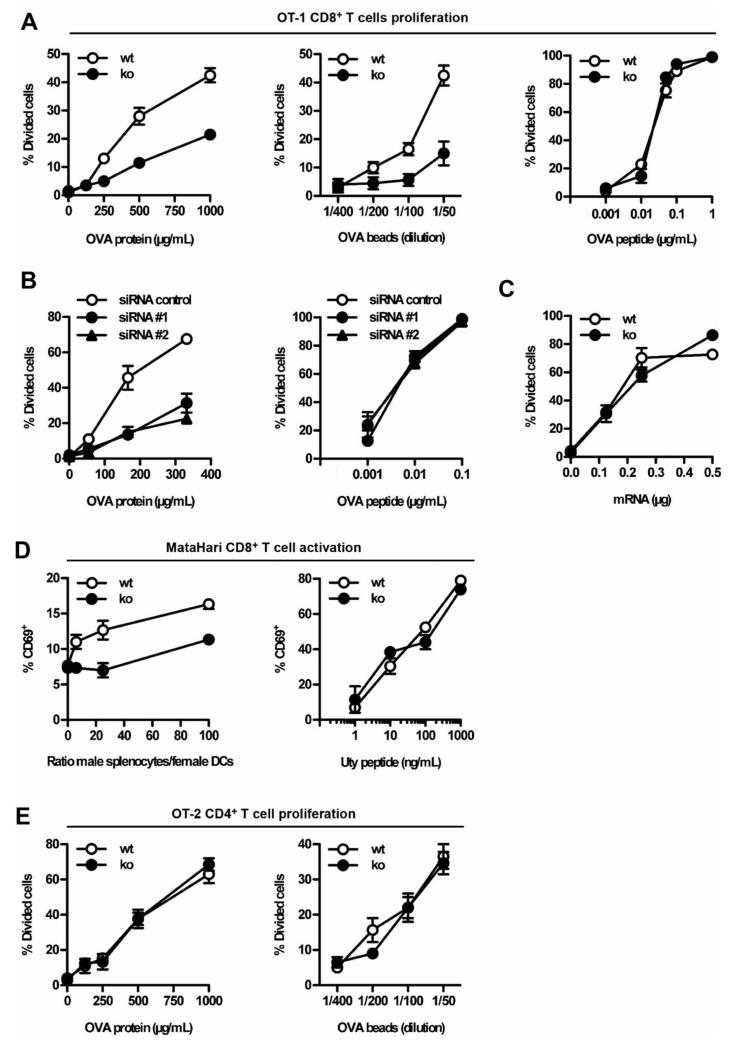
We then directly assessed whether KO ATDCs failed to cross-present exogenous antigens in vitro. Cross-presentation of both soluble OVA and OVA-coated latex beads to OVA-specific OT-1 CD8+ T cells was impaired in KO ATDCs compared to WT ATDCs (Figure 2A). However, KO and WT ATDCs were equally effective at stimulating the proliferation of OT-1 cells in the presence of the preprocessed, minimal peptide SIINFEKL (Figure 2A). Knock-down of Tmem176b expression in WT ATDCs using two different siRNAs (13) also resulted in reduced OVA cross-presentation (Figure 2B). Thus, TMEM176B is required for the cross-presentation of soluble and particulate OVA by ATDCs. When OVA was expressed endogenously after OVA mRNA electroporation of Tmem176b-sufficient or deficient ATDCs, the levels of presentation to OT-1 cells were similar, indicating that TMEM176B is not involved in the endogenous pathway of MHC class I-restricted antigen presentation (Figure 2C). We then determined whether TMEM176B could regulate the processing of other exogenous antigens. We reasoned that cross-presentation of male antigens from live cells may be a pertinent system to test in sight of our in vivo results described above. Female WT or KO ATDCs were incubated with live male β2-microglobulin−/− splenocytes and then co-cultured with MataHari transgenic CD8+ T cells, which are specific for the male antigen, Uty. KO ATDCs failed to cross-present the cell-associated male antigens as efficiently as the control cells (Figure 2D, left panel). In contrast, when the ATDCs were loaded with the minimal Uty peptide, WT and KO ATDCs exhibited identical capacities to stimulate antigen-specific CD8+ T cells (Figure 2D, right panel). Importantly, KO ATDCs were as efficient as WT ATDCs in stimulating the OVA-specific CD4+ OT-2 T cells (Figure 2E). Similar results were obtained using WT and KO splenic DCs ex vivo (Figure S6). Therefore, the absence of TMEM176B impairs the cross-presentation of two different model antigens but spares both the presentation of these antigens to CD4+ T cells and the endogenous presentation to CD8+ T cells.
Figure 2. Defective in vitro antigen cross-presentation by Tmem176b−/− (KO) ATDCs.
ATDCs from Tmem176b−/− (KO) mice and WT controls were examined in vitro to compare their capacities to present antigens to CD8+ and CD4+ T cells. (A) Soluble OVA (left panel), OVA-coated latex beads (center panel) and OVA-minimal peptide SIINFEKL (right panel) were used at different concentrations to treat WT (open symbols) and KO (full symbols) ATDCs. CD8+ T cell proliferation was assessed as described in the Materials and Methods section. One experiment representative of 10 is shown for both the OVA protein and the minimal peptide. n = 5 for the OVA-coated beads. (B) Similar experiments were performed using WT ATDCs treated with control siRNA (open symbols) or two different anti Tmem176b-specific siRNAs (filled symbols). The OVA protein-treated ATDCs are depicted in the left panel whereas the right panel shows proliferation upon treatment with the SIINFEKL peptide (n = 3). (C) To study OVA presentation when expressed as an endogenous antigen, mRNA coding for the GFP fused to the SIINFEKL peptide was electroporated into WT (open symbols) and KO (filled symbols) ATDCs (n = 2). (D) Left panel: Male β2-microglobulin−/− splenocytes were incubated for 2 h at 37°C with different ratios of adherent female WT or KO ATDCs. The splenocytes were extensively washed, and MataHari CD8+ T cells were then co-cultured with the ATDCs for 20 h. CD69 expression on CD8+ T cells was determined by flow cytometry. Right panel: WT or KO ATDCs were treated for 45 min at 37°C with different doses of the minimal Uty peptide. Upon extensive washing, MataHari CD8+ T cells were co-cultured with ATDCs for 20 h. CD69 expression on CD8+ T cells was determined by flow cytometry (n = 3). (E) Similar experiments to those described in (A) were performed using OT-2 CD4+ T cells (n = 3). ATDCs, autologous tolerogenic dendritic cells; GFP, green fluorescent protein; siRNA, small interfering RNA.
Taken together, our data indicate that Tmem176b is required to cross-present antigens to CD8+ T cells, whereas it is dispensable for presentation to CD4+ T cells through the MHC class II pathway.
TMEM176B is responsible for a cation conductance that regulates phagosomal pH in DCs
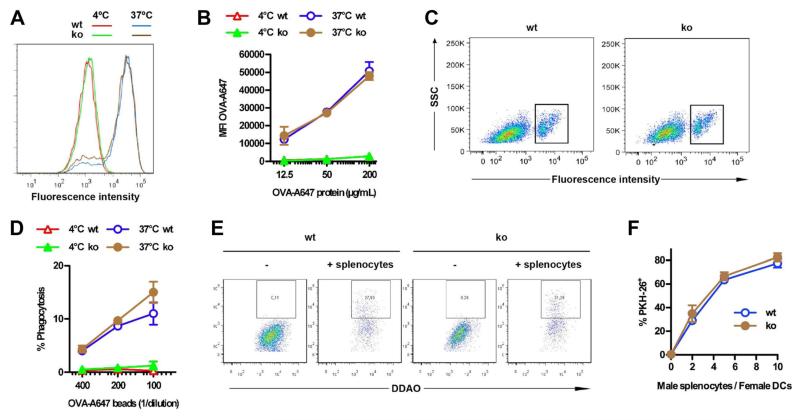
The observation that TMEM176B is involved in antigen processing in the cross-presentation pathway prompted us to interrogate the molecular mechanisms mediating this effect. Proteomic studies suggested a phagosomal localization for TMEM176B in macrophages (26,27). TMEM176B co-localized with soluble OVA in a population of scattered vesicular compartments (Figure S7A). In addition, TMEM176B expression was observed surrounding phagocytosed latex beads and in purified phagosomes (Figure S7B and data not shown), indicating that TMEM176B is indeed localized to the phagosomal membrane. To understand the mechanism by which TMEM176B could regulate antigen cross-presentation, we next investigated its function in endosomes and phagosomes. Internalization of soluble OVA (Figure 3A and B) and phagocytosis of fluorescent latex beads (Figure 3C and D) were not affected in KO ATDCs compared to WT counterparts. Similarly, internalization of antigenic material from live cells was not affected (Figure 3E and F). Thus, TMEM176B does not regulate antigen internalization.
Figure 3. Tmem176b deficiency does not alter antigen internalization by ATDCs.
(A,B) Soluble antigen endocytosis was compared between WT and Tmem176b−/− (KO) cells. ATDCs were pulsed for 15 min at 4°C or 37°C with OVA-Alexa 647. Sixty-minute chase was then performed before analyzing fluorescence intensity by flow cytometry. A representative histogram using 200 μg/mL OVA-Alexa 647 is shown (n = 3) in (A). Quantification ± SD is shown in (B). (C,D) Phagocytosis of particulate antigens was compared between WT and KO cells. ATDCs were pulsed for 15 min with fluorescent beads and then extensively washed and chased for 30 min. The percentage of ATDCs that internalized beads was quantified by flow cytometry. Representative dot plots of ATDCs treated with beads at 37°C are shown (n = 3) in (C). Quantification ± SD is shown in (D). (E,F) Male β2-microglobulin−/− splenocytes were labeled with PKH-26 and co-cultured at 37°C with DDAO-labeled WT or Tmem176b−/− ATDCs at different ratios. Upon a 2-h culture, the percentage of PKH-26+ cells among DDAO+ ATDCs was determined by flow cytometry. A representative experiment at a 2:1 ratio is shown in (E). Quantification ± SD is shown in (F). ATDCs, autologous tolerogenic dendritic cells.
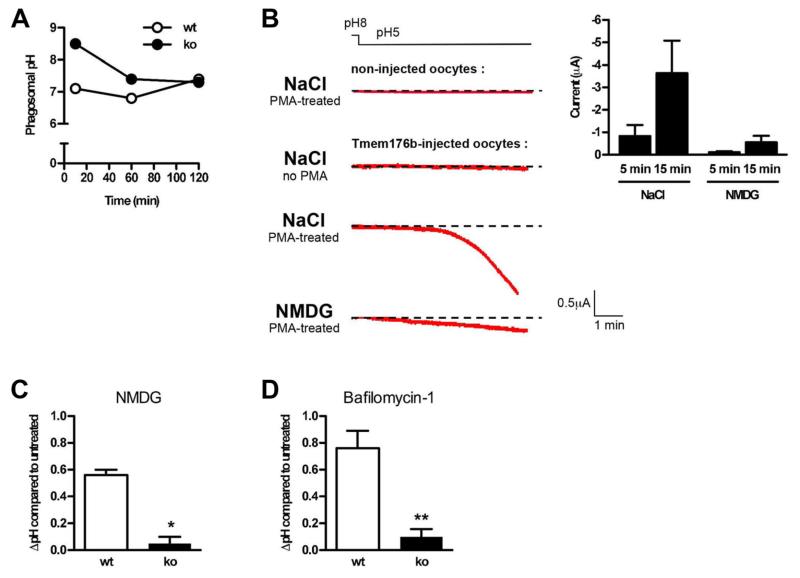
We then analyzed phagosomal pH, one of the upstream mechanisms that controls antigen processing at the phagosomal lumen. Indeed, acidification of the phagosomal lumen in DCs has been linked to excessive antigen degradation (14). Moreover, neutralization of the phagosomal lumen pH using chloroquine has been shown to promote antigen export to the cytosol and thus cross-presentation (28). We measured the phagosomal pH in WT and KO ATDCs. Consistent with our previous work (14,15), WT DCs displayed a near-neutral pH even 2 h after phagocytosis (Figure 4A). However, in clear contrast, we observed that KO ATDCs displayed a striking, although transient, alkalinized phagosomal pH. In fact, the pH values were at least 1.5 pH units higher in KO ATDCs compared to WT ATDCs during the first hour following phagocytosis. DCs are known to actively alkalinize phagosomes. The alkalinized phagosomal pH in KO ATDCs is therefore compatible with deficient acidification mechanisms.
Figure 4. TMEM176B is responsible for a cation conductance that regulates phagosomal pH in ATDCs.
(A) Phagosomal pH was measured in WT and Tmem176b−/− (KO) ATDCs at different time points using flow cytometry analysis as described in the Materials and Methods section (n = 5). (B) Xenopus oocytes were injected with Tmem176b mRNA, and currents were recorded in voltage-clamp 2–4 days later as detailed in the Materials and Methods section. Before recording, translocation of TMEM176B to the plasma membrane was induced by a 30-min treatment with PMA (100 nM). The left panel shows typical current recordings in extracellular buffers containing NaCl or NMDG. The right panel shows the means ± SD of the currents recorded in the presence of Na+ or NMDG. The currents were quantified 5–15 min after holding the extracellular pH at 5 (n = 5–7). (C) Phagosomal pH was measured by flow cytometry in WT and KO ATDCs. Na+ was substituted with NMDG in the extracellular buffer. The Na+ buffer contained 140 mM NaCl, 5 mM KCl, 2 mM CaCl2, 1.2 mM MgCl2, 3 mM glucose, and 10 mM HEPES, pH 7.4. In the NMDG buffer, 140 mM NMDG was substituted for Na+. WT but not KO DCs exhibited a pH increase. (D) In phagosomal pH experiments, ATDCs were treated with the V-ATPase inhibitor, bafilomycin-1 (400 ng/mL). The phagosomal pH increased in WT but not in KO DCs. ATDCs, autologous tolerogenic dendritic cells; DCs, dendritic cells; PMA, phorbol myristate acetate; V-ATPase, vacuolar ATPase.
It has been suggested that TMEM176B could be involved in ion flux (29,30). In this regard, we and others have previously shown that Tmem176b presumably shares a common ancestral gene with the MS4A gene family that includes CD20, a B cell specific protein proposed to function as a store-operated calcium channel (12,31). We directly tested the hypothesis that TMEM176B could function as an ion channel. We expressed TMEM176B in Xenopus oocytes and recorded the electric activity under whole-cell patch clamp. Because TMEM176B is localized in intracellular compartments, surface expression was achieved by a 20−30 min pretreatment with PMA (data not shown) as described for other ion channels (32). This treatment resulted in the development of an inward current at a holding potential of −40 mV that was activated by acidification of the extracellular solution to pH 5 (Figure 4B). Further analysis revealed that TMEM176B forms a nonselective monovalent cation channel because the current was abolished by substitution of extracellular Na+ with NMDG (N-methyl-D-glucamine) but not K+ and was not affected by substitution of intracellular Cl− with gluconate. This conductance was not blocked by Cd2+ (0.5 mM), La3+ (1 mM), Gd3+ (1 mM), nifedipine (10 μM), niflumic acid (100 mM) or amiloride (20 μM). Additionally, this conductance could not be activated directly by intracellular acidification because injection of methane sulfonic acid (40 nL, 150 mM) into the oocyte had no effect.
Our electrophysiological results define TMEM176B as an acid-sensitive, nonselective cation channel. Because the phagosomes from KO ATDCs showed excessive alkalinization, we questioned whether TMEM176B might provide a phagosomal Na+ efflux conductance that permits greater proton pumping by the vacuolar ATPase (V-ATPase) through charge compensation. Consistent with this hypothesis, removal of Na+ from the phagosomal lumen by incubation of cells in extracellular NMDG solution during phagocytosis led to the alkalinization of WT but not KO phagosomes (Figure 4C). In these experiments, the mean fluorescence intensity of FITC was not saturated at the pH observed in the KO phagosomes, ruling out a lack of alkalinization due to technical reasons. Furthermore, KO phagosomes had profoundly impaired phagosomal V-ATPase activity, which was assessed by measuring luminal pH changes following bafilomycin treatment. Inhibition of the V-ATPase increased the pH of WT but not KO ATDC phagosomes (Figure 4D). This result suggested that the cationic conductance mediated by TMEM176B promotes V-ATPase activity and phagosomal acidification in DCs.
Discussion
The injection of donor-derived tolerogenic DCs before transplantation has been shown to prolong allograft survival (2,33). However, Divito et al (34) elegantly showed that endogenous DCs mediate this effect, presenting alloantigens on self MHCs. Injecting autologous DCs appears as a different approach and a clinically innovative and feasible strategy to control pathogenic immune responses. In humans, a pioneering study by Dhodapkar et al (35) demonstrated the feasibility and safety of injecting ATDCs in healthy volunteers. Injection of DCs was associated with antigen-specific inhibition of effector T cell function and induction of antigen-specific CD8+ Tregs in vivo (35,36). We are currently developing a protocol to inject ATDCs into kidney graft recipients in a phase I/II clinical trial (The ONE Study, 7th Frame Program, European Commission). We have shown that human monocyte-derived DCs do express TMEM176B. Cross-presenting human DCs such as blood BDCA3+ DCs and tonsil-resident BDCA1+ DCs, BDCA3+ DCs and pDCs may express this cation channel (37).
Humanized anti-CD3 antibodies are undergoing clinical evaluation in the field of autoimmunity with encouraging results (38). In mice, anti-CD3 treatment induces apoptosis of activated T cells. Phagocytosis of apoptotic cells by macrophages and immature DCs triggers TGF-β production, leading to induction of CD4+Foxp3+ Tregs (39). In type 1 diabetes patients undergoing anti-CD3 treatment, increased CD8+CD25+FOXP3+ Tregs were associated with clinical response (40). We have shown in the present study that CD8+ cells purified from grafted mice treated only with anti-CD3 antibodies were not able to prolong allograft survival when transferred into grafted mice. Thus, in agreement with the pentamer staining experiments, anti-CD3 treatment alone is not able to trigger CD8+ Tregs. Moreover, we studied whether ATDC injection alone could generate Tregs. We observed that ATDC injection alone failed to accumulate donor-specific CD8+ T cells (n = 3, data not shown). These results strongly suggest that ATDC and anti-CD3 injections synergize to generate Tregs. We propose that T cell death might create a tolerogenic environment allowing ATDCs to generate Tregs in this model and prolong allograft survival.
Herein, we showed that injected ATDCs need to cross-present donor antigens in a Tmem176b-dependent manner to generate CD8+ Tregs and prolong allograft survival in the mouse. These observations may explain the challenging observation that autologous (not donor-derived) DCs prolong allograft survival in a donor-specific manner (3). These observations may have practical consequences when setting up clinical protocols. For example, if ATDCs are to be used with calcineurin inhibitors, specific precautions should be taken since several of these drugs are known to interfere with antigen processing by DCs (41).
Although cross-presentation triggers immunity, in some circumstances cross-presentation can lead to immunological tolerance. Abortive proliferation of CD8+ T cells is one of the best studied mechanisms by which cross-presentation can regulate immune responses (42). A much less characterized mechanism is the possibility that cross-presentation may lead to generation of CD8+ Tregs. We have previously suggested that rat plasmacytoid DCs may generate CD8+ Tregs through cross-presentation in a transplantation model (43). Herein, we show novel evidence supporting this concept. We performed cell transfer experiments to determine whether the donor-specific CD8+ T cells have regulatory properties. Due to the low percentage of donor-specific cells in the graft-draining lymph node and the total amount of cells recovered, we did not succeed in purifying sufficient numbers of donor-specific CD8+ T cells or CD8+CD11c+ T cells to be used in adoptive transfer experiments. However, we believe that our results strongly support the interpretation that donor-specific CD8+CD11c+ T cells are indeed Tregs and not effectors. In fact, adoptive transfer of total CD8+ cells from WT ATDC-treated recipients but not from untreated ones or from Tmem176b−/−-treated ATDC mice significantly prolonged skin allograft survival. The mechanisms by which donor-specific CD8+CD11c+ Tregs impair graft rejection deserve a deep characterization, which is beyond the scope of this study. CD8+CD11c+ Tregs have been previously characterized in other models. Indeed, Seo et al (25) have shown that injection of anti-4-1BB (CD137) antibodies suppressed collagen-induced arthritis in mice by expanding CD8+CD11c+ Tregs.
We have recently shown that phagosomal pH is actively regulated in DCs and impacts antigen cross-presentation (14-16,18-20). In DCs, two multiprotein complexes tightly regulate the phagosomal pH. The NADPH-oxidase 2 promotes alkalinization, whereas the proton pump, V-ATPase, acidifies the phagosomal lumen (14-16,18-20). Proton pumping by V-ATPase generates a voltage across the membrane of the phagosome, which is intraluminally positive, antagonizing further inward transport of proton equivalents (44-47). Therefore, neutralizing counterion conductances are needed to prevent the generation of charge imbalances across the phagosomal membrane and to allow vesicular acidification. However, the molecular identities of these neutralizing counterion conductances remain unknown.
Intracellular ion metabolism has been linked with important biological processes in DCs such as migration (48) and inflammasome activation (49-51). To the best of our knowledge, this is the first time that intracellular ions are suggested to play a role in antigen processing by DCs. The acid-activated cation channel function described here for TMEM176B provides a mechanistic explanation for the transiently alkalinized phagosomal pH observed in KO DCs. This evidence is consistent with a role for TMEM176B as a cation channel responsible for the counterion conductances involved in regulating phagosomal pH. Notably, counterion conductances are postulated to regulate the rate, rather than the extent, at which vesicular organelles are acidified.
Casey et al proposed that given sufficient time, even small conductances can cause V-ATPases to reach the maximal theoretical pH gradient (47). According to this suggestion, the presence of anionic counterionic conductances, such as chloride channel transporters present in KO phagosomes, may explain why the phagosomes were able to achieve normal pH values at late time points.
In conclusion, here we characterized cellular and molecular mechanisms required by a clinically relevant therapeutic approach. TMEM176B arises as a novel partner for tolerogenic DCs. Its function in controlling ionic homeostasis may be relevant to understand the role played by this protein in other cells.
Supplementary Material
Acknowledgments
This work was funded by Fondation Progreffe, Vaincre la Mucoviscidose and IMBIO. MH was supported by a Junior Basic Science grant from ESOT. We are grateful to Philippe Hulin from the IFR26 MicroPIcell imagery core facility Nantes, France, for excellent assistance with confocal microscopy, and to Claire Usal and Emmanuel Merieau for their great help in mouse experimentation. Tmem176b−/− (KO) mice were generated by “La Clinique de la souris,” Strasbourg, France. Jeffrey Bluestone and Giovanna Lombardi critically read the manuscript.
Abbreviations
- ATDC
autologous tolerogenic dendritic cell
- BMDCs
bone marrow dendritic cells
- DCs
dendritic cells
- FITC
fluorescein isothiocyanate
- GFP
green fluorescent protein
- PMA
phorbol myristate acetate
- Treg
regulatory T cell
- V-ATPase
vacuolar ATPase
Footnotes
Additional Supporting Information may be found in the online version of this article.
Disclosure
The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
References
- 1.Bluestone JA, Thomson AW, Shevach EM, Weiner HL. What does the future hold for cell-based tolerogenic therapy? Nat Rev Immunol. 2007;7:650–654. doi: 10.1038/nri2137. [DOI] [PubMed] [Google Scholar]
- 2.Morelli AE, Thomson AW. Tolerogenic dendritic cells and the quest for transplant tolerance. Nat Rev Immunol. 2007;7:610–621. doi: 10.1038/nri2132. [DOI] [PubMed] [Google Scholar]
- 3.Beriou G, Peche H, Guillonneau C, Merieau E, Cuturi MC. Donor-specific allograft tolerance by administration of recipient-derived immature dendritic cells and suboptimal immunosuppression. Transplantation. 2005;79:969–972. doi: 10.1097/01.tp.0000158277.50073.35. [DOI] [PubMed] [Google Scholar]
- 4.Peche H, Trinite B, Martinet B, Cuturi MC. Prolongation of heart allograft survival by immature dendritic cells generated from recipient type bone marrow progenitors. Am J Transplant. 2005;5:255–267. doi: 10.1111/j.1600-6143.2004.00683.x. [DOI] [PubMed] [Google Scholar]
- 5.Moreau A, Hill M, Thebault P, et al. Tolerogenic dendritic cells actively inhibit T cells through heme oxygenase-1 in rodents and in nonhuman primates. FASEB J. 2009;23:3070–3077. doi: 10.1096/fj.08-128173. [DOI] [PubMed] [Google Scholar]
- 6.Segovia M, Cuturi MC, Hill M. Preparation of mouse bone marrow-derived dendritic cells with immunoregulatory properties. Methods Mol Biol. 2011;677:161–168. doi: 10.1007/978-1-60761-869-0_11. [DOI] [PubMed] [Google Scholar]
- 7.Hill M, Cuturi MC. Negative vaccination by tolerogenic dendritic cells in organ transplantation. Curr Opin Organ Transplant. 2010;15:738–743. doi: 10.1097/MOT.0b013e32833f7114. [DOI] [PubMed] [Google Scholar]
- 8.Hill M, Thebault P, Segovia M, et al. Cell therapy with autologous tolerogenic dendritic cells induces allograft tolerance through interferon-gamma and Epstein–Barr virus-induced gene 3. Am J Transplant. 2011;11:2036–2045. doi: 10.1111/j.1600-6143.2011.03651.x. [DOI] [PubMed] [Google Scholar]
- 9.Beriou G, Moreau A, Cuturi MC. Tolerogenic dendritic cells: Applications for solid organ transplantation. Curr Opin Organ Transplant. 2012;17:42–47. doi: 10.1097/MOT.0b013e32834ee662. [DOI] [PubMed] [Google Scholar]
- 10.Giannoukakis N, Phillips B, Finegold D, Harnaha J, Trucco M. Phase I (safety) study of autologous tolerogenic dendritic cells in type 1 diabetic patients. Diabetes Care. 2011;34:2026–2032. doi: 10.2337/dc11-0472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moreau A, Varey E, Beriou G, et al. Tolerogenic dendritic cells and negative vaccination in transplantation: From rodents to clinical trials. Front Immunol. 2012;3:218. doi: 10.3389/fimmu.2012.00218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Louvet C, Chiffoleau E, Heslan M, et al. Identification of a new member of the CD20/FcepsilonRIbeta family overexpressed in tolerated allografts. Am J Transplant. 2005;5:2143–2153. doi: 10.1111/j.1600-6143.2005.01007.x. [DOI] [PubMed] [Google Scholar]
- 13.Condamine T, Le Texier L, Howie D, et al. Tmem176B and Tmem176A are associated with the immature state of dendritic cells. J Leukoc Biol. 2010;88:507–515. doi: 10.1189/jlb.1109738. [DOI] [PubMed] [Google Scholar]
- 14.Savina A, Jancic C, Hugues S, et al. NOX2 controls phagosomal pH to regulate antigen processing during crosspresentation by dendritic cells. Cell. 2006;126:205–218. doi: 10.1016/j.cell.2006.05.035. [DOI] [PubMed] [Google Scholar]
- 15.Jancic C, Savina A, Wasmeier C, et al. Rab27a regulates phagosomal pH and NADPH oxidase recruitment to dendritic cell phagosomes. Nat Cell Biol. 2007;9:367–378. doi: 10.1038/ncb1552. [DOI] [PubMed] [Google Scholar]
- 16.Mantegazza AR, Savina A, Vermeulen M, et al. NADPH oxidase controls phagosomal pH and antigen cross-presentation in human dendritic cells. Blood. 2008;112:4712–4722. doi: 10.1182/blood-2008-01-134791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Joffre OP, Segura E, Savina A, Amigorena S. Cross-presentation by dendritic cells. Nat Rev Immunol. 2012;12:557–569. doi: 10.1038/nri3254. [DOI] [PubMed] [Google Scholar]
- 18.Savina A, Peres A, Cebrian I, et al. The small GTPase Rac2 controls phagosomal alkalinization and antigen crosspresentation selectively in CD8(+) dendritic cells. Immunity. 2009;30:544–555. doi: 10.1016/j.immuni.2009.01.013. [DOI] [PubMed] [Google Scholar]
- 19.Savina A, Amigorena S. Phagocytosis and antigen presentation in dendritic cells. Immunol Rev. 2007;219:143–156. doi: 10.1111/j.1600-065X.2007.00552.x. [DOI] [PubMed] [Google Scholar]
- 20.Amigorena S, Savina A. Intracellular mechanisms of antigen cross presentation in dendritic cells. Curr Opin Immunol. 2010;22:109–117. doi: 10.1016/j.coi.2010.01.022. [DOI] [PubMed] [Google Scholar]
- 21.De Wilde V, Van Rompaey N, Hill M, et al. Endotoxin-induced myeloid-derived suppressor cells inhibit alloimmune responses via heme oxygenase-1. Am J Transplant. 2009;9:2034–2047. doi: 10.1111/j.1600-6143.2009.02757.x. [DOI] [PubMed] [Google Scholar]
- 22.James E, Scott D, Chai JG, Millrain M, Chandler P, Simpson E. HY peptides modulate transplantation responses to skin allografts. Int Immunol. 2002;14:1333–1342. doi: 10.1093/intimm/dxf093. [DOI] [PubMed] [Google Scholar]
- 23.Bienvenu B, Martin B, Auffray C, Cordier C, Becourt C, Lucas B. Peripheral CD8+CD25+ T lymphocytes from MHC class II-deficient mice exhibit regulatory activity. J Immunol. 2005;175:246–253. doi: 10.4049/jimmunol.175.1.246. [DOI] [PubMed] [Google Scholar]
- 24.Najafian N, Chitnis T, Salama AD, et al. Regulatory functions of CD8+CD28− T cells in an autoimmune disease model. J Clin Invest. 2003;112:1037–1048. doi: 10.1172/JCI17935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Seo SK, Choi JH, Kim YH, et al. 4-1BB-mediated immunotherapy of rheumatoid arthritis. Nat Med. 2004;10:1088–1094. doi: 10.1038/nm1107. [DOI] [PubMed] [Google Scholar]
- 26.Shui W, Sheu L, Liu J, et al. Membrane proteomics of phagosomes suggests a connection to autophagy. Proc Natl Acad Sci USA. 2008;105:16952–16957. doi: 10.1073/pnas.0809218105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Trost M, English L, Lemieux S, Courcelles M, Desjardins M, Thibault P. The phagosomal proteome in interferon-gamma-activated macrophages. Immunity. 2009;30:143–154. doi: 10.1016/j.immuni.2008.11.006. [DOI] [PubMed] [Google Scholar]
- 28.Accapezzato D, Visco V, Francavilla V, et al. Chloroquine enhances human CD8+ T cell responses against soluble antigens in vivo. J Exp Med. 2005;202:817–828. doi: 10.1084/jem.20051106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Maeda Y, Fujimura L, O-Wang J, et al. Role of Clast1 in development of cerebellar granule cells. Brain Res. 2006;1104:18–26. doi: 10.1016/j.brainres.2006.05.068. [DOI] [PubMed] [Google Scholar]
- 30.Pfeufer A, Sanna S, Arking DE, et al. Common variants at ten loci modulate the QT interval duration in the QTSCD Study. Nat Genet. 2009;41:407–414. doi: 10.1038/ng.362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zuccolo J, Bau J, Childs SJ, Goss GG, Sensen CW, Deans JP. Phylogenetic analysis of the MS4A and TMEM176 gene families. PLoS ONE. 2010;5:e9369. doi: 10.1371/journal.pone.0009369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lan JY, Skeberdis VA, Jover T, et al. Protein kinase C modulates NMDA receptor trafficking and gating. Nat Neurosci. 2001;4:382–390. doi: 10.1038/86028. [DOI] [PubMed] [Google Scholar]
- 33.Lutz MB, Suri RM, Niimi M, et al. Immature dendritic cells generated with low doses of GM-CSF in the absence of IL-4 are maturation resistant and prolong allograft survival in vivo. Eur J Immunol. 2000;30:1813–1822. doi: 10.1002/1521-4141(200007)30:7<1813::AID-IMMU1813>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 34.Divito SJ, Wang Z, Shufesky WJ, et al. Endogenous dendritic cells mediate the effects of intravenously injected therapeutic immunosuppressive dendritic cells in transplantation. Blood. 2010;116:2694–2705. doi: 10.1182/blood-2009-10-251058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dhodapkar MV, Steinman RM, Krasovsky J, Munz C, Bhardwaj N. Antigen-specific inhibition of effector T cell function in humans after injection of immature dendritic cells. J Exp Med. 2001;193:233–238. doi: 10.1084/jem.193.2.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dhodapkar MV, Steinman RM. Antigen-bearing immature dendritic cells induce peptide-specific CD8(+) regulatory T cells in vivo in humans. Blood. 2002;100:174–177. doi: 10.1182/blood.v100.1.174. [DOI] [PubMed] [Google Scholar]
- 37.Segura E, Touzot M, Bohineust A, et al. Human inflammatory dendritic cells induce Th17 cell differentiation. Immunity. 2013;38:336–348. doi: 10.1016/j.immuni.2012.10.018. [DOI] [PubMed] [Google Scholar]
- 38.You S, Chatenoud L. New generation CD3 monoclonal antibodies: Are we ready to have them back in clinical transplantation? Curr Opin Organ Transplant. 2010;15:720–724. doi: 10.1097/MOT.0b013e3283402bd8. [DOI] [PubMed] [Google Scholar]
- 39.Perruche S, Zhang P, Liu Y, Saas P, Bluestone JA, Chen W. CD3-specific antibody-induced immune tolerance involves transforming growth factor-beta from phagocytes digesting apoptotic T cells. Nat Med. 2008;14:528–535. doi: 10.1038/nm1749. [DOI] [PubMed] [Google Scholar]
- 40.Bisikirska B, Colgan J, Luban J, Bluestone JA, Herold KC. TCR stimulation with modified anti-CD3 mAb expands CD8+ T cell population and induces CD8+CD25+ Tregs. J Clin Invest. 2005;115:2904–2913. doi: 10.1172/JCI23961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lee YR, Yang IH, Lee YH, et al. Cyclosporin A and tacrolimus, but not rapamycin, inhibit MHC-restricted antigen presentation pathways in dendritic cells. Blood. 2005;105:3951–3955. doi: 10.1182/blood-2004-10-3927. [DOI] [PubMed] [Google Scholar]
- 42.Albert ML, Jegathesan M, Darnell RB. Dendritic cell maturation is required for the cross-tolerization of CD8+ T cells. Nat Immunol. 2001;2:1010–1017. doi: 10.1038/ni722. [DOI] [PubMed] [Google Scholar]
- 43.Li XL, Menoret S, Bezie S, et al. Mechanism and localization of CD8 regulatory T cells in a heart transplant model of tolerance. J Immunol. 2010;185:823–833. doi: 10.4049/jimmunol.1000120. [DOI] [PubMed] [Google Scholar]
- 44.Fuchs R, Male P, Mellman I. Acidification and ion permeabilities of highly purified rat liver endosomes. J Biol Chem. 1989;264:2212–2220. [PubMed] [Google Scholar]
- 45.Lukacs GL, Rotstein OD, Grinstein S. Determinants of the phagosomal pH in macrophages. In situ assessment of vacuolar H(+)-ATPase activity, counterion conductance, and H+ “leak”. J Biol Chem. 1991;266:24540–24548. [PubMed] [Google Scholar]
- 46.Steinberg BE, Touret N, Vargas-Caballero M, Grinstein S. In situ measurement of the electrical potential across the phagosomal membrane using FRET and its contribution to the proton-motive force. Proc Natl Acad Sci USA. 2007;104:9523–9528. doi: 10.1073/pnas.0700783104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Casey JR, Grinstein S, Orlowski J. Sensors and regulators of intracellular pH. Nat Rev Mol Cell Biol. 2010;11:50–61. doi: 10.1038/nrm2820. [DOI] [PubMed] [Google Scholar]
- 48.Barbet G, Demion M, Moura IC, et al. The calcium-activated nonselective cation channel TRPM4 is essential for the migration but not the maturation of dendritic cells. Nat Immunol. 2008;9:1148–1156. doi: 10.1038/ni.1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mariathasan S, Weiss DS, Newton K, et al. Cryopyrin activates the inflammasome in response to toxins and ATP. Nature. 2006;440:228–232. doi: 10.1038/nature04515. [DOI] [PubMed] [Google Scholar]
- 50.Compan V, Baroja-Mazo A, Lopez-Castejon G, et al. Cell volume regulation modulates NLRP3 inflammasome activation. Immunity. 2012;37:487–500. doi: 10.1016/j.immuni.2012.06.013. [DOI] [PubMed] [Google Scholar]
- 51.Latz E, Xiao TS, Stutz A. Activation and regulation of the inflammasomes. Nat Rev Immunol. 2013;13:397–411. doi: 10.1038/nri3452. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.